An Anthocyanin- and Anti-Ageing Amino Acids-Enriched Pigmented Rice Innovation Promotes Healthy Ageing Through the Modulation of Telomere, Oxidative Stress and Inflammation Reduction: A Randomized Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Effect of “Zuper Rice” on Cognition
2.2. Effect of “Zuper Rice” on Facial Wrinkles
2.3. Safety and Adverse Effects Evaluation
2.4. Cardiovascular Risk
2.5. Changes in Oxidative Stress and Inflammatory Markers
2.6. Changes in Telomere Length and Telomerase Activity
3. Discussion
4. Materials and Methods
4.1. Preparation of a Capsule Containing Heated “Zuper Rice”
4.2. Ethical Statement
4.3. Study Participants
4.4. Experimental Protocol
4.5. Cognitive Assessment
4.6. Facial Wrinkle Assessment
4.7. Biochemical Assessments
4.8. Assessments of Telomere Length and Telomerase Enzyme Activity
4.9. Safety Evaluation and Adverse Effect Assessment
4.10. Cardiovascular Risk Assessment
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solhi, M.; Pirouzeh, R.; Zanjari, N. Middle-aged preparation for healthy aging: A qualitative study. BMC Public Health 2022, 22, 274. [Google Scholar] [CrossRef]
- Amarya, S.; Singh, K.; Sabharwal, M. Ageing Process and Physiological Changes. In Gerontology; D’Onofrio, G., Greco, A., Sancarlo, D., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–24. [Google Scholar]
- Langmann, E. Vulnerability, ageism, and health: Is it helpful to label older adults as a vulnerable group in health care? Med. Health Care Philos. 2023, 26, 133–142. [Google Scholar] [CrossRef]
- de Meijer, C.; Wouterse, B.; Polder, J.; Koopmanschap, M. The effect of population aging on health expenditure growth: A critical review. Eur. J. Ageing 2013, 10, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Stefanacci, R.G. Overview of Ageing. Available online: https://www.msdmanuals.com/home/older-people-s-health-issues/the-aging-body/overview-of-aging (accessed on 18 January 2025).
- Hartmann, J.; Roßmeier, C.; Riedl, L.; Dorn, B.; Fischer, J.; Slawik, T.; Fleischhaker, M.; Hartmann, F.; Egert-Schwender, S.; Kehl, V.; et al. Quality of life in Advanced Dementia with Late Onset, Young Onset, and very young onset. J. Alzheimer’s Dis. 2021, 80, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Song, S.; Jin, Y.; Zheng, Z.J. Prospective association between social engagement and cognitive impairment among middle-aged and older adults: Evidence from the China Health and Retirement Longitudinal Study. BMJ Open 2020, 10, e040936. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jigeer, G.; Gunn, D.A.; Liu, Y.; Chen, X.; Guo, Y.; Li, Y.; Gu, X.; Ma, Y.; Wang, J.; et al. Facial aging, cognitive impairment, and dementia risk. Alzheimer’s Res. Ther. 2024, 16, 245. [Google Scholar] [CrossRef]
- Pujos, M.; Chamayou-Robert, C.; Parat, M.; Bonnet, M.; Couret, S.; Robiolo, A.; Doucet, O. Impact of Chronic Moderate Psychological Stress on Skin Aging: Exploratory Clinical Study and Cellular Functioning. J. Cosmet. Dermatol. 2025, 24, e16634. [Google Scholar] [CrossRef]
- Chen, P.Y.; Shen, M.; Cai, S.Q.; Tang, Z.W. Association Between Atopic Dermatitis and Aging: Clinical Observations and Underlying Mechanisms. J. Inflamm. Res. 2024, 17, 3433–3448. [Google Scholar] [CrossRef]
- Flanagan, E.; Lamport, D.; Brennan, L.; Burnet, P.; Calabrese, V.; Cunnane, S.C.; de Wilde, M.C.; Dye, L.; Farrimond, J.A.; Emerson Lombardo, N.; et al. Nutrition and the ageing brain: Moving towards clinical applications. Ageing Res. Rev. 2020, 62, 62–101079. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Vlase, L.; Popa, D.-S. Antioxidants in Age-Related Diseases and Anti-Aging Strategies. Antioxidants 2022, 11, 1868. [Google Scholar] [CrossRef]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. in Primary Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2019, 2019, 2089817, Erratum in Oxidative Med. Cell. Longev. 2020, 2020, 6306104. [Google Scholar] [CrossRef] [PubMed]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Tong-Un, T.; Thukham-Mee, W.; Paholpak, P.; Rangseekhajee, P. A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations. Nutrients 2023, 15, 3499. [Google Scholar] [CrossRef]
- Aarsland, D.; Khalifa, K.; Bergland, A.K.; Soennesyn, H.; Oppedal, K.; Holteng, L.B.A.; Oesterhus, R.; Nakling, A.; Jarholm, J.A.; de Lucia, C.; et al. A Randomised Placebo-Controlled Study of Purified Anthocyanins on Cognition in Individuals at Increased Risk for Dementia. Am. J. Geriatr. Psychiatry 2023, 31, 141–151. [Google Scholar] [CrossRef]
- Kent, K.; Yousefi, M.; do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Visentin, D.; Roodenrys, S.; Walton, K.; Charlton, K.E. Anthocyanin intake is associated with improved memory in older adults with mild cognitive impairment. Nutr. Res. 2022, 104, 36–43. [Google Scholar] [CrossRef]
- Guo, X.; He, L.; Sun, J.; Ye, H.; Yin, C.; Zhang, W.; Han, H.; Jin, W. Exploring the Potential of Anthocyanins for Repairing Photoaged Skin: A Comprehensive Review. Foods 2024, 13, 3506. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweet potatoes cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of acute consumption of pigmented rice in humans. Food Funct. 2019, 10, 8230–8239. [Google Scholar] [CrossRef] [PubMed]
- Callcott, E.T.; Blanchard, C.L.; Snell, P.; Santhakumar, A.B. The anti-inflammatory and antioxidant effects of pigmented rice consumption in an obese cohort. Food Funct. 2019, 10, 8016–8025. [Google Scholar] [CrossRef]
- Pannangrong, W.; Wattanathorn, J.; Muchimapura, S.; Tiamkao, S.; Tong-Un, T. Purple rice berry is neuroprotective and enhances cognition in a rat model of Alzheimer’s disease. J. Med. Food 2011, 14, 688–694. [Google Scholar] [CrossRef]
- Thummayot, S.; Tocharus, C.; Pinkaew, D.; Viwatpinyo, K.; Sringarm, K.; Tocharus, J. Neuroprotective effect of purple rice extract and its constituent against amyloid beta-induced neuronal cell death in SK-N-SH cells. Neurotoxicology 2014, 45, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Natthee, P.; Wattanathorn, J.; Thukham-mee, W.; Paholpak, P.; Rangseekajee, P.; Piyavhatkul, N.; Wattanathorn, S.; Muchimapura, S.; Tong-Un, T. A Black Sticky Rice-Derived Functional Ingredient Improves Anxiety, Depression, and Stress Perception in Adult Volunteers. Foods 2024, 13, 3884. [Google Scholar] [CrossRef]
- Manosroi, J.; Chankhampan, C.; Kitdamrongtham, W.; Zhang, J.; Abe, M.; Akihisa, T.; Manosroi, W.; Manosroi, A. In vivo anti-ageing activity of cream containing niosomes loaded with purple glutinous rice (Oryza sativa Linn.) extract. Int. J. Cosmet. Sci. 2020, 42, 622–631. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Zhang, Z.; Li, H.; Sun, G.; Yin, N.; Wen, J. Anti-ageing peptides and proteins for topical applications: A review. Pharm. Dev. Technol. 2022, 27, 108–125. [Google Scholar] [CrossRef] [PubMed]
- Canfield, C.A.; Bradshaw, P.C. Amino acids in the regulation of aging and aging-related diseases. Transl. Med. Aging 2019, 3, 70–89. [Google Scholar] [CrossRef]
- Nagao, K. Cognition and nutrition: The role of dietary protein and amino acids in cognitive health. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 40–46. [Google Scholar] [CrossRef]
- Suzuki, H.; Yamashiro, D.; Ogawa, S.; Kobayashi, M.; Cho, D.; Iizuka, A.; Tsukamoto-Yasui, M.; Takada, M.; Isokawa, M.; Nagao, K.; et al. Intake of Seven Essential Amino Acids Improves Cognitive Function and Psychological and Social Function in Middle-Aged and Older Adults: A Double-Blind, Randomized, Placebo-Controlled Trial. Front. Nutr. 2020, 7, 586166. [Google Scholar] [CrossRef]
- Van Ngo, T.; Kunyanee, K.; Luangsakul, N. Insight into the nutritional, physicochemical, functional, antioxidative properties and in vitro gastrointestinal digestibility of selected Thai rice: Comparative and multivariate studies. Curr. Res. Food Sci. 2024, 8, 100735. [Google Scholar] [CrossRef]
- File, S.E.; Fluck, E.; Fernandes, C. Beneficial effects of glycine (bioglycin) on memory and attention in young and middle-aged adults. Clin. Psychopharmacol. 1999, 19, 506–512. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Madiha, S.; Khaliq, S.; Shahzad, S.; Batool, Z.; Haider, S. Impact of oral supplementation of Glutamate and GABA on memory performance and neurochemical profile in hippocampus of rats. Pak. J. Pharm. Sci. 2017, 30 (Suppl. 3), 1013–1021. [Google Scholar]
- Mone, P.; Pansini, A.; Jankauskas, S.S.; Varzideh, F.; Kansakar, U.; Lombardi, A.; Trimarco, V.; Frullone, S.; Santulli, G. L-Arginine Improves Cognitive Impairment in Hypertensive Frail Older Adults. Front. Cardiovasc. Med. 2022, 9, 868521. [Google Scholar] [CrossRef]
- Hosseini, M.; Headari, R.; Oryan, S.; Hadjzadeh, M.A.; Saffarzadeh, F.; Khazaei, M. The effect of chronic administration of L-arginine on the learning and memory of estradiol-treated ovariectomized rats tested in the morris water maze. Clinics 2010, 65, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Xie, F.; Sun, Z.; Guo, B.; Li, F.; Wang, S.; Wang, Y.; Tian, Y.; Zhao, Y.; et al. Leucine mediates cognitive dysfunction in early life stress-induced mental disorders by activating autophagy. Front. Cell. Neurosci. 2023, 16, 1060712. [Google Scholar] [CrossRef] [PubMed]
- Zachar, G.; Kemecsei, R.; Papp, S.M.; Wéber, K.; Kisparti, T.; Tyler, T.; Gáspár, G.; Balázsa, T.; Csillag, A. D-Aspartate consumption selectively promotes intermediate-term spatial memory and the expression of hippocampal NMDA receptor subunits. Sci. Rep. 2021, 11, 6166. [Google Scholar] [CrossRef]
- Dos Santos Quaresma, M.; Souza, W.; Lemos, V.A.; Caris, A.V.; Thomatieli-Santos, R.V. The Possible Importance of Glutamine Supplementation to Mood and Cognition in Hypoxia from High Altitude. Nutrients 2020, 12, 3627. [Google Scholar] [CrossRef]
- Takaoka, M.; Okumura, S.; Seki, T.; Ohtani, M. Effect of amino-acid intake on physical conditions and skin state: A randomized, double-blind, placebo-controlled, crossover trial. J. Clin. Biochem. Nutr. 2019, 65, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Jauhiainen, R.; Vangipurapu, J.; Laakso, A.; Kuulasmaa, T.; Kuusisto, J.; Laakso, M. The Association of 9 Amino Acids with Cardiovascular Events in Finnish Men in a 12-Year Follow-up Study. J. Clin. Endocrinol. Metab. 2021, 106, 3448–3454. [Google Scholar] [CrossRef]
- Harris, S.; DePalma, J.; Barkoukis, H. Protein and Aging: Practicalities and Practice. Nutrients 2025, 17, 2461. [Google Scholar] [CrossRef]
- Lumu, W.; Bahendeka, S.; Wesonga, R.; Kibirige, D.; Kasoma, R.M.; Ssendikwanawa, E. Atherogenic index of plasma and its cardiovascular risk factor correlates among patients with type 2 diabetes in Uganda. Afr. Heal. Sci. 2023, 23, 515–527. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, B.; Sánchez-Benavides, G.; Genius, P.; Minguillon, C.; Fauria, K.; De Vivo, I.; Navarro, A.; Molinuevo, J.L.; Gispert, J.D.; Sala-Vila, A.; et al. Association between telomere length and cognitive function among cognitively unimpaired individuals at risk of Alzheimer’s disease. Neurobiol. Aging 2024, 141, 140–150. [Google Scholar] [CrossRef]
- Sheikh-Wu, S.F.; Liang, Z.; Downs, C.A. The Relationship Between Telomeres, Cognition, Mood, and Physical Function: A Systematic Review. Biol. Res. Nurs. 2022, 25, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.J.; Koh, S.H. Is Telomere Length Shortening a Risk Factor for Neurodegenerative Disorders? Dement. Neurocogn. Disord. 2022, 21, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Tsatsakis, A.; Renieri, E.; Tsoukalas, D.; Buga, A.M.; Sarandi, E.; Vakonaki, E.; Fragkiadaki, P.; Alegakis, A.; Nikitovic, D.; Calina, D.; et al. A novel nutraceutical formulation increases telomere length and activates telomerase activity in middle-aged rats. Mol. Med. Rep. 2023, 28, 232. [Google Scholar] [CrossRef]
- Yang, D.; Su, J.; Chen, Y.; Chen, G. The NF-κB pathway: Key players in neurocognitive functions and related disorders. Eur. J. Pharmacol. 2024, 984, 177038. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Ndiaye, D.; Korte, M.; Pothion, S.; Arbibe, L.; Prüllage, M.; Pfeiffer, J.; Lindecke, A.; Staiger, V.; Israël, A.; et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol. Cell. Biol. 2006, 26, 2936–2946. [Google Scholar] [CrossRef]
- Mekhora, C.; Lamport, D.J.; Spencer, J.P.E. An overview of the relationship between inflammation and cognitive function in humans, molecular pathways and the impact of nutraceuticals. Neurochem. Int. 2024, 181, 105900. [Google Scholar] [CrossRef]
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med. Cell. Longev. 2015, 2015, 804198. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Wang, C.; Chen, P.; Yang, Q.; Hu, R.; Zhang, H.; Weng, Q.; Xu, M. Expressions of IL-6, TNF-α and NF-κB in the skin of Chinese brown frog (Rana dybowskii). Eur. J. Histochem. 2017, 61, 2834. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhang, Z.; Zhang, H.; Wang, N.; Chen, X.; Han, X.; Lu, Q.; Chi, S. Effects of Dietary Intervention on Inflammatory Markers in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 846591. [Google Scholar] [CrossRef] [PubMed]
- D’Esposito, V.; Di Tolla, M.F.; Lecce, M.; Cavalli, F.; Libutti, M.; Misso, S.; Cabaro, S.; Ambrosio, M.R.; Parascandolo, A.; Covelli, B.; et al. Lifestyle and Dietary Habits Affect Plasma Levels of Specific Cytokines in Healthy Subjects. Front. Nutr. 2022, 9, 913176. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL-6 signaling in acute exercise and chronic training: Potential consequences for health and athletic performance. Scand. J. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Ljungman, P.; Bellander, T.; Schneider, A.; Breitner, S.; Forastiere, F.; Hampel, R.; Illig, T.; Jacquemin, B.; Katsouyanni, K.; von Klot, S.; et al. Modification of the interleukin-6 response to air pollution by interleukin-6 and fibrinogen polymorphisms. Environ. Health Perspect. 2009, 117, 1373–1379. [Google Scholar] [CrossRef]
- Gao, J.; Zhao, S.; Halstensen, T.S. Increased interleukin-6 expression is associated with poor prognosis and acquired cisplatin resistance in head and neck squamous cell carcinoma. Oncol. Rep. 2016, 35, 3265–3274. [Google Scholar] [CrossRef][Green Version]
- Sakunkoo, P.; Thonglua, T.; Sangkham, S.; Jirapornkul, C.; Limmongkon, Y.; Daduang, S.; Tessiri, T.; Rayubkul, J.; Thongtip, S.; Maneenin, N.; et al. Human health risk assessment of PM2.5-bound heavy metal of anthropogenic sources in the Khon Kaen Province of Northeast Thailand. Heliyon 2022, 8, e09572. [Google Scholar] [CrossRef]
- Haß, U.; Herpich, C.; Kochlik, B.; Weber, D.; Grune, T.; lNorman, K. Dietary Inflammatory Index and Cross-Sectional Associations with Inflammation, Muscle Mass and Function in Healthy Old Adults. J. Nutr. Health Aging 2022, 26, 346–351. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Płaczkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Sozański, R.; Grajeta, H. Association of Dietary Inflammatory Index with Serum IL-6, IL-10, and CRP Concentration during Pregnancy. Nutrients 2020, 12, 2789. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Huang, J.; Zhang, J.; Duan, J.; Guo, X.; Wu, S.; Sun, Z. Impact of PM2.5 exposure on plasma metabolome in healthy adults during air pollution waves: A randomized, crossover trial. J. Hazard. Mater. 2022, 436, 129180. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Barman, B.; Thakur, M.K. Oxidative stress-mediated memory impairment during aging and its therapeutic intervention by natural bioactive compounds. Front. Aging Neurosci. 2022, 14, 944697. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Czlapka-Matyasik, M.; Wadolowska, L.; Gut, P.; Gramza-Michałowska, A. Changes in Oxidative Stress, Inflammatory Markers, and Lipid Profile After a 6-Week High-Antioxidant-Capacity Dietary Intervention in CVD Patients. Nutrients 2025, 17, 806. [Google Scholar] [CrossRef]
- Dalby, A.B.; Hofr, C.; Cech, T.R. Contributions of the TEL-patch amino acid cluster on TPP1 to telomeric DNA synthesis by human telomerase. J. Mol. Biol. 2015, 427 Pt B, 1291–1303. [Google Scholar] [CrossRef]
- van Meijl, L.E.; Popeijus, H.E.; Mensink, R.P. Amino acids stimulate Akt phosphorylation, and reduce IL-8 production and NF-κB activity in HepG2 liver cells. Mol. Nutr. Food Res. 2010, 54, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Yohannessa, W.; Diana, S. Systematic review of branched-chain amino acid supplementation high in leucine on interleukin-6. Int. J. Nutr. Pharmacol. Neurol. Dis. 2025, 15, 1–9. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef]
- Ngamsamer, C.; Sirivarasai, J.; Sutjarit, N. The Benefits of Anthocyanins against Obesity-Induced Inflammation. Biomolecules 2022, 12, 852. [Google Scholar] [CrossRef]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and Human Health-A Focus on Oxidative Stress, Inflammation and Disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Zhao, H.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox. Biol. 2020, 32, 101474. [Google Scholar] [CrossRef]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef]
- Da Silvam, C.S.M.; Costa, G.A.M.; Aguiar, A.F.; Carmargo, M.Z.; Fernandes, K.B.S.; Oliviera, M.R.; Da Silva, R.A. Effect of the Use of a Cream with Leucine and Lactic Acid Associated with Electrostimulation in Contouring and Facial Tonus: A Randomized Clinical Controlled Trial. Cosmetics 2022, 9, 36. [Google Scholar] [CrossRef]
- Mozos, I.; Flangea, C.; Vlad, D.C.; Gug, C.; Mozos, C.; Stoian, D.; Luca, C.T.; Horbańczuk, J.O.; Horbańczuk, O.K.; Atanasov, A.G. Effects of Anthocyanins on Vascular Health. Biomolecules 2021, 11, 811. [Google Scholar] [CrossRef]
- Zaric, B.L.; Radovanovic, J.N.; Gluvic, Z.; Stewart, A.J.; Essack, M.; Motwalli, O.; Gojobori, T.; Isenovic, E.R. Atherosclerosis Linked to Aberrant Amino Acid Metabolism and Immunosuppressive Amino Acid Catabolizing Enzymes. Front. Immunol. 2020, 11, 551758. [Google Scholar] [CrossRef] [PubMed]
- Wattanathorn, J.; Thukham-Mee, W.; Tong-Un, T.; Sangartit, W.; Somboonporn, W.; Paholpak, P. A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, 8-Week Pilot Study of Tuna-Byproduct-Derived Novel Supplements for Managing Cellular Senescence and Cognitive Decline in Perimenopausal and Postmenopausal Women. Antioxidants 2025, 14, 520. [Google Scholar] [CrossRef]
- Sim, J.; Lewis, M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef]
- Browne, R.H. On the use of a pilot sample for sample size determination. Stat. Med. 1995, 14, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- In, J. Introduction of a pilot study. Korean J. Anesthesiol. 2017, 70, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Michaels, B.M.; Csank, G.A.; Ryb, G.E.; Eko, F.N.; Rubin, A. Prospective randomized comparison of onabotulinumtoxinA (Botox) and abobotulinumtoxinA (Dysport) in the treatment of forehead, glabellar, and periorbital wrinkles. Aesthetic Surg. J. 2012, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zaa, C.A.; Marcelo, Á.J.; An, Z.; Medina-Franco, J.L.; Velasco-Velázquez, M.A. Anthocyanins: Molecular Aspects on Their Neuroprotective Activity. Biomolecules 2023, 13, 1598. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, R.E.; Goldman, M.P.; Satur, N.M.; Tope, W.D. Pulsed carbon dioxide laser resurfacing of photoaged facial skin. Arch. Dermatol. 1996, 132, 395–402. [Google Scholar] [CrossRef]
- Turapra, B.; Daduang, J.; Wongwattanakul, M.; Bae, C.-Y.; Jearanaikoon, P. Establishment of in-house telomere length measurement using qPCR. Arch. AHS 2022, 34, 44–52. [Google Scholar]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef]
- Wang, Y.; Savage, S.A.; Alsaggaf, R.; Aubert, G.; Dagnall, C.L.; Spellman, S.R.; Lee, S.J.; Hicks, B.; Jones, K.; Katki, H.A.; et al. Telomere Length Calibration from qPCR Measurement: Limitations of Current Method. Cells 2018, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islam. Repub. Iran 2015, 29, 240. [Google Scholar]
- Chan, A.-W.; Boutron, I.; Hopewell, S.; Moher, D.; Schulz, K.F.; Collins, G.S.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. SPIRIT 2025 statement: Updated guideline for protocols of randomised trials. BMJ 2025, 389, e081477. [Google Scholar] [CrossRef]
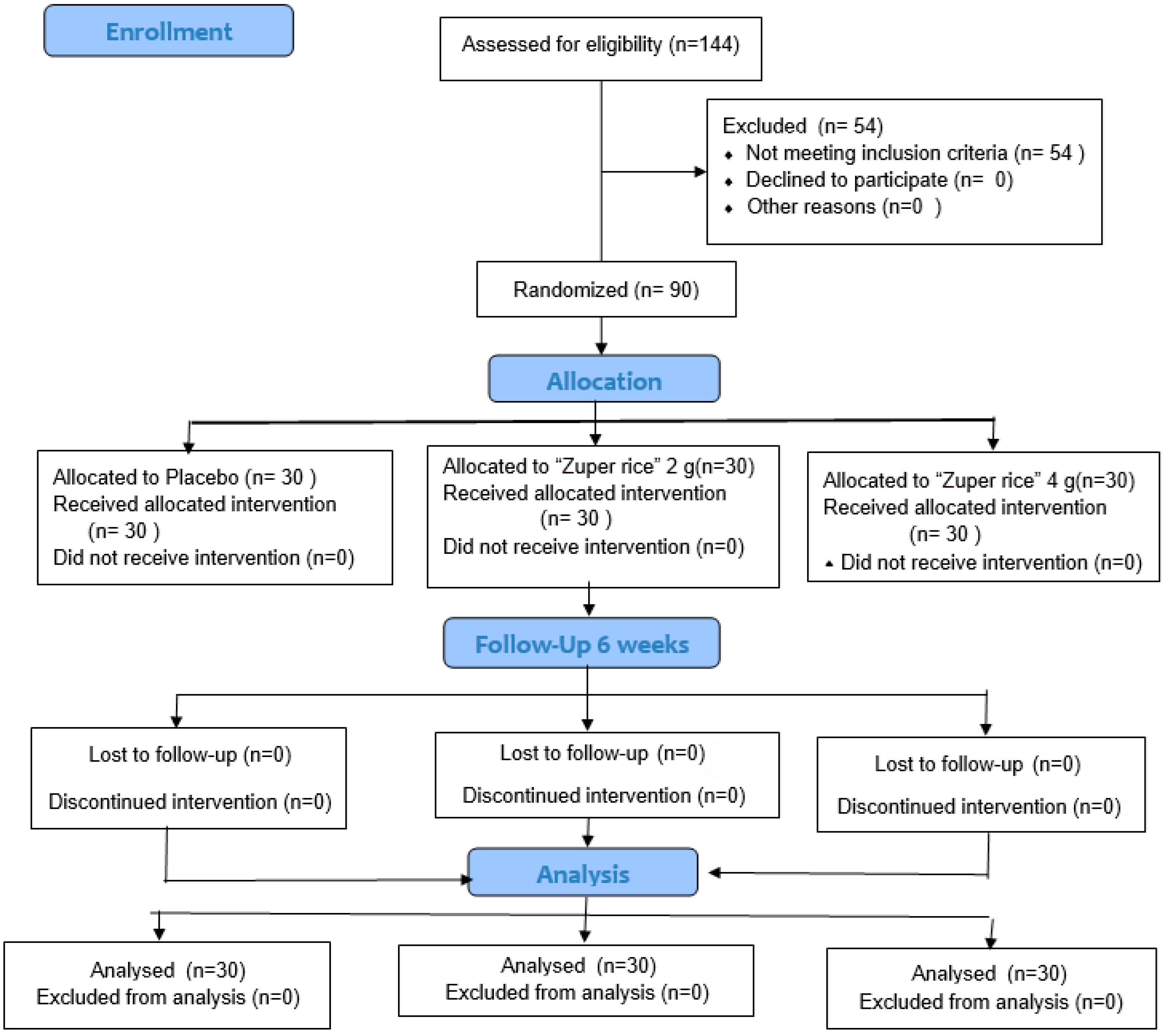
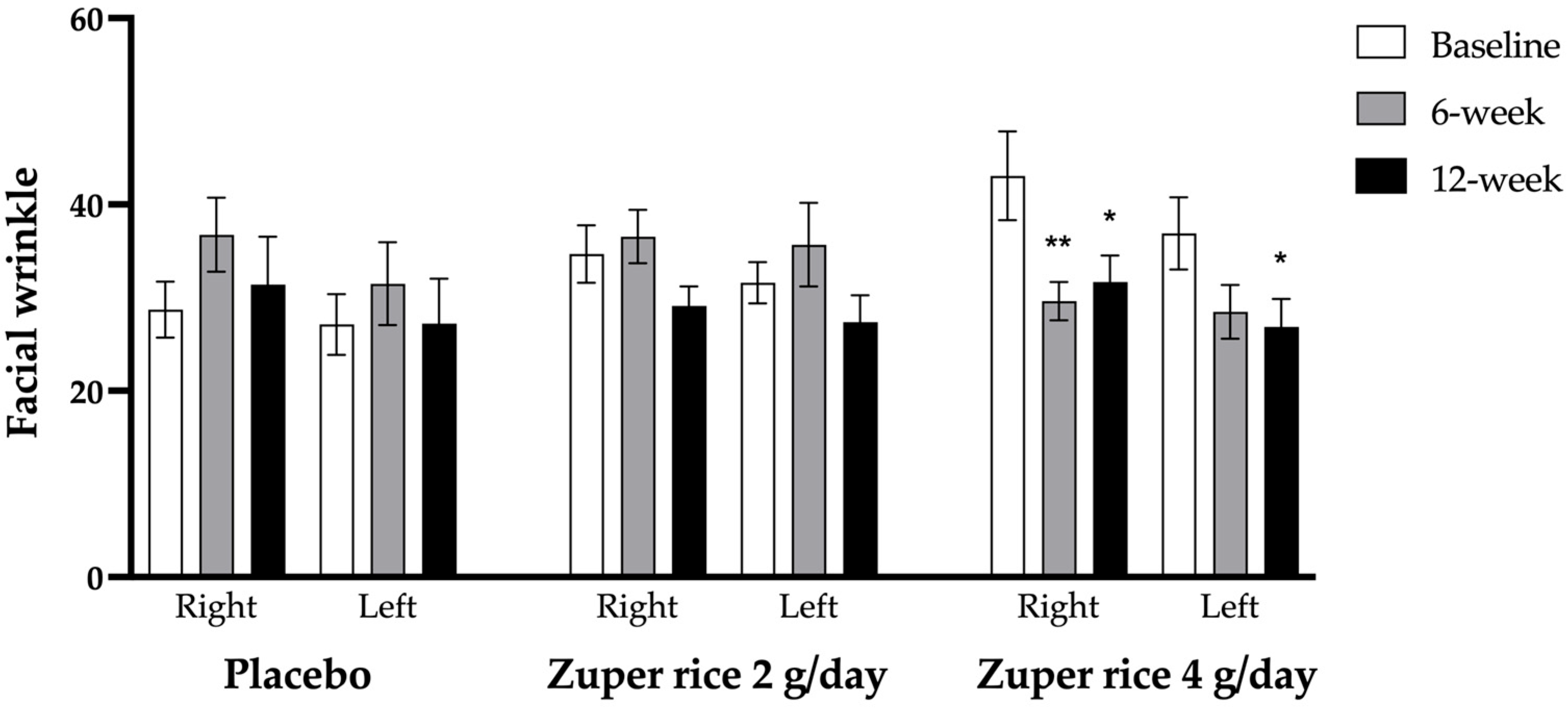
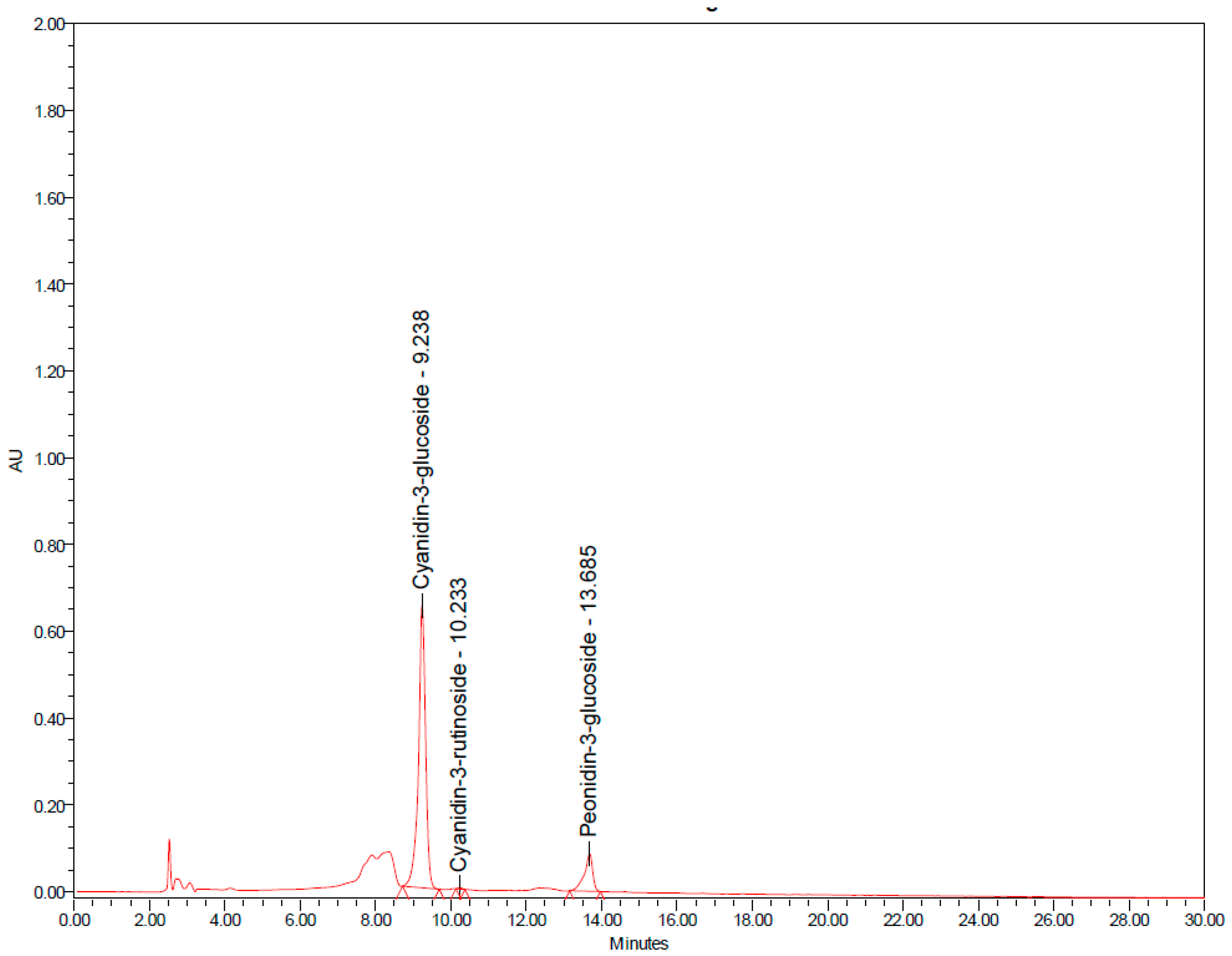
| Parameters | Baseline | 6-Week | 12-Week |
|---|---|---|---|
| Placebo (n = 30) | |||
| Age (years) | 52.80 ± 0.99 | 52.80 ± 0.99 (p = 1.000) | 53.00 ± 1.01 (p = 1.000) |
| Gender (male/female) | 3/27 | 3/27 | 3/27 |
| Blood temperature (°C) | 36.60 ± 0.02 | 36.66 ± 0.02 (p = 0.547) | 36.61 ± 0.02 (p = 1.000) |
| Heart rate (beats/min) | 71.53 ± 1.48 | 71.93 ± 1.84 (p = 1.000) | 70.62 ± 1.13 (p = 0.739) |
| Respiratory rate (breaths/min) | 17.03 ± 0.09 | 17.03 ± 0.06 (p = 1.000) | 17.34 ± 0.10 (p = 0.143) |
| Systolic BP (mmHg) | 119.23 ± 2.10 | 117.10 ± 1.96 (p = 0.645) | 117.62 ± 1.91 (p = 0.266) |
| Diastolic BP (mmHg) | 70.83 ± 2.08 | 71.93 ± 1.92 (p = 1.000) | 72.83 ± 2.04 (p = 0.352) |
| Body weight (kg) | 57.22 ± 1.18 | 56.86 ± 1.16 (p = 0.344) | 57.26 ± 1.10 (p = 0.318) |
| Body height (cm) | 156.93 ± 1.02 | 156.93 ± 1.02 (p = 1.000) | 157.17 ± 1.03 (p = 1.000) |
| BMI (kg/m2) | 23.21 ± 0.39 | 23.08 ± 0.04 (p = 0.379) | 23.18 ± 0.40 (p = 0.339) |
| Zuper rice 2 g/day (n = 30) | |||
| Age (years) | 52.03 ± 0.88 | 52.03 ± 0.88 (p = 1.000) | 52.03 ± 0.88 (p = 1.000) |
| Gender (male/female) | 3/27 | 3/27 | 3/27 |
| Blood temperature (°C) | 36.58 ± 0.02 | 36.60 ± 0.02 (p = 1.000) | 36.61 ± 0.03 (p = 1.000) |
| Heart rate (beats/min) | 71.00 ± 1.05 | 72.67 ± 1.27 (p = 0.669) | 73.37 ± 1.09 (p = 0.161) |
| Respiratory rate (breaths/min) | 17.00 ± 0.07 | 17.10 ± 0.09 (p = 0.791) | 16.97 ± 0.14 (p = 1.000) |
| Systolic BP (mmHg) | 115.00 ± 1.85 | 111.10 ± 1.92 (p = 0.063) | 110.90 ± 1.93 * (p = 0.031) |
| Diastolic BP (mmHg) | 67.70 ± 1.79 | 69.33 ± 1.57 (p = 0.371) | 66.80 ± 1.37 (p = 1.000) |
| Body weight (kg) | 56.90 ± 1.17 | 56.90 ± 1.14 (p = 1.000) | 57.11 ± 1.15 (p = 0.505) |
| Body height (cm) | 157.13 ± 0.90 | 157.13 ± 0.90 (p = 1.000) | 157.13 ± 0.90 (p = 1.000) |
| BMI (kg/m2) | 23.05 ± 0.45 | 23.06 ± 0.44 (p = 1.000) | 23.15 ± 0.45 (p = 0.449) |
| Zuper rice 4 g/day (n = 30) | |||
| Age (years) | 52.27 ± 0.99 | 52.27 ± 0.99 (p = 1.000) | 52.27 ± 0.99 (p = 1.000) |
| Gender (male/female) | 3/27 | 3/27 | 3/27 |
| Blood temperature (°C) | 36.63 ± 0.03 | 36.62 ± 0.02 (p = 1.000) | 36.62 ± 0.02 (p = 1.000) |
| Heart rate (beats/min) | 69.63 ± 1.22 | 69.83 ± 1.35(p = 1.000) | 69.77 ± 1.36 (p = 1.000) |
| Respiratory rate (breaths/min) | 16.97 ± 0.08 | 17.23 ± 0.09(p = 0.054) | 17.23 ± 0.10 (p = 0.090) |
| Systolic BP (mmHg) | 117.23 ± 2.25 | 114.00 ± 2.16(p = 0.271) | 113.50 ± 2.15 (p = 0.105) |
| Diastolic BP (mmHg) | 70.53 ± 1.66 | 69.17 ± 1.67 (p = 0.860) | 69.77 ± 1.17 (p = 1.000) |
| Body weight (kg) | 55.69 ± 0.97 | 55.31 ± 1.02 (p = 0.247) | 55.24 ± 1.03 (p = 0.075) |
| Body height (cm) | 154.73 ± 0.91 | 154.73 ± 0.91 (p = 1.000) | 154.73 ± 0.91 (p = 1.000) |
| BMI (kg/m2) | 23.26 ± 0.35 | 23.10 ± 0.37 (p = 0.252) | 23.06 ± 0.37 (p = 0.062) |
| Parameters | Placebo (n = 30) | Zuper Rice (2 g/day) (n = 30) | Zuper Rice (4 g/day) (n = 30) | |
|---|---|---|---|---|
| Baseline | ||||
| Word Recognition | Time | 1345.90 ± 81.37 | 1360.34 ± 77.05 (p = 0.826) | 1361.39 ± 82.38 (p = 0.826) |
| %Accuracy | 85.73 ± 2.04 | 86.74 ± 1.85 (p = 0.740) | 86.79 ± 1.64 (p = 0.953) | |
| Picture Recognition | Time | 1425.40 ± 79.63 | 1389.78 ± 64.60 (p = 0.940) | 1412.89 ± 71.94 (p = 0.983) |
| %Accuracy | 86.80 ± 1.43 | 84.46 ± 1.61 (p = 0.272) | 83.40 ± 1.77 (p = 0.165) | |
| Simple Reaction | Time | 664.49 ± 26.76 | 659.13 ± 23.20 (p = 1.000) | 681.48 ± 25.99 (p = 0.734) |
| Digit Vigilance | Time | 663.19 ± 10.09 | 669.03 ± 8.75 (p = 0.735) | 660.58 ± 11.32 (p = 0.778) |
| %Accuracy | 92.61 ± 0.91 | 94.23 ± 0.82 (p = 0.179) | 93.33 ± 1.04 (p = 0.459) | |
| Choice Reaction Time | Time | 835.89 ± 20.80 | 836.95 ± 17.92 (p = 0.967) | 826.47 ± 15.14 (p = 0.719) |
| %Accuracy | 98.24 ± 0.46 | 98.14 ± 0.41 (p = 0.702) | 97.68 ± 0.86 (p = 1.000) | |
| Spatial Memory | Time | 1451.34 ± 57.83 | 1351.73 ± 45.77 (p = 0.186) | 1469.67 ± 58.10 (p = 0.815) |
| %Accuracy | 90.99 ± 2.18 | 91.76 ± 1.87 (p = 0.747) | 84.77 ± 1.48 (p = 0.213) | |
| Numeric Working Memory | Time | 1169.06 ± 43.17 | 1114.13 ± 37.86 (p = 0.376) | 1171.36 ± 33.95 (p = 0.798) |
| %Accuracy | 92.53 ± 2.09 | 96.66 ± 1.13 (p = 0.072) | 95.60 ± 1.25 (p = 0.309) | |
| 6 weeks | ||||
| Word Recognition | Time | 1145.57 ± 34.68 | 1153.29 ± 39.92 (p = 0.889) | 1193.42 ± 39.29 (p = 0.376) |
| %Accuracy | 86.01 ± 2.06 | 90.52 ± 1.75 (p = 0.086) | 89.09 ± 1.64 (p = 0.340) | |
| Picture Recognition | Time | 1394.51 ± 82.71 | 1266.91 ± 40.87 (p = 0.339) | 1382.52 ± 77.40 (p = 0.706) |
| %Accuracy | 87.75 ± 1.79 | 88.68 ± 1.86 (p = 0.730) | 86.59 ± 1.98 (p = 0.739) | |
| Simple Reaction | Time | 688.08 ± 38.58 | 660.53 ± 30.02 (p = 0.536) | 648.74 ± 16.19 (p = 0.378) |
| Digit Vigilance | Time | 674.27 ± 11.58 | 675.71 ± 9.89 (p = 0.966) | 677.60 ± 7.15 (p = 0.772) |
| %Accuracy | 90.25 ± 1.22 | 93.45 ± 1.34 (p = 0.083) | 93.53 ± 0.85 * (p = 0.036) | |
| Choice Reaction Time | Time | 907.73 ± 48.24 | 825.00 ± 21.65 (p = 0.071) | 840.50 ± 14.75 (p = 0.127) |
| %Accuracy | 96.80 ± 0.63 | 98.52 ± 0.47 * (p = 0.035) | 98.00 ± 0.39 (p = 0.164) | |
| Spatial Memory | Time | 1428.15 ± 67.40 | 1411.57 ± 90.03 (p = 0.555) | 1437.83 ± 51.67 (p = 0.687) |
| %Accuracy | 95.14 ± 1.31 | 96.63 ± 1.07 (p = 0.476) | 95.57 ± 1.24 (p = 0.843) | |
| Numeric Working Memory | Time | 1205.42 ± 87.48 | 1122.24 ± 43.40 (p = 0.329) | 1147.51 ± 32.35 (p = 0.480) |
| %Accuracy | 94.50 ± 1.95 | 97.36 ± 1.09 (p = 0.429) | 95.15 ± 1.70 (p = 1.000) | |
| 12 weeks | ||||
| Word Recognition | Time | 1236.37± 81.34 | 1170.18 ± 70.53 (p = 0.765) | 1281.69± 66.01 (p = 0.451) |
| %Accuracy | 89.55 ± 1.83 | 88.71 ± 2.34 (p = 0.871) | 90.63 ± 1.46 (p = 0.636) | |
| Picture Recognition | Time | 1397.87 ± 81.59 | 1189.39 ± 61.84 * (p = 0.018) | 1287.25 ± 48.33 (p = 0.255) |
| %Accuracy | 88.33 ± 1.59 | 86.15 ± 2.66 (p = 0.603) | 87.61 ± 1.64 (p = 0.908) | |
| Simple Reaction | Time | 753.56 ± 74.11 | 663.18 ± 45.56 (p = 0.259) | 676.90 ± 25.02 (p = 0.395) |
| Digit Vigilance | Time | 677.55 ± 14.10 | 660.62 ± 14.70 (p = 0.279) | 670.76 ± 9.57 (p = 0.422) |
| %Accuracy | 91.36 ± 1.46 | 94.59 ± 1.33 (p = 0.129) | 92.67 ± 1.09 (p = 0.487) | |
| Choice Reaction Time | Time | 893.01 ± 49.60 | 831.73 ± 28.73 (p = 0.420) | 869.68 ± 21.26 (p = 0.692) |
| %Accuracy | 98.40 ± 0.40 | 98.15 ± 0.47 (p = 0.713) | 98.28 ± 0.39 (p = 0.959) | |
| Spatial Memory | Time | 1409.67 ± 95.82 | 1273.87 ± 75.84 (p = 0.475) | 1497.62 ± 76.97 (p = 0.328) |
| %Accuracy | 96.10 ± 1.29 | 96.36 ± 1.45 (p = 0.785) | 94.84 ± 1.50 (p = 0.825) | |
| Numeric Working Memory | Time | 1194.36 ± 73.14 | 1093.55 ± 47.35 (p = 0.596) | 1127.09 ± 30.52 (p = 0.885) |
| %Accuracy | 94.00 ± 1.92 | 95.38 ± 1.75 (p = 0.612) | 94.76 ± 1.18 (p = 0.987) |
| Parameters | Reference | Baseline | 6-Week | 12-Week |
|---|---|---|---|---|
| Placebo (n = 30) | ||||
| RBC | 4.7–6.2 106/μL | 4.57 ± 0.07 | 4.68 ± 0.06 (p = 0.154) | 4.57 ± 0.06 (p = 1.000) |
| MCV | 80.0–97.8 fL | 86.27 ± 1.25 | 85.83 ± 1.18 (p = 0.962) | 87.49 ± 1.27 * (p = 0.035) |
| MCH | 25.2–32.0 pg | 27.60 ± 0.46 | 27.33 ± 0.42 (p = 0.096) | 27.63 ± 0.44 (p = 1.000) |
| MCHC | 31.3–33.4 g/dL | 31.96 ± 0.12 | 31.84 ± 0.15 (p = 1.000) | 31.57 ± 0.15 ** (p = 0.004) |
| RDW | 11.9–14.8% | 13.66 ± 0.22 | 13.50 ± 0.19 (p = 0.253) | 13.63 ± 0.22 (p = 1.000) |
| Zuper rice 2 g/day (n = 30) | ||||
| RBC | 4.7–6.2 106/μL | 4.70 ± 0.10 | 4.75 ± 0.12 (p = 1.000) | 4.81 ± 0.11 (p = 0.107) |
| MCV | 80.0–97.8 fL | 81.49 ± 2.02 | 81.33 ± 2.08 (p = 1.000) | 81.12 ± 2.06 (p = 0.504) |
| MCH | 25.2–32.0 pg | 25.43 ± 0.69 | 25.49 ± 0.71 (p = 1.000) | 25.30 ± 0.71 (p = 1.000) |
| MCHC | 31.3–33.4 g/dL | 31.16 ± 0.21 | 31.29 ± 0.18 (p = 1.000) | 31.15 ± 0.24 (p = 1.000) |
| RDW | 11.9–14.8% | 14.68 ± 0.43 | 14.70 ± 0.43 (p = 1.000) | 14.51 ± 0.42 (p = 0.310) |
| Zuper rice 4 g/day (n = 30) | ||||
| RBC | 8.7–12.5 fL | 4.58 ± 0.09 | 4.67 ± 0.09 (p = 0.346) | 4.63 ± 0.10 (p = 1.000) |
| MCV | 4.7–6.2 106/μL | 84.11 ± 1.95 | 83.34 ± 1.93 (p = 0.665) | 84.06 ± 1.93 (p = 0.755) |
| MCH | 80.0–97.8 fL | 26.66 ± 0.68 | 26.32 ± 0.67 (p = 0.504) | 26.43 ± 0.67 (p = 1.000) |
| MCHC | 25.2–32.0 pg | 31.65 ± 0.22 | 31.57 ± 0.22 (p = 1.000) | 31.41 ± 0.22 (p = 0.317) |
| RDW | 31.3–33.4 g/dL | 14.38 ± 0.46 | 14.24 ± 0.42 (p = 0.665) | 14.19 ± 0.46 (p = 0.394) |
| Parameters | Reference | Baseline | 6-Week | 12-Week |
|---|---|---|---|---|
| Placebo (n = 30) | ||||
| BUN | 5.8–19.1 mg/dL | 12.14 ± 0.57 | 11.85 ± 0.56 (p = 1.000) | 11.65 ± 0.45 (p = 0.896) |
| Creatinine | 0.5–1.5 mg/dL | 0.83 ± 0.02 | 0.81 ± 0.02 (p = 0.994) | 0.80 ± 0.02 (p = 0.215) |
| Sodium | 130–147 mEq/L | 139.07 ± 0.30 | 139.40 ± 0.37 (p = 0.264) | 139.07 ± 0.34 (p = 1.000) |
| Potassium | 3.4–4.7 mEq/L | 4.50 ± 0.06 | 4.38 ± 0.08 (p = 0.360) | 4.51 ± 0.10 (p = 1.000) |
| Bicarbonate | 20.6–28.3 mEq/L | 22.40 ± 0.54 | 21.94 ± 0.30 (p = 1.000) | 22.96 ± 0.38 (p = 0.653) |
| Chloride | 96–107 mEq/L | 101.27 ± 0.37 | 101.30 ± 0.50 (p = 1.000) | 101.79 ± 0.40 (p = 0.465) |
| Albumin | 3.8–5.4 g/dL | 4.37 ± 0.04 | 4.47 ± 0.04 ** (p = 0.003) | 4.39 ± 0.04 (p = 1.000) |
| Total Bilirubin | 0.3–1.5 mg/dL | 0.49 ± 0.07 | 0.61 ± 0.08 (p = 0.053) | 0.52 ± 0.07 (p = 1.000) |
| ALT | 4–36 U/L | 16.47 ± 1.21 | 15.77 ± 1.15 (p = 1.000) | 17.90 ± 1.92 (p = 1.000) |
| AST | 12–32 U/L | 21.47 ± 1.04 | 22.43 ± 1.10 (p = 0.647) | 23.48 ± 1.75 (p = 0.406) |
| ALP | 42–121 U/L | 78.60 ± 7.28 | 74.23 ± 3.98 (p = 1.000) | 78.24 ± 4.52 (p = 1.000) |
| Thyroxine (T4) | 4.5–11.7 μg/dL | 7.30 ± 0.31 | 7.00 ± 0.24 (p = 0.838) | 6.61 ± 0.22 * (p = 0.011) |
| Triiodothyronine (T3) | 80–200 ng/dL | 108.25 ± 3.22 | 110.33 ± 4.17 (p = 1.000) | 99.44 ± 3.00 * (p = 0.012) |
| Cholesterol | Less than 200 mg/dL | 206.47 ± 5.23 | 216.20 ± 7.44 (p = 0.093) | 212.34 ± 6.66 (p = 0.173) |
| Triglyceride | 10–200 mg/dL | 120.83 ± 11.42 | 114.60 ± 7.08 (p = 1.000) | 124.59 ± 11.19 (p = 1.000) |
| HDL-Chol | >35 mg/dL | 59.37 ± 2.63 | 61.87 ± 3.04 (p = 0.206) | 61.62 ± 3.05 (p = 0.241) |
| AI | - | 2.48 ± 0.16 | 2.49 ± 0.14 (p = 1.000) | 2.44 ± 0.15 (p = 1.000) |
| LDL-Chol (DIRECT) | 10–150 mg/dL | 134.50 ± 5.04 | 143.77 ± 6.75 * (p = 0.038) | 137.14 ± 5.97 (p = 0.833) |
| Zuper rice 2 g/day (n = 30) | ||||
| BUN | 5.8–19.1 mg/dL | 10.47 ± 0.56 | 10.53 ± 0.53 (p = 1.000) | 10.73 ± 0.51 (p = 1.000) |
| Creatinine | 0.5–1.5 mg/dL | 0.78 ± 0.01 | 0.79 ± 0.02 (p = 1.000) | 0.77 ± 0.01 (p = 0.641) |
| Sodium | 130–147 mEq/L | 138.87 ± 0.44 | 139.40 ± 0.37 (p = 0.765) | 138.80 ± 0.42 (p = 1.000) |
| Potassium | 3.4–4.7 mEq/L | 4.55 ± 0.09 | 4.51 ± 0.07 (p = 1.000) | 4.47 ± 0.06 (p = 1.000) |
| Bicarbonate | 20.6–28.3 mEq/L | 22.62 ± 0.35 | 21.03 ± 0.37 ** (p = 0.001) | 21.43 ± 0.35 * (p = 0.016) |
| Chloride | 96–107 mEq/L | 101.23 ± 0.37 | 101.80 ± 0.35 (p = 0.596) | 101.67 ± 0.32 (p = 1.000) |
| Albumin | 3.8–5.4 g/dL | 4.33 ± 0.05 | 4.35 ± 0.05 (p = 1.000) | 4.31 ± 0.05 (p = 1.000) |
| Total Bilirubin | 0.3–1.5 mg/dL | 0.47 ± 0.04 | 0.49 ± 0.04 (p = 0.795) | 0.51 ± 0.04 (p = 0.482) |
| ALT | 4–36 U/L | 16.60 ± 1.35 | 19.23 ± 1.59 (p = 0.053) | 21.57 ± 2.69 (p = 0.102) |
| AST | 12–32 U/L | 22.07 ± 1.35 | 24.93 ± 1.36 ** (p = 0.007) | 24.73 ± 1.78 (p = 0.139) |
| ALP | 42–121 U/L | 72.10 ± 4.23 | 74.87 ± 4.50 (p = 0.193) | 79.505 ± 5.22 ** (p = 0.004) |
| Thyroxine (T4) | 4.5–11.7 μg/dL | 7.30 ± 0.32 | 6.78 ± 0.30 (p = 0.207) | 6.57 ± 0.22 ** (p = 0.001) |
| Triiodothyronine (T3) | 80–200 ng/dL | 107.52 ± 3.98 | 108.35 ± 4.12 (p = 1.000) | 102.08 ± 3.43 (p = 0.158) |
| Cholesterol | Less than 200 mg/dL | 206.93 ± 5.94 | 209.43 ± 6.80 (p = 1.000) | 210.37 ± 6.17 (p = 0.765) |
| Triglyceride | 10–200 mg/dL | 113.77 ± 7.70 | 115.87 ± 8.94 (p = 1.000) | 112.33 ± 7.45 (p = 1.000) |
| HDL-Chol | >35 mg/dL | 60.70 ± 2.71 | 63.37 ± 3.38 (p = 0.202) | 63.63 ± 3.25 (p = 0.084) |
| AI | - | 2.45 ± 0.13 | 2.30 ± 0.16 p = 1.000) | 2.31 ± 0.16 (p = 1.000) |
| LDL-Chol (DIRECT) | 10–150 mg/dL | 135.40 ± 5.52 | 135.67 ± 5.95 (p = 1.000) | 138.00 ± 5.73 (p = 1.000) |
| Zuper rice 4 g/day (n = 30) | ||||
| BUN | 5.8–19.1 mg/dL | 11.59 ± 0.67 | 11.09 ± 0.62 (p = 0.958) | 11.09 ± 0.59 (p = 0.855) |
| Creatinine | 0.5–1.5 mg/dL | 0.78 ± 0.01 | 0.76 ± 0.01 * (p = 0.012) | 0.75 ± 0.01 ** (p = 0.002) |
| Sodium | 130–147 mEq/L | 138.93 ± 0.30 | 138.93 ± 0.26 (p = 1.000) | 138.73 ± 0.34 (p = 1.000) |
| Potassium | 3.4–4.7 mEq/L | 4.63 ± 0.09 | 4.44 ± 0.05 (p = 0.186) | 4.38 ± 0.06 (p = 0.071) |
| Bicarbonate | 20.6–28.3 mEq/L | 21.39 ± 0.54 | 21.43 ± 0.39 (p = 1.000) | 22.72 ± 0.41 (p = 0.193) |
| Chloride | 96–107 mEq/L | 102.10 ± 0.29 | 101.50 ± 0.39 (p = 0.178) | 101.27 ± 0.34 (p = 0.195) |
| Albumin | 3.8–5.4 g/dL | 4.29 ± 0.04 | 4.33 ± 0.04 (p = 0.950) | 4.32 ± 0.04 (p = 0.989) |
| Total Bilirubin | 0.3–1.5 mg/dL | 0.40 ± 0.04 | 0.47 ± 0.03 (p = 0.151) | 0.46 ± 0.03 (p = 0.457) |
| ALT | 4–36 U/L | 17.13 ± 1.07 | 16.13 ± 1.16 (p = 0.999) | 17.17 ± 1.76 (p = 1.000) |
| AST | 12–32 U/L | 23.27 ± 1.00 | 22.90 ± 0.86 (p = 1.000) | 23.13 ± 1.06 (p = 1.000) |
| ALP | 42–121 U/L | 72.10 ± 4.76 | 72.47 ± 4.23 (p = 1.000) | 74.73 ± 4.33 (p = 1.000) |
| Thyroxine (T4) | 4.5–11.7 μg/dL | 6.74 ± 0.21 | 6.84 ± 0.23 (p = 1.000) | 6.46 ± 0.19 (p = 0.226) |
| Triiodothyronine (T3) | 80–200 ng/dL | 106.05 ± 3.08 | 112.83 ± 4.45 (p = 0.177) | 109.90 ± 4.63 (p = 0.796) |
| Cholesterol | Less than 200 mg/dL | 200.87 ± 6.16 | 203.73 ± 7.22 (p = 1.000) | 206.43 ± 6.22 (p = 0.662) |
| Triglyceride | 10–200 mg/dL | 115.97 ± 11.46 | 117.07 ± 14.32 (p = 1.000) | 103.00 ± 10.52 (p = 0.131) |
| HDL-Chol | >35 mg/dL | 57.50 ± 2.69 | 59.30 ± 2.78 (p = 0.489) | 61.57 ± 2.90 * (p = 0.039) |
| AI | - | 2.49 ± 0.19 | 2.43 ± 0.20 (p = 0.785) | 2.35 ± 0.16 (p = 0.117) |
| LDL-Chol (DIRECT) | 10–150 mg/dL | 129.77 ± 5.54 | 131.43 ± 5.96 (p = 1.000) | 134.07 ± 5.35 (p = 0.869) |
| Atherogenic Index In Plasma (AIP) | References | Placebo | “Zuper Rice” 2 g/day | “Zuper Rice” 4 g/day |
|---|---|---|---|---|
| Baseline | <0.11 | 0.31 ± 0.07 | 0.27 ± 0.05 | 0.30 ± 0.06 |
| 6-week | <0.11 | 0.27 ± 0.05 (p = 1.000) | 0.26 ± 0.09 (p = 0.950) | 0.26 ± 0.06 (p = 0.334) |
| 12-week | <0.11 | 0.30 ± 0.06 (p = 0.666) | 0.24 ± 0.07 (p = 1.000) | 0.22 ± 0.06 * (p = 0.030) |
| 8-OHdG (ng/mL) | Baseline | 6-Week | 12-Week |
|---|---|---|---|
| Placebo (n = 30) | 199.71 ± 13.51 | 199.20 ± 12.33 (p = 1.000) | 140.43 ± 20.76 (p = 0.059) |
| Zuper rice 2 g/day (n = 30) | 204.91 ± 17.02 | 199.07 ± 18.87 (p = 0.330) | 172.20 ± 20.63 (p = 0.433) |
| Zuper rice 4 g/day (n = 30) | 243.62 ± 17.47 | 244.85 ± 18.56 (p = 0.109) | 157.62 ± 17.18 * (p = 0.031) |
| Parameters | Baseline | 6-Week | 12-Week |
|---|---|---|---|
| NF-kB (ng/mL) | |||
| Placebo (n = 30) | 3.37 ± 0.29 | 4.29± 0.50 (p = 0.108) | 2.34 ± 0.18 (p = 0.071) |
| Zuper rice 2 g/day (n = 30) | 3.67 ± 0.26 | 4.92 ± 0.53 * (p = 0.031) | 2.29 ± 0.16 ** (p = 0.004) |
| Zuper rice 4 g/day (n = 30) | 3.14 ± 0.33 | 3.42 ± 0.63 (p = 1.000) | 2.31 ± 0.19 (p = 0.295) |
| TNF-α(ng/mL) | |||
| Placebo (n = 30) | 3.25 ± 0.72 | 4.56 ± 1.69 (p = 0.464) | 4.73 ± 0.40 (p = 0.565) |
| Zuper rice 2 g/day (n = 30) | 5.70 ± 3.12 | 5.19 ± 1.67 (p = 1.000) | 4.91 ± 0.42 (p = 0.306) |
| Zuper rice 4 g/day (n = 30) | 1.56 ± 0.65 | 2.35 ± 0.68 (p = 0.890) | 4.64 ± 0.25 (p = 0.451) |
| IL-6(pg/mL) | |||
| Placebo (n = 30) | 11.43 ± 3.99 | 10.57 ± 2.31 (p = 1.000) | 38.24 ± 14.76 (p = 0.389) |
| Zuper rice 2 g/day (n = 30) | 12.73 ± 2.44 | 15.53 ± 2.35 (p = 0.639) | 21.70 ± 1.00 ** (p = 0.006) |
| Zuper rice 4 g/day (n = 30) | 7.53 ± 1.76 | 8.40 ± 1.82 (p = 1.000) | 24.30 ± 1.59 *** (p < 0.001) |
| Parameters | Placebo (n = 30) | Zuper Rice 2 g/day (n = 30) | Zuper Rice 4 g/day (n = 30) |
|---|---|---|---|
| Telomere Length | |||
| Baseline | 4.96 ± 0.05 | 4.98 ± 0.07 | 4.98 ± 0.06 |
| 6-week | 5.02 ± 0.08 (p = 1.000) | 4.95 ± 0.05 (p = 1.000) | 4.97 ± 0.08 (p = 1.000) |
| 12-week | 5.23 ± 0.31 (p = 1.000) | 5.46 ± 0.24 ** (p = 0.006) | 4.55 ± 0.35 (p = 0.629) |
| Telomerase (ng/mL) | |||
| Baseline | 13.29 ± 0.89 | 14.27 ± 0.67 | 12.32 ± 0.84 |
| 6-week | 11.83 ± 1.08 (p = 0.165) | 12.80 ± 1.05 (p = 0.326) | 10.51 ± 1.46 (p = 0.681) |
| 12-week | 16.09 ± 0.90 (p = 0.102) | 17.57 ± 0.87 * (p = 0.035) | 15.70 ± 0.87 (p = 0.078) |
| PCR Primers | Oligomer Sequence (5′–3′) | Amplicon Size |
|---|---|---|
| teloF | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGG TTTGGGTT | >76 bp |
| teloR | GGCTTGCCTTACCCTTACCCTTACCC TTACCCTTACCCT | |
| 36B4F | CAGCAAGTGGGAAGGTGTAATCC | 75 bp |
| 36B4R | CCCATTCTATCATCAACGGGTACAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanathorn, J.; Thukham-mee, W.; Phuthong, S.; Sangartit, W.; Thong-un, T.; Kotruchin, P.; Mitsungnern, T.; Im-uan, S.; Sirijun, N.; Muchimapura, S. An Anthocyanin- and Anti-Ageing Amino Acids-Enriched Pigmented Rice Innovation Promotes Healthy Ageing Through the Modulation of Telomere, Oxidative Stress and Inflammation Reduction: A Randomized Clinical Trial. Int. J. Mol. Sci. 2025, 26, 10911. https://doi.org/10.3390/ijms262210911
Wattanathorn J, Thukham-mee W, Phuthong S, Sangartit W, Thong-un T, Kotruchin P, Mitsungnern T, Im-uan S, Sirijun N, Muchimapura S. An Anthocyanin- and Anti-Ageing Amino Acids-Enriched Pigmented Rice Innovation Promotes Healthy Ageing Through the Modulation of Telomere, Oxidative Stress and Inflammation Reduction: A Randomized Clinical Trial. International Journal of Molecular Sciences. 2025; 26(22):10911. https://doi.org/10.3390/ijms262210911
Chicago/Turabian StyleWattanathorn, Jintanaporn, Wipawee Thukham-mee, Sophida Phuthong, Weerapon Sangartit, Terdthai Thong-un, Praew Kotruchin, Thapanawong Mitsungnern, Suphap Im-uan, Nitiwat Sirijun, and Supaporn Muchimapura. 2025. "An Anthocyanin- and Anti-Ageing Amino Acids-Enriched Pigmented Rice Innovation Promotes Healthy Ageing Through the Modulation of Telomere, Oxidative Stress and Inflammation Reduction: A Randomized Clinical Trial" International Journal of Molecular Sciences 26, no. 22: 10911. https://doi.org/10.3390/ijms262210911
APA StyleWattanathorn, J., Thukham-mee, W., Phuthong, S., Sangartit, W., Thong-un, T., Kotruchin, P., Mitsungnern, T., Im-uan, S., Sirijun, N., & Muchimapura, S. (2025). An Anthocyanin- and Anti-Ageing Amino Acids-Enriched Pigmented Rice Innovation Promotes Healthy Ageing Through the Modulation of Telomere, Oxidative Stress and Inflammation Reduction: A Randomized Clinical Trial. International Journal of Molecular Sciences, 26(22), 10911. https://doi.org/10.3390/ijms262210911








