Pathophysiology of Pulmonary Arterial Hypertension: Focus on Vascular Endothelium as a Potential Therapeutic Target
Abstract
1. Introduction
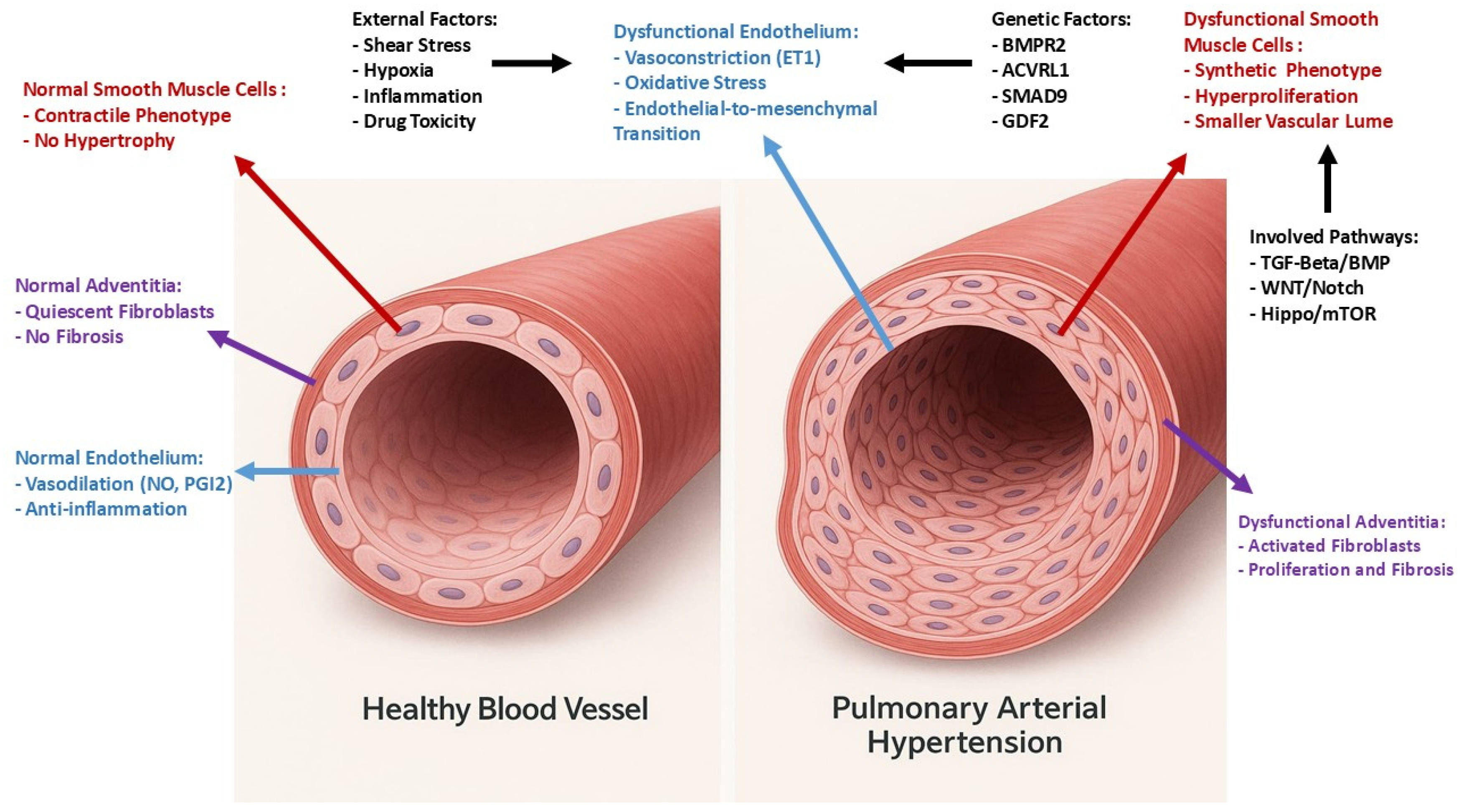
2. Pathophysiology of Pulmonary Hypertension
3. Vascular Endothelium
- Vascular tone regulation: ECs produce substances that modulate vessel diameter and tone, controlling the degree of dilation and constriction of blood vessels. Key molecules include NO and prostaglandins, which promote vasodilation, and endothelin-1 (ET-1), which causes vasoconstriction. This careful regulation of vascular tone ensures a normal blood pressure.
- Anti-inflammatory and antithrombotic properties: These cells help suppress inflammation and prevent blood clot formation by releasing anti-inflammatory and antithrombotic agents, such as prostacyclin and thromboresistant factors. ECs play a pivotal role in the inflammatory response by checking the passage of leukocytes into the lung tissue. These cells present adhesion molecules that facilitate the binding and transmigration of leukocytes during inflammation and infection.
- Angiogenesis: The pulmonary endothelium is involved in the process of angiogenesis. This is crucial for tissue repair and regeneration, as well as for adapting to changes in oxygen demand and blood flow.
- Barrier Function: The endothelium acts as a selective barrier, preventing potentially harmful substances from entering the bloodstream and allowing the passage of those necessary.
- Gas Exchange Facilitation: The endothelium ensures that oxygen and carbon dioxide can efficiently pass between the lungs and the blood. This process is crucial for maintaining the body’s oxygen supply and removing carbon dioxide.
- Cellular Signaling: The pulmonary endothelium is involved in a complex network of signaling pathways that regulate cell growth, differentiation, and responses to injury. This is particularly important in maintaining the structural integrity of the blood vessels and responding to pathological conditions [58,63].
- Endothelial function is regulated by different and numerous factors such as neurotransmitters, catecholamines, and endocrine factors [64]. In relation to the important functions that the pulmonary endothelium performs, its alteration and, in particular, the destruction of its glycocalyx seem to play an essential role in the etiopathogenesis of PAH [58,61,65,66].
4. Role of Endothelium in the Pathophysiology of Pulmonary Hypertension
4.1. Molecular Imbalances
4.2. Endothelial Injury and Inflammation
4.3. Plexiform Lesions (Angioproliferative Foci)
4.4. Preclinical Evidence—Endothelium as the Driver
4.4.1. Monocrotaline Toxicity
4.4.2. SU5416/Hypoxia (“Two-Hit”) Model
4.5. Therapeutic Implications
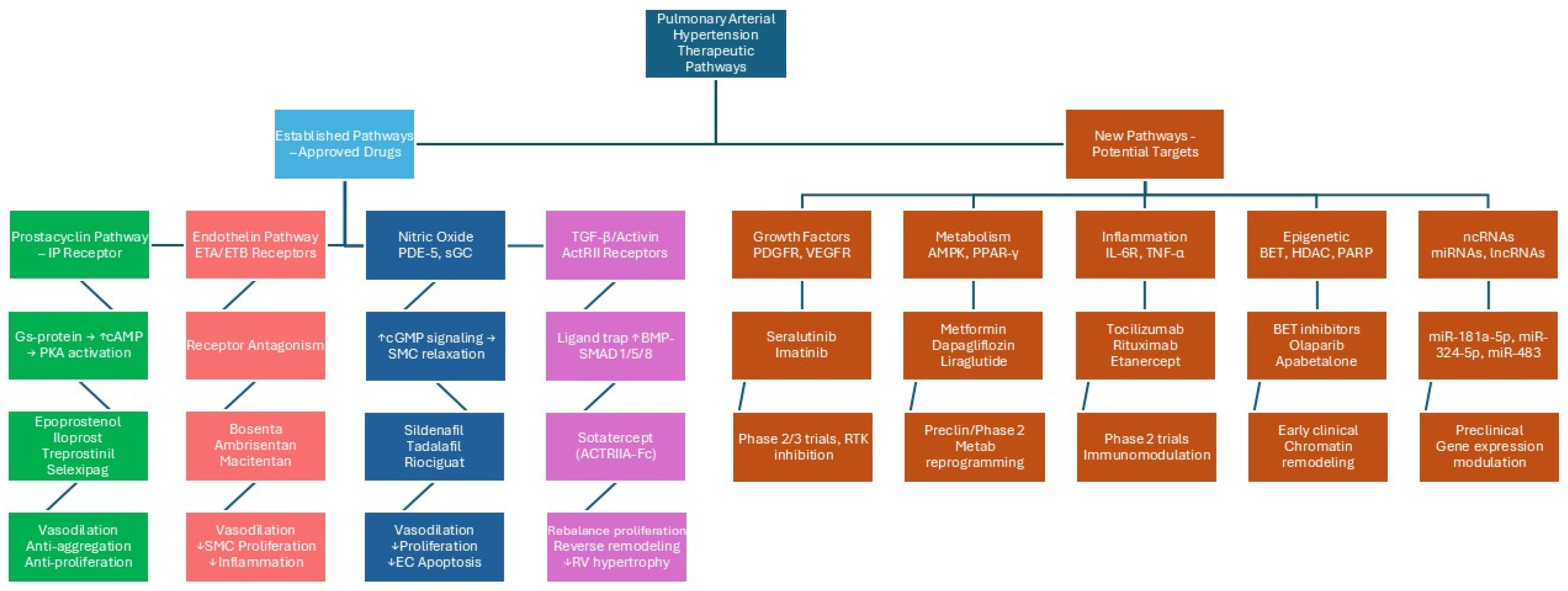
5. Vascular Endothelium as Potential Therapeutic Target in PAH
6. Established Specific PAH Drugs
6.1. Drugs Acting on Prostacyclin Pathway
6.2. Drugs Acting on Endothelin 1 Pathway
6.3. Drugs Acting on Nitric Oxide Pathway
6.4. Drugs Acting on Transforming Growth Factor-Beta Superfamily Pathway
7. Novel Specific PAH Drugs (Figure 2)
7.1. Drugs Acting on Inflammatory System
7.2. Drugs Acting on Growth Factors
7.3. Drugs Acting on Metabolic Pathways
7.4. Drugs Acting on ncRNA
- Although it represents an intriguing perspective, the clinical application of ncRNA-based therapies presents several challenges [164], such as the complex systemic delivery of ncRNAs, the need for repeated applications, the potential toxicity and interactions with commonly used drugs, and the absence of an antidote.
- Nevertheless, attempts have been made in recent years with interesting outcomes, although current trials are on miRNAs predominantly active in muscle vascular cells.
- Olaparib, a PARP-1 (a protein involved in the processes of DNA repair) inhibitor already used in cancer therapy [169], targets a DNA repair enzyme that is overactivated in PAH as a consequence of DNA damage due to chronic inflammation. The overactivation in turn reduces miRNA-204 levels [170] that promotes abnormal smooth muscle cell proliferation and survival by enhancing expression of bromodomain-containing protein 4 (BRD4) [171]. Olaparib is currently being evaluated in PAH patients within the OPTION phase 1b trial [172]. Additionally, Apabetalone, an oral BRD4 inhibitor, has shown preliminary promise in improving pulmonary vascular resistance and cardiac output when combined with standard PAH treatments [173].
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731, Erratum in Eur. Heart J. 2023, 44, 1312. https://doi.org/10.1093/eurheartj/ehad005. [Google Scholar] [CrossRef] [PubMed]
- Vatrano, M.; Manzi, G.; Picariello, C.; D’Alto, M.; Enea, I.; Ghio, S.; Caravita, S.; Argiento, P.; Garascia, A.; Vitulo, P.; et al. Documento di consenso ANMCO/SIC sull’ipertensione arteriosa polmonare. G. Ital. Cardiol. 2024, 25, 192–201. [Google Scholar] [CrossRef]
- Palazzini, M.; Branzi, A.; Conficoni, E.; Beciani, E.; Bachetti, C.; Gambetti, S.; Leci, E.; Marinelli, A.; Negro, L.; Manes, A.; et al. L’ipertensione arteriosa polmonare. Parte I: Patobiologia, fisiopatologia, aspetti clinici e diagnostici. G. Ital. Cardiol. 2009, 10, 271–300. [Google Scholar] [CrossRef]
- Correale, M.; Tricarico, L.; Bevere, E.M.L.; Chirivì, F.; Croella, F.; Severino, P.; Mercurio, V.; Magrì, D.; Dini, F.; Licordari, R.; et al. Circulating Biomarkers in Pulmonary Arterial Hypertension: An Update. Biomolecules 2024, 14, 552. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vonk-Noordegraaf, A.; Haddad, F.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right heart adaptation to pulmonary arterial hypertension: Physiology and pathobiology. J. Am. Coll. Cardiol. 2013, 62 (Suppl. S25), D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, S.; Mukherjee, D.; Banerjee, S.; Islam, A.M.; Daggubati, R.; Paul, T.K. Current Trends and Future Perspectives in the Treatment of Pulmonary Hypertension: WHO Group II-V. Curr. Probl. Cardiol. 2018, 43, 217–231. [Google Scholar] [CrossRef]
- Zolty, R. Novel Experimental Therapies for Treatment of Pulmonary Arterial Hypertension. J. Exp. Pharmacol. 2021, 13, 817–857. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correale, M.; Chirivì, F.; Bevere, E.M.L.; Tricarico, L.; D’Alto, M.; Badagliacca, R.; Brunetti, N.D.; Vizza, C.D.; Ghio, S. Endothelial Function in Pulmonary Arterial Hypertension: From Bench to Bedside. J. Clin. Med. 2024, 13, 2444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and Pathobiology of Pulmonary Hypertension: State of the Art and Research Perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef]
- Ghigna, M.R.; Guignabert, C.; Montani, D.; Girerd, B.; Jaïs, X.; Savale, L.; Hervé, P.; De Montpréville, V.T.; Mercier, O.; Sitbon, O.; et al. BMPR2 Mutation Status Influences Bronchial Vascular Changes in Pulmonary Arterial Hypertension. Eur. Respir. J. 2016, 48, 1668–1681. [Google Scholar] [CrossRef]
- Huertas, A.; Perros, F.; Tu, L.; Cohen-Kaminsky, S.; Montani, D.; Dorfmüller, P.; Guignabert, C.; Humbert, M. Immune Dysregulation and Endothelial Dysfunction in Pulmonary Arterial Hypertension: A Complex Interplay. Circulation 2014, 129, 1332–1340. [Google Scholar] [CrossRef]
- Huertas, A.; Tu, L.; Humbert, M.; Guignabert, C. Chronic Inflammation within the Vascular Wall in Pulmonary Arterial Hypertension: More than a Spectator. Cardiovasc. Res. 2020, 116, 885–893. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and Immunity in the Pathogenesis of Pulmonary Arterial Hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Stacher, E.; Graham, B.B.; Hunt, J.M.; Gandjeva, A.; Groshong, S.D.; McLaughlin, V.V.; Jessup, M.; Grizzle, W.E.; Aldred, M.A.; Cool, C.D.; et al. Modern Age Pathology of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Sitbon, O.; Guignabert, C.; Savale, L.; Boucly, A.; Gallant-Dewavrin, M.; McLaughlin, V.; Hoeper, M.M.; Weatherald, J. Treatment of Pulmonary Arterial Hypertension: Recent Progress and a Look to the Future. Lancet Respir. Med. 2023, 11, 804–819. [Google Scholar] [CrossRef]
- Tuder, R.M.; Gandjeva, A.; Williams, S.; Kumar, S.; Kheyfets, V.O.; Hatton-Jones, K.M.; Starr, J.R.; Yun, J.; Hong, J.; West, N.P.; et al. Digital Spatial Profiling Identifies Distinct Molecular Signatures of Vascular Lesions in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2024, 210, 329–342. [Google Scholar] [CrossRef]
- Westoo, C.; Norvik, C.; Peruzzi, N.; van der Have, O.; Lovric, G.; Jeremiasen, I.; Tran, P.K.; Mokso, R.; de Jesus Perez, V.; Brunnstrom, H.; et al. Distinct Types of Plexiform Lesions Identified by Synchrotron-Based Phase-Contrast Micro-CT. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L17–L28. [Google Scholar] [CrossRef]
- Sakao, S.; Voelkel, N.F.; Tatsumi, K. The Vascular Bed in COPD: Pulmonary Hypertension and Pulmonary Vascular Alterations. Eur. Respir. Rev. 2014, 23, 350–355. [Google Scholar] [CrossRef]
- Trip, P.; Nossent, E.J.; de Man, F.S.; van den Berk, I.A.H.; Boonstra, A.; Groepenhoff, H.; Leter, E.M.; Westerhof, N.; Grünberg, K.; Bogaard, H.-J.; et al. Severely Reduced Diffusion Capacity in Idiopathic Pulmonary Arterial Hypertension: Patient Characteristics and Treatment Responses. Eur. Respir. J. 2013, 42, 1575–1585. [Google Scholar] [CrossRef]
- Welch, C.L.; Aldred, M.A.; Balachandar, S.; Dooijes, D.; Eichstaedt, C.A.; Gräf, S.; Houweling, A.C.; Machado, R.D.; Pandya, D.; Prapa, M.; et al. Defining the Clinical Validity of Genes Reported to Cause Pulmonary Arterial Hypertension. Genet. Med. 2023, 25, 100925. [Google Scholar] [CrossRef]
- Montani, D.; Girerd, B.; Jaïs, X.; Laveneziana, P.; Lau, E.M.T.; Bouchachi, A.; Hascoët, S.; Günther, S.; Godinas, L.; Parent, F.; et al. Screening for Pulmonary Arterial Hypertension in Adults Carrying a BMPR2 Mutation. Eur. Respir. J. 2021, 58, 2004229. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Shao, N.Y.; Sa, S.; Li, D.; Termglinchan, V.; Ameen, M.; Karakikes, I.; Sosa, G.; Grubert, F.; Lee, J.; et al. Patient-Specific IPSC-Derived Endothelial Cells Uncover Pathways That Protect against Pulmonary Hypertension in BMPR2 Mutation Carriers. Cell Stem Cell 2017, 20, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.; Vasilaki, E.; Aman, J.; Chen, C.N.; Wu, Y.; Liang, O.D.; Ashek, A.; Dubois, O.; Zhao, L.; Sabrin, F.; et al. SOX17 Enhancer Variants Disrupt Transcription Factor Binding and Enhancer Inactivity Drives Pulmonary Hypertension. Circulation 2023, 147, 1606–1621. [Google Scholar] [CrossRef]
- Mercurio, V.; Cuomo, A.; Naranjo, M.; Hassoun, P.M. Inflammatory Mechanisms in the Pathogenesis of Pulmonary Arterial Hypertension: Recent Advances. Compr. Physiol. 2021, 11, 1805–1829. [Google Scholar] [CrossRef]
- Guignabert, C.; Phan, C.; Seferian, A.; Huertas, A.; Tu, L.; Thuillet, R.; Sattler, C.; Le Hiress, M.; Tamura, Y.; Jutant, E.-M.; et al. Dasatinib Induces Lung Vascular Toxicity and Predisposes to Pulmonary Hypertension. J. Clin. Investig. 2016, 126, 3207–3218. [Google Scholar] [CrossRef]
- Nagaraj, C.; Tang, B.; Bálint, Z.; Wygrecka, M.; Hrzenjak, A.; Kwapiszewska, G.; Stacher, E.; Lindenmann, J.; Weir, E.K.; Olschewski, H.; et al. Src Tyrosine Kinase Is Crucial for Potassium Channel Function in Human Pulmonary Arteries. Eur. Respir. J. 2013, 41, 85–95. [Google Scholar] [CrossRef]
- Guignabert, C.; Humbert, M. Targeting Transforming Growth Factor-β Receptors in Pulmonary Hypertension. Eur. Respir. J. 2021, 57, 2002341, Erratum in Eur. Respir. J. 2024, 64, 2052341. [Google Scholar] [CrossRef]
- Guignabert, C.; Savale, L.; Boucly, A.; Thuillet, R.; Tu, L.; Ottaviani, M.; Rhodes, C.J.; De Groote, P.; Prévot, G.; Bergot, E.; et al. Serum and Pulmonary Expression Profiles of the Activin Signaling System in Pulmonary Arterial Hypertension. Circulation 2023, 147, 1809–1822. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Badesch, D.B.; Ghofrani, H.A.; Gibbs, J.S.R.; Gomberg-Maitland, M.; McLaughlin, V.V.; Preston, I.R.; Souza, R.; Waxman, A.B.; Grünig, E.; et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023, 388, 1478–1490. [Google Scholar] [CrossRef]
- Humbert, M.; McLaughlin, V.; Gibbs, J.S.R.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.; Escribano Subias, P.; Feldman, J.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021, 384, 1204–1215. [Google Scholar] [CrossRef]
- Humbert, M.; McLaughlin, V.; Gibbs, J.R.S.; Gomberg-Maitland, M.; Hoeper, M.M.; Preston, I.R.; Souza, R.; Waxman, A.B.; Ghofrani, H.A.; Subias, P.E.; et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension: PULSAR Open-Label Extension. Eur. Respir. J. 2023, 61, 2201347. [Google Scholar] [CrossRef]
- Antigny, F.; Hautefort, A.; Meloche, J.; Belacel-Ouari, M.; Manoury, B.; Rucker-Martin, C.; Péchoux, C.; Potus, F.; Nadeau, V.; Tremblay, E.; et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 2016, 133, 1371–1385. [Google Scholar] [CrossRef]
- Lambert, M.; Capuano, V.; Boet, A.; Tesson, L.; Bertero, T.; Nakhleh, M.K.; Remy, S.; Anegon, I.; Pechoux, C.; Hautefort, A.; et al. Characterization of Kcnk3-Mutated Rat, a Novel Model of Pulmonary Hypertension. Circ. Res. 2019, 125, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Le Ribeuz, H.; To, L.; Ghigna, M.R.; Martin, C.; Nagaraj, C.; Dreano, E.; Rucker-Martin, C.; Girerd, B.; Bouliguan, J.; Pechoux, C.; et al. Involvement of CFTR in the Pathogenesis of Pulmonary Arterial Hypertension. Eur. Respir. J. 2021, 58, 2000653. [Google Scholar] [CrossRef]
- Papp, R.; Nagaraj, C.; Zabini, D.; Nagy, B.M.; Lengyel, M.; Maurer, D.S.; Sharma, N.; Egemnazarov, B.; Kovacs, G.; Kwapiszewska, G.; et al. Targeting TMEM16A to Reverse Vasoconstriction and Remodelling in Idiopathic Pulmonary Arterial Hypertension. Eur. Respir. J. 2019, 53, 1800965. [Google Scholar] [CrossRef]
- Grobs, Y.; Romanet, C.; Lemay, S.-E.; Bourgeois, A.; Voisine, P.; Theberge, C.; Sauvaget, M.; Breuils-Bonnet, S.; Martineau, S.; El Kabbout, R.; et al. ATP Citrate Lyase Drives Vascular Remodeling in Systemic and Pulmonary Vascular Diseases through Metabolic and Epigenetic Changes. Sci. Transl. Med. 2024, 16, eado7824. [Google Scholar] [CrossRef]
- Li, D.; Shao, N.Y.; Moonen, J.R.M.D.; Zhao, Z.; Shi, M.; Otsuki, S.; Wang, L.; Elaine Yan, T.N.; Marciano, D.P.; Contrepois, K.; et al. ALDH1A3 Coordinates Metabolism with Gene Regulation in Pulmonary Arterial Hypertension. Circulation 2021, 143, 2074–2090. [Google Scholar] [CrossRef]
- Van Der Feen, D.E.; Kurakula, K.; Tremblay, E.; Boucherat, O.; Bossers, G.P.L.; Szulcek, R.; Bourgeois, A.; Lampron, M.C.; Habbout, K.; Martineau, S.; et al. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2019, 200, 910–920. [Google Scholar] [CrossRef]
- Chen, C.-N.; Hajji, N.; Yeh, F.-C.; Rahman, S.; Ali, S.; Wharton, J.; Baxan, N.; Zhao, L.; Xie, C.-Y.; Chen, Y.-G.; et al. Restoration of Foxp3+ Regulatory T Cells by HDAC-Dependent Epigenetic Modulation Plays a Pivotal Role in Resolving Pulmonary Arterial Hypertension Pathology. Am. J. Respir. Crit. Care Med. 2023, 208, 879–895. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, Y.; Yang, Y.; Fan, H.; Deng, Y.; Chen, W.; Liu, J.; Yang, F.; Li, W.; Xiao, Y. Comprehensive Analysis of M6A Methylomes in Idiopathic Pulmonary Arterial Hypertension. Epigenetics 2023, 18, 2242225. [Google Scholar] [CrossRef]
- Gao, G.; Chen, A.; Gong, J.; Lin, W.; Wu, W.; Mohammad Ismail Hajary, S.; Lian, G.; Luo, L.; Xie, L. Comprehensive Analyses of M6A RNA Methylation Patterns and Related Immune Microenvironment in Idiopathic Pulmonary Arterial Hypertension. Front. Genet. 2023, 14, 1222368. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Zhu, B.; Lin, Y.; Lin, K.; Sun, Y.; Yao, Z.; Yuan, L. Identification of a Potentially Novel LncRNA-MiRNA-MRNA Competing Endogenous RNA Network in Pulmonary Arterial Hypertension via Integrated Bioinformatic Analysis. Life Sci. 2021, 277, 119455. [Google Scholar] [CrossRef] [PubMed]
- Omura, J.; Habbout, K.; Shimauchi, T.; Wu, W.H.; Breuils-Bonnet, S.; Tremblay, E.; Martineau, S.; Nadeau, V.; Gagnon, K.; Mazoyer, F.; et al. Identification of Long Noncoding RNA H19 as a New Biomarker and Therapeutic Target in Right Ventricular Failure in Pulmonary Arterial Hypertension. Circulation 2020, 142, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Lemay, S.E.; Grobs, Y.; Romanet, C.; Martineau, S.; Salem, M.; Shimauchi, T.; Breuils-Bonnet, S.; Bourgeois, A.; Théberge, C.; Pelletier, A.; et al. Hypusine Signaling Promotes Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2024, 209, 1376–1391. [Google Scholar] [CrossRef]
- Eyries, M.; Montani, D.; Nadaud, S.; Girerd, B.; Levy, M.; Bourdin, A.; Trésorier, R.; Chaouat, A.; Cottin, V.; Sanfiorenzo, C.; et al. Widening the Landscape of Heritable Pulmonary Hypertension Mutations in Paediatric and Adult Cases. Eur. Respir. J. 2019, 53, 1801371. [Google Scholar] [CrossRef]
- Prapa, M.; Lago-Docampo, M.; Swietlik, E.M.; Montani, D.; Eyries, M.; Humbert, M.; Welch, C.L.; Chung, W.K.; Berger, R.M.F.; Bogaard, H.J.; et al. First Genotype-Phenotype Study in TBX4 Syndrome: Gain-of-Function Mutations Causative for Lung Disease. Am. J. Respir. Crit. Care Med. 2022, 206, 1522–1533. [Google Scholar] [CrossRef]
- Moonen, J.R.; Chappell, J.; Shi, M.; Shinohara, T.; Li, D.; Mumbach, M.R.; Zhang, F.; Nair, R.V.; Nasser, J.; Mai, D.H.; et al. KLF4 Recruits SWI/SNF to Increase Chromatin Accessibility and Reprogram the Endothelial Enhancer Landscape under Laminar Shear Stress. Nat. Commun. 2022, 13, 4941. [Google Scholar] [CrossRef]
- Frascarelli, C.; Zanetti, N.; Nasca, A.; Izzo, R.; Lamperti, C.; Lamantea, E.; Legati, A.; Ghezzi, D. Nanopore Long-Read next-Generation Sequencing for Detection of Mitochondrial DNA Large-Scale Deletions. Front. Genet. 2023, 14, 1089956. [Google Scholar] [CrossRef]
- Jacobs, W.; van de Veerdonk, M.C.; Trip, P.; de Man, F.; Heymans, M.W.; Marcus, J.T.; Kawut, S.M.; Bogaard, H.-J.; Boonstra, A.; Vonk Noordegraaf, A. The Right Ventricle Explains Sex Differences in Survival in Idiopathic Pulmonary Arterial Hypertension. Chest 2014, 145, 1230–1236. [Google Scholar] [CrossRef]
- van Wezenbeek, J.; Groeneveldt, J.A.; Llucià-Valldeperas, A.; van der Bruggen, C.E.; Jansen, S.M.A.; Smits, A.J.; Smal, R.; van Leeuwen, J.W.; Remedios, C.D.; Keogh, A.; et al. Interplay of Sex Hormones and Long-Term Right Ventricular Adaptation in a Dutch PAH-Cohort. J. Heart Lung Transplant. 2022, 41, 445–457. [Google Scholar] [CrossRef]
- Tello, K.; Richter, M.J.; Yogeswaran, A.; Ghofrani, H.A.; Naeije, R.; Vanderpool, R.; Gall, H.; Tedford, R.J.; Seeger, W.; Lahm, T. Sex Differences in Right Ventricular⇓pulmonary Arterial Coupling in Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2020, 202, 1042–1046. [Google Scholar] [CrossRef]
- Kawut, S.M.; Feng, R.; Ellenberg, S.S.; Zamanian, R.; Bull, T.; Chakinala, M.; Mathai, S.C.; Hemnes, A.; Lin, G.; Doyle, M.; et al. Pulmonary Hypertension and Anastrozole (PHANTOM): A Randomized, Double-Blind, Placebo-Controlled Trial. Am. J. Respir. Crit. Care Med. 2024, 210, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Sangam, S.; Sun, X.; Schwantes-An, T.-H.; Yegambaram, M.; Lu, Q.; Shi, Y.; Cook, T.; Fisher, A.; Frump, A.L.; Coleman, A.; et al. SOX17 Deficiency Mediates Pulmonary Hypertension: At the Crossroads of Sex, Metabolism, and Genetics. Am. J. Respir. Crit. Care Med. 2023, 207, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. X Chromosome Agents of Sexual Differentiation. Nat. Rev. Endocrinol. 2022, 18, 574–583. [Google Scholar] [CrossRef]
- Qin, S.; Predescu, D.; Carman, B.; Patel, P.; Chen, J.; Kim, M.; Lahm, T.; Geraci, M.; Predescu, S.A. Up-Regulation of the Long Noncoding RNA X-Inactive–Specific Transcript and the Sex Bias in Pulmonary Arterial Hypertension. Am. J. Pathol. 2021, 191, 1135–1150. [Google Scholar] [CrossRef]
- Cunningham, C.M.; Li, M.; Ruffenach, G.; Doshi, M.; Aryan, L.; Hong, J.; Park, J.; Hrncir, H.; Medzikovic, L.; Umar, S.; et al. Y-Chromosome Gene, Uty, Protects Against Pulmonary Hypertension by Reducing Proinflammatory Chemokines. Am. J. Respir. Crit. Care Med. 2022, 206, 186–196. [Google Scholar] [CrossRef]
- Yan, L.; Cogan, J.D.; Hedges, L.K.; Nunley, B.; Hamid, R.; Austin, E.D. The Y Chromosome Regulates BMPR2 Expression via SRY: A Possible Reason “Why” Fewer Males Develop Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2018, 198, 1581–1583. [Google Scholar] [CrossRef]
- Alturaif, N.; Attanasio, U.; Mercurio, V. Pulmonary Arterial Hypertension: Sex-Specific Differences and Outcomes. Ther. Adv. Respir. Dis. 2025, 19, 17534666251350493. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Riley, J.T.; Dardik, A. Cells in focus: Endothelial cell. Int. J. Biochem. Cell Biol. 2002, 34, 1508–1512. [Google Scholar] [CrossRef]
- Amraoui, F.; Olde Engberink, R.H.; van Gorp, J.; Ramdani, A.; Vogt, L.; van den Born, B.J. Microvascular glycocalyx dimension estimated by automated SDF imaging is not related to cardiovascular disease. Microcirculation 2014, 21, 499–505. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.C.; Liu, Y. Attenuating Pulmonary Hypertension by Protecting the Integrity of Glycocalyx in Rats Model of Pulmonary Artery Hypertension. Inflammation 2019, 42, 1951–1956. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic. Biol. Med. 2017, 109, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Tricarico, L.; Chirivì, F.; Bevere, E.M.L.; Ruggeri, D.; Migliozzi, C.; Rossi, L.; Vitullo, A.; Granatiero, M.; Granato, M.; et al. Endothelial Function Correlates With Pulmonary Pressures in Subjects With Clinically Suspected Pulmonary Hypertension. Am. J. Cardiol. 2024, 225, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Haensel, M.; Wojciak-Stothard, B. The role of endothelial cells in pulmonary hypertension: Old concepts and new science. Curr. Opin. Physiol. 2023, 34, 100667. [Google Scholar] [CrossRef]
- Guignabert, C.; Aman, J.; Bonnet, S.; Dorfmüller, P.; Olschewski, A.J.; Pullamsetti, S.; Rabinovitch, M.; Schermuly, R.T.; Humbert, M.; Stenmark, K.R. Pathology and Pathobiology of Pulmonary Hypertension: Current Insights and Future Directions. Eur. Respir. J. 2024, 64, 2401095. [Google Scholar] [CrossRef]
- Christou, H.; Khalil, R.A. Mechanisms of pulmonary vascular dysfunction in pulmonary hypertension and implications for novel therapies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H702–H724. [Google Scholar] [CrossRef]
- Dai, J.; Chen, H.; Fang, J.; Wu, S.; Jia, Z. Vascular Remodeling: The Multicellular Mechanisms of Pulmonary Hypertension. Int. J. Mol. Sci. 2025, 26, 4265. [Google Scholar] [CrossRef] [PubMed]
- Cober, N.D.; VandenBroek, M.M.; Ormiston, M.L.; Stewart, D.J. Evolving Concepts in Endothelial Pathobiology of Pulmonary Arterial Hypertension. Hypertension 2022, 79, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, A.A.; Aldhahir, A.M.; Alghamdi, S.A.; Alqahtani, J.S.; Siraj, R.A.; Alwafi, H.; AlGarni, A.A.; Majrshi, M.S.; Alshehri, S.M.; Pang, L. Role of prostanoids, nitric oxide and endothelin pathways in pulmonary hypertension due to COPD. Front. Med. 2023, 10, 1275684. [Google Scholar] [CrossRef] [PubMed]
- Corrado, A.; Mansueto, N.; Correale, M.; Rella, V.; Tricarico, L.; Altomare, A.; Brunetti, N.D.; Cantatore, F.P.; Rotondo, C. Flow Mediated Dilation in Systemic Sclerosis: Association with clinical findings, capillaroscopic patterns and endothelial circulating markers. Vasc. Pharmacol. 2024, 154, 107252. [Google Scholar] [CrossRef] [PubMed]
- Correale, M.; Rotondo, C.; Bevere, E.M.L.; Tricarico, L.; Rella, V.; Villani, D.; Granato, M.; Migliozzi, C.; Cantatore, F.P.; Brunetti, N.D.; et al. Combined peripheral and central ultrasound for the diagnosis of PAH-SSc patients. Echocardiography 2024, 41, e15853. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Pan, Q.; Zhang, J.; Gong, S.; Zhang, F.; Liang, N.; Yang, Y.; Jiang, Z. Role of inflammation in endothelial responses in Pulmonary Hypertension. Biomed. Pharmacother. 2025, 188, 118206. [Google Scholar] [CrossRef] [PubMed]
- Gorelova, A.; Berman, M.; Al Ghouleh, I. Endothelial-to-Mesenchymal Transition in Pulmonary Arterial Hypertension. Antioxid. Redox Signal. 2021, 34, 891–914. [Google Scholar] [CrossRef] [PubMed]
- Cool, C.D.; Stewart, J.S.; Werahera, P.; Miller, G.J.; Williams, R.L.; Voelkel, N.F.; Tuder, R.M. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am. J. Pathol. 1999, 155, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Tuder, R.M.; Groves, B.; Badesch, D.B.; Voelkel, N.F. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am. J. Pathol. 1994, 144, 275–285. [Google Scholar] [PubMed]
- Xiao, R.; Su, Y.; Feng, T.; Sun, M.; Liu, B.; Zhang, J.; Lu, Y.; Li, J.; Wang, T.; Zhu, L.; et al. Monocrotaline Induces Endothelial Injury and Pulmonary Hypertension by Targeting the Extracellular Calcium-Sensing Receptor. J. Am. Heart Assoc. 2017, 6, e004865. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L363–L369. [Google Scholar] [CrossRef]
- Taraseviciene-Stewart, L.; Kasahara, Y.; Alger, L.; Hirth, P.; Mc Mahon, G.; Waltenberger, J.; Voelkel, N.F.; Tuder, R.M. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001, 15, 427–438. [Google Scholar] [CrossRef] [PubMed]
- de Raaf, M.A.; Schalij, I.; Gomez-Arroyo, J.; Rol, N.; Happé, C.; de Man, F.S.; Vonk-Noordegraaf, A.; Westerhof, N.; Voelkel, N.F.; Bogaard, H.J. SuHx rat model: Partly reversible pulmonary hypertension and progressive intima obstruction. Eur. Respir. J. 2014, 44, 160–168. [Google Scholar] [CrossRef]
- Dai, Z.; Thorp, E.B. New Way to Study Pulmonary Hypertension in HFpEF. Circ. Res. 2023, 133, 899–901. [Google Scholar] [CrossRef]
- Jasińska-Stroschein, M. An updated review of experimental rodent models of pulmonary hypertension and left heart disease. Front. Pharmacol. 2024, 14, 1308095. [Google Scholar] [CrossRef]
- Dhoble, S.; Patravale, V.; Weaver, E.; Lamprou, D.A.; Patravale, T. Comprehensive review on novel targets and emerging therapeutic modalities for pulmonary arterial Hypertension. Int. J. Pharm. 2022, 621, 121792. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Bowman, L.; D’Arsigny, C.L.; Archer, S.L. Soluble guanylate cyclase: A new therapeutic target for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Clin. Pharmacol. Ther. 2015, 97, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Ghofrani, A.H. New horizons in pulmonary arterial hypertension therapies. Eur. Respir. Rev. 2013, 22, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.J.; Wu, Q.; He, W.N.; Wang, S.; Zhao, Y.L.; Huang, J.X.; Yan, X.S.; Jiang, R. Interleukin-6 and pulmonary hypertension: From physiopathology to therapy. Front. Immunol. 2023, 14, 1181987. [Google Scholar] [CrossRef]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Anti-TNFα therapy in inflammatory lung diseases. Pharmacol. Ther. 2017, 180, 90–98. [Google Scholar] [CrossRef]
- Sun, Q.W.; Sun, Z. Stem cell therapy for pulmonary arterial hypertension: An update. J. Heart Lung Transplant. 2022, 41, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Suen, C.M.; Stewart, D.J.; Montroy, J.; Welsh, C.; Levac, B.; Wesch, N.; Zhai, A.; Fergusson, D.; McIntyre, L.; Lalu, M.M. Regenerative cell therapy for pulmonary arterial hypertension in animal models: A systematic review. Stem Cell Res. Ther. 2019, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Granton, J.; Langleben, D.; Kutryk, M.B.; Camack, N.; Galipeau, J.; Courtman, D.W.; Stewart, D.J. Endothelial NO-Synthase Gene-Enhanced Progenitor Cell Therapy for Pulmonary Arterial Hypertension: The PHACeT Trial. Circ. Res. 2015, 117, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.L.; Reynolds, A.M.; Bonder, C.S.; Reynolds, P.N. BMPR2 gene therapy for PAH acts via Smad and non-Smad signalling. Respirology 2016, 21, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mubarak, K.K. A review of prostaglandin analogs in the management of patients with pulmonary arterial hypertension. Respir. Med. 2010, 104, 9–21. [Google Scholar] [CrossRef]
- Clapp, L.H.; Gurung, R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015, 120, 56–71. [Google Scholar] [CrossRef]
- Biscetti, F.; Gaetani, E.; Flex, A.; Straface, G.; Pecorini, G.; Angelini, F.; Stigliano, E.; Aprahamian, T.; Smith, R.C.; Castellot, J.J.; et al. Peroxisome proliferator-activated receptor alpha is crucial for iloprost-induced in vivo angiogenesis and vascular endothelial growth factor upregulation. J. Vasc. Res. 2009, 46, 103–108. [Google Scholar] [CrossRef]
- He, H.; Venema, V.J.; Gu, X.; Venema, R.C.; Marrero, M.B.; Caldwell, R.B. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem. 1999, 274, 25130–25135. [Google Scholar] [CrossRef]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef]
- Zardi, E.M.; Zardi, D.M.; Cacciapaglia, F.; Dobrina, A.; Amoroso, A.; Picardi, A.; Afeltra, A. Endothelial dysfunction and activation as an expression of disease: Role of prostacyclin analogs. Int. Immunopharmacol. 2005, 5, 437–459. [Google Scholar] [CrossRef]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galiè, N.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Preiss, R.; et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef]
- Ataya, A.; Coons, J.C.; Patzlaff, N.; Broderick, M.; Seaman, S.; Sood, N.; Patricia George, M.; Chakinala, M.M. Safety and pharmacokinetics of ralinepag, a novel oral prostacyclin receptor agonist. JHLT Open 2025, 9, 100270. [Google Scholar] [CrossRef]
- Torres, F.; Farber, H.; Ristic, A.; McLaughlin, V.; Adams, J.; Zhang, J.; Klassen, P.; Shanahan, W.; Grundy, J.; Hoffmann, I.; et al. Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: Results from a phase 2 randomised, parallel group, placebo-controlled trial. Eur. Respir. J. 2019, 54, 1901030, Erratum in Eur. Respir. J. 2024, 63, 1951030. [Google Scholar] [CrossRef]
- Therapeutics United. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Ralinepag When Added to PAH Standard of Care or PAH Specific Background Therapy in Subjects with WHO Group 1 PAH. clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT03626688 (accessed on 27 July 2018).
- Correale, M.; Ferraretti, A.; Monaco, I.; Grazioli, D.; Di Biase, M.; Brunetti, N.D. Endothelin-receptor antagonists in the management of pulmonary arterial hypertension: Where do we stand? Vasc. Health Risk Manag. 2018, 14, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Iglarz, M.; Steiner, P.; Wanner, D.; Rey, M.; Hess, P.; Clozel, M. Vascular effects of endothelin receptor antagonists depends on their selectivity for ETA versus ETB receptors and on the functionality of endothelial ETB receptors. J. Cardiovasc. Pharmacol. 2015, 66, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Elisa, T.; Antonio, P.; Giuseppe, P.; Alessandro, B.; Giuseppe, A.; Federico, C.; Marzia, D.; Ruggero, B.; Giacomo, M.; Andrea, O.; et al. Endothelin Receptors Expressed by Immune Cells Are Involved in Modulation of Inflammation and in Fibrosis: Relevance to the Pathogenesis of Systemic Sclerosis. J. Immunol. Res. 2015, 2015, 147616. [Google Scholar] [CrossRef]
- Sfikakis, P.P.; Papamichael, C.; Stamatelopoulos, K.S.; Tousoulis, D.; Fragiadaki, K.G.; Katsichti, P.; Stefanadis, C.; Mavrikakis, M. Improvement of vascular endothelial function using the oral endothelin receptor antagonist bosentan in patients with systemic sclerosis. Arthritis Rheum. 2007, 56, 1985–1993. [Google Scholar] [CrossRef]
- Rubin, L.J.; Badesch, D.B.; Barst, R.J.; Galiè, N.; Black, C.M.; Keogh, A.; Pulido, T.; for the Bosentan Randomized Trial of Endothelin Antagonist Therapy Study Group. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2002, 346, 896–903. [Google Scholar] [CrossRef]
- Galiè, N.; Olschewski, H.; Oudiz, R.J.; Torres, F.; Frost, A.; Ghofrani, H.A.; Badesch, D.B.; McGoon, M.D.; McLaughlin, V.V.; Roecker, E.B.; et al. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008, 117, 3010–3019. [Google Scholar] [CrossRef]
- Pulido, T.; Adzerikho, I.; Channick, R.N.; Delcroix, M.; Galiè, N.; Ghofrani, H.A.; Jansa, P.; Jing, Z.C.; Le Brun, F.O.; Mehta, S.; et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 809–818. [Google Scholar] [CrossRef]
- Galiè, N.; Barberà, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.L.; Grünig, E.; et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, H.; Yao, C.; Pan, H.; Guo, Y.; Fan, K.; Jing, S. Therapeutic Monoclonal Antibody Antagonizing Endothelin Receptor A for Pulmonary Arterial Hypertension. J. Pharmacol. Exp. Ther. 2019, 370, 54–61. [Google Scholar] [CrossRef]
- Hye, T.; Hossain, M.R.; Saha, D.; Foyez, T. Emerging biologics for the treatment of pulmonary arterial hypertension. J. Drug Target. 2023, 31, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Bin-Nun, A.; Schreiber, M.D. Role of iNO in the modulation of pulmonary vascular resistance. J. Perinatol. 2008, 28 (Suppl. S3), S84–S92. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tettey, A.; Jiang, Y.; Li, X.; Li, Y. Therapy for Pulmonary Arterial Hypertension: Glance on Nitric Oxide Pathway. Front. Pharmacol. 2021, 12, 767002. [Google Scholar] [CrossRef]
- Galiè, N.; Ghofrani, H.A.; Torbicki, A.; Barst, R.J.; Rubin, L.J.; Badesch, D.; Fleming, T.; Parpia, T.; Burgess, G.; Branzi, A.; et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 2005, 353, 2148–2157, Erratum in N. Engl. J. Med. 2006, 354, 2400–2401. [Google Scholar] [CrossRef]
- Sastry, B.K.; Narasimhan, C.; Reddy, N.K.; Raju, B.S. Clinical efficacy of sildenafil in primary pulmonary hypertension: A randomized, placebo-controlled, double-blind, crossover study. J. Am. Coll. Cardiol. 2004, 43, 1149–1153. [Google Scholar] [CrossRef]
- Simonneau, G.; Rubin, L.J.; Galiè, N.; Barst, R.J.; Fleming, T.R.; Frost, A.E.; Engel, P.J.; Kramer, M.R.; Burgess, G.; Collings, L.; et al. Addition of sildenafil to long term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: A randomized trial. Ann. Intern. Med. 2008, 149, 521–530, Erratum in Ann. Intern. Med. 2009, 151, 435. [Google Scholar] [CrossRef]
- Galiè, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A.; et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009, 119, 2894–2903, Erratum in Circulation 2011, 124, e279. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Stasch, J.P.; Schmidt, P.; Alonso-Alija, C.; Apeler, H.; Dembowsky, K.; Haerter, M.; Heil, M.; Minuth, T.; Perzborn, E.; Pleiss, U.; et al. NO- and haem-independent activation of soluble guanylyl cyclase: Molecular basis and cardiovascular implications of a new pharmacological principle. Br. J. Pharmacol. 2002, 136, 773–783. [Google Scholar] [CrossRef]
- Becker-Pelster, E.M.; Hahn, M.G.; Delbeck, M.; Dietz, L.; Hüser, J.; Kopf, J.; Kraemer, T.; Marquardt, T.; Mondritzki, T.; Nagelschmitz, J.; et al. Inhaled mosliciguat (BAY 1237592): Targeting pulmonary vasculature via activating apo-sGC. Respir. Res. 2022, 23, 272. [Google Scholar] [CrossRef]
- Guglielmi, G.; Dimopoulos, K.; Wort, S.J. New therapies in pulmonary arterial hypertension: Recent insights. Int. J. Cardiol. Congenit. Heart Dis. 2025, 19, 100571. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Stewart, S.; Upton, P.D.; Machado, R.; Thomson, J.R.; Trembath, R.C.; Morrell, N.W. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation 2002, 105, 1672–1678. [Google Scholar] [CrossRef]
- Yung, L.M.; Yang, P.; Joshi, S.; Augur, Z.M.; Kim, S.S.J.; Bocobo, G.A.; Dinter, T.; Troncone, L.; Chen, P.S.; McNeil, M.E.; et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci Transl Med. 2020, 12, eaaz5660. [Google Scholar] [CrossRef]
- Waxman, A.B.; Systrom, D.M.; Manimaran, S.; de Oliveira Pena, J.; Lu, J.; Rischard, F.P. SPECTRA phase 2b study: Impact of sotatercept on exercise tolerance and right ventricular function in pulmonary arterial hypertension. Circ. Heart Fail. 2024, 17, e011227. [Google Scholar] [CrossRef]
- Acceleron Pharma, Inc. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Sotatercept When Added to Background Pulmonary Arterial Hypertension (PAH) Therapy in Newly Diagnosed Intermediate- and High-Risk PAH Patients. A Wholly-Owned Subsidiary of Merck & Co., Inc.: Rahway, NJ, USA, clinicaltrials.gov; 2024. Available online: https://clinicaltrials.gov/study/NCT04811092 (accessed on 23 March 2021).
- Acceleron Pharma, Inc. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Sotatercept When Added to Maximum Tolerated Background Therapy in Participants with Pulmonary Arterial Hypertension (PAH) World Health Organization (WHO) Functional Class (FC) III or FC IV at High Risk Mortality. A Wholly-Owned Subsidiary of Merck & Co., Inc.: Rahway, NJ, USA, clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT04896008 (accessed on 21 May 2021).
- Acceleron Pharma, Inc. A Phase 2, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Effects of Sotatercept Versus Placebo for the Treatment of Combined Postcapillary and Precapillary Pulmonary Hypertension (Cpc-PH) Due to Heart Failure with Preserved Ejection Fraction (HFpEF). A Wholly-Owned Subsidiary of Merck & Co., Inc.: Rahway, NJ, USA, clinicaltrials.gov; 2024. Available online: https://clinicaltrials.gov/study/NCT04945460 (accessed on 30 June 2021).
- Reddy, Y. Effect of Sotatercept on Central Cardiopulmonary Performance and Peripheral Oxygen Transport During Exercise in Pulmonary Arterial Hypertension. clinicaltrials.gov; 2024. Available online: https://clinicaltrials.gov/study/NCT06409026 (accessed on 10 May 2024).
- Study Details|A Study to Investigate the Safety and Efficacy of KER-012 in Combination with Background Therapy in Adult Participants with Pulmonary Arterial Hypertension (PAH) (TROPOS Study). clinicaltrials.gov. Available online: https://clinicaltrials.gov/study/NCT05975905 (accessed on 4 August 2023).
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Invest. 2013, 123, 3600–3613. [Google Scholar] [CrossRef]
- Spiekerkoetter, E.; Sung, Y.K.; Sudheendra, D.; Scott, V.; Del Rosario, P.; Bill, M.; Haddad, F.; Long-Boyle, J.; Hedlin, H.; Zamanian, R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir J. 2017, 50, 1602449. [Google Scholar] [CrossRef]
- Soon, E.; Holmes, A.M.; Treacy, C.M.; Doughty, N.J.; Southgate, L.; Machado, R.D.; Trembath, R.C.; Jennings, S.; Barker, L.; Nicklin, P.; et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010, 122, 920–927. [Google Scholar] [CrossRef]
- Hashimoto-Kataoka, T.; Hosen, N.; Sonobe, T.; Arita, Y.; Yasui, T.; Masaki, T.; Minami, M.; Inagaki, T.; Miyagawa, S.; Sawa, Y.; et al. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2015, 112, E2677–E2686. [Google Scholar] [CrossRef]
- Dayer, J.M.; Choy, E. Therapeutic targets in rheumatoid arthritis: The interleukin-6 receptor. Rheumatology 2010, 49, 15–24. [Google Scholar] [CrossRef]
- De Benedetti, F.; Brunner, H.I.; Ruperto, N.; Kenwright, A.; Wright, S.; Calvo, I.; Cuttica, R.; Ravelli, A.; Schneider, R.; Woo, P.; et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N. Engl. J. Med. 2012, 367, 2385–2395, Erratum in N. Engl. J. Med. 2015, 372, 887. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Church, C.; Harbaum, L.; Rhodes, C.; Villar Moreschi, S.S.; Liley, J.; Jones, R.; Arora, A.; Batai, K.; Desai, A.A.; et al. Mendelian randomisation and experimental medicine approaches to interleukin-6 as a drug target in pulmonary arterial hypertension. Eur. Respir. J. 2022, 59, 2002463, Erratum in Eur. Respir. J. 2022, 60, 2052463. [Google Scholar] [CrossRef]
- Tamura, Y.; Takeyasu, R.; Takata, T.; Miyazaki, N.; Takemura, R.; Wada, M.; Tamura, Y.; Abe, K.; Shigeta, A.; Taniguchi, Y.; et al. Kuwana MSATISFY-JP, a phase II multicenter openlabel study on Satralizumab, an anti-IL-6 receptor antibody, use for the treatment of pulmonary arterial hypertension in patients with an immune-responsive phenotype: Study protocol. Pulm. Circ. 2023, 13, e122. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Ishii, S.; Minatsuki, S.; Hatano, M.; Takeda, N. Exploring Novel Therapeutics for Pulmonary Arterial Hypertension. Int. Heart J. 2025, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.A.; Dunmore, B.J.; Long, L.; Crosby, A.; Al-Lamki, R.; Deighton, J.; Southwood, M.; Yang, X.; Nikolic, M.Z.; Herrera, B.; et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017, 8, 14079. [Google Scholar] [CrossRef]
- Zamanian, R.T.; Badesch, D.; Chung, L.; Domsic, R.T.; Medsger, T.; Pinckney, A.; Keyes-Elstein, L.; D’Aveta, C.; Spychala, M.; White, R.J.; et al. Safety and efficacy of B-cell depletion with Rituximab for the treatment of systemic sclerosis-associated pulmonary arterial hypertension: A multicenter, double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2021, 204, 209–221. [Google Scholar] [CrossRef]
- Chinese SLE Treatment and Research Group. Efficacy and Mechanism of Anti-CD20 Antibodies in Systemic Lupus Erythematosus Associated Pulmonary Arterial Hypertension Based on Multiomics Studies. Available online: https://clinicaltrials.gov/study/NCT05828147 (accessed on 25 April 2024).
- Middleton, R.C.; Fournier, M.; Xu, X.; Marbàn, E.; Lewis, M.I. Therapeutic benefits of intravenous cardiosphere-derived cell therapy in rats with pulmonary hypertension. PLoS ONE 2017, 12, e0183557. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Cheng, K.; Marban, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Lewis, M.I.; Shapiro, S.; Oudiz, R.J.; Nakamura, M.; Geft, D.; Matusov, Y.; Hage, A.; Tapson, V.F.; Henry, T.D.; Azizad, P.; et al. The ALPHA phase 1 study: Pulmonary ArteriaL hypertension treated with CardiosPHere-Derived allogeneic stem cells. EBioMedicine 2023, 100, 104900. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Barst, R.J.; Bourge, R.C.; Feldman, J.; Frost, A.E.; Galié, N.; Gómez-Sánchez, M.A.; Grimminger, F.; Grünig, E.; Hassoun, P.M.; et al. Imatinib mesylate as add-on therapy for pulmonary arterial hypertension: Results of the randomized IMPRES study. Circulation 2013, 127, 1128–1138. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Mckie, M.A.; Law, M.; Roussakis, A.A.; Harbaum, L.; Church, C.; Coghlan, J.G.; Condliffe, R.; Howard, L.S.; Kiely, D.G.; et al. Positioning imatinib for pulmonary arterial hypertension: A phase I/II design comprising dose finding and single-arm efficacy. Pulm. Circ. 2021, 11, 20458940211052823. [Google Scholar] [CrossRef]
- Frantz, R.P.; Benza, R.L.; Channick, R.N.; Chin, K.; Howard, L.S.; McLaughlin, V.V.; Sitbon, O.; Zamanian, R.T.; Hemnes, A.R.; Cravets, M.; et al. TORREY, a Phase 2 study to evaluate the efficacy and safety of inhaled seralutinib for the treatment of pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 20458940211057071. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Sahay, S.; Escribano Subías, P.; P. Escribano Subías, R.L.; Zolty, J.F.; Kingrey, B.; Penn, I.; Sobol, N.; Sood, R.L.; Benza, R.N.; et al. Ghofrani, and on behalf of the TORREY Study Investigators. Interim Results From the Phase 1B and Phase 2 TORREY Open-label Extension Study of Seralutinib in Pulmonary Arterial Hypertension (PAH) [abstract]. Am. J. Respir. Crit. Care Med. 2024, 209, A1011. [Google Scholar] [CrossRef]
- Gillies, H.; Chakinala, M.M.; Dake, B.T.; Feldman, J.P.; Hoeper, M.M.; Humbert, M.; Jing, Z.C.; Langley, J.; McLaughlin, V.V.; Niven, R.W.; et al. IMPAHCT: A randomized phase 2b/3 study of inhaled imatinib for pulmonary arterial hypertension. Pulm. Circ. 2024, 14, e12352. [Google Scholar] [CrossRef]
- GB002, Inc. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of oral Inhalation of Seralutinib for the Treatment of Pulmonary Arterial Hypertension (PAH). clinicaltrials.gov; 2024. Available online: https://clinicaltrials.gov/study/NCT05934526 (accessed on 7 July 2023).
- Ameshima, S.; Golpon, H.; Cool, C.D.; Chan, D.; Vandivier, R.W.; Gardai, S.J.; Wick, M.; Nemenoff, R.A.; Geraci, M.W.; Voelkel, N.F. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003, 92, 1162–1169. [Google Scholar] [CrossRef]
- Nisbet, R.E.; Bland, J.M.; Kleinhenz, D.J.; Mitchell, P.O.; Walp, E.R.; Sutliff, R.L.; Hart, C.M. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am. J. Respir. Cell Mol. Biol. 2010, 42, 482–490. [Google Scholar] [CrossRef]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003, 108, 2941–2948. [Google Scholar] [CrossRef]
- Omura, J.; Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Nogi, M.; Otsuki, T.; Kozu, K.; Numano, K.; Suzuki, K.; et al. Protective Roles of Endothelial AMP-Activated Protein Kinase Against Hypoxia-Induced Pulmonary Hypertension in Mice. Protective Roles of Endothelial AMP-Activated Protein Kinase Against Hypoxia-Induced Pulmonary Hypertension in Mice. Circ. Res. 2016, 119, 197–209. [Google Scholar] [CrossRef]
- Brittain, E.L.; Niswender, K.; Agrawal, V.; Chen, X.; Fan, R.; Pugh, M.E.; Rice, T.W.; Robbins, I.M.; Song, H.; Thompson, C.; et al. Mechanistic Phase II Clinical Trial of Metformin in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2020, 9, e018349. [Google Scholar] [CrossRef]
- Tang, Y.; Tan, S.; Li, M.; Tang, Y.; Xu, X.; Zhang, Q.; Fu, Q.; Tang, M.; He, J.; Zhang, Y.; et al. Dapagliflozin, sildenafil and their combination in monocrotaline-induced pulmonary arterial hypertension. BMC Pulm. Med. 2022, 22, 142. [Google Scholar] [CrossRef]
- Chowdhury, B.; Luu, A.Z.; Luu, V.Z.; Kabir, M.G.; Pan, Y.; Teoh, H.; Quan, A.; Sabongui, S.; Al-Omran, M.; Bhatt, D.L.; et al. The SGLT2 inhibitor empagliflozin reduces mortality and prevents progression in experimental pulmonary hypertension. Biochem. Biophys. Res. Commun. 2020, 524, 50–56. [Google Scholar] [CrossRef]
- Li, X.C.; Zhu, X.Y.; Wang, Y.Y.; Tong, S.L.; Chen, Z.L.; Lu, Z.Y.; Zhang, J.H.; Song, L.L.; Wang, X.H.; Zhang, C.; et al. Canagliflozin alleviates pulmonary hypertension by activating PPARgamma and inhibiting its S225 phosphorylation. Acta Pharmacol. Sin. 2024, 45, 1861–1878. [Google Scholar] [CrossRef]
- Lee, M.Y.; Tsai, K.B.; Hsu, J.H.; Shin, S.J.; Wu, J.R.; Yeh, J.L. Liraglutide prevents and reverses monocrotaline-induced pulmonary arterial hypertension by suppressing ET-1 and enhancing eNOS/sGC/PKG pathways. Sci. Rep. 2016, 6, 31788. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wang, W.T.; Lee, S.S.; Kuo, Y.R.; Wang, Y.C.; Yen, S.J.; Lee, M.Y.; Yeh, J.L. Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3beta Pathways. Int. J. Mol. Sci. 2019, 20, 3435. [Google Scholar] [CrossRef]
- Luna-Marco, C.; Iannantuoni, F.; Hermo-Argibay, A.; Devos, D.; Salazar, J.D.; Víctor, V.M.; Rovira-Llopis, S. Cardiovascular benefits of SGLT2 inhibitors and GLP-1 receptor agonists through effects on mitochondrial function and oxidative stress. Free Radic. Biol. Med. 2024, 213, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Sanz, R.L.; Menéndez, S.G.; Inserra, F.; Ferder, L.; Manucha, W. Cellular and Mitochondrial Pathways Contribute to SGLT2 Inhibitors-mediated Tissue Protection: Experimental and Clinical Data. Curr. Pharm. Des. 2024, 30, 969–974. [Google Scholar] [CrossRef]
- Bernardi, N.; Bianconi, E.; Vecchi, A.; Ameri, P. Noncoding RNAs in pulmonary arterial hypertension: Current knowledge and translational perspectives. Heart Fail. Clin. 2023, 19, 137–152. [Google Scholar] [CrossRef]
- Mei, J.; Huang, W.; Meng, Z.; Wen, S.; Ou, L.; Bai, J.; Wang, X.; Yuan, H.; Li, Y.; Zhang, L.; et al. Super-Enhancer-Driven HCG20 Promotes Pulmonary Hypertension Through U2AF2 Splicing. Circ. Res. 2025, 137, e19–e39. [Google Scholar] [CrossRef]
- Kumar, S.; Kim, C.W.; Simmons, R.D.; Jo, H. Role of flowsensitive microRNAs in endothelial dysfunction and atherosclerosis mechanosensitive athero-miRs. Arter. Thromb. Vasc. Biol. 2014, 34, 2206–2216. [Google Scholar] [CrossRef]
- Sindi, H.A.; Russomanno, G.; Satta, S.; Abdul-Salam, V.B.; Jo, K.B.; Qazi-Chaudhry, B.; Ainscough, A.J.; Szulcek, R.; Bogaard, H.J.; Morgan, C.C.; et al. Therapeutic potential of KLF2-induced exosomal microRNAs in pulmonary hypertension. Nat. Commun. 2020, 11, 1185, Erratum in Nat. Commun. 2020, 11, 3300. [Google Scholar] [CrossRef]
- Zhang, J.; He, Y.; Yan, X.; Chen, S.; He, M.; Lei, Y.; Zhang, J.; Gongol, B.; Gu, M.; Miao, Y.; et al. MicroRNA-483 amelioration of experimental pulmonary hypertension. EMBO Mol. Med. 2020, 12, e11303. [Google Scholar] [CrossRef]
- Meloche, J.; Pflieger, A.; Vaillancourt, M.; Paulin, R.; Potus, F.; Zervopoulos, S.; Graydon, C.; Courboulin, A.; Breuils-Bonnet, S.; Tremblay, E.; et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 2014, 129, 786–797. [Google Scholar] [CrossRef]
- Courboulin, A.; Paulin, R.; Giguère, N.J.; Saksouk, N.; Perreault, T.; Meloche, J.; Paquet, E.R.; Biardel, S.; Provencher, S.; Côté, J.; et al. Role for miR-204 in human pulmonary arterial hypertension. J. Exp. Med. 2011, 208, 535–548. [Google Scholar] [CrossRef]
- Meloche, J.; Potus, F.; Vaillancourt, M.; Bourgeois, A.; Johnson, I.; Deschamps, L.; Chabot, S.; Ruffenach, G.; Henry, S.; Breuils-Bonnet, S.; et al. Bromodomain-containing protein 4: The epigenetic origin of pulmonary arterial hypertension. Circ. Res. 2015, 117, 525–535. [Google Scholar] [CrossRef]
- Provencher, S. Olaparib for Pulmonary Arterial Hypertension: A Multicenter Clinical Trial. clinicaltrials.gov; 2023. Available online: https://clinicaltrials.gov/study/NCT03782818 (accessed on 20 December 2018).
- Provencher, S.; Potus, F.; Blais-Lecours, P.; Bernard, S.; Martineau, S.; Breuils-Bonnet, S.; Weatherald, J.; Sweeney, M.; Kulikowski, E.; Boucherat, O.; et al. BET protein inhibition for pulmonary arterial hypertension: A pilot clinical trial. Am. J. Respir. Crit. Care Med. 2022, 205, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.P.; Roberts, J.T.; Kuebler, W.M.; Lee, J.Y.; Suresh, K.; Hough, R.F. Bioenergetics and Metabolism of the Pulmonary Endothelium: Scientific Session I, ReSPIRE 2025. Am. J. Physiol. Lung Cell. Mol. Physiol. 2025, 329, L389–L396. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Yun, X.; Pi, H.; Murray, S.; Hill, Z.; Fonticella, J.; Perez, P.; Zhang, C.; Pathmasiri, W.; Sumner, S.; et al. Fatty acid 372 metabolism promotes TRPV4 activity in lung microvascular endothelial cells in pulmonary 373 arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2024, 326, L252–L265. [Google Scholar] [CrossRef] [PubMed]
- Philip, N.; Pi, H.; Gadkari, M.; Yun, X.; Huetsch, J.; Zhang, C.; Harlan, R.; Roux, A.; Graham, D.; Shimoda, L.; et al. Transpulmonary amino acid metabolism in the sugen hypoxia model of pulmonary hypertension. Pulm. Circ. 2023, 13, e12205. [Google Scholar] [CrossRef]
- Simpson, C.E.; Coursen, J.; Hsu, S.; Gough, E.K.; Harlan, R.; Roux, A.; Aja, S.; Graham, D.; Kauffman, M.; Suresh, K.; et al. Metabolic profiling of in vivo right ventricular function and exercise performance in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 324, L836–L848. [Google Scholar] [CrossRef]
- Ghio, S.; Badagliacca, R.; D’Alto, M.; Scelsi, L.; Argiento, P.; Brunetti, N.D.; Casu, G.; Cedrone, N.; Confalonieri, M.; Corda, M.; et al. Right ventricular phenotyping in incident patients with idiopathic pulmonary arterial hypertension. J. Heart Lung Transplant. 2024, 43, 1668–1676. [Google Scholar] [CrossRef]
- Badagliacca, R.; Ghio, S.; D’Alto, M.; Ameri, P.; Correale, M.; Filomena, D.; Raineri, C.; Stolfo, D.; Naeije, R.; Vizza, C.D. Relevance of Echocardiography-derived Phenotyping in Patients with Pulmonary Arterial Hypertension Treated with Initial Oral Combination Therapy: An Italian Pulmonary Hypertension Network (iPHNET) Study. Am. J. Respir. Crit. Care Med. 2024, 210, 362–365. [Google Scholar] [CrossRef]
- Rafikov, R.; de Jesus Perez, V.; Dekan, A.; Kudryashova, T.V.; Rafikova, O. Deciphering the Complexities of Pulmonary Hypertension: The Emergent Role of Single-Cell Omics. Am. J. Respir. Cell Mol. Biol. 2024, 72, 32–40. [Google Scholar] [CrossRef]
| Function | Description | Mechanisms/Key Molecules | Approved Therapeutic Agents | Experimental Therapeutic Agents |
|---|---|---|---|---|
| Vascular tone regulation [63] | Modulator of vessel diameter to maintain blood pressure and proper blood flow [63] | NO Prostaglandins ET-1 [63] | Sildenafil, Tadalafil, Bosentan, Ambrisentan, Macitentan, Riociguat, Epoprostenol, Iloprost, Treprostinil, Selexipag | Ralinepag, miRNAs |
| Anti-inflammatory and antithrombotic properties [62,63] | Preventer of inflammation and thrombus formation, regulator of leukocyte migration into lung tissue [62,63] | Prostacyclin Thromboprotective factors Adhesion molecules (e.g., ICAM-1, VCAM-1) [62,63] | Tocilizumab, Satralizumab, Etanercept, Dapagliflozin, Empagliflozin, Canagliflozin, Liraglutide | |
| Angiogenesis [62,63] | Promotes new vessel formation, essential for tissue repair and adaptation to oxygen demand changes [62,63] | PDGF and VEGF Angiopoietin-2 Other proangiogenic factors [62,63] | Imatinib, Seralutinib, miRNAs | |
| Barrier Function [12,13] | Acts as a selective barrier, preventing harmful substances from entering the bloodstream while allowing essential molecules to pass [62,63] | Endothelium glycocalyx Tight junction proteins [62,63] | ||
| Gas Exchange Facilitation [63] | Ensures efficient oxygen and carbon dioxide transfer between alveoli and bloodstream [63] | Alveolar-capillary endothelium [63] | ||
| Cellular Signaling [58,63,64] | Involved in signaling pathways regulating cell growth, differentiation, and responses to injury [58,63,64] | Signaling cascade (e.g., MAPK, PI3K/AKT pathways) Regulatory molecules [58,63,64] | Sotatercept | KER-012, FK506 (Tacrolimus), Rosiglitazone, Imatinib, mTOR, Tamoxifen, Anastrazole |
| Response to shear stress [11] | Detects blood flow changes, promotes NO production to preserve vascular homeostasis [60,61] | eNOS [62] Endothelial glycocalyx [60,61] | Metformin, mechano-miRs, miRNAs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correale, M.; Mercurio, V.; Bevere, E.M.L.; Pezzuto, B.; Tricarico, L.; Attanasio, U.; Raucci, A.; Ferrara, A.L.; Loffredo, S.; Puteo, C.; et al. Pathophysiology of Pulmonary Arterial Hypertension: Focus on Vascular Endothelium as a Potential Therapeutic Target. Int. J. Mol. Sci. 2025, 26, 9631. https://doi.org/10.3390/ijms26199631
Correale M, Mercurio V, Bevere EML, Pezzuto B, Tricarico L, Attanasio U, Raucci A, Ferrara AL, Loffredo S, Puteo C, et al. Pathophysiology of Pulmonary Arterial Hypertension: Focus on Vascular Endothelium as a Potential Therapeutic Target. International Journal of Molecular Sciences. 2025; 26(19):9631. https://doi.org/10.3390/ijms26199631
Chicago/Turabian StyleCorreale, Michele, Valentina Mercurio, Ester Maria Lucia Bevere, Beatrice Pezzuto, Lucia Tricarico, Umberto Attanasio, Angela Raucci, Anne Lise Ferrara, Stefania Loffredo, Claudio Puteo, and et al. 2025. "Pathophysiology of Pulmonary Arterial Hypertension: Focus on Vascular Endothelium as a Potential Therapeutic Target" International Journal of Molecular Sciences 26, no. 19: 9631. https://doi.org/10.3390/ijms26199631
APA StyleCorreale, M., Mercurio, V., Bevere, E. M. L., Pezzuto, B., Tricarico, L., Attanasio, U., Raucci, A., Ferrara, A. L., Loffredo, S., Puteo, C., Iacoviello, M., Margaglione, M., Brunetti, N. D., Tocchetti, C. G., Agostoni, P., Mussolino, C., & Vinci, M. C. (2025). Pathophysiology of Pulmonary Arterial Hypertension: Focus on Vascular Endothelium as a Potential Therapeutic Target. International Journal of Molecular Sciences, 26(19), 9631. https://doi.org/10.3390/ijms26199631










