The Role of Toll-like Receptors and Viral Infections in the Pathogenesis and Progression of Pulmonary Arterial Hypertension—A Narrative Review
Abstract
1. Introduction
| TLR | PAMP | DAMP |
|---|---|---|
| TLR1/2 | Triacylated bacterial lipopeptides (e.g., Pam3CSK4; TLR1/2 heterodimer). | No specific, reliable DAMPs for TLR1 itself; DAMP signaling usually via TLR2-dependent heterodimers. |
| TLR2 | Gram(+) bacteria: lipopeptides, lipoteichoic acid, peptidoglycan; zymosan (fungi); often as TLR2/6. | HMGB1, hyaluronan fragments, biglycan, HSP; |
| TLR3 | dsRNA viral; agonist: poly(I:C). | Endogenous dsRNA/exosomal RNA from necrotic cancer cells/tissues. |
| TLR4 | LPS (Gram−); classic endotoxin receptor. | HMGB1, S100A8/A9, heme, ECM fragments (e.g., hyaluronan), and fibronectin-EDA are the TLRs with the most significant number of proposed DAMPs (some of which are still under discussion). |
| TLR5 | Bacterial flagellin. | There are no widely accepted endogenous DAMPs for humans. |
| TLR6/2 | Diacylated bacterial lipopeptides (e.g., Pam2CSK4; TLR2/6 heterodimer). | As for TLR2, HMGB1/ECM/HSP has been reported, but data for TLR6 alone are sparse. |
| TLR7 | viral ssRNA (GU-rich); agonists: imidazoquinolines. | Endogenous miRNAs (e.g., miR-21, let-7, miR-154-5p); RNA–LL37 complexes facilitating self-RNA recognition. |
| TLR8 | Viral ssRNA; R848 and other small molecules. | Extracellular miRNAs (e.g., miR-21/miR-29a in tumor exosomes); RNA–LL37 complexes activating TLR8 in neutrophils. |
| TLR9 | Unmethylated CpG bacterial/viral DNA; agonist: CpG ODN. | mtDNA (also oxidized) and DNA–LL37 complexes facilitating the recognition of one’s own DNA. |
| TLR10 | Function/ligands remain ambiguous; lipopeptides are suggested (often as TLR2/10); the role is more likely to be modulatory/inhibitory. | No reliably confirmed DAMPs; receptor still considered “orphan”. |
2. The Role of Toll-like Receptors in the Pathogenesis and Progression of Pulmonary Arterial Hypertension
2.1. TLR1 and TLR2
2.2. TLR3
2.3. TLR4
2.4. TLR5 and TLR6
2.5. TLR7 and TLR8
2.6. TLR9
2.7. TLR10
3. The Role of Viral Infections in the Development and Progression of Pulmonary Arterial Hypertension
3.1. Epstein–Barr Virus (EBV) Infection
- Identification of target cells and TLR pathways activated by EBV in the lung.
- Validation of biomarkers, such as plasma EBV DNA and IL-6/TGF-β signatures.
- Assessment of the therapeutic potential of strategies combining antiviral treatment with modulation of the innate immune response and blocking the pro-remodeling cytokine and factor axes.
3.2. Human Immunodeficiency Virus (HIV) Infection
3.3. Hepatitis C Virus (HCV) Infection
3.4. Human Endogenous Retrovirus K (HERV-K) Infection
3.5. SARS-CoV-2 Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Expansion |
| ACE2 | angiotensin-converting enzyme 2 |
| ANA | antinuclear antibodies |
| AP-1 | Activator Protein-1 |
| ARDS | acute respiratory distress syndrome |
| BMPR2 | bone morphogenetic protein receptor type 2 |
| CD281–CD290 | CD designations corresponding to TLR1–TLR10 |
| CNS | central nervous system |
| COX-2 | cyclooxygenase-2 |
| CpG DNA | unmethylated CpG motif–containing DNA |
| CpG ODN | CpG oligodeoxynucleotide |
| CRRT | continuous renal replacement therapy |
| CTEPH | chronic thromboembolic pulmonary hypertension |
| DAA | direct-acting antiviral |
| DAMP | damage-associated molecular pattern |
| dsDNA | double-stranded DNA |
| dsRNA | double-stranded RNA |
| EBV | Epstein–Barr virus |
| EC | endothelial cell(s) |
| ECM | extracellular matrix |
| ECMO | extracorporeal membrane oxygenation |
| E6446 | selective TLR9 antagonist |
| EndMT | endothelial-to-mesenchymal transition |
| ET-1 | endothelin-1 |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
| gp120 | HIV-1 envelope glycoprotein 120 |
| gp96 | endoplasmin/GRP94 (heat-shock protein HSP90B1) |
| HAART | highly active antiretroviral therapy |
| Hb | hemoglobin |
| HCC | hepatocellular carcinoma |
| HCV | hepatitis C virus |
| HERV-K | human endogenous retrovirus K |
| HIF-1α | hypoxia-inducible factor-1 alpha |
| HIV-1 | human immunodeficiency virus type 1 |
| HMGB1 | high-mobility group box 1 |
| HSP | heat-shock protein |
| Hsp70 | heat-shock protein 70 |
| ICAM-1 | intercellular adhesion molecule-1 |
| Id1 | inhibitor of DNA binding 1 |
| IFN | interferon (general) |
| IFN-I | type I interferons |
| IFN-α | interferon-alpha |
| IFN-β | interferon-beta |
| IFN-γ | interferon-gamma |
| IFNA1 | interferon alpha 1 gene |
| IFNB1 | interferon beta 1 gene |
| IL-1 | interleukin-1 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| IRAK | interleukin-1 receptor–associated kinase |
| IRF3 | interferon regulatory factor 3 |
| LDH | lactate dehydrogenase |
| LL-37 | human cathelicidin antimicrobial peptide |
| LPS | lipopolysaccharide |
| MALP-2 | macrophage-activating lipopeptide-2 (TLR2/6 agonist) |
| miRNA | microRNA |
| MRI | magnetic resonance imaging |
| mtDNA | mitochondrial DNA |
| MyD88 | myeloid differentiation primary response 88 |
| NOX (NADPH oxidase) | nicotinamide adenine dinucleotide phosphate oxidase system |
| NETs | neutrophil extracellular traps |
| NF-κB | nuclear factor kappa-B |
| NLR | NOD-like receptor |
| NO | nitric oxide |
| Nox1 | NOX family isoform 1 |
| Nox4 | NOX family isoform 4 |
| NS3 | nonstructural protein 3 (HCV) |
| NS5A | nonstructural protein 5A (HCV) |
| NS5B | nonstructural protein 5B (HCV) |
| Nef | HIV-1 negative factor protein |
| PAMP | pathogen-associated molecular pattern |
| PAEC | pulmonary artery endothelial cell(s) |
| PAH | pulmonary arterial hypertension |
| PASMC | pulmonary arterial smooth muscle cell(s) |
| PDE-5 | phosphodiesterase type 5 |
| PDGF | platelet-derived growth factor |
| PGE2 | prostaglandin E2 |
| PH | pulmonary hypertension |
| poly(I:C) | polyinosinic:polycytidylic acid (synthetic dsRNA; TLR3 agonist) |
| p53 | tumor protein p53 |
| pDC | plasmacytoid dendritic cell(s) |
| RAAS | renin–angiotensin–aldosterone system |
| R848 | resiquimod (TLR7/8 agonist) |
| R840 | imidazoquinoline TLR7/8 agonist (experimental) |
| RANTES (CCL5) | Regulated upon Activation, Normal T-cell Expressed and Secreted |
| RIPK3 | receptor-interacting protein kinase 3 |
| RNA-seq | RNA sequencing |
| ROS | reactive oxygen species |
| RV | right ventricle |
| RVEF | right ventricular ejection fraction |
| RVSP | right ventricular systolic pressure |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SLE | systemic lupus erythematosus |
| Smad1/5/8 | SMAD proteins downstream of BMP/TGF-β signaling |
| SMC | smooth muscle cell(s) |
| ssRNA | single-stranded RNA |
| STAT3 | signal transducer and activator of transcription 3 |
| SSc | systemic sclerosis |
| SU5416 | VEGFR inhibitor used to induce experimental PAH |
| Tat | HIV trans-activator of transcription protein |
| TGF-β | transforming growth factor-beta |
| Th17 | T helper 17 cells |
| TIR | Toll/Interleukin-1 receptor (domain) |
| TLR | Toll-like receptor(s) |
| TMAO | trimethylamine-N-oxide |
| TNF | tumor necrosis factor |
| TNF-α | tumor necrosis factor-alpha |
| TPVR | total pulmonary vascular resistance (index) |
| TRIF | TIR-domain–containing adapter-inducing interferon-β |
| Treg | regulatory T cell(s) |
| TXA2 | thromboxane A2 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| VEGF | vascular endothelial growth factor |
| VEGFR | vascular endothelial growth factor receptor |
| WHO | World Health Organization |
| mPAP | mean pulmonary arterial pressure |
References
- Montani, D.; Günther, S.; Dorfmüller, P.; Perros, F.; Girerd, B.; Garcia, G.; Jaïs, X.; Savale, L.; Artaud-Macari, E.; Price, L.C.; et al. Pulmonary Arterial Hypertension. Orphanet J. Rare Dis. 2013, 8, 97. [Google Scholar] [CrossRef]
- Boucly, A.; Gerges, C.; Savale, L.; Jaïs, X.; Jevnikar, M.; Montani, D.; Sitbon, O.; Humbert, M. Pulmonary Arterial Hypertension. Presse Med. 2023, 52, 104168. [Google Scholar] [CrossRef] [PubMed]
- Manek, G.; Bhardwaj, A. Pulmonary Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Rosenkranz, S.; Howard, L.S.; Gomberg-Maitland, M.; Hoeper, M.M. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation 2020, 141, 678–693. [Google Scholar] [CrossRef] [PubMed]
- Funk-Hilsdorf, T.C.; Behrens, F.; Grune, J.; Simmons, S. Dysregulated Immunity in Pulmonary Hypertension: From Companion to Composer. Front. Physiol. 2022, 13, 819145. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhang, T.; Yu, J.; Lu, X.; Xiao, S.; Zhang, T.; Qing, T.; Xiao, Z.; Zeng, L.; Luo, L. A New Perspective on Targeting Pulmonary Arterial Hypertension: Programmed Cell Death Pathways (Autophagy, Pyroptosis, Ferroptosis). Biomed. Pharmacother. 2024, 181, 117706. [Google Scholar] [CrossRef]
- DeVaughn, H.; Rich, H.E.; Shadid, A.; Vaidya, P.K.; Doursout, M.-F.; Shivshankar, P. Complement Immune System in Pulmonary Hypertension-Cooperating Roles of Circadian Rhythmicity in Complement-Mediated Vascular Pathology. Int. J. Mol. Sci. 2024, 25, 12823. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, S.; Zhou, Y.; Zhang, T.; Sun, L. Immunotherapy for Pulmonary Arterial Hypertension: From the Pathogenesis to Clinical Management. Int. J. Mol. Sci. 2024, 25, 8427. [Google Scholar] [CrossRef]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The Interaction of Innate Immune and Adaptive Immune System. MedComm 2024, 5, e714. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Wu, K.-H.; Wu, H.-P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zou, J.; Chen, J.; Zhong, X.; Kang, R.; Tang, D. Pattern Recognition Receptors: Function, Regulation and Therapeutic Potential. Signal Transduct. Target. Ther. 2025, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Tanekhy, M. The Role of Toll-like Receptors in Innate Immunity and Infectious Diseases of Teleost. Aquac. Res. 2016, 47, 1369–1391. [Google Scholar] [CrossRef]
- Bhagwani, A.; Thompson, A.A.R.; Farkas, L. When Innate Immunity Meets Angiogenesis—The Role of Toll-Like Receptors in Endothelial Cells and Pulmonary Hypertension. Front. Med. 2020, 7, 352. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Ahmad, S.; Irshad, R.; Goyal, Y.; Rafat, S.; Siddiqui, N.; Dev, K.; Husain, M.; Ali, S.; Mohan, A.; et al. TLRs in Pulmonary Diseases. Life Sci. 2019, 233, 116671. [Google Scholar] [CrossRef]
- George, P.M.; Badiger, R.; Shao, D.; Edwards, M.R.; Wort, S.J.; Paul-Clark, M.J.; Mitchell, J.A. Viral Toll Like Receptor Activation of Pulmonary Vascular Smooth Muscle Cells Results in Endothelin-1 Generation; Relevance to Pathogenesis of Pulmonary Arterial Hypertension. Biochem. Biophys. Res. Commun. 2012, 426, 486–491. [Google Scholar] [CrossRef]
- Lafferty, E.I.; Qureshi, S.T.; Schnare, M. The Role of Toll-like Receptors in Acute and Chronic Lung Inflammation. J. Inflamm. 2010, 7, 57. [Google Scholar] [CrossRef]
- Ma, L.; Ambalavanan, N.; Liu, H.; Sun, Y.; Jhala, N.; Bradley, W.E.; Dell’Italia, L.J.; Michalek, S.; Wu, H.; Steele, C.; et al. TLR4 Regulates Pulmonary Vascular Homeostasis and Remodeling via Redox Signaling. Front. Biosci. 2016, 21, 397–409. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Savai, R.; Seeger, W.; Goncharova, E.A. Translational Advances in the Field of Pulmonary Hypertension.From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am. J. Respir. Crit. Care Med. 2017, 195, 425–437. [Google Scholar] [CrossRef]
- Farkas, D.; Thompson, A.A.R.; Bhagwani, A.R.; Hultman, S.; Ji, H.; Kotha, N.; Farr, G.; Arnold, N.D.; Braithwaite, A.; Casbolt, H.; et al. Toll-like Receptor 3 is a Therapeutic Target for Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2019, 199, 199–210. [Google Scholar] [CrossRef]
- Bhagwani, A.R.; Ali, M.; Piper, B.; Liu, M.; Hudson, J.; Kelly, N.; Bogamuwa, S.; Yang, H.; Londino, J.D.; Bednash, J.S.; et al. A P53-TLR3 Axis Ameliorates Pulmonary Hypertension by Inducing BMPR2 via IRF3. iScience 2023, 26, 105935. [Google Scholar] [CrossRef]
- Willcocks, S.; Offord, V.; Seyfert, H.-M.; Coffey, T.J.; Werling, D. Species-Specific PAMP Recognition by TLR2 and Evidence for Species-Restricted Interaction with Dectin-1. J. Leukoc. Biol. 2013, 94, 449–458. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening Inflammation by Modulating TLR Signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-Sensing Receptors in Inflammation and Diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Decoding Toll-like Receptors: Recent Insights and Perspectives in Innate Immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.F.; Liu, N.; Candolfi, M.; Xiong, W.; Assi, H.; Yagiz, K.; Edwards, M.R.; Michelsen, K.S.; Kroeger, K.M.; Liu, C.; et al. HMGB1 Mediates Endogenous TLR2 Activation and Brain Tumor Regression. PLoS Med. 2009, 6, e1000010. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.L.; Nishizaki, D.; Adashek, J.J.; Kato, S.; Kurzrock, R. Toll-like Receptor 3: A Double-Edged Sword. Biomark. Res. 2025, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Di, B. Endogenous Ligands of TLR4 in Microglia: Potential Targets for Related Neurological Diseases. Curr. Drug Targets 2024, 25, 953–970. [Google Scholar] [CrossRef]
- Kim, J.; Ha, S.; Son, M.; Kim, D.; Kim, M.-J.; Kim, B.; Kim, D.; Chung, H.Y.; Chung, K.W. TLR7 Activation by miR-21 Promotes Renal Fibrosis by Activating the pro-Inflammatory Signaling Pathway in Tubule Epithelial Cells. Cell Commun. Signal. 2023, 21, 215. [Google Scholar] [CrossRef]
- Fabbri, M.; Paone, A.; Calore, F.; Galli, R.; Gaudio, E.; Santhanam, R.; Lovat, F.; Fadda, P.; Mao, C.; Nuovo, G.J.; et al. MicroRNAs Bind to Toll-like Receptors to Induce Prometastatic Inflammatory Response. Proc. Natl. Acad. Sci. USA 2012, 109, E2110–E2116. [Google Scholar] [CrossRef]
- Rebetz, J.; Cederholm, H.; McGauran, D.; Moore, E.; Chi, C.; Tabak, K.; Allhorn, M.; Olsson, M.L.; Egesten, A.; Semple, J.W.; et al. Mitochondrial DNA via Recipient TLR9 Acts as a Potent First-Hit in Murine Transfusion-Related Acute Lung Injury (TRALI). Blood 2025, 146, 2479–2490. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Balachandran, Y.; Aulakh, G.K.; Singh, B. TLR10: An Intriguing Toll-Like Receptor with Many Unanswered Questions. J. Innate Immun. 2024, 16, 96–104. [Google Scholar] [CrossRef]
- Almodovar, S.; Hsue, P.Y.; Morelli, J.; Huang, L.; Flores, S.C. Pathogenesis of HIV-Associated Pulmonary Hypertension. Proc. Am. Thorac. Soc. 2011, 8, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Butrous, G. Global Landscape of Infection-Induced Pulmonary Hypertension. Infect. Dis. Rep. 2025, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Cool, C.D.; Voelkel, N.F.; Bull, T. Viral Infection and Pulmonary Hypertension: Is There an Association? Expert Rev. Respir. Med. 2011, 5, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Galán, S.; Parra, V.; Cuenca, J. Unraveling the Pathogenesis of Viral-Induced Pulmonary Arterial Hypertension: Possible New Therapeutic Avenues with Mesenchymal Stromal Cells and Their Derivatives. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2025, 1871, 167519. [Google Scholar] [CrossRef]
- Wang, D.; Gomes, M.T.; Mo, Y.; Prohaska, C.C.; Zhang, L.; Chelvanambi, S.; Clauss, M.A.; Zhang, D.; Machado, R.F.; Gao, M.; et al. Human Endogenous Retrovirus, SARS-CoV-2, and HIV Promote PAH via Inflammation and Growth Stimulation. Int. J. Mol. Sci. 2023, 24, 7472. [Google Scholar] [CrossRef]
- Nie, L.; Cai, S.-Y.; Shao, J.-Z.; Chen, J. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Front. Immunol. 2018, 9, 1523. [Google Scholar] [CrossRef]
- Mertowski, S.; Lipa, P.; Morawska, I.; Niedźwiedzka-Rystwej, P.; Bębnowska, D.; Hrynkiewicz, R.; Grywalska, E.; Roliński, J.; Załuska, W. Toll-Like Receptor as a Potential Biomarker in Renal Diseases. Int. J. Mol. Sci. 2020, 21, 6712. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q15399/entry (accessed on 11 September 2025).
- Xiao, G.; Zhuang, W.; Wang, T.; Lian, G.; Luo, L.; Ye, C.; Wang, H.; Xie, L. Transcriptomic Analysis Identifies Toll-like and Nod-like Pathways and Necroptosis in Pulmonary Arterial Hypertension. J. Cell Mol. Med. 2020, 24, 11409–11421. [Google Scholar] [CrossRef]
- Broen, J.C.A.; Bossini-Castillo, L.; van Bon, L.; Vonk, M.C.; Knaapen, H.; Beretta, L.; Rueda, B.; Hesselstrand, R.; Herrick, A.; Worthington, J.; et al. A Rare Polymorphism in the Gene for Toll-like Receptor 2 is Associated with Systemic Sclerosis Phenotype and Increases the Production of Inflammatory Mediators. Arthritis Rheum. 2012, 64, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. Toll-like Receptors. Curr. Protoc. Immunol. 2015, 109, 14.12.1–14.12.10. [Google Scholar] [CrossRef] [PubMed]
- de Kivit, S.; Tobin, M.C.; Forsyth, C.B.; Keshavarzian, A.; Landay, A.L. Regulation of Intestinal Immune Responses through TLR Activation: Implications for Pro- and Prebiotics. Front. Immunol. 2014, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/O60603/entry (accessed on 11 September 2025).
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.-G.; Lee, H.; Lee, J.-O. Crystal Structure of the TLR1-TLR2 Heterodimer Induced by Binding of a Tri-Acylated Lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Sabroe, I.; Dower, S.K.; Whyte, M.K.B. The Role of Toll-like Receptors in the Regulation of Neutrophil Migration, Activation, and Apoptosis. Clin. Infect. Dis. 2005, 41 (Suppl. S7), S421–S426. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. The Instructive Role of Dendritic Cells on T-Cell Responses. Arthritis Res. 2002, 4 (Suppl. S3), S127–S132. [Google Scholar] [CrossRef]
- Ma, D.; Wang, X.; Liu, X.; Li, Z.; Liu, J.; Cao, J.; Wang, G.; Guo, Y.; Zhao, S. Macrophage Infiltration Initiates RIP3/MLKL-Dependent Necroptosis in Paclitaxel-Induced Neuropathic Pain. Mediat. Inflamm. 2022, 2022, 1567210. [Google Scholar] [CrossRef]
- Lian, G.; You, J.; Lin, W.; Gao, G.; Xu, C.; Wang, H.; Luo, L. Bioinformatics Analysis of the Immune Cell Infiltration Characteristics and Correlation with Crucial Diagnostic Markers in Pulmonary Arterial Hypertension. BMC Pulm. Med. 2023, 23, 300. [Google Scholar] [CrossRef]
- Adu-Amankwaah, J.; Shi, Y.; Song, H.; Ma, Y.; Liu, J.; Wang, H.; Yuan, J.; Sun, K.; Hu, Q.; Tan, R. Signaling Pathways and Targeted Therapy for Pulmonary Hypertension. Signal Transduct. Target. Ther. 2025, 10, 207. [Google Scholar] [CrossRef]
- Wang, J.; Uddin, M.N.; Wang, R.; Gong, Y.; Wu, Y. Comprehensive Analysis and Validation of Novel Immune and Vascular Remodeling Related Genes Signature Associated with Drug Interactions in Pulmonary Arterial Hypertension. Front. Genet. 2022, 13, 922213. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/O15455/entry (accessed on 11 September 2025).
- Kim, J.H.; Song, D.H.; Youn, S.-J.; Kim, J.W.; Cho, G.; Kim, S.C.; Lee, H.; Jin, M.S.; Lee, J.-O. Crystal Structures of Mono- and Bi-Specific Diabodies and Reduction of Their Structural Flexibility by Introduction of Disulfide Bridges at the Fv Interface. Sci. Rep. 2016, 6, 34515. [Google Scholar] [CrossRef]
- Jiang, Z.; Mak, T.W.; Sen, G.; Li, X. Toll-like Receptor 3-Mediated Activation of NF-kappaB and IRF3 Diverges at Toll-IL-1 Receptor Domain-Containing Adapter Inducing IFN-Beta. Proc. Natl. Acad. Sci. USA 2004, 101, 3533–3538. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhao, Y.; Ma, X.; Yi, H. Toll-like Receptor 3 (TLR3) Regulation Mechanisms and Roles in Antiviral Innate Immune Responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Han, B.; Zhang, C.; Wang, X.; Song, H.; Zhang, L.; Li, T.; He, J.; Zhao, H. The Functional Mechanisms of Toll-Like Receptor 3 and Its Implications in Digestive System Tumors. FBL 2023, 28, 297. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. TLR Signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef]
- Li, B.; Xia, Y.; Hu, B. Infection and Atherosclerosis: TLR-Dependent Pathways. Cell Mol. Life Sci. 2020, 77, 2751–2769. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Sayers, S.; D’Armiento, J.M.; Tall, A.R.; Welch, C.L. TLR3 Deficiency Protects against Collagen Degradation and Medial Destruction in Murine Atherosclerotic Plaques. Atherosclerosis 2013, 229, 52–61. [Google Scholar] [CrossRef]
- Pan, L.; Zhu, W.; Li, Y.; Xu, X.; Guo, L.; Lu, Q.; Wang, J. Astrocytic Toll-Like Receptor 3 is Associated with Ischemic Preconditioning- Induced Protection against Brain Ischemia in Rodents. PLoS ONE 2014, 9, e99526. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.E.; Navin, T.J.; Cross, A.J.; Goddard, M.E.; Alexopoulou, L.; Mitra, A.T.; Davies, A.H.; Flavell, R.A.; Feldmann, M.; Monaco, C. Unexpected Protective Role for Toll-like Receptor 3 in the Arterial Wall. Proc. Natl. Acad. Sci. USA 2011, 108, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Squillace, S.; Salvemini, D. Toll-like Receptor-Mediated Neuroinflammation: Relevance for Cognitive Dysfunctions. Trends Pharmacol. Sci. 2022, 43, 726–739. [Google Scholar] [CrossRef]
- Negishi, H.; Osawa, T.; Ogami, K.; Ouyang, X.; Sakaguchi, S.; Koshiba, R.; Yanai, H.; Seko, Y.; Shitara, H.; Bishop, K.; et al. A Critical Link between Toll-like Receptor 3 and Type II Interferon Signaling Pathways in Antiviral Innate Immunity. Proc. Natl. Acad. Sci. USA 2008, 105, 20446–20451. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhu, B.; Chen, D. Type I Interferon-Mediated Tumor Immunity and Its Role in Immunotherapy. Cell. Mol. Life Sci. 2022, 79, 191. [Google Scholar] [CrossRef] [PubMed]
- Kopeć, G.; Skride, A.; Ereminiene, E.; Simkova, I.; Enache, R.; Samarzija, M.; Salobir, B.; Jansa, P. Emerging Therapies and New Directions in the Treatment of Pulmonary Arterial Hypertension. Pol. Heart J. 2025, 83, 18–26. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/O00206/entry (accessed on 12 September 2025).
- Kim, H.-J.; Kim, H.; Lee, J.-H.; Hwangbo, C. Toll-like Receptor 4 (TLR4): New Insight Immune and Aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Li, J.; Yang, F.; Wei, F.; Ren, X. The Role of Toll-like Receptor 4 in Tumor Microenvironment. Oncotarget 2017, 8, 66656–66667. [Google Scholar] [CrossRef]
- Oda, M.; Yamamoto, H.; Kawakami, T. Maintenance of Homeostasis by TLR4 Ligands. Front. Immunol. 2024, 15, 1286270. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like Receptor 4: A Potential Therapeutic Target for Multiple Human Diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef]
- Mansouri, A.; Akthar, I.; Miyamoto, A. TLR2 and TLR4 Bridge Physiological and Pathological Inflammation in the Reproductive System. Commun. Biol. 2025, 8, 1008. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, B.; Zhang, L.; Zhang, Z.; Wang, L.; Tang, L.; Li, S.; Yang, Y.; Yang, F.; Zhang, P.; et al. MyD88-Dependent Toll-like Receptor 4 Signal Pathway in Intervertebral Disc Degeneration. Exp. Ther. Med. 2016, 12, 611–618. [Google Scholar] [CrossRef]
- Chettab, K.; Fitzsimmons, C.; Novikov, A.; Denis, M.; Phelip, C.; Mathé, D.; Choffour, P.A.; Beaumel, S.; Fourmaux, E.; Norca, P.; et al. A Systemically Administered Detoxified TLR4 Agonist Displays Potent Antitumor Activity and an Acceptable Tolerance Profile in Preclinical Models. Front. Immunol. 2023, 14, 1066402. [Google Scholar] [CrossRef]
- Richert, I.; Berchard, P.; Abbes, L.; Novikov, A.; Chettab, K.; Vandermoeten, A.; Dumontet, C.; Karanian, M.; Kerzerho, J.; Caroff, M.; et al. A TLR4 Agonist Induces Osteosarcoma Regression by Inducing an Antitumor Immune Response and Reprogramming M2 Macrophages to M1 Macrophages. Cancers 2023, 15, 4635. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.M.; Saeed, I.; Miles, J.A.; Ross, B.P.; Shaw, P.N.; Hollmann, M.W.; Parat, M.-O. Interaction of Opioids with TLR4—Mechanisms and Ramifications. Cancers 2021, 13, 5274. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Zhou, Q.; Lee, A.J.; Wang, L.; Zhang, R.; Wang, S. Effects of Opioid Drugs on Immune Function in Cancer Patients. Biomed. Pharmacother. 2024, 175, 116665. [Google Scholar] [CrossRef] [PubMed]
- Dabi, Y.T.; Ajagbe, A.O.; Degechisa, S.T. Toll-like Receptors in Pathogenesis of Neurodegenerative Diseases and Their Therapeutic Potential. Immun. Inflamm. Dis. 2023, 11, e839. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of Astroglial Toll-like Receptors (TLRs) in Central Nervous System Infections, Injury and Neurodegenerative Diseases. Brain Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef]
- Fu, Y.; Gong, T.; Loughran, P.A.; Li, Y.; Billiar, T.R.; Liu, Y.; Wen, Z.; Fan, J. Roles of TLR4 in Macrophage Immunity and Macrophage-Pulmonary Vascular/Lymphatic Endothelial Cell Interactions in Sepsis. Commun. Biol. 2025, 8, 469. [Google Scholar] [CrossRef]
- Stierschneider, A.; Wiesner, C. Shedding Light on the Molecular and Regulatory Mechanisms of TLR4 Signaling in Endothelial Cells under Physiological and Inflamed Conditions. Front. Immunol. 2023, 14, 1264889. [Google Scholar] [CrossRef]
- Sun, Z. Platelet TLR4, a Critical Link in Pulmonary Arterial Hypertension. Circ. Res. 2014, 114, 1551–1553. [Google Scholar] [CrossRef]
- Dai, J.; Chen, H.; Fang, J.; Wu, S.; Jia, Z. Vascular Remodeling: The Multicellular Mechanisms of Pulmonary Hypertension. Int. J. Mol. Sci. 2025, 26, 4265. [Google Scholar] [CrossRef]
- Ye, Y.; Xu, Q.; Wuren, T. Inflammation and Immunity in the Pathogenesis of Hypoxic Pulmonary Hypertension. Front. Immunol. 2023, 14, 1162556. [Google Scholar] [CrossRef]
- Peri, F.; Calabrese, V. Toll-like Receptor 4 (TLR4) Modulation by Synthetic and Natural Compounds: An Update. J. Med. Chem. 2014, 57, 3612–3622. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Yang, Z.; Fan, B.; Wang, C.; Tian, Z. Cinnamaldehyde Alleviates Pulmonary Hypertension by Affecting Vascular Remodeling through the TLR4/NF-kB/HIF-1a Pathway. Clin. Exp. Hypertens. 2025, 47, 2486829. [Google Scholar] [CrossRef] [PubMed]
- Vallance, T.M.; Zeuner, M.-T.; Williams, H.F.; Widera, D.; Vaiyapuri, S. Toll-Like Receptor 4 Signalling and Its Impact on Platelet Function, Thrombosis, and Haemostasis. Mediat. Inflamm. 2017, 2017, 9605894. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.M.; Chanthaphavong, R.S.; Sodhi, C.P.; Hackam, D.J.; Billiar, T.R.; Bauer, P.M. Genetic Deletion of Toll-like Receptor 4 on Platelets Attenuates Experimental Pulmonary Hypertension. Circ. Res. 2014, 114, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, X.-T.; Peng, Z.; Li, W.-Q.; Cao, Y.-Y.; Li, Y.; Li, X.-H. HMGB1/TLR4 Promotes Hypoxic Pulmonary Hypertension via Suppressing BMPR2 Signaling. Vasc. Pharmacol. 2019, 117, 35–44. [Google Scholar] [CrossRef]
- Grinnan, D.; Farkas, L. A Novel Peptide for Immunomodulation in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 199, 1460–1461. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Liu, Y.; Long, Y. The Role of Gut and Airway Microbiota in Pulmonary Arterial Hypertension. Front. Microbiol. 2022, 13, 929752. [Google Scholar] [CrossRef]
- Wedgwood, S.; Gerard, K.; Halloran, K.; Hanhauser, A.; Monacelli, S.; Warford, C.; Thai, P.N.; Chiamvimonvat, N.; Lakshminrusimha, S.; Steinhorn, R.H.; et al. Intestinal Dysbiosis and the Developing Lung: The Role of Toll-Like Receptor 4 in the Gut-Lung Axis. Front. Immunol. 2020, 11, 357. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/O60602/entry (accessed on 12 September 2025).
- Feuillet, V.; Medjane, S.; Mondor, I.; Demaria, O.; Pagni, P.P.; Galán, J.E.; Flavell, R.A.; Alexopoulou, L. Involvement of Toll-like Receptor 5 in the Recognition of Flagellated Bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 12487–12492. [Google Scholar] [CrossRef]
- Mizel, S.B.; West, A.P.; Hantgan, R.R. Identification of a Sequence in Human Toll-like Receptor 5 Required for the Binding of Gram-Negative Flagellin. J. Biol. Chem. 2003, 278, 23624–23629. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, K.; Dankmeyer, J.L.; Bernhards, R.C.; Fetterer, D.P.; Waag, D.M.; Worsham, P.L.; DeShazer, D. Activation of Toll-Like Receptors by Live Gram-Negative Bacterial Pathogens Reveals Mitigation of TLR4 Responses and Activation of TLR5 by Flagella. Front. Cell. Infect. Microbiol. 2021, 11, 745325. [Google Scholar] [CrossRef] [PubMed]
- Tallant, T.; Deb, A.; Kar, N.; Lupica, J.; de Veer, M.J.; DiDonato, J.A. Flagellin Acting via TLR5 is the Major Activator of Key Signaling Pathways Leading to NF-κB and Proinflammatory Gene Program Activation in Intestinal Epithelial Cells. BMC Microbiol. 2004, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Buschard, K.; Krogvold, L.; Pociot, F.; Gerling, I.; Thea, R.; Dahl-Jørgensen, K.; Hansen, C.H.F. TLR5 Influences the Development of Type 1 Diabetes. BMJ Open Diabetes Res. Care 2025, 13, e005111. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, C.; Chen, S.; He, R.; Chao, G.; Zhang, S. TLR5 Signaling in the Regulation of Intestinal Mucosal Immunity. J. Inflamm. Res. 2023, 16, 2491–2501. [Google Scholar] [CrossRef]
- Chamberlain, N.D.; Vila, O.M.; Volin, M.V.; Volkov, S.; Pope, R.M.; Swedler, W.; Mandelin, A.M.; Shahrara, S. TLR5; a Novel and Unidentified Inflammatory Mediator in Rheumatoid Arthritis That Correlates with Disease Activity Score and Joint TNF-α Levels. J. Immunol. 2012, 189, 475–483. [Google Scholar] [CrossRef]
- Vijay, K. Toll-like Receptors in Immunity and Inflammatory Diseases: Past, Present, and Future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef]
- Wang, K.; Huang, H.; Zhan, Q.; Ding, H.; Li, Y. Toll-like Receptors in Health and Disease. MedComm 2024, 5, e549. [Google Scholar] [CrossRef]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-Like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef]
- McGinty, M.T.; Putelo, A.M.; Kolli, S.H.; Feng, T.-Y.; Dietl, M.R.; Hatzinger, C.N.; Bajgai, S.; Poblete, M.K.; Azar, F.N.; Mohammad, A.; et al. TLR5 Signaling Causes Dendritic Cell Dysfunction and Orchestrates Failure of Immune Checkpoint Therapy against Ovarian Cancer. Cancer Immunol. Res. 2025, 13, 696–711. [Google Scholar] [CrossRef]
- Kim, J.; Yoo, J.-Y.; Suh, J.M.; Park, S.; Kang, D.; Jo, H.; Bae, Y.S. The Flagellin-TLR5-Nox4 Axis Promotes the Migration of Smooth Muscle Cells in Atherosclerosis. Exp. Mol. Med. 2019, 51, 78. [Google Scholar] [CrossRef]
- Kim, J.; Seo, M.; Kim, S.K.; Bae, Y.S. Flagellin-Induced NADPH Oxidase 4 Activation is Involved in Atherosclerosis. Sci. Rep. 2016, 6, 25437. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, G.H.J.M.; van Puijvelde, G.H.M.; Anas, A.A.; Bot, M.; Asbach, M.; Schoneveld, A.; van Santbrink, P.J.; Foks, A.C.; Timmers, L.; Doevendans, P.A.; et al. Leukocyte TLR5 Deficiency Inhibits Atherosclerosis by Reduced Macrophage Recruitment and Defective T-Cell Responsiveness. Sci. Rep. 2017, 7, 42688. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Zhao, S.; Jiang, W.; Zhang, C.; Liang, T.; Hou, G. TLR5: A Prognostic and Monitoring Indicator for Triple-Negative Breast Cancer. Cell Death Dis. 2019, 10, 954. [Google Scholar] [CrossRef] [PubMed]
- Pfirschke, C.; Garris, C.; Pittet, M.J. Common TLR5 Mutations Control Cancer Progression. Cancer Cell 2015, 27, 1–3. [Google Scholar] [CrossRef][Green Version]
- Rohm, I.; Grün, K.; Müller, L.M.; Bäz, L.; Förster, M.; Schrepper, A.; Kretzschmar, D.; Pistulli, R.; Yilmaz, A.; Bauer, R.; et al. Cellular Inflammation in Pulmonary Hypertension: Detailed Analysis of Lung and Right Ventricular Tissue, Circulating Immune Cells and Effects of a Dual Endothelin Receptor Antagonist. Clin. Hemorheol. Microcirc. 2019, 73, 497–522. [Google Scholar] [CrossRef]
- Sakamachi, Y.; Wiley, E.; Solis, A.; Johnson, C.G.; Meng, X.; Hussain, S.; Lipinski, J.H.; O’Dwyer, D.N.; Randall, T.; Malphurs, J.; et al. Toll-Like-Receptor 5 Protects against Pulmonary Fibrosis by Reducing Lung Dysbiosis. bioRxiv 2024. bioRxiv:2024.04.30.591719. [Google Scholar] [CrossRef]
- Oh, S.; Jang, A.Y.; Chae, S.; Choi, S.; Moon, J.; Kim, M.; Spiekerkoetter, E.; Zamanian, R.T.; Yang, P.C.; Hwang, D.; et al. Comparative Analysis on the Anti-Inflammatory/Immune Effect of Mesenchymal Stem Cell Therapy for the Treatment of Pulmonary Arterial Hypertension. Sci. Rep. 2021, 11, 2012. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9Y2C9/entry (accessed on 12 September 2025).
- Jang, T.-H.; Park, H.H. Crystal Structure of TIR Domain of TLR6 Reveals Novel Dimeric Interface of TIR-TIR Interaction for Toll-like Receptor Signaling Pathway. J. Mol. Biol. 2014, 426, 3305–3313. [Google Scholar] [CrossRef]
- Misch, E.A.; Verbon, A.; Prins, J.M.; Skerrett, S.J.; Hawn, T.R. A TLR6 Polymorphism is Associated with Increased Risk of Legionnaires’ Disease. Genes Immun. 2013, 14, 420–426. [Google Scholar] [CrossRef][Green Version]
- Hoffjan, S.; Stemmler, S.; Parwez, Q.; Petrasch-Parwez, E.; Arinir, U.; Rohde, G.; Reinitz-Rademacher, K.; Schultze-Werninghaus, G.; Bufe, A.; Epplen, J.T. Evaluation of the Toll-like Receptor 6 Ser249Pro Polymorphism in Patients with Asthma, Atopic Dermatitis and Chronic Obstructive Pulmonary Disease. BMC Med. Genet. 2005, 6, 34. [Google Scholar] [CrossRef]
- Grote, K.; Petri, M.; Liu, C.; Jehn, P.; Spalthoff, S.; Kokemüller, H.; Luchtefeld, M.; Tschernig, T.; Krettek, C.; Haasper, C.; et al. Toll-like Receptor 2/6-Dependent Stimulation of Mesenchymal Stem Cells Promotes Angiogenesis by Paracrine Factors. Eur. Cell Mater. 2013, 26, 66–79; discussion 79. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9NYK1/entry (accessed on 12 September 2025).
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9NR97/entry (accessed on 12 September 2025).
- Rathinam, V.A.K.; Fitzgerald, K.A. Innate Immune Sensing of DNA Viruses. Virology 2011, 411, 153–162. [Google Scholar] [CrossRef]
- Bello, M.B.; Usman, D. Identification of Single Stranded RNA Fragments Recognised by TLR7/8 in Dengue and Other Mosquito-Borne Flaviviral Genomes: A Bioinformatics Approach. Microbe 2025, 7, 100406. [Google Scholar] [CrossRef]
- Soleiman-Meigooni, S.; Yarahmadi, A.; Kheirkhah, A.-H.; Afkhami, H. Recent Advances in Different Interactions between Toll-like Receptors and Hepatitis B Infection: A Review. Front. Immunol. 2024, 15, 1363996. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, B.; Wu, X.; Liu, R.; Fan, H.; Han, L.; Zhang, Z.; Ma, X.; Chu, C.-Q.; Shi, X. Toll-like Receptors 7 and 9 Regulate the Proliferation and Differentiation of B Cells in Systemic Lupus Erythematosus. Front. Immunol. 2023, 14, 1093208. [Google Scholar] [CrossRef]
- Sakata, K.; Nakayamada, S.; Miyazaki, Y.; Kubo, S.; Ishii, A.; Nakano, K.; Tanaka, Y. Up-Regulation of TLR7-Mediated IFN-α Production by Plasmacytoid Dendritic Cells in Patients With Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1957. [Google Scholar] [CrossRef] [PubMed]
- Sakref, C.; Saby, A.; Rodriguez, C.; Ardin, M.; Moudombi, L.; Doffin, A.-C.; Gobbini, E.; Voissiere, A.; Besson, L.; Laoubi, L.; et al. Type III Interferon Primes pDCs for TLR7 Activation and Antagonizes Immune Suppression Mediated by TGF-β and PGE2. Nat. Commun. 2025, 16, 3045. [Google Scholar] [CrossRef]
- Lee, J.; Hayashi, M.; Lo, J.-F.; Fearns, C.; Chu, W.-M.; Luo, Y.; Xiang, R.; Chuang, T.-H. NF-κB Activation Primes Cells to a pro-Inflammatory Polarized Response to a TLR7 Agonist. Biochem. J. 2009, 421, 301–310. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Pottle, J.; Ni, M.; He, Y.; Zhang, Z.-Y.; Buckner, J.H. Negative Regulation of TLR Signaling in Myeloid Cells—Implications for Autoimmune Diseases. Immunol. Rev. 2016, 269, 212–227. [Google Scholar] [CrossRef]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The Forgotten Relative Revindicated. Cell Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef]
- Iwasaki, A.; Pillai, P.S. Innate Immunity to Influenza Virus Infection. Nat. Rev. Immunol. 2014, 14, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A.M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 Independently Confer Responsiveness to the Antiviral Compound R-848. Nat. Immunol. 2002, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- de Groot, N.G.; Bontrop, R.E. COVID-19 Pandemic: Is a Gender-Defined Dosage Effect Responsible for the High Mortality Rate among Males? Immunogenetics 2020, 72, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, M.; Takaishi, M.; Nakajima, K.; Kamijima, R.; Fujimoto, C.; Kataoka, S.; Terada, Y.; Sano, S. Epicutaneous Application of Toll-like Receptor 7 Agonists Leads to Systemic Autoimmunity in Wild-Type Mice: A New Model of Systemic Lupus Erythematosus. Arthritis Rheumatol. 2014, 66, 694–706. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Chen, C.-N.; Xie, C.-Y.; Baxan, N.; Zhao, L.; Ashek, A.; Sabrin, F.; Lawrie, A.; Wilkins, M.; Zhao, L. TLR7/8 Activation Induces Autoimmune Vasculopathy and Causes Severe Pulmonary Arterial Hypertension. Eur. Respir. J. 2023, 62, 2300204. [Google Scholar] [CrossRef]
- Hubert, F.-X.; Voisine, C.; Louvet, C.; Heslan, M.; Josien, R. Rat Plasmacytoid Dendritic Cells Are an Abundant Subset of MHC Class II+ CD4+CD11b-OX62- and Type I IFN-Producing Cells That Exhibit Selective Expression of Toll-like Receptors 7 and 9 and Strong Responsiveness to CpG. J. Immunol. 2004, 172, 7485–7494. [Google Scholar] [CrossRef]
- Hautefort, A.; Girerd, B.; Montani, D.; Cohen-Kaminsky, S.; Price, L.; Lambrecht, B.N.; Humbert, M.; Perros, F. T-Helper 17 Cell Polarization in Pulmonary Arterial Hypertension. Chest 2015, 147, 1610–1620. [Google Scholar] [CrossRef]
- Seki, N.; Tsujimoto, H.; Tanemura, S.; Ishigaki, S.; Takei, H.; Sugahara, K.; Yoshimoto, K.; Akiyama, M.; Kaneko, Y.; Chiba, K.; et al. Th17/IL-17A Axis is Critical for Pulmonary Arterial Hypertension (PAH) in Systemic Sclerosis (SSc): SSc Patients with High Levels of Serum IL-17A Exhibit Reduced Lung Functions and Increased Prevalence of PAH. Cytokine 2024, 176, 156534. [Google Scholar] [CrossRef]
- Huertas, A.; Phan, C.; Bordenave, J.; Tu, L.; Thuillet, R.; Le Hiress, M.; Avouac, J.; Tamura, Y.; Allanore, Y.; Jovan, R.; et al. Regulatory T Cell Dysfunction in Idiopathic, Heritable and Connective Tissue-Associated Pulmonary Arterial Hypertension. Chest 2016, 149, 1482–1493. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Visitación, N.D.L.; Toral, M.; Sánchez, M.; Gómez-Guzmán, M.; O’valle, F.; Jiménez, R.; Duarte, J.; Romero, M. Toll-like Receptor 7-Driven Lupus Autoimmunity Induces Hypertension and Vascular Alterations in Mice. J. Hypertens. 2020, 38, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Margery-Muir, A.A.; Bundell, C.; Nelson, D.; Groth, D.M.; Wetherall, J.D. Gender Balance in Patients with Systemic Lupus Erythematosus. Autoimmun. Rev. 2017, 16, 258–268. [Google Scholar] [CrossRef] [PubMed]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9NR96/entry (accessed on 12 September 2025).
- Bauer, S.; Wagner, H. Bacterial CpG-DNA Licenses TLR9. Curr. Top. Microbiol. Immunol. 2002, 270, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Ohto, U.; Shibata, T.; Tanji, H.; Ishida, H.; Krayukhina, E.; Uchiyama, S.; Miyake, K.; Shimizu, T. Structural Basis of CpG and Inhibitory DNA Recognition by Toll-like Receptor 9. Nature 2015, 520, 702–705. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, S.; Wang, X.; Khaidakov, M.; Dai, Y.; Mehta, J.L. Oxidant Stress in Mitochondrial DNA Damage, Autophagy and Inflammation in Atherosclerosis. Sci. Rep. 2013, 3, 1077. [Google Scholar] [CrossRef]
- Suresh, M.V.; Thomas, B.; Dolgachev, V.A.; Sherman, M.A.; Goldberg, R.; Johnson, M.; Chowdhury, A.; Machado-Aranda, D.; Raghavendran, K. Toll-Like Receptor-9 (TLR9) Is Requisite for Acute Inflammatory Response and Injury Following Lung Contusion. Shock 2016, 46, 412–419. [Google Scholar] [CrossRef]
- Tsuji, N.; Tsuji, T.; Ohashi, N.; Kato, A.; Fujigaki, Y.; Yasuda, H. Role of Mitochondrial DNA in Septic AKI via Toll-Like Receptor 9. J. Am. Soc. Nephrol. 2016, 27, 2009–2020. [Google Scholar] [CrossRef]
- Schumacker, P.T.; Gillespie, M.N.; Nakahira, K.; Choi, A.M.K.; Crouser, E.D.; Piantadosi, C.A.; Bhattacharya, J. Mitochondria in Lung Biology and Pathology: More than Just a Powerhouse. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L962–L974. [Google Scholar] [CrossRef]
- Garcia-Martinez, I.; Santoro, N.; Chen, Y.; Hoque, R.; Ouyang, X.; Caprio, S.; Shlomchik, M.J.; Coffman, R.L.; Candia, A.; Mehal, W.Z. Hepatocyte Mitochondrial DNA Drives Nonalcoholic Steatohepatitis by Activation of TLR9. J. Clin. Investig. 2016, 126, 859–864. [Google Scholar] [CrossRef]
- Han, S.J.; Li, H.; Kim, M.; Shlomchik, M.J.; Lee, H.T. Kidney Proximal Tubular TLR9 Exacerbates Ischemic Acute Kidney Injury. J. Immunol. 2018, 201, 1073–1085. [Google Scholar] [CrossRef]
- McCarthy, C.G.; Wenceslau, C.F.; Goulopoulou, S.; Ogbi, S.; Baban, B.; Sullivan, J.C.; Matsumoto, T.; Webb, R.C. Circulating Mitochondrial DNA and Toll-like Receptor 9 Are Associated with Vascular Dysfunction in Spontaneously Hypertensive Rats. Cardiovasc. Res. 2015, 107, 119–130. [Google Scholar] [CrossRef]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA That Escapes from Autophagy Causes Inflammation and Heart Failure. Nature 2012, 485, 251–255. [Google Scholar] [CrossRef]
- Loomis, Z.; Eigenberger, P.; Redinius, K.; Lisk, C.; Karoor, V.; Nozik-Grayck, E.; Ferguson, S.K.; Hassell, K.; Nuss, R.; Stenmark, K.; et al. Hemoglobin Induced Cell Trauma Indirectly Influences Endothelial TLR9 Activity Resulting in Pulmonary Vascular Smooth Muscle Cell Activation. PLoS ONE 2017, 12, e0171219. [Google Scholar] [CrossRef]
- Ishikawa, T.; Abe, K.; Takana-Ishikawa, M.; Yoshida, K.; Watanabe, T.; Imakiire, S.; Hosokawa, K.; Hirano, M.; Hirano, K.; Tsutsui, H. Chronic Inhibition of Toll-Like Receptor 9 Ameliorates Pulmonary Hypertension in Rats. J. Am. Heart Assoc. 2021, 10, e019247. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Shinoda, M.; Tanaka, M.; Kuwabara, Y.; Yoshida, K.; Hirooka, Y.; McMurtry, I.F.; Oka, M.; Sunagawa, K. Haemodynamic Unloading Reverses Occlusive Vascular Lesions in Severe Pulmonary Hypertension. Cardiovasc. Res. 2016, 111, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T. Revisiting the Hyperhemolysis Paradigm. Blood 2015, 126, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T.; Vichinsky, E. Pulmonary Complications of Sickle Cell Disease. N. Engl. J. Med. 2008, 359, 2254–2265. [Google Scholar] [CrossRef]
- Kato, G.J.; Taylor, J.G. Pleiotropic Effects of Intravascular Hemolysis on Vascular Homeostasis. Br. J. Haematol. 2010, 148, 690–701. [Google Scholar] [CrossRef]
- Kato, G.J. Novel Small Molecule Therapeutics for Sickle Cell Disease: Nitric Oxide, Carbon Monoxide, Nitrite, and Apolipoprotein A-I. Hematol. Am. Soc. Hematol. Educ. Program 2008, 2008, 186–192. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Haase-Fielitz, A. Novel Biomarkers, Oxidative Stress, and the Role of Labile Iron Toxicity in Cardiopulmonary Bypass-Associated Acute Kidney Injury. J. Am. Coll. Cardiol. 2010, 55, 2024–2033. [Google Scholar] [CrossRef]
- Lisk, C.; Kominsky, D.; Ehrentraut, S.; Bonaventura, J.; Nuss, R.; Hassell, K.; Nozik-Grayck, E.; Irwin, D.C. Hemoglobin-Induced Endothelial Cell Permeability is Controlled, in Part, via a Myeloid Differentiation Primary Response Gene-88-Dependent Signaling Mechanism. Am. J. Respir. Cell Mol. Biol. 2013, 49, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.; Jeney, V.; Chora, A.; Larsen, R.; Balla, J.; Soares, M.P. Oxidized Hemoglobin is an Endogenous Proinflammatory Agonist That Targets Vascular Endothelial Cells. J. Biol. Chem. 2009, 284, 29582–29595. [Google Scholar] [CrossRef] [PubMed]
- Shaver, C.M.; Upchurch, C.P.; Janz, D.R.; Grove, B.S.; Putz, N.D.; Wickersham, N.E.; Dikalov, S.I.; Ware, L.B.; Bastarache, J.A. Cell-Free Hemoglobin: A Novel Mediator of Acute Lung Injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L532–L541. [Google Scholar] [CrossRef] [PubMed]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, Hemopexin, and Related Defense Pathways-Basic Science, Clinical Perspectives, and Drug Development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef]
- Schaer, D.J.; Buehler, P.W. Cell-Free Hemoglobin and Its Scavenger Proteins: New Disease Models Leading the Way to Targeted Therapies. Cold Spring Harb. Perspect. Med. 2013, 3, a013433. [Google Scholar] [CrossRef]
- Smith, A.; McCulloh, R.J. Hemopexin and Haptoglobin: Allies against Heme Toxicity from Hemoglobin Not Contenders. Front. Physiol. 2015, 6, 187. [Google Scholar] [CrossRef]
- UniProt. Available online: https://www.uniprot.org/uniprotkb/Q9BXR5/entry (accessed on 12 September 2025).
- Oosting, M.; Cheng, S.-C.; Bolscher, J.M.; Vestering-Stenger, R.; Plantinga, T.S.; Verschueren, I.C.; Arts, P.; Garritsen, A.; van Eenennaam, H.; Sturm, P.; et al. Human TLR10 is an Anti-Inflammatory Pattern-Recognition Receptor. Proc. Natl. Acad. Sci. USA 2014, 111, E4478–E4484. [Google Scholar] [CrossRef]
- Su, S.-B.; Tao, L.; Deng, Z.-P.; Chen, W.; Qin, S.-Y.; Jiang, H.-X. TLR10: Insights, Controversies and Potential Utility as a Therapeutic Target. Scand. J. Immunol. 2021, 93, e12988. [Google Scholar] [CrossRef]
- Henrick, B.M.; Yao, X.-D.; Zahoor, M.A.; Abimiku, A.; Osawe, S.; Rosenthal, K.L. TLR10 Senses HIV-1 Proteins and Significantly Enhances HIV-1 Infection. Front. Immunol. 2019, 10, 482. [Google Scholar] [CrossRef]
- Nyman, T.; Stenmark, P.; Flodin, S.; Johansson, I.; Hammarström, M.; Nordlund, P. The Crystal Structure of the Human Toll-like Receptor 10 Cytoplasmic Domain Reveals a Putative Signaling Dimer. J. Biol. Chem. 2008, 283, 11861–11865. [Google Scholar] [CrossRef]
- Guan, Y.; Ranoa, D.R.E.; Jiang, S.; Mutha, S.K.; Li, X.; Baudry, J.; Tapping, R.I. Human TLRs 10 and 1 Share Common Mechanisms of Innate Immune Sensing but Not Signaling. J. Immunol. 2010, 184, 5094–5103. [Google Scholar] [CrossRef]
- Chuang, T.; Ulevitch, R.J. Identification of hTLR10: A Novel Human Toll-like Receptor Preferentially Expressed in Immune Cells. Biochim. Biophys. Acta 2001, 1518, 157–161. [Google Scholar] [CrossRef]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-like Receptor 1-10 mRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed]
- Regan, T.; Nally, K.; Carmody, R.; Houston, A.; Shanahan, F.; Macsharry, J.; Brint, E. Identification of TLR10 as a Key Mediator of the Inflammatory Response to Listeria Monocytogenes in Intestinal Epithelial Cells and Macrophages. J. Immunol. 2013, 191, 6084–6092. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Iwatani, S.; Cruz, M.; Jiménez Abreu, J.A.; Uchida, T.; Mahachai, V.; Vilaichone, R.; Graham, D.Y.; Yamaoka, Y. Toll-like Receptor 10 in Helicobacter Pylori Infection. J. Infect. Dis. 2015, 212, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Wu, Y.; Lian, S.; Yan, L.; Meng, X.; Duan, Q.; Zhu, G. Research Progress on Toll-like Receptor Signal Transduction and Its Roles in Antimicrobial Immune Responses. Appl. Microbiol. Biotechnol. 2021, 105, 5341–5355. [Google Scholar] [CrossRef]
- Neuper, T.; Frauenlob, T.; Sarajlic, M.; Posselt, G.; Wessler, S.; Horejs-Hoeck, J. TLR2, TLR4 and TLR10 Shape the Cytokine and Chemokine Release of H. Pylori-Infected Human DCs. Int. J. Mol. Sci. 2020, 21, 3897. [Google Scholar] [CrossRef]
- Pullamsetti, S.S.; Savai, R.; Janssen, W.; Dahal, B.K.; Seeger, W.; Grimminger, F.; Ghofrani, H.A.; Weissmann, N.; Schermuly, R.T. Inflammation, Immunological Reaction and Role of Infection in Pulmonary Hypertension. Clin. Microbiol. Infect. 2011, 17, 7–14. [Google Scholar] [CrossRef]
- Tomaszewski, M.; Bębnowska, D.; Hrynkiewicz, R.; Dworzyński, J.; Niedźwiedzka-Rystwej, P.; Kopeć, G.; Grywalska, E. Role of the Immune System Elements in Pulmonary Arterial Hypertension. J. Clin. Med. 2021, 10, 3757. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, L.; Shi, F.; Tang, M.; Li, Y.; Hu, J.; Zhao, L.; Zhao, L.; Yu, X.; Luo, X.; et al. Targeting the Signaling in Epstein–Barr Virus-Associated Diseases: Mechanism, Regulation, and Clinical Study. Signal Transduct. Target. Ther. 2021, 6, 15. [Google Scholar] [CrossRef]
- Huang, W.; Bai, L.; Tang, H. Epstein-Barr Virus Infection: The Micro and Macro Worlds. Virol. J. 2023, 20, 220. [Google Scholar] [CrossRef]
- Silva, J.D.M.; Alves, C.E.d.C.; Pontes, G.S. Epstein-Barr Virus: The Mastermind of Immune Chaos. Front. Immunol. 2024, 15, 1297994. [Google Scholar] [CrossRef]
- Kliszczewska, E.; Jarzyński, A.; Boguszewska, A.; Pasternak, J.; Polz-Dacewicz, M. Epstein-Barr Virus—Pathogenesis, Latency and Cancers. J. Pre-Clin. Clin. Res. 2017, 11, 142–146. [Google Scholar] [CrossRef]
- Pisano, G.; Roy, A.; Ahmed Ansari, M.; Kumar, B.; Chikoti, L.; Chandran, B. Interferon-γ-Inducible Protein 16 (IFI16) is Required for the Maintenance of Epstein-Barr Virus Latency. Virol. J. 2017, 14, 221. [Google Scholar] [CrossRef]
- Ansari, M.A.; Singh, V.V.; Dutta, S.; Veettil, M.V.; Dutta, D.; Chikoti, L.; Lu, J.; Everly, D.; Chandran, B. Constitutive Interferon-Inducible Protein 16-Inflammasome Activation during Epstein-Barr Virus Latency I, II, and III in B and Epithelial Cells. J. Virol. 2013, 87, 8606–8623, Erratum in J. Virol. 2017, 91, e01519-17. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, K.; Smolders, V.F.E.D.; Tura-Ceide, O.; Jukema, J.W.; Quax, P.H.A.; Goumans, M.-J. Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence? Biomedicines 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Sakata, Y.; Fukushima, K.; Maeda, T.; Arita, Y.; Shioyama, W.; Nakaoka, Y.; Hori, Y.; Morii, E.; Aozasa, K.; et al. Pulmonary Arterial Hypertension Associated with Chronic Active Epstein-Barr Virus Infection. Intern. Med. 2011, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Misaki, Y.; Minakata, D.; Ibe, T.; Gomyo, A.; Yoshimura, K.; Kimura, S.; Nakamura, Y.; Kawamura, M.; Kawamura, S.; Takeshita, J.; et al. Chronic Active Epstein-Bar Virus Infection Complicated by Pulmonary Artery Hypertension. J. Infect. Chemother. 2023, 29, 212–218. [Google Scholar] [CrossRef]
- Fukuda, Y.; Momoi, N.; Akaihata, M.; Nagasawa, K.; Mitomo, M.; Aoyagi, Y.; Endoh, K.; Hosoya, M. Pulmonary Arterial Hypertension Associated with Chronic Active Epstein-Barr Virus Infection. Pediatr. Int. 2015, 57, 731–734. [Google Scholar] [CrossRef]
- Egan, J.J.; Adamali, H.I.; Lok, S.S.; Stewart, J.P.; Woodcock, A.A. Ganciclovir Antiviral Therapy in Advanced Idiopathic Pulmonary Fibrosis: An Open Pilot Study. Pulm. Med. 2011, 2011, 240805. [Google Scholar] [CrossRef]
- Calabrese, F.; Kipar, A.; Lunardi, F.; Balestro, E.; Perissinotto, E.; Rossi, E.; Nannini, N.; Marulli, G.; Stewart, J.P.; Rea, F. Herpes Virus Infection is Associated with Vascular Remodeling and Pulmonary Hypertension in Idiopathic Pulmonary Fibrosis. PLoS ONE 2013, 8, e55715. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, A.; Longhurst, H.J.; Paxton, J.K.; Scotton, C.J. The Role of Herpes Viruses in Pulmonary Fibrosis. Front. Med. 2021, 8, 704222. [Google Scholar] [CrossRef] [PubMed]
- Savale, L.; Lador, F.; Jais, X.; Montani, D.; Simonneau, G.; Humbert, M.; Sitbon, O. HIV-related pulmonary arterial hypertension. Rev. Mal. Respir. 2012, 29, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.; Barjatya, H.C.; Bhakar, B.L.; Gothwal, S.K.; Jangir, T. To Estimate Prevalence of Pulmonary Arterial Hypertension in HIV Patients and Its Association with CD4 Cell Count. Clin. Epidemiol. Glob. Health 2024, 25, 101479. [Google Scholar] [CrossRef]
- Șoldea, S.; Iovănescu, M.; Berceanu, M.; Mirea, O.; Raicea, V.; Beznă, M.C.; Rocșoreanu, A.; Donoiu, I. Cardiovascular Disease in HIV Patients: A Comprehensive Review of Current Knowledge and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 1837. [Google Scholar] [CrossRef]
- Kim, K.K.; Factor, S.M. Membranoproliferative Glomerulonephritis and Plexogenic Pulmonary Arteriopathy in a Homosexual Man with Acquired Immunodeficiency Syndrome. Hum. Pathol. 1987, 18, 1293–1296. [Google Scholar] [CrossRef]
- HIV Data and Statistics. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 12 September 2025).
- Tseng, A.; Seet, J.; Phillips, E.J. The Evolution of Three Decades of Antiretroviral Therapy: Challenges, Triumphs and the Promise of the Future. Br. J. Clin. Pharmacol. 2015, 79, 182–194. [Google Scholar] [CrossRef]
- Masters, M.C.; Krueger, K.M.; Williams, J.L.; Morrison, L.; Cohn, S.E. Beyond One Pill, Once Daily: Current Challenges of Antiretroviral Therapy Management in the United States. Expert Rev. Clin. Pharmacol. 2019, 12, 1129–1143. [Google Scholar] [CrossRef]
- Woldegeorgis, B.Z.; Asgedom, Y.S.; Habte, A.; Kassie, G.A.; Badacho, A.S. Highly Active Antiretroviral Therapy is Necessary but Not Sufficient. A Systematic Review and Meta-Analysis of Mortality Incidence Rates and Predictors among HIV-Infected Adults Receiving Treatment in Ethiopia, a Surrogate Study for Resource-Poor Settings. BMC Public Health 2024, 24, 1735. [Google Scholar] [CrossRef]
- Kumar, A.; Mahajan, A.; Salazar, E.A.; Pruitt, K.; Guzman, C.A.; Clauss, M.A.; Almodovar, S.; Dhillon, N.K. Impact of Human Immunodeficiency Virus on Pulmonary Vascular Disease. Glob. Cardiol. Sci. Pract. 2021, 2021, e202112. [Google Scholar] [CrossRef]
- Chan, S.Y.; Loscalzo, J. Pathogenic Mechanisms of Pulmonary Arterial Hypertension. J. Mol. Cell Cardiol. 2008, 44, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Bigna, J.J.R.; Sime, P.S.D.; Koulla-Shiro, S. HIV Related Pulmonary Arterial Hypertension: Epidemiology in Africa, Physiopathology, and Role of Antiretroviral Treatment. AIDS Res. Ther. 2015, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Swami Vetha, B.S.; Chakraborty, R.; Wenegieme, T.-Y.; Masenga, S.K.; Muthian, G.; Balasubramaniam, M.; Wanjalla, C.N.; Hinton, A.O.; Kirabo, A.; et al. HIV-Associated Hypertension: Risks, Mechanisms, and Knowledge Gaps. Circ. Res. 2024, 134, e150–e175. [Google Scholar] [CrossRef] [PubMed]
- Palakeel, J.J.; Ali, M.; Chaduvula, P.; Chhabra, S.; Lamsal Lamichhane, S.; Ramesh, V.; Opara, C.O.; Khan, F.Y.; Kabiraj, G.; Kauser, H.; et al. An Outlook on the Etiopathogenesis of Pulmonary Hypertension in HIV. Cureus 2022, 14, e27390. [Google Scholar] [CrossRef]
- Singh, T.; Motes, A.; Vinan-Vega, M.; Nugent, K. Pulmonary Arterial Hypertension in Human Immunodeficiency Virus Infections. Southwest Respir. Crit. Care Chron. 2024, 12, 12–18. [Google Scholar] [CrossRef]
- Chandran, S.; Adler, M.; Chen, L.; Kaur, S.; Dhillon, N.K. HIV-Tat and Vascular Endothelium: Implications in the HIV Associated Brain, Heart, and Lung Complications. Front. Immunol. 2025, 16, 1621338. [Google Scholar] [CrossRef]
- Kovacs, L.; Khakina, B.N.; de Chantemèle, E.J.B. Role of HIV-Encoded Proteins in Cardiovascular Disease. Am. J. Physiol. Cell Physiol. 2025, 329, C592–C598. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- Butrous, G. Human Immunodeficiency Virus–Associated Pulmonary Arterial Hypertension. Circulation 2015, 131, 1361–1370. [Google Scholar] [CrossRef]
- Henriques-Forsythe, M.; Annangi, S.; Farber, H.W. Prevalence and Hospital Discharge Status of Human Immunodeficiency Virus–Associated Pulmonary Arterial Hypertension in the United States. Pulm. Circ. 2015, 5, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.M.; Walp, E.R.; Elms, S.C.; Raynor, R.; Mitchell, P.O.; Guidot, D.M.; Sutliff, R.L. Human Immunodeficiency Virus-1 Transgene Expression Increases Pulmonary Vascular Resistance and Exacerbates Hypoxia-Induced Pulmonary Hypertension Development. Pulm. Circ. 2013, 3, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Marecki, J.C.; Cool, C.D.; Parr, J.E.; Beckey, V.E.; Luciw, P.A.; Tarantal, A.F.; Carville, A.; Shannon, R.P.; Cota-Gomez, A.; Tuder, R.M.; et al. HIV-1 Nef Is Associated with Complex Pulmonary Vascular Lesions in SHIV-Nef–Infected Macaques. Am. J. Respir. Crit. Care Med. 2006, 174, 437–445. [Google Scholar] [CrossRef]
- Garcia, A.K.; Almodovar, S. The Intersection of HIV and Pulmonary Vascular Health: From HIV Evolution to Vascular Cell Types to Disease Mechanisms. J. Vasc. Dis. 2024, 3, 174–200. [Google Scholar] [CrossRef]
- Morozov, V.A.; Lagaye, S. Hepatitis C Virus: Morphogenesis, Infection and Therapy. World J. Hepatol. 2018, 10, 186–212. [Google Scholar] [CrossRef]
- Suhail, M.; Sohrab, S.S.; Kamal, M.A.; Azhar, E.I. Role of Hepatitis C Virus in Hepatocellular Carcinoma and Neurological Disorders: An Overview. Front. Oncol. 2022, 12, 913231. [Google Scholar] [CrossRef]
- Topi, S.; Gaxhja, E.; Charitos, I.A.; Colella, M.; Santacroce, L. Hepatitis C Virus: History and Current Knowledge. Gastroenterol. Insights 2024, 15, 676–707. [Google Scholar] [CrossRef]
- Mazumder, H.; Hossain, M.F.; Shrestha, P.; Mahmud, S.; Husain, M.; Ahmed, R. Prevalence and Associated Risk Factors of Current Hepatitis C Infection among U.S. General Population and Injection Drug Users Aged 20–59 Years: NHANES 2009–2018. PLoS ONE 2024, 19, e0309345. [Google Scholar] [CrossRef]
- Stroffolini, T.; Stroffolini, G. Prevalence and Modes of Transmission of Hepatitis C Virus Infection: A Historical Worldwide Review. Viruses 2024, 16, 1115. [Google Scholar] [CrossRef]
- Huang, D.Q.; Terrault, N.A.; Tacke, F.; Gluud, L.L.; Arrese, M.; Bugianesi, E.; Loomba, R. Global Epidemiology of Cirrhosis—Aetiology, Trends and Predictions. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 388–398. [Google Scholar] [CrossRef]
- Kwong, A.; Kim, W.R.; Mannalithara, A.; Heo, N.-Y.; Udompap, P.; Kim, D. Decreasing Mortality and Disease Severity in Hepatitis C Patients Awaiting Liver Transplantation in the United States. Liver Transpl. 2018, 24, 735–743. [Google Scholar] [CrossRef]
- Renard, S.; Borentain, P.; Salaun, E.; Benhaourech, S.; Maille, B.; Darque, A.; Bregigeon, S.; Colson, P.; Laugier, D.; Gaubert, M.R.; et al. Severe Pulmonary Arterial Hypertension in Patients Treated for Hepatitis C With Sofosbuvir. Chest 2016, 149, e69–e73. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Kioka, H.; Ozu, K.; Ohtani, T.; Yamaguchi, O.; Yazaki, Y.; Yamauchi-Takihara, K.; Sakata, Y. Interferon Therapy Exacerbated Pulmonary Hypertension in a Patient with Hepatitis C Virus Infection: Pathogenic Interplay among Multiple Risk Factors. Intern. Med. 2017, 56, 1061–1065. [Google Scholar] [CrossRef][Green Version]
- Doherty, M.; Bhindi, A.; Kilpin, M.; Jeganathan, V.; Fisher, L.; Chimunda, T. A Case of Pulmonary Arterial Hypertension Secondary to Hepatitis C Direct Antiviral Treatment with Harvoni® (Ledipasvir/Sofosbuvir Fixed-Dose Combination). Aust. Crit. Care 2020, 33, S37. [Google Scholar] [CrossRef]
- Savale, L.; Sattler, C.; Günther, S.; Montani, D.; Chaumais, M.-C.; Perrin, S.; Jaïs, X.; Seferian, A.; Jovan, R.; Bulifon, S.; et al. Pulmonary Arterial Hypertension in Patients Treated with Interferon. Eur. Respir. J. 2014, 44, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Waris, G.; Tanveer, R.; Siddiqui, A. Human Hepatitis C Virus NS5A Protein Alters Intracellular Calcium Levels, Induces Oxidative Stress, and Activates STAT-3 and NF-Kappa B. Proc. Natl. Acad. Sci. USA 2001, 98, 9599–9604. [Google Scholar] [CrossRef] [PubMed]
- Paulin, R.; Courboulin, A.; Meloche, J.; Mainguy, V.; Dumas de la Roque, E.; Saksouk, N.; Côté, J.; Provencher, S.; Sussman, M.A.; Bonnet, S. Signal Transducers and Activators of Transcription-3/Pim1 Axis Plays a Critical Role in the Pathogenesis of Human Pulmonary Arterial Hypertension. Circulation 2011, 123, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Guedj, J.; Pang, P.S.; Denning, J.; Rodriguez-Torres, M.; Lawitz, E.; Symonds, W.; Perelson, A.S. Analysis of Hepatitis C Viral Kinetics during Administration of Two Nucleotide Analogues: Sofosbuvir (GS-7977) and GS-0938. Antivir. Ther. 2014, 19, 211–220. [Google Scholar] [CrossRef]
- Garcia-Montojo, M.; Doucet-O’Hare, T.; Henderson, L.; Nath, A. Human Endogenous Retrovirus-K (HML-2): A Comprehensive Review. Crit. Rev. Microbiol. 2018, 44, 715–738. [Google Scholar] [CrossRef]
- Lavie, L.; Medstrand, P.; Schempp, W.; Meese, E.; Mayer, J. Human Endogenous Retrovirus Family HERV-K(HML-5): Status, Evolution, and Reconstruction of an Ancient Betaretrovirus in the Human Genome. J. Virol. 2004, 78, 8788–8798. [Google Scholar] [CrossRef]
- Contreras-Galindo, R.; Kaplan, M.H.; Dube, D.; Gonzalez-Hernandez, M.J.; Chan, S.; Meng, F.; Dai, M.; Omenn, G.S.; Gitlin, S.D.; Markovitz, D.M. Human Endogenous Retrovirus Type K (HERV-K) Particles Package and Transmit HERV-K–Related Sequences. J. Virol. 2015, 89, 7187–7201. [Google Scholar] [CrossRef] [PubMed]
- Simula, E.R.; Jasemi, S.; Cossu, D.; Fais, M.; Cossu, I.; Chessa, V.; Canu, M.; Sechi, L.A. Human Endogenous Retroviruses as Novel Therapeutic Targets in Neurodegenerative Disorders. Vaccines 2025, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Johanning, G.L.; Malouf, G.G.; Zheng, X.; Esteva, F.J.; Weinstein, J.N.; Wang-Johanning, F.; Su, X. Expression of Human Endogenous Retrovirus-K is Strongly Associated with the Basal-like Breast Cancer Phenotype. Sci. Rep. 2017, 7, 41960. [Google Scholar] [CrossRef]
- Dou, A.; Xu, J.; Zhou, C. The Relationship between HERVs and Exogenous Viral Infections: A Focus on the Value of HERVs in Disease Prediction and Treatment. Virulence 2025, 16, 2523888. [Google Scholar] [CrossRef]
- Mantovani, F.; Kitsou, K.; Magiorkinis, G. HERVs: Expression Control Mechanisms and Interactions in Diseases and Human Immunodeficiency Virus Infection. Genes 2024, 15, 192. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, S.; Liang, J.Q. Transcriptional and Reverse Transcriptional Regulation of Host Genes by Human Endogenous Retroviruses in Cancers. Front. Microbiol. 2022, 13, 946296. [Google Scholar] [CrossRef]
- Stricker, E.; Peckham-Gregory, E.C.; Scheurer, M.E. HERVs and Cancer—A Comprehensive Review of the Relationship of Human Endogenous Retroviruses and Human Cancers. Biomedicines 2023, 11, 936. [Google Scholar] [CrossRef]
- Costa, B.; Vale, N. Exploring HERV-K (HML-2) Influence in Cancer and Prospects for Therapeutic Interventions. Int. J. Mol. Sci. 2023, 24, 14631. [Google Scholar] [CrossRef]
- Kozubek, P.; Kuźniar, J.; Czaja, M.; Sitka, H.; Kochman, U.; Leszek, J. Human Endogenous Retroviruses and Their Putative Role in Pathogenesis of Alzheimer’s Disease, Inflammation, and Senescence. Biomedicines 2025, 13, 59. [Google Scholar] [CrossRef]
- Saito, T.; Miyagawa, K.; Chen, S.-Y.; Tamosiuniene, R.; Wang, L.; Sharpe, O.; Samayoa, E.; Harada, D.; Moonen, J.-R.A.J.; Cao, A.; et al. Upregulation of Human Endogenous Retrovirus-K is Linked to Immunity and Inflammation in Pulmonary Arterial Hypertension. Circulation 2017, 136, 1920–1935. [Google Scholar] [CrossRef]
- Culley, M.K.; Chan, S.Y. Human Endogenous Retrovirus K and Pulmonary Arterial Hypertension. Circulation 2017, 136, 1936–1938. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, W.; Gu, S.; Rabinovitch, M.; Nicolls, M.R.; Snyder, M.P. Microbiome–Immune Interaction in Pulmonary Arterial Hypertension: What Have We Missed? Research 2025, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Isobe, S.; Cao, A.; Contrepois, K.; Benayoun, B.A.; Jiang, L.; Wang, L.; Melemenidis, S.; Ozen, M.O.; Otsuki, S.; et al. Endogenous Retroviral Elements Generate Pathologic Neutrophils in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2022, 206, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, S.; Saito, T.; Taylor, S.; Li, D.; Moonen, J.-R.; Marciano, D.P.; Harper, R.L.; Cao, A.; Wang, L.; Ariza, M.E.; et al. Monocyte-Released HERV-K dUTPase Engages TLR4 and MCAM Causing Endothelial Mesenchymal Transition. JCI Insight 2021, 6, e146416. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.L.; Zhou, X.; Marciano, D.P.; Cao, A.; Wang, L.; Chen, G.; Adil, M.S.; Zhou, W.; Maguire, P.; Deivanayagam, S.; et al. Altered Maturation and Activation State of Circulating Monocytes is Associated with Their Enhanced Recruitment in Pulmonary Arterial Hypertension. Respir. Res. 2025, 26, 148. [Google Scholar] [CrossRef]
- Hinojosa, W.; Cristo-Ropero, M.J.; Cruz-Utrilla, A.; Segura de la Cal, T.; López-Medrano, F.; Salguero-Bodes, R.; Pérez-Olivares, C.; Navarro, B.; Ochoa, N.; Arribas Ynsurriaga, F.; et al. The Impact of COVID-19 Pandemic on Pulmonary Hypertension: What Have We Learned? Pulm. Circ. 2022, 12, e12142. [Google Scholar] [CrossRef]
- Farha, S.; Heresi, G.A. COVID-19 and Pulmonary Arterial Hypertension: Early Data and Many Questions. Ann. Am. Thorac. Soc. 2020, 17, 1528–1530. [Google Scholar] [CrossRef]
- Bertici, N.S.; Dobrescu, L.; Pislaru, L.; Marincu, I. Controversies over the Impact of the COVID-19 Pandemic on Patients with Pulmonary Arterial Hypertension. Eur. Respir. J. 2021, 58, PA3587. [Google Scholar] [CrossRef]
- Tudoran, C.; Tudoran, M.; Lazureanu, V.E.; Marinescu, A.R.; Pop, G.N.; Pescariu, A.S.; Enache, A.; Cut, T.G. Evidence of Pulmonary Hypertension after SARS-CoV-2 Infection in Subjects without Previous Significant Cardiovascular Pathology. J. Clin. Med. 2021, 10, 199. [Google Scholar] [CrossRef]
- Soliman, Y.M.A.; Elkorashy, R.I.M.; Aziz, A.A.; Abdelnaby, A.; Magdy, S. Impact and Predictors of Outcome of COVID-19 in Pulmonary Hypertension Patients. Egypt. J. Bronchol. 2022, 16, 56. [Google Scholar] [CrossRef]
- Charif, F.; Dakroub, F.; Bou Akl, I.; Kularatne, M.; Montani, D. Pulmonary Arterial Hypertension and COVID-19: Piecing the Puzzle. Respir. Med. Res. 2023, 84, 101053. [Google Scholar] [CrossRef] [PubMed]
- Mamzer, A.; Waligora, M.; Kopec, G.; Ptaszynska-Kopczynska, K.; Kurzyna, M.; Darocha, S.; Florczyk, M.; Mroczek, E.; Mularek-Kubzdela, T.; Smukowska-Gorynia, A.; et al. Impact of the COVID-19 Pandemic on Pulmonary Hypertension Patients: Insights from the BNP-PL National Database. Int. J. Environ. Res. Public Health 2022, 19, 8423. [Google Scholar] [CrossRef] [PubMed]
- Al-Jahdhami, I.; Al-Mawali, A.; Bennji, S.M. Respiratory Complications after COVID-19. Oman Med. J. 2022, 37, e343. [Google Scholar] [CrossRef]
- Ambardar, S.R.; Hightower, S.L.; Huprikar, N.A.; Chung, K.K.; Singhal, A.; Collen, J.F. Post-COVID-19 Pulmonary Fibrosis: Novel Sequelae of the Current Pandemic. J. Clin. Med. 2021, 10, 2452. [Google Scholar] [CrossRef]
- Cueto-Robledo, G.; Porres-Aguilar, M.; Puebla-Aldama, D.; del Pilar Barragan-Martinez, M.; Jurado-Hernández, M.Y.; García-César, M.; Rojas, M.B.T.; García-Treminio, C.; Roldan-Valadez, E. Severe Pulmonary Hypertension: An Important Sequel After Severe Post-Acute COVID-19 Pneumonia. Curr. Probl. Cardiol. 2022, 47, 101004. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Larson, C.; Hammond, T.C.; Oronsky, A.; Kesari, S.; Lybeck, M.; Reid, T.R. A Review of Persistent Post-COVID Syndrome (PPCS). Clin. Rev. Allergy Immunol. 2023, 64, 66–74. [Google Scholar] [CrossRef]
- Zheng, M.; Karki, R.; Williams, E.P.; Yang, D.; Fitzpatrick, E.; Vogel, P.; Jonsson, C.B.; Kanneganti, T.-D. TLR2 Senses the SARS-CoV-2 Envelope Protein to Produce Inflammatory Cytokines. Nat. Immunol. 2021, 22, 829–838. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.W.; Savani, R.C.; Zaki, H. SARS-CoV-2 Spike Protein Induces Inflammation via TLR2-Dependent Activation of the NF-κB Pathway. eLife 2021, 10, e68563. [Google Scholar] [CrossRef]
- Halajian, E.A.; LeBlanc, E.V.; Gee, K.; Colpitts, C.C. Activation of TLR4 by Viral Glycoproteins: A Double-Edged Sword? Front. Microbiol. 2022, 13, 1007081. [Google Scholar] [CrossRef]
- Asano, T.; Boisson, B.; Onodi, F.; Matuozzo, D.; Moncada-Velez, M.; Maglorius Renkilaraj, M.R.L.; Zhang, P.; Meertens, L.; Bolze, A.; Materna, M.; et al. X-Linked Recessive TLR7 Deficiency in ~1% of Men under 60 Years Old with Life-Threatening COVID-19. Sci. Immunol. 2021, 6, eabl4348. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial Cell Infection and Endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Ejikeme, C.; Safdar, Z. Exploring the Pathogenesis of Pulmonary Vascular Disease. Front. Med. 2024, 11, 1402639. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, P.K.; Chatterjee, S. COVID-19 and Dys-Regulation of Pulmonary Endothelium: Implications for Vascular Remodeling. Cytokine Growth Factor Rev. 2022, 63, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Haberecker, M.; Schwarz, E.I.; Steiger, P.; Frontzek, K.; Scholkmann, F.; Zeng, X.; Höller, S.; Moch, H.; Varga, Z. Autopsy-Based Pulmonary and Vascular Pathology: Pulmonary Endotheliitis and Multi-Organ Involvement in COVID-19 Associated Deaths. Respiration 2022, 101, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.G.; da Cruz Rattis, B.A.; Ottaviani, G.; Celes, M.R.N.; Dias, E.P. ACE2 Down-Regulation May Act as a Transient Molecular Disease Causing RAAS Dysregulation and Tissue Damage in the Microcirculatory Environment Among COVID-19 Patients. Am. J. Pathol. 2021, 191, 1154–1164. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting Enzyme 2 (ACE2), SARS-CoV-2 and the Pathophysiology of Coronavirus Disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Gagliardi, S.; Hotchkin, T.; Tibebe, H.; Hillmer, G.; Marquez, D.; Izumi, C.; Chang, J.; Diggs, A.; Ezaki, J.; Suzuki, Y.J.; et al. The Renin–Angiotensin System Modulates SARS-CoV-2 Entry via ACE2 Receptor. Viruses 2025, 17, 1014. [Google Scholar] [CrossRef]
- Tiwari, N.R.; Phatak, S.; Sharma, V.R.; Agarwal, S.K. COVID-19 and Thrombotic Microangiopathies. Thromb. Res. 2021, 202, 191–198. [Google Scholar] [CrossRef]
- Safak, S.; Aksoy, E.; Dirim, A.B.; Demir, E.; Garayeva, N.; Oto, O.A.; Artan, A.S.; Yazici, H.; Besisik, S.; Turkmen, A. Successful Treatment of a COVID-19 Patient with Thrombotic Microangiopathy. Clin. Kidney J. 2021, 14, 1287–1288. [Google Scholar] [CrossRef]
- Baykara, Y.; Sevgi, K.; Akgun, Y. COVID-19 Microangiopathy: Insights into plasma exchange as a therapeutic strategy. Rev. Bras. Hematol. Hemoter. 2025, 47, 103963. [Google Scholar] [CrossRef]
- Smadja, D.M.; Mentzer, S.J.; Fontenay, M.; Laffan, M.A.; Ackermann, M.; Helms, J.; Jonigk, D.; Chocron, R.; Pier, G.B.; Gendron, N.; et al. COVID-19 is a Systemic Vascular Hemopathy: Insight for Mechanistic and Clinical Aspects. Angiogenesis 2021, 24, 755–788. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Schwartz, M.A.; Simons, M. Endothelial-to-Mesenchymal Transition, Vascular Inflammation, and Atherosclerosis. Front. Cardiovasc. Med. 2020, 7, 53. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, C.; Zhang, M.; Tong, X.; Xie, Y.; Yan, R.; Wang, X.; Zhang, X.; Liu, D.; Li, S. Single Cell Meta-Analysis of EndMT and EMT State in COVID-19. Front. Immunol. 2022, 13, 976512. [Google Scholar] [CrossRef]
- Ciszewski, W.M.; Woźniak, L.A.; Sobierajska, K. Diverse Roles of SARS-CoV-2 Spike and Nucleocapsid Proteins in EndMT Stimulation through the TGF-β-MRTF Axis Inhibited by Aspirin. Cell Commun. Signal 2024, 22, 296. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Alastra, G.; Cescatti, M.; Quadalti, C.; Lorenzini, L.; Giardino, L.; Calzà, L. SARS-CoV-2-Related Peptides Induce Endothelial-to-Mesenchymal Transition in Endothelial Capillary Cells Derived from Different Body Districts: Focus on Membrane (M) Protein. Cell Tissue Res. 2024, 397, 241–262. [Google Scholar] [CrossRef]
- Yamada, T.; Takaoka, A. Innate Immune Recognition against SARS-CoV-2. Inflamm. Regen. 2023, 43, 7. [Google Scholar] [CrossRef]
- Asaba, C.N.; Ekabe, C.J.; Ayuk, H.S.; Gwanyama, B.N.; Bitazar, R.; Bukong, T.N. Interplay of TLR4 and SARS-CoV-2: Unveiling the Complex Mechanisms of Inflammation and Severity in COVID-19 Infections. JIR 2024, 17, 5077–5091. [Google Scholar] [CrossRef]
- Xu, S.; Ilyas, I.; Weng, J. Endothelial Dysfunction in COVID-19: An Overview of Evidence, Biomarkers, Mechanisms and Potential Therapies. Acta Pharmacol. Sin. 2023, 44, 695–709. [Google Scholar] [CrossRef]
- Aljadah, M.; Khan, N.; Beyer, A.M.; Chen, Y.; Blanker, A.; Widlansky, M.E. Clinical Implications of COVID-19-Related Endothelial Dysfunction. JACC Adv. 2024, 3, 101070. [Google Scholar] [CrossRef]
- Oliveira, R.K.F.; Nyasulu, P.S.; Iqbal, A.A.; Hamdan Gul, M.; Ferreira, E.V.M.; Leclair, J.W.; Htun, Z.M.; Howard, L.S.; Mocumbi, A.O.; Bryant, A.J.; et al. Cardiopulmonary Disease as Sequelae of Long-Term COVID-19: Current Perspectives and Challenges. Front. Med. 2022, 9, 1041236. [Google Scholar] [CrossRef] [PubMed]
- Kauppert, C.A.; Dvorak, I.; Kollert, F.; Heinemann, F.; Jörres, R.A.; Pfeifer, M.; Budweiser, S. Pulmonary Hypertension in Obesity-Hypoventilation Syndrome. Respir. Med. 2013, 107, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Certain, M.-C.; Weatherald, J.; Jaïs, X.; Bulifon, S.; Noel-Savina, E.; Nieves, A.; Renard, S.; Traclet, J.; Bouvaist, H.; et al. COVID-19 in Patients with Pulmonary Hypertension: A National Prospective Cohort Study. Am. J. Respir. Crit. Care Med. 2022, 206, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Riou, M.; Coste, F.; Meyer, A.; Enache, I.; Talha, S.; Charloux, A.; Reboul, C.; Geny, B. Mechanisms of Pulmonary Vasculopathy in Acute and Long-Term COVID-19: A Review. Int. J. Mol. Sci. 2024, 25, 4941. [Google Scholar] [CrossRef]
- Eroume, À. Egom, E.; Shiwani, H.A.; Nouthe, B. From Acute SARS-CoV-2 Infection to Pulmonary Hypertension. Front. Physiol. 2022, 13, 1023758. [Google Scholar] [CrossRef]
- Ciurzyński, M.; Kurzyna, M.; Kopeć, G.; Błaszczak, P.; Chrzanowski, Ł.; Kamiński, K.; Mizia-Stec, K.; Mularek-Kubzdela, T.; Biederman, A.; Zieliński, D.; et al. An Expert Opinion of the Polish Cardiac Society Working Group on Pulmonary Circulation on Screening for Chronic Thromboembolic Pulmonary Hypertension Patients after Acute Pulmonary Embolism: Update. Pol. Heart J. 2022, 80, 723–732. [Google Scholar] [CrossRef]
- Taha, H.A.; Elshafey, B.I.; Abdullah, T.M.; Salem, H.A. Study of Pulmonary Hypertension in Post-COVID-19 Patients by Transthoracic Echocardiography. Egypt. J. Bronchol. 2023, 17, 27. [Google Scholar] [CrossRef]
- Puk, O.; Nowacka, A.; Smulewicz, K.; Mocna, K.; Bursiewicz, W.; Kęsy, N.; Kwiecień, J.; Wiciński, M. Pulmonary Artery Targeted Therapy in Treatment of COVID-19 Related ARDS. Literature Review. Biomed. Pharmacother. 2022, 146, 112592. [Google Scholar] [CrossRef]
- Badagliacca, R.; Sciomer, S.; Petrosillo, N. Endothelin Receptor Antagonists for Pulmonary Arterial Hypertension and COVID-19: Friend or Foe? J. Heart Lung Transplant. 2020, 39, 729–730. [Google Scholar] [CrossRef]
- Nuche, J.; Pérez-Olivares, C.; de la Cal, T.S.; López-Guarch, C.J.; Ynsaurriaga, F.A.; Subías, P.E. Clinical Course of COVID-19 in Pulmonary Arterial Hypertension Patients. Rev. Española Cardiol. 2020, 73, 775–778. [Google Scholar] [CrossRef]
- Pęksa, J.W. Diagnosis and Treatment of Pulmonary Hypertension—Based on the 2022 ESC Guidelines. Lek. POZ 2023, 9, 7–15. [Google Scholar]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension: Developed by the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on Rare Respiratory Diseases (ERN-LUNG). Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef]
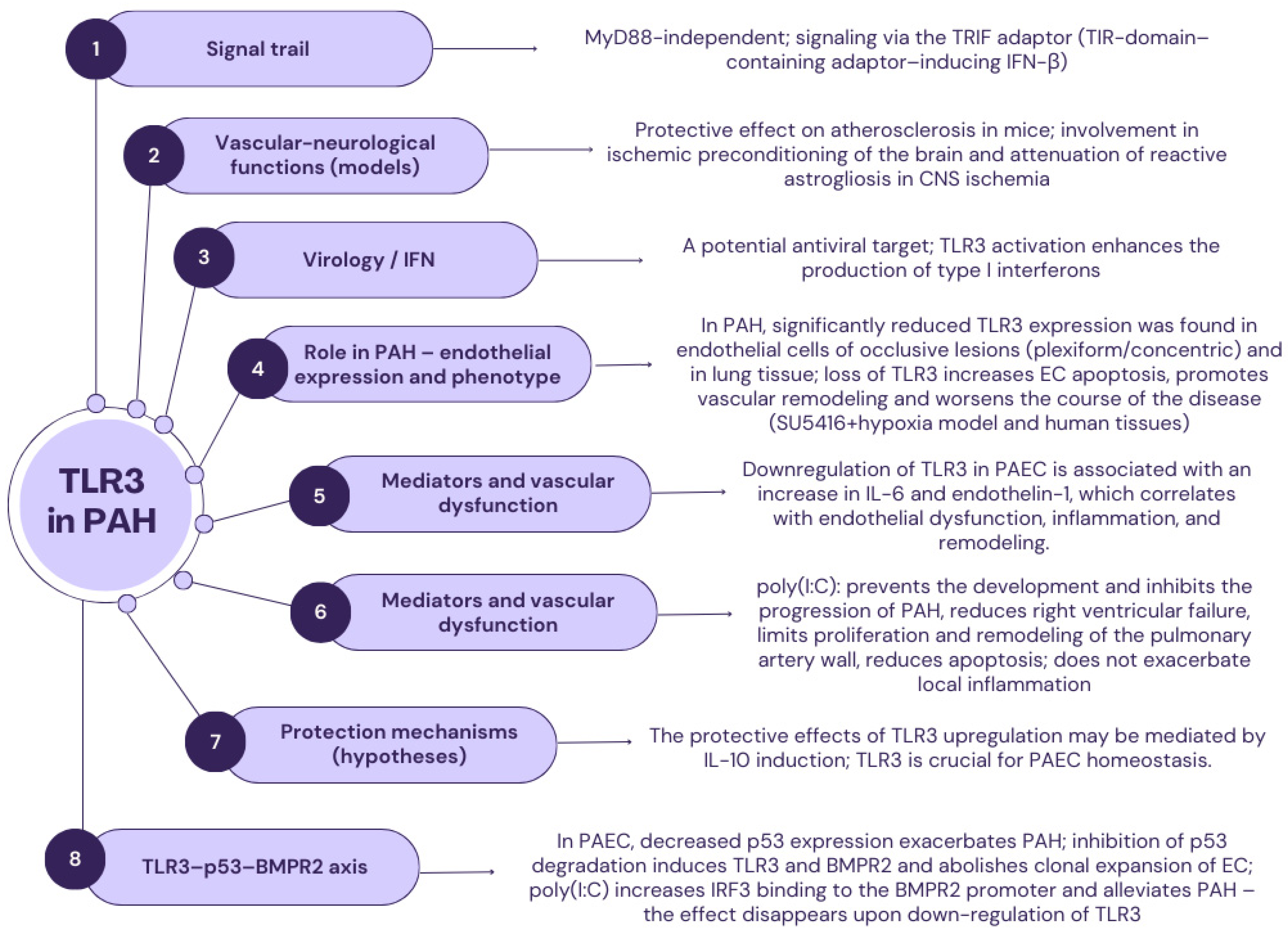
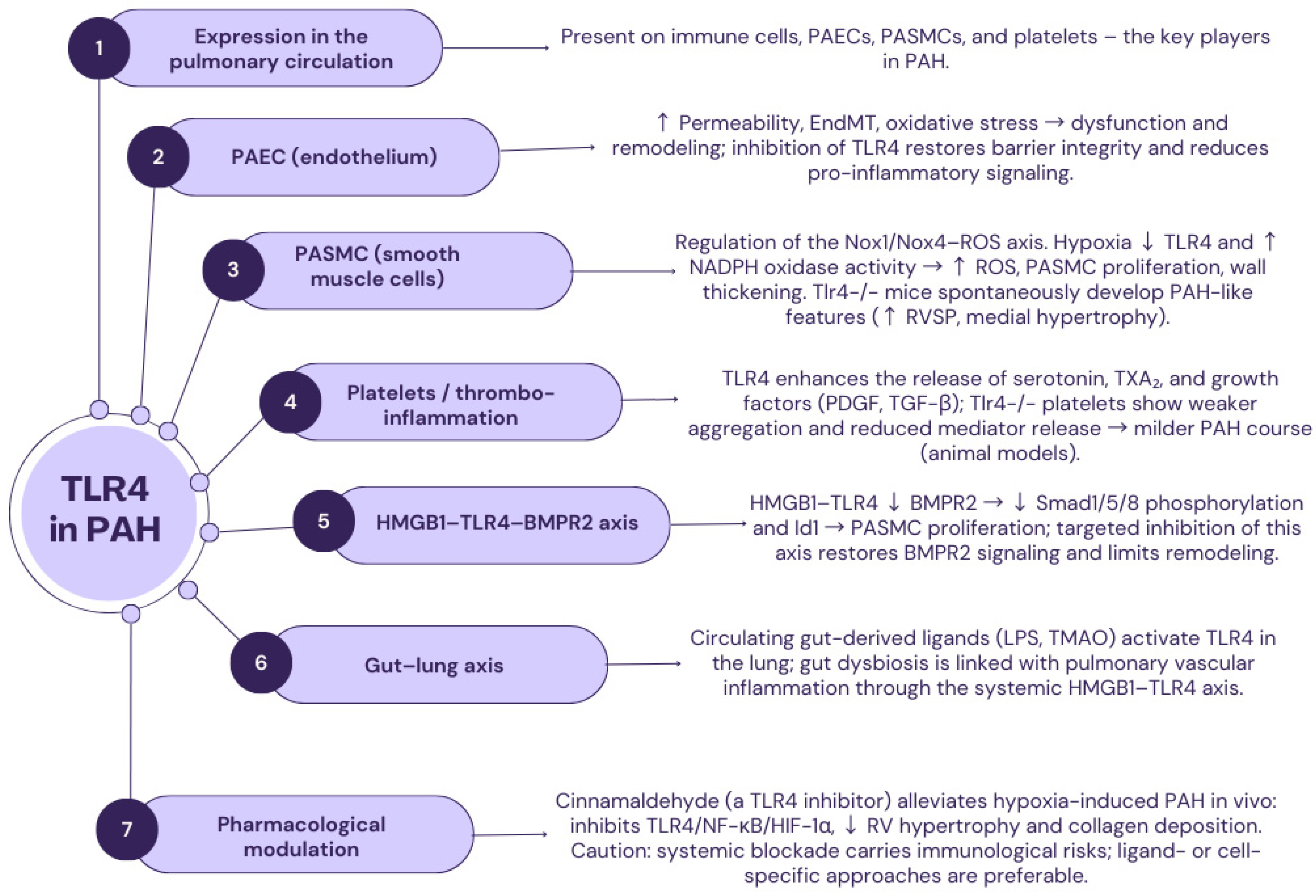
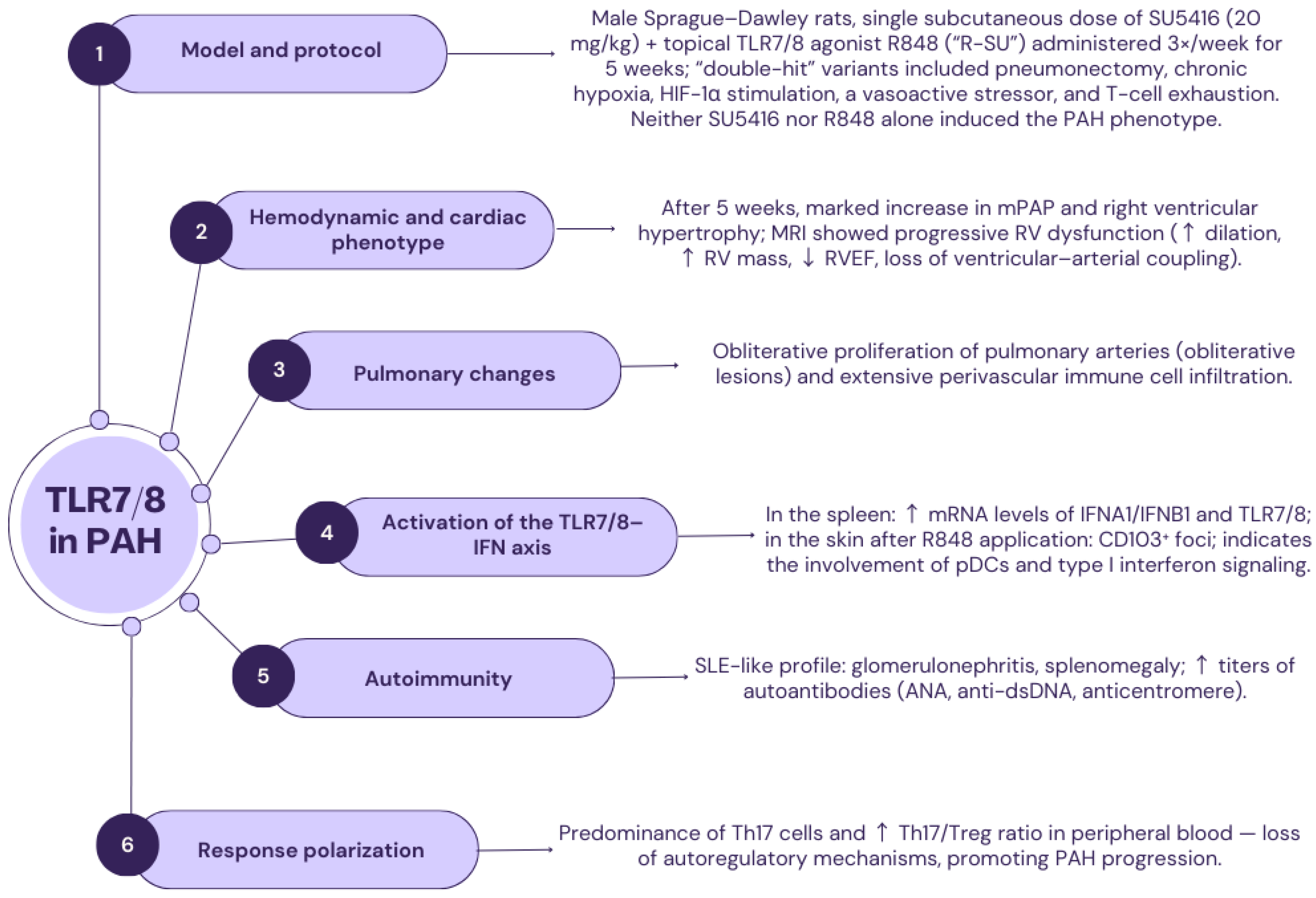
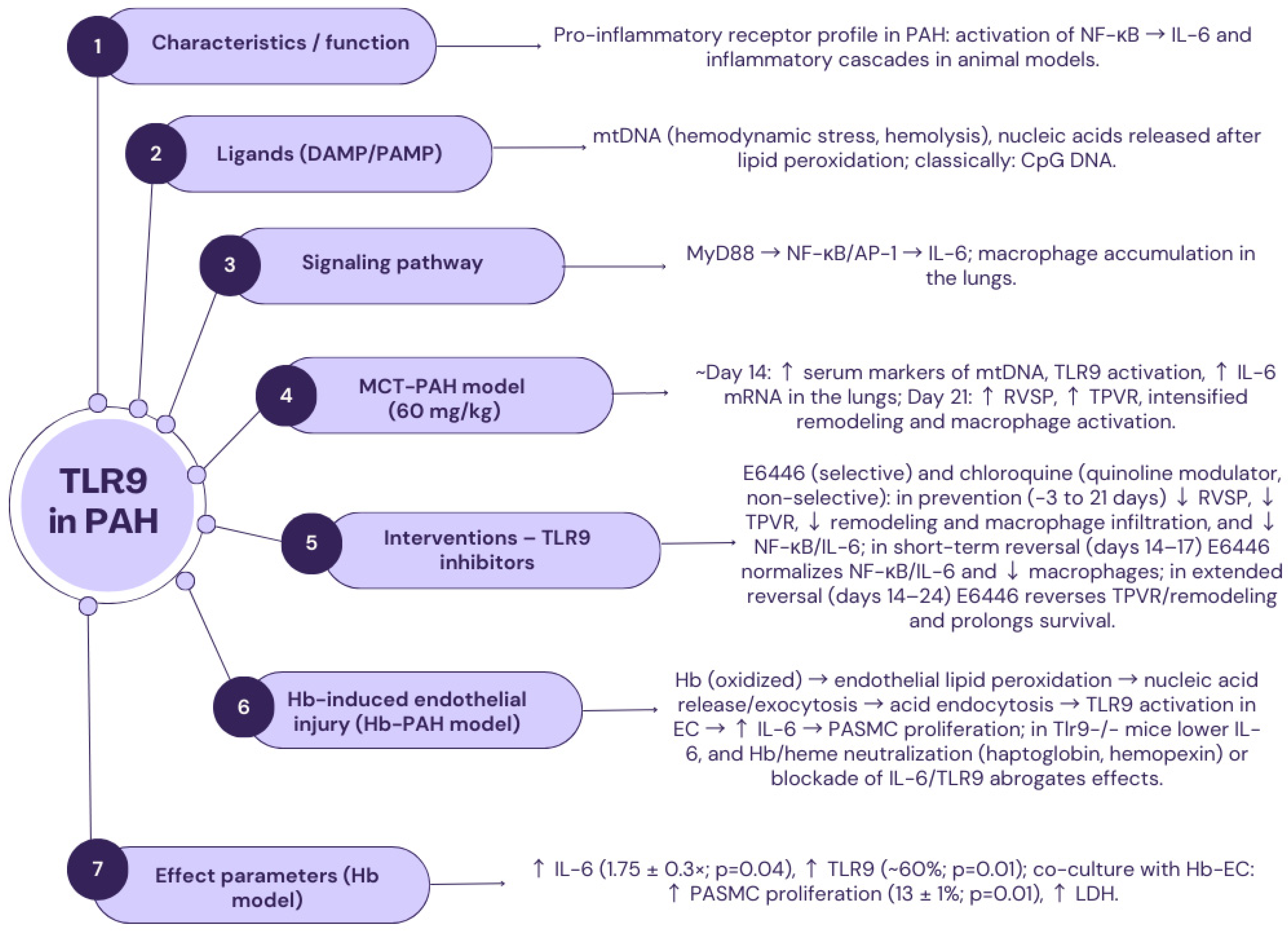
| Aspect | TLR1 | TLR2 |
|---|---|---|
| Regulation/expression in PAH (pre-clinical models) | ↓ Expression in the lungs of rats with MT-PAH; positive correlation with neutrophil and monocyte counts, negative correlation with resting CD4+ lymphocytes | ↑ Lung expression in MT-PAH; macrophage infiltration, activation of the TLR–RIPK3 axis, and dysregulation of TLR/NLR pathways promote progression of vascular remodeling |
| Compartments/target cells (inference from data) | Modulation of the innate component (neutrophils, monocytes) and, indirectly, T lymphocytes; role in sensing pathogenic stimuli | Endothelial cells (EC), pulmonary artery smooth muscle (PASMC), fibroblasts, leukocytes; high activity in the pulmonary vascular microenvironment |
| Signal paths | Mainly MyD88 → NF-κB, induction of inflammatory mediators (inferred from TLR1/2 function) | MyD88 → NF-κB; induction of IL-1, IL-6, TNF-α; association with RIPK3 and interactions with NLRs |
| Impact on key mechanisms for PAH | Indirectly enhancing innate effector cell recruitment; potentially driving neutrophil/monocyte-dependent inflammatory responses | ↓ PASMC apoptosis; ↑ PASMC proliferation; ↑ TGF-β/PDGF; fibroblast activation and ECM deposition; ↑ oxidative stress; ↑ ICAM-1/VCAM-1 (facilitated leukocyte migration); EC dysfunction (permeability, ↓ NO) |
| Vascular/hemodynamic consequences | Potential involvement in the initiation and maintenance of inflammation affecting vascular remodeling | Increased vascular remodeling and inflammation; correlation with severity and deterioration of right ventricular function in animal models |
| Biomarkers and therapeutic implications | Candidate immunoinfiltration biomarker (requires translational validation) | Potential biomarker of inflammatory activity; modification of TLR2 activity considered a therapeutic target in PAH |
| Aspect | TLR5 | TLR6 (with TLR2) |
|---|---|---|
| Status of evidence in PAH | No dedicated functional studies have been conducted in PAH models; however, transcriptomic analyses indicate a significant induction of TLR5 in the lungs of patients and mice with PH. | No direct clinical/animal studies in PAH; available RNA-seq analyses and data from stimulation of the TLR2/6 heterodimer in PASMCs. |
| Ligand(s)/recognition | Bacterial flagellin. | The TLR2/6 heterodimer recognizes diacylated bacterial lipopeptides (e.g., MALP-2). |
| Canonical signaling pathway | MyD88 → IRAK → NF-κB → TNF-α, IL-6, IL-8. | MyD88 → NF-κB (via TLR2/6 heterodimer) → IL-1β, IL-6, TNF-α. |
| Cellular/vascular effects (inferred) | Induction of inflammation similar to TLR2/TLR4: leukocyte recruitment, oxidative stress, vascular wall remodeling. | Promotion of proliferation and vascular remodeling, an increase in endothelin-1, and persistence of inflammation in the pulmonary microenvironment. |
| Angiogenesis/repair | No data available. | The TLR2/6 complex enhances angiogenesis (via GM-CSF) both in vitro and in vivo, supporting blood flow, immune cell recruitment, and tissue regeneration (e.g., in the liver). |
| Genetic associations | No reports in the cited material. | TLR6 A359T>C: predisposition to Legionnaires’ disease; Ser249Pro: possible association with asthma in selected populations. |
| Implications for PAH | Potential pro-inflammatory and pro-remodeling role; requires verification in in vivo/in vitro studies. | Potential therapeutic target: antagonism of signaling via the TLR2/6 → NF-κB axis to reduce inflammation and remodeling. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Styczeń, A.; Krysa, M.; Mertowska, P.; Grywalska, E.; Urbanowicz, T.; Krasiński, M.; Grobelna, M.; Topyła-Putowska, W.; Rahnama-Hezavah, M.; Tomaszewski, M. The Role of Toll-like Receptors and Viral Infections in the Pathogenesis and Progression of Pulmonary Arterial Hypertension—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 11143. https://doi.org/10.3390/ijms262211143
Styczeń A, Krysa M, Mertowska P, Grywalska E, Urbanowicz T, Krasiński M, Grobelna M, Topyła-Putowska W, Rahnama-Hezavah M, Tomaszewski M. The Role of Toll-like Receptors and Viral Infections in the Pathogenesis and Progression of Pulmonary Arterial Hypertension—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(22):11143. https://doi.org/10.3390/ijms262211143
Chicago/Turabian StyleStyczeń, Agnieszka, Martyna Krysa, Paulina Mertowska, Ewelina Grywalska, Tomasz Urbanowicz, Maciej Krasiński, Malwina Grobelna, Weronika Topyła-Putowska, Mansur Rahnama-Hezavah, and Michał Tomaszewski. 2025. "The Role of Toll-like Receptors and Viral Infections in the Pathogenesis and Progression of Pulmonary Arterial Hypertension—A Narrative Review" International Journal of Molecular Sciences 26, no. 22: 11143. https://doi.org/10.3390/ijms262211143
APA StyleStyczeń, A., Krysa, M., Mertowska, P., Grywalska, E., Urbanowicz, T., Krasiński, M., Grobelna, M., Topyła-Putowska, W., Rahnama-Hezavah, M., & Tomaszewski, M. (2025). The Role of Toll-like Receptors and Viral Infections in the Pathogenesis and Progression of Pulmonary Arterial Hypertension—A Narrative Review. International Journal of Molecular Sciences, 26(22), 11143. https://doi.org/10.3390/ijms262211143






