β-Sitosterol Enhances the Anticancer Efficacy of Oxaliplatin in COLO-205 Cells via Apoptosis and Suppression of VEGF-A, NF-κB-p65, and β-Catenin
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Docking Results
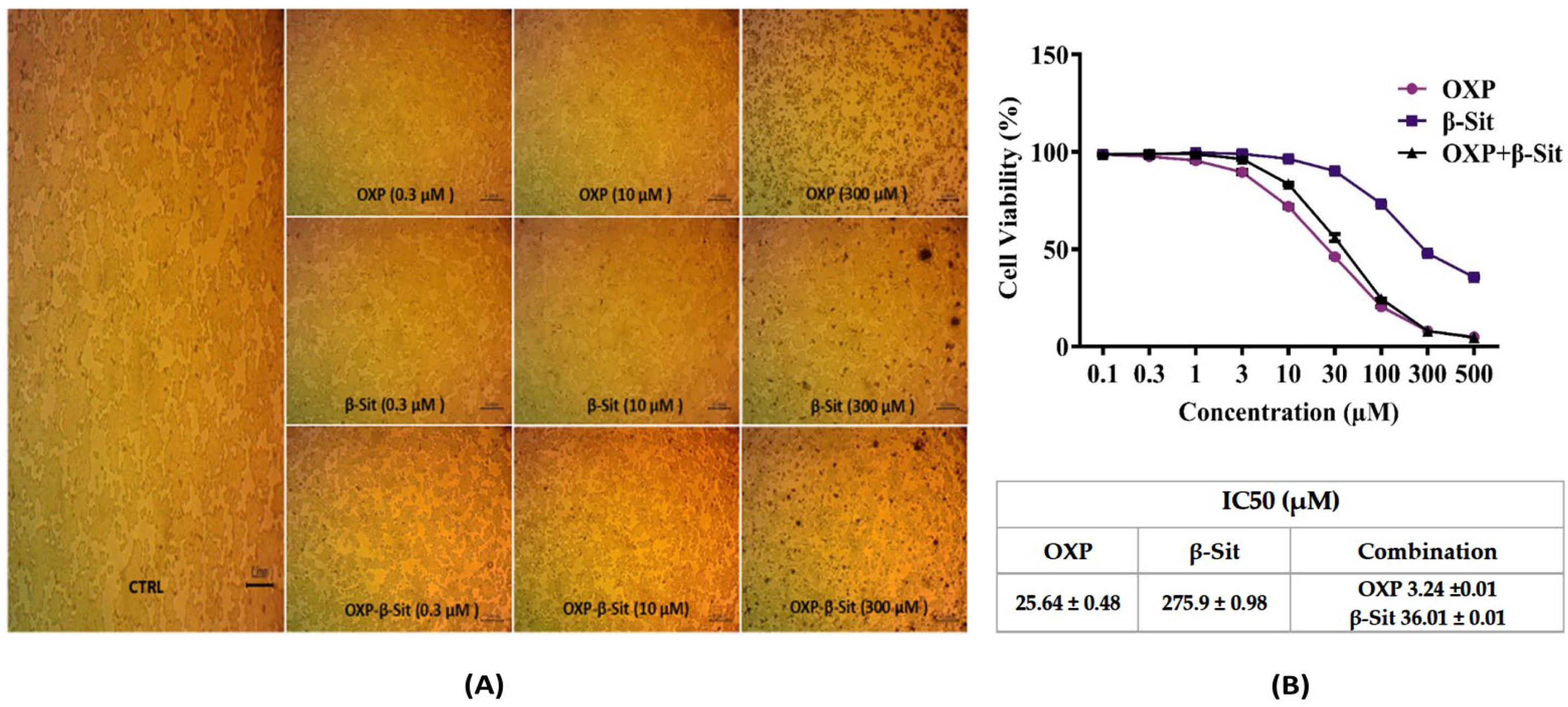
2.2. Cytotoxic Evaluation
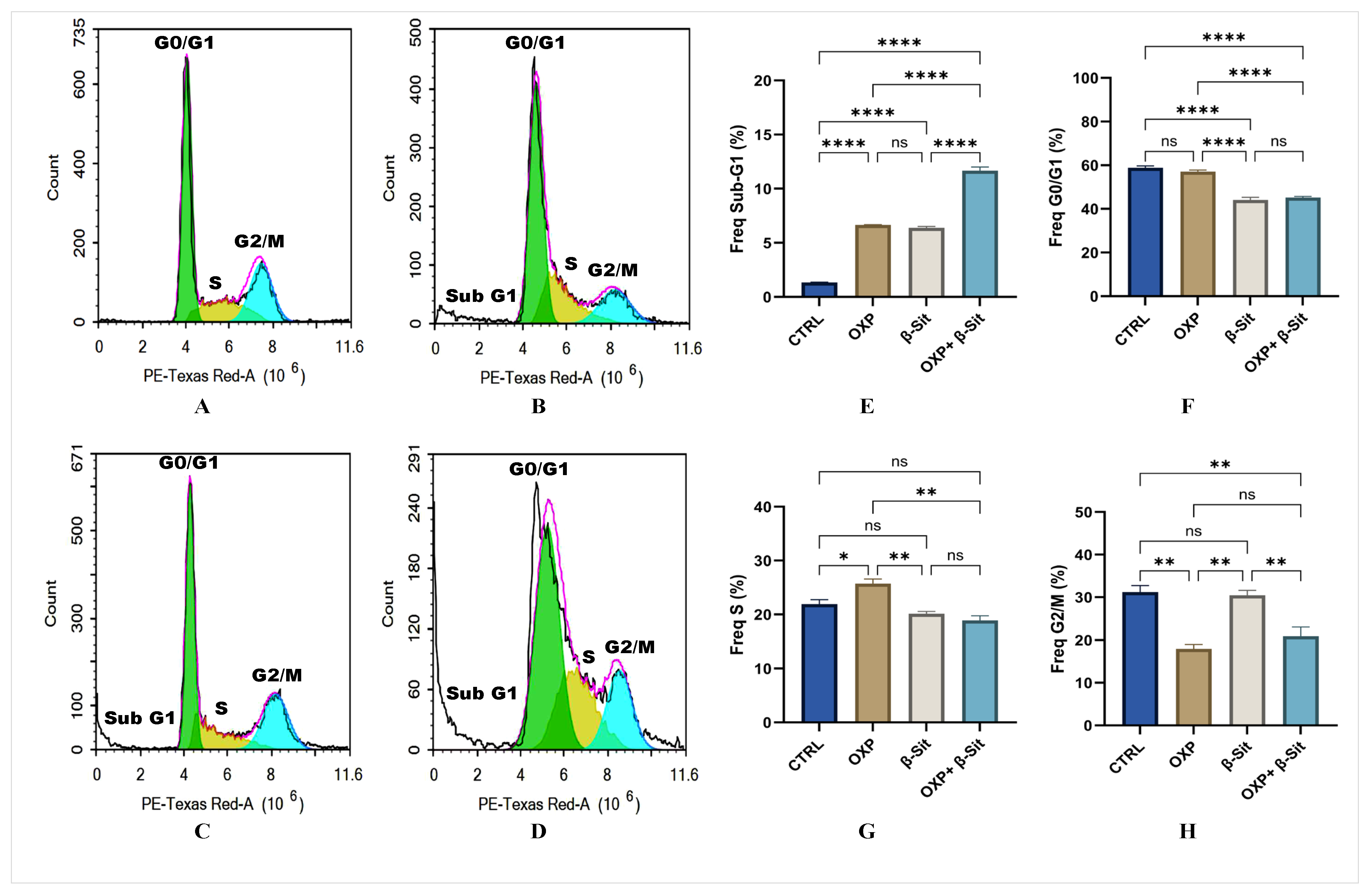
2.3. Effect of OXP, β-Sit, or Their Combination on Cell Cycle Distribution
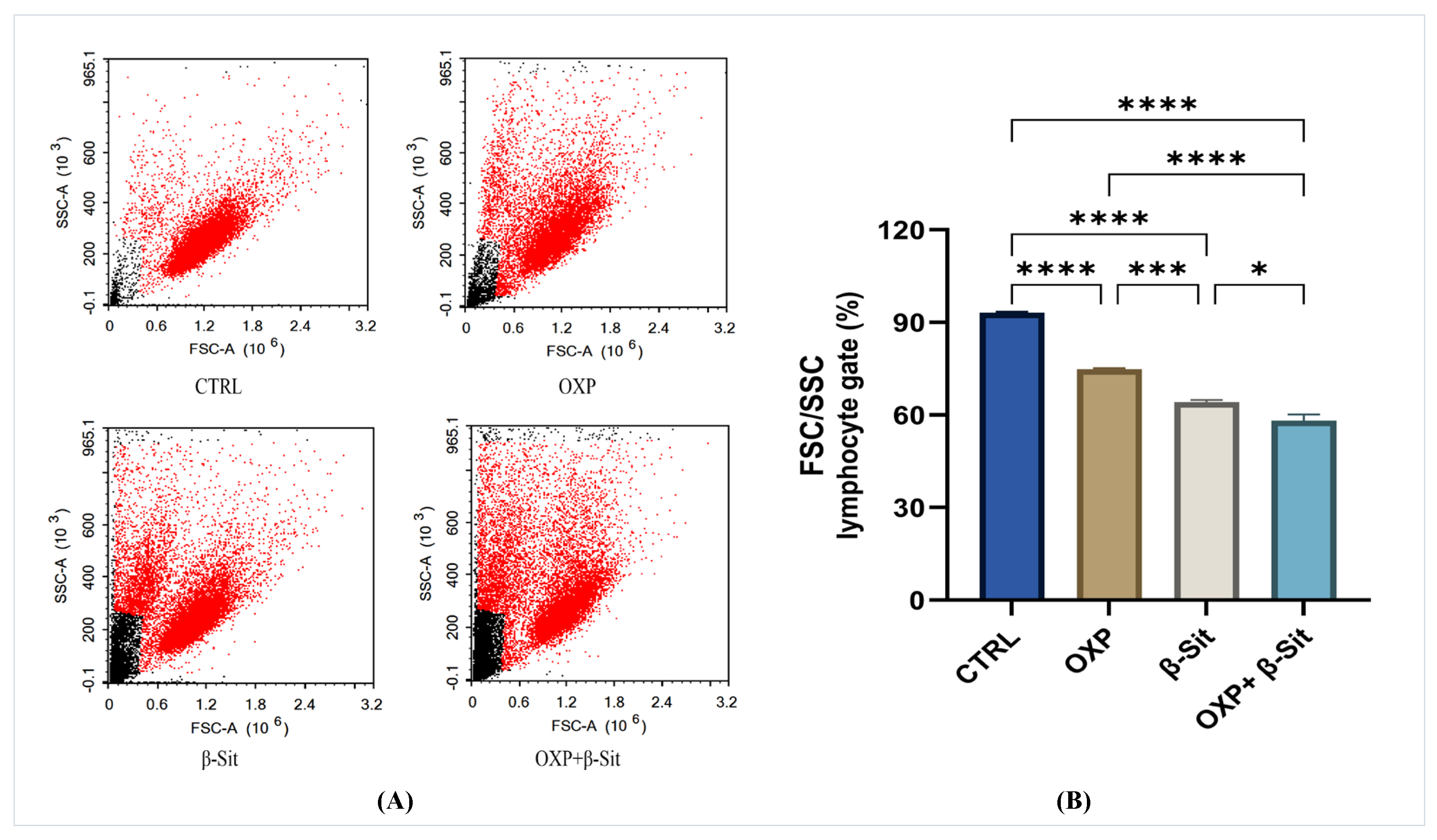
2.4. Assessment of Lymphocyte Forward and Side Scatter (FSC and SSC)
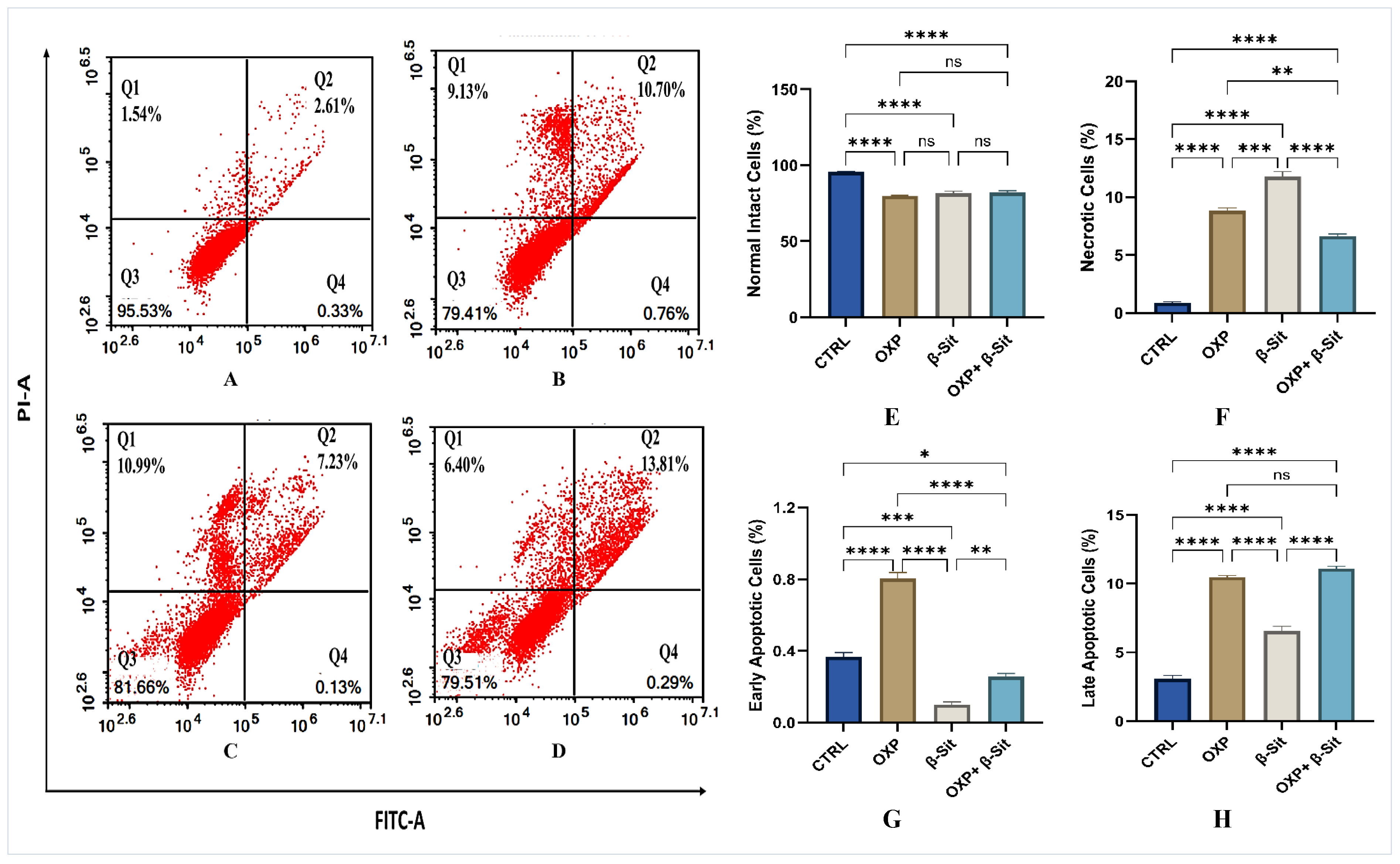
2.5. Apoptosis Evaluation
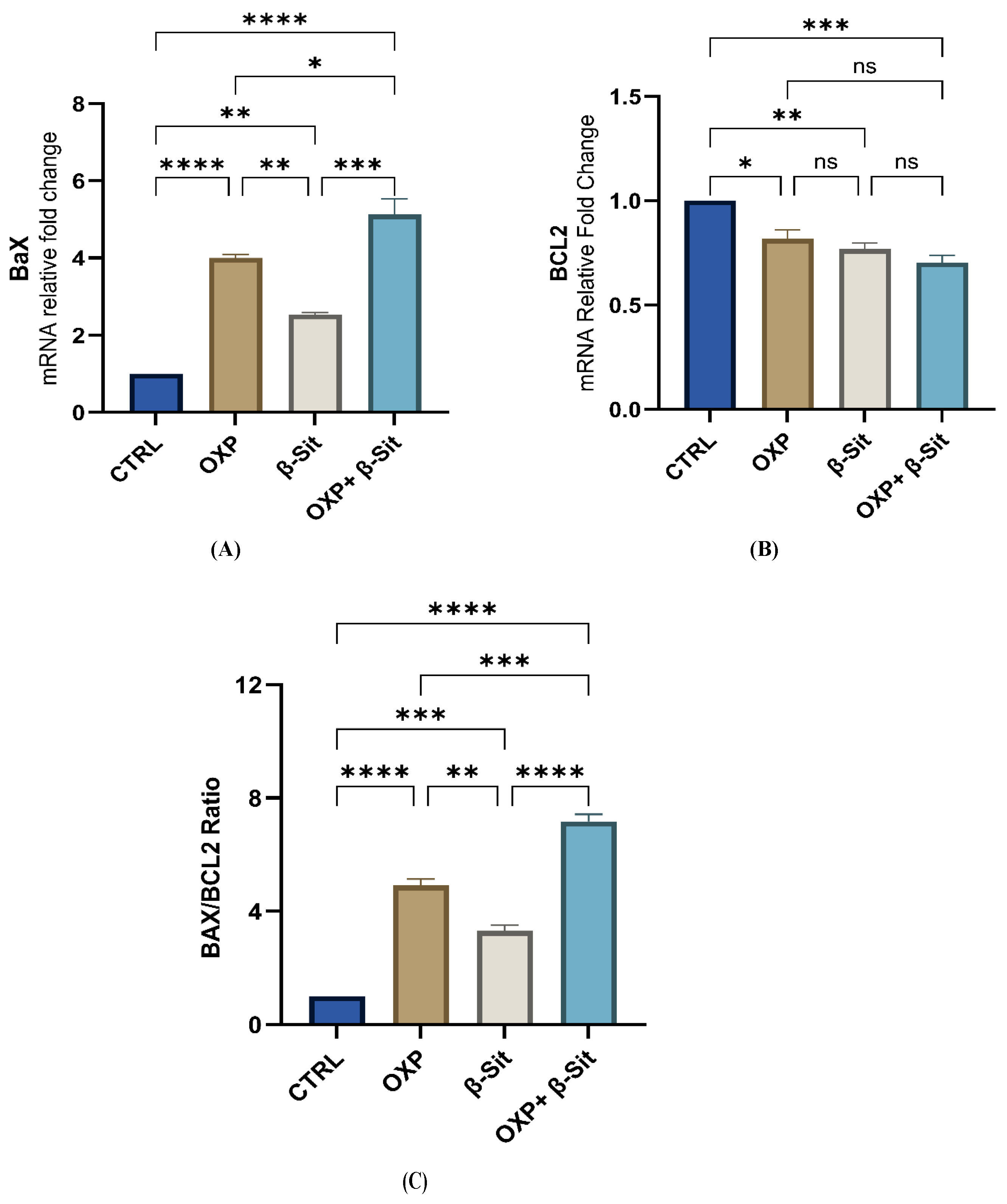
2.6. Modulation of BAX, BCL2, and BAX/BCL2 Ratio by Treatments
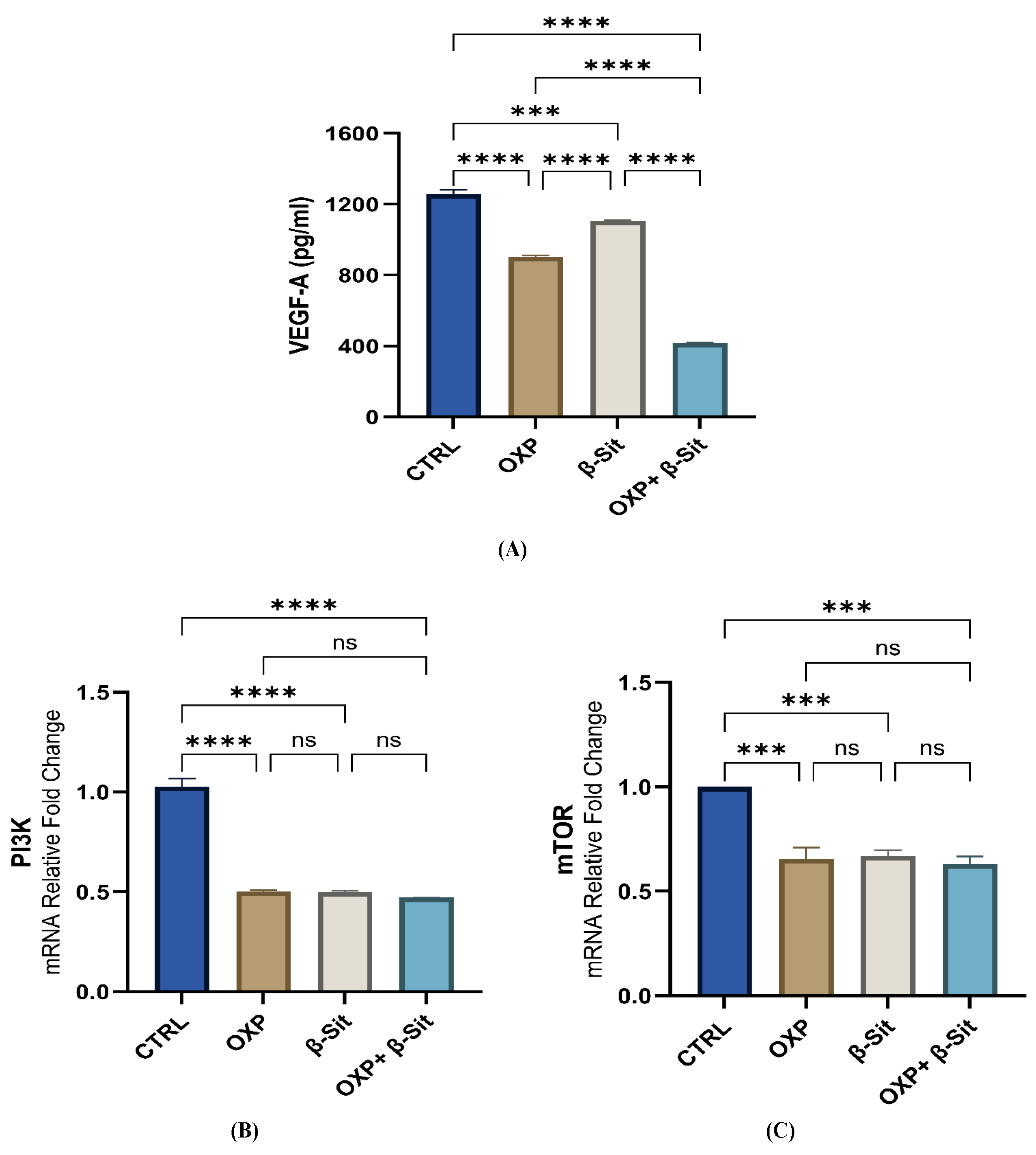
2.7. Impact of OXP, β-Sit, and Their Combination on the VEGF-A and PI3K/mTOR Pathways
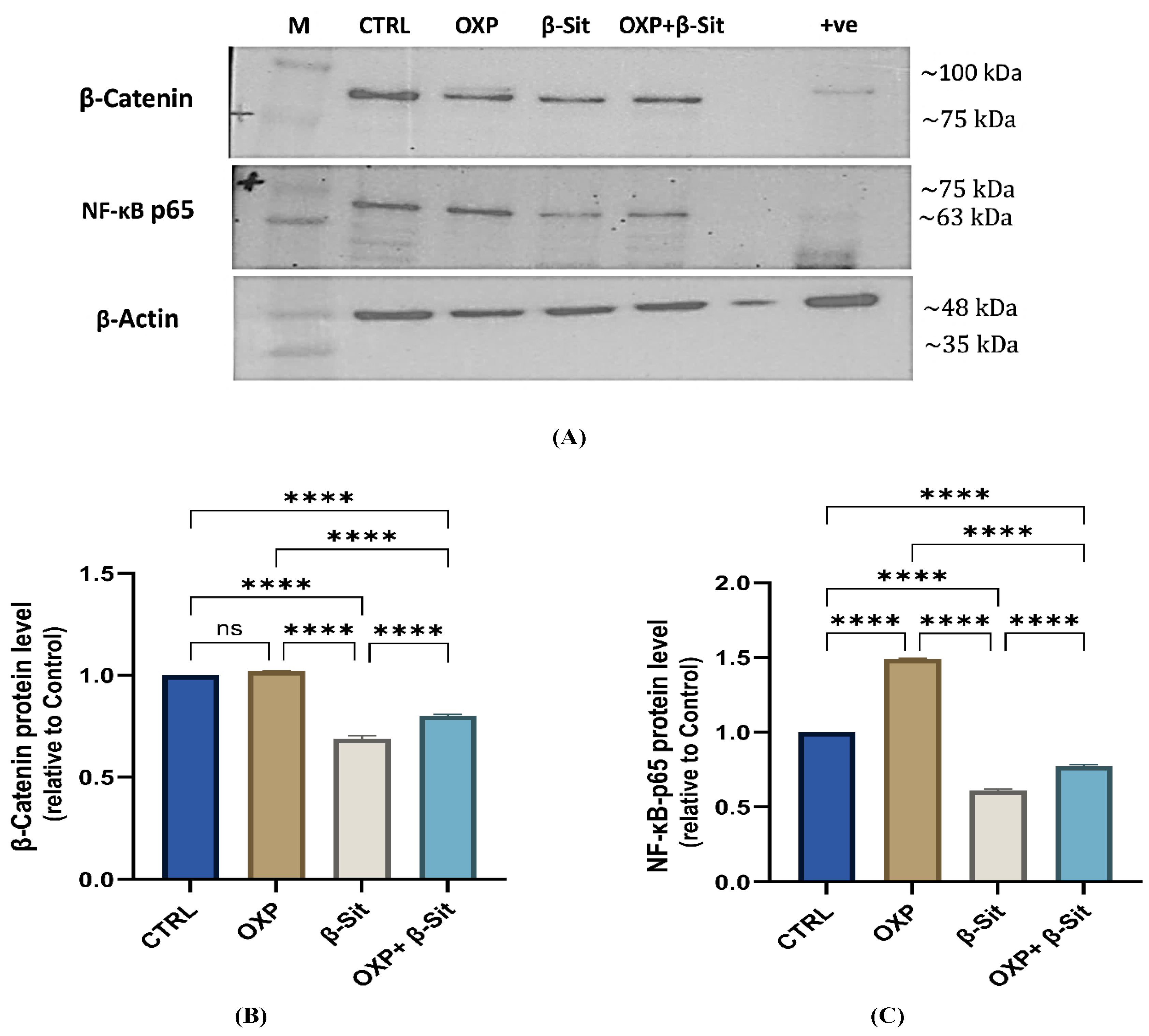
2.8. Impact of OXP, β-Sit, and Their Combination on β-Catenin and NF-κB-p65 Protein Expression
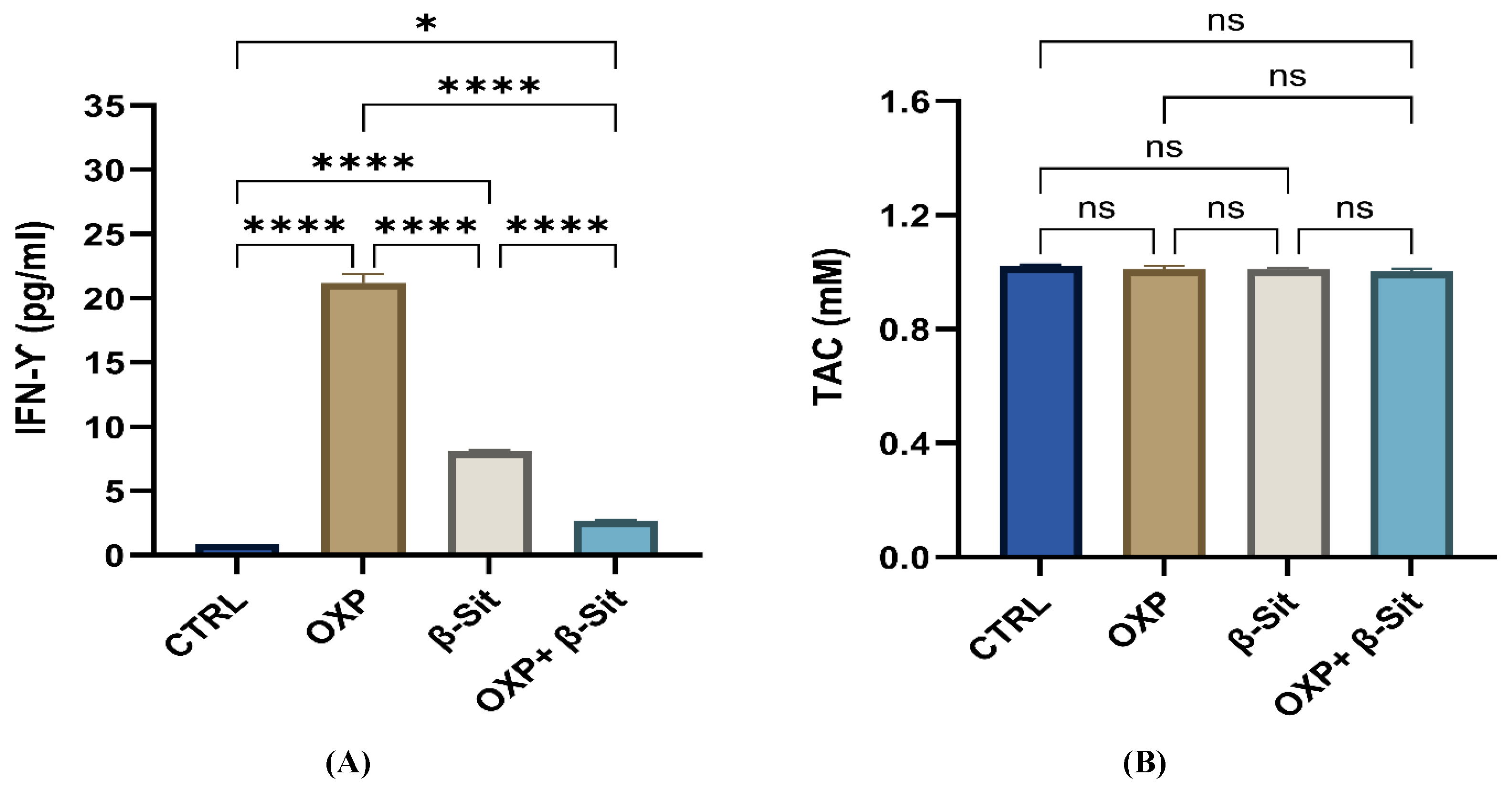
2.9. Effect of Treatment on Interferon Gamma (IFN-γ) and Total Antioxidant Capacity (TAC)
2.10. Study Limitations and Future Perspectives
3. Materials and Methods
3.1. Materials
3.2. Molecular Docking Analysis
3.3. Cell Culture
3.4. Evaluation of Cytotoxicity
3.5. Cell Cycle Analysis
3.6. Analysis of Apoptosis
3.7. Quantitative RT-PCR Analysis
3.8. Colorimetric and Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
3.9. Western Blotting Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Cedermark, B.; Dahlberg, M.; Glimelius, B.; Pahlman, L.; Rutqvist, L.E.; Wilking, N. Improved Survival with Pre-Operative Radiotherapy in Resectable Rectal Cancer. N. Engl. J. Med. 1997, 336, 980–987, Erratum in N. Engl. J. Med. 1997, 336, 1539. [Google Scholar]
- Ektate, K.; Munteanu, M.C.; Ashar, H.; Malayer, J.; Ranjan, A. Chemo-Immunotherapy of Colon Cancer with Focused Ultrasound and Salmonella-Laden Temperature Sensitive Liposomes (Thermobots). Sci. Rep. 2018, 8, 13062. [Google Scholar] [CrossRef]
- Asmis, T.R.; Saltz, L. Systemic Therapy for Colon Cancer. Gastroenterol. Clin. N. Am. 2008, 37, 287–295. [Google Scholar]
- Tournigand, C.; André, T.; Bonnetain, F.; Chibaudel, B.; Lledo, G.; Hickish, T.; Tabernero, J.; Boni, C.; Bachet, J.B.; Teixeira, L.; et al. Adjuvant Therapy with Fluorouracil and Oxaliplatin in Stage II and Elderly Patients (Aged 70–75 Years) with Colon Cancer: Subgroup Analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer. J. Clin. Oncol. 2012, 30, 3353–3360. [Google Scholar]
- Capdevila, J.; Elez, E.; Peralta, S.; Macarulla, T.; Ramos, F.J.; Tabernero, J. Oxaliplatin-Based Chemotherapy in the Management of Colorectal Cancer. Expert Rev. Anticancer Ther. 2008, 8, 1223–1236. [Google Scholar] [CrossRef]
- Martin, L.P.; Hamilton, T.C.; Schilder, R.J. Platinum Resistance: The Role of DNA Repair Pathways. Clin. Cancer Res. 2008, 14, 1291–1295. [Google Scholar] [CrossRef]
- Martínez-Balibrea, E.; Martínez-Cardús, A.; Ginés, A.; Ruiz de Porras, V.; Moutinho, C.; Layos, L.; Manzano, J.L.; Bugés, C.; Bystrup, S.; Esteller, M.; et al. Tumor-Related Molecular Mechanisms of Oxaliplatin Resistance. Mol. Cancer Ther. 2015, 14, 1767–1776. [Google Scholar] [CrossRef]
- Van der Jeught, K.; Xu, H.-C.; Li, Y.-J.; Lu, X.-B.; Ji, G. Drug Resistance and New Therapies in Colorectal Cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Combes, E.; Andrade, A.F.; Tosi, D.; Michaud, H.A.; Coquel, F.; Garambois, V.; Desigaud, D.; Jarlier, M.; Coquelle, A.; Pasero, P.; et al. Inhibition of Ataxia-Telangiectasia Mutated and RAD3-Related (ATR) Overcomes Oxaliplatin Resistance and Promotes Antitumor Immunity in Colorectal Cancer. Cancer Res. 2019, 79, 2933–2946. [Google Scholar] [CrossRef]
- Ruiz de Porras, V.; Bystrup, S.; Martínez-Cardús, A.; Pluvinet, R.; Sumoy, L.; Howells, L.; James, M.I.; Iwuji, C.; Manzano, J.L.; Layos, L.; et al. Curcumin Mediates Oxaliplatin-Acquired Resistance Reversion in Colorectal Cancer Cell Lines through Modulation of the CXC-Chemokine/NF-κB Signaling Pathway. Sci. Rep. 2016, 6, 24675. [Google Scholar] [CrossRef]
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Jadoon, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics, Epigenetics, and Cancer Prevention: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.C.; Lai, M.H.; Hsu, K.P.; Kuo, Y.H.; Chen, J.; Tsai, M.C.; Li, C.X.; Yin, X.J.; Jeyashoke, N.; Chao, L.K. Identification of β-Sitosterol as an In Vitro Anti-Inflammatory Constituent in Moringa oleifera. J. Agric. Food Chem. 2018, 66, 10748–10759. [Google Scholar] [CrossRef]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and Anti-Inflammatory Activity of β-Sitosterol Isolated from Nyctanthes arbor-tristis Leaves. Inflammopharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Bin Sayeed, M.S.; Ameen, S.S. β-Sitosterol: A Promising but Orphan Nutraceutical to Fight Against Cancer. Nutr. Cancer 2015, 67, 1214–1220. [Google Scholar] [CrossRef]
- Bae, H.; Park, S.; Ham, J.; Song, J.; Hong, T.; Choi, J.H.; Song, G.; Lim, W. ER–Mitochondria Calcium Flux by β-Sitosterol Promotes Cell Death in Ovarian Cancer. Antioxidants 2021, 10, 1583. [Google Scholar] [CrossRef]
- Rajavel, T.; Packiyaraj, P.; Suryanarayanan, V.; Singh, S.K.; Ruckmani, K.; Pandima Devi, K. β-Sitosterol Targets Trx/Trx1 Reductase to Induce Apoptosis in A549 Cells via ROS-Mediated Mitochondrial Dysregulation and p53 Activation. Sci. Rep. 2018, 8, 2071. [Google Scholar] [CrossRef]
- Qian, K.; Fu, D.; Jiang, B.; Wang, Y.; Tian, F.; Song, L.; Li, L. Mechanism of Hedyotis diffusa in the Treatment of Cervical Cancer. Front. Pharmacol. 2021, 12, 808144. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, T.; Liao, X.; Li, Z.; Lin, R.; Qi, X.; Chen, G.; Sun, L.; Lin, L. Network Pharmacology-Based Research into the Effect and Mechanism of Yinchenhao Decoction Against Cholangiocarcinoma. Chin. Med. 2021, 16, 13. [Google Scholar] [CrossRef]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium Salts and Formazan Products in Cell Biology: Viability Assessment, Fluorescence Imaging, and Labeling Perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef]
- Vanzyl, E.J.; Rick, K.R.; Blackmore, A.B.; MacFarlane, E.M.; McKay, B.C. Flow Cytometric Analysis Identifies Changes in S and M Phases as Novel Cell Cycle Alterations Induced by the Splicing Inhibitor Isoginkgetin. PLoS ONE 2018, 13, e0191178, Erratum in PLoS ONE 2025, 20, e0320500. [Google Scholar] [CrossRef]
- Choi, Y.H.; Kong, K.R.; Kim, Y.A.; Jung, K.O.; Kil, J.H.; Rhee, S.H.; Park, K.Y. Induction of Bax and Activation of Caspases during β-Sitosterol-Mediated Apoptosis in Human Colon Cancer Cells. Int. J. Oncol. 2003, 23, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Beyond Annexin V: Fluorescence Response of Cellular Membranes to Apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Muqbil, I.; Lowe, L.; Yedjou, C.; Hsu, H.Y.; Lin, L.T.; Siegelin, M.D.; Fimognari, C.; Kumar, N.B.; Dou, Q.P.; et al. Broad Targeting of Resistance to Apoptosis in Cancer. Semin. Cancer Biol. 2015, 35, S78–S103. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Luna-Vargas, M.P.; Chipuk, J.E. The Deadly Landscape of Pro-Apoptotic BCL-2 Proteins in the Outer Mitochondrial Membrane. FEBS J. 2016, 283, 2676–2689. [Google Scholar] [CrossRef]
- Campbell, K.J.; Tait, S.W.G. Targeting BCL-2–Regulated Apoptosis in Cancer. Open Biol. 2018, 8, 180002. [Google Scholar] [CrossRef]
- Tai, Y.T.L.S.; Niloff, E.; Weisman, C.; Strobel, T.; Cannistra, S.A. BAX Protein Expression and Clinical Outcome in Epithelial Ovarian Cancer. J. Clin. Oncol. 1998, 16, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, R.; Zhang, P.; Wang, Y. Songorine Suppresses Cell Growth and Metastasis in Epithelial Ovarian Cancer via the Bcl-2/Bax and GSK3β/β-Catenin Signaling Pathways. Oncol. Rep. 2019, 41, 3069–3079. [Google Scholar] [CrossRef]
- Gu, S.; Liu, F.; Xie, X.; Ding, M.; Wang, Z.; Xing, X.; Xiao, T.; Sun, X. β-Sitosterol Blocks the LEF-1–Mediated Wnt/β-Catenin Pathway to Inhibit Proliferation of Human Colon Cancer Cells. Cell Signal. 2023, 104, 110585. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Wang, X.X.; Lu, L.; Xu, J.W.; Li, X.B.; Zhang, G.R.; Ma, Z.J.; Shi, A.C.; Wang, Y.; Song, Y.J. β-Sitosterol and Gemcitabine Exhibit Synergistic Anti-Pancreatic Cancer Activity by Modulating Apoptosis and Inhibiting Epithelial-Mesenchymal Transition through Deactivating Akt/GSK-3β Signaling. Front. Pharmacol. 2019, 9, 1525, Erratum in Front Pharmacol. 2020, 11, 565535. [Google Scholar] [CrossRef]
- Guo, L.Y.; Zhu, P.; Jin, X.P. Association between the Expression of HIF-1α and VEGF and Prognostic Implications in Primary Liver Cancer. Genet. Mol. Res. 2016, 15, e8107. [Google Scholar] [CrossRef]
- Flak, B.; Wawrzyniec, K.; Kwiatek, S.; Kawczyk-Krupka, A.; Czuba, Z.; Sieroń-Stołtny, K.; Sieroń, A. Vascular Endothelial Growth Factor (VEGF) as a Marker for Cancer Progression: A Review. Acta Bio. Opt. Inf. Med. Inż. Biom. 2013, 19, 205–209. [Google Scholar]
- Ferroni, P.; Spila, A.; Martini, F.; D’Alessandro, R.; Mariotti, S.; Del Monte, G.; Graziano, P.; Buonomo, O.; Guadagni, F.; Roselli, M. Prognostic Value of Vascular Endothelial Growth Factor Tumor Tissue Content in Colorectal Cancer. Oncology 2005, 69, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Ouyang, Z.; Tang, H.H. Inhibiting the Proliferation and Metastasis of Hilar Cholangiocarcinoma Cells by Blocking the Expression of Vascular Endothelial Growth Factor with Small Interfering RNA. Oncol. Lett. 2018, 16, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.C.; Zeng, M.Q.; Huang, L.; Li, Y.L.; Gao, B.M.; Chen, J.J.; Xue, R.Z.; Tang, Z.Y. Downregulation of VEGFA Inhibits Proliferation, Promotes Apoptosis, and Suppresses Migration and Invasion of Renal Clear Cell Carcinoma. OncoTargets Ther. 2016, 9, 2131–2141. [Google Scholar] [CrossRef]
- Zahid, S.; Malik, A.; Waqar, S.; Zahid, F.; Tariq, N.; Khawaja, A.I.; Safir, W.; Gulzar, F.; Iqbal, J.; Ali, Q. Countenance and Implication of β-Sitosterol, β-Amyrin and Epiafzelechin in Nickel-Exposed Rats: In Silico and In Vivo Approach. Sci. Rep. 2023, 13, 21351. [Google Scholar] [CrossRef]
- Bilanges, B.; Posor, Y.; Vanhaesebroeck, B. PI3K Isoforms in Cell Signalling and Vesicle Trafficking. Nat. Rev. Mol. Cell Biol. 2019, 20, 515–534. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR Signalling and Cellular Metabolism Are Mutual Determinants in Cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Majumder, P.K.; Febbo, P.G.; Bikoff, R.; Berger, R.; Xue, Q.; McMahon, L.M.; Manola, J.; Brugarolas, J.; McDonnell, T.J.; Golub, T.R.; et al. mTOR Inhibition Reverses Akt-Dependent Prostate Intraepithelial Neoplasia through Regulation of Apoptotic and HIF-1–Dependent Pathways. Nat. Med. 2004, 10, 594–601. [Google Scholar] [CrossRef]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef]
- Li, X.; Shang, D.; Shen, H.; Song, J.; Hao, G.; Tian, Y. ZSCAN16 Promotes Proliferation, Migration and Invasion of Bladder Cancer via Regulating NF-κB, AKT, mTOR, p38 and Other Genes. Biomed. Pharmacother. 2020, 126, 110066, Erratum in Biomed. Pharmacother. 2020, 130, 110858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Xi, S. Expression of Concern: Upregulation of lncRNA HAGLROS Enhances the Development of Nasopharyngeal Carcinoma via Modulating miR-100/ATG14 Axis-Mediated PI3K/AKT/mTOR Signals. Artif. Cells Nanomed. Biotechnol. 2020, 48, 717. [Google Scholar]
- Sook, S.H.; Lee, H.J.; Kim, J.H.; Sohn, E.J.; Jung, J.H.; Kim, B.; Kim, J.H.; Jeong, S.J.; Kim, S.H. Reactive Oxygen Species-Mediated Activation of AMP-Activated Protein Kinase and c-Jun N-Terminal Kinase Plays a Critical Role in β-Sitosterol-Induced Apoptosis in Multiple Myeloma U266 Cells. Phytother. Res. 2014, 28, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Lee, K.J.; Choi, Y.H.; Kim, G.Y. β-Sitosterol-Induced Apoptosis Is Mediated by the Activation of ERK and the Downregulation of Akt in MCA-102 Murine Fibrosarcoma Cells. Int. Immunopharmacol. 2007, 7, 1044–1053. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, Q.; Yang, X.; Yu, Y.; Shen, X.; Sun, G. Combination of Sorafenib and Valproic Acid Synergistically Induces Cell Apoptosis and Inhibits Hepatocellular Carcinoma Growth via Downregulating Notch3 and pAkt. Am. J. Cancer Res. 2017, 7, 2503–2514. [Google Scholar]
- Jiang, L.; Zhao, X.; Xu, J.; Li, C.; Yu, Y.; Wang, W.; Zhu, L. The Protective Effect of Dietary Phytosterols on Cancer Risk: A Systematic Meta-Analysis. J. Oncol. 2019, 2019, 7479518. [Google Scholar] [CrossRef]
- Yu, W.K.; Xu, Z.Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.D.; Qin, J.J. Targeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef]
- Baskar, A.A.; Ignacimuthu, S.; Paulraj, G.M.; Al Numair, K.S. Chemopreventive Potential of β-Sitosterol in an Experimental Colon Cancer Model: In Vitro and In Vivo Study. BMC Complement. Altern. Med. 2010, 10, 24. [Google Scholar] [CrossRef]
- Shen, C.Y.; Lee, C.F.; Chou, W.T.; Hwang, J.J.; Tyan, Y.S.; Chuang, H.Y. Liposomal β-Sitosterol Suppresses Metastasis of CT26/luc Colon Carcinoma via Inhibition of MMP-9 and Activation of the Immune System. Pharmaceutics 2022, 14, 1214. [Google Scholar] [CrossRef]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and Interferons: Role of Interferon-Stimulated Genes as Mediators of Apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef]
- Coughlin, C.M.; Salhany, K.E.; Gee, M.S.; LaTemple, D.C.; Kotenko, S.; Ma, X.; Gri, G.; Wysocka, M.; Kim, J.E.; Liu, L.; et al. Tumor Cell Responses to IFN-γ Affect Tumorigenicity and Response to IL-12 Therapy and Antiangiogenesis. Immunity 1998, 9, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yu, T.; Liu, X.; He, Y.; Deng, L.; Guo, J.; Hua, Y.; Luo, T.; Gao, X. Improved Anti-Tumor Efficacy via Combination of Oxaliplatin and Fibrin Glue in Colorectal Cancer. Oncotarget 2018, 9, 2515–2526, Erratum in Oncotarget 2020, 11, 3484–3485. [Google Scholar] [CrossRef] [PubMed]
- Casara, P.; Davidson, J.; Claperon, A.; Le Toumelin-Braizat, G.; Vogler, M.; Bruno, A.; Chanrion, M.; Lysiak-Auvity, G.; Le Diguarher, T.; Starck, J.B.; et al. S55746 Is a Novel Orally Active BCL-2–Selective and Potent Inhibitor That Impairs Hematological Tumor Growth. Oncotarget 2018, 9, 20075–20088. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Huang, M.; Song, K.; Li, X.; Huang, M.; Meng, L.; Zhang, J. New Insights into PI3K Inhibitor Design Using X-ray Structures of PI3Kα Complexed with a Potent Lead Compound. Sci. Rep. 2017, 7, 14572. [Google Scholar] [CrossRef]
- Weiss, M.M.; Harmange, J.C.; Polverino, A.J.; Bauer, D.; Berry, L.; Berry, V.; Borg, G.; Bready, J.; Chen, D.; Choquette, D.; et al. Evaluation of a Series of Naphthamides as Potent, Orally Active Vascular Endothelial Growth Factor Receptor-2 Tyrosine Kinase Inhibitors. J. Med. Chem. 2008, 51, 1668–1680. [Google Scholar] [CrossRef]
- El-Demerdash, A.S.; Alfaraj, R.; Farid, F.A.; Yassin, M.H.; Saleh, A.M.; Dawwam, G.E. Essential Oils as Capsule Disruptors: Enhancing Antibiotic Efficacy Against Multidrug-Resistant Klebsiella pneumoniae. Front. Microbiol. 2024, 15, 1467460. [Google Scholar] [CrossRef]
- Bu, H.; Liu, D.; Cui, J.; Cai, K.; Shen, F. Wnt/β-catenin signaling pathway is involved in induction of apoptosis by oridonin in colon cancer COLO-205 cells. Transl. Cancer Res. 2019, 8, 1782–1794. [Google Scholar] [CrossRef]
- Ditty, M.J.; Ezhilarasan, D. β-sitosterol induces reactive oxygen species-mediated apoptosis in human hepatocellular carcinoma cells. Avicenna J. Phytomed. 2021, 11, 541–549. [Google Scholar]
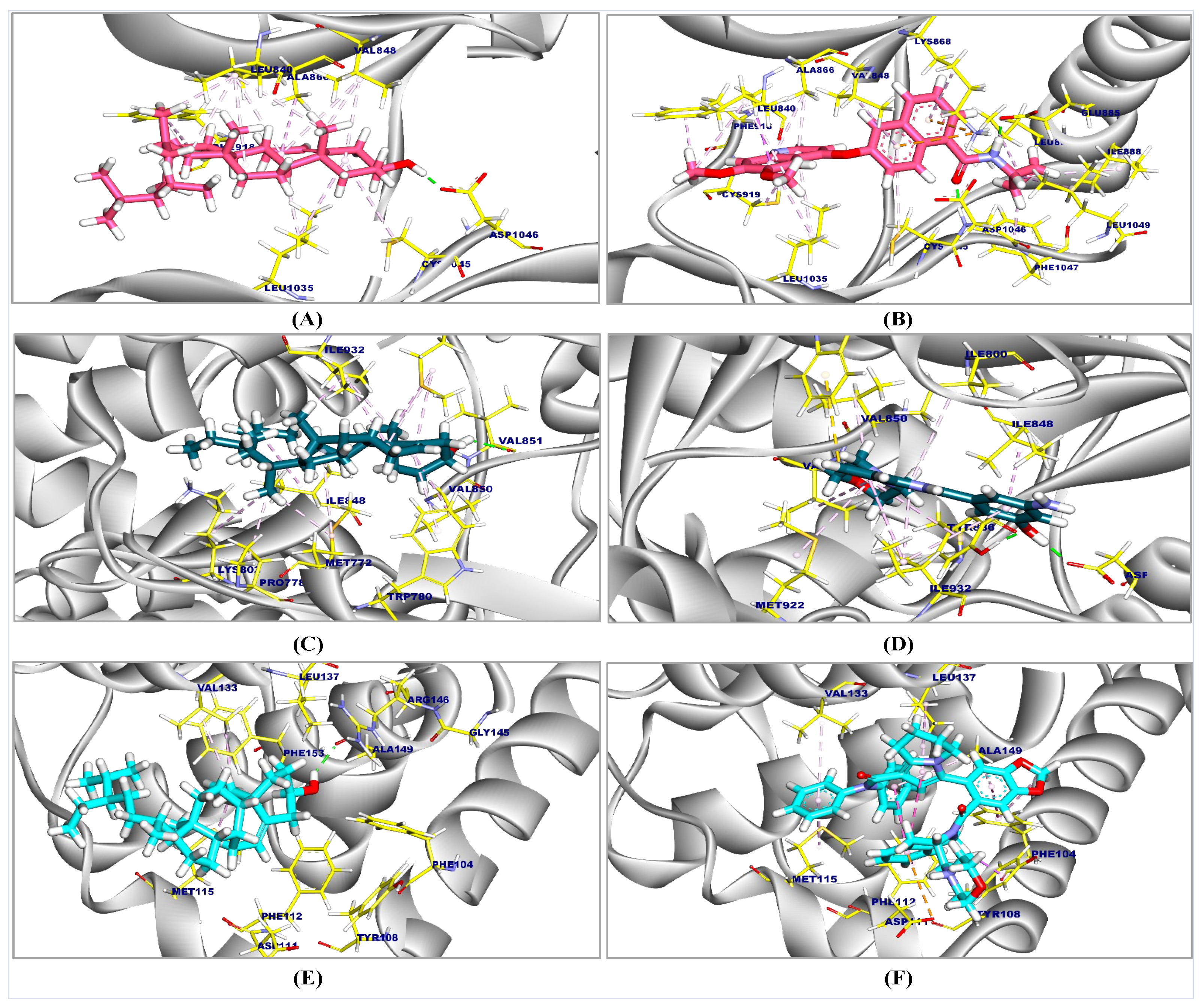
| Tested Compounds | Compounds | RMSD Value (Å) | Docking (Affinity) Score (kcal/mol) |
|---|---|---|---|
| VEGFR-2 | β-Sit | 1.41 | −7.45 |
| Co-crystalized ligand (887) | 0.79 | 8.13 | |
| PI3K | β-Sit | 1.26 | −7.98 |
| Co-crystalized ligand (84R) | 0.85 | −8.02 | |
| BCL2 | β-Sit | 1.85 | −6.88 |
| Co-crystalized ligand (F3Q) | 1.44 | 7.11 |
| Forward Sequence | Reverse Sequence | Genes Accession Numbers | |
|---|---|---|---|
| GAPDH | GTC TCC TCT GAC TTC AAC AGC G | ACC ACC CTG TTG CTG TAG CCA A | NM_002046 |
| Bcl2 | ATC GCC CTG TGG ATG ACT GAG T | GCC AGG AGA AAT CAA ACA GAG GC | NM_000633 |
| Bax | TCA GGA TGC GTC CAC CAA GAA G | TGT GTC CAC GGC GGC AAT CAT C | NM_138761 |
| mTOR | AGC ATC GGA TGC TTA GGA GTG G | CAG CCA ATC TTT GGA GAC C | NM_004958 |
| PI3K | GAA GCA CCT GAA TAG GCA AGT CG | GAG CAST CCA TGA AAT CTG GTC GC | NM_006218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khateeb, S.; Almutairi, F.M.; Alalawy, A.I.; Obidan, A.; Albalawi, M.; Al-Massabi, R.; Sagini, H.A.; Abuzahrah, S.S.; Taha, E.F.S. β-Sitosterol Enhances the Anticancer Efficacy of Oxaliplatin in COLO-205 Cells via Apoptosis and Suppression of VEGF-A, NF-κB-p65, and β-Catenin. Int. J. Mol. Sci. 2025, 26, 10897. https://doi.org/10.3390/ijms262210897
Khateeb S, Almutairi FM, Alalawy AI, Obidan A, Albalawi M, Al-Massabi R, Sagini HA, Abuzahrah SS, Taha EFS. β-Sitosterol Enhances the Anticancer Efficacy of Oxaliplatin in COLO-205 Cells via Apoptosis and Suppression of VEGF-A, NF-κB-p65, and β-Catenin. International Journal of Molecular Sciences. 2025; 26(22):10897. https://doi.org/10.3390/ijms262210897
Chicago/Turabian StyleKhateeb, Sahar, Fahad M. Almutairi, Adel I. Alalawy, Amnah Obidan, Mody Albalawi, Rehab Al-Massabi, Hanan Abdulrahman Sagini, Samah S. Abuzahrah, and Eman F. S. Taha. 2025. "β-Sitosterol Enhances the Anticancer Efficacy of Oxaliplatin in COLO-205 Cells via Apoptosis and Suppression of VEGF-A, NF-κB-p65, and β-Catenin" International Journal of Molecular Sciences 26, no. 22: 10897. https://doi.org/10.3390/ijms262210897
APA StyleKhateeb, S., Almutairi, F. M., Alalawy, A. I., Obidan, A., Albalawi, M., Al-Massabi, R., Sagini, H. A., Abuzahrah, S. S., & Taha, E. F. S. (2025). β-Sitosterol Enhances the Anticancer Efficacy of Oxaliplatin in COLO-205 Cells via Apoptosis and Suppression of VEGF-A, NF-κB-p65, and β-Catenin. International Journal of Molecular Sciences, 26(22), 10897. https://doi.org/10.3390/ijms262210897







