Advance in Managing Indoor Cat Allergen Proteins: Molecular Insights, Detection, and Control
Abstract
1. Introduction
2. Allergens from Cats
2.1. Molecular Insights of the Major Cat Allergen Fel d 1
2.2. Other Cat Allergens and Their Cross-Reactivity
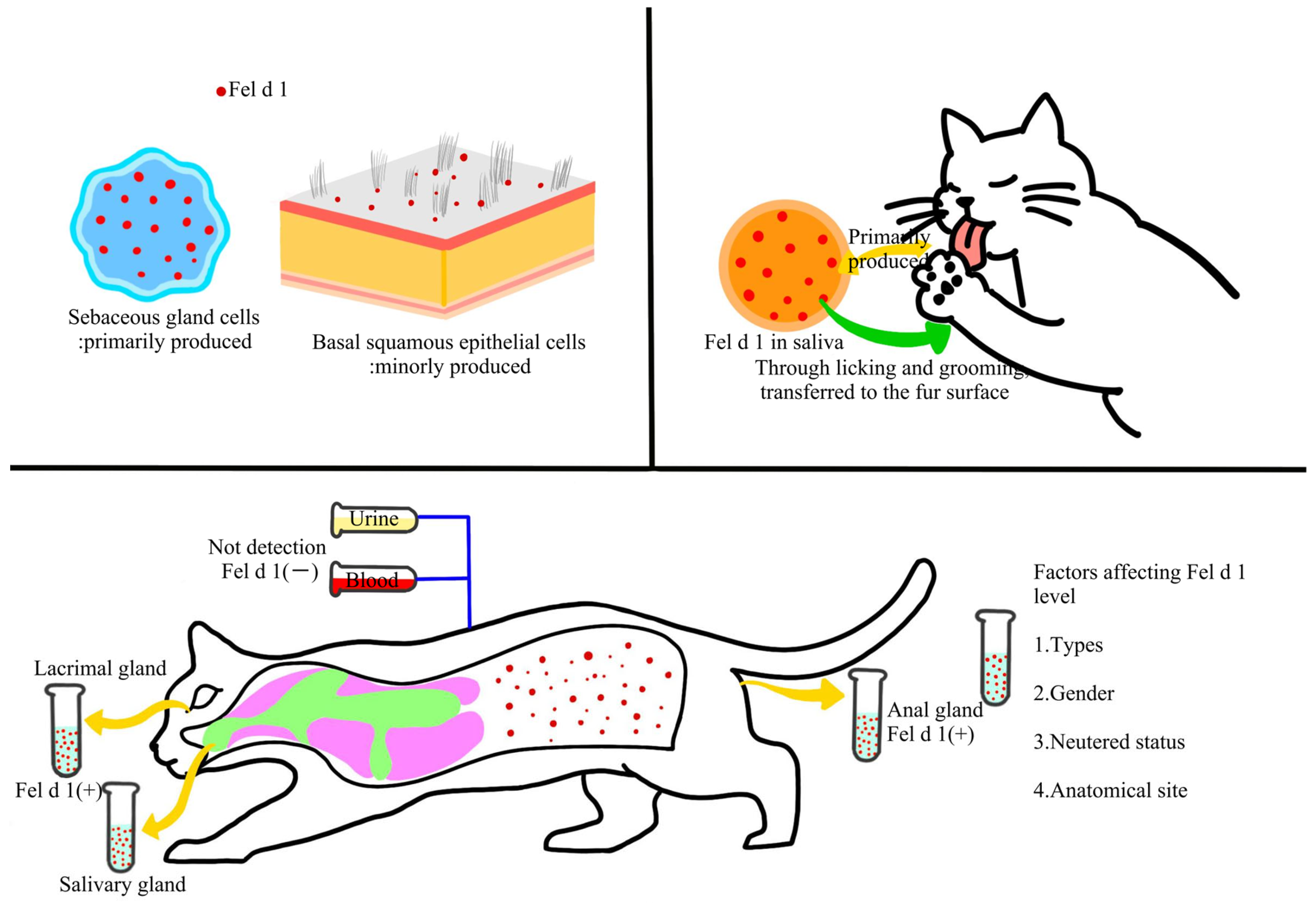
2.3. Distribution and Transmission of Cat Allergens in Indoor Environments
2.4. Exposure Routes of Human to Cat Allergens and Sensitization Mechanisms
3. Indoor Cat Allergen Testing Methods
3.1. Immunological Assay
3.2. High-Throughput Technologies for the Multiplex Detection
3.3. Real-Time Monitoring and Emerging Immunosensing Technologies
4. Factors Influencing Indoor Cat Allergen Concentration
4.1. Cat-Related Factors
4.2. Environmental and Architectural Factors
4.3. Human Activity Factors
4.4. Spatiotemporal Variation Patterns
5. Methods for Preventing and Managing Allergies
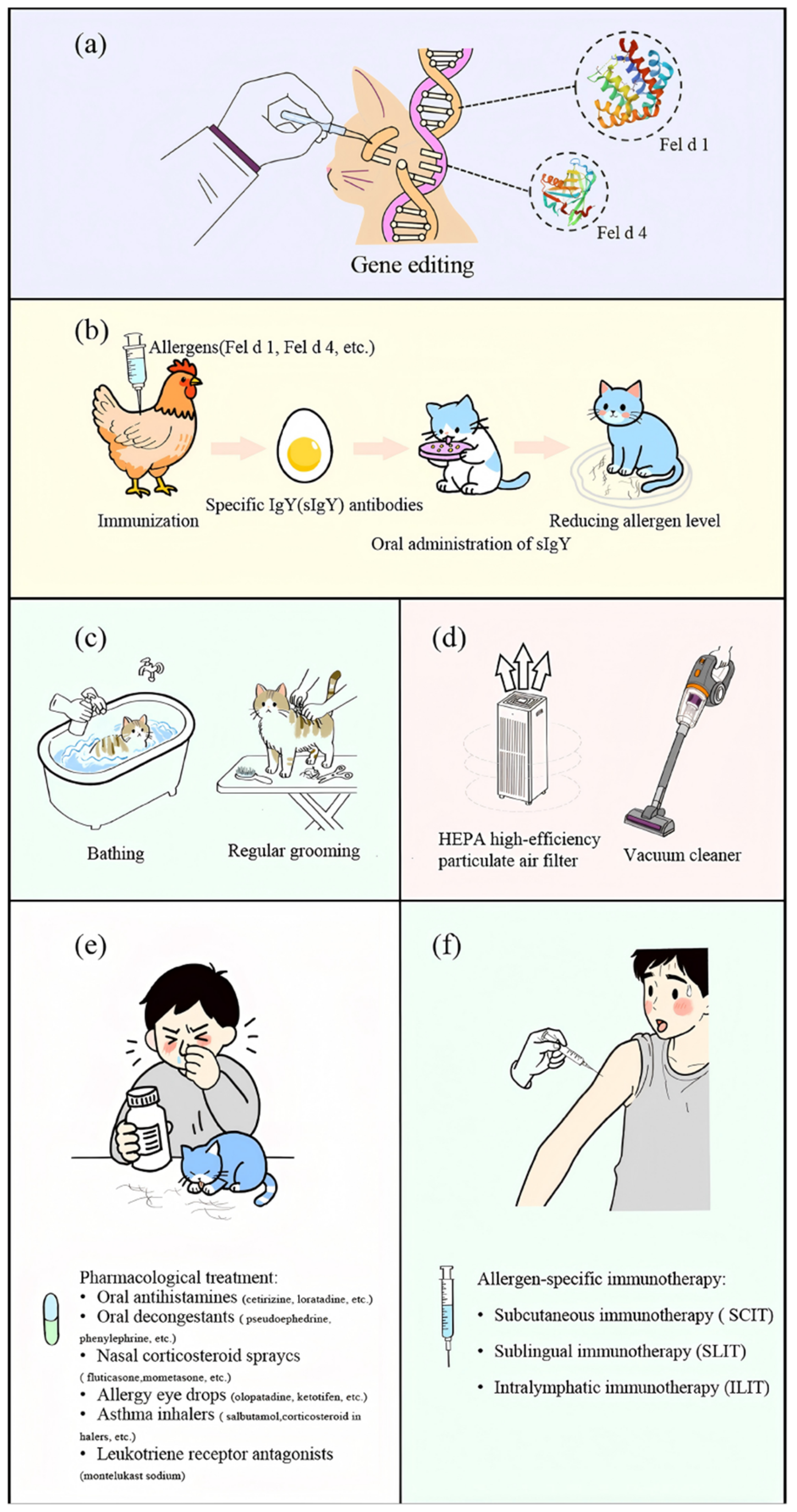
5.1. Source Control of Allergens
5.1.1. Gene Editing
5.1.2. Immunological Methods
5.1.3. Physical Methods
5.2. Environmental Interventions
5.3. Personal Protection and Medical Intervention
5.3.1. Chemical Drug Therapy
5.3.2. Immunotherapy
6. Prospects for Future Managing Indoor Cat Allergens
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| IUIS | International Union of Immunological Societies |
| CRD | component-resolved diagnosis |
| IL-4 | interleukin-4 |
| IL-5 | interleukin-5 |
| IL-9 | interleukin-9 |
| IL-13 | interleukin-13 |
| ELISA | the enzyme-linked immunosorbent assay |
| CARD | catalytic reported deposition |
| qPCR | quantitative PCR |
| EDC | electrostatic dust collectors |
| HPLC-MS/MS | high-performance liquid chromatography-tandem mass spectrometry |
| QCM | Quartz Crystal Microbalance |
| SAW | Surface Acoustic Wave |
| LPC | Laser Particle Counters |
| OPC | Optical Particle Counters |
| IoT | Internet of Things |
| VC | Vacuum cleaners |
| IgY | Immunoglobulin Y |
| BLG | β-lactoglobulin |
| AHR | aryl hydrocarbon receptor |
| HEPA | High-efficiency particulate air |
| PECO | photo-electrochemical oxidation |
| ASIT | allergen-specific immunotherapy |
| SCIT | encompassing subcutaneous immunotherapy |
| SLIT | sublingual immunotherapy |
| ILIT | intralymphatic immunotherapy |
| AI | Artificial intelligence |
References
- Dramburg, S.; Hilger, C.; Santos, F.A.; Vecillas, L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI molecular allergology user’s guide 2.0. Egypt. J. Pediatr. Allergy 2023, 34, e13854. [Google Scholar] [CrossRef]
- Holgate, S.T.; Polosa, R. Treatment strategies for allergy and asthma. Nat. Rev. Immunol. 2008, 8, 218–230. [Google Scholar] [CrossRef]
- Kay, A.B. Allergy and allergic diseases. N. Engl. J. Med. 2001, 344, 30–37. [Google Scholar] [CrossRef]
- Asher, M.I.; Montefort, S.; Björkstén, B.; Lai, C.K.W.; Strachan, D.M.; Weiland, S.K. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, E.; Yoon, D.; Lee, J.K.; Chang, W.-S.; Lim, Y.-M.; Park, J.-W.; Lee, J.-S. Prevalence of self-reported allergic diseases and IgE levels: A 2010 KNHANES analysis. Allergy Asthma Immunol. 2017, 9, 329. [Google Scholar] [CrossRef]
- Edwards-Salmon, S.E.; Padmanabhan, S.L.; Kuruvilla, M.; Levy, J.M. Increasing prevalence of allergic disease and its impact on current practice. Curr. Otorhinolaryngol. Rep. 2022, 10, 278–284. [Google Scholar] [CrossRef]
- Merhej, T.; Zein, J.G. Epidemiology of asthma: Prevalence and burden of disease. Adv. Exp. Med. Biol. 2023, 1426, 3–23. [Google Scholar] [PubMed]
- GBD 2021 Asthma and Allergic Diseases Collaborators. Global, regional, and national burden of asthma and atopic dermatitis, 1990–2021, and projections to 2050: A systematic analysis of the global burden of disease study 2021. Lancet Respir. Med. 2025, 13, 425–446. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Hwang, J.; Kwon, R.; Lee, S.W.; Kim, M.S.; GBD 2019 Allergic Disorders Collaborators; Shin, J.I.; Yon, D.K. Global, regional, and national burden of allergic disorders and their risk factors in 204 countries and territories, from 1990 to 2019: A systematic analysis for the global burden of disease study 2019. Allergy 2023, 78, 2232–2254. [Google Scholar] [CrossRef]
- Melén, E.; Garcia-Aymerich, J. Predicting the future global burden of asthma and atopic dermatitis: Identifying strategies for prevention. Lancet Respir. Med. 2025, 13, 376–378. [Google Scholar] [CrossRef]
- Takkouche, B.; González-Barcala, F.-J.; Etminan, M.; FitzGerald, M. Exposure to furry pets and the risk of asthma and allergic rhinitis: A meta-analysis. Allergy 2008, 63, 857–864. [Google Scholar] [CrossRef]
- Chan, S.K.; Leung, D.Y. Dog and cat allergies: Current state of diagnostic approaches and challenges. Allergy Asthma Immunol. 2018, 10, 97. [Google Scholar] [CrossRef]
- Yin, W.; Zeng, X.L.; Du, W.J.; Yang, L.; Ye, X.; Jiang, Q.; Huang, N.; Li, W.J.; Yang, Y.Q.; Ma, D.X.; et al. Sensitization profiles of aeroallergens among allergic rhinitis patients in China: A 13-year multicenter retrospective study. Allergy 2023, 79, 1329–1332. [Google Scholar] [CrossRef]
- Schoos, A.M.; Nwaru, B.I.; Borres, M.P. Component-resolved diagnostics in pet allergy: Current perspectives and future directions. J. Allergy Clin. Immunol. 2021, 147, 1164–1173. [Google Scholar] [CrossRef]
- Liang, H.; Ouyang, Z.; Liu, J.; Zhu, G. The development trend analysis of pet industry. In Proceedings of the 2025 7th International Conference on Software Engineering and Computer Science (CSECS), Taicang, China, 21–23 March 2025. [Google Scholar]
- Shen, R.; Shen, F.; Zhao, Y. Pet industry analysis based on multiple linear regression and aeima models. In Proceedings of the 2025 IEEE 3rd International Conference on Image Processing and Computer Applications (ICIPCA), Shenyang, China, 28–30 June 2025. [Google Scholar]
- Zahradnik, E.; Raulf, M. Animal allergens and their presence in the environment. Front. Immunol. 2014, 5, 76. [Google Scholar] [CrossRef]
- Konradsen, J.R.; Fujisawa, T.; Van Hage, M.; Hedin, G.; Hilger, C.; Kleine-Tebbe, J.; Matsui, E.C.; Roberts, G.; Ronnmark, E.; Platts-Mills, T.A.E. Allergy to furry animals: New insights, diagnostic approaches, and challenges. Allergy Clin. Immunol. 2015, 135, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Arbes Jr, S.J.; Cohn, R.D.; Yin, M.; Muilenberg, M.L.; Friedman, W.; Zeldin, D. Dog allergen (Can f 1) and cat allergen (Fel d 1) in US homes: Results from the national survey of lead and allergens in housing. Ann. Allergy Asthma Immunol. 2008, 101, 517. [Google Scholar] [CrossRef] [PubMed]
- An, W.; Li, T.; Tian, X.; Fu, X.; Li, C.; Wang, Z.; Wang, J.; Wang, X. Allergies to allergens from cats and dogs: A review and update on sources, pathogenesis, and strategies. Int. J. Mol. Sci. 2024, 25, 10520. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.K.; Grammer, L.C. An overview of allergens. Allergy Asthma Proc. 2019, 40, 362–365. [Google Scholar] [CrossRef]
- Chapman, M.D.; Wood, R.A. The role and remediation of animal allergens in allergic diseases. Allergy Clin. Immunol. 2001, 107, S414–S421. [Google Scholar] [CrossRef]
- Nadeau, K.C. Allergen-specific IgG antibodies for cat allergy? Am. J. Respir. Crit. Care Med. 2021, 204, 1–2. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; BPharm, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic rhinitis and its impact on asthma (ARIA) guidelines—2016 revision. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Dávila, I.; Domínguez-Ortega, J.; Navarro-Pulido, A.; Alonso, A.; Antolín-Amerigo, D.; González-Mancebo, E.; Martín-García, C.; Núñez-Acevedo, B.; Prior, N.; Reche, M.; et al. Consensus document on dog and cat allergy. Allergy 2018, 73, 1206–1222. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.M.; Giovannini, M.; Escribese, M.M.; Prior, G.; Heffler, E.; Lonzano, M.A.; Bargber, D.; Canonica, G.W.; Pfaar, O. Mechanisms of allergen immunotherapy and potential biomarkers for clinical evaluation. J. Pers. Med. 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Ohman Jr, J.L.; Lowell, F.C.; Bloch, K.J. Allergens of mammalian origin: III. Properties of a major feline allergen. J. Immunol. 1974, 113, 1668–1677. [Google Scholar] [CrossRef]
- Klug, J.; Beier, H.M.; Bernard, A.; Chilton, B.S.; Fleming, T.P.; Lehrer, R.I.; Miele, L.; Pattabiraman, N.; Singh, G. Uteroglobin/Clara cell 10-kDa family of proteins: Nomenclature committee report. Ann. N. Y. Acad. Sci. 2000, 923, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Grönlund, H.; Sandalova, T.; Ljunggren, H.-G.; Hage-Hamsten, M.; Achour, A.; Schneider, G. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J. Biol. Chem. 2003, 278, 37730–37735. [Google Scholar] [CrossRef]
- Duffort, O.; Carreira, J.E.; Lombardero, M. Monoclonal antibodies against Fel d I and other clinically relevant cat allergens. Immunol. Lett. 1988, 17, 71–77. [Google Scholar] [CrossRef]
- Kaiser, L.; Velickovic, T.C.; Badia-Martinez, D.; Adedoyin, J.; Thunberg, S.; Hallén, D.; Berndt, K.; Grönlund, H.; Gafvelin, G.; Hage, M.; et al. Structural characterization of the tetrameric form of the major cat allergen Fel d 1. J. Mol. Biol. 2007, 370, 714–727. [Google Scholar] [CrossRef]
- Karn, R.C. The mouse salivary androgen-binding protein (ABP) alpha subunit closely resembles chain 1 of the cat allergen Fel dI. Biochem. Genet. 1994, 32, 271–277. [Google Scholar] [CrossRef]
- Bonnet, B.; Messaoudi, K.; Jacomet, F.; Michaud, E.; Fauquert, J.L.; Caillaud, D.; Evrard, B. An update on molecular cat allergens: Fel d 1 and what else? Chapter 1: Fel d 1, the major cat allergen. Allergy Asthma Clin. Immunol. 2018, 14, 14. [Google Scholar] [CrossRef]
- van Ree, R.; van Leeuwen, W.A.; Bulder, I.; Bond, J.; Aalberse, R.C. Purified natural and recombinant Fel d 1 and cat albumin in in vitro diagnostics for cat allergy. J. Allergy Clin. Immunol. 1999, 104, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Cleveland III, C.W.; Davis, B.W.; Khatri, K.; Pomés, A.; Chapman, M.D.; Brackett, N.F. Genetic diversity of the major cat allergen, Fel d 1. PNAS Nexus 2024, 3, pgae447. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R. The advent of recombinant allergens and allergen cloning. J. Allergy Clin. Immunol. 2011, 127, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Charpin, C.; Mata, P.; Charpin, D.; Lavaut, M.N.; Allasia, C.; Vervloet, D. Fel d I allergen distribution in cat fur and skin. J. Allergy Clin. Immunol. 1991, 88, 77–82. [Google Scholar] [CrossRef]
- Dabrowski, A.J.; Van der Brempt, X.; Soler, M.; Lucciani, P.; Charpin, D.; Vervloet, D. Cat skin as an important source of Fel d I allergen. J. Allergy Clin. Immunol. 1990, 86, 462–465. [Google Scholar] [CrossRef]
- Bartholome, K.; Kissler, W.; Baer, H.; Kopietz-Schulte, E.; Wahn, U. Where does cat allergen 1 come from? J. Allergy Clin. Immunol. 1985, 76, 503–506. [Google Scholar] [CrossRef]
- Brown, P.R.; Leitermann, K.; Ohman Jr, J.L. Distribution of cat allergen 1 in cat tissues and fluids. Int. Arch. Allergy Immunol. 1984, 74, 67–70. [Google Scholar] [CrossRef]
- De Andrade, A.D.; Birnbaum, J.; Magalon, C.; Magnol, J.P.; Lanteaume, A.; Charpin, D.; Vervloet, D. Fel d I levels in cat anal glands. Clin. Exp. Allergy 1996, 26, 178–180. [Google Scholar] [CrossRef]
- Anderson, M.C.; Baer, H.; Ohman Jr, J.L. A comparative study of the allergens of cat urine, serum, saliva, and pelt. J. Allergy Clin. Immunol. 1985, 76, 563–569. [Google Scholar] [CrossRef]
- Siebers, R.; Healy, B.; Holt, S.; Peters, S.; Crane, J.; Fitzharris, P. Fel d 1 levels in domestic living rooms are not related to cat color or hair length. J. Allergy Clin. Immunol. 2001, 108, 652–653. [Google Scholar] [CrossRef] [PubMed]
- Jalil-Colome, J.E.; de Andrade, A.E.L.D.; Birnbaum, J.E.L.; Casanova, D.; Mège, J.L.; Lanteaume, A.; Charpin, D.; Vervloet, D. Sex difference in Fel d 1 allergen production. J. Allergy Clin. Immunol. 1996, 98, 165–168. [Google Scholar] [CrossRef]
- Zielonka, T.M.; Charpin, D.; Berbis, P.; Luciani, P.; Casanova, D.; Vervloet, D. Effects of castration and testosterone on Fel d I production by sebaceous glands of male cats: I—Immunological assessment. Rev. Fr. D’Allergologie D’Immunologie Clin. 1995, 35, 359–363. [Google Scholar] [CrossRef]
- Carayol, N.; Birnbaum, J.; Magnan, A.; Ramadour, M.; Lanteaume, A.; Vervloet, D.; Tessier, Y.; Pageat, P. Fel d 1 production in the cat skin varies according to anatomical sites. Allergy 2000, 55, 570–573. [Google Scholar] [CrossRef]
- Trifonova, D.; Curin, M.; Riabova, K.; Karsonova, A.; Keller, W.; Grönlund, H.; Käck, U.; Konradsen, J.J.R.; Hage, M.V.; Karaulov, A. Allergenic activity of individual cat allergen molecules. Int. J. Mol. Sci. 2023, 24, 16729. [Google Scholar] [CrossRef]
- Popescu, F.; Ganea, C.S.; Panaitescu, C.; Panaitescu, C.; Vieru, M. Molecular diagnosis in cat allergy. World J. Methodol. 2021, 11, 46–60. [Google Scholar] [CrossRef]
- Ichikawa, K.; Vailes, L.D.; Pomes, A.; Chapman, M.D. Molecular cloning, expression and modelling of cat allergen, cystatin (Fel d 3), a cysteine protease inhibitor. Clin. Exp. Allergy 2001, 31, 1279–1286. [Google Scholar] [CrossRef]
- Smith, W.; Butler, A.; Hazell, L.A.; Chapman, M.D.; Pomés, A.; Nickels, D.G.; Thomas, W.R. Fel d 4, a cat lipocalin allergen. Clin. Exp. Allergy 2004, 34, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.; ONeil, S.E.; Hales, B.J.; Chai, T.L.Y.; Hazell, L.A.; Tanyaratsrisakul, S.; Piboonpocanum, S.; Thomas, W.R. Two newly identified cat allergens: The von Ebner gland protein Fel d 7 and the latherin-like protein Fel d 8. Int. Arch. Allergy Immunol. 2011, 156, 159–170. [Google Scholar] [CrossRef]
- Riabova, K.; Karsonova, A.V.; van Hage, M.; Käck, U.; Konradsen, J.R.; Grönlund, H.; Fomina, D.; Beltyukov, E.; Glazkova, P.A.; Semenov, D.Y.; et al. Molecular allergen-specific IgE recognition profiles and cumulative specific IgE levels associated with phenotypes of cat allergy. Int. J. Mol. Sci. 2022, 23, 6984. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Valenta, R.; Puerta, L.; Pomés, A.; Zakzuk, J.; Fernandez-Caldas, E.; Acevedo, N.; Sanchez-Borges, M.; Ansotegui, I.; Zhang, L.; et al. The allergenic activity and clinical impact of individual IgE-antibody binding molecules from indoor allergen sources. World Allergy Organ. 2020, 13, 100118. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, H.; Yang, Q.; Xu, Y.; Huang, Z.; Sun, B. Associations of protein classes with cross-reactivity and cross-sensitization in furry animal allergens: A component-resolved diagnostics study. J. Asthma Allergy 2025, 18, 363–375. [Google Scholar] [CrossRef]
- Saarelainen, S.; Rytk ONen-Nissinen, M.; Rouvinen, J.; Taivainen, A.; Auriola, S.; Kauppinen, A.; Kinnunen, T.; Virtanen, T. Animal-derived lipocalin allergens exhibit immunoglobulin E cross-reactivity. Clin. Exp. Allergy 2008, 38, 374–381. [Google Scholar] [CrossRef]
- Popescu, F.; Vieru, M. Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules. World J. Methodol. 2018, 8, 17–36. [Google Scholar] [CrossRef]
- Hilger, C.; Swiontek, K.; Arumugam, K.; Lehners, C.; Hentges, F. Identification of a new major dog allergen highly cross-reactive with Fel d 4 in a population of cat-and dog-sensitized patients. J. Allergy Clin. Immunol. 2012, 129, 1149–1151. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ishibashi, O.; Sugiura, K.; Ubatani, M.; Sakaguchi, M.; Nakatsuji, M.; Shimamoto, S.; Noda, M.; Uchiyama, S.; Fukutomi, Y.; et al. Crystal structure of the dog allergen Can f 6 and structure-based implications of its cross-reactivity with the cat allergen Fel d 4. Sci. Rep. 2019, 9, 1503. [Google Scholar] [CrossRef]
- Liu, Z.; Trifonova, D.; Tulaeva, I.; Riabova, K.; Karsonova, A.; Kozlov, E.; Elisyutina, O.; Khaitov, M.; Focke-Tejkl, M.; Chen, T.-H.; et al. Albumins represent highly cross-reactive animal allergens. Front. Immunol. 2023, 14, 1241518. [Google Scholar] [CrossRef] [PubMed]
- Luczynska, C.M.; Li, Y.; Chapman, M.D.; Platts-Mills, T.A. Airborne concentrations and particle size distribution of allergen derived from domestic cats (Felis domesticus). Am. Rev. Respir. Dis. 1990, 141, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Laheri, A.N.; Eggleston, P.A. The aerodynamic characteristics of cat allergen. Clin. Exp. Allergy 1993, 23, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.C.; Agarwal, M.K.; Reed, C.E. An immunochemical approach to indoor aeroallergen quantitation with a new volumetric air sampler: Studies with mite, roach, cat, mouse, and guinea pig antigens. J. Allergy Clin. Immunol. 1985, 76, 724–729. [Google Scholar] [CrossRef]
- De Blay, F.; Heymann, P.W.; Chapman, M.D.; Platts-Mills, T.A. Airborne dust mite allergens: Comparison of group II allergens with group I mite allergen and cat-allergen Fel d I. J. Allergy Clin. Immunol. 1991, 88, 919–926. [Google Scholar] [CrossRef]
- Wood, R.A.; Eggleston, P.A.; Lind, P.; Ingemann, L.; Schwartz, B.; Graveson, S.; Terry, D.; Wheeler, B.; Adkinson, N.F. Antigenic analysis of household dust samples1-4. Am. Rev. Respir. Dis. 1988, 137, 358–363. [Google Scholar] [CrossRef]
- Dallongeville, A.; Le Cann, P.; Zmirou-Navier, D.; Chevrier, C.; Costet, N.; Annesi-Maesano, I.; Blanchard, O. Concentration and determinants of molds and allergens in indoor air and house dust of French dwelling. Sci. Total Environ. 2015, 536, 964–972. [Google Scholar] [CrossRef]
- Ritz, B.R.; Hoelscher, B.; Frye, C.; Meyer, I.; Heinrich, J. Allergic sensitization owing to ‘second-hand’cat exposure in schools. Allergy 2002, 57, 357–361. [Google Scholar] [CrossRef]
- Custovic, A.; Green, R.; Taggart, S.; Smith, A.; Pickering, C.A.; Chapman, M.D.; Woodcock, A. Domestic allergens in public places II: Dog (Can f 1) and cockroach (Bla g 2) allergens in dust and mite, cat, dog and cockroach allergens in the air in public buildings. Clin. Exp. Allergy 1996, 26, 1246–1252. [Google Scholar] [CrossRef]
- Partti-Pellinen, K.; Marttila, O.; MAKinen-Kiljunen, S.; Haahtela, T. Occurrence of dog, cat, and mite allergens in public transport vehicles. Allergy 2000, 55, 65–68. [Google Scholar] [CrossRef]
- Enberg, R.N.; Shamie, S.M.; McCullough, J.; Haahtela, T. Ubiquitous presence of cat allergen in cat-free buildings: Probable dispersal from human clothing. Ann. Allergy 1993, 70, 471–474. [Google Scholar] [PubMed]
- De Lucca, S.D.; OMeara, T.J.; Tovey, E.R. Exposure to mite and cat allergens on a range of clothing items at home and the transfer of cat allergen in the workplace. J. Allergy Clin. Immunol. 2000, 106, 874–879. [Google Scholar] [CrossRef]
- Karlsson, A.; Renstr, O.M.A. Human hair is a potential source of cat allergen contamination of ambient air. Allergy 2005, 60, 961–964. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.; Renstr, O.M.A.; Hedren, M.; Larsson, K. Allergen avoidance does not alter airborne cat allergen levels in classrooms. Allergy 2004, 59, 661–667. [Google Scholar] [CrossRef] [PubMed]
- van Hage, M.; Käck, U.; Asarnoj, A.; Konradsen, J.R. An update on the prevalence and diagnosis of cat and dog allergy-emphasizing the role of molecular allergy diagnostics. Mol. Immunol. 2023, 157, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Akiyama, K. Anaphylaxis after a cat bite. Allergol. Int. 2012, 61, 511–512. [Google Scholar] [CrossRef]
- Custovic, A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin. Exp. Allergy 2015, 45, 54–62. [Google Scholar] [CrossRef]
- Reinero, C.R. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet. J. 2011, 190, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.; Woodfolk, J.A.; Erwin, E.A.; Aalberse, R. Mechanisms of tolerance to inhalant allergens: The relevance of a modified Th2 response to allergens from domestic animals. Springer Semin. Immunopathol. 2004, 25, 271–279. [Google Scholar] [CrossRef]
- Leo, G.; Incorvaia, C.; Arasi, S. Could a bite trigger the onset of cat allergy? Pediatr. Allergy Immunol. 2022, 33, e13841. [Google Scholar] [CrossRef]
- Jones, M. Understanding of the molecular mechanisms of allergy. Methods Mol. Med. 2008, 138, 1–15. [Google Scholar]
- Han, X.; Krempski, J.W.; Nadeau, K. Advances and novel developments in mechanisms of allergic inflammation. Allergy 2020, 75, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.H.; Valenta, R.; Jardetzky, T.; Verhasselt, V.; Durham, S.R.; Würtzen, P.A.; Neerven, R.J.J. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy 2021, 76, 3627–3641. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Suzuki, S.; Ekerljung, L.; Sjölander, S.; Mincheva, R.; Rönmark, E.P.; Rådinger, M.; Rönmark, E.; Borres, M.P.; Lundbäck, B.; et al. Furry animal allergen component sensitization and clinical outcomes in adult asthma and rhinitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 1230–1238. [Google Scholar] [CrossRef]
- Özuygur Ermis, S.S.; Norouzi, A.; Borres, M.P.; Basna, R.; Ekerljung, L.; Malmhäll, C.; Goksör, E.; Wennergren, G.; Rådinger, M.; Lötvall, J. Sensitization patterns to cat molecular allergens in subjects with allergic sensitization to cat dander. Clin. Transl. Allergy 2023, 13, e12294. [Google Scholar] [CrossRef]
- van Ree, F. Analytic aspects of the standardization of allergenic extracts. Allergy 1997, 52, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, M.E.; Wood, R.A.; Chen, P.; Eggleston, P.A. Measurement of cat allergen levels in the home by use of an amplified ELISA. J. Allergy Clin. Immunol. 1998, 101, 124–125. [Google Scholar] [CrossRef]
- Tasaniyananda, N.; Tungtrongchitr, A.; Seesuay, W.; Sakolvaree, Y.; Aiumurai, P.; Indrawattana, N.; Chaicumpa, W.; Sookrung, N. Quantification of Fel d 1 in house dust samples of cat allergic patients by using monoclonal antibody specific to a novel IgE-binding epitope. Asian Pac. J. Allergy 2018, 36, 8–15. [Google Scholar]
- Lau, S.; Schulz, G.; Sommerfeld, C.; Wahn, C.S. Comparison of quantitative ELISA and semiquantitative dustscreen™ for determination of Der p 1, Der f 1, and Fel d 1 in domestic dust samples. Allergy 2001, 56, 993–995. [Google Scholar] [CrossRef]
- Kelly, S.M.; Karsh, J.; Marcelo, J.; Boeckh, D.; Stepner, N.; Santone, B.; Yang, J.; Yang, W.H. Fel d 1 and Fel d 4 levels in cat fur, saliva, and urine. J. Allergy Clin. Immunol. 2018, 142, 1990–1992. [Google Scholar] [CrossRef]
- Huynh, S.; Menzies, S.; Khurana, T.; Lin, T.; Yan, L.; deVore, N.; Slater, J.E.; Rabin, R.L. Radial Immunodiffusion (RID) to sandwich ELISA for the quantitation of Fel d 1 and Amb a 1 in cat and short ragweed pollen allergenic extracts. J. Allergy Clin. Immunol. 2012, 129, AB88. [Google Scholar] [CrossRef]
- Rabin, R.L.; Croote, D.; Chen, A.; Dobrovolskaia, E.; Wong, J.J.W.; Grossman, J.; Hamilton, R.G. A human monoclonal antibody based immunoenzymetric assay to measure Fel d 1 concentrations in cat hair and pelt allergenic extracts. Front. Allergy 2024, 5, 1417879. [Google Scholar] [CrossRef] [PubMed]
- King, E.M.; Filep, S.; Smith, B.; Platts-Mills, T.; Hamilton, R.G.; Schmechel, D.; Sordillo, J.E.; Milton, D.; Ree, R.V.; Krop, E.J.M.; et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J. Immunol. Methods 2013, 387, 89–95. [Google Scholar] [CrossRef]
- Filep, S.C.; Black, K.R.; Smith, B.R.; Block, D.S.; Kuklinska-Pijanka, A.; Bermingham, M.; Oliver, M.A.; Thorpe, C.M.; Schuhmacher, Z.P.; Agah, S.; et al. Simultaneous quantification of specific food allergen proteins using a fluorescent multiplex array. Food Chem. 2022, 389, 132986. [Google Scholar] [CrossRef]
- Earle, C.D.; King, E.M.; Tsay, A.; Pittman, K.; Saric, B.; Vailes, L.; Godbout, R.; Oliver, K.G.; Chapman, M.D. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J. Allergy Clin. Immunol. 2007, 119, 428–433. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 4611–4622. [Google Scholar] [CrossRef]
- Scherer, E.; Valot, B.; Vacheyrou, M.; Naegele, A.; Knapp, J.; Rocchi, S.; Roussel, S.; Millon, L.; Reboux, G. Assessment of pets (cats and dogs) in homes using electrostatic dust collectors and QPCR: New tools to evaluate exposure and risk of allergies. Int. J. Environ. Heal. Res. 2016, 26, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Strasser, L.; Dang, H.-H.; Schwarz, H.; Asam, C.; Ferreria, F.; Horejs-Hoeck, J.; Huber, C.G. Unbiased quantitative proteomics reveals a crucial role of the allergen context for the activation of human dendritic cells. Sci. Rep. 2017, 7, 16638. [Google Scholar] [CrossRef] [PubMed]
- Krutz, N.L.; Kimber, I.; Winget, J.; Nguyen, M.N.; Limviphuvadh, V.; Maurer-Stroh, S.; Mahony, C.; Gerberick, G.F. Identification and semi-quantification of protein allergens in complex mixtures using proteomic and AllerCatPro 2.0 bioinformatic analyses: A proof-of-concept investigation. J. Immunotoxicol. 2024, 21, 2305452. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Dios Alché, J.D.; Moreira, R.; Franco, D. Advanced proteomic and bioinformatic tools for predictive analysis of allergens in novel foods. Biology 2023, 12, 714. [Google Scholar] [CrossRef]
- Wéry, N.; Galès, A.; Brunet, Y. Bioaerosol sources. In Microbiology of Aerosols; Wiley-VCH: Cham, Switzerland, 2017; pp. 115–135. [Google Scholar]
- Haig, C.W.; Mackay, W.G.; Walker, J.T.; Williams, C. Bioaerosol sampling: Sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016, 93, 242. [Google Scholar] [CrossRef]
- Kabir, E.; Azzouz, A.; Raza, N.; Bhardwaj, S.K.; Kim, K.-H.; Tabatabaei, M.; Kukkar, D. Recent advances in monitoring, sampling, and sensing techniques for bioaerosols in the atmosphere. ACS Sens. 2020, 5, 1254–1267. [Google Scholar] [CrossRef]
- Morris, D.R.; Fatisson, J.; Olsson, A.L.; Tufenkji, N.; Ferro, A.R. Real-time monitoring of airborne cat allergen using a QCM-based immunosensor. Sens. Actuat. B Chem. 2014, 190, 851–857. [Google Scholar] [CrossRef]
- Toma, K.; Horibe, M.; Kishikawa, C.; Yoshimura, N.; Arakawa, T.; Yatsuda, H.; Shimomura, H.; Mitsubayashi, K. Rapid and repetitive immunoassay with a surface acoustic wave device for monitoring of dust mite allergens. Sens. Actuat. B-Chem. 2017, 248, 924–929. [Google Scholar] [CrossRef]
- Hossenbaccus, L.; Walker, T.; Ellis, A.K. Technical validation of controlled exposure to cat dander in the specialized particulate control environmental exposure unit (SPaC-EEU). Allergy Asthma Clin. Immunol. 2025, 21, 6. [Google Scholar] [CrossRef]
- Salimifard, P.; Rim, D.; Freihaut, J.D. Evaluation of low-cost optical particle counters for monitoring individual indoor aerosol sources. Aerosol Sci. Technol. 2020, 54, 217–231. [Google Scholar] [CrossRef]
- Fong, A.; Fong, B. Indoor air quality control for asthma patients using smart home technology. In Proceedings of the 2011 IEEE 15th International Symposium on Consumer Electronics (ISCE), Singapore, 14–17 June 2011. [Google Scholar]
- Bastien, B.C.; Gardner, C.; Satyaraj, E. Influence of time and phenotype on salivary Fel d1 in domestic shorthair cats. J. Feline Med. Surg. 2019, 21, 867–874. [Google Scholar] [CrossRef]
- Bienboire-Frosini, C.E.C.; Lebrun, R.E.G.; Vervloet, D.; Pageat, P.; Ronin, C. Distribution of core fragments from the major cat allergen Fel d 1 is maintained among the main anatomical sites of production. Int. Arch. Allergy Immunol. 2010, 152, 197–206. [Google Scholar] [CrossRef]
- Peterson, E.L.; Ownby, D.R.; Johnson, C.C. The relationship of housing and household characteristics to the indoor concentrations of Der f 1, Der p 1, and Fel d 1 measured in dust and air samples. Ann. Allergy Asthma Immunol. 2003, 90, 564–571. [Google Scholar] [CrossRef]
- Neal, J.S.; Arlian, L.G.; Morgan, M.S. Relationship among house-dust mites, Der 1, Fel d 1, and Can f 1 on clothing and automobile seats with respect to densities in houses. Ann. Allergy Asthma Immunol. 2002, 88, 410–415. [Google Scholar] [CrossRef]
- Montoya, L.D.; Hildemann, L.M. Evolution of the mass distribution of resuspended cat allergen (Fel d 1) indoors following a disturbance. Atmos. Environ. 2001, 35, 859–866. [Google Scholar] [CrossRef]
- Corsi, R.L.; Siegel, J.A.; Chiang, C. Particle resuspension during the use of vacuum cleaners on residential carpet. J. Occup. Environ. Hyg. 2008, 5, 232–238. [Google Scholar] [CrossRef] [PubMed]
- De Blay, F.; Spirlet, F.; Gries, P.; Casel, S.; Ott, M.; Pauli, G. Effects of various vacuum cleaners on the airborne content of major cat allergen (Fel d 1). Allergy 1998, 53, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, J.M.; Rouhbakhsh, S.; Warner, J.A.; Warner, J.O. A comparison of the effect of conventional and filter vacuum cleaners on airborne house dust mite allergen. Resp. Med. 1995, 89, 279–284. [Google Scholar] [CrossRef]
- Chew, G.L.; Higgins, K.M.; Gold, D.R.; Muilenberg, M.L.; Burge, H.A. Monthly measurements of indoor allergens and the influence of housing type in a northeastern US city. Allergy 1999, 54, 1058–1066. [Google Scholar] [CrossRef]
- Chew, G.L.; Saha, S. Impacts of climate change on indoor allergens. Impacts of climate change on allergens and allergic diseases. In Impacts of Climate Change on Allergens and Allergic Diseases; Cambridge University Press: Cambridge, UK, 2016; p. 119. [Google Scholar]
- Egmar, A.; Almqvist, C.; Emenius, G.; Lilja, G.; Wickman, M. Deposition of cat (Fel d 1), dog (Can f 1), and horse allergen over time in public environments--a model of dispersion. Allergy 1998, 53, 957–961. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, K.; Song, S.; Joo, M.-D.; Lee, S.-H.; Kang, J.-S.; Kang, S.-M.; Idrees, M.; Kim, J.-W.; Kong, I.-K. Generation and cloning of CH2 knockout cats by CRISPR-Cas9 system: Hypoallergenic cats. Authorea Preprints 2023. [Google Scholar] [CrossRef]
- Brackett, N.F.; Davis, B.W.; Adli, M.; Pomés, A.; Chapman, M.D. Evolutionary biology and gene editing of cat allergen, Fel d 1. CRISPR J. 2022, 5, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Brackett, N.; Riedy, J.; Adli, M.; Pomés, A.; Chapman, M.D. CRISPR gene editing of the major cat allergen, Fel d 1. Allergy 2020, 75, 59–60. [Google Scholar]
- Lee, S.R.; Lee, K.; Song, S.; Joo, M.-D.; Lee, S.-H.; Kang, J.-S.; Kang, S.-M.; Idrees, M.; Kim, J.-W.; Kong, I.-K. Generation of Fel d 1 chain 2 genome-edited cats by CRISPR-Cas9 system. Sci. Rep. 2024, 14, 4987. [Google Scholar] [CrossRef]
- Zhu, D.; Kepley, C.L.; Zhang, K.; Terada, T.; Yamada, T.; Saxon, A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat. Med. 2005, 11, 446–449. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Q.; Lin, J. Egg yolk antibody for passive immunization: Status, challenges, and prospects. J. Agric. Food Chem. 2023, 71, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Calvert, R.A.; Sutton, B.J.; Doré, K.A. IgY: A key isotype in antibody evolution. Biol. Rev. 2017, 92, 2144–2156. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012, 3, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Satyaraj, E.; Li, Q.; Sun, P.; Sherrill, S. Anti-Fel d1 immunoglobulin Y antibody-containing egg ingredient lowers allergen levels in cat saliva. J. Feline Med. Surg. 2019, 21, 875–881. [Google Scholar] [CrossRef]
- Satyaraj, E.; Wedner, H.J.; Bousquet, J. Keep the cat, change the care pathway: A transformational approach to managing Fel d 1, the major cat allergen. Allergy 2019, 74, 5–17. [Google Scholar] [CrossRef]
- Matulka, R.A.; Thompson, L.; Corley, D. Multi-level safety studies of anti Fel d 1 IgY ingredient in cat food. Front. Vet. Sci. 2020, 6, 477. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, E.D.; Matulka, R.A.; Conboy-Schmidt, L.; May, K.A. Evaluation of anti-Fel d 1 IgY ingredient for pet food on growth performance in kittens. Front. Vet. Sci. 2024, 11, 1355390. [Google Scholar] [CrossRef] [PubMed]
- Satyaraj, E.; Gardner, C.; Filipi, I.; Cramer, K.; Sherrill, S. Reduction of active Fel d1 from cats using an antiFel d1 egg IgY antibody. Immun. Inflamm. Dis. 2019, 7, 68–73. [Google Scholar] [CrossRef]
- Thoms, F.; Jennings, G.T.; Maudrich, M.; Vogel, M.; Haas, S.; Zeltins, A.; Hofmann-Lehmann, R.; Riond, B.; Grossmann, J.; Hunziker, P.; et al. Immunization of cats to induce neutralizing antibodies against Fel d 1, the major feline allergen in human subjects. J. Allergy Clin. Immunol. 2019, 144, 193–203. [Google Scholar] [CrossRef]
- Thoms, F.; Haas, S.; Erhart, A.; Nett, C.S.; Rüfenacht, S.; Graf, N.; Strods, A.; Patil, G.; Leenadevi, T.; Fontaine, M.C.; et al. Immunization of cats against Fel d 1 results in reduced allergic symptoms of owners. Viruses 2020, 12, 288. [Google Scholar] [CrossRef]
- Bergmann, K.; Graessel, A.; Raab, J.; Banghard, W.; Krause, L.; Becker, S.; Kugler, S.; Zuberbier, T.; Ott, V.B.; Kramer, M.F.; et al. Targeted micronutrition via holo-BLG based on the farm effect in house dust mite allergic rhinoconjunctivitis patients—First evaluation in a standardized allergen exposure chamber. Allergo J. Int. 2021, 30, 141–149. [Google Scholar] [CrossRef]
- Bergmann, K.; Raab, J.; Krause, L.; Becker, S.; Kugler, S.; Zuberbier, T.; Roth-Walter, F.; Jensen-Jarolim, E.; Kramer, M.; Graessel, A. Long-term benefits of targeted micronutrition with the holoBLG lozenge in house dust mite allergic patients. Allergo J. Int. 2022, 31, 161–171. [Google Scholar] [CrossRef]
- Jensen-Jarolim, E.; Jensen, S.A.; Bergmann, K. Allergy to the cat—From diagnosis to management. Allergo J. Int. 2023, 32, 130–137. [Google Scholar] [CrossRef]
- Bergmann, K.; Raab, J.; Graessel, A.; Zwingers, T.; Becker, S.; Kugler, S.; Zuberbier, T.; Roth-Walter, F.; Kramer, M.F.; Jensen-Jarolim, E. The holo beta-lactoglobulin lozenge reduces symptoms in cat allergy—Evaluation in an allergen exposure chamber and by titrated nasal allergen challenge. Clin. Transl. Allergy 2023, 13, e12274. [Google Scholar] [CrossRef]
- Wood, R.A.; Chapman, M.D.; Adkinson, N.F., Jr.; Eggleston, P.A. The effect of cat removal on allergen content in household-dust samples. J. Allergy Clin. Immunol. 1989, 83, 730–734. [Google Scholar] [CrossRef]
- Matsui, E.; Kagey-Sobotka, A.; Chichester, K.; Eggleston, P.A. Allergic potency of recombinant Fel d 1 is reduced by low concentrations of chlorine bleach. J. Allergy Clin. Immunol. 2003, 111, 396–401. [Google Scholar] [CrossRef]
- Avner, D.B.; Perzanowski, M.S.; Platts-Mills, T.A.; Woodfolk, J.A. Evaluation of different techniques for washing cats: Quantitation of allergen removed from the cat and the effect on airborne Fel d 1. J. Allergy Clin. Immunol. 1997, 100, 307–312. [Google Scholar] [CrossRef]
- Nageotte, C.; Park, M.; Havstad, S.; Zoratti, E.; Ownby, D. Duration of airborne Fel d 1 reduction after cat washing. J. Allergy Clin. Immunol. 2006, 118, 521–522. [Google Scholar] [CrossRef]
- Maya-Manzano, J.E.M.I.; Pusch, G.; Ebner Von Eschenbach, C.; Bartusel, E.; Belzner, T.; Karg, E.; Bardolatzy, U.; Scheja, M.; Schmidt-Weber, C.; Buters, J. Effect of air filtration on house dust mite, cat and dog allergens and particulate matter in homes. Clin. Transl. Allergy 2022, 12, e12137. [Google Scholar] [CrossRef]
- Gherasim, A.; de Blay, F.E.D.E. Does air filtration work for cat allergen exposure? Curr. Allergy Asthma Rep. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, D.; Surbaugh, K.; Manevski, M.; Wickramaratne, C.; Chaput, D.; Chung, A.; Leon, F.; Chand, H.S.; Dhau, J.S. Indoor-air purification by photoelectrochemical oxidation mitigates allergic airway responses to aerosolized cat dander in a murine model. Sci. Rep. 2023, 13, 10980. [Google Scholar] [CrossRef]
- Golightly, L.K.; Greos, L.S. Second-generation antihistamines: Actions and efficacy in the management of allergic disorders. Drugs 2005, 65, 341–384. [Google Scholar] [CrossRef] [PubMed]
- Linton, S.; Hossenbaccus, L.; Ellis, A.K. Evidence-based use of antihistamines for treatment of allergic conditions. Ann. Allergy Asthma Immunol. 2023, 131, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, P.J.; Berlin, R.G. Efficacy of phenylephrine. Br. J. Clin. Pharmaco. 2007, 64, 555. [Google Scholar] [CrossRef]
- Krause, H.F. Antihistamines and decongestants. Otolaryngol. Head Neck 1992, 107, 835–840. [Google Scholar] [CrossRef]
- Paggiaro, P.L.; Dahle, R.; Bakran, I.; Frith, L.; Hollingworth, K.; Efthimiou, J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. Lancet 1998, 351, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Penagos, M.; Compalati, E.; Tarantini, F.; Baena-Cagnani, C.E.; Passalacqua, G.; Canonica, G.W. Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta-analysis of randomized, double-blind, placebo-controlled, clinical trials. Allergy 2008, 63, 1280–1291. [Google Scholar] [CrossRef]
- Hoang, M.P.; Chitsuthipakorn, W.; Seresirikachorn, K.; Snidvongs, K. As-needed intranasal corticosteroid spray for allergic rhinitis: A systematic review and meta-analysis. Rhinology 2022, 60, 242–251. [Google Scholar] [CrossRef]
- Abelson, M.B. A review of olopatadine for the treatment of ocular allergy. Expert Opin. Pharmacother. 2004, 5, 1979–1994. [Google Scholar] [CrossRef]
- Grant, S.M.; Goa, K.L.; Fitton, A.; Sorkin, E.M. Ketotifen: A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in asthma and allergic disorders. Drugs 1990, 40, 412–448. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Pedersen, S.O.R.; Busse, W.W. Efficacy and safety of inhaled corticosteroids: New developments. Am. J. Crit. Care 1998, 157, S1–S53. [Google Scholar] [CrossRef] [PubMed]
- Marques, L.; Vale, N. Salbutamol in the management of asthma: A review. Int. J. Mol. Sci. 2022, 23, 14207. [Google Scholar] [CrossRef]
- Nayak, A.; Langdon, R.B. Montelukast in the treatment of allergic rhinitis: An evidence-based review. Drugs 2007, 67, 887–901. [Google Scholar] [CrossRef]
- Noonan, M.J.; Chervinsky, P.; Brandon, M.; Zhang, J.; Kundu, S.; McBurney, J.; Reiss, T.F. Montelukast, a potent leukotriene receptor antagonist, causes dose-related improvements in chronic asthma. Eur. Respir. J. 1998, 11, 1232–1239. [Google Scholar] [CrossRef]
- Lipworth, B.J. Leukotriene-receptor antagonists. Lancet 1999, 353, 57–62. [Google Scholar] [CrossRef]
- Montuschi, P.; Peters-Golden, M.L. Leukotriene modifiers for asthma treatment. Clin. Exp. Allergy 2010, 40, 1732–1741. [Google Scholar] [CrossRef]
- Arshad, S.H. An update on allergen immunotherapy. Clin. Med. Res. 2016, 16, 584–587. [Google Scholar] [CrossRef]
- Adlany, Y.K.; Šošić, L.; Senti, G.; Lang, C.C.V.; Wüthrich, B.; Kündig, T.M.; Johansen, P. Quality of life in allergic rhinitis patients treated with intralymphatic immunotherapy (ILIT): A 19-year follow-up. J. Allergy Clin. Immunol. 2023, 2, 43–50. [Google Scholar] [CrossRef]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Hu, S.; Li, M.; Yang, L.; Liu, L.; Zheng, M.; Guo, Z.; Song, Z.; Zhang, C.; Diao, Q.; et al. Efficacy and safety of omalizumab in Chinese patients with anti-histamine refractory chronic spontaneous urticaria. Dermatol. Ther. 2022, 35, e15303. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, D.; Curin, M.; Focke-Tejkl, M.; Liu, Z.; Borochova, K.; Gattinger, P.; Hage, M.; Grönlund, H.; Kiss, R.; Huang, H.-J.; et al. Recombinant hypoallergenic cat allergy vaccines. Allergy 2025, 80, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, M.; Votto, M.; Caminiti, L.; Panasiti, I.; Carella, F.; Castro, G.D.; Landi, M.; Olcese, R.; Vernich, M.; Marseglia, G.L.; et al. Safety of allergen-specific immunotherapy in children. Pediatr. Allergy Immunol. 2022, 33, 27–30. [Google Scholar] [CrossRef]
- Remes, S.T.; Castro-Rodriguez, J.A.; Holberg, C.J.; Martinez, F.D.; Wright, A.L. Dog exposure in infancy decreases the subsequent risk of frequent wheeze but not of atopy. J. Allergy Clin. Immunol. 2001, 108, 509–515. [Google Scholar] [CrossRef]
- Celed, O.N.J.C.; Litonjua, A.A.; Ryan, L.; Platts-Mills, T.; Weiss, S.; Gold, D.R. Exposure to cat allergen, maternal history of asthma, and wheezing in first 5 years of life. Lancet 2002, 360, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.; Torkamani, A. Artificial intelligence in clinical and genomic diagnostics. Genome Med. 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- MacMath, D.; Chen, M.; Khoury, P. Artificial intelligence: Exploring the future of innovation in allergy immunology. Curr. Allergy Asthma Rep. 2023, 23, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, H.H. Role of artificial intelligence in advancing immunology. Immunol. Res. 2025, 73, 76. [Google Scholar] [CrossRef]
- Indolfi, C.; Klain, A.; Dinardo, F.; Giudice, M.M.D. Artificial intelligence in the transition of allergy: A valuable tool from childhood to adulthood. Front. Med. 2024, 11, 1469161. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.F.; Hu, Z.M.; Li, K.; Xu, Z.-Q.; Sun, J.-L.; Wei, J.-F. Artificial intelligence-driven design of the assembled major cat allergen Fel d 1 to improve its spatial folding and IgE-reactivity. Int. Immunopharmacol. 2024, 128, 111488. [Google Scholar] [CrossRef]
| Countries | Methods | Number of Subjects Investigated | Cats | Dogs |
|---|---|---|---|---|
| China | Blood testing | 16,664 | 15.47% | 10.50% |
| Russia | Blood testing | 513 | 24.10% | 21.40% |
| South Korea | Skin prick test | 7504 | 20.60% | 15.20% |
| Germany | Blood testing | 356 | 34.80% | 31.70% |
| Japan | Blood testing | 12,205,097 | 18.20% | 18.90% |
| America | Blood testing | 478 | 54.40% | 64.70% |
| Canada | Skin prick test | 623 | 53.10% | 17.30% |
| Qatar | Skin prick test | 473 | 6.18% | 0.50% |
| Lebanon | Skin prick test | 919 | 29.90% | 21.90% |
| Thailand | Skin prick test | 1516 | 12.90% | 10.00% |
| Nepal | Skin prick test | 170 | 15.30% | 14.10% |
| Mexico | Skin prick test | 761 | 26.70% | 33.90% |
| Allergens | Protein Molecular Category | Cross-Reactivity | References |
|---|---|---|---|
| Fel d 2 | Serum albumin | Can f 3 from dogs | [55,56] |
| Fel d 3 | Cystatin | Can f 8 | [49] |
| Fel d 4 | Lipocalin family | Can f 1, Can f 2, Can f 4, and Can f 6 from dogs | [57,58] |
| Fel d 5 | Immunoglobulins | NA | [51] |
| Fel d 6 | Immunoglobulins | NA | [51] |
| Fel d 7 | Lipocalin family | Can f 1, Can f 2, and Can f 4 from dogs | [57] |
| Fel d 8 | Latherin-like protein | NA | [51] |
| Methods | Technical Features | Application Scenarios | Sensitivity | References |
| Signal amplification ELISA | Employing catalytic reported deposition (CARD) technology for the cascade amplification of enzyme-substrate reactions | Ultra-low concentration air samples | 156 pg Fel d 1/mL | [85] |
| Double-point sandwich ELISA | MAbC48 targets conserved IgE epitopes, avoiding interference from Fel d 1 polymorphisms | Indoor dust samples | 390 pg Fel d 1/mL | [86] |
| Immunodot assay (Dustscreen™) | Semi-quantitative, 4 h results, capable of multiple allergen testing | Rapid screening for allergens in public areas | 100 pg Fel d 1/mL | [87] |
| Double antibody sandwich ELISA | Quantified using manufacturer standard curves, between plates CV < 10% | For detecting allergen content in clinical or research settings | 80,000 pg Fel d 1 | [88] |
| scFv-Sandwich ELISA | The 96-well high-throughput format replaces traditional radial diffusion, eliminating the need for subjective interpretation | Specific for the potency verification of commercially available standardized allergen extracts | 500 pg Fel d 1/mL | [89] |
| Human IgG4-tweezed sandwich ELISA | Using high-affinity humanized monoclonal antibodies, between plates CV < 9% | Suitable for manufacturers calibration of cat allergen concentrations | 250 pg Fel d 1/mL | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Tian, X.; Fu, X.; Ma, B.; Wang, Z.; Han, B.; Tao, H.; Wang, J.; Wang, X. Advance in Managing Indoor Cat Allergen Proteins: Molecular Insights, Detection, and Control. Int. J. Mol. Sci. 2025, 26, 10913. https://doi.org/10.3390/ijms262210913
Jiang Y, Tian X, Fu X, Ma B, Wang Z, Han B, Tao H, Wang J, Wang X. Advance in Managing Indoor Cat Allergen Proteins: Molecular Insights, Detection, and Control. International Journal of Molecular Sciences. 2025; 26(22):10913. https://doi.org/10.3390/ijms262210913
Chicago/Turabian StyleJiang, Yuxin, Xinya Tian, Xiaoxin Fu, Baichuan Ma, Zhenlong Wang, Bing Han, Hui Tao, Jinquan Wang, and Xiumin Wang. 2025. "Advance in Managing Indoor Cat Allergen Proteins: Molecular Insights, Detection, and Control" International Journal of Molecular Sciences 26, no. 22: 10913. https://doi.org/10.3390/ijms262210913
APA StyleJiang, Y., Tian, X., Fu, X., Ma, B., Wang, Z., Han, B., Tao, H., Wang, J., & Wang, X. (2025). Advance in Managing Indoor Cat Allergen Proteins: Molecular Insights, Detection, and Control. International Journal of Molecular Sciences, 26(22), 10913. https://doi.org/10.3390/ijms262210913








