Recent Advances in Nanoparticle-Mediated Antibacterial Photodynamic Therapy
Abstract
1. Introduction
2. Mechanism of Photodynamic Therapy
3. Novel Nanoparticle Materials
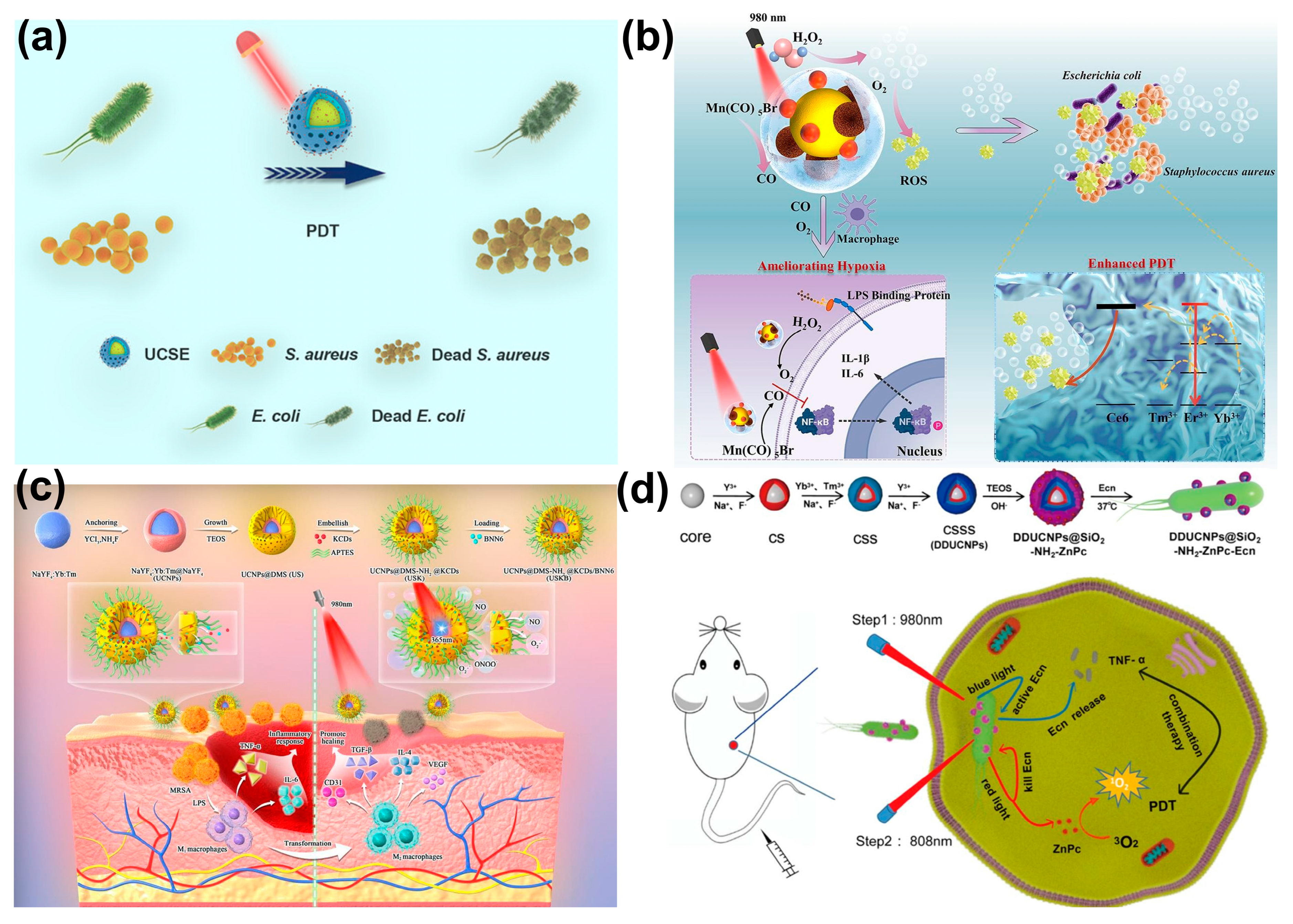
3.1. Upconverting Nanoparticles
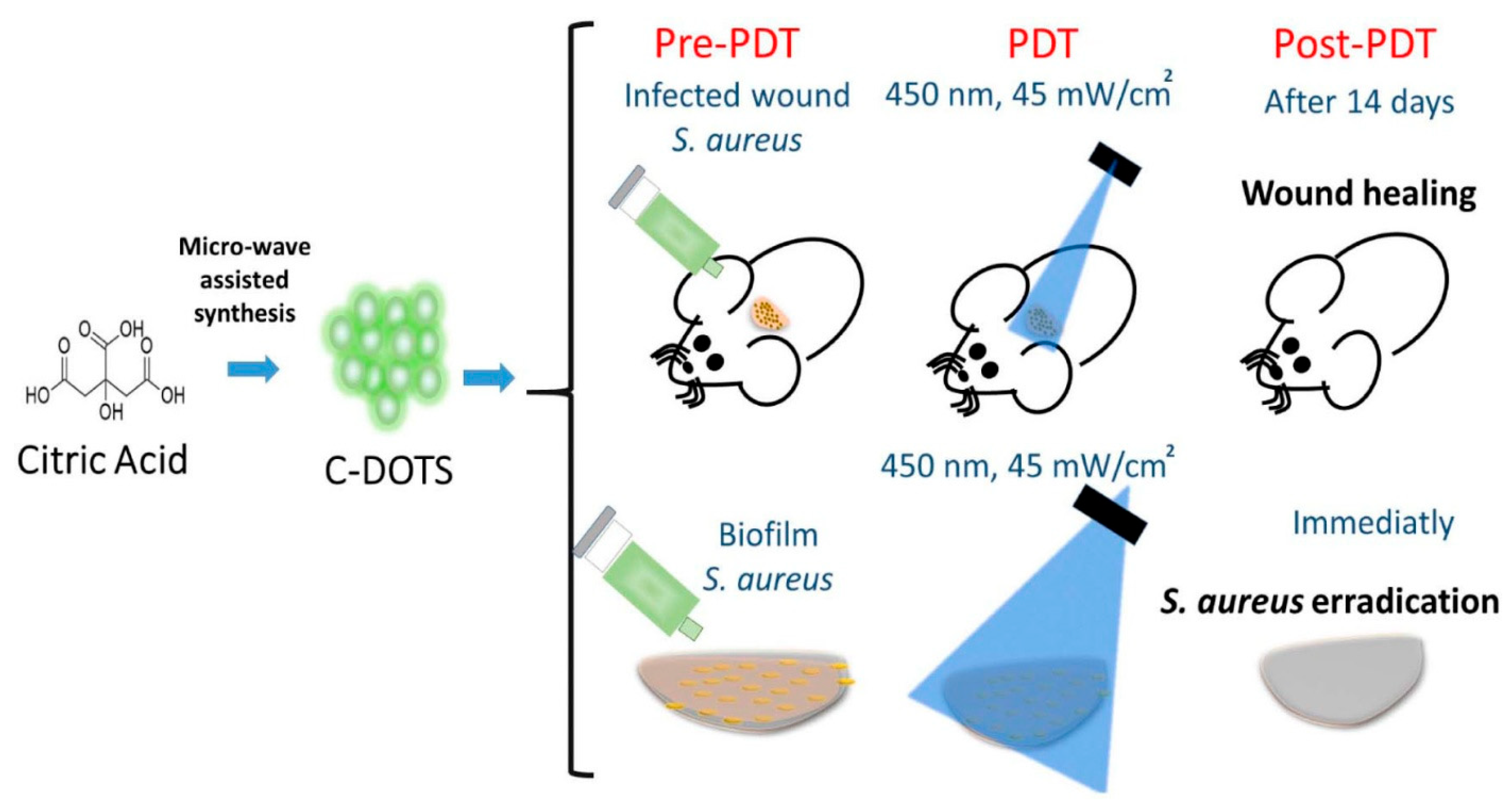
3.2. Carbon Dots (CDs)
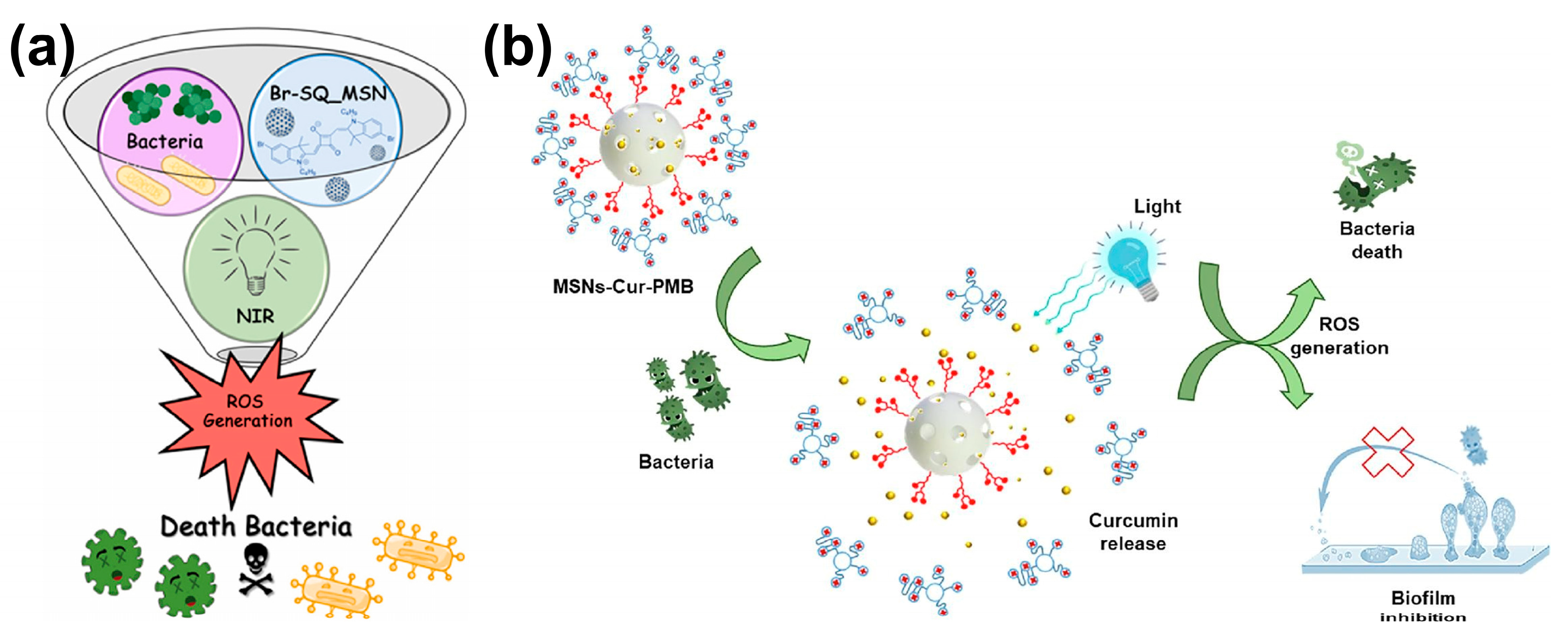
3.3. Mesoporous Silica Nanoparticles
3.4. Polymer-Based Nanoparticles
3.5. Liposomes
4. Combination Therapies
5. Recent Patents in Antibacterial Photodynamic Therapy
6. In Vivo Applications of Different Nanoparticles in the Photodynamic Therapy
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, C.S.; Wong, C.T.H.; Aung, T.T.; Lakshminarayanan, R.; Mehta, J.S.; Rauz, S.; McNally, A.; Kintses, B.; Peacock, S.J.; de la Fuente-Nunez, C.; et al. Antimicrobial resistance: A concise update. Lancet Microbe 2025, 6, 100947. [Google Scholar]
- Draviana, H.T.; Fitriannisa, I.; Khafid, M.; Krisnawati, D.I.; Widodo; Lai, C.-H.; Fan, Y.-J.; Kuo, T.-R. Size and charge effects of metal nanoclusters on antibacterial mechanisms. J. Nanobiotechnol. 2023, 21, 428. [Google Scholar] [CrossRef]
- Mutalik, C.; Lin, I.-H.; Krisnawati, D.I.; Khaerunnisa, S.; Khafid, M.; Hsiao, Y.-C.; Kuo, T.-R. Antibacterial pathways in transition metal-based nanocomposites: A mechanistic overview. Int. J. Nanomed. 2022, 17, 6821. [Google Scholar] [CrossRef]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-based nanostructures for antibacterial application. Int. J. Mol. Sci. 2023, 24, 10006. [Google Scholar] [CrossRef]
- Afonso, J.; Fastl, C.; Chaters, G.; Hoza, A.; Shirima, G.; Nyasebwa, O.; Rushton, J. Burden assessment of antimicrobial use and resistance in livestock in data-scarce contexts. Rev. Sci. Tech. (Int. Off. Epizoot.) 2024, 43, 168–176. [Google Scholar]
- Bo, L.; Sun, H.; Li, Y.-D.; Zhu, J.; Wurpel, J.N.; Lin, H.; Chen, Z.-S. Combating antimicrobial resistance: The silent war. Front. Pharmacol. 2024, 15, 1347750. [Google Scholar] [CrossRef] [PubMed]
- Draviana, H.T.; Fitriannisa, I.; Jazidie, A.; Krisnawati, D.I.; Khafid, M.; Kuo, T.-R. Antibacterial Mechanisms of Negatively and Positively Charged Ligands on Gold Nanoclusters. ACS Appl. Nano Mater. 2025, 8, 6380–6390. [Google Scholar] [CrossRef]
- Saukani, M.; Lai, C.-H.; Mutalik, C.; Krisnawati, D.I.; Chu, H.-Y.; Kuo, T.-R. Copper Sulfide Nanorod-Embedded Urinary Catheter with Hydrophobicity and Photothermal Sterilization. Int. J. Mol. Sci. 2024, 25, 11440. [Google Scholar] [CrossRef] [PubMed]
- Aebisher, D.; Szpara, J.; Bartusik-Aebisher, D. Advances in medicine: Photodynamic therapy. Int. J. Mol. Sci. 2024, 25, 8258. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Rayis, M.; Rizvi, A.; Alam, M.M.; Rizvi, M.; Naseem, I. ROS mediated antibacterial activity of photoilluminated riboflavin: A photodynamic mechanism against nosocomial infections. Toxicol. Rep. 2019, 6, 136–142. [Google Scholar] [CrossRef]
- Khorsandi, K.; Hosseinzadeh, R.; Esfahani, H.; Zandsalimi, K.; Shahidi, F.K.; Abrahamse, H. Accelerating skin regeneration and wound healing by controlled ROS from photodynamic treatment. Inflamm. Regen. 2022, 42, 40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-Y.; Chiu, Y.-C.; Chen, K.-H.; Chen, Y.-J.; Huang, C.-C. Galactosylated silver nanoparticles as a biocompatible intrinsic SERS probe for bladder cancer imaging and ex vivo tumor detection. J. Mater. Chem. B 2025, 13, 11232–11241. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Yu, H.; Shu, Y.; Ma, M.; Chen, H. A robust ROS generation strategy for enhanced chemodynamic/photodynamic therapy via H2O2/O2 self-supply and Ca2+ overloading. Adv. Funct. Mater. 2021, 31, 2106106. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Wang, K.-P.; Mo, J.-G.; Xiong, L.; Wen, Y. Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species. World J. Stem Cells 2020, 12, 562. [Google Scholar] [CrossRef]
- Rajendran, M. Quinones as photosensitizer for photodynamic therapy: ROS generation, mechanism and detection methods. Photodiagn. Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Tada, D.B.; Baptista, M.S. Photosensitizing nanoparticles and the modulation of ROS generation. Front. Chem. 2015, 3, 33. [Google Scholar] [CrossRef]
- Nifantiev, N.E.; Gitter, B.; Yashunsky, D.V. Anti-Microbial Photodynamic Therapy—Google Patents. US20090326434A1, 22 December 2015. [Google Scholar]
- Chen, S.; Zeng, Q.; Tan, X.; Ye, M.; Zhang, Y.; Zou, L.; Liu, S.; Yang, Y.; Liu, A.; He, L.; et al. Photodynamic antibacterial chitosan/nitrogen-doped carbon dots composite packaging film for food preservation applications. Carbohydr. Polym. 2023, 314, 120938. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, P.; Wang, Y.; Sun, B.; Liu, Y.; Zhang, Q.; Feng, W.; Li, Z.; Li, K.; Zhou, N.; et al. Near-infrared carbon dot-based platform for bioimaging and photothermal/photodynamic/quaternary ammonium triple synergistic sterilization triggered by single NIR light source. Carbon 2021, 176, 126–138. [Google Scholar] [CrossRef]
- Dandamudi, M.; McLoughlin, P.; Behl, G.; Rani, S.; Coffey, L.; Chauhan, A.; Kent, D.; Fitzhenry, L. Chitosan-Coated PLGA Nanoparticles Encapsulating Triamcinolone Acetonide as a Potential Candidate for Sustained Ocular Drug Delivery. Pharmaceutics 2021, 13, 1590. [Google Scholar] [CrossRef]
- Jiang, J.; Lv, X.; Cheng, H.; Yang, D.; Xu, W.; Hu, Y.; Song, Y.; Zeng, G. Type I photodynamic antimicrobial therapy: Principles, progress, and future perspectives. Acta Biomater. 2024, 177, 1–19. [Google Scholar] [CrossRef]
- Karimi, M.; Homayoonfal, M.; Zahedifar, M.; Ostadian, A.; Adibi, R.; Mohammadzadeh, B.; Raisi, A.; Ravaei, F.; Rashki, S.; Khakbraghi, M.; et al. Development of a novel nanoformulation based on aloe vera-derived carbon quantum dot and chromium-doped alumina nanoparticle (Al2O3:Cr@Cdot NPs): Evaluating the anticancer and antimicrobial activities of nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2024, 15, 26. [Google Scholar] [CrossRef]
- Lai, W.; Liu, H.; Yan, J.; Tian, L.; Shi, Y.; Xi, Z.; Lin, B. Photosensitizer of phthalocyanine-conjugated chitosan-doped nano-silver for inactivation against bacteria and promote wound healing. Biomed. Eng. Adv. 2025, 9, 100147. [Google Scholar] [CrossRef]
- Li, Y.; Su, X.; Wang, X.; Leung, A.W.; Xu, C.; Wang, P.; Liu, Q. Cytotoxic effect of protoporphyrin IX to human Leukemia U937 cells under ultrasonic irradiation. Cell. Physiol. Biochem. 2014, 33, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, J.; Rahban, D.; Aghamiri, S.; Teymouri, A.; Bahador, A. Photosensitizers in antibacterial photodynamic therapy: An overview. Laser Ther. 2018, 27, 293–302. [Google Scholar] [CrossRef]
- Hsu, F.-T.; Chen, Y.-T.; Chin, Y.-C.; Chang, L.-C.; Chiang, S.-C.; Yang, L.-X.; Liu, H.-S.; Yueh, P.-F.; Tu, H.-L.; He, R.-Y.; et al. Harnessing the Power of Sugar-Based Nanoparticles: A Drug-Free Approach to Enhance Immune Checkpoint Inhibition against Glioblastoma and Pancreatic Cancer. ACS Nano 2024, 18, 28764–28781. [Google Scholar] [CrossRef]
- Chia, Z.-C.; Chen, Y.-L.; Chuang, C.-H.; Hsieh, C.-H.; Chen, Y.-J.; Chen, K.-H.; Huang, T.-C.; Chen, M.-C.; Huang, C.-C. Polyphenol-assisted assembly of Au-deposited polylactic acid microneedles for SERS sensing and antibacterial photodynamic therapy. Chem. Commun. 2023, 59, 6339–6342. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.; Breukink, E.; Heger, M. Antibacterial photodynamic therapy: Overview of a promising approach to fight antibiotic-resistant bacterial infections. J. Clin. Transl. Res. 2015, 1, 140. [Google Scholar]
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated photodynamic therapy for bacterial infections. Adv. Healthc. Mater. 2019, 8, 1900608. [Google Scholar] [CrossRef]
- Gao, Y.; Lin, H.; Luo, Y.; Li, J.; Gong, C.; Chen, H.; Gong, R. Nanomaterial-based photodynamic therapy for antibacterial applications: A comprehensive review. Front. Mater. 2023, 10, 1260887. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Abrahamse, H. Can light-based approaches overcome antimicrobial resistance? Drug Dev. Res. 2019, 80, 48–67. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, H.; Wang, Y.; Shiu, B.-C.; Lin, J.-H.; Zhang, S.; Lou, C.-W.; Li, T.-T. Synergistic antibacterial strategy based on photodynamic therapy: Progress and perspectives. Chem. Eng. J. 2022, 450, 138129. [Google Scholar] [CrossRef]
- Kumar, J.; Roy, I. Upconverting nanophosphors for various sensing applications. Talanta Open 2024, 9, 100302. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Upconversion nanoparticles for photodynamic therapy and other cancer therapeutics. Theranostics 2013, 3, 317–330. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, H.; Chen, Q. Near-Infrared Light-Mediated Antibacterial Photodynamic Therapy Based on Erythrosine-Functionalized Mesoporous Silica-Coated Upconversion Nanoplatform. ACS Omega 2024, 9, 34799–34807. [Google Scholar] [CrossRef]
- Liu, W.; Sun, Y.; Zhou, B.; Chen, Y.; Liu, M.; Wang, L.; Qi, M.; Liu, B.; Dong, B. Near-infrared light triggered upconversion nanocomposites with multifunction of enhanced antimicrobial photodynamic therapy and gas therapy for inflammation regulation. J. Colloid Interface Sci. 2024, 663, 834–846. [Google Scholar] [CrossRef]
- Chen, M.; Han, Q.; Zhang, M.; Liu, Y.; Wang, L.; Yang, F.; Li, Q.; Cao, Z.; Fan, C.; Liu, J. Upconversion dual-photosensitizer–expressing bacteria for near-infrared monochromatically excitable synergistic phototherapy. Sci. Adv. 2024, 10, eadk9485. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, X.; Dai, C.; Yang, B.; Wang, W.; Fan, C.; Zhang, P.; Sun, J.; Sun, D. NIR II-triggered core-shell upconversion nanocomposites for peroxynitrite-boosted anti-infection against diabetic wound. Chem. Eng. J. 2024, 480, 148271. [Google Scholar] [CrossRef]
- Chen, F.; Qiu, Y.; Liu, J. NIR responsive nanosystem based on upconversion nanoparticle-engineered bacteria for immune/photodynamic combined therapy with bacteria self-clearing capability. J. Colloid Interface Sci. 2025, 678, 583–594. [Google Scholar] [CrossRef]
- Pan, H.; Li, L.; Pang, G.; Han, C.; Liu, B.; Zhang, Y.; Shen, Y.; Sun, T.; Liu, J.; Chang, J. Engineered NIR light-responsive bacteria as anti-tumor agent for targeted and precise cancer therapy. Chem. Eng. J. 2021, 426, 130842. [Google Scholar] [CrossRef]
- Jia, H.-R.; Zhu, Y.-X.; Chen, Z.; Wu, F.-G. Cholesterol-assisted bacterial cell surface engineering for photodynamic inactivation of gram-positive and gram-negative bacteria. ACS Appl. Mater. Interfaces 2017, 9, 15943–15951. [Google Scholar] [CrossRef]
- Wu, M.; Wu, W.; Duan, Y.; Li, X.; Qi, G.; Liu, B. Photosensitizer-bacteria biohybrids promote photodynamic cancer cell ablation and intracellular protein delivery. Chem. Mater. 2019, 31, 7212–7220. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Y.; Xu, S.; Li, G. A wrinkle to sub-100 nm yolk/shell Fe3O4@ SiO2 nanoparticles. Nano Res. 2016, 9, 3632–3643. [Google Scholar] [CrossRef]
- Wang, S.; McCoy, C.P.; Li, P.; Li, Y.; Zhao, Y.; Andrews, G.P.; Wylie, M.P.; Ge, Y. Carbon Dots in Photodynamic/Photothermal Antimicrobial Therapy. Nanomaterials 2024, 14, 1250. [Google Scholar] [CrossRef] [PubMed]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomed. 2019, 14, 2655–2665. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Alves, F.; Stringasci, M.D.; Buzzá, H.H.; Ciol, H.; Inada, N.M.; Bagnato, V.S. One-Pot Microwave-Assisted Synthesis of Carbon Dots and in vivo and in vitro Antimicrobial Photodynamic Applications. Front. Microbiol. 2021, 12, 662149. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.-V.; Shim, K.; Vo Thi, T.-T.; Kook, J.-K.; An, S.S.A.; Lee, S.-W. Targeted and controlled drug delivery by multifunctional mesoporous silica nanoparticles with internal fluorescent conjugates and external polydopamine and graphene oxide layers. Acta Biomater. 2018, 74, 397–413. [Google Scholar] [CrossRef]
- Mahmoud Abd-Alaziz, D.; Mansour, M.; Nasr, M.; Sammour, O. Tailored green synthesized silymarin-selenium nanoparticles: Topical nanocarrier of promising antileishmanial activity. Int. J. Pharm. 2024, 660, 124275. [Google Scholar] [CrossRef]
- Fatima, R.; Katiyar, P.; Kushwaha, K. Recent advances in mesoporous silica nanoparticle: Synthesis, drug loading, release mechanisms, and diverse applications. Front. Nanotechnol. 2025, 7, 1564188. [Google Scholar] [CrossRef]
- Nady, D.S.; Hassan, A.; Amin, M.U.; Bakowsky, U.; Fahmy, S.A. Recent Innovations of Mesoporous Silica Nanoparticles Combined with Photodynamic Therapy for Improving Cancer Treatment. Pharmaceutics 2024, 16, 14. [Google Scholar] [CrossRef]
- Dereje, D.M.; García, A.; Pontremoli, C.; González, B.; Colilla, M.; Vallet-Regí, M.; Izquierdo-Barba, I.; Barbero, N. Squaraine-loaded mesoporous silica nanoparticles for antimicrobial Photodynamic Therapy against bacterial infection. Microporous Mesoporous Mater. 2024, 372, 113096. [Google Scholar] [CrossRef]
- Medaglia, S.; Otri, I.; Bernardos, A.; Marcos, M.D.; Aznar, E.; Sancenón, F.; Martínez-Máñez, R. Synergistic antimicrobial photodynamic therapy using gated mesoporous silica nanoparticles containing curcumin and polymyxin B. Int. J. Pharm. 2024, 654, 123947. [Google Scholar] [CrossRef]
- Vaara, M. Polymyxin derivatives that sensitize Gram-negative bacteria to other antibiotics. Molecules 2019, 24, 249. [Google Scholar] [CrossRef] [PubMed]
- Newton, B. The properties and mode of action of the polymyxins. Bacteriol. Rev. 1956, 20, 14–27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshida, T.; Hiramatsu, K. Potent in vitro bactericidal activity of polymyxin B against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Immunol. 1993, 37, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Trimble, M.J.; Mlynárčik, P.; Kolář, M.; Hancock, R.E. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef]
- Dai, C.; Lin, J.; Li, H.; Shen, Z.; Wang, Y.; Velkov, T.; Shen, J. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martinez-Manez, R.; Sancenon, F. Gated materials for on-command release of guest molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Beagan, A.M.; Alghamdi, A.A.; Lahmadi, S.S.; Halwani, M.A.; Almeataq, M.S.; Alhazaa, A.N.; Alotaibi, K.M.; Alswieleh, A.M. Folic acid-terminated poly (2-diethyl amino ethyl methacrylate) brush-gated magnetic mesoporous nanoparticles as a smart drug delivery system. Polymers 2020, 13, 59. [Google Scholar] [CrossRef]
- Aznar, E.; Villalonga, R.; Giménez, C.; Sancenón, F.; Marcos, M.D.; Martínez-Máñez, R.; Díez, P.; Pingarrón, J.M.; Amorós, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef]
- Ruiz-Rico, M.; Daubenschüz, H.; Pérez-Esteve, É.; Marcos, M.D.; Amorós, P.; Martínez-Máñez, R.; Barat, J.M. Protective effect of mesoporous silica particles on encapsulated folates. Eur. J. Pharm. Biopharm. 2016, 105, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Guarí-Borràs, G.; Ruiz-Rico, M.; Morellá-Aucejo, Á.; Aznar, E.; Barat, J.M.; Martínez-Máñez, R.; Marcos, M.D.; Bernardos, A. Towards the Enhancement of Essential Oil Components’ Antimicrobial Activity Using New Zein Protein-Gated Mesoporous Silica Microdevices. Int. J. Mol. Sci. 2021, 22, 3795. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Ng, S.-F.; Khan, S.; Katas, H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: A review of new trends and state-of-the-art. Colloids Surf. B Biointerfaces 2017, 150, 223–241. [Google Scholar] [PubMed]
- Qi, C.; Tan, G.; Hu, H.; Zhang, Y.; Chen, J.; Zhang, Q.; Tu, J. Prussian blue-decorated indocyanine green-loaded mesoporous silica nanohybrid for synergistic photothermal-photodynamic-chemodynamic therapy against methicillin-resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2024, 241, 114065. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Arruda, L.M.; Moreira, I.P.; Scacchetti, F.A.P.; de Oliveira, H.P.M.; Samulewski, R.B.; Fangueiro, R.; Tessaro, A.L. Functionalization of fibrous substrates with mesoporous silica nanoparticles as a strategy to obtain photodynamic antibacterial textiles. Dye. Pigment. 2024, 230, 112342. [Google Scholar] [CrossRef]
- Huang, X.; Zhan, Y.; Xiao, Z.; He, S.; Hu, L.; Zhu, H.; Guo, H.; Sun, H.; Liu, M. Photodynamic antibacterial research on hypericin-loaded PEGylated mesoporous silica delivery system. J. Biomater. Sci. Polym. Ed. 2024, 35, 1795–1818. [Google Scholar] [CrossRef]
- Yan, R.; Zhan, M.; Xu, J.; Peng, Q. Functional nanomaterials as photosensitizers or delivery systems for antibacterial photodynamic therapy. Biomater. Adv. 2024, 159, 213820. [Google Scholar] [CrossRef]
- Yuan, Q.; Yin, J.; Li, L.; Bao, B.; Zhang, X.; Li, M.; Tang, Y. Conjugated Polymer Composite Nanoparticles Augmenting Photosynthesis-Based Light-Triggered Hydrogel Promotes Chronic Wound Healing. Adv. Sci. 2024, 11, 2304048. [Google Scholar] [CrossRef]
- Elian, C.; Méallet, R.; Versace, D.-L. Photoactive Dye-Loaded Polymer Materials: A New Cutting Edge for Antibacterial Photodynamic Therapy. Adv. Funct. Mater. 2024, 34, 2407228. [Google Scholar]
- Zhou, W.; Chen, L.; Li, H.; Wu, M.; Liang, M.; Liu, Q.; Wu, W.; Jiang, X.; Zhen, X. Membrane Disruption-Enhanced Photodynamic Therapy against Gram-Negative Bacteria by a Peptide-Photosensitizer Conjugate. ACS Nano 2024, 18, 19771–19782. [Google Scholar]
- Guo, J.; Feng, K.; Wu, W.; Ruan, Y.; Liu, H.; Han, X.; Shao, G.; Sun, X. Smart 131I-Labeled Self-Illuminating Photosensitizers for Deep Tumor Therapy. Angew. Chem. Int. Ed. 2021, 60, 21884–21889. [Google Scholar] [CrossRef]
- Qu, H.; Li, L.; Chen, H.; Tang, M.; Cheng, W.; Lin, T.Y.; Li, L.; Li, B.; Xue, X. Drug-drug conjugates self-assembled nanomedicines triggered photo-/immuno- therapy for synergistic cancer treatments. J. Control. Release 2023, 363, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, S.; Sun, Y.; Zou, X.; Zheng, L.; Duan, S.; Wang, J.; Yu, B.; Sui, R.; Xu, F.-J. Bacteria-Targeting Photodynamic Nanoassemblies for Efficient Treatment of Multidrug-Resistant Biofilm Infected Keratitis. Adv. Funct. Mater. 2022, 32, 2111066. [Google Scholar]
- Hu, J.; Liu, N.; Fan, Q.; Gu, Y.; Chen, S.; Zhu, F.; Cheng, Y. A Fluorous Peptide Amphiphile with Potent Antimicrobial Activity for the Treatment of MRSA-induced Sepsis and Chronic Wound Infection. Angew. Chem. Int. Ed. 2024, 63, e202403140. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, Z.; Zou, Z.; Qian, M.; Wang, X.; Zhang, X.; Yin, Z.; Wang, J.; Ye, X.; Liu, D.; et al. Combating Escherichia coli O157:H7 with Functionalized Chickpea-Derived Antimicrobial Peptides. Adv. Sci. 2023, 10, 2205301. [Google Scholar]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880, Erratum in Chem. Soc. Rev. 2022, 51, 792. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, W.; Sun, L.; Ma, L.; Shang, D. Insights into the membrane interaction mechanism and antibacterial properties of chensinin-1b. Biomaterials 2015, 37, 299–311. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef]
- Imani, S.M.; Ladouceur, L.; Marshall, T.; Maclachlan, R.; Soleymani, L.; Didar, T.F. Antimicrobial Nanomaterials and Coatings: Current Mechanisms and Future Perspectives to Control the Spread of Viruses Including SARS-CoV-2. ACS Nano 2020, 14, 12341–12369. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, Y.; Gao, Y.; Wei, X.; Li, J.; Zhang, Y.; Wu, Z.; Zhang, X. Oxygen Self-Supplying Nanotherapeutic for Mitigation of Tissue Hypoxia and Enhanced Photodynamic Therapy of Bacterial Keratitis. ACS Appl. Mater. Interfaces 2021, 13, 33790–33801. [Google Scholar] [CrossRef]
- Pujari, A.K.; Kaur, R.; Reddy, Y.N.; Paul, S.; Gogde, K.; Bhaumik, J. Design and Synthesis of Metalloporphyrin Nanoconjugates for Dual Light-Responsive Antimicrobial Photodynamic Therapy. J. Med. Chem. 2024, 67, 2004–2018. [Google Scholar] [CrossRef]
- Xu, D.; Zhu, W.; Ding, C.; Mei, J.; Zhou, J.; Cheng, T.; Guo, G.; Zhang, X. Self-Homeostasis Immunoregulatory Strategy for Implant-Related Infections through Remodeling Redox Balance. ACS Nano 2023, 17, 4574–4590. [Google Scholar] [CrossRef] [PubMed]
- Soury, R.; Chaabene, M.; Jabli, M.; Rousselin, Y. Synthesis, characterization, and computational study of a new zinc derivative (4.4′ diaminodiphenylmethane)(meso-tetratolylporphyrinato) zinc {[Zn (TTP)(DADMP)2]}n. J. Solid. State Chem. 2021, 295, 121920. [Google Scholar] [CrossRef]
- Chandna, S.; Thakur, N.S.; Kaur, R.; Bhaumik, J. Lignin–Bimetallic Nanoconjugate Doped pH-Responsive Hydrogels for Laser-Assisted Antimicrobial Photodynamic Therapy. Biomacromolecules 2020, 21, 3216–3230. [Google Scholar] [CrossRef]
- Du, H.; Zhao, S.; Wang, Y.; Wang, Z.; Chen, B.; Yan, Y.; Yin, Q.; Liu, D.; Wan, F.; Zhang, Q. pH/Cathepsin B hierarchical-responsive nanoconjugates for enhanced tumor penetration and chemo-immunotherapy. Adv. Funct. Mater. 2020, 30, 2003757. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z. A pH-responsive hydrogel based on a tumor-targeting mesoporous silica nanocomposite for sustained cancer labeling and therapy. Macromol. Rapid Commun. 2016, 37, 1533–1539. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Kirar, S.; Thakur, N.S.; Patil, M.D.; Bhaumik, J. Sunlight Assisted Photocatalytic Valorization of Lignin Using Recyclable Light Harvesters. ACS Sustain. Chem. Eng. 2023, 11, 4568–4579. [Google Scholar] [CrossRef]
- Marydasan, B.; Nair, A.K.; Ramaiah, D. Optimization of triplet excited state and singlet oxygen quantum yields of picolylamine–porphyrin conjugates through zinc insertion. J. Phys. Chem. B 2013, 117, 13515–13522. [Google Scholar] [CrossRef]
- Kee, H.L.; Bhaumik, J.; Diers, J.R.; Mroz, P.; Hamblin, M.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Photophysical characterization of imidazolium-substituted Pd (II), In (III), and Zn (II) porphyrins as photosensitizers for photodynamic therapy. J. Photochem. Photobiol. A Chem. 2008, 200, 346–355. [Google Scholar] [CrossRef]
- Büchau, A.S.; Hassan, M.; Kukova, G.; Lewerenz, V.; Kellermann, S.; Würthner, J.U.; Wolf, R.; Walz, M.; Gallo, R.L.; Ruzicka, T. S100A15, an antimicrobial protein of the skin: Regulation by E. coli through Toll-like receptor 4. J. Investig. Dermatol. 2007, 127, 2596–2604. [Google Scholar] [CrossRef]
- Izquierdo, N.; Gamez, E.; Alejo, T.; Mendoza, G.; Arruebo, M. Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens. Materials 2024, 17, 1717. [Google Scholar] [CrossRef]
- Crow, J.P. Dichlorodihydrofluorescein and Dihydrorhodamine 123 Are Sensitive Indicators of Peroxynitritein Vitro:Implications for Intracellular Measurement of Reactive Nitrogen and Oxygen Species. Nitric Oxide 1997, 1, 145–157. [Google Scholar] [CrossRef]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical properties of protoporphyrin IX, pyropheophorbide-a, and Photofrin® in different conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Gerega, A.; Zolek, N.; Soltysinski, T.; Milej, D.; Sawosz, P.; Toczylowska, B.; Liebert, A. Wavelength-resolved measurements of fluorescence lifetime of indocyanine green. J. Biomed. Opt. 2011, 16, 067010. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Moan, J.; Ma, L.; Iani, V.; Juzeniene, A. Influence of light exposure on the kinetics of protoporphyrin IX formation in normal skin of hairless mice after application of 5-aminolevulinic acid methyl ester. J. Investig. Dermatol. 2005, 125, 1039–1044. [Google Scholar] [CrossRef][Green Version]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagn. Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-Tetraphenylporphyrin Metal Complex-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef]
- Löw, K.; Knobloch, T.; Wagner, S.; Wiehe, A.; Engel, A.; Langer, K.; Briesen, H.v. Comparison of intracellular accumulation and cytotoxicity of free mTHPC and mTHPC-loaded PLGA nanoparticles in human colon carcinoma cells. Nanotechnology 2011, 22, 245102. [Google Scholar] [CrossRef]
- Bœuf-Muraille, G.; Rigaux, G.; Callewaert, M.; Zambrano, N.; Van Gulick, L.; Roullin, V.G.; Terryn, C.; Andry, M.C.; Chuburu, F.; Dukic, S.; et al. Evaluation of mTHPC-loaded PLGA nanoparticles for in vitro photodynamic therapy on C6 glioma cell line. Photodiagn. Photodyn. Ther. 2019, 25, 448–455. [Google Scholar] [CrossRef]
- Hongcharu, W.; Taylor, C.R.; Aghassi, D.; Suthamjariya, K.; Anderson, R.R.; Chang, Y. Topical ALA-Photodynamic Therapy for the Treatment of Acne Vulgaris. J. Investig. Dermatol. 2000, 115, 183–192. [Google Scholar] [CrossRef]
- Hamblin Michael, R.; Viveiros, J.; Yang, C.; Ahmadi, A.; Ganz Robert, A.; Tolkoff, M.J. Helicobacter pylori Accumulates Photoactive Porphyrins and Is Killed by Visible Light. Antimicrob. Agents Chemother. 2005, 49, 2822–2827. [Google Scholar] [CrossRef] [PubMed]
- Dellambra, E.; Dimri, G.P. Chapter 7—Cellular Senescence and Skin Aging. In Skin Aging Handbook; Dayan, N., Ed.; William Andrew Publishing: Norwich, NY, USA, 2009. [Google Scholar]
- de Oliveira Miguel, J.; da Silva, D.B.; da Silva, G.C.C.; Corrêa, R.J.; de Oliveira Miguel, N.C.; Lione, V.d.O.F.; Riemma Pierre, M.B. Polymeric nanoparticles favor the in vitro dermal accumulation of Protoporphyrin IX (PpIX) with optimal biocompatibility and cellular recovery in culture of healthy dermal fibroblasts after Photodynamic Therapy. J. Photochem. Photobiol. A Chem. 2020, 386, 112109. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127. [Google Scholar] [CrossRef] [PubMed]
- Dereje, D.M.; Pontremoli, C.; García, A.; Galliano, S.; Colilla, M.; González, B.; Vallet-Regí, M.; Izquierdo-Barba, I.; Barbero, N. Poly Lactic-co-Glycolic Acid (PLGA) Loaded with a Squaraine Dye as Photosensitizer for Antimicrobial Photodynamic Therapy. Polymers 2024, 16, 1962. [Google Scholar] [CrossRef]
- Serpe, L.; Ellena, S.; Barbero, N.; Foglietta, F.; Prandini, F.; Gallo, M.P.; Levi, R.; Barolo, C.; Canaparo, R.; Visentin, S. Squaraines bearing halogenated moieties as anticancer photosensitizers: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2016, 113, 187–197. [Google Scholar] [CrossRef]
- Bordignon, N.; Köber, M.; Chinigò, G.; Pontremoli, C.; Sansone, E.; Vargas-Nadal, G.; Moran Plata, M.J.; Fiorio Pla, A.; Barbero, N.; Morla-Folch, J.; et al. Quatsomes Loaded with Squaraine Dye as an Effective Photosensitizer for Photodynamic Therapy. Pharmaceutics 2023, 15, 902. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ghitman, J.; Biru, E.I.; Stan, R.; Iovu, H. Review of hybrid PLGA nanoparticles: Future of smart drug delivery and theranostics medicine. Mater. Des. 2020, 193, 108805. [Google Scholar] [CrossRef]
- Ricci-Júnior, E.; Marchetti, J.M. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 2006, 310, 187–195. [Google Scholar] [CrossRef]
- Todaro, B.; Moscardini, A.; Luin, S. Pioglitazone-Loaded PLGA Nanoparticles: Towards the Most Reliable Synthesis Method. Int. J. Mol. Sci. 2022, 23, 2522. [Google Scholar] [CrossRef]
- Sarcan, E.T.; Silindir-Gunay, M.; Ozer, A.Y. Theranostic polymeric nanoparticles for NIR imaging and photodynamic therapy. Int. J. Pharm. 2018, 551, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Agarwal, P.; Zhao, S.; Xu, R.X.; Yu, J.; Lu, X.; He, X. Hyaluronic acid-decorated dual responsive nanoparticles of Pluronic F127, PLGA, and chitosan for targeted co-delivery of doxorubicin and irinotecan to eliminate cancer stem-like cells. Biomaterials 2015, 72, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Simmen, H.-P.; Blaser, J. Analysis of pH and pO2 in abscesses, peritoneal fluid, and drainage fluid in the presence or absence of bacterial infection during and after abdominal surgery. Am. J. Surg. 1993, 166, 24–27. [Google Scholar] [CrossRef]
- Zolnik, B.S.; Burgess, D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Control. Release 2007, 122, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.-l.; Tang, D.-y.; Yan, L.-l.; Deng, L.-l.; Wang, X.-h.; Liu, X.-y.; Zhou, Q.-h. Poly-l-lysine modified MOF nanoparticles with pH/ROS sensitive CIP release and CUR triggered photodynamic therapy against drug-resistant bacterial infection. Int. J. Biol. Macromol. 2024, 266, 131330. [Google Scholar] [CrossRef]
- Cressey, P.; Bronstein, L.-G.; Benmahmoudi, R.; Rosilio, V.; Regeard, C.; Makky, A. Novel liposome-like assemblies composed of phospholipid-porphyrin conjugates with photothermal and photodynamic activities against bacterial biofilms. Int. J. Pharm. 2022, 623, 121915. [Google Scholar] [CrossRef]
- Kassab, G.; Manav, N.; Pires, L.; Cheng, M.H.Y.; Mo, Y.; Buzzá, H.H.; Gupta, I.; Chen, J.; Zheng, G. Structural Effect of Rhenium- and Iridium-Complex Liposome Composition on Their Selectivity for Antimicrobial Photodynamic Therapy. Small Sci. 2024, 4, 2300131. [Google Scholar] [CrossRef]
- Cui, Z.; Li, Y.; Qin, Y.; Li, J.; Shi, L.; Wan, M.; Hu, M.; Chen, Y.; Ji, Y.; Hou, Y.; et al. Polymyxin B-targeted liposomal photosensitizer cures MDR A. baumannii burn infections and accelerates wound healing via M1/M2 macrophage polarization. J. Control. Release 2024, 366, 297–311. [Google Scholar] [CrossRef]
- Wang, Y.; Harrington, O.D.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Dai, T. In Vivo Investigation of Antimicrobial Blue Light Therapy for Multidrug-resistant Acinetobacter baumannii Burn Infections Using Bioluminescence Imaging. J. Vis. Exp. 2017, 122, 54997. [Google Scholar]
- Dai, T.; Tegos, G.P.; Lu, Z.; Huang, L.; Zhiyentayev, T.; Franklin, M.J.; Baer, D.G.; Hamblin, M.R. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob. Agents Chemother. 2009, 53, 3929–3934. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Chu, G.; Jin, J.; Liu, C.; Cheng, L.; Guo, Y.; Deng, Z.; Wang, Y. A Combined Cyanine/Carbomer Gel Enhanced Photodynamic Antimicrobial Activity and Wound Healing. Nanomaterials 2022, 12, 2173. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Rout, L.; Mohanty, N.; Satpathy, A.; Sankar Satapathy, B.; Rath, S.; Gopinath, D. Exploring the photosensitizing potential of Nanoliposome Loaded Improved Toluidine Blue O (NLITBO) Against Streptococcus mutans: An in-vitro feasibility study. PLoS ONE 2024, 19, e0312521. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Wang, D.; Huang, L.; Chen, X.; Wang, C. Multifunctional nanomaterials composed entirely of active pharmaceutical ingredients for synergistically enhanced antitumor and antibacterial effects. Front. Pharmacol. 2024, 15, 1498728. [Google Scholar] [CrossRef]
- Zhao, Z.; Pang, J.; Zhao, D.; Guo, N.; Guo, Y.; Kong, F.; Yang, H.; Zhao, J. Exploring the efficacy of photodynamic antimicrobial chemotherapy on diabetic foot ulcers in rats. J. Biophotonics 2024, 17, e202300568. [Google Scholar] [CrossRef]
- Zheng, J.; Meng, W.; Chen, S.; Cui, Z.; Xian, X.; Tian, J.; Krysko, D.V.; Li, B.; Zhang, W. A near-infrared broad-spectrum antimicrobial nanoplatform powered by bacterial metabolic activity for enhanced antimicrobial photodynamic-immune therapy. Acta Biomater. 2024, 184, 335–351. [Google Scholar] [CrossRef]
- Chen, S.; Xie, J.; Weng, S.; Meng, W.; Zheng, J.; Huang, B.; Zhan, R.; Zhang, W.; Tian, J. A supramolecular photosensitizer for combating multiple antibiotic resistance via photodynamic biofilm dispersion. Chem. Eng. J. 2024, 496, 153951. [Google Scholar] [CrossRef]
- Bu, Q.; Jiang, D.; Yu, Y.; Deng, Y.; Chen, T.; Xu, L. Surface chemistry engineered selenium nanoparticles as bactericidal and immuno-modulating dual-functional agents for combating methicillin-resistant Staphylococcus aureus Infection. Drug Resist. Updates 2024, 76, 101102. [Google Scholar] [CrossRef]
- Chen, W. Antibacterial Photodynamic Therapy Using Copper-Cysteamine Nanoparticles—Google Patents. US12121580B2, 22 October 2024. [Google Scholar]
- Handa, H.; Kumar, A.; Brisbois, E.J. Antibacterial Porphyrin Nanoparticles and Methods for Making and Using the Same—Google Patents. US20240366545A1, 7 November 2024. [Google Scholar]
- Bourke, F.A., Jr.; Fathi, Z.; Walder, H. Non-Invasive Systems and Methods for Selective Activation of Photoreactive Responses—Google Patents. US20230109074A1, 6 April 2023. [Google Scholar]
- Vo-Dinh, T.; Scaffidi, J.P.; Chada, V.G.R.; Lauly, B.; Zhang, Y.; Gregas, M.K.; Stanton, I.N.; Stecher, J.T.; Therien, M.J.; Bourke, F.A., Jr.; et al. Non-Invasive Energy Upconversion Methods and Systems—Google Patents. US9526914B2, 27 December 2016. [Google Scholar]
- Zhang, P.; Wijesiri, N.; Tang, H. Silver Nanoparticle-Enhanced Photosensitizers—Google Patents. US10420346B2, 24 September 2019. [Google Scholar]
- Albrecht, V.; Gitter, B.; Graefe, S.; Wiehe, A.; Epple, M.; Schwiertz, J.; Kathinvel, G. Calcium Phosphate Nanoparticles as Dye Carrier for Photodynamic Therapy. EP2198885B1, 8 February 2012. [Google Scholar]
- Friedman, J.M.; Navati, M.S.; Friedman, A.J.; Nacharaju, P. Therapeutic Nanoparticles and Methods Thereof—Google Patents. US20180055877A1, 1 March 2018. [Google Scholar]
- Decker, E.-M.; von Ohle, C.; Bartha, V. Antimicrobial Photodynamic Therapy—Google Patents. DE102014117532A1, 2 June 2016. [Google Scholar]
- Kert, J. Composition for antimicrobial photodynamic therapy—Google Patents. US20220409729A1, 29 December 2022. [Google Scholar]
- Zhang, H.; Jiang, W.; Peng, Y.; Yang, J.; Chu, X.; Long, Z.; Li, R.; Liang, Q.; Suo, H.; Wang, S.; et al. Killing three birds with one stone: Near-infrared light triggered nitric oxide release for enhanced photodynamic and anti-inflammatory therapy in refractory keratitis. Biomaterials 2022, 286, 121577. [Google Scholar] [CrossRef]
- Sun, J.; Fan, Y.; Ye, W.; Tian, L.; Niu, S.; Ming, W.; Zhao, J.; Ren, L. Near-infrared light triggered photodynamic and nitric oxide synergistic antibacterial nanocomposite membrane. Chem. Eng. J. 2021, 417, 128049. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Liu, W.; Dai, T.; Ke, J.; Li, X.; Li, R.; Zhang, Y.; Chen, Z.; Chen, X. Synergistic Lysozyme-Photodynamic Therapy Against Resistant Bacteria based on an Intelligent Upconversion Nanoplatform. Angew. Chem. Int. Ed. 2021, 60, 19201–19206. [Google Scholar] [CrossRef]
- Zhou, K.; Qiu, X.; Xu, L.; Li, G.; Rao, B.; Guo, B.; Pei, D.; Li, A.; He, G. Poly(selenoviologen)-Assembled Upconversion Nanoparticles for Low-Power Single-NIR Light-Triggered Synergistic Photodynamic and Photothermal Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 26432–26443. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Ren, X.; Li, W.; Sun, Y.; Sun, X.; Li, C.; Yu, S.; Xu, L.; Zhou, Y.; Song, S.; et al. NIR responsive nitric oxide nanogenerator for enhanced biofilm eradication and inflammation immunotherapy against periodontal diseases. Nano Today 2022, 43, 101447. [Google Scholar] [CrossRef]
- Wang, X.; Shi, W.; Jin, Y.; Li, Z.; Deng, T.; Su, T.; Zheng, A.; Cao, L. Photodynamic and photothermal bacteria targeting nanosystems for synergistically combating bacteria and biofilms. J. Nanobiotechnol. 2025, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Yan, H.; Li, P.; Zhang, B.; Zhang, Y.; Su, W. Photo-enhanced antibacterial activity of polydopamine-curcumin nanocomposites with excellent photodynamic and photothermal abilities. Photodiagn. Photodyn. Ther. 2021, 35, 102417. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, B.; Zhang, Y.; Su, R.; Li, P.; Su, W. Fluorescent Carbon Dot-Curcumin Nanocomposites for Remarkable Antibacterial Activity with Synergistic Photodynamic and Photothermal Abilities. ACS Appl. Bio Mater. 2021, 4, 6703–6718. [Google Scholar] [CrossRef]
- Vijeata, A.; Chaudhary, G.R.; Chaudhary, S. Photosensitizer conjugated carbon dots@dopamine-copper sulfide nanocomposites: Advanced photoactive agents for antibacterial applications through experimental and theoretical insights. J. Environ. Chem. Eng. 2024, 12, 114406. [Google Scholar] [CrossRef]
- Shen, J.; Li, L.; Kang, X.; Lin, D.; Xiao, Y.; Yang, L.; Jiang, C. Multilayered Upconversion Nanocomposite-Based Photodynamic Hydrogel Dressings for Wound Sterilizing and Healing. ACS Appl. Nano Mater. 2023, 6, 12726–12735. [Google Scholar] [CrossRef]
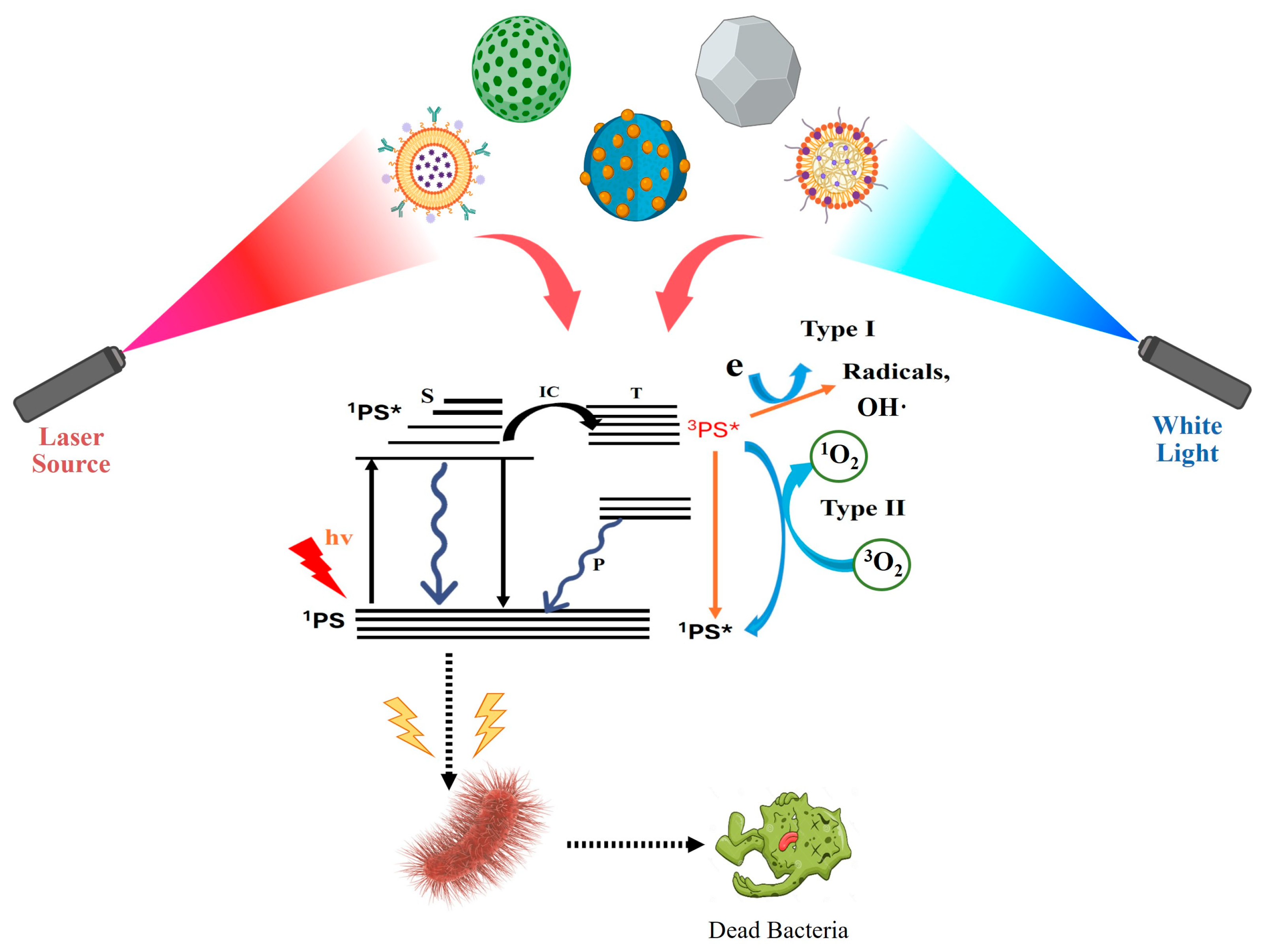
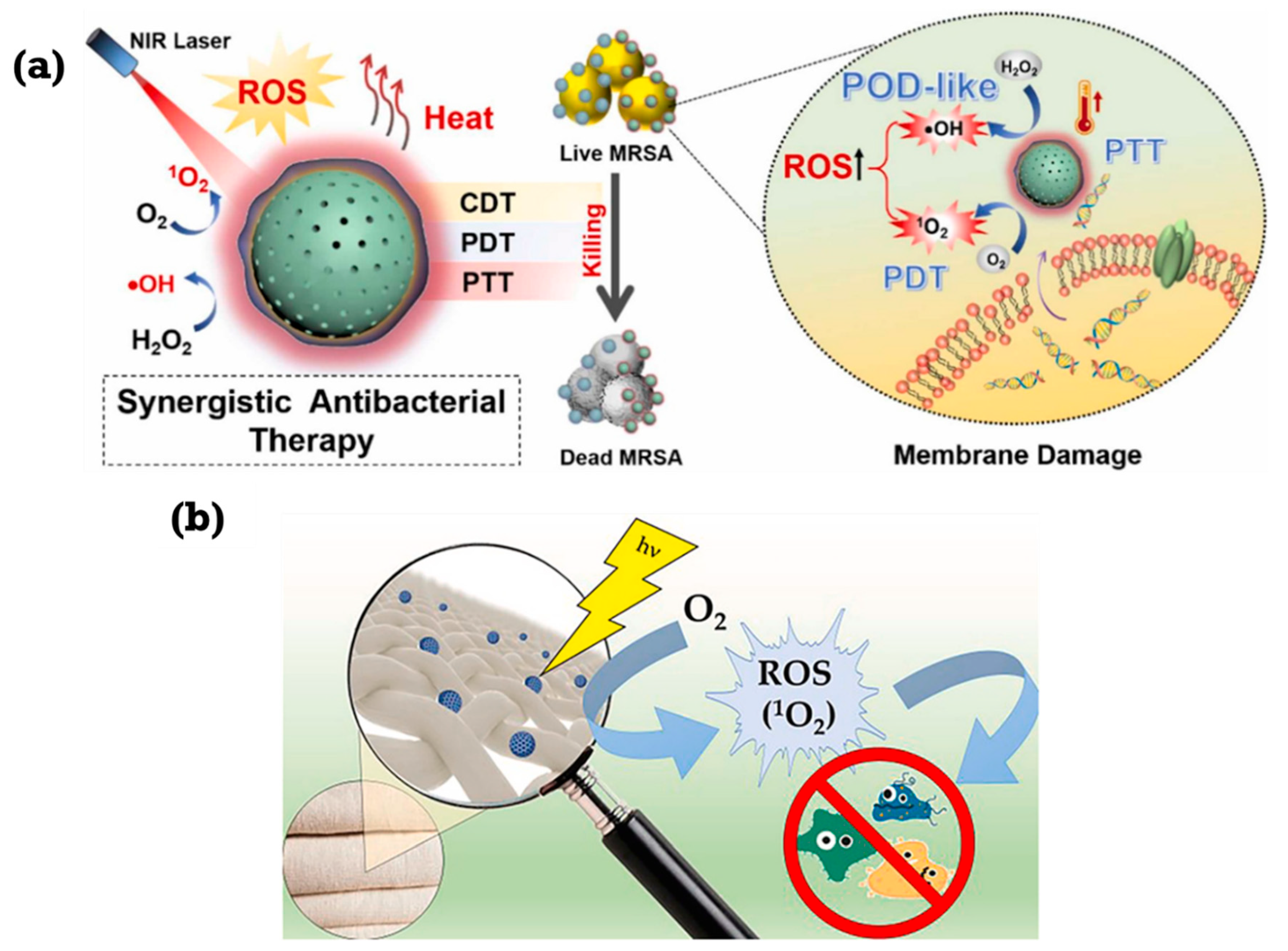
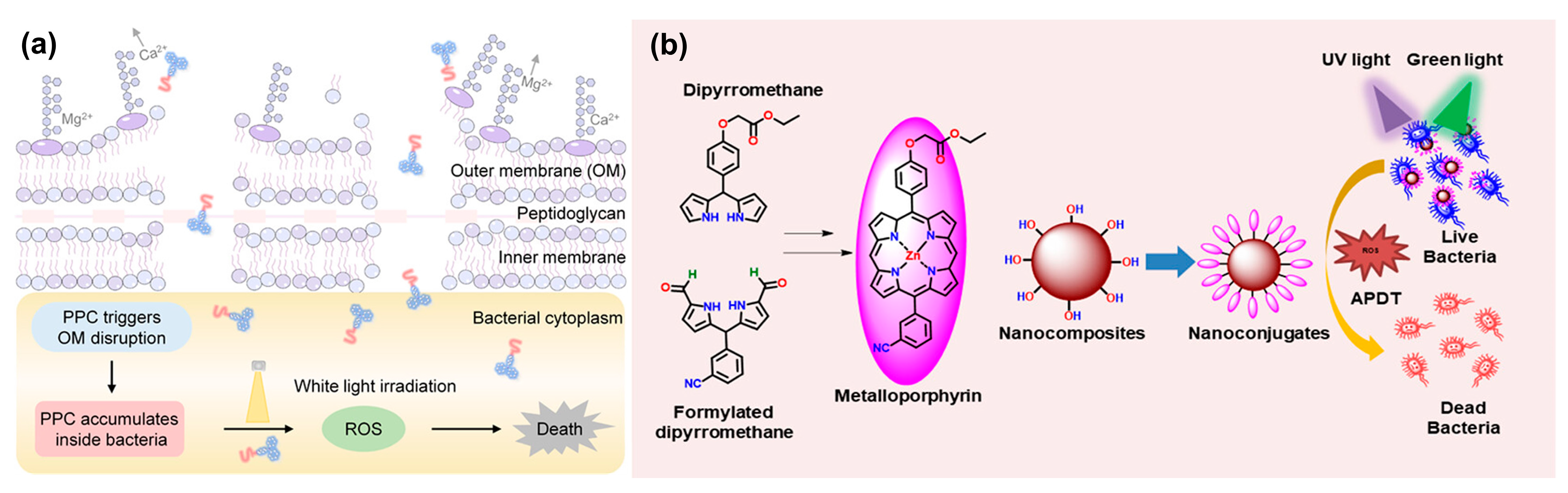
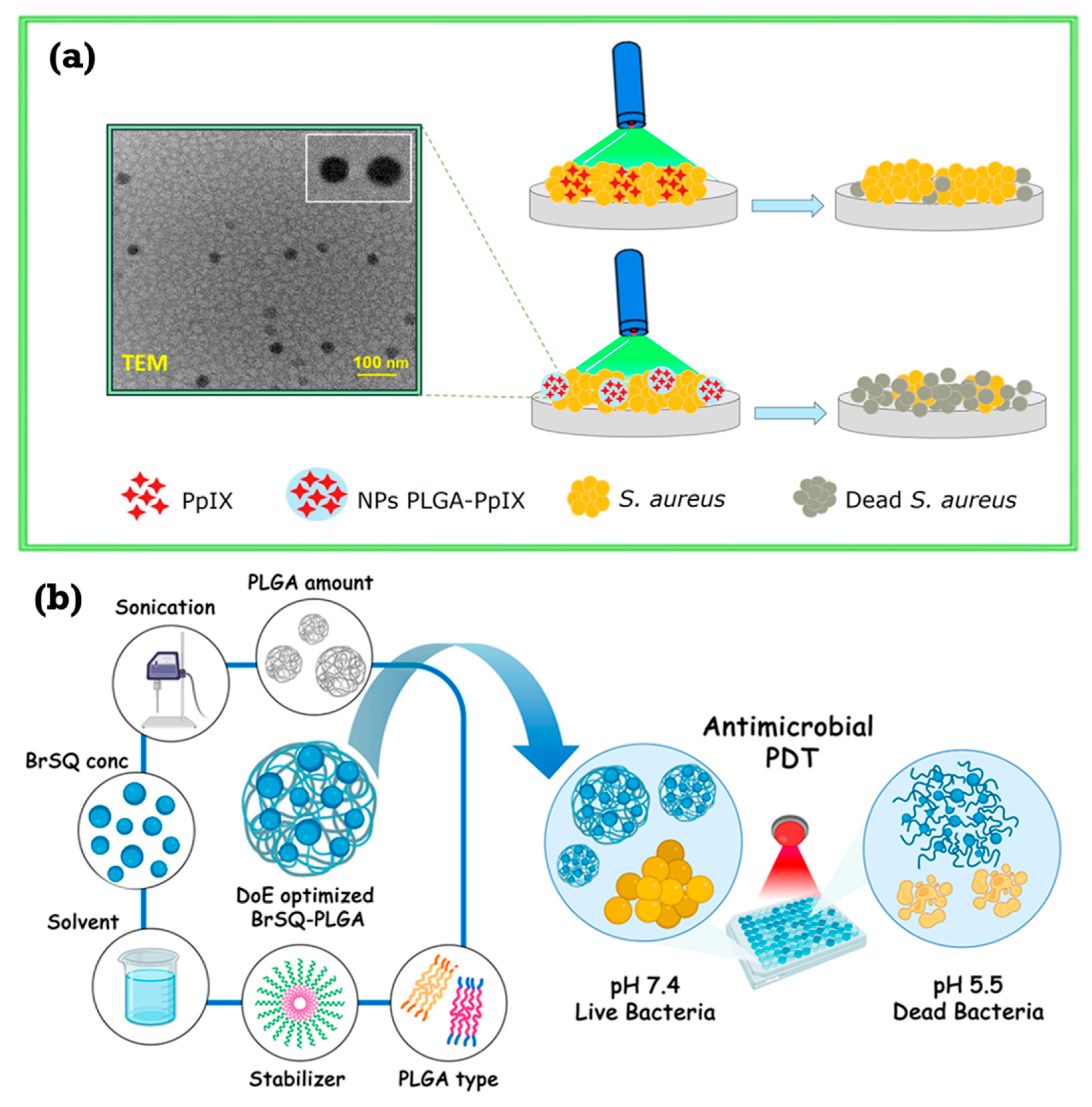
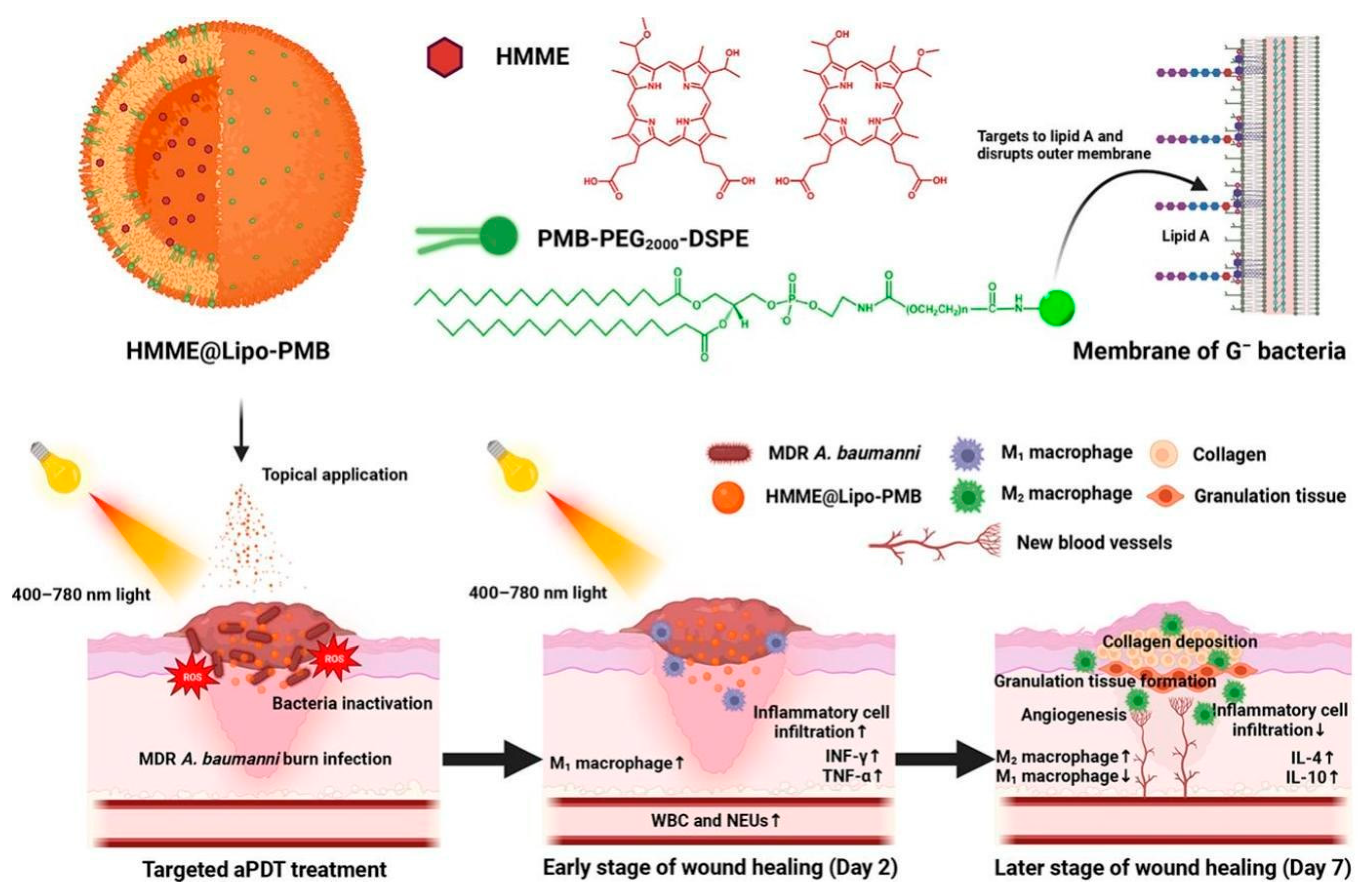
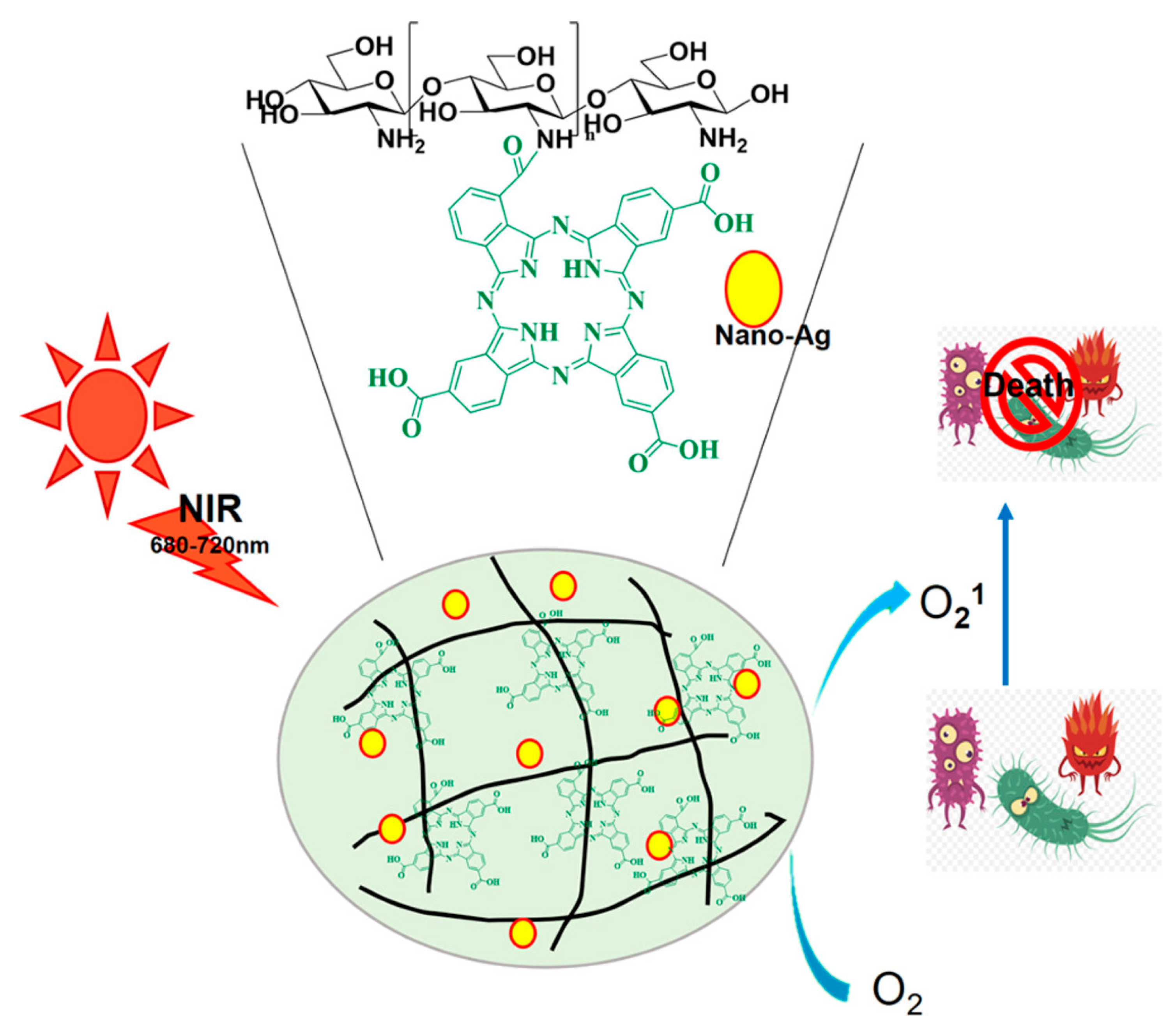
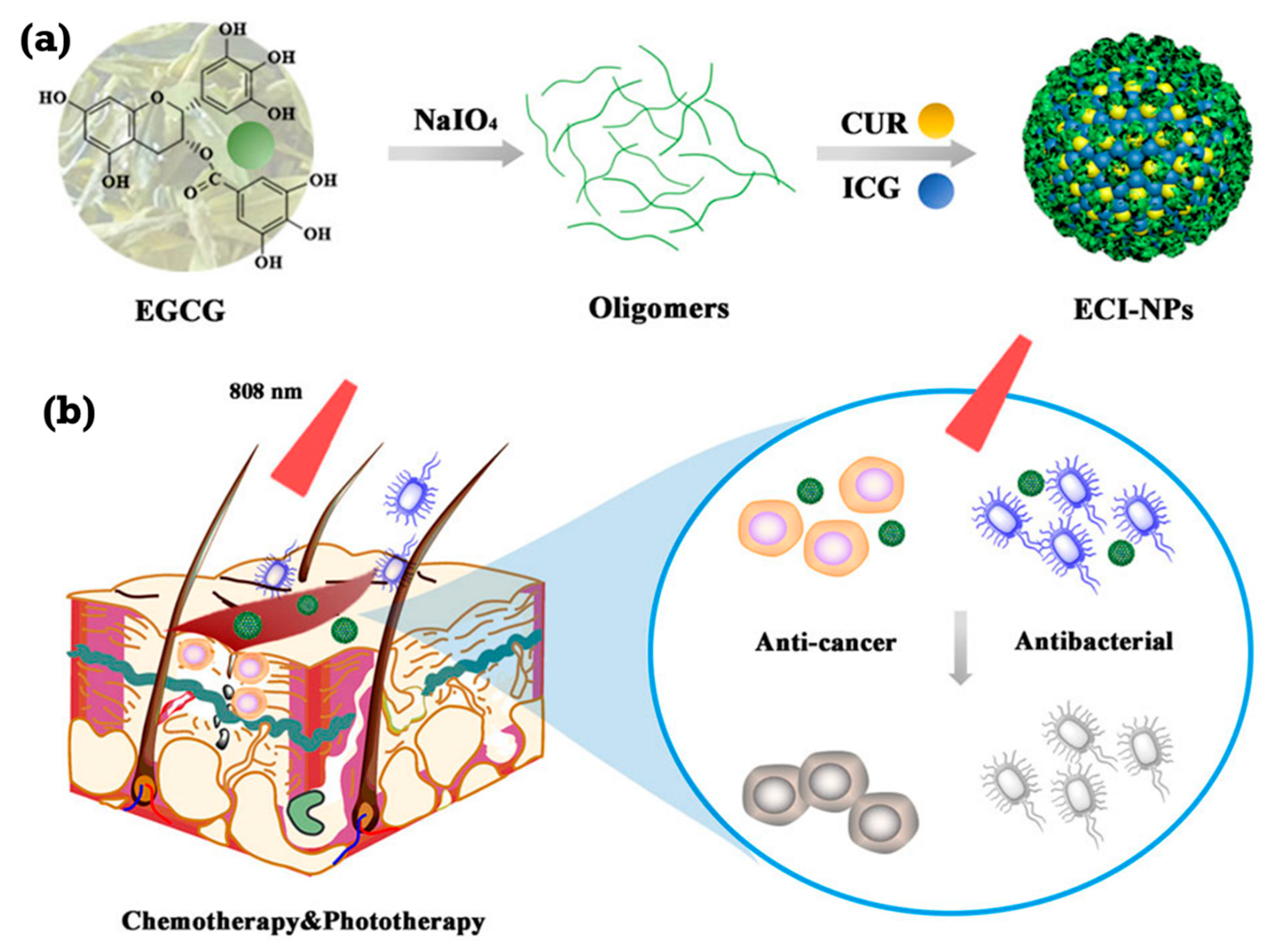
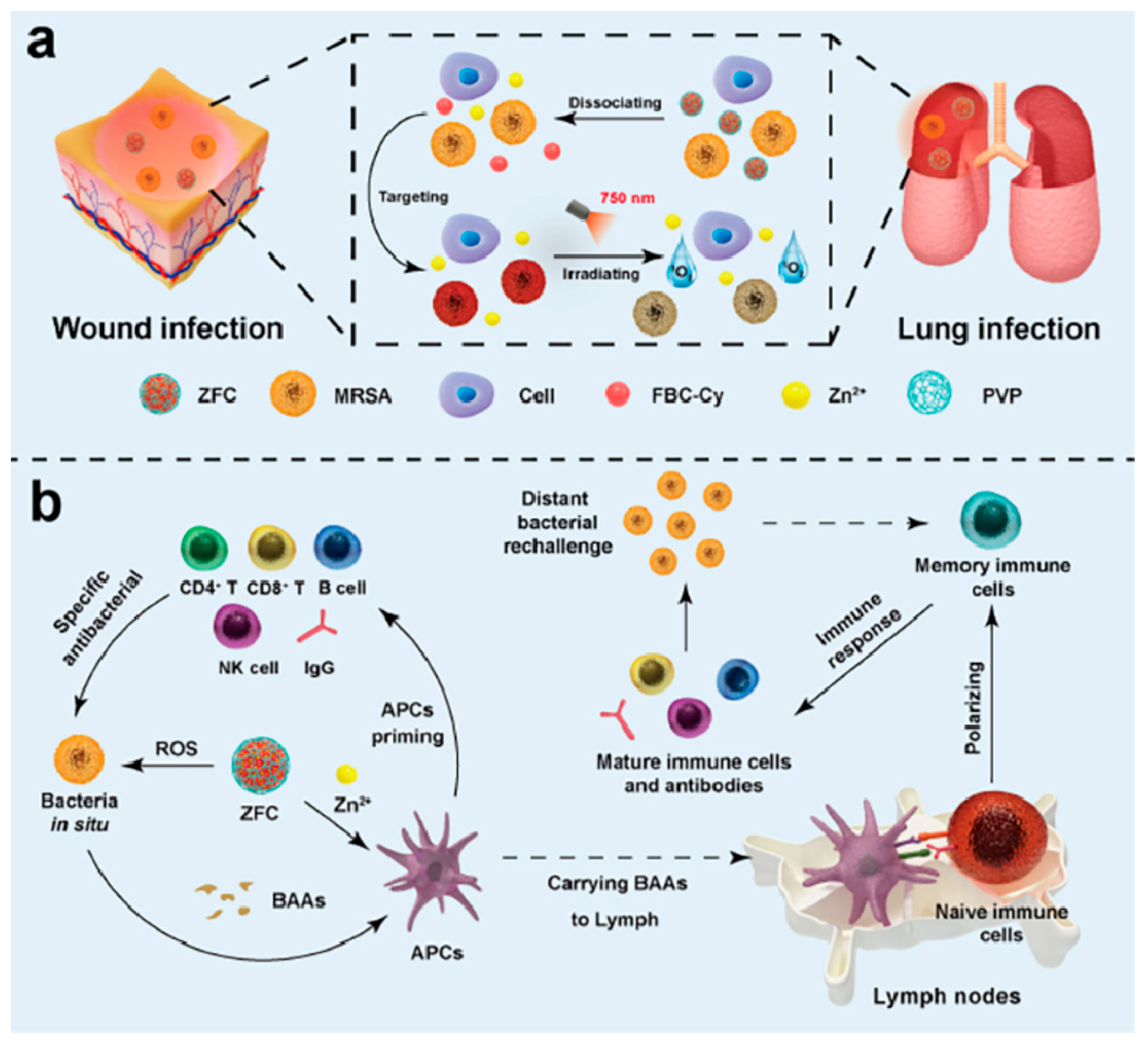
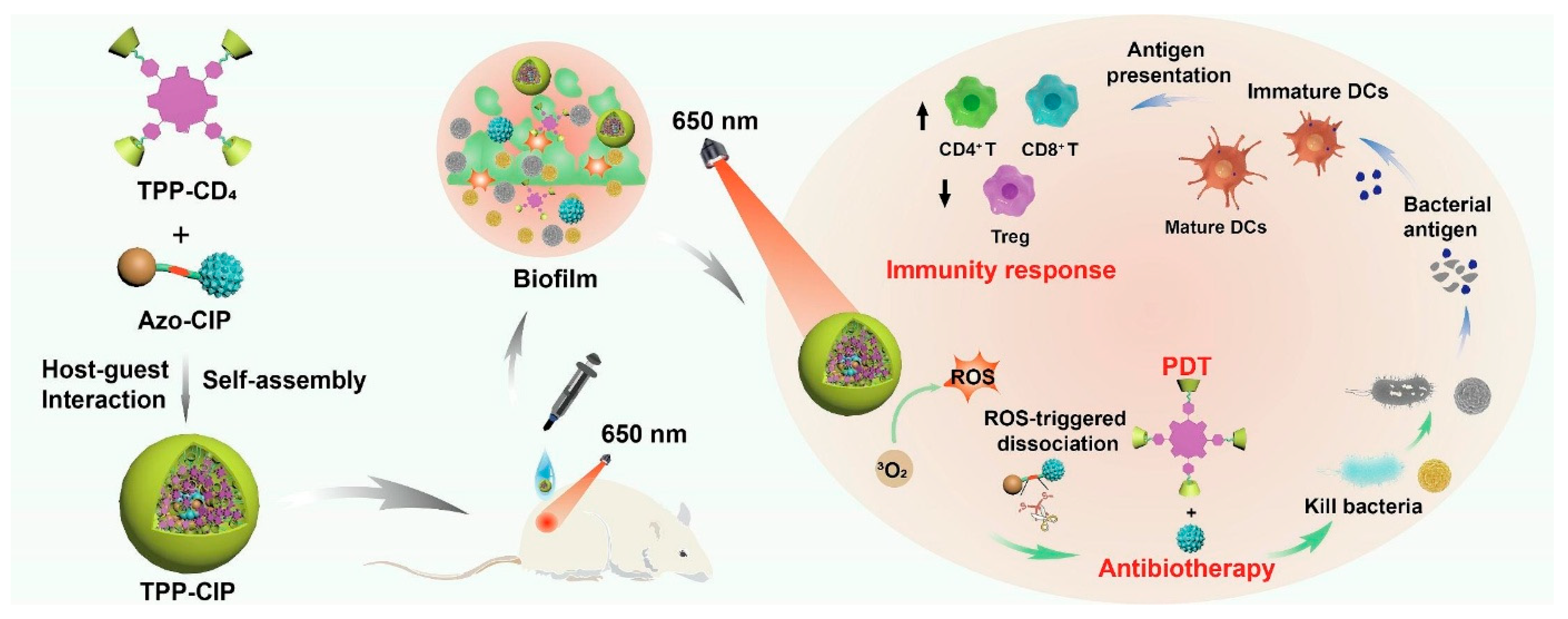
| Type | ROS | Generation Mechanisms | Antibacterial Mechanisms |
|---|---|---|---|
| Type II | 1O2 | Generated via energy transfer from excited photosensitizers or enzymatic oxidation (e.g., flavin-containing oxidases). | Induces oxidative damage to nucleic acids (purine oxidation), lipids (peroxidation of unsaturated fatty acids), and proteins (oxidation of amino acid residues). |
| Type I | OH· | Produced via Fenton/Haber-Weiss reactions, water radiolysis, or photocatalytic oxidation. | Highly reactive, causing DNA strand breaks, lipid peroxidation, and protein inactivation via amino acid oxidation. |
| O2− | Formed by single-electron reduction of O2 via NADPH oxidase, mitochondrial respiration, or redox cycling. | Oxidizes thiol groups, disrupts Fe-S cluster enzymes, and propagates lipid peroxidation. | |
| H2O2 | Generated via superoxide dismutation, oxidase-catalyzed reactions, or metabolic oxidative bursts. | Permeates bacterial membranes, oxidizes proteins, disrupts redox homeostasis, and generates ·OH via Fenton reactions. |
| Patent Number | Title | Assignee(s) | Inventor(s) | Publication Date | Ref. |
|---|---|---|---|---|---|
| US-12121580-B2 | Antibacterial photodynamic therapy using copper-cysteamine nanoparticles | Board Of Regents, the University of Texas System | Wei Chen | 22 October 2024 | [131] |
| US-2024366545-A1 | Antibacterial porphyrin nanoparticles and methods for making and using the same | University of Georgia Research Foundation, Inc. | Hitesh Handa, Anil Kumar, Elizabeth J. Brisbois | 7 November 2024 | [132] |
| US-2023109074-A1 | Non-invasive systems and methods for selective activation of photoreactive responses | Immunolight, Llc | Frederic A. Bourke, Jr., Zakaryae Fathi, Harold Walder | 6 April 2023 | [133] |
| US-9526914-B2 | Non-invasive energy upconversion methods and systems | Duke University, Immunolight, Llc | Tuan Vo-Dinh, Jonathan P. SCAFFIDI, Venkata Gopal Reddy | 27 December 2016 | [134] |
| US-10420346-B2 | Silver nanoparticle-enhanced photosensitizers | University of Cincinnati | Peng Zhang, Niranga Wijesiri, Hong Tang | 24 September 2019 | [135] |
| EP-2198885-B1 | Calcium phosphate nanoparticles as dye carrier for photodynamic therapy | Biolitec AG, Universität Duisburg-Essen | Volker Dr. Prof. Albrecht, Burkhard Dr. Gitter, Susanna | 8 February 2012 | [136] |
| US-2018055877-A1 | Therapeutic nanoparticles and methods thereof | Albert Einstein College of Medicine, Inc. | Joel M. Friedman, Mahantesh S. Navati, Adam J. Friedman, Parimala Nacharaju | 1 March 2018 | [137] |
| US20090326434A1 | Anti-Microbial Photodynamic Therapy | n/a | Nikolay E. Nifantiev, et al. | 22 December 2015 | [18] |
| DE102014117532A1 | Antimicrobial Photodynamic Therapy | n/a | Eva-Maria Decker, et al. | 2 June 2016 | [138] |
| US20220409729A1 | Composition for Antimicrobial Photodynamic Therapy | n/a | Jimmie Kert | 29 December 2022 | [139] |
| Nanoparticle | Advantages | Limitations | Applications in Photodynamic Antibacterial Therapy |
|---|---|---|---|
| Upconverting Nanoparticles (UCNPs) | NIR excitation for deep tissue penetration. Reduced photodamage to healthy tissue. | Low photothermal efficiency. Complex synthesis. Potential toxicity from rare-earth elements. | Used for deep tissue PDT, enhancing bacterial eradication in hard-to-reach infections via NIR excitation. |
| Carbon Dots (CDs) | High biocompatibility and low toxicity. Superior fluorescence and functionalization. | Moderate photodynamic efficiency. Stability issues. Scaling challenges. | Applied in antibacterial PDT, often functionalized for targeted therapy against specific bacterial strains. |
| Mesoporous Silica Nanoparticles (MSNs) | High surface area for drug loading. Biocompatible and biodegradable. | Limited photodynamic activity. Slow biodegradation. | Used as carriers for photosensitizers in PDT, enhancing drug delivery and multi-functional antibacterial therapy. |
| Polymer-Based Nanoparticles | Biodegradable, flexible design. High drug loading capacity. | Stability concerns. Costly synthesis. | Common in drug delivery systems for PDT, enabling targeted antibacterial treatment. |
| Liposomes | Biocompatible and biodegradable. Can encapsulate both hydrophilic and hydrophobic agents. | Expensive production. Premature leakage of encapsulated agents. | Used to encapsulate photosensitizers for efficient bacterial targeting and controlled release in PDT. |
| Nanoparticle | Size (nm) | Photosensitizer | Synthesis Method | Design Strategy | In Vivo Model | Target Microorganism(s) | Laser Strength | Inhibition Values (%) | Novelty | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | UCNP@SiO2 | Hexagonal-phased (64.04 ± 1.81 nm) | TPE-Ph-DCM | Thermal decomposition | NaYF4:Yb3+, Tm3+@NaYF4:Nd3+, Yb3 doped with Mesoporous Silica. | SD rat cornea (Refractory keratitis) | S. aureus P. aeruginosa | 808 nm at 0.4 W/cm−2 for 30 min | 99% for both P. aeruginosa and S. aureus | Performed bacterial eradication with dual action of nitric oxide release and anti-inflammation. | [140] |
| 2 | UCNP@PCN@LA-PVDF | Hierarchical structure (130 nm) | Not Available | Electrospinning | NaYF4:Yb,Er nanorods doped with PCN-224 (MOF) in polyvinylidene fluoride. | Subcutaneous BALB/c mice model | P. aeruginosa S. aureus | 980 nm at 0.5 W/cm−2 for 5 min | 99.64% and 99.63% for P. aeruginosa and S. aureus | Nitric Oxide-assisted PDT. | [141] |
| 3 | UCMB-LYZ-HP | Crystalline hexanal 239 nm | Methylene blue | Solid liquid-thermal decomposition and silica sol–gel reaction | β-NaYF 4:Yb,Er@NaYF4 was doped with mesoporous silica and Lyzosome. | Murine Model | MRSA | 980 nm, 0.5 W.cm−2, 10 min | 5.2 log10 reduction in MRSA viability | Utilized lysozyme as an advanced antibacterial agent. | [142] |
| 4 | UCNPs/PSeV | hexagonal-phase 67 nm | Poly(selenoviologen) (PSeV) | Thermal decomposition-based self-assembly | PSeV was synthesized and further self-assembled with core–shell NaYF4:Yb/Tm@NaYF4. | MRSA-based Inflammation mouse model. | MRSA | 980 nm, 0.150 W/cm−2, 4 min | 98% CFU reduction | Synergistic PDT/PTT, accounted for a single NIR treatment. | [143] |
| 5 | UCNPs-Ce6-Mn(CO)5Br@Silane (UCM@Si) | Hexagonal Phase 78 ± 2 nm | Chlorin e6 (Ce6) | Solvothermal synthesis | NaErF4:Tm3+@NaYF4:Yb3+, was first synthesized, which was further doped with Ce6 and Silane. | Subcutaneous abscess mouse model | S. aureus, E. coli | 980 nm, 1 W·cm−2, 10 min | 80% inhibition of E. coli and S. aureus | Synergistic (CO) mediated inflammation regulation and ROS production. | [37] |
| 6 | UCNP@mSiO2 | Monodisperse orthohexagonal | Erythrosine (UCSE) | Solvothermal synthesis | NaYF4:Yb,Er@NaYF4 was first synthesized, which was further doped with erythrosine. | No in vivo studies | S. aureus E. coli | 808 nm, 1 W/cm2, for 10 min | 97.41 and 97.69% inhibition of E. coli and S. aureus | Erythrosine aided the photosensitization and ROS production. | [36] |
| 7 | CS/N-CDs | (2.34 nm–5.88 nm), diameter (3.80 ± 0.73 nm). | Nitrogen-doped carbon dots (N-CDs) | Solvent casting method, compounding N-CDs with chitosan (CS) | N-CDs were synthesized by adding different concentrations of Nitrogen to chitosan films. | No in vivo studies | E. coli S. aureus | White light for 6 h. | 91.2% and 99.9% for E. coli and S. aureus | Enhanced antibacterial activity under UV-A light exposure; potential application in food packaging. | [19] |
| 8 | Cu-RCDs-C35 | 4.2 nm, graphene-like structure | (N-Cu-N) complex in Cu-RCDs-C35 | Acid amine coupling of carbon dots and betaine | RCDs were first synthesized, followed by the Carbodiimide crosslinking with CAB-35. | Female Balb/c based (S. aureus) wound healing model. | E. coli S. aureus | 808 nm irradiation (2.0 W/cm2, 5 min) | 99.36% and 99.98%, for E. coli and S. aureus. 96% wound healing ratio | Triple synergistic sterilization combining QACs, PTT, and PDT under a single NIR light source | [20] |
| 9 | CDs/Cur | 3 nm, spherical and uniform distribution | N, S-doped Carbon dots with curcumin | Hydrothermal route followed by dialysis-based purification | N, S-doped Carbon dots were first synthesized by the hydrothermal route, followed by the attachment of curcumin. | No in vivo studies | E. coli S. aureus | 808 nm; 500 mW/cm2, 30 min | 100% inhibition of E. coli for 1 μM CD/Cur | Dual PDT and PTT irradiation with fluorescent tracking and ROS effects. | [147] |
| 10 | CDs@Dop-CuS | Spherical, carbon dots of average particle size (6 nm) | Methylene blue | Microwave-assisted green synthesis, followed by solvothermal synthesis | Microwave irradiation facilitates rapid co-precipitation, yielding dopamine-functionalized copper sulfide nanoparticles, offering enhanced surface properties for potential applications. | No in vivo studies | E. coli S. aureus | laser diode (660 nm) and 808 nm 1.0 W/cm2 | Enhanced singlet oxygen production, i.e., 79% | Synergistic photothermal and photodynamic effects; molecular docking-based theoretical insights provided. | [148] |
| 11 | GNRs@mSiO2-SNO | Cylindrical rods with an average size of 13.5 nm | Indocyanine green | Seed-mediated growth followed surface functionalisation and nitrosation. | Gold nanorods were synthesized, silica-coated for stability, functionalized with thiols, nitrosated for nitric oxide release, and drug-loaded for therapy. | Wistar rats based Periodontal inflammation model | P. gingivalis S. aureus | 808 nm, 1 W cm−2, 5 min | 4-log CFU reduction in biofilms | Triple-functional NO nanogenerator enabling synergistic aPDT/PTT/gas therapy for biofilm eradication. | [144] |
| 12 | UCNPs@mSiO2 (ZnPc) | High-temperature coprecipitation method | zinc phthalocyanine | Solvothermal and Stöber methods | Core–shell UCNPs@mSiO2 were synthesized via solvothermal and Stöber methods, then photosensitizer-loaded through adsorption. | No in vivo studies | S. aureus E. coli | 980 nm, 1 W cm−2, 5 min | S. aureus and E. coli survival rates were 5.8% and 10.1%, respectively. | Combines chitosan for antibacterial, photodynamic wound healing therapy. | [149] |
| 13 | Ce6@ZIF-8-PDA/UBI | polyhedral shaped (99.9 ± 18.8 nm) | Chlorin e6 (Ce6) | Solvothermal method | Chlorin e6 (Ce6) was first absorbed on the surface of the ZIF structure, leading to the attachment of the PDA and UBI. | Rat femur peri-implantitis model [male SD rats (8 weeks old)] | S. aureus E. coli F. nucleatum P. gingivalis | 660 nm, 5 min, 1.3 W/cm2 | Significant decrese in 71.1% ± 0.9% (F. nucleatum) and 73.0% ± 2.0% (P. gingivalis) while | Synergistic photothermal and photodynamic effects with advanced photosensitizers like chlorin e6. | [145] |
| 14 | PDA-Cur (polydopamine-curcumin) | cluster-like structures (376 nm) | Curcumin (Cur) | Oxidative polymerization of dopamine followed by the adsorption of Curcumin | Cur adsorbed onto PDA through π-π stacking interactions and hydrogen bonding to form stable PDA-Cur complexes. | No in vivo studies | E. coli S. aureus | 405 nm, 10 s, 0.5 mW/cm2 | 100% (E. coli and S. aureus) | Dual PDT and PTT irradiation with Cur-based fluorescent tracking and ROS effects. | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivedita; Sharma, S.; Krisnawati, D.I.; Cheng, T.-M.; Kuo, T.-R. Recent Advances in Nanoparticle-Mediated Antibacterial Photodynamic Therapy. Int. J. Mol. Sci. 2025, 26, 10949. https://doi.org/10.3390/ijms262210949
Nivedita, Sharma S, Krisnawati DI, Cheng T-M, Kuo T-R. Recent Advances in Nanoparticle-Mediated Antibacterial Photodynamic Therapy. International Journal of Molecular Sciences. 2025; 26(22):10949. https://doi.org/10.3390/ijms262210949
Chicago/Turabian StyleNivedita, Shashwat Sharma, Dyah Ika Krisnawati, Tsai-Mu Cheng, and Tsung-Rong Kuo. 2025. "Recent Advances in Nanoparticle-Mediated Antibacterial Photodynamic Therapy" International Journal of Molecular Sciences 26, no. 22: 10949. https://doi.org/10.3390/ijms262210949
APA StyleNivedita, Sharma, S., Krisnawati, D. I., Cheng, T.-M., & Kuo, T.-R. (2025). Recent Advances in Nanoparticle-Mediated Antibacterial Photodynamic Therapy. International Journal of Molecular Sciences, 26(22), 10949. https://doi.org/10.3390/ijms262210949







