Defensin-Rich Platelets Drive Pro-Tumorigenic Programs in Pancreatic Adenocarcinoma
Abstract
1. Introduction
2. Results
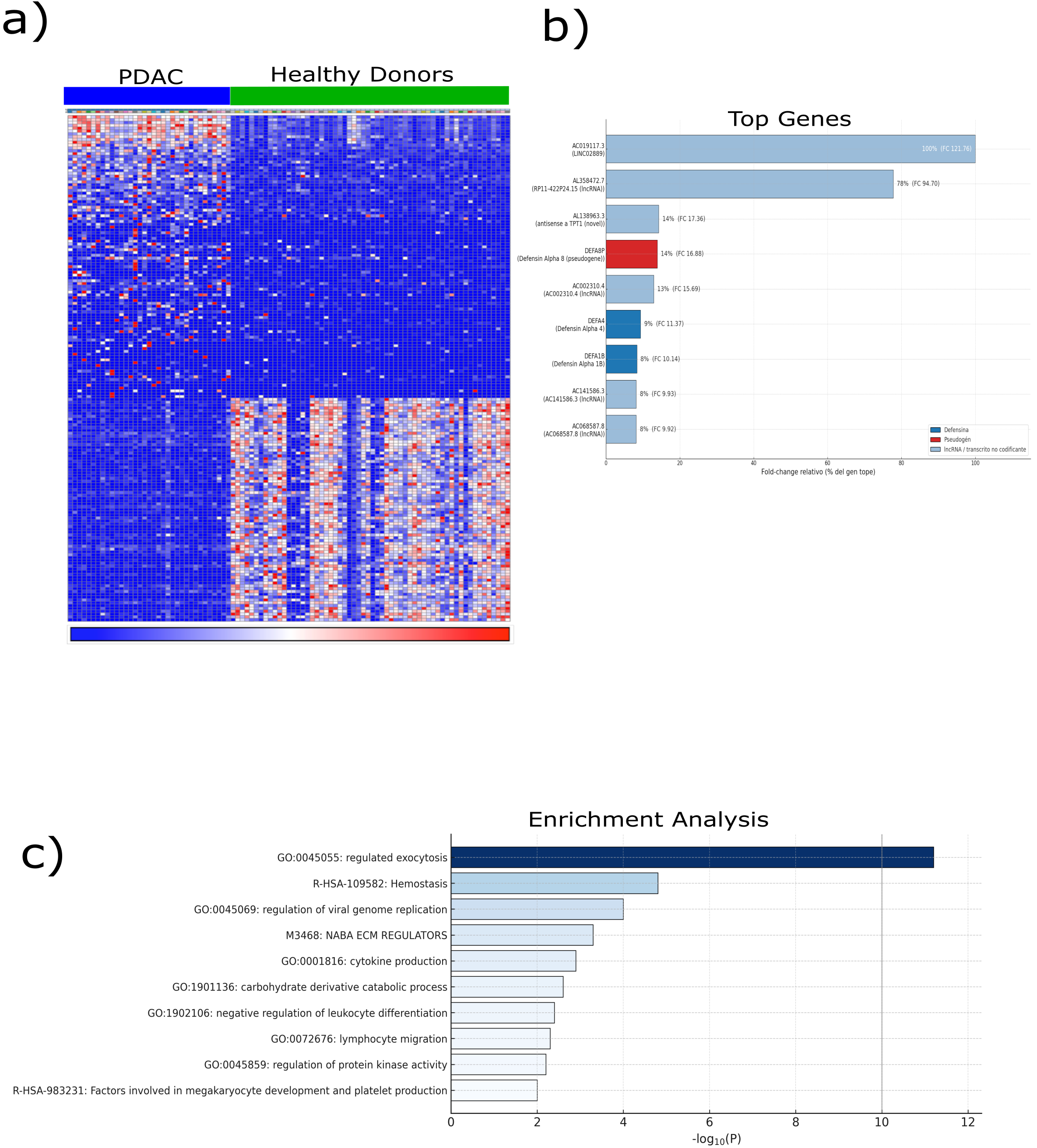
2.1. Transcriptomic Alterations in PDAC-Derived Platelets
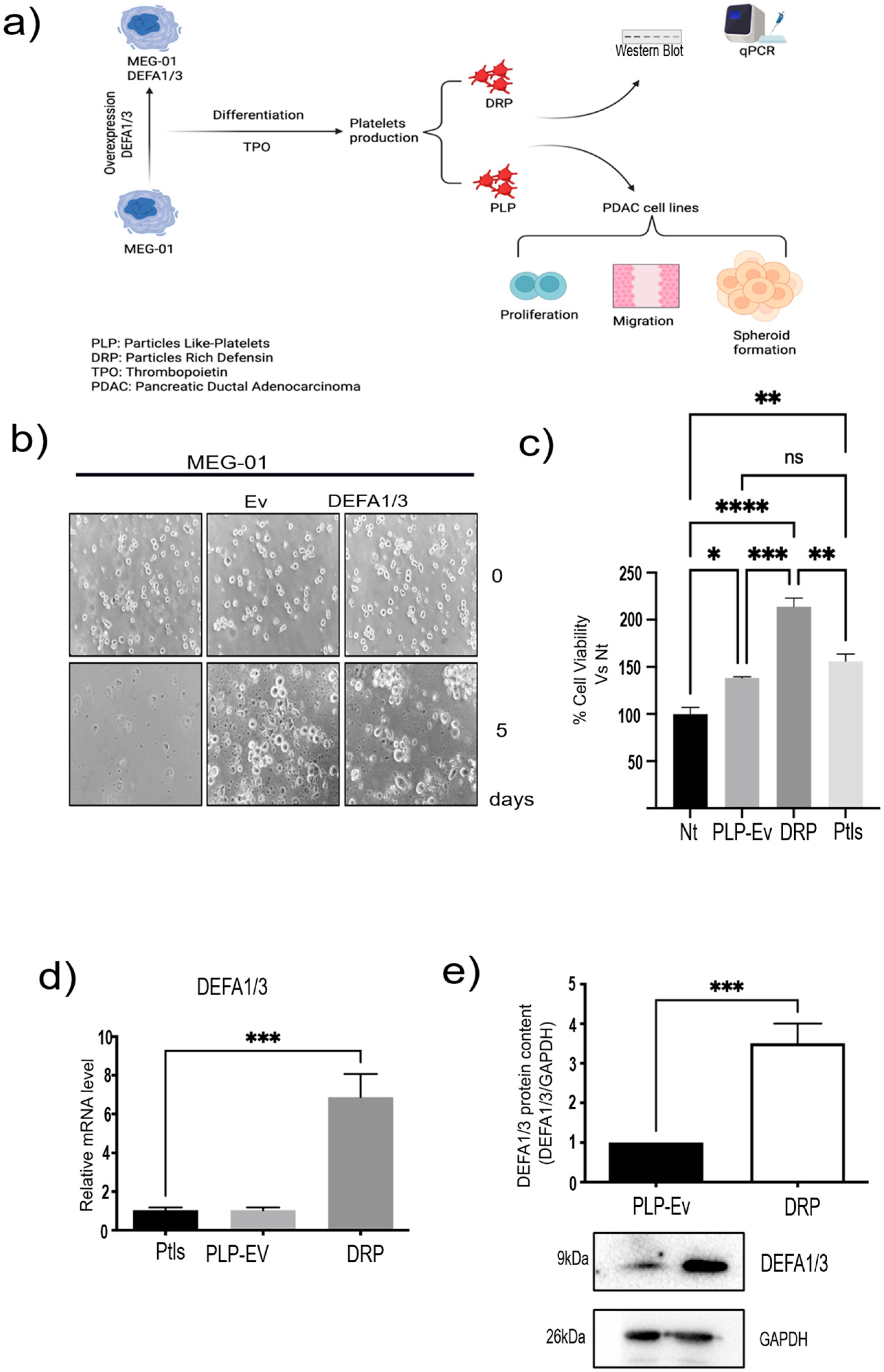
2.2. Generation of an In Vitro Model of DRPs
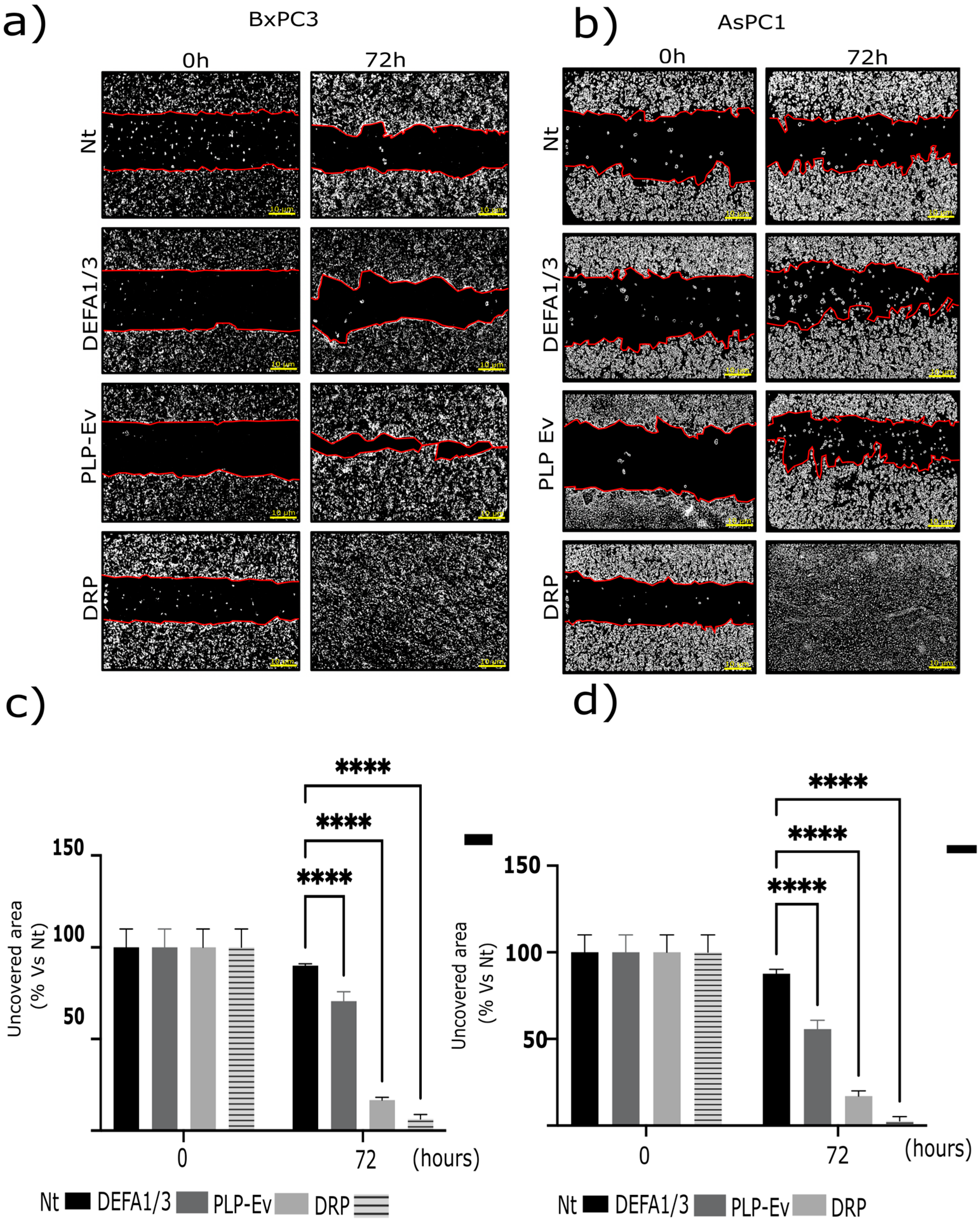
2.3. DRPs Enhance the Migratory Capacity of Pancreatic Cancer Cells
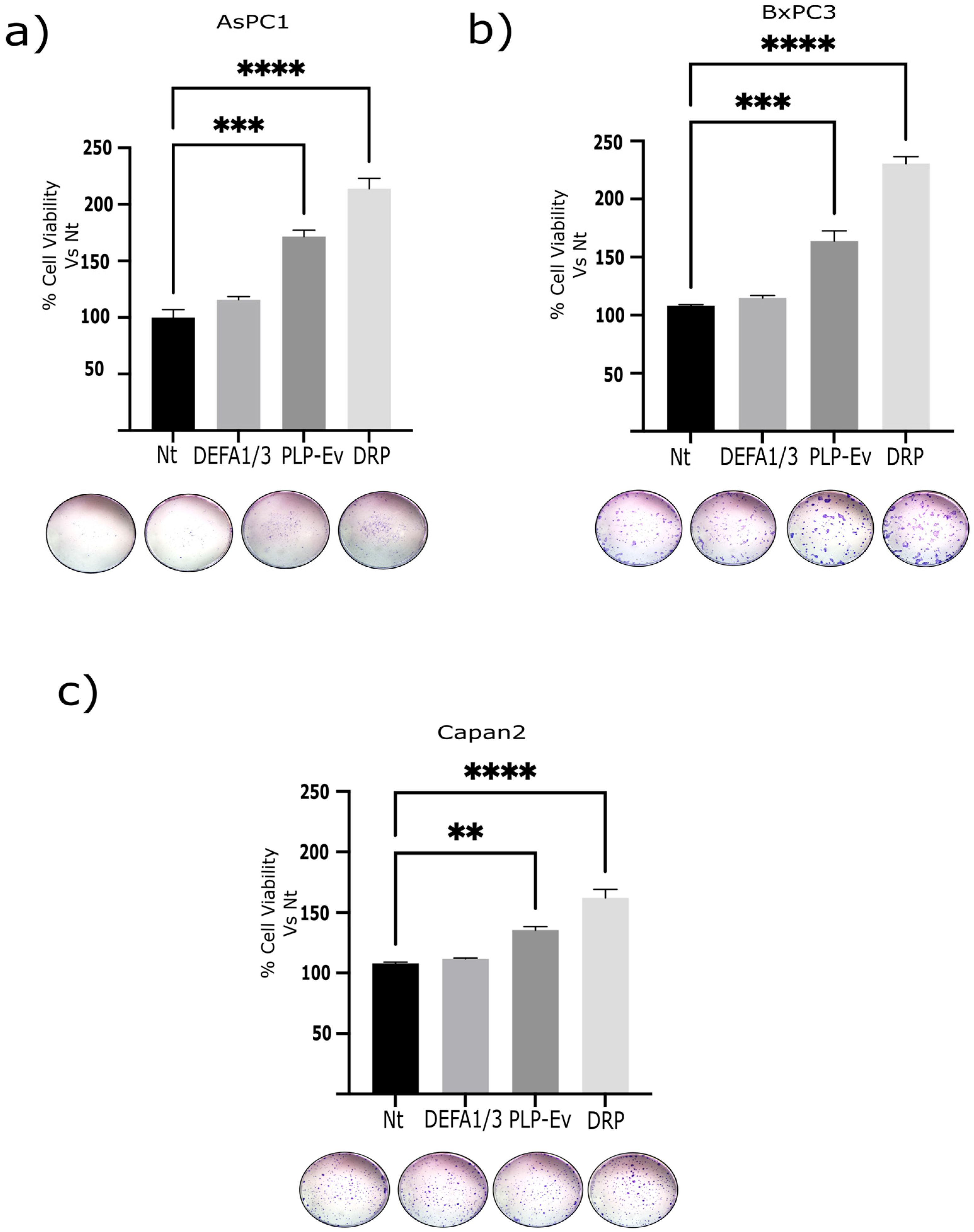
2.4. DRPs Promote Proliferation, 3d Spheroid Growth, and Clonogenic Potential in Pancreatic Cancer Cells
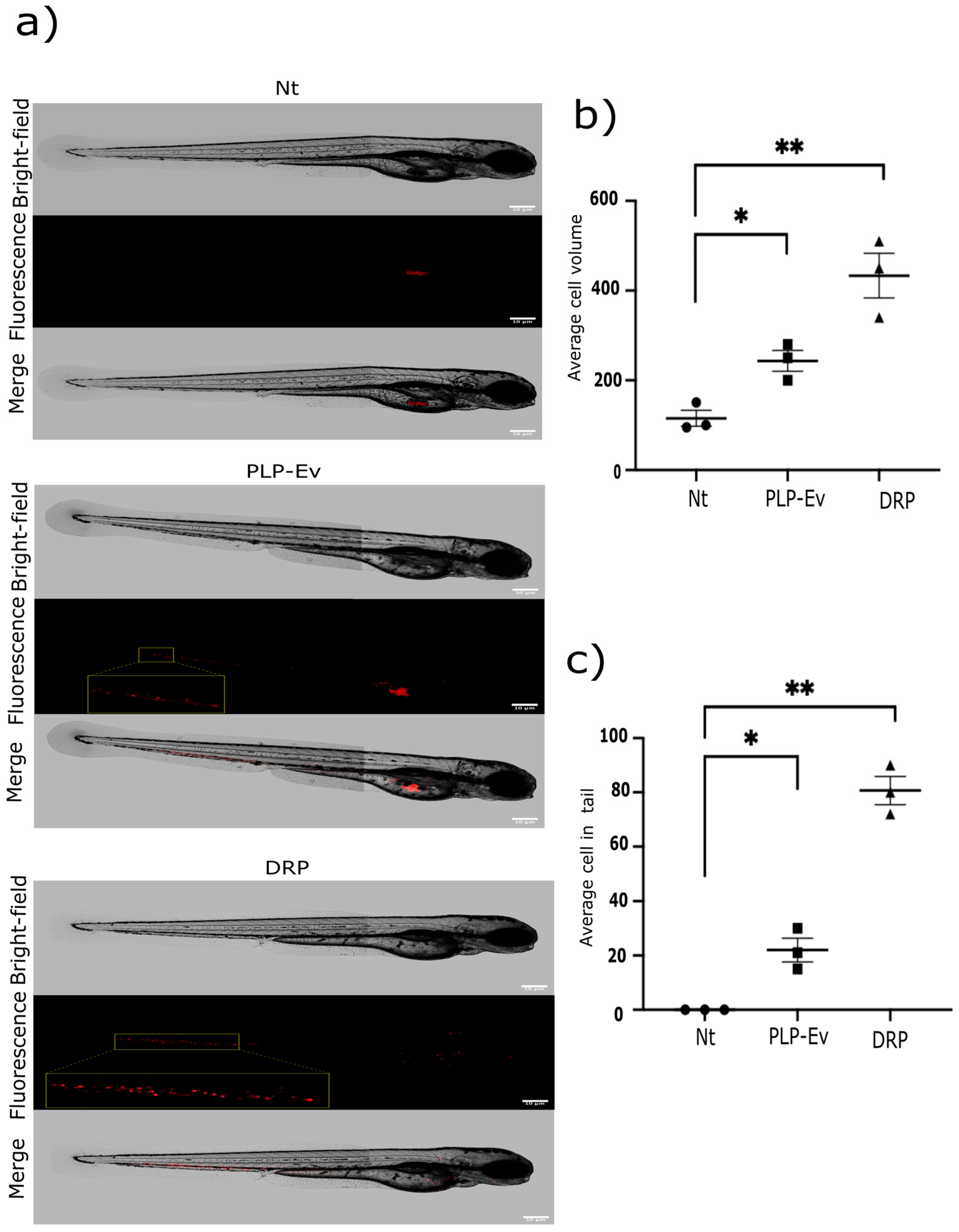
2.5. DRPs Enhance Tumor Growth and Dissemination In Vivo
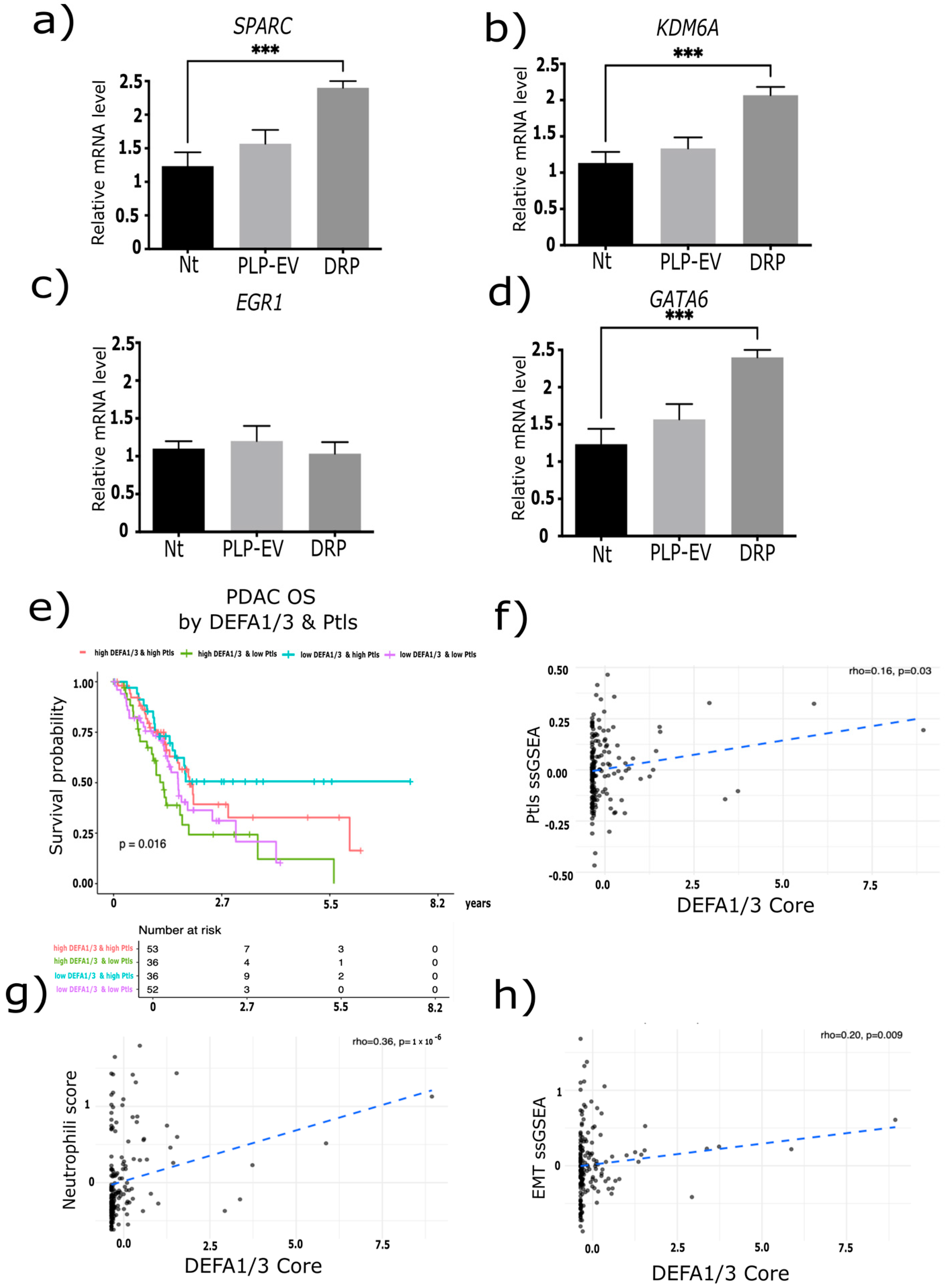
2.6. DRPs Induce Transcriptional Reprogramming in PDAC Cells
2.7. Correlation of SPARC, GATA6, and KDM6A with Aggressive Transcriptional Programs in TCGA-PDAC
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Cell Culture
4.2. Sample Classification
4.3. Differentiation of Mks (MEG-01) Cells into Platelet-like Particles
4.4. Isolation of PTLS from Healthy Donor Samples
4.5. Generation of DEFA1/3-Rich Platelet-Derived Particles
4.6. Genomic Studies
4.7. Transfection
4.8. Cell Migration Assays
4.9. Spheroid Culture
4.10. Viability Assay
4.11. Clonogenicity Assay
4.12. Western Blot Analysis
4.13. Zebrafish Husbandry and Ethical Compliance
4.14. Embryo Preparation, Microinjection, and Migration Assays
4.15. Transcriptomic Analyses and Survival Modeling
4.16. Single-Sample Gene Set Enrichment Analysis
4.17. Correlation Analyses with Aggressiveness-Related Genes
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine Diphosphate |
| ANOVA | Analysis of Variance |
| ATP | Adenosine Triphosphate |
| ATCC | American Type Culture Collection |
| AVMA | American Veterinary Medical Association |
| BSA | Bovine Serum Albumin |
| cDNA | Complementary DNA |
| CICUAL | Comité Interno para el Cuidado y Uso de Animales de Laboratorio |
| CPM | Counts Per Million |
| DEFA1/3 | Defensin Alpha 1/3 |
| DNA | Deoxyribonucleic Acid |
| DPF | Days Post Fertilization |
| DRPs | Defensin-Rich Platelets |
| EMT | Epithelial–Mesenchymal Transition |
| FBS | Fetal Bovine Serum |
| GDC | Genomic Data Commons |
| GO | Gene Ontology |
| GSVA | Gene Set Variation Analysis |
| HNPs | Human Neutrophil Peptides |
| IACUC | Institutional Animal Care and Use Committee |
| INMEGEN | Instituto Nacional de Medicina Genómica |
| Mks | Megakaryocytes |
| NES | normalized enrichment score |
| Nt | Non-treated Control |
| OS | Overall Survival |
| PBS | Phosphate-Buffered Saline |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PF4 | Platelet factor 4 |
| PLP-Ev | Platelet-Like Particles Empty Vector |
| PLPs | Platelet-Like Particles |
| PLTs | Platelets |
| PRP | Platelet-Rich Plasma |
| PCR | Quantitative Polymerase Chain Reaction |
| qPCR | Quantitative Polymerase Chain Reaction |
| S.E.M. | Standard Error of the Mean |
| ssGSEA | Single-Sample Gene Set Enrichment Analysis |
| SVM | Support Vector Machine |
| TCGA | The Cancer Genome Atlas |
| TEPs | Tumor-Educated Platelets |
| TGF-β | Transforming growth factor-beta |
| TPO | Thrombopoietin |
| VEGF | Vascular Endothelial Growth Factor |
References
- Antunes-Ferreira, M.; D’Ambrosi, S.; Arkani, M.; Post, E.; In’t Veld, S.G.J.G.; Ramaker, J.; Zwaan, K.; Kucukguzel, E.D.; Wedekind, L.E.; Griffioen, A.W.; et al. Tumor-educated platelet blood tests for Non-Small Cell Lung Cancer detection and management. Sci. Rep. 2023, 13, 9359. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Y.; Yang, F.; Zhu, L.; Zhu, X.Q.; Wang, Z.F.; Wu, X.L.; Zhou, C.H.; Yan, J.Y.; Hu, B.Y.; et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021, 6, 249. [Google Scholar] [CrossRef] [PubMed]
- Leiphrakpam, P.D.; Chowdhury, S.; Zhang, M.; Bajaj, V.; Dhir, M.; Are, C. Trends in the Global Incidence of Pancreatic Cancer and a Brief Review of its Histologic and Molecular Subtypes. J. Gastrointest. Cancer 2025, 56, 71. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Sherman, R.L.; Firth, A.U.; Henley, S.J.; Siegel, R.L.; Negoita, S.; Sung, H.; Kohler, B.A.; Anderson, R.N.; Cucinelli, J.; Scott, S.; et al. Annual Report to the Nation on the Status of Cancer, featuring state-level statistics after the onset of the COVID-19 pandemic. Cancer 2025, 131, e35833. [Google Scholar] [CrossRef]
- Li, N.; Yu, Z.; Zhang, X.; Liu, T.; Sun, Y.X.; Wang, R.T.; Yu, K.J. Elevated mean platelet volume predicts poor prognosis in colorectal cancer. Sci. Rep. 2017, 7, 10261. [Google Scholar] [CrossRef]
- Tian, Y.; Zong, Y.; Pang, Y.; Zheng, Z.; Ma, Y.; Zhang, C.; Gao, J. Platelets and diseases: Signal transduction and advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 159. [Google Scholar] [CrossRef]
- Li, H.; Jiang, W.; Zhang, S.R.; Li, P.C.; Li, T.J.; Jin, W.; Xu, H.X.; Yu, X.J.; Liu, L. The platelet pannexin 1-IL-1β axis orchestrates pancreatic ductal adenocarcinoma invasion and metastasis. Oncogene 2023, 42, 1453–1465. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, S.; Fathy, A.H.; Qian, H.; Zhao, Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: A comprehensive meta-analysis of 17 cohort studies. OncoTargets Ther. 2018, 11, 1899–1908. [Google Scholar] [CrossRef]
- Walke, V.; Das, S.; Mittal, A.; Agrawal, A. Tumor Educated Platelets as a Biomarker for Diagnosis of Lung cancer: A Systematic Review. Asian Pac. J. Cancer Prev. 2024, 25, 1911–1920. [Google Scholar] [CrossRef]
- Xing, S.; Zeng, T.; Xue, N.; He, Y.; Lai, Y.Z.; Li, H.L.; Huang, Q.; Chen, S.L.; Liu, W.L. Development and Validation of Tumor-educated Blood Platelets Integrin Alpha 2b (ITGA2B) RNA for Diagnosis and Prognosis of Non-small-cell Lung Cancer through RNA-seq. Int. J. Biol. Sci. 2019, 15, 1977–1992. [Google Scholar] [CrossRef]
- Asghar, S.; Waqar, W.; Umar, M.; Manzoor, S. Tumor educated platelets, a promising source for early detection of hepatocellular carcinoma: Liquid biopsy an alternative approach to tissue biopsy. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 836–844. [Google Scholar] [CrossRef]
- Fu, J.; Zong, X.; Jin, M.; Min, J.; Wang, F.; Wang, Y. Mechanisms and regulation of defensins in host defense. Signal Transduct. Target. Ther. 2023, 8, 300. [Google Scholar] [CrossRef]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef]
- Sabit, H.; Pawlik, T.M.; Abdel-Ghany, S.; Arneth, B. Defensins: Exploring Their Opposing Roles in Colorectal Cancer Progression. Cancers 2024, 16, 2622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Valle-Jimenez, X.; Ramirez-Cosmes, A.; Aquino-Dominguez, A.S.; Sanchez-Pena, F.; Bustos-Arriaga, J.; Romero-Tlalolini, M.D.L.A.; Torres-Aguilar, H.; Serafin-Lopez, J.; Aguilar Ruiz, S.R. Human platelets and megakaryocytes express defensin alpha 1. Platelets 2020, 31, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Best, M.G.; Sol, N.; Kooi, I.; Tannous, J.; Westerman, B.A.; Rustenburg, F.; Schellen, P.; Verschueren, H.; Post, E.; Koster, J.; et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell 2015, 28, 666–676. [Google Scholar] [CrossRef]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Bonamy, C.; Sechet, E.; Amiot, A.; Alam, A.; Mourez, M.; Fraisse, L.; Sansonetti, P.J.; Sperandio, B. Expression of the human antimicrobial peptide β-defensin-1 is repressed by the EGFR-ERK-MYC axis in colonic epithelial cells. Sci. Rep. 2018, 8, 18043. [Google Scholar] [CrossRef]
- Ling, Y.M.; Chen, J.Y.; Guo, L.; Wang, C.Y.; Tan, W.T.; Wen, Q.; Zhang, S.D.; Deng, G.H.; Lin, Y.; Kwok, H.F. β-defensin 1 expression in HCV infected liver/liver cancer: An important role in protecting HCV progression and liver cancer development. Sci. Rep. 2017, 7, 13404. [Google Scholar] [CrossRef]
- Li, S.; Mu, R.; Guo, X. Defensins regulate cell cycle: Insights of defensins on cellular proliferation and division. Life Sci. 2024, 349, 122740. [Google Scholar] [CrossRef]
- Gagandeep, K.R.; Balenahalli Narasingappa, R.; Vishnu Vyas, G. Unveiling mechanisms of antimicrobial peptide: Actions beyond the membranes disruption. Heliyon 2024, 10, e38079. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Asemani, Y.; Majidpoor, J.; Mahmoudi, R.; Aghaei-Zarch, S.M.; Mortezaee, K. Tumor-educated platelets. Clin. Chim. Acta 2024, 552, 117690. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Ge, X.; Yuan, L.; Cheng, B.; Dai, K. Identification of seven tumor-educated platelets RNAs for cancer diagnosis. J. Clin. Lab. Anal. 2021, 35, e23791. [Google Scholar] [CrossRef]
- Karp, J.M.; Modrek, A.S.; Ezhilarasan, R.; Zhang, Z.Y.; Ding, Y.; Graciani, M.; Sahimi, A.; Silvestro, M.; Chen, T.; Li, S.; et al. Deconvolution of the tumor-educated platelet transcriptome reveals activated platelet and inflammatory cell transcript signatures. JCI Insight 2024, 9, e178719. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wang, W.; Yao, Y.; Lian, S.; Xie, X.; Xu, J.; He, S.; Luo, L.; Ye, Z.; Zhang, J.; et al. TGF-β secreted by cancer cells-platelets interaction activates cancer metastasis potential by inducing metabolic reprogramming and bioenergetic adaptation. J. Cancer 2025, 16, 1310–1323. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, X.; Dong, S.; Han, F.; He, R.; Zhou, W. Challenges and Opportunities Associated With Platelets in Pancreatic Cancer. Front. Oncol. 2022, 12, 850485. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, S.; Wang, Z.; Gao, T. Platelets involved tumor cell EMT during circulation: Communications and interventions. Cell Commun. Signal. 2022, 20, 82. [Google Scholar] [CrossRef]
- Martínez-López, M.F.; Lopez-Gil, J.F. Small Fish, Big Answers: Zebrafish and the Molecular Drivers of Metastasis. Int. J. Mol. Sci. 2025, 26, 871. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Z.; Tian, Y.; Li, Z.; Liu, Z.; Zhu, S. The critical role of platelet in cancer progression and metastasis. Eur. J. Med. Res. 2023, 28, 385. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X.; Yan, L.; Yang, W.; Wu, F.Q.; Ling, Y.; Chen, S.Z.; Tang, L.; Tan, Y.X.; Cao, D.; Wu, M.C.; et al. Platelets promote tumour metastasis via interaction between TLR4 and tumour cell-released high-mobility group box1 protein. Nat. Commun. 2014, 5, 5256. [Google Scholar] [CrossRef]
- Haemmerle, M.; Taylor, M.L.; Gutschner, T.; Pradeep, S.; Cho, M.S.; Sheng, J.; Lyons, Y.M.; Nagaraja, A.S.; Dood, R.L.; Wen, Y.; et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 2017, 8, 310. [Google Scholar] [CrossRef] [PubMed]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Ormanns, S.; Haas, M.; Baechmann, S.; Altendorf-Hofmann, A.; Remold, A.; Quietzsch, D.; Clemens, M.R.; Bentz, M.; Geissler, M.; Lambertz, H.; et al. Impact of SPARC expression on outcome in patients with advanced pancreatic cancer not receiving nab-paclitaxel: A pooled analysis from prospective clinical and translational trials. Br. J. Cancer 2016, 115, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, A.; Malik, K.; Mohamedi, F.; Moaraf, S.; Kocher, H.; Jones, L.; Hill, N.J. Fibronectin acts as a molecular switch to determine SPARC function in pancreatic cancer. Cancer Lett. 2020, 477, 88–96. [Google Scholar] [CrossRef]
- Qiu, H.; Makarov, V.; Bolzenius, J.K.; Halstead, A.; Parker, Y.; Wang, A.; Iyer, G.V.; Wise, H.; Kim, D.; Thayaparan, V.; et al. KDM6A Loss Triggers an Epigenetic Switch That Disrupts Urothelial Differentiation and Drives Cell Proliferation in Bladder Cancer. Cancer Res. 2023, 83, 814–829. [Google Scholar] [CrossRef]
- Chang, Y.S.; Tu, S.J.; Chen, Y.C.; Liu, T.Y.; Lee, Y.T.; Yen, J.C.; Fang, H.Y.; Chang, J.G. Mutation profile of non-small cell lung cancer revealed by next generation sequencing. Respir. Res. 2021, 22, 3. [Google Scholar] [CrossRef]
- Yi, Z.; Wei, S.; Jin, L.; Jeyarajan, S.; Yang, J.; Gu, Y.; Kim, H.S.; Schechter, S.; Lu, S.; Paulsen, M.T.; et al. KDM6A Regulates Cell Plasticity and Pancreatic Cancer Progression by Noncanonical Activin Pathway. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 643–667. [Google Scholar] [CrossRef]
- Yang, J.; Jin, L.; Kim, H.S.; Tian, F.; Yi, Z.; Bedi, K.; Ljungman, M.; Pasca di Magliano, M.; Crawford, H.; Shi, J. KDM6A Loss Recruits Tumor-Associated Neutrophils and Promotes Neutrophil Extracellular Trap Formation in Pancreatic Cancer. Cancer Res. 2022, 82, 4247–4260. [Google Scholar] [CrossRef] [PubMed]
- Sivapalan, L.; Kocher, H.M.; Ross-Adams, H.; Chelala, C. The molecular landscape of pancreatic ductal adenocarcinoma. Pancreatology 2022, 22, 925–936. [Google Scholar] [CrossRef]
- Matsumoto, K.; Fujimori, N.; Ichihara, K.; Takeno, A.; Murakami, M.; Ohno, A.; Kakehashi, S.; Teramatsu, K.; Ueda, K.; Nakata, K.; et al. Patient-derived organoids of pancreatic ductal adenocarcinoma for subtype determination and clinical outcome prediction. J. Gastroenterol. 2024, 59, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Virolle, T.; Krones-Herzig, A.; Baron, V.; De Gregorio, G.; Adamson, E.D.; Mercola, D. Egr1 promotes growth and survival of prostate cancer cells: Identification of novel Egr1 target genes. J. Biol. Chem. 2003, 278, 11802–11810. [Google Scholar] [CrossRef]
- Ferraro, B.; Bepler, G.; Sharma, S.; Cantor, A.; Haura, E.B. EGR1 predicts PTEN and survival in patients with non–small-cell lung cancer. J. Clin. Oncol. 2005, 23, 1921–1926. [Google Scholar] [CrossRef]
- Risitano, A.; Beaulieu, L.M.; Vitseva, O.; Freedman, J.E. Platelets and platelet-like particles mediate intercellular RNA transfer. Blood 2012, 119, 6288–6295. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M. Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author’s Perspective. J. Cutan. Aesthet. Surg. 2014, 7, 189–197. [Google Scholar] [CrossRef]
- NOM-012-SSA3-2012; Que Establece los Criterios para la Ejecución de Proyectos de Investigación para la Salud en Seres Humanos. Diario Oficial de la Federación: Mexico City, Mexico, 2013.
- Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-Related Research Involving Humans; CIOMS: Geneva, Switzerland, 2016. [Google Scholar]
- Skalamera, D.; Dahmer, M.; Purdon, A.S.; Wilson, B.M.; Ranall, M.V.; Blumenthal, A.; Gabrielli, B.; Gonda, T.J. Generation of a genome scale lentiviral vector library for EF1α promoter-driven expression of human ORFs and identification of human genes affecting viral titer. PLoS ONE 2012, 7, e51733. [Google Scholar] [CrossRef] [PubMed]
- Bandala, E.; Espinosa, M.; Maldonado, V.; Melendez-Zajgla, J. Inhibitor of apoptosis-1 (IAP-1) expression and apoptosis in non-small-cell lung cancer cells exposed to gemcitabine. Biochem. Pharmacol. 2001, 62, 13–19. [Google Scholar] [CrossRef]
- Schwarz-Cruz y Celis, A.; Ceballos-Cancino, G.; Vazquez-Santillan, K.; Espinosa, M.; Zampedri, C.; Bahena, I.; Ruiz, V.; Maldonado, V.; Melendez-Zajgla, J. Basal-Type Breast Cancer Stem Cells Over-Express Chromosomal Passenger Complex Proteins. Cells 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, M.; Ceballos-Cancino, G.; Callaghan, R.; Maldonado, V.; Patino, N.; Ruiz, V.; Melendez-Zajgla, J. Survivin isoform Delta Ex3 regulates tumor spheroid formation. Cancer Lett. 2012, 318, 61–67. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087379. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The zebrafish book. In A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; Eugene University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals, 2020 ed.; AVMA: Anaheim, CA, USA, 2020. [Google Scholar]
- Martínez-López, M.; Povoa, V.; Fior, R. Generation of Zebrafish Larval Xenografts and Tumor Behavior Analysis. J. Vis. Exp. 2021, 172, e62373. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Zhai, J.; Li, C.Y.; Tan, A.M.; Wei, P.; Shen, L.Z.; He, M.F. Patient-derived xenograft in zebrafish embryos: A new platform for translational research in gastric cancer. J. Exp. Clin. Cancer Res. 2017, 36, 160. [Google Scholar] [CrossRef]
- Li, C.Y.; Rajapakshe, K.I.; Maitra, A. Integrative transcriptomic analysis identifies a novel gene signature to predict prognosis of pancreatic cancer in different subtypes. Pancreatology 2022, 22, 965–972. [Google Scholar] [CrossRef]
- Hanzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Wang, W.; Yan, L.; Guan, X.; Dong, B.; Zhao, M.; Wu, J.; Tian, X.; Hao, C. Identification of an Immune-Related Signature for Predicting Prognosis in Patients With Pancreatic Ductal Adenocarcinoma. Front. Oncol. 2020, 10, 618215. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.; Zhao, Y.; Zhang, Q.; Wu, X.; Miao, B.; Cao, J.; Fei, S. FN1, SPARC, and SERPINE1 are highly expressed and significantly related to a poor prognosis of gastric adenocarcinoma revealed by microarray and bioinformatics. Sci. Rep. 2019, 9, 7827. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Ruiz, J.; Sarmiento-Casas, M.; Bahena-Ocampo, I.; Espinosa, M.; Ceballos-Cancino, G.; Vazquez-Santillan, K.; Maldonado, V.; Melendez-Zajgla, J. Defensin-Rich Platelets Drive Pro-Tumorigenic Programs in Pancreatic Adenocarcinoma. Int. J. Mol. Sci. 2025, 26, 10898. https://doi.org/10.3390/ijms262210898
Gonzalez-Ruiz J, Sarmiento-Casas M, Bahena-Ocampo I, Espinosa M, Ceballos-Cancino G, Vazquez-Santillan K, Maldonado V, Melendez-Zajgla J. Defensin-Rich Platelets Drive Pro-Tumorigenic Programs in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences. 2025; 26(22):10898. https://doi.org/10.3390/ijms262210898
Chicago/Turabian StyleGonzalez-Ruiz, Jonathan, Miryam Sarmiento-Casas, Ivan Bahena-Ocampo, Magali Espinosa, Gisela Ceballos-Cancino, Karla Vazquez-Santillan, Vilma Maldonado, and Jorge Melendez-Zajgla. 2025. "Defensin-Rich Platelets Drive Pro-Tumorigenic Programs in Pancreatic Adenocarcinoma" International Journal of Molecular Sciences 26, no. 22: 10898. https://doi.org/10.3390/ijms262210898
APA StyleGonzalez-Ruiz, J., Sarmiento-Casas, M., Bahena-Ocampo, I., Espinosa, M., Ceballos-Cancino, G., Vazquez-Santillan, K., Maldonado, V., & Melendez-Zajgla, J. (2025). Defensin-Rich Platelets Drive Pro-Tumorigenic Programs in Pancreatic Adenocarcinoma. International Journal of Molecular Sciences, 26(22), 10898. https://doi.org/10.3390/ijms262210898




