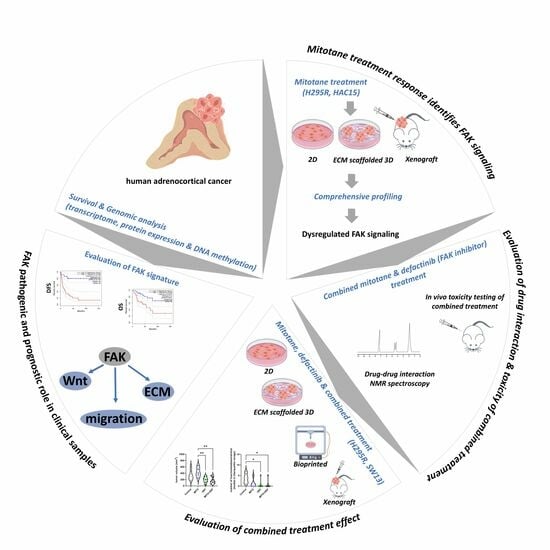
Defactinib in Combination with Mitotane Can Be an Effective Treatment in Human Adrenocortical Carcinoma
Abstract
1. Introduction
2. Results
2.1. Transcriptome Sequencing Identifies FAK Signalling in Mitotane-Treated In Vitro ACC Models
2.2. Functional and Prognostic Relevance of Focal Adhesion Signalling in Human Adrenocortical Carcinoma Samples
2.3. Defactinib Combined with Mitotane Shows Efficacy in ACC In Vitro Models
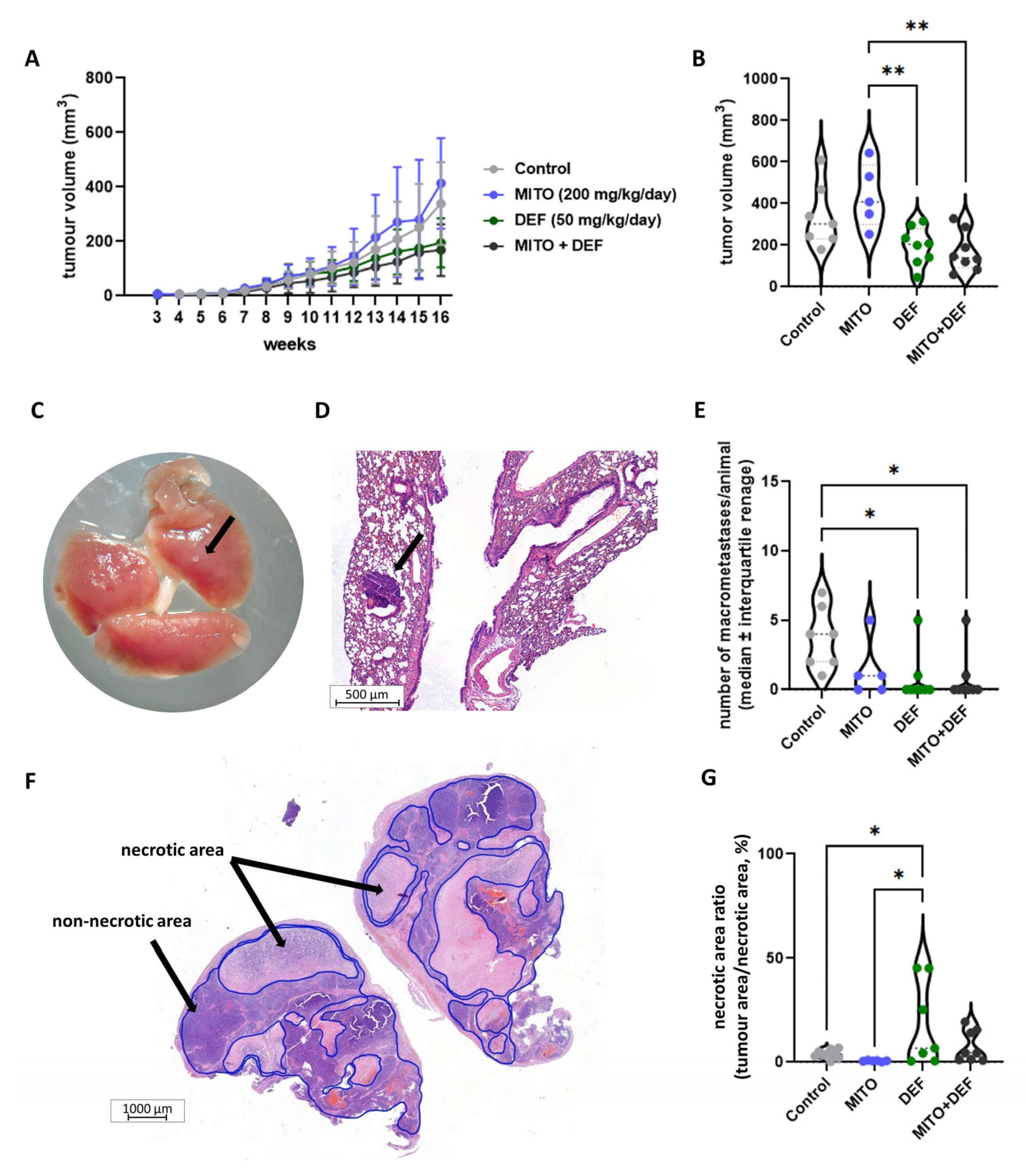
2.4. Defactinib Combined with Mitotane Is Effective in ACC Xenograft Model
3. Discussion
4. Materials and Methods
4.1. In Vitro 2D and 3D ACC Models, Bioprinting, Treatments and Functional Assays
4.2. Xenograft Model and Dose Testing with Non-Lethal Outcomes
4.3. Steroid Hormone Measurements and Nuclear Magnetic Resonance (NMR) Spectroscopy
4.4. Transcriptome Sequencing and Bioinformatics
4.5. In Silico Datasets, Genomic Characterisation, and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | Adrenocortical carcinoma |
| CNV | Copy number variation |
| DMSO | Dimethyl sulfoxide |
| ECM | Extracellular matrix |
| ENSAT | European Network for Study of Adrenal Tumours |
| ESMO | European Society for Medical Oncology |
| FAK | Focal adhesion kinase |
| FDA | Food and Drug Administration |
| HPLC-MS/MS | High-performance liquid chromatography–tandem mass spectrometry |
| NMR | Nuclear magnetic resonance |
References
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical Carcinomas and Malignant Phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Gratian, L.; Pura, J.; Dinan, M.; Reed, S.; Scheri, R.; Roman, S.; Sosa, J.A. Treatment Patterns and Outcomes for Patients with Adrenocortical Carcinoma Associated with Hospital Case Volume in the United States. Ann. Surg. Oncol. 2014, 21, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Sbiera, S.; Leich, E.; Liebisch, G.; Sbiera, I.; Schirbel, A.; Wiemer, L.; Matysik, S.; Eckhardt, C.; Gardill, F.; Gehl, A.; et al. Mitotane Inhibits Sterol-O-Acyl Transferase 1 Triggering Lipid-Mediated Endoplasmic Reticulum Stress and Apoptosis in Adrenocortical Carcinoma Cells. Endocrinology 2015, 156, 3895–3908. [Google Scholar] [CrossRef] [PubMed]
- Lerario, A.M.; Worden, F.P.; Ramm, C.A.; Hasseltine, E.A.; Stadler, W.M.; Else, T.; Shah, M.H.; Agamah, E.; Rao, K.; Hammer, G.D. The Combination of Insulin-Like Growth Factor Receptor 1 (IGF1R) Antibody Cixutumumab and Mitotane as a First-Line Therapy for Patients with Recurrent/Metastatic Adrenocortical Carcinoma: A Multi-Institutional NCI-Sponsored Trial. Horm. Cancer 2014, 5, 232–239. [Google Scholar] [CrossRef]
- Landwehr, L.-S.; Altieri, B.; Sbiera, I.; Remde, H.; Kircher, S.; Olabe, J.; Sbiera, S.; Kroiss, M.; Fassnacht, M. Expression and Prognostic Relevance of PD-1, PD-L1 and CTLA-4 Immune Checkpoints in Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2024, 109, 2325–2334. [Google Scholar] [CrossRef]
- Remde, H.; Schmidt-Pennington, L.; Reuter, M.; Landwehr, L.-S.; Jensen, M.; Lahner, H.; Kimpel, O.; Altieri, B.; Laubner, K.; Schreiner, J.; et al. Outcome of Immunotherapy in Adrenocortical Carcinoma: A Retrospective Cohort Study. Eur. J. Endocrinol. 2023, 188, 485–493. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Dawson, J.C.; Serrels, A.; Stupack, D.G.; Schlaepfer, D.D.; Frame, M.C. Targeting FAK in Anticancer Combination Therapies. Nat. Rev. Cancer 2021, 21, 313–324. [Google Scholar] [CrossRef]
- Aakriti, J.; Vithalkar, M.P.; Maity, S.; Baby, K.; Nagareddy, P.R.; Nayak, Y. Focal Adhesion Kinase (FAK): Emerging Target for Drug-Resistant Malignant Tumors. Mol. Biol. Rep. 2025, 52, 248. [Google Scholar] [CrossRef]
- Banerjee, S.; Aghajanian, C.; Van Nieuwenhuysen, E.; Santin, A.; Ring, K.; Colombo, N.; Thaker, P.; Prendergast, E.; Moore, K.; Chon, H.S.; et al. LB007/#1548 Efficacy and Safety of Avutometinib ± Defactinib in Recurrent Low Grade Serous Ovarian Cancer: Primary Analysis of ENGOT-OV60/GOG-3052/RAMP 201. Int. J. Gynecol. Cancer 2024, 34, A13. [Google Scholar] [CrossRef]
- Symeonides, S.; Evans, T.R.J.; Coyle, V.; Serrels, A.; Thomson, F.; Currie, D.; Dillon, S.; Paul, J.; Fennell, D.A.; Ottensmeier, C. FAK-PD1: A Phase I/IIa Trial of FAK (Defactinib) & PD-1 (Pembrolizumab) Inhibition. Ann. Oncol. 2017, 28, v427. [Google Scholar] [CrossRef]
- Liu, F.; Wu, Q.; Dong, Z.; Liu, K. Integrins in Cancer: Emerging Mechanisms and Therapeutic Opportunities. Pharmacol. Ther. 2023, 247, 108458. [Google Scholar] [CrossRef] [PubMed]
- Pittaway, J.F.H.; Guasti, L. Pathobiology and Genetics of Adrenocortical Carcinoma. J. Mol. Endocrinol. 2019, 62, R105–R119. [Google Scholar] [CrossRef] [PubMed]
- Wörthmüller, J.; Rüegg, C. The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. Int. J. Mol. Sci. 2020, 21, 9107. [Google Scholar] [CrossRef] [PubMed]
- Penny, M.K.; Lerario, A.M.; Basham, K.J.; Chukkapalli, S.; Mohan, D.R.; LaPensee, C.; Converso-Baran, K.; Hoenerhoff, M.J.; Suárez-Fernández, L.; del Rey, C.G.; et al. Targeting Oncogenic Wnt/β-Catenin Signaling in Adrenocortical Carcinoma Disrupts ECM Expression and Impairs Tumor Growth. Cancers 2023, 15, 3559. [Google Scholar] [CrossRef]
- Seidel, E.; Walenda, G.; Messerschmidt, C.; Obermayer, B.; Peitzsch, M.; Wallace, P.; Bahethi, R.; Yoo, T.; Choi, M.; Schrade, P.; et al. Generation and Characterization of a Mitotane-Resistant Adrenocortical Cell Line. Endocr. Connect. 2020, 9, 122–134. [Google Scholar] [CrossRef]
- Nagy, Z.; Baghy, K.; Hunyadi-Gulyás, É.; Micsik, T.; Nyírő, G.; Rácz, G.; Butz, H.; Perge, P.; Kovalszky, I.; Medzihradszky, K.F.; et al. Evaluation of 9-Cis Retinoic Acid and Mitotane as Antitumoral Agents in an Adrenocortical Xenograft Model. Am. J. Cancer Res. 2015, 5, 3645–3658. [Google Scholar]
- Tan, X.; Yan, Y.; Song, B.; Zhu, S.; Mei, Q.; Wu, K. Focal Adhesion Kinase: From Biological Functions to Therapeutic Strategies. Exp. Hematol. Oncol. 2023, 12, 83. [Google Scholar] [CrossRef]
- Halder, J.; Lin, Y.G.; Merritt, W.M.; Spannuth, W.A.; Nick, A.M.; Honda, T.; Kamat, A.A.; Han, L.Y.; Kim, T.J.; Lu, C.; et al. Therapeutic Efficacy of a Novel Focal Adhesion Kinase Inhibitor TAE226 in Ovarian Carcinoma. Cancer Res. 2007, 67, 10976–10983. [Google Scholar] [CrossRef]
- Le Large, T.Y.S.; Bijlsma, M.F.; El Hassouni, B.; Mantini, G.; Lagerweij, T.; Henneman, A.A.; Funel, N.; Kok, B.; Pham, T.V.; De Haas, R.; et al. Focal Adhesion Kinase Inhibition Synergizes with Nab-Paclitaxel to Target Pancreatic Ductal Adenocarcinoma. J. Exp. Clin. Cancer Res. 2021, 40, 91. [Google Scholar] [CrossRef]
- Zhong, B.; Shingyoji, M.; Hanazono, M.; Nguyễn, T.T.; Morinaga, T.; Tada, Y.; Shimada, H.; Hiroshima, K.; Tagawa, M. Combination of a P53-Activating CP-31398 and an MDM2 or a FAK Inhibitor Produces Growth Suppressive Effects in Mesothelioma with Wild-Type P53 Genotype. Apoptosis 2020, 25, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Kanteti, R.; Mirzapoiazova, T.; Riehm, J.J.; Dhanasingh, I.; Mambetsariev, B.; Wang, J.; Kulkarni, P.; Kaushik, G.; Seshacharyulu, P.; Ponnusamy, M.P.; et al. Focal Adhesion Kinase a Potential Therapeutic Target for Pancreatic Cancer and Malignant Pleural Mesothelioma. Cancer Biol. Ther. 2018, 19, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Lee, B.Y.; Castillo, L.; Spielman, C.; Grogan, J.; Yeung, N.K.; Kench, J.G.; Stricker, P.D.; Haynes, A.; Centenera, M.M.; et al. Effect of FAK Inhibitor VS-6063 (Defactinib) on Docetaxel Efficacy in Prostate Cancer. Prostate 2018, 78, 308–317. [Google Scholar] [CrossRef] [PubMed]
- François, R.A.; Maeng, K.; Nawab, A.; Kaye, F.J.; Hochwald, S.N.; Zajac-Kaye, M. Targeting Focal Adhesion Kinase and Resistance to mTOR Inhibition in Pancreatic Neuroendocrine Tumors. J. Natl. Cancer Inst. 2015, 107, djv123. [Google Scholar] [CrossRef]
- Zsippai, A.; Szabó, D.R.; Tömböl, Z.; Szabó, P.M.; Éder, K.; Pállinger, É.; Gaillard, R.C.; Patócs, A.; Tóth, S.; Falus, A.; et al. Effects of Mitotane on Gene Expression in the Adrenocortical Cell Line NCI-H295R: A Microarray Study. Pharmacogenomics 2012, 13, 1351–1361. [Google Scholar] [CrossRef]
- Creemers, S.G.; Van Koetsveld, P.M.; Van Den Dungen, E.S.R.; Korpershoek, E.; Van Kemenade, F.J.; Franssen, G.J.H.; De Herder, W.W.; Feelders, R.A.; Hofland, L.J. Inhibition of Human Adrenocortical Cancer Cell Growth by Temozolomide in Vitro and the Role of the MGMT Gene. J. Clin. Endocrinol. Metab. 2016, 101, 4574–4584. [Google Scholar] [CrossRef]
- Schiavon, A.; Saba, L.; Evaristo, C.; Petiti, J.; Pignochino, Y.; Ferrero, G.; Giordano, G.; Tucciarello, C.; Puglisi, S.; Reimondo, G.; et al. Mitotane Activates ATF4/ATF3 Axis Triggering Endoplasmic Reticulum Stress in Adrenocortical Carcinoma Cells. Biomed. Pharmacother. 2025, 184, 117917. [Google Scholar] [CrossRef]
- Lindhe, Ö.; Skogseid, B. Mitotane Effects in a H295R Xenograft Model of Adjuvant Treatment of Adrenocortical Cancer. Horm. Metab. Res. 2010, 42, 725–730. [Google Scholar] [CrossRef]
- Howe, G.A.; Xiao, B.; Zhao, H.; Al-Zahrani, K.N.; Hasim, M.S.; Villeneuve, J.; Sekhon, H.S.; Goss, G.D.; Sabourin, L.A.; Dimitroulakos, J.; et al. Focal Adhesion Kinase Inhibitors in Combination with Erlotinib Demonstrate Enhanced Anti-Tumor Activity in Non-Small Cell Lung Cancer. PLoS ONE 2016, 11, e0150567. [Google Scholar] [CrossRef]
- Fukami, S.; Tomioka, D.; Murakami, Y.; Honda, T.; Hatakeyama, S. Pharmacological Profiling of a Dual FAK/IGF-1R Kinase Inhibitor TAE226 in Cellular and in Vivo Tumor Models. BMC Res. Notes 2019, 12, 347. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, W.; Puszyk, W.M.; Wallet, S.; Hochwald, S.; Robertson, K.; Liu, C. Focal Adhesion Kinase Inhibitor PF573228 and Death Receptor 5 Agonist Lexatumumab Synergistically Induce Apoptosis in Pancreatic Carcinoma. Tumour Biol. 2017, 39, 1010428317699120. [Google Scholar] [CrossRef] [PubMed]
- Wang-Gillam, A.; Lim, K.-H.; McWilliams, R.; Suresh, R.; Lockhart, A.C.; Brown, A.; Breden, M.; Belle, J.I.; Herndon, J.; Bogner, S.J.; et al. Defactinib, Pembrolizumab, and Gemcitabine in Patients with Advanced Treatment Refractory Pancreatic Cancer: A Phase I, Dose Escalation, and Expansion Study. Clin. Cancer Res. 2022, 28, 5254–5262. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.-H.; Spencer, K.R.; Safyan, R.A.; Picozzi, V.J.; Varghese, A.M.; Siolas, D.; Perez, K.; Clift, S.; Denis, L.J.; Bhambhani, V.; et al. Avutometinib/Defactinib and Gemcitabine/Nab-Paclitaxel Combination in First-Line Metastatic Pancreatic Ductal Adenocarcinoma: Initial Safety and Efficacy of Phase 1b/2 Study (RAMP 205). J. Clin. Oncol. 2024, 42, 4140. [Google Scholar] [CrossRef]
- Verastem Oncology. Verastem Oncology Receives Orphan Drug Designation from FDA for Avutometinib Alone or in Combination With Defactinib in Recurrent Low-Grade Serous Ovarian Cancer. Verastem, Inc. Available online: https://investor.verastem.com/news-releases/news-release-details/verastem-oncology-receives-orphan-drug-designation-fda/ (accessed on 19 August 2024).
- Banerjee, S.N.; Ring, K.L.; Van Nieuwenhuysen, E.; Fabbro, M.; Aghajanian, C.; Oaknin, A.; Colombo, N.; Santin, A.; Clamp, A.R.; Moore, K.N.; et al. Initial Efficacy and Safety Results from ENGOT-Ov60/GOG-3052/RAMP 201: A Phase 2 Study of Avutometinib (VS-6766) ± Defactinib in Recurrent Low-Grade Serous Ovarian Cancer (LGSOC). J. Clin. Oncol. 2023, 41, 5515. [Google Scholar] [CrossRef]
- Soria, J.C.; Gan, H.K.; Blagden, S.P.; Plummer, R.; Arkenau, H.T.; Ranson, M.; Evans, T.R.J.; Zalcman, G.; Bahleda, R.; Hollebecque, A.; et al. A Phase I, Pharmacokinetic and Pharmacodynamic Study of GSK2256098, a Focal Adhesion Kinase Inhibitor, in Patients with Advanced Solid Tumors. Ann. Oncol. 2016, 27, 2268–2274. [Google Scholar] [CrossRef]
- Shimizu, T.; Fukuoka, K.; Takeda, M.; Iwasa, T.; Yoshida, T.; Horobin, J.; Keegan, M.; Vaickus, L.; Chavan, A.; Padval, M.; et al. A First-in-Asian Phase 1 Study to Evaluate Safety, Pharmacokinetics and Clinical Activity of VS-6063, a Focal Adhesion Kinase (FAK) Inhibitor in Japanese Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2016, 77, 997–1003. [Google Scholar] [CrossRef]
- Pan, M.-R.; Wu, C.-C.; Kan, J.-Y.; Li, Q.-L.; Chang, S.-J.; Wu, C.-C.; Li, C.-L.; Ou-Yang, F.; Hou, M.-F.; Yip, H.-K.; et al. Impact of FAK Expression on the Cytotoxic Effects of CIK Therapy in Triple-Negative Breast Cancer. Cancers 2019, 12, 94. [Google Scholar] [CrossRef]
- Li, S.; Xiao, S.; Situ, Y. Apolipoprotein C1 and Apoprotein E as Potential Therapeutic and Prognostic Targets for Adrenocortical Carcinoma. Cancer Biomark. 2025, 42, 18758592241308440. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, Y.; Liu, C.; Li, Y.; Lin, C.; Chen, H.; Hou, J.; Gao, G.; Liu, Z.; Yan, Q.; et al. Identification of CENPM as a Key Gene Driving Adrenocortical Carcinoma Metastasis via Physical Interaction with Immune Checkpoint Ligand FGL1. Clin. Transl. Med. 2025, 15, e70182. [Google Scholar] [CrossRef]
- Lai, Q.; Su, S.; He, P.; Yang, H.; Huang, Z.; Lu, D.; Luo, Z. Role of Endoplasmic Reticulum Stress in Melatonin-Induced Apoptosis and Inhibition of Invasion and Migration in Adrenocortical Carcinoma Cells. Discov. Med. 2024, 36, 2214–2223. [Google Scholar] [CrossRef]
- Situ, Y.; Deng, L.; Huang, Z.; Jiang, X.; Zhao, L.; Zhang, J.; Lu, L.; Liang, Q.; Xu, Q.; Shao, Z.; et al. CDH2 and CDH13 as Potential Prognostic and Therapeutic Targets for Adrenocortical Carcinoma. Cancer Biol. Ther. 2024, 25, 2428469. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Rubin, B.; Monticelli, H.; Barollo, S.; Redaelli, M.; Bertazza, L.; Mian, C.; Zorzan, M.; Garzoli, S.; Iriti, M.; et al. Biological Activities of the Aerial and Undergound Parts of Gymnadenia Nigra Rchb.f. (Syn. Nigritella Nigra (L.) Rchb. f.) from the Italian Alps. Nat. Prod. Res. 2024, 38, 3687–3692. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef]
- Laha, D.; Grant, R.R.C.; Mishra, P.; Boufraqech, M.; Shen, M.; Zhang, Y.-Q.; Hall, M.D.; Quezado, M.; De Melo, M.S.; Del Rivero, J.; et al. Preclinical Assessment of Synergistic Efficacy of MELK and CDK Inhibitors in Adrenocortical Cancer. J. Exp. Clin. Cancer Res. 2022, 41, 282. [Google Scholar] [CrossRef]
- Cerquetti, L.; Bucci, B.; Carpinelli, G.; Lardo, P.; Proietti, A.; Saporito, R.; Rindi, G.; Petrangeli, E.; Toscano, V.; Stigliano, A. Antineoplastic Effect of a Combined Mitotane Treatment/Ionizing Radiation in Adrenocortical Carcinoma: A Preclinical Study. Cancers 2019, 11, 1768. [Google Scholar] [CrossRef]
- Cerquetti, L.; Bucci, B.; Marchese, R.; Misiti, S.; De Paula, U.; Miceli, R.; Muleti, A.; Amendola, D.; Piergrossi, P.; Brunetti, E.; et al. Mitotane Increases the Radiotherapy Inhibitory Effect and Induces G2-Arrest in Combined Treatment on Both H295R and SW13 Adrenocortical Cell Lines. Endocr. Relat. Cancer 2008, 15, 623–634. [Google Scholar] [CrossRef]
- Sigala, S.; Rossini, E.; Abate, A.; Tamburello, M.; Bornstein, S.R.; Hantel, C. An Update on Adrenocortical Cell Lines of Human Origin. Endocrine 2022, 77, 432–437. [Google Scholar] [CrossRef]
- KEGG Drug Interaction List. Defactinib Drug Interaction List. Available online: https://www.kegg.jp/kegg-bin/ddi_list?drug=D10618 (accessed on 24 June 2025).
- Krokker, L.; Szabó, B.; Németh, K.; Tóháti, R.; Sarkadi, B.; Mészáros, K.; Patócs, A.; Butz, H. Three Dimensional Cell Culturing for Modeling Adrenal and Pituitary Tumors. Pathol. Oncol. Res. 2021, 27, 640676. [Google Scholar] [CrossRef]
- Sztankovics, D.; Moldvai, D.; Petővári, G.; Gelencsér, R.; Krencz, I.; Raffay, R.; Dankó, T.; Sebestyén, A. 3D Bioprinting and the Revolution in Experimental Cancer Model Systems—A Review of Developing New Models and Experiences with in Vitro 3D Bioprinted Breast Cancer Tissue-Mimetic Structures. Pathol. Oncol. Res. 2023, 29, 1610996. [Google Scholar] [CrossRef]
- Dankó, T.; Petővári, G.; Raffay, R.; Sztankovics, D.; Moldvai, D.; Vetlényi, E.; Krencz, I.; Rókusz, A.; Sipos, K.; Visnovitz, T.; et al. Characterisation of 3D Bioprinted Human Breast Cancer Model for In Vitro Drug and Metabolic Targeting. Int. J. Mol. Sci. 2022, 23, 7444. [Google Scholar] [CrossRef]
- Wolfensohn, S.; Lloyd, M. (Eds.) Pain, Stress and Humane End Points. In Handbook of Laboratory Animal Management and Welfare; Wiley: Hoboken, NJ, USA, 2003; pp. 59–73. ISBN 978-1-4051-1159-1. [Google Scholar]
- Mészáros, K.; Karvaly, G.; Márta, Z.; Magda, B.; Tőke, J.; Szücs, N.; Tóth, M.; Rácz, K.; Patócs, A. Diagnostic Performance of a Newly Developed Salivary Cortisol and Cortisone Measurement Using an LC–MS/MS Method with Simple and Rapid Sample Preparation. J. Endocrinol. Investig. 2018, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Szabó, B.; Németh, K.; Mészáros, K.; Krokker, L.; Likó, I.; Saskői, É.; Németh, K.; Szabó, P.T.; Szücs, N.; Czirják, S.; et al. Aspirin Mediates Its Antitumoral Effect Through Inhibiting PTTG1 in Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2022, 107, 3066–3079. [Google Scholar] [CrossRef] [PubMed]
- Pósa, S.P.; Saskői, É.; Bársony, L.; Pongor, L.; Fekete, F.; Papp, J.; Bozsik, A.; Patócs, A.; Butz, H. The Impact of Glucocorticoid Receptor Transactivation on Context-Dependent Cell Migration Dynamics. Sci. Rep. 2025, 15, 4163. [Google Scholar] [CrossRef]
- Mercatelli, D.; Lopez-Garcia, G.; Giorgi, F.M. Corto: A Lightweight R Package for Gene Network Inference and Master Regulator Analysis. Bioinformatics 2020, 36, 3916–3917. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Baruzzo, G.; Hayer, K.E.; Kim, E.J.; Di Camillo, B.; FitzGerald, G.A.; Grant, G.R. Simulation-Based Comprehensive Benchmarking of RNA-Seq Aligners. Nat. Methods 2017, 14, 135–139. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An Efficient General Purpose Program for Assigning Sequence Reads to Genomic Features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex Heatmaps Reveal Patterns and Correlations in Multidimensional Genomic Data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
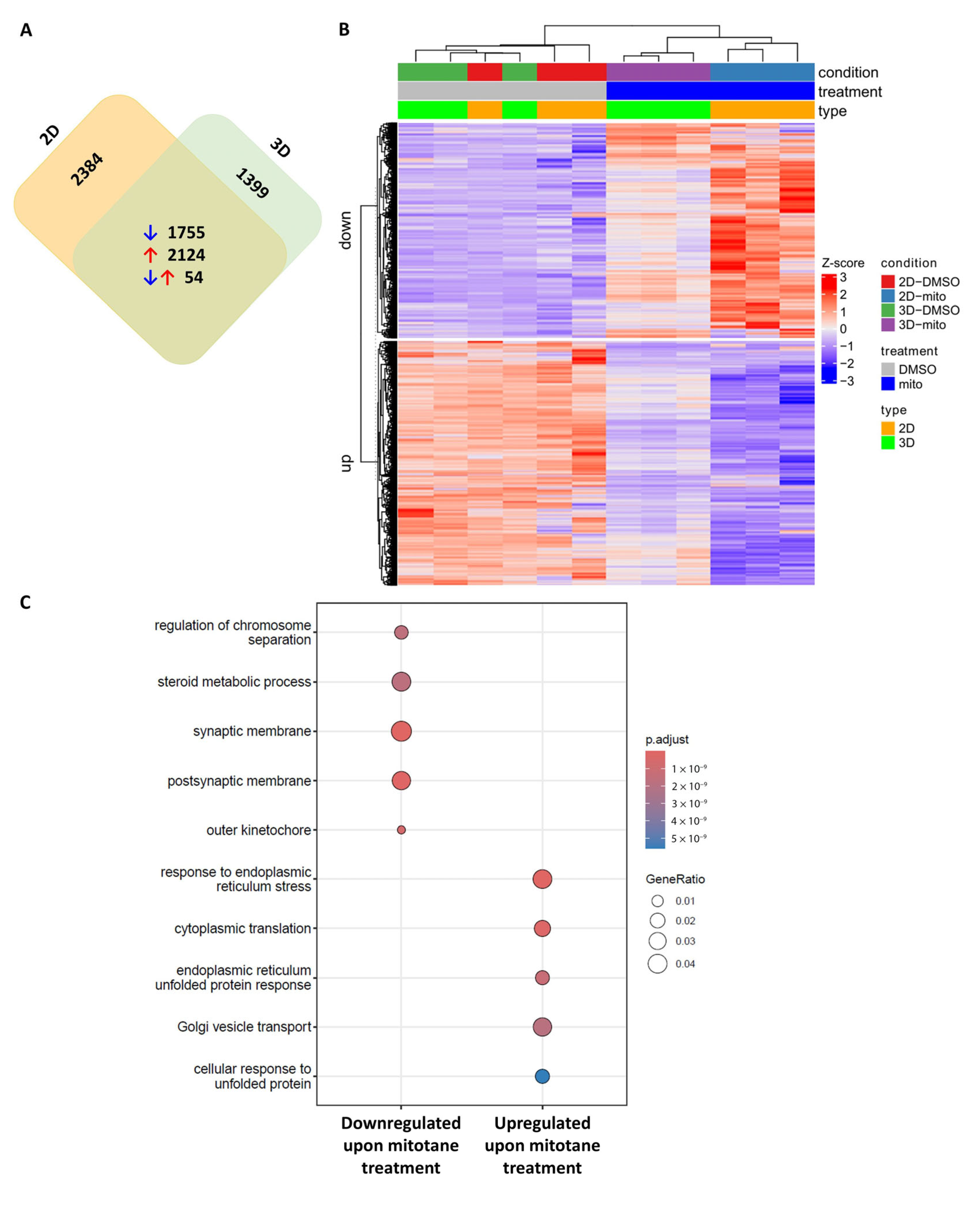
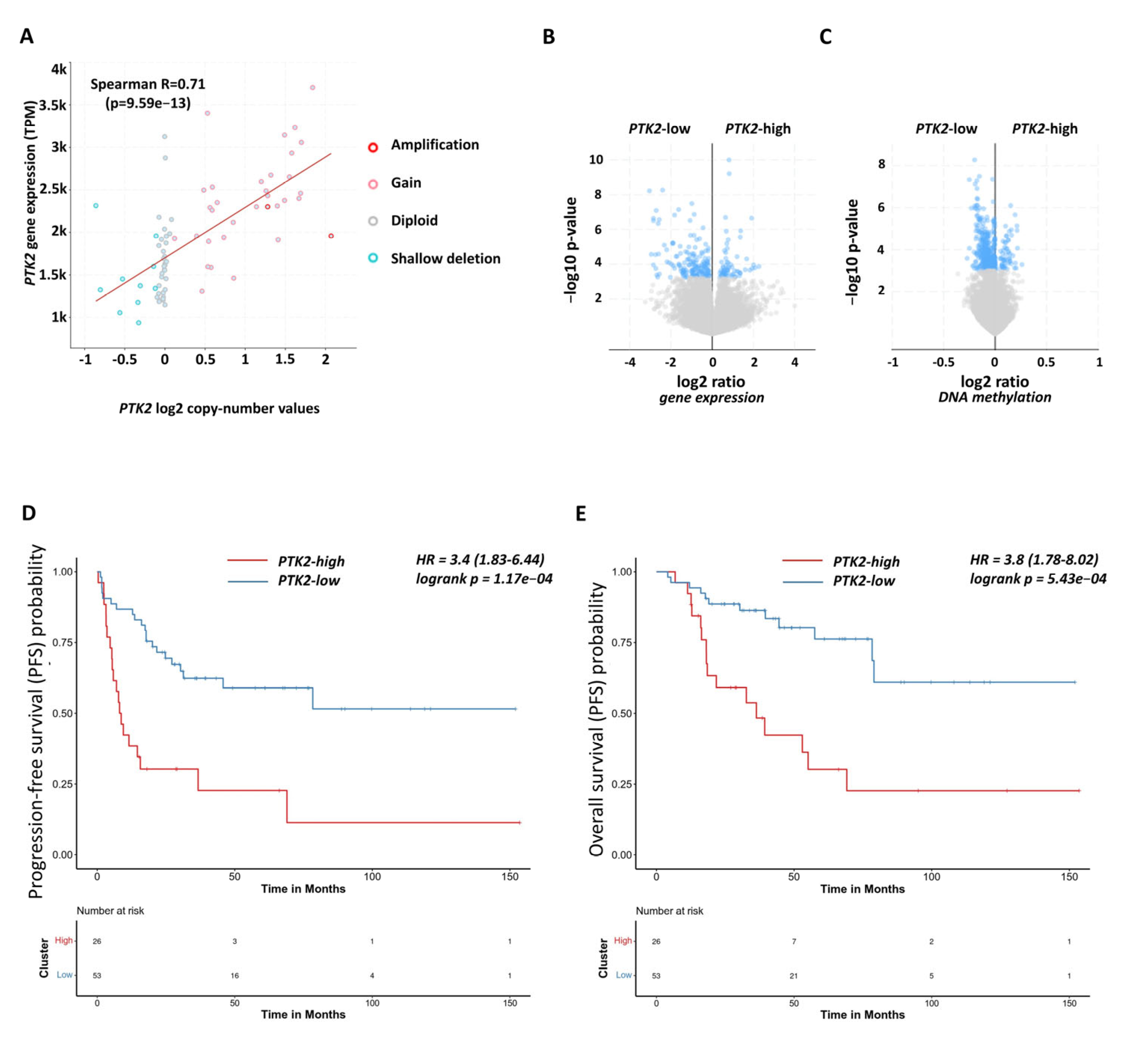
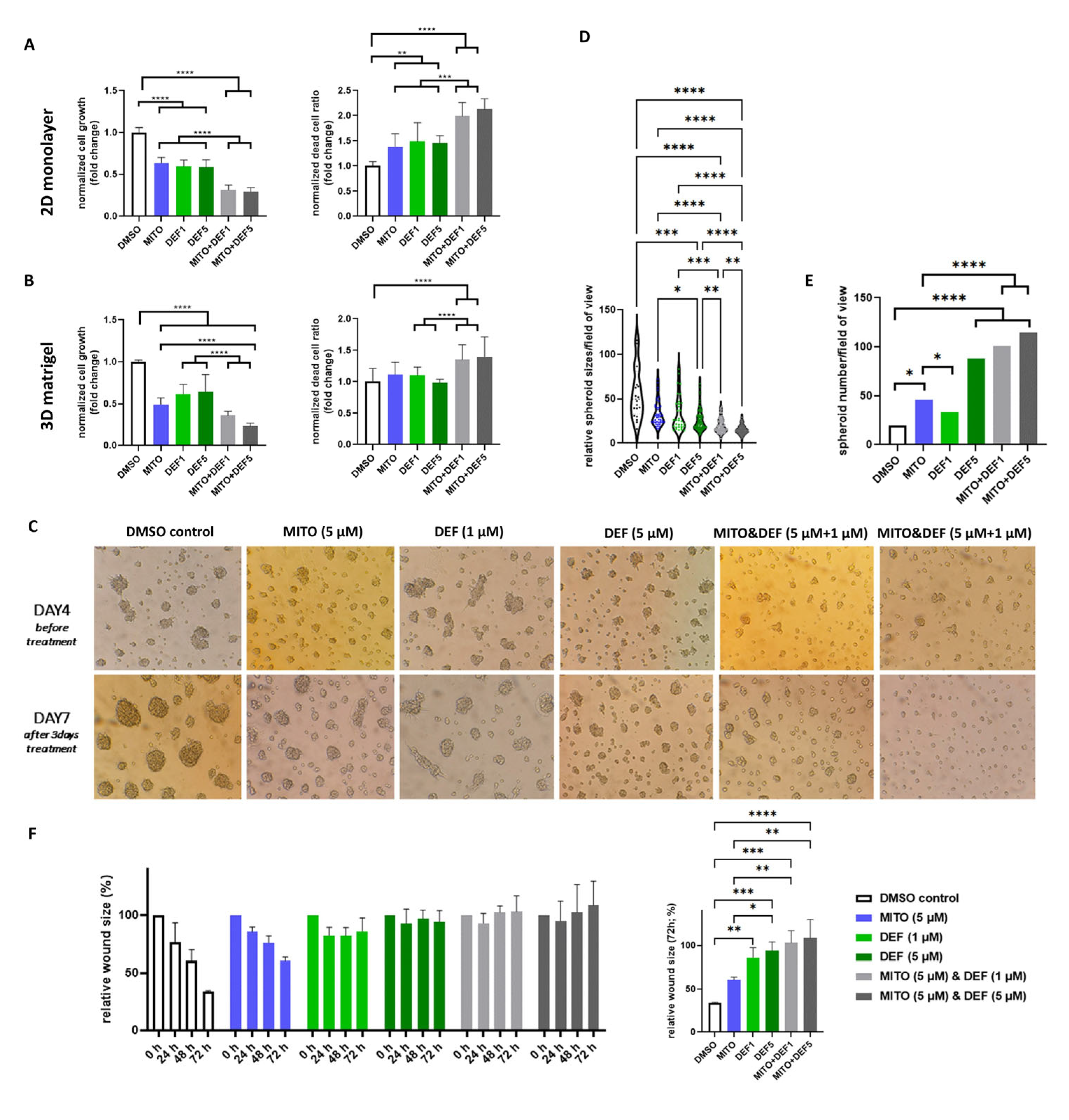

| ID | Ontology | Description | p.Adjusted | q Value |
|---|---|---|---|---|
| GO:0007267 | BP | cell–cell signalling | 0.00025 | 0.028 |
| GO:0031012 | CC | extracellular matrix | 0.00087 | 0.058 |
| GO:0098742 | BP | cell–cell adhesion via plasma membrane adhesion molecules | 0.00137 | 0.078 |
| GO:0098640 | MF | integrin binding involved in cell–matrix adhesion | 0.003010 | 0.129 |
| GO:0007155 | BP | cell adhesion | 0.01168 | 0.251 |
| GO:0062023 | CC | collagen-containing extracellular matrix | 0.01543 | 0.286 |
| GO:0099560 | BP | synaptic membrane adhesion | 0.01856 | 0.311 |
| GO:0086019 | BP | cell–cell signalling involved in cardiac conduction | 0.02338 | 0.343 |
| GO:0007156 | BP | homophilic cell adhesion via plasma membrane adhesion molecules | 0.02461 | 0.349 |
| GO:0098609 | BP | cell–cell adhesion | 0.02524 | 0.356 |
| GO:0030020 | MF | extracellular matrix structural constituent conferring tensile strength | 0.03141 | 0.385 |
| GO:0030198 | BP | extracellular matrix organization | 0.03805 | 0.415 |
| GO:0005925 | CC | focal adhesion | 0.04605 | 0.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butz, H.; Pongor, L.; Krokker, L.; Szabó, B.; Dezső, K.; Dankó, T.; Sebestyén, A.; Sztankovics, D.; Tóvári, J.; Surguta, S.E.; et al. Defactinib in Combination with Mitotane Can Be an Effective Treatment in Human Adrenocortical Carcinoma. Int. J. Mol. Sci. 2025, 26, 6539. https://doi.org/10.3390/ijms26136539
Butz H, Pongor L, Krokker L, Szabó B, Dezső K, Dankó T, Sebestyén A, Sztankovics D, Tóvári J, Surguta SE, et al. Defactinib in Combination with Mitotane Can Be an Effective Treatment in Human Adrenocortical Carcinoma. International Journal of Molecular Sciences. 2025; 26(13):6539. https://doi.org/10.3390/ijms26136539
Chicago/Turabian StyleButz, Henriett, Lőrinc Pongor, Lilla Krokker, Borbála Szabó, Katalin Dezső, Titanilla Dankó, Anna Sebestyén, Dániel Sztankovics, József Tóvári, Sára Eszter Surguta, and et al. 2025. "Defactinib in Combination with Mitotane Can Be an Effective Treatment in Human Adrenocortical Carcinoma" International Journal of Molecular Sciences 26, no. 13: 6539. https://doi.org/10.3390/ijms26136539
APA StyleButz, H., Pongor, L., Krokker, L., Szabó, B., Dezső, K., Dankó, T., Sebestyén, A., Sztankovics, D., Tóvári, J., Surguta, S. E., Likó, I., Mészáros, K., Deák, A., Fekete, F., Vida, R., Báthory-Fülöp, L., Tóth, E., Igaz, P., & Patócs, A. (2025). Defactinib in Combination with Mitotane Can Be an Effective Treatment in Human Adrenocortical Carcinoma. International Journal of Molecular Sciences, 26(13), 6539. https://doi.org/10.3390/ijms26136539