Coenzyme Q10 and Intracellular Signalling Pathways: Clinical Relevance
Abstract
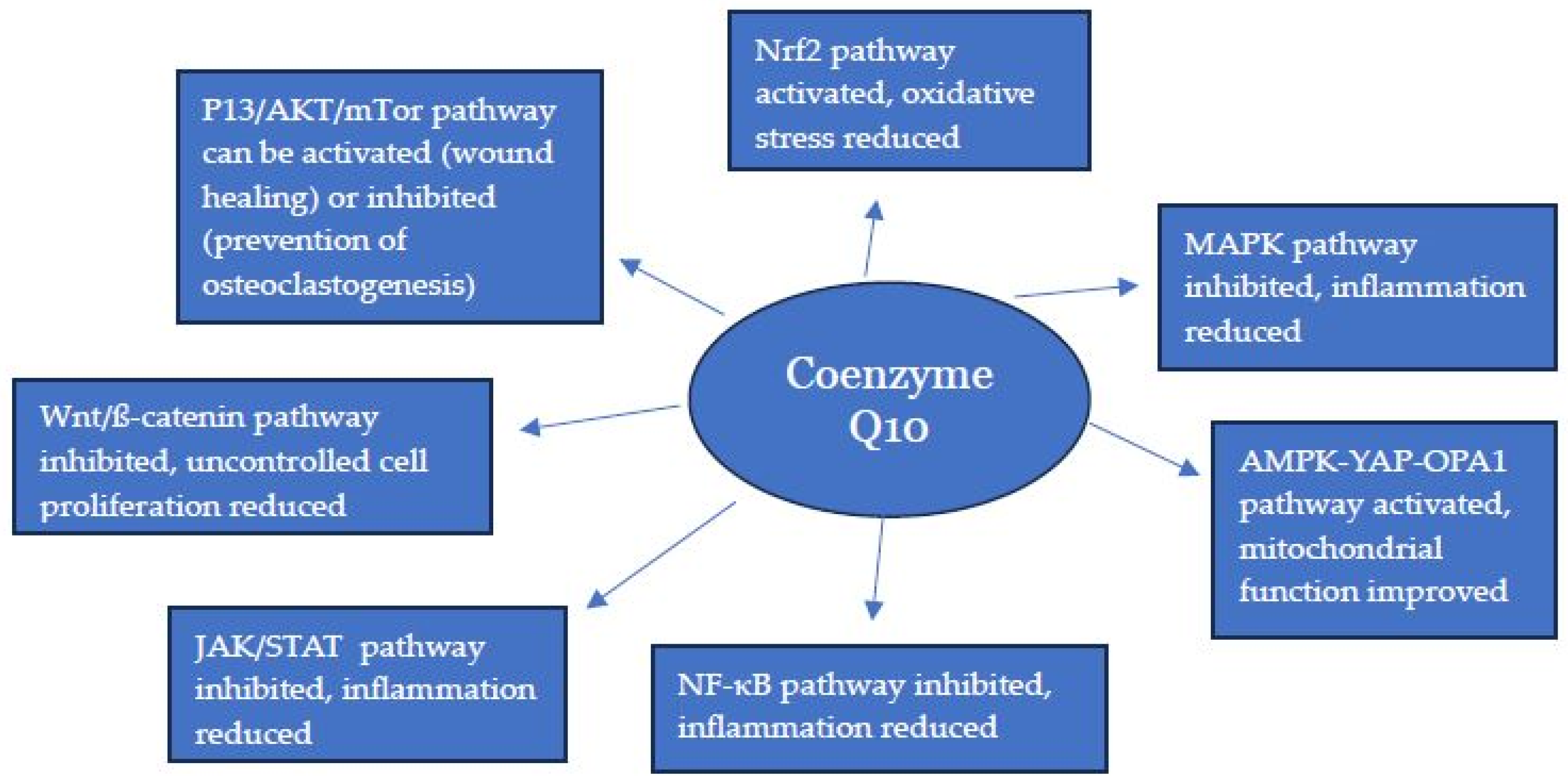
1. Introduction
2. The Nrf2/NQO1 Pathway
3. The NF-κB Pathway
4. The PI3K/AKT/mTOR Pathway
5. The MAPK Pathway
6. The JAK/STAT Pathway
7. The Wnt/β-Catenin Pathway
8. The AMPK-YAP-OPA1 Pathway
9. The Hedgehog Pathway
10. Discussion
| Pathway | Cellular Function of Pathway | Principal Clinical Consequence of Pathway Dysfunction |
|---|---|---|
| Nrf2/NQO1/ | Protection against oxidative stress | Neurodegenerative disorders, including Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, and amyotrophic lateral sclerosis [90] |
| Nf-κB | Regulation of immune response and inflammation | Autoimmune disorders, including rheumatoid arthritis, lupus erythematous, and IBS [91] |
| P13K/AKT/mTOR | Regulation of cell growth and proliferation | Cancer, including breast, colon, and skin cancers [92] |
| MAPK | Control of cell growth, differentiation and survival | Cancer, including colon, pancreatic, lung, and skin cancers [93] |
| JAK/STAT | Regulation of immune response and inflammation; cell growth and proliferation | Autoimmune disorders (e.g., rheumatoid arthritis, IBS) and cancer (e.g., haematological cancers, breast and lung cancer) [94] |
| Wnt/β-catenin | Normal embryonic development; control of cell growth | Embryonic abnormalities; cancer [95] |
| AMPK/YAP/OPA1 | Control of energy metabolism | Cardiovascular disease; metabolic disorders (diabetes, obesity) [96] |
| Hedgehog | Normal embryonic development | Developmental disorders (e.g., craniofacial defects); cancer (e.g., basal cell carcinoma) [97] |
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antebi, Y.E.; Nandagopal, N.; Elowitz, M.B. An operational view of intercellular signaling pathways. Curr. Opin. Syst. Biol. 2017, 1, 16–24. [Google Scholar] [CrossRef]
- Valdespino-Gómez, V.M.; Valdespino-Castillo, P.M.; Valdespino-Castillo, V.E. Cell signaling pathways interaction in cellular proliferation: Potential target for therapeutic interventionism. Cirugía Cir. 2015, 83, 165–174. [Google Scholar] [CrossRef]
- Crane, F.L. Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Turton, N.; Hargreaves, I.P. Depletion and supplementation of Coenzyme Q10 in Secondary Deficiency Disorders. Front. Biosci. Landmark Ed. 2022, 27, 322. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Chu, Q.; Shi, Q.; Zeng, Y.; Lu, J.; Li, L. Wnt signaling pathways in biology and disease: Mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020, 25, 5474. [Google Scholar] [CrossRef]
- Esteras, N.; Abramov, A.Y. Nrf2 as a regulator of mitochondrial function: Energy metabolism and beyond. Free Radic. Biol. Med. 2022, 189, 136–153. [Google Scholar] [CrossRef]
- Dodson, M.; Anandhan, A.; Zhang, D.D.; Madhavan, L. An NRF2 Perspective on stem cells and ageing. Front. Aging 2021, 2, 690686. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Sun, J.H.; Wang, L.; Cheng, Y.Y. Genetic polymorphism of NQO1 influences susceptibility to coronary heart disease in a chinese population: A cross-sectional study and meta-anaylsis. Pharmgenom. Pers. Med. 2023, 16, 825–833. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Samimi, F.; Baazm, M.; Nadi, Z.; Dastghaib, S.; Rezaei, M.; Jalali-Mashayekhi, F. Evaluation of antioxidant effects of Coenzyme Q10 against hyperglycemia-mediated oxidative stress by focusing on Nrf2/Keap1/HO-1 signaling pathway in the liver of diabetic rats. Iran. J. Med. Sci. 2024, 49, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Lotem, J.; Kama, R.; Sachs, L.; Shaul, Y. NQO1 stabilizes p53 through a distinct pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 3099–3104. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhan, J.; Hou, Y.; Chen, S.; Hou, Y.; Xiao, Z.; Luo, D.; Lin, D. Coenzyme Q10 suppresses oxidative stress and apoptosis via activating the Nrf-2/NQO-1 and NF-κB signaling pathway after spinal cord injury in rats. Am. J. Transl. Res. 2019, 11, 6544–6552. [Google Scholar]
- Choi, H.K.; Pokharel, Y.R.; Lim, S.C.; Han, H.K.; Ryu, C.S.; Kim, S.K.; Kwak, M.K.; Kang, K.W. Inhibition of liver fibrosis by solubilized coenzyme Q10: Role of Nrf2 activation in inhibiting transforming growth factor-beta1 expression. Toxicol. Appl. Pharmacol. 2009, 240, 377–384. [Google Scholar] [CrossRef]
- Pala, R.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ali, S.; Cinar, V.; Atalay, M.; Sahin, K. Coenzyme Q10 supplementation modulates NFκB and Nrf2 pathways in exercise training. J. Sports Sci. Med. 2016, 15, 196–203. [Google Scholar]
- Yang, H.L.; Lin, M.W.; Korivi, M.; Wu, J.J.; Liao, C.H.; Chang, C.T.; Liao, J.W.; Hseu, Y.C. Coenzyme Q0 regulates NFκB/AP-1 activation and enhances Nrf2 stabilization in attenuation of LPS-induced inflammation and redox imbalance: Evidence from in vitro and in vivo studies. Biochim. Biophys. Acta. 2016, 1859, 246–261. [Google Scholar] [CrossRef]
- Li, L.; Du, J.; Lian, Y.; Zhang, Y.; Li, X.; Liu, Y.; Zou, L.; Wu, T. Protective effects of Coenzyme Q10 against hydrogen peroxide-Induced oxidative stress in PC12 cell: The role of Nrf2 and antioxidant enzymes. Cell. Mol. Neurobiol. 2016, 36, 103–111. [Google Scholar] [CrossRef]
- Kabel, A.M.; Elkhoely, A.A. Ameliorative effect of Coenzyme Q10 and/or Candesartan on carboplatin-Induced nephrotoxicity: Roles of apoptosis, transforming growth factor-Β1, Nuclear Factor Kappa-B and the Nrf2/HO-1 pathway. Asian Pac. J. Cancer Prev. 2017, 18, 1629–1636. [Google Scholar] [CrossRef]
- Khodir, A.E.; Atef, H.; Said, E.; ElKashef, H.A.; Salem, H.A. Implication of Nrf2/HO-1 pathway in the coloprotective effect of coenzyme Q10 against experimentally induced ulcerative colitis. Inflammopharmacology 2017, 25, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Samimi, F.; Baazm, M.; Eftekhar, E.; Rajabi, S.; Goodarzi, M.T.; Jalali Mashayekhi, F. Possible antioxidant mechanism of coenzyme Q10 in diabetes: Impact on Sirt1/Nrf2 signaling pathways. Res. Pharm. Sci. 2019, 14, 524–533. [Google Scholar] [CrossRef]
- Yousef, A.O.; AFahad, A.; Abdel Moneim, A.E.; Metwally, D.M.; El-Khadragy, M.F.; Kassab, R.B. The neuroprotective role of Coenzyme Q10 against lead acetate-Induced neurotoxicity Is mediated by antioxidant, anti-Inflammatory and anti-apoptotic activities. Int. J. Environ. Res. Public Health 2019, 16, 2895. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L. Protective effects of trimetazidine and coenzyme Q10 on cisplatin-induced cardiotoxicity by alleviating oxidative stress and mitochondrial dysfunction. Anatol. J. Cardiol. 2019, 22, 232–239. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef]
- Mahmoud, A.R.; Ali, F.E.M.; Abd-Elhamid, T.H.; Hassanein, E.H.M. Coenzyme Q10 protects hepatocytes from ischemia reperfusion-induced apoptosis and oxidative stress via regulation of Bax/Bcl-2/PUMA and Nrf-2/FOXO-3/Sirt-1 signaling pathways. Tissue Cell 2019, 60, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; Zhou, Q.; Lu, Y.; Chen, P.; Wang, X.; Zhao, H. Implication of nuclear factor-erythroid 2-like 2/heme oxygenase 1 pathway in the protective effects of coenzyme Q10 against preeclampsia-like in a rat model. Microcirculation 2020, 27, e12651. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Soliman, D.; Kassab, R.B.; Metwally, D.M.; Ahmed EAbdel Moneim El-Khadragy, M.F. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 2020, 11, 64. [Google Scholar] [CrossRef]
- Hussein, R.M.; Sawy, D.M.; Kandeil, M.A.; Farghaly, H.S. Chlorogenic acid, quercetin, coenzyme Q10 and silymarin modulate Keap1-Nrf2/heme oxygenase-1 signaling in thioacetamide-induced acute liver toxicity. Life Sci. 2021, 277, 119460. [Google Scholar] [CrossRef]
- Du, Q.; Meng, W.; Athari, S.S.; Wang, R. The effect of Co-Q10 on allergic rhinitis and allergic asthma. Allergy Asthma Clin. Immunol. 2021, 17, 32. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, H.; You, L.; Zhang, J.; Jiang, Z. Coenzyme Q10 inhibits intracranial aneurysm formation and progression in a mouse model. Pediatr. Res. 2022, 91, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.M.; Salem, O.M.; Safa, M.A.E.; Barhoma, R.A.E.; Eltabaa, E.F.; Shalaby, A.M.; Alabiad, M.A.; Arakeeb, H.M.; Mohamed, H.A. Possible protective effects of CO Q10 against vincristine-induced peripheral neuropathy: Targeting oxidative stress, inflammation, and sarmoptosis. J. Biochem. Mol. Toxicol. 2022, 36, e22976. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Fhatima, S.; Parmar, D.; Singh, D.; Mishra, R.; Singh, G. Therapeutic effects of CoenzymeQ10, Biochanin A and Phloretin against arsenic and chromium induced oxidative stress in mouse (Mus musculus) brain. 3Biotech 2022, 121, 116. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Ahuja, P.; Kapil, L.; Sharma, D.; Singh, C.; Singh, A. Coenzyme Q10 ameliorates chemotherapy-induced cognitive impairment in mice: A preclinical study. Mol. Biol. Rep. 2024, 51, 930. [Google Scholar] [CrossRef]
- Deppe, L.; Mueller-Buehl, A.M.; Tsai, T.; Erb, C.; Dick, H.B.; Joachim, S.C. Protection against oxidative stress by Coenzyme Q10 in a porcine retinal degeneration model. J. Pers. Med. 2024, 14, 437. [Google Scholar] [CrossRef]
- Arafa, E.A.; Hassanein, E.H.M.; Ibrahim, N.A.; Buabeid, M.A.; Mohamed, W.R. Involvement of Nrf2-PPAR-γ signaling in Coenzyme Q10 protecting effect against methotrexate-induced testicular oxidative damage. Int. Immunopharmacol. 2024, 129, 111566. [Google Scholar] [CrossRef]
- Tripathi, S.; Parmar, D.; Singh, D.; Singh, G. Attenuation of chromium (VI) and arsenic (III)-induced oxidative stress and hepatic apoptosis by phloretin, biochanin-A, and coenzyme Q10 via activation of SIRT1/Nrf2/HO-1/NQO1 signaling. J. Biochem. Mol. Toxicol. 2024, 38, e23817. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Li, G.; Zou, L.Y.; Cao, C.M.; Yang, E.S. Coenzyme Q10 protects SHSY5Y neuronal cells from beta amyloid toxicity and oxygen-glucose deprivation by inhibiting the opening of the mitochondrial permeability transition pore. Biofactors 2005, 25, 97–107. [Google Scholar] [CrossRef]
- Brea-Calvo, G.; Siendones, E.; Sánchez-Alcázar, J.A.; de Cabo, R.; Navas, P. Cell survival from chemotherapy depends on NF-kappaB transcriptional up-regulation of coenzyme Q biosynthesis. PLoS ONE 2009, 4, e5301. [Google Scholar] [CrossRef]
- Kooncumchoo, P.; Sharma, S.; Porter, J.; Govitrapong, P.; Ebadi, M. Coenzyme Q(10) provides neuroprotection in iron-induced apoptosis in dopaminergic neurons. J. Mol. Neurosci. 2006, 28, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Jresat, I. Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ. Toxicol. Pharmacol. 2012, 33, 158–167. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Song, C.Y.; Yuan, Y.; Eber, A.; Rodriguez, Y.; Levitt, R.C.; Takacs, P.; Yang, Z.; Goldberg, R.; Candiotti, K.A. Diabetic neuropathic pain development in type 2 diabetic mouse model and the prophylactic and therapeutic effects of coenzyme Q10. Neurobiol. Dis. 2013, 58, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Al-Mulhim, A.S.; Jresat, I. Therapeutic effect of coenzyme Q10 against experimentally-induced hepatocellular carcinoma in rats. Environ. Toxicol. Pharmacol. 2013, 35, 100–108. [Google Scholar] [CrossRef]
- Yoneda, T.; Tomofuji, T.; Kawabata, Y.; Ekuni, D.; Azuma, T.; Kataoka, K.; Kunitomo, M.; Morita, M. Application of coenzyme Q10 for accelerating soft tissue wound healing after tooth extraction in rats. Nutrients 2014, 6, 5756–5769. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Xu, X.; Wang, W.; Song, S.; Liang, K.; Yang, M.; Guo, L.; Zhao, Y.; Li, R. Coenzyme Q10 suppresses TNF-α-Induced inflammatory reaction In vitro and attenuates severity of dermatitis in mice. Inflammation 2016, 39, 281–289. [Google Scholar] [CrossRef]
- Li, L.; Xu, D.; Lin, J.; Zhang, D.; Wang, G.; Sui, L.; Ding, H.; Du, J. Coenzyme Q10 attenuated β-amyloid25–35-induced inflammatory responses in PC12 cells through regulation of the NF-κB signaling pathway. Brain Res. Bull. 2017, 131, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Boroujeni, M.B.; Khayat, Z.K.; Anbari, K.; Niapour, A.; Gholami, M.; Gharravi, A.M. Coenzyme Q10 protects skeletal muscle from ischemia-reperfusion through the NF-kappa B pathway. Perfusion 2017, 32, 372–377. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Cassano, P.; Sadigh-Eteghad, S.; Erfani, M.; Hamblin, M.R.; Salimi, M.M.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Near-infrared photobiomodulation combined with coenzyme Q10 for depression in a mouse model of restraint stress: Reduction in oxidative stress, neuroinflammation, and apoptosis. Brain Res. Bull. 2019, 144, 213–222. [Google Scholar] [CrossRef]
- Nyariki, J.N.; Ochola, L.A.; Jillani, N.E.; Nyamweya, N.O.; Amwayi, P.E.; Yole, D.S.; Azonvide, L.; Isaac, A.O. Oral administration of Coenzyme Q10 protects mice against oxidative stress and neuro-inflammation during experimental cerebral malaria. Parasitol. Int. 2019, 71, 106–120. [Google Scholar] [CrossRef]
- Quagliariello, V.; Vecchione, R.; De Capua, A.; Lagreca, E.; Iaffaioli, R.V.; Botti, G.; Netti, P.A.; Maurea, N. Nano-encapsulation of Coenzyme Q10 in secondary and tertiary nano-emulsions for enhanced cardioprotection and hepatoprotection in human cardiomyocytes and hepatocytes during exposure to anthracyclines and Ttastuzumab. Int. J. Nanomed. 2020, 15, 4859–4876. [Google Scholar] [CrossRef]
- Ali, F.E.M.; Ahmed, S.F.; Eltrawy, A.H.; Yousef, R.S.; Ali, H.S.; Mahmoud, A.R.; Abd-Elhamid, T.H. Pretreatment with Coenzyme Q10 combined with aescin protects against sepsis-Induced acute lung injury. Cells Tissues Organs 2021, 210, 195–217. [Google Scholar] [CrossRef]
- Mohamed, H.A.; Said, R.S. Coenzyme Q10 attenuates inflammation and fibrosis implicated in radiation enteropathy through suppression of NF-κB/TGF-β/MMP-9 pathways. Int. Immunopharmacol. 2021, 92, 107347. [Google Scholar] [CrossRef]
- Alhusaini, A.; Sarawi, W.; Mattar, D.; Abo-Hamad, A.; Almogren, R.; Alhumaidan, S.; Alsultan, E.; Alsaif, S.; Hasan, I.; Hassanein, E.; et al. Acetyl-L-carnitine and/or liposomal co-enzyme Q10 prevent propionic acid-induced neurotoxicity by modulating oxidative tissue injury, inflammation, and ALDH1A1-RA-RARα signaling in rats. Biomed. Pharmacother. 2022, 153, 113360, Corrigendum in Biomed. Pharmacother. 2023, 162, 114645. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Hashem, M.M.M.; Abo-El-Sooud, K.; Mousa, M.R.; Soliman, A.M.; Mouneir, S.M.; Ismail, S.H.; Hassan, B.A.; El-Nour, H.H.M. Interactive effects of cadmium and titanium dioxide nanoparticles on hepatic tissue in rats: Ameliorative role of coenzyme 10 via modulation of the NF-κB and TNFα pathway. Food Chem. Toxicol. 2023, 182, 114191. [Google Scholar] [CrossRef]
- Fakharaldeen, Z.A.; Al-Mudhafar, A.; Gany, S.N.; Radhi, A.N.; Hadi, N.R. Neuroprotective effects of Coenzyme Q10 in ischemia-reperfusion injury via inflammation and oxidative stress reduction in adult male rats. J. Med. Life. 2023, 16, 1534–1539. [Google Scholar] [CrossRef]
- Frontiñán-Rubio, J.; Llanos-González, E.; García-Carpintero, S.; Peinado, J.R.; Ballesteros-Yáñez, I.; Rayo, M.V.; de la Fuente, J.; Pérez-García, V.M.; Perez-Romasanta, L.A.; Malumbres, M.; et al. CoQ10 reduces glioblastoma growth and infiltration through proteome remodeling and inhibition of angiogenesis and inflammation. Cell. Oncol. 2023, 46, 65–77, Correction in Cell. Oncol. 2023, 46, 1543. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.F.; Hashad, I.M.; Rady, M.; Abd-El Razik, A.N.; Saleh, D.O. Diosmin and Coenzyme q10: Synergistic histopathological and functional protection against doxorubicin-induced hepatorenal injury in rats. Toxicol. Rep. 2024, 13, 101848. [Google Scholar] [CrossRef]
- Antar, S.A.; Abdo, W.; Helal, A.I.; Abduh, M.S.; Hakami, Z.H.; Germoush, M.O.; Alsulimani, A.; Al-Noshokaty, T.M.; El-Dessouki, A.M.; ElMahdy, M.K.; et al. Coenzyme Q10 mitigates cadmium cardiotoxicity by downregulating NF-κB/NLRP3 inflammasome axis and attenuating oxidative stress in mice. Life Sci. 2024, 348, 122688. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Yu, S.; Chi, L.; Cai, Y. Coenzyme Q10 alleviates neurological deficits in a mouse model of intracerebral hemorrhage by reducing inflammation and apoptosis. Exp. Biol. Med. 2025, 250, 10321. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, L.; Wu, J.; Ai, D.; Zhang, J.Q.; Chen, T.G.; Wang, L. Targeting the PI3K/AKT/mTOR signaling pathway in the treatment of human diseases: Current status, trends, and solutions. J. Med. Chem. 2022, 65, 16033–16061. [Google Scholar] [CrossRef]
- Xue, R.; Yang, J.; Wu, J.; Meng, Q.; Hao, J. Coenzyme Q10 inhibits the activation of pancreatic stellate cells through PI3K/AKT/mTOR signaling pathway. Oncotarget 2017, 8, 92300–92311. [Google Scholar] [CrossRef]
- Kurashiki, T.; Horikoshi, Y.; Kamizaki, K.; Sunaguchi, T.; Hara, K.; Morimoto, M.; Kitagawa, Y.; Nakaso, K.; Otsuki, A.; Matsura, T. Molecular mechanisms underlying the promotion of wound repair by coenzyme Q10: PI3K/Akt signal activation via alterations to cell membrane domains. J. Clin. Biochem. Nutr. 2022, 70, 222–230. [Google Scholar] [CrossRef]
- Choi, H.; Park, H.H.; Koh, S.H.; Choi, N.Y.; Yu, H.J.; Park, J.; Lee, Y.J.; Lee, K.Y. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology 2012, 33, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, B.; Tang, S.; Sun, J.; Bao, E. Co-enzyme Q10 protects primary chicken myocardial cells from heat stress by upregulating autophagy and suppressing the PI3K/AKT/mTOR pathway. Cell Stress Chaperones 2019, 24, 1067–1078. [Google Scholar] [CrossRef]
- Zheng, D.; Cui, C.; Shao, C.; Wang, Y.; Ye, C.; Lv, G. Coenzyme Q10 inhibits RANKL-induced osteoclastogenesis by regulation of mitochondrial apoptosis and oxidative stress in RAW264.7 cells. J. Biochem. Mol. Toxicol. 2021, 35, e22778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yan, B.; Yu, S.; Zhang, C.; Wang, B.; Wang, Y.; Wang, J.; Yuan, Z.; Zhang, L.; Pan, J. Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxidative Med. Cell Longev. 2015, 2015, 867293. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A comprehensive review on MAPK: A promising therapeutic target in cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef]
- Li, X.; Guo, Y.; Huang, S.; He, M.; Liu, Q.; Chen, W.; Liu, M.; Xu, D.; He, P. Coenzyme Q10 prevents the interleukin-1 beta induced inflammatory response via inhibition of MAPK signaling pathways in rat articular chondrocytes. Drug Dev. Res. 2017, 78, 403–410. [Google Scholar] [CrossRef]
- Zheng, D.; Cui, C.; Ye, C.; Shao, C.; Zha, X.; Xu, Y.; Liu, X.; Wang, C. Coenzyme Q10 prevents RANKL-induced osteoclastogenesis by promoting autophagy via inactivation of the PI3K/AKT/mTOR and MAPK pathways. Braz. J. Med. Biol. Res. 2024, 57, e13474. [Google Scholar] [CrossRef]
- Allam, E.A.; Ibrahim, H.F.; Abdulmalek, S.A.; Abdelmeniem, I.M.; Basta, M. Coenzyme Q10 alleviates testicular endocrine and spermatogenic dysfunction induced by high-fat diet in male Wistar rats: Role of adipokines, oxidative stress and MAPK/ERK/JNK pathway. Andrologia 2022, 54, e14544. [Google Scholar] [CrossRef]
- Nawar, N.F.; Beltagy, D.M.; Tousson, E.; El-Keey, M.M.; Mohamed, T.M. Coenzyme Q10 alleviates AlCl3 and D-galactose induced Alzheimer via modulating oxidative burden and TLR-4/MAPK pathways and regulation microRNA in rat brain. Toxicol. Res. Camb 2025, 14, tfaf031. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tang, S.; Yin, B.; Sun, J.; Bao, E. Co-enzyme Q10 upregulates Hsp70 and protects chicken primary myocardial cells under in vitro heat stress via PKC/MAPK. Mol. Cell. Biochem. 2018, 449, 195–206. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.S.; Zeng, J.; Mei, J.; Wang, P.Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.; Lindner, I.; Vock, C.; Fujii, K.; Döring, F. Functional connections and pathways of coenzyme Q10-inducible genes: An in-silico study. IUBMB Life 2007, 59, 628–633. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Hussein, Z.; Michel, H.E.; El-Naga, R.N.; El-Demerdash, E.; Mantawy, E.M. Coenzyme Q10 ameliorates cyclophosphamide-induced chemobrain by repressing neuronal apoptosis and preserving hippocampal neurogenesis: Mechanistic roles of Wnt/ β-catenin signaling pathway. Neurotoxicology 2024, 105, 21–33. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Jin, J.; Nan, Q.Y.; Ding, J.; Cui, S.; Xuan, M.Y.; Piao, M.H.; Piao, S.G.; Zheng, H.L.; Jin, J.Z.; et al. Coenzyme Q10 attenuates renal fibrosis by inhibiting RIP1-RIP3-MLKL-mediated necroinflammation via Wnt3α/β-catenin/GSK-3β signaling in unilateral ureteral obstruction. Int. Immunopharmacol. 2022, 108, 108868. [Google Scholar] [CrossRef] [PubMed]
- Ozhan, G.; Weidinger, G. Wnt/β-catenin signaling in heart regeneration. Cell Regen. 2015, 4, 3. [Google Scholar] [CrossRef]
- Mortensen, S.A.; Rosenfeldt, F.; Kumar, A.; Dolliner, P.; Filipiak, K.J.; Pella, D.; Alehagen, U.; Steurer, G.; Littarru, G.P.; Q-SYMBIO Study Investigators. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014, 2, 641–649. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Wang, C.; Jin, Y.; Meng, Q.; Liu, Q.; Wu, J.; Sun, H. CoenzymeQ10-induced activation of AMPK-YAP-OPA1 pathway alleviates atherosclerosis by improving mitochondrial function, inhibiting oxidative stress and promoting energy metabolism. Front. Pharmacol. 2020, 11, 1034. [Google Scholar] [CrossRef]
- Robbins, D.J.; Fei, D.L.; Riobo, N.A. The Hedgehog signal transduction network. Sci. Signal. 2012, 5, re6. [Google Scholar] [CrossRef]
- Patel, S.; Armbruster, H.; Pardo, G.; Archambeau, B.; Kim, N.H.; Jeter, J.; Wu, R.; Kendra, K.; Contreras, C.M.; Spaccarelli, N.; et al. Hedgehog pathway inhibitors for locally advanced and metastatic basal cell carcinoma: A real-world single-center retrospective review. PLoS ONE 2024, 19, e0297531. [Google Scholar] [CrossRef]
- Govorova, I.A.; Nikitochkina, S.Y.; Vorotelyak, E.A. Influence of intersignaling crosstalk on the intracellular localization of YAP/TAZ in lung cells. Cell Commun. Signal. 2024, 22, 289. [Google Scholar] [CrossRef]
- Zeke, A.; Lukács, M.; Lim, W.A.; Reményi, A. Scaffolds: Interaction platforms for cellular signalling circuits. Trends Cell Biol. 2009, 19, 364–374. [Google Scholar] [CrossRef]
- Aksamitiene, E.; Kiyatkin, A.; Kholodenko, B.N. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: A fine balance. Biochem. Soc. Trans. 2012, 40, 139–146. [Google Scholar] [CrossRef]
- Mantle, D.; Rowbottom, H.; Jones, J.; Potts, I.M.; Turton, N.; Dewsbury, M.; Lopez-Lluch, G.; Hargreaves, I.P. Energy metabolism as a therapeutic target in cancer: The role of coenzyme Q10. Oxygen 2024, 4, 122–138. [Google Scholar] [CrossRef]
- Lee, S.K.; Lee, J.O.; Kim, J.H.; Kim, N.; You, G.Y.; Moon, J.W.; Sha, J.; Kim, S.J.; Lee, Y.W.; Kang, H.J.; et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARα induction in 3T3-L1 preadipocytes. Cell Signal. 2012, 24, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88 Pt B, 253–267. [Google Scholar] [CrossRef]
- Sun, S.C.; Chang, J.H.; Jin, J. Regulation of nuclear factor-κB in autoimmunity. Trends Immunol. 2013, 34, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Rascio, F.; Spadaccino, F.; Rocchetti, M.T.; Castellano, G.; Stallone, G.; Netti, G.S.; Ranieri, E. The pathogenic role of PI3K/AKT pathway in cancer onset and drug resistance: An updated review. Cancers 2021, 13, 3949. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Fatma, M.; Mir, S.S.; Dermime, S.; Uddin, S. JAK-STAT signaling in autoimmunity and cancer. Immunotargets Ther. 2025, 14, 523–554. [Google Scholar] [CrossRef]
- Sidrat, T.; Rehman, Z.U.; Joo, M.D.; Lee, K.L.; Kong, I.K. Wnt/β-catenin pathway-mediated PPARδ expression during embryonic development differentiation and disease. Int. J. Mol. Sci. 2021, 22, 1854. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.H. AMPK, Mitochondrial function, and cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef]
- Abramyan, J. Hedgehog signaling and embryonic craniofacial disorders. J. Dev. Biol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in neurodegenerative diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Yang, X.X.; Yang, R.; Zhang, F. Role of Nrf2 in Parkinson’s Disease: Toward new perspectives. Front. Pharmacol. 2022, 13, 919233. [Google Scholar] [CrossRef] [PubMed]
- Sarlette, A.; Krampfl, K.; Grothe, C.; Neuhoff Nv Dengler, R.; Petri, S. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2008, 67, 1055–1062. [Google Scholar] [CrossRef]
- Quinti, L.; Naidu, S.D.; Träger, U.; Chen, X.; Kegel-Gleason, K.; Llères, D.; Connolly, C.; Chopra, V.; Low, C.; Moniot, S.; et al. KEAP1-modifying small molecule reveals muted NRF2 signaling responses in neural stem cells from Huntington’s disease patients. Proc. Natl. Acad. Sci. USA 2017, 114, E4676–E4685. [Google Scholar] [CrossRef]
- Petrillo, S.; D’Amico, J.; La Rosa, P.; Bertini, E.S.; Piemonte, F. Targeting NRF2 for the treatment of Friedreich’s Ataxia: A comparison among drugs. Int. J. Mol. Sci. 2019, 20, 5211. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, S.U.; Suleman, M.; Khan, M.U.; Khan, M.S.; Arbi, F.M.; Hussain, T.; Mohammed Alsuhaibani, A.; SRefat, M. Natural allies for heart health: Nrf2 activation and cardiovascular disease management. Curr. Probl. Cardiol. 2024, 49 Pt B, 102084. [Google Scholar] [CrossRef]
- Liu, P.; Anandhan, A.; Chen, J.; Shakya, A.; Dodson, M.; Ooi, A.; Chapman, E.; White, E.; Garcia, J.G.; Zhang, D.D. Decreased autophagosome biogenesis, reduced NRF2, and enhanced ferroptotic cell death are underlying molecular mechanisms of non-alcoholic fatty liver disease. Redox Biol. 2023, 59, 102570. [Google Scholar] [CrossRef] [PubMed]
- Juul-Nielsen, C.; Shen, J.; Stenvinkel, P.; Scholze, A. Systematic review of the nuclear factor erythroid 2-related factor 2 (NRF2) system in human chronic kidney disease: Alterations, interventions and relation to morbidity. Nephrol. Dial. Transplant. 2022, 37, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Ban, W.H.; Rhee, C.K. Role of Nuclear Factor Erythroid 2–Related Factor 2 in chronic obstructive pulmonary disease. Tuberc. Respir. Dis. Seoul 2022, 85, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A protective role of the NRF2-Keap1 pathway in maintaining Intestinal barrier function. Oxidative Med. Cell. Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Chen, L.; Wu, Y.; Li, Y. NRF2 in age-related musculoskeletal diseases: Role and treatment prospects. Genes Dis. 2023, 11, 101180. [Google Scholar] [CrossRef]
- Paplomata, E.; Zelnak, A.; Santa-Maria, C.A.; Liu, Y.; Gogineni, K.; Li, X.; Moreno, C.S.; Chen, Z.; Kaklamani, V.; O’Regan, R.M. Use of Everolimus and Trastuzumab in addition to endocrine therapy in hormone-refractory metastatic breast cancer. Clin. Breast Cancer 2019, 19, 188–196. [Google Scholar] [CrossRef]
- Lynch, D.R.; Chin, M.P.; Delatycki, M.B.; Subramony, S.H.; Corti, M.; Hoyle, J.C.; Boesch, S.; Nachbauer, W.; Mariotti, C.; Mathews, K.D.; et al. Safety and efficacy of Omaveloxolone in Friedreich Ataxia (MOXIe Study). Ann. Neurol. 2021, 89, 212–225, Correction in Ann. Neurol. 2023, 94, 1190. [Google Scholar] [CrossRef]
- Okuda, D.T.; Kantarci, O.; Lebrun-Frénay, C.; Sormani, M.P.; Azevedo, C.J.; Bovis, F.; Hua, L.H.; Amezcua, L.; Mowry, E.M.; Hotermans, C.; et al. Dimethyl fumarate delays multiple sclerosis in radiologically isolated syndrome. Ann. Neurol. 2023, 93, 604–614. [Google Scholar] [CrossRef]
- Cengiz Seval, G.; Beksac, M. The safety of bortezomib for the treatment of multiple myeloma. Expert Opin. Drug Saf. 2018, 17, 953–962. [Google Scholar] [CrossRef]
- Kavanaugh, S.M.; White, L.A.; Kolesar, J.M. Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. Am. J. Health Syst. Pharm. 2010, 67, 793–797. [Google Scholar] [CrossRef]
- Copur, M.S. Alpelisib to treat breast cancer. Drugs Today 2020, 56, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.D.; Filiaci, V.; Bellone, S.; Ratner, E.S.; Mathews, C.A.; Cantuaria, G.; Gunderson, C.C.; Rutledge, T.; Buttin, B.M.; Lankes, H.A.; et al. Phase II evaluation of copanlisib, a selective inhibitor of Pi3kca, in patients with persistent or recurrent endometrial carcinoma harboring PIK3CA hotspot mutations: An NRG Oncology study (NRG-GY008). Gynecol. Oncol. Rep. 2020, 31, 100532, Corrigendum in Gynecol. Oncol. Rep. 2020, 33, 100590. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in hormone receptor-positive advanced breast cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Sutaria, D.S.; Rasuo, G.; Harris, A.; Johnson, R.; Miles, D.; Gallo, J.D.; Sane, R. Drug-drug interaction study to evaluate the pharmacokinetics, safety, and tolerability of Ipatasertib in combination with Darolutamide in patients with advanced prostate cancer. Pharmaceutics 2022, 14, 2101. [Google Scholar] [CrossRef]
- Amato, R.; Stepankiw, M. Evaluation of everolimus in renal cell cancer. Expert Opin. Pharmacother. 2013, 14, 1229–1240. [Google Scholar] [CrossRef]
- Goudarzi, Z.; Mostafavi, M.; Salesi, M.; Jafari, M.; Mirian, I.; Hashemi Meshkini, A.; Keshavarz, K.; Ghasemi, Y. Everolimus and temsirolimus are not the same second-line in metastatic renal cell carcinoma: A systematic review and meta-analysis. Cost. Eff. Resour. Alloc. 2023, 21, 10. [Google Scholar] [CrossRef]
- Aires-Lopes, B.; Justo, G.Z.; Cordeiro, H.G.; Durán, N.; Azevedo-Martins, J.M.; Ferreira Halder, C.V. Violacein improves vemurafenib response in melanoma spheroids. Nat. Prod. Res. 2024, 38, 3417–3420. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Hauschild, A.; Santinami, M.; Kirkwood, J.M.; Atkinson, V.; Mandala, M.; Merelli, B.; Sileni, V.C.; Nyakas, M.; Haydon, A.; et al. Final results for adjuvant Dabrafenib plus Trametinib in Stage III melanoma. N. Engl. J. Med. 2024, 391, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Thota, R.; Johnson, D.B.; Sosman, J.A. Trametinib in the treatment of melanoma. Expert Opin. Biol. Ther. 2015, 15, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yoon, H.M.; Kim, E.K.; Ra, Y.S.; Kim, H.W.; Yum, M.S.; Kim, M.J.; Baek, J.S.; Sung, Y.S.; Lee, S.M.; et al. Safety and efficacy of selumetinib in pediatric and adult patients with neurofibromatosis type 1 and plexiform neurofibroma. Neuro Oncol. 2024, 26, 2352–2363. [Google Scholar] [CrossRef]
- Urits, I.; Israel, J.; Hakobyan, H.; Yusin, G.; Lassiter, G.; Fackler, N.; Berger, A.A.; Kassem, H.; Kaye, A.; Viswanath, O. Baricitinib for the treatment of rheumatoid arthritis. Reumatologia 2020, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and safety of Upadacitinib in patients with moderate to severe atopic dermatitis: Analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, S.H.; Han, S.W.; Kim, M.J.; Kim, T.M.; Kim, T.Y.; Heo, D.S.; Yuasa, M.; Yanagihara, Y.; Bang, Y.J. Phase I study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res. Treat. 2015, 47, 607–615. [Google Scholar] [CrossRef]
- Aguilar-Recarte, D.; Barroso, E.; Zhang, M.; Rada, P.; Pizarro-Delgado, J.; Peña, L.; Palomer, X.; Valverde, Á.M.; Wahli, W.; Vázquez-Carrera, M. A positive feedback loop between AMPK and GDF15 promotes metformin antidiabetic effects. Pharmacol. Res. 2023, 187, 106578. [Google Scholar] [CrossRef]
- Zhang, J.; Nannapaneni, S.; Wang, D.; Liu, F.; Wang, X.; Jin, R.; Liu, X.; Rahman, M.A.; Peng, X.; Qian, G.; et al. Phenformin enhances the therapeutic effect of selumetinib in KRAS-mutant non-small cell lung cancer irrespective of LKB1 status. Oncotarget 2017, 8, 59008–59022. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef]
- Fersing, C.; Mathias, F. Update on glasdegib in acute myeloid leukemia—Broadening horizons of Hedgehog pathway inhibitors. Acta Pharm. 2021, 72, 9–34. [Google Scholar] [CrossRef]
- Wang, Q.; Chaerkady, R.; Wu, J.; Hwang, H.J.; Papadopoulos, N.; Kopelovich, L.; Maitra, A.; Matthaei, H.; Eshleman, J.R.; Hruban, R.H.; et al. Mutant proteins as cancer-specific biomarkers. Proc. Natl. Acad. Sci. USA 2011, 108, 2444–2449. [Google Scholar] [CrossRef]
- Abarca-Zabalía, J.; García, M.I.; Lozano Ros, A.; Marín-Jiménez, I.; Martínez-Ginés, M.L.; López-Cauce, B.; Martín-Barbero, M.L.; Salvador-Martín, S.; Sanjurjo-Saez, M.; García-Domínguez, J.M.; et al. Differential expression of SMAD genes and S1PR1 on circulating CD4+ T cells in multiple sclerosis and Crohn’s Disease. Int. J. Mol. Sci. 2020, 21, 676. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- Scuto, M.C.; Anfuso, C.D.; Lombardo, C.; Di Fatta, E.; Ferri, R.; Musso, N.; Zerbo, G.; Terrana, M.; Majzúnová, M.; Lupo, G.; et al. Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic. Int. J. Mol. Sci. 2025, 26, 9391. [Google Scholar] [CrossRef]
- Ferrante, K.L.; Shefner, J.; Zhang, H.; Betensky, R.; O’Brien, M.; Yu, H.; Fantasia, M.; Taft, J.; Beal, M.F.; Traynor, B.; et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology 2005, 65, 1834–1836. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Hargreaves, I.P. Mitochondrial dysfunction and neurodegenerative disorders: Role of nutritional supplementation. Int. J. Mol. Sci. 2022, 23, 12603. [Google Scholar] [CrossRef]
- Mantle, D.; Hargreaves, I.P. Disorders of human Coenzyme Q10 metabolism: An overview. Int. J. Mol. Sci. 2024, 25, 4576. [Google Scholar] [CrossRef]
- Mantle, D.; Heaton, R.A.; Hargreaves, I.P. Coenzyme Q10 and immune function: An overview. Antioxidants 2021, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Mantle, D.; Kozhevnikova, S.; Larsen, S. Coenzyme Q10 and obesity: An overview. Antioxidants 2025, 14, 871. [Google Scholar] [CrossRef] [PubMed]
| Outcome of CoQ10 Supplementation | System Studied | Study |
|---|---|---|
| Nrf2 increased, antioxidant enzymes increased, fibrosis decreased. | Fibrogenesis in mouse liver | Choi et al. [16] |
| Nrf2 increased, inflammation decreased. | Exercise training in rats | Pala et al. [17] |
| Nrf2 increased, oxidative stress reduced, inflammation reduced. | Liver inflammation in mice | Yang et al. [18] |
| Nrf2 increased, antioxidant enzymes increased, cell viability increased. | Neurotoxicity in PC12 cells | Li et al. [19] |
| Nrf2 increased, antioxidant enzymes increased, improved renal function. | Nephrotoxicity in mice | Kabel & Elkhoely [20] |
| Nrf2 increased, decreased oxidative stress, inflammation, and apoptosis. | Ulcerative colitis in rats | Khodir et al. [21] |
| Nrf2 increased, oxidative stress decreased. | Diabetes in rats | Samimi et al. [22] |
| Nrf2 increased, oxidative stress and apoptosis decreased. | Spinal cord injury in rats | Li et al. [15] |
| Nrf2 increased, oxidative stress, inflammation, and apoptosis decreased. | Lead induced neurotoxicity in rats | Yousef et al. [23] |
| Nrf2 increased, oxidative stress, and apoptosis decreased, improved cell viability. | Cisplatin induced toxicity in rat cardiomyocytes | Zhao [24] |
| Nrf2 increased, oxidative stress decreased. | Diabetic nephropathy in mice | Sun et al. [25] |
| Nrf2 increased, oxidative stress, inflammation, and apoptosis reduced. | Hepatic ischaemia–reperfusion injury in rats | Mahmoud et al. [26] |
| Nrf2 increased, oxidative stress and inflammation reduced, decreased blood pressure. | Pre-eclampsia in rats | Li et al. [27] |
| Nrf2 increased, oxidative stress, inflammation and apoptosis reduced. | Lead induced nephrotoxicity in rats | Al-Megrin et al. [28] |
| Nrf2 increased, oxidative stress, inflammation, and apoptosis reduced, improved liver function. | Thioacetamide induced liver toxicity in rats | Hussein et al. [29] |
| Nrf2 increased, oxidative stress, inflammation, and apoptosis reduced, allergy status improved. | Allergic rhinitis/asthma in mice | Du et al. [30] |
| Nrf2 increased, oxidative stress and apoptosis decreased, aneurysm formation reduced. | Intracranial aneurysm in mice | Huang et al. [31] |
| Nrf2 increased, oxidative stress and inflammation reduced. | Vincristine induced peripheral neuropathy in rats | Elsamy et al. [32] |
| Nrf2 increased, oxidative stress reduced. | Arsenic/chromium neurotoxicity in mice | Tripathy et al. [33] |
| Nrf2 increased, oxidative stress and inflammation decreased, cognition improved. | Chemotherapy induced cognitive impairment in mice | Kaur et al. [34] |
| Nrf2 increased, oxidative stress decreased, loss of retinal cells reduced. | Porcine retinal explant degeneration | Deppe et al. [35] |
| Nrf2 increased, oxidative stress decreased. | Diabetes in rats | Samimi et al. [13] |
| Nrf2 increased, oxidative stress, inflammation, and apoptosis reduced. | Testicular damage in rats | Arafa et al. [36] |
| Nrf2 increased, oxidative stress and apoptosis decreased. | Arsenic/chromium hepatotoxicity in mice | Tripathi et al. [37] |
| Outcome of CoQ10 Supplementation | System Studied | Study |
|---|---|---|
| NF-κB downregulated, oxidative stress, inflammation and apoptosis reduced | Mouse model of Parkinson’s disease | Kooncumchoo et al. [41] |
| NF-κB downregulated, oxidative stress, inflammation and apoptosis reduced | Acetaminophen induced liver toxicity in rats | Fouad et al. [42] |
| NF-κB downregulated, oxidative stress reduced | Neuropathic pain in diabetic mice | Zhang et al. [43] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Trichloroacetic acid induced hepatocellular carcinoma in rats | Fouad et al. [44] |
| NF-κB downregulated, inflammation reduced | Wound healing in rats | Yoneda et al. [45] |
| NF-κB downregulated, inflammation reduced | Oxazolone induced dermatitis in mice | Li et al. [46] |
| NF-κB downregulated, inflammation reduced | Amyloid induced inflammation in PC12 cells | Li et al. [47] |
| NF-κB downregulated, inflammation reduced | Ischaemia–reperfusion injury in skeletal muscle of rats | Boroujeni et al. [48] |
| NF-κB downregulated, oxidative stress, inflammation and apoptosis reduced | Restraint induced depression in mice | Salehpour et al. [49] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Cerebral malaria in mice | Nyariki et al. [50] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Anthracycline induced toxicity in human cardiomyocytes | Quagliariello et al. [51] |
| NF-κB downregulated, mitochondrial function improved, inflammation reduced | Lipopolysaccharide induced lung injury in rats | Ali et al. [52] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Radiation induced enteropathy in rats | Mohamed & Said [53] |
| NF-κB downregulated, oxidative stress, inflammation and apoptosis reduced | Propionic acid induced cerebral injury in rats | Alhusaini et al. [54] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Cadmium/titanium induced liver toxicity in rats | Abd-Elkahim et al. [55] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Cerebral ischaemia–reperfusion injury in rats | Fakharaldeen et al. [56] |
| NF-κB downregulated, tumour cell invasiveness decreased | Glioblastoma in mice | Frontinan-Rubio et al. [57] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Doxorubicin induced liver toxicity in rats | Mansour et al. [58] |
| NF-κB downregulated, oxidative stress and inflammation reduced | Cadmium cardiotoxicity in rats | Antar et al. [59] |
| NF-κB downregulated, inflammation and apoptosis reduced | Intracerebral haemorrhage in mice | Yang et al. [60] |
| Pathway | Medicine | Disorder | Reference |
|---|---|---|---|
| Nrf2/NQO1 | Nrf2 activators: omaveloxolone dimethyl fumarate | Friedreich’s ataxia multiple sclerosis | Lynch et al. [110] Okuda et al. [111] |
| NF-κB | NF-κB inhibitors: bortezomib vorinostat | myeloma cutaneous T-cell lymphoma | Cengiz-Seval et al. [112] Kavanaugh et al. [113] |
| P13K/AKT/mTOR | P13K inhibitors: alpelisib copanlisib AKT inhibitors: capivasertib ipatasertib mTOR inhibitors: everolimus temsirolimus | breast cancer endometrial cancer breast, prostate cancer prostate cancer renal cell cancer renal cell cancer | Copur [114] Santin et al. [115] Turner et al. [116] Sutaria et al. [117] Amato [118] Goudarzi et al. [119] |
| MAPK | RAF inhibitors: vemurafenib dabrafenib MEK inhibitors: trametinib selumetinib | melanoma melanoma melanoma neurofibroma | Aires-Lopez [120] Long et al. [121] Thota et al. [122] Kim et al. [123] |
| JAK/STAT | JAK inhibitors: baricitinib upadacitinib STAT inhibitors: OBP-31121 | rheumatoid arthritis atopic dermatitis various cancers | Urits et al. [124] Simpson et al. [125] Oh et al. [126] |
| AMPK/YAP/OPA1 | AMPK activators: metformin phenformin | type II diabetes cancer | Aguilar-Recarte [127] Zhang et al. [128] |
| Hedgehog | SMO inhibitors: vismodegib glasdegib | basal cell carcinoma leukaemia | Sekulic et al. [129] Fersing [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mantle, D. Coenzyme Q10 and Intracellular Signalling Pathways: Clinical Relevance. Int. J. Mol. Sci. 2025, 26, 11024. https://doi.org/10.3390/ijms262211024
Mantle D. Coenzyme Q10 and Intracellular Signalling Pathways: Clinical Relevance. International Journal of Molecular Sciences. 2025; 26(22):11024. https://doi.org/10.3390/ijms262211024
Chicago/Turabian StyleMantle, David. 2025. "Coenzyme Q10 and Intracellular Signalling Pathways: Clinical Relevance" International Journal of Molecular Sciences 26, no. 22: 11024. https://doi.org/10.3390/ijms262211024
APA StyleMantle, D. (2025). Coenzyme Q10 and Intracellular Signalling Pathways: Clinical Relevance. International Journal of Molecular Sciences, 26(22), 11024. https://doi.org/10.3390/ijms262211024





