Does the Relationship Between Microelements (Copper, Zinc and Selenium) and Proinflammatory Proteins (IL-6, IL-8 and Tissue Factor) Have Diagnostic Value in Equine Medicine?
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Horses
4.2. Blood Sampling and Analyses
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beghelli, D.; Giacconi, R.; Mocchegiani, E.; Cipriano, C.; Malavolta, M.; Renieri, C. A genetic variant near the equine interleukin 6 gene associated with copper:zinc ratio. Vet. J. 2011, 190, e143–e145. [Google Scholar] [CrossRef] [PubMed]
- Fedorka, C.E.; Scoggin, K.E.; El-Sheikh Ali, H.; Troedsson, M.H.T. Evaluating the IL-6 family of cytokines throughout equine gestation. Am. J. Reprod. Immunol. 2024, 92, e13910. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.; Chałabis-Mazurek, A.; Bełkot, Z.; Janczarek, I.; Kowalik, S. Zinc, copper and selenium deficiencies in broodmares in south-eastern Poland. Pol. J. Vet. Sci. 2025, 28, 475. [Google Scholar] [CrossRef]
- Jacobs, R.D.; Grum, D.; Trible, B.; Ayala, D.I.; Karnezos, T.P.; Gordon, M.E. Oral probiotic administration attenuates postexercise inflammation in horses. Transl. Anim. Sci. 2024, 8, 4–10. [Google Scholar] [CrossRef]
- Barton, M.H.; Collatos, C. Tumor necrosis factor and interleukin-6 activity and endotoxin concentration in peritoneal fluid and blood of horses with acute abdominal disease. J. Vet. Intern. Med. 1999, 13, 457–464. [Google Scholar] [CrossRef]
- Wessely-Szponder, J.; Bełkot, Z.; Bobowiec, R.; Wójcik, M.; Kosior-Korzecka, U. Crosstalk between adiponectin and cytokines (IL-6 and IL-8) during transportation stress in horses. Med. Weter. 2014, 70, 546–549. [Google Scholar]
- Zhang, C.; Xu, L.; Zhao, Y.; Wang, Y. Changes in serum heavy metals in polycystic ovary syndrome and their association with endocrine, lipid-metabolism, inflammatory characteristics and pregnancy outcomes. Reprod. Toxicol. 2022, 111, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tong, J.; Liang, C.; Wang, X.; Ma, Y.; Tao, S.; Liu, M.; Wang, Y.; Liu, J.; Yan, S.; et al. Trimester-specific effects of maternal exposure to single and mixed metals on cord serum inflammatory cytokines levels: A prospective birth cohort study. Sci. Total Environ. 2023, 895, 165086. [Google Scholar] [CrossRef]
- Bernhard, S.; Hug, S.; Stratmann, A.E.P.; Erber, M.; Vidoni, L.; Knapp, C.L.; Thomaß, B.D.; Fauler, M.; Nilsson, B.; Nilsson Ekdahl, K.; et al. Interleukin 8 elicits rapid physiological changes in neutrophils that are altered by inflammatory conditions. J. Innate Immun. 2021, 13, 225–241. [Google Scholar] [CrossRef]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef]
- Gale, J.; Aizenman, E. The physiological and pathophysiological roles of copper in the nervous system. Eur. J. Neurosci. 2024, 60, 3505–3543. [Google Scholar] [CrossRef]
- Crutchley, D.J.; Que, B.G. Copper-induced tissue factor expression in human monocytic THP-1 cells and its inhibition by antioxidants. Circulation 1995, 92, 238–243. [Google Scholar] [CrossRef]
- Musgrave, K.M.; Scott, J.; Sendama, W.; Gardner, A.I.; Dewar, F.; Lake, C.J.; Spronk, H.M.H.; van Oerle, R.; Visser, M.; Ten Cate, H.; et al. Tissue factor expression in monocyte subsets during human immunothrombosis, endotoxemia and sepsis. Thromb. Res. 2023, 228, 10–20. [Google Scholar] [CrossRef]
- Prochazkova, J.; Slavik, L.; Ulehlova, J.; Prochazka, M. The role of tissue factor in normal pregnancy and in the development of preeclampsia: A review. Biomed. Pap. 2015, 159, 192–196. [Google Scholar] [CrossRef]
- Girardi, G.; Mackman, N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: A pro-inflammatory molecule. Lupus 2008, 17, 931–936. [Google Scholar] [CrossRef]
- Jawa, R.S.; Anillo, S.; Huntoon, K.; Baumann, H.; Kulaylat, M. Analytic Review: Interleukin-6 in Surgery, Trauma, and Critical Care: Part I: Basic Science. J. Intensive Care Med. 2011, 26, 3–12. [Google Scholar] [CrossRef]
- Hjortoe, G.M.; Petersen, L.C.; Albrektsen, T.; Sorensen, B.B.; Norby, P.L.; Manda, S.K.; Pendurthi, U.R.; Rao, L.V.M. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood 2004, 103, 3029–3037. [Google Scholar] [CrossRef]
- Kiouri, D.P.; Chasapis, C.T.; Mavromoustakos, T.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and its binding proteins: Essential roles and therapeutic potential. Arch. Toxicol. 2025, 99, 23–41. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Pal, S.; Prasad, S.; Dang, A.K. Effect of micronutrient supplementation on the immune function of crossbred dairy cows under semi-arid tropical environment. Trop. Anim. Health Prod. 2014, 46, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Li, F.; Yu, X.W.; Sheng, Q.; Shi, X.W.; Zhang, X.W. Trace elements and cytokine profile in cytomegalovirus-infected pregnancies: A controlled study. Gynecol. Obstet. Investig. 2008, 65, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Malavolta, M.; Giacconi, R.; Piacenza, F.; Santarelli, L.; Cipriano, C.; Costarelli, L.; Tesei, S.; Pierpaoli, S.; Basso, A.; Galeazzi, R.; et al. Plasma copper/zinc ratio: An inflammatory/nutritional biomarker as predictor of all-cause mortality in elderly population. Biogerontology 2010, 11, 309–319. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Tomas, E.; Kelly, M.; Xiang, X.; Tsao, T.-S.; Keller, C.; Keller, P.; Luo, Z.; Lodish, H.; Saha, A.K.; Unger, R.; et al. Metabolic and hormonal interactions between muscle and adipose tissue. Proc. Nutr. Soc. 2004, 63, 381–385. [Google Scholar] [CrossRef]
- Akdas, S.; Turan, B.; Durak, A.; Aribal Ayral, P.; Yazihan, N. The relationship between metabolic syndrome development and tissue trace elements status and inflammatory markers. Biol. Trace Elem. Res. 2020, 198, 16–24. [Google Scholar] [CrossRef]
- Schneider, T.; Caviezel, D.; Korcan Ayata, C.; Kiss, C.; Niess, J.H.; Hruz, P. The copper/zinc ratio correlates with markers of disease activity in patients with inflammatory bowel disease. Crohn’s Colitis 360 2020, 2, otaa001. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Barrado, E.; Parodi-Román, J.; Escobedo-Monge, M.A.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Copper and copper/Zn ratio in a series of children with chronic diseases: A cross-sectional study. Nutrients 2021, 13, 3578. [Google Scholar] [CrossRef]
- Cappelli, K.; Felicetti, M.; Capomaccio, S.; Pieramati, C.; Silvestrelli, M.; Verini-Supplizi, A. Exercise-induced up-regulation of MMP-1 and IL-8 genes in endurance horses. BMC Physiol. 2009, 9, 12. [Google Scholar] [CrossRef]
- Cywińska, A.; Turło, A.; Witkowski, L.; Szarska, E.; Winnicka, A. Changes in blood cytokine concentrations in horses after long-distance endurance rides. Med. Weter. 2014, 70, 568–571. [Google Scholar]
- Lewandowski, Ł.; Kepinska, M.; Milnerowicz, H. The copper-zinc superoxide dismutase activity in selected diseases. Eur. J. Clin. Investig. 2019, 49, e13036. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, R.; Penning, L.C. COMMD1, a multi-potent intracellular protein involved in copper homeostasis, protein trafficking, inflammation, and cancer. J. Trace Elem. Med. Biol. 2021, 65, 126712. [Google Scholar] [CrossRef]
- Lappas, M. Copper metabolism domain-containing 1 represses the mediators involved in the terminal effector pathways of human labour and delivery. Mol. Hum. Reprod. 2016, 22, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Xue, Q.; Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.; Guralnik, J.; Fried, L.P. Serum antioxidants, inflammation, and total mortality in older women. Am. J. Epidemiol. 2006, 163, 18–26. [Google Scholar] [CrossRef]
- Ershler, W.B.; Keller, E.T. Age-Associated Increased Interleukin-6 Gene Expression, Late-Life Diseases, and Frailty. Annu. Rev. Med. 2000, 51, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.; Wałkuska, G. Effectiveness of copper supplementation in mares during reproduction season according to the feed zinc: Copper ratio. Ann. UMCS Sect. DD 2007, 62, 62–69. [Google Scholar]
- Erez, O.; Romero, R.; Vaisbuch, E.; Than, N.G.; Kusanovic, J.P.; Mazaki-Tovi, S.; Gotsch, F.; Mittal, P.; Dong, Z.; Chaiworapongsa, T.; et al. Tissue factor activity in women with preeclampsia or SGA: A potential explanation for the excessive thrombin generation in these syndromes. J. Matern. Neonatal Med. 2018, 31, 1568–1577. [Google Scholar] [CrossRef]
- Hedia, M.; Ibrahim, S.; Mahmoud, K.; Ahmed, Y.; Ismail, S.; El-Belely, M. Hemodynamic changes in cytokines, chemokines, acute phase proteins and prostaglandins in mares with subclinical endometritis. Theriogenology 2021, 171, 38–43. [Google Scholar] [CrossRef]
- Liu, I.K.M.; Troedsson, M.H.T. The diagnosis and treatment of endometritis in the mare: Yesterday and today. Theriogenology 2008, 70, 415–420. [Google Scholar] [CrossRef]
- Sikorska, U.; Maśko, M.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Role of Cortisol in Horse’s Welfare and Health. Agriculture 2023, 13, 2219. [Google Scholar] [CrossRef]
- Antoniak, S.; Mackman, N. Editorial Commentary: Tissue factor expression by the endothelium: Coagulation or inflammation? Trends Cardiovasc. Med. 2016, 26, 304–305. [Google Scholar] [CrossRef]
- Witkowski, M.; Landmesser, U.; Rauch, U. Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc. Med. 2016, 26, 297–303. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Al-Fadhel, S.Z.; Al-Dujaili, A.H. Correlation of serum Cu and Zn with some cytokines in major depressive disorder. J. Glob. Pharma Technol. 2020, 12, 115–121. [Google Scholar]
- Maśko, M.; Chałabis-Mazurek, A.; Sikorska, U.; Ciesielska, A.; Zdrojkowski, Ł.; Domino, M. Monthly and Pregnancy-Related Concentration of Cu and Zn in Serum of Mares in an Equine Breeding Herd. Agriculture 2024, 14, 35. [Google Scholar] [CrossRef]
- Janasik, B.; Trzcinka-Ochocka, M.; Brodzka, R. Oznaczanie selenu w surowicy/osoczu technika̧ spektrometrii mas z plazma̧ indukcyjnie sprzȩżona̧ (ICP-MS): Porównanie z technika̧ bezpłomieniowej absorpcyjnej spektrometrii atomowej (GF-AAS). Med. Pr. 2011, 62, 489–498. [Google Scholar] [PubMed]
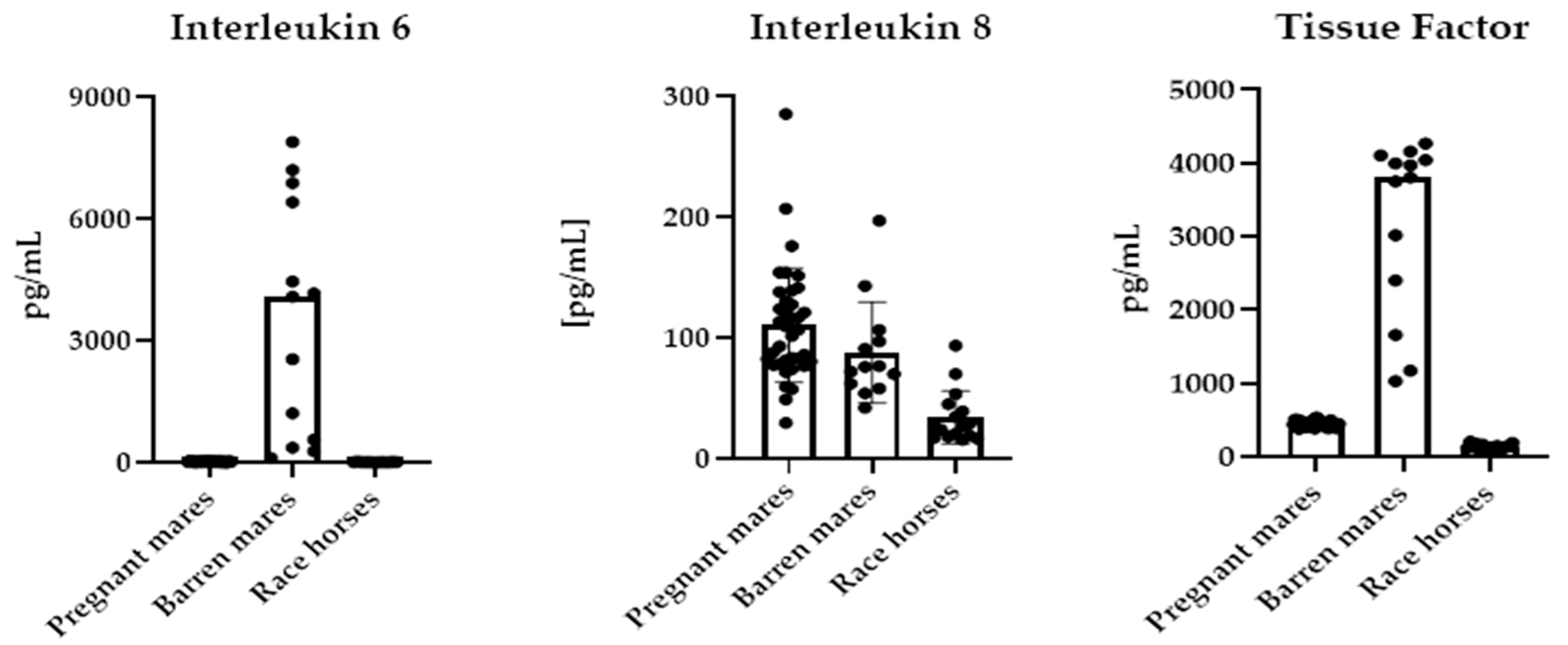

| Parameters | Pregnant Mares n = 37 | Barren Mares n = 13 | Race Thoroughbreds n = 16 | p Value |
|---|---|---|---|---|
| Medians ± QD | ||||
| IL-6 [pg/mL] | 15.6 ± 8.60 a | 4085 ± 2793 b | 8.75 ± 3.45 a | ≤0.0001 |
| TF [pg/mL] | 486 ± 135 a | 3804 ± 1155 b | 142 ± 36.9 c | ≤0.0001 |
| Means ± SD | ||||
| Il-8 [pg/mL] | 103 ± 56.5 a | 88.0 ± 39.9 a | 35.1 ± 19.8 b | ≤0.0001 |
| Cortisol [ng/mL] | 93.1 ± 50.9 | 101 ± 60.2 | 73.4 ± 12.7 | ≤0.0001 |
| Cu [µmol/L] | 17.9 ± 4.21 a | 20.9 ± 3.04 a | 7.84 ± 1.80 b | ≤0.0001 |
| Zn [µmol/L] | 10.2 ± 1.22 a | 9.01 ± 1.34 a | 70.6 ± 51.5 b | ≤0.0001 |
| Se [µmol/L] | 0.77 ± 0.42 a | 1.49 ± 0.60 b | 0.84 ± 0.11 a | ≤0.0001 |
| Cu:Zn ratio | 1.78 ± 0.44 a | 2.37 ± 0.45 b | 0.11 ± 0.09 c | ≤0.0001 |
| Correlation Coefficient | Cu | Zn | Cu:Zn Ratio | Se |
|---|---|---|---|---|
| IL-6 | 0.342 | −0.213 | 0.418 ** | 0.433 *** |
| IL-8 | 0.422 *** | −0.415 *** | 0.490 **** | 0.079 |
| TF | 0.487 *** | −0.327 | 0.571 **** | 0.541 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mojsym, W.; Kowalik, S.; Chałabis-Mazurek, A.; Janczarek, I.; Kędzierski, W. Does the Relationship Between Microelements (Copper, Zinc and Selenium) and Proinflammatory Proteins (IL-6, IL-8 and Tissue Factor) Have Diagnostic Value in Equine Medicine? Int. J. Mol. Sci. 2025, 26, 10429. https://doi.org/10.3390/ijms262110429
Mojsym W, Kowalik S, Chałabis-Mazurek A, Janczarek I, Kędzierski W. Does the Relationship Between Microelements (Copper, Zinc and Selenium) and Proinflammatory Proteins (IL-6, IL-8 and Tissue Factor) Have Diagnostic Value in Equine Medicine? International Journal of Molecular Sciences. 2025; 26(21):10429. https://doi.org/10.3390/ijms262110429
Chicago/Turabian StyleMojsym, Wioleta, Sylwester Kowalik, Agnieszka Chałabis-Mazurek, Iwona Janczarek, and Witold Kędzierski. 2025. "Does the Relationship Between Microelements (Copper, Zinc and Selenium) and Proinflammatory Proteins (IL-6, IL-8 and Tissue Factor) Have Diagnostic Value in Equine Medicine?" International Journal of Molecular Sciences 26, no. 21: 10429. https://doi.org/10.3390/ijms262110429
APA StyleMojsym, W., Kowalik, S., Chałabis-Mazurek, A., Janczarek, I., & Kędzierski, W. (2025). Does the Relationship Between Microelements (Copper, Zinc and Selenium) and Proinflammatory Proteins (IL-6, IL-8 and Tissue Factor) Have Diagnostic Value in Equine Medicine? International Journal of Molecular Sciences, 26(21), 10429. https://doi.org/10.3390/ijms262110429





