Hyaluronan-Based Hybrid Systems as Growth Factor Carriers in the Treatment of Chronic Wounds
Abstract
1. Introduction
2. Hyaluronic Acid—Properties and Biomedical Potential
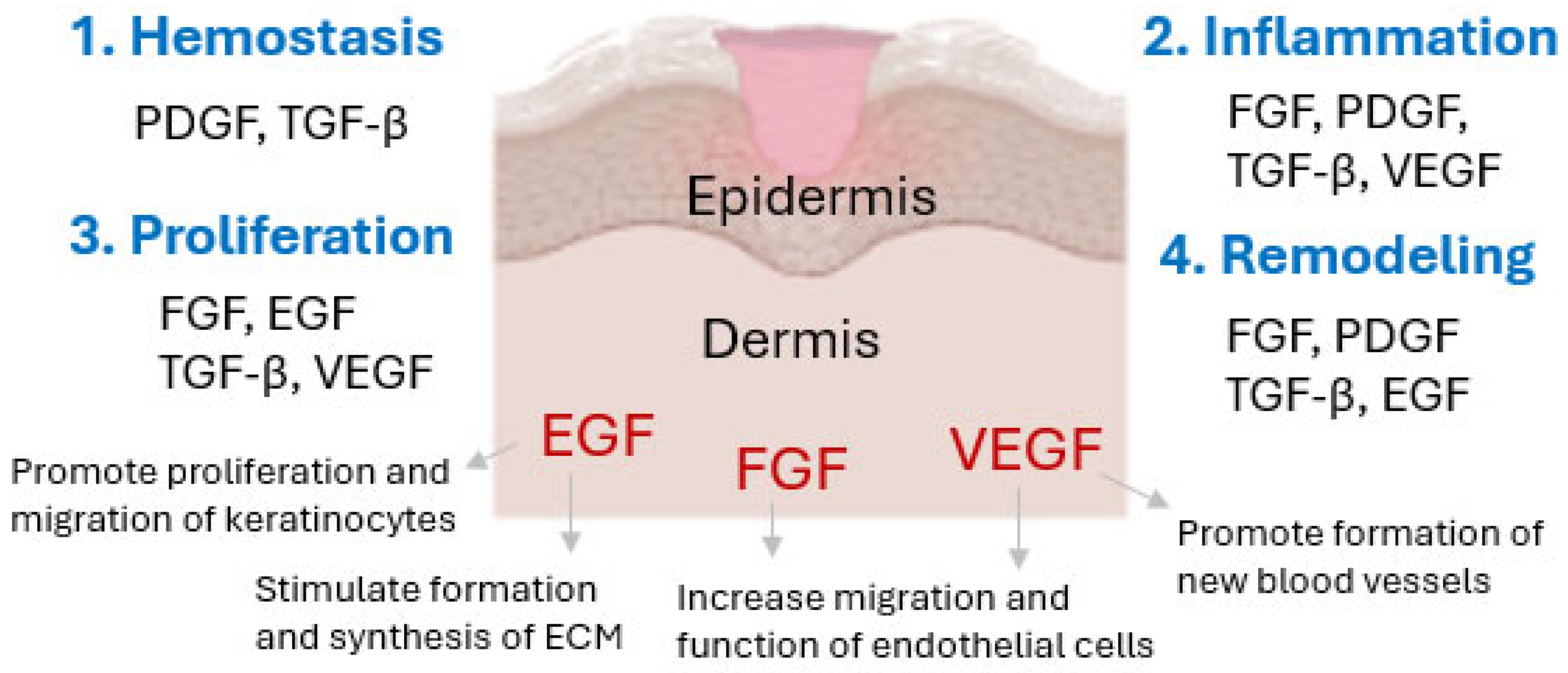
3. Growth Factors and Their Importance in Skin Regeneration and Wound Healing Processes
4. Review of Studies on Hyaluronan-Based Hybrid Systems with Growth Factors in the Treatment of Chronic Wounds
4.1. Mechanisms Responsible for the Beneficial Effects of Hybrid Systems Containing Growth Factors
4.2. Significance and Limitations of Research Based on Animal Models
5. Materials and Methods
5.1. Focused Questions
5.2. Eligibility Criteria
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rana, M.M.; De la Hoz Siegler, H. Evolution of Hybrid Hydrogels: Next-Generation Biomaterials for Drug Delivery and Tissue Engineering. Gels 2024, 10, 216. [Google Scholar] [CrossRef]
- Choudhary, A.; Sharma, A.; Singh, A.; Han, S.S.; Sood, A. Strategy and Advancement in Hybrid Hydrogel and Their Applications: Recent Progress and Trends. Adv. Eng. Mater. 2024, 26, 2400944. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, X.; Liu, J.; Zhang, H.; Fu, D. Advances in the application of natural/synthetic hybrid hydrogels in tissue engineering and delivery systems: A comprehensive review. Int. J. Pharm. 2025, 672, 125323. [Google Scholar] [CrossRef]
- Cai, M.-H.; Chen, X.-Y.; Fu, L.-Q.; Du, W.-L.; Yang, X.; Mou, X.-Z.; Hu, P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 630943. [Google Scholar] [CrossRef] [PubMed]
- Secco, J.; Spinazzola, E.; Pittarello, M.; Ricci, E.; Pareschi, F. Clinically validated classification of chronic wounds method with memristor-based cellular neural network. Sci. Rep. 2024, 14, 30839. [Google Scholar] [CrossRef] [PubMed]
- Bowers, S.; Franco, E. Chronic wounds: Evaluation and management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar] [PubMed]
- Macedo, A.S.; Mendes, F.; Filipe, P.; Reis, S.; Fonte, P. Nanocarrier-Mediated Topical Insulin Delivery for Wound Healing. Materials 2021, 14, 4257. [Google Scholar] [CrossRef]
- Sen, C.K. Human Wound and Its Burden: Updated 2022 Compendium of Estimates; Mary Ann Liebert, Inc.: Larchmont, NY, USA, 2023; Volume 12, pp. 657–670. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R.; Uspenskaya, M. Hyaluronic Acid: The Influence of Molecular Weight on Structural, Physical, Physico-Chemical, and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef]
- Palenčárová, K.; Köszagová, R.; Nahálka, J. Hyaluronic Acid and Its Synthases—Current Knowledge. Int. J. Mol. Sci. 2025, 26, 7028. [Google Scholar] [CrossRef]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 4281. [Google Scholar] [CrossRef]
- Kogan, G.; Soltes, L.; Stern, R.; Gemeiner, P. Hyaluronic acid: A natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol. Lett. 2007, 29, 17–25. [Google Scholar] [CrossRef]
- Grabowski, M.; Gmyrek, D.; Zurawska, M.; Trusek, A. Hyaluronic Acid: Production Strategies, Gel-Forming Properties, and Advances in Drug Delivery Systems. Gels 2025, 11, 424. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic Acid and Its Biomedical Applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Monslow, J.; Govindaraju, P.; Puré, E. Hyaluronan—A functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 2015, 6, 231. [Google Scholar] [CrossRef]
- Lee, B.M.; Park, S.J.; Noh, I.; Kim, C.H. The Effects of the Molecular Weights of Hyaluronic Acid on the Immune Responses. Biomater. Res. 2021, 25, 27. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Gholamali, I.; Vu, T.T.; Jo, S.-H.; Park, S.-H.; Lim, K.T. Exploring the Progress of Hyaluronic Acid Hydrogels: Synthesis, Characteristics, and Wide-Ranging Applications. Materials 2024, 17, 2439. [Google Scholar] [CrossRef]
- Juhaščik, M.; Kováčik, A.; Huerta-Ángeles, G. Recent Advances of Hyaluronan for Skin Delivery: From Structure to Fabrication Strategies and Applications. Polymers 2022, 14, 4833. [Google Scholar] [CrossRef]
- Tiwari, S.; Bahadur, P. Modified hyaluronic acid-based materials for biomedical applications. Int. J. Biol. Macromol. 2019, 121, 556–571. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Badoiu, S.C.; Stanescu-Spinu, I.-I.; Totan, A.R.; Stefani, C.; Greabu, M. Growth Factors, Reactive Oxygen Species, and Metformin—Promoters of the Wound Healing Process in Burns? Int. J. Mol. Sci. 2021, 22, 9512. [Google Scholar] [CrossRef]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef]
- Pastore, S.; Mascia, F.; Mariani, V.; Girolomoni, G. The Epidermal Growth Factor Receptor System in Skin Repair and Inflammation. J. Investig. Dermatol. 2008, 128, 1365–1374. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, S.R.; Yoon, I.S. Advanced Growth Factor Delivery Systems in Wound Management and Skin Regeneration. Molecules 2017, 22, 1259. [Google Scholar] [CrossRef]
- Sawa, M.; Kuroyanagi, Y. Potential of a cryopreserved cultured dermal substitute composed of hyaluronic acid and collagen to release angiogenic cytokine. J. Biomater. Sci. 2013, 24, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Harris, R.C. Epidermal growth factor, from gene organization to bedside. Semin. Cell Dev. Biol. 2014, 28, 2–11. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Stojadinovic, O.K.A.; Golinko, M.; Tomic-Canic, M.; Brem, H. A novel, non-angiogenic mechanism of VEGF: Stimulation of keratinocyte and fibroblast migration. Wound Repair Regen. 2007, 15, A30. [Google Scholar]
- Bodnar, R.J. Epidermal Growth Factor and Epidermal Growth Factor Receptor: The Yin and Yang in the Treatment of Cutaneous Wounds and Cancer. Adv. Wound Care 2013, 2, 24–29. [Google Scholar] [CrossRef]
- Choi, S.M.; Ryu, H.A.; Lee, K.-M.; Kim, H.J.; Park, I.K.; Shin, H.-C.; Choi, W.J.; Lee, J.W. Development of Stabilized Growth Factor-Loaded Hyaluronate—Collagen Dressing (HCD) matrix for impaired wound healing. Biomater. Res. 2016, 20, 9. [Google Scholar] [CrossRef]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast Growth Factor 2—A Review of Stabilisation Approaches for Clinical Applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.M.; Han, S.H.; Ko, E.A.; Yoon, D.S.; Park, I.K.; Shin, H.C.; Park, K.H.; Lee, J.W. Development of stabilized dual growth factor-loaded hyaluronate collagen dressing matrix. J. Tissue Eng. 2021, 12, 2041731421999750. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Martino, M.M. Growth Factor and Cytokine Delivery Systems for Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041234. [Google Scholar] [CrossRef]
- Kondo, S.; Kuroyanagi, Y. Development of a Wound Dressing Composed of Hyaluronic Acid and Collagen Sponge with Epidermal Growth Factor. J. Biomater. Sci. Polym. Ed. 2012, 23, 629–643. [Google Scholar] [CrossRef]
- Hwang, H.S.; Lee, C.-S. Recent Progress in Hyaluronic-Acid-Based Hydrogels for Bone Tissue Engineering. Gels 2023, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking method of hyaluronic-based hydrogel for biomedical applications. J. Tissue Eng. 2017, 8, 2041731417726464. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Ostróżka-Cieślik, A. Hyaluronan-Based Hydrogel Hybrid Insulin Carriers—Preformulation Studies. Polymers 2025, 17, 2661. [Google Scholar] [CrossRef]
- Choi, S.M.; Lee, K.M.; Kim, H.J.; Park, I.K.; Kang, H.J.; Shin, H.C.; Baek, D.; Choi, Y.; Park, K.H.; Lee, J.W. Effects of structurally stabilized EGF and bFGF on wound healing in type I and type II diabetic mice. Acta Biomater. 2018, 66, 325–334. [Google Scholar] [CrossRef]
- Kondo, S.; Niiyama, H.; Yu, A.; Kuroyanagi, Y. Evaluation of a wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor in diabetic mice. J. Biomater. Sci. Polym. Ed. 2012, 23, 1729–1740. [Google Scholar] [CrossRef]
- Yu, A.; Niiyama, H.; Kondo, S.; Yamamoto, A.; Suzuki, R.; Kuroyanagi, Y. Wound dressing composed of hyaluronic acid and collagen containing EGF or bFGF: Comparative culture study. J. Biomater. Sci. Polym. Ed. 2013, 24, 1015–1026. [Google Scholar] [CrossRef]
- Yu, A.; Takeda, A.; Kumazawa, K.; Miyoshi, H.; Kuroyanagi, M.; Yoshitake, T.; Uchinuma, E.; Suzuki, R.; Kuroyanagi, Y. Preliminary clinical study using a novel wound dressing composed of hyaluronic acid and collagen containing EGF. Open J. Regen. Med. 2015, 4, 6–13. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kuroyanagi, Y. Development of a wound dressing composed of hyaluronic acid sponge containing arginine and epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2010, 21, 715–726. [Google Scholar] [CrossRef]
- Niiyama, H.; Kuroyanagi, Y. Development of novel wound dressing composed of hyaluronic acid and collagen sponge containing epidermal growth factor and vitamin C derivative. J. Artif. Organs 2014, 17, 81–87. [Google Scholar] [CrossRef]
- Mineo, A.; Suzuki, R.; Kuroyanagi, Y. Development of an artificial dermis composed of hyaluronic acid and collagen. J. Biomater. Sci. Polym. Ed. 2013, 24, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Yamamoto, A.; Shimizu, N.; Ishihara, E.; Ohno, H.; Takeda, A.; Kuroyanagi, Y. Development of cultured dermal substitute composed of hyaluronic acid and collagen spongy sheet containing fibroblasts and epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2014, 25, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Iijima, E.D.; Toyoda, D.; Yamamoto, A.; Kuroyanagi, M.; Kuroyanagi, Y. In vitro analysis of VEGF and HGF production by fibroblast in cultured dermal substitute combined with EGF-incorporating top dressing. Open J. Regen. Med. 2014, 3, 13–21. [Google Scholar] [CrossRef]
- Thönes, S.; Rother, S.; Wippold, T.; Blaszkiewicz, J.; Balamurugan, K.; Moeller, S.; Ruiz-Gómez, G.; Schnabelrauch, M.; Scharnweber, D.; Saalbach, A.; et al. Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of Heparin-Binding EGF-like growth factor. Acta Biomater. 2019, 86, 135–147. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Ren, D.Y.; Feng, Z.X.; Zhang, L.Y.; Zhong, Y.F.; Jin, M.Y.; Xu, F.W.; Feng, C.Y.; Du, Y.Z.; et al. Mussel-inspired collagen-hyaluronic acid composite scaffold with excellent antioxidant properties and sustained release of a growth factor for enhancing diabetic wound healing. Mater. Today Biol. 2022, 15, 100320. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, L.J.; Kim, M.J.; Kim, J.-H.; Chung, D.J. In situ sodium alginate-hyaluronic acid hydrogel coating method for clinical applications. Macromol. Res. 2014, 22, 240–247. [Google Scholar] [CrossRef]
- Ali, M.; Kwak, S.H.; Lee, B.-T.; Choi, H.J. Controlled release of vascular endothelial growth factor (VEGF) in alginate and hyaluronic acid (ALG–HA) bead system to promote wound healing in punch-induced wound rat model. J. Biomater. Sci. Polym. Ed. 2023, 34, 612–631. [Google Scholar] [CrossRef]
- Ali, M.; Kwak, S.H.; Byeon, J.Y.; Choi, H.J. In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing. J. Funct. Biomater. 2023, 14, 403. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Wang, X.; Ge, J.; Tredget, E.E.; Wu, Y. The Mouse Excisional Wound Splinting Model, Including Applications for Stem Cell Transplantation. Nat. Protoc. 2013, 8, 302–309. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Little, M.O. Nutrition and skin ulcers. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 39–49, Erratum in Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 373. [Google Scholar] [CrossRef]
| Author, Year | Hybrid Matrix/Preparation Method | Growth Factor(s) | Research Model | Therapeutic Efficacy |
|---|---|---|---|---|
| Choi S.M., 2016 [38] | hyaluronate–collagen dressing (HCD) Mw HA: n.d. Physical blending | EGF bFGF (S-EGF, S-bFGF: 0.1, 0.3, 1.0, 2.5 μg/cm2). | in vitro | extended release; no toxic effects on cells observed; no inflammatory response occurred |
| Choi S.M., 2018 [47] | hyaluronate–collagen dressing (HCD) Mw HA: n.d. Physical blending | EGF bFGF (S-EGF: 0.3 μg/cm2; S-bFGF: 1 μg/cm2) | in vitro/in vivo type I and II diabetic mouse models | accelerated wound healing through stimulation of re-epithelialisation, neovascularisation, and collagen deposition |
| Kim M.S., 2021 [40] | hyaluronate–collagen dressing (HCD) Mw HA: n.d. Physical blending | EGF bFGF (S-EGF: S-bFGF, 1:2, 1 μg/cm2) | in vitro/in vivo type I diabetic mouse models | Dual-HCD exhibits a synergistic effect; the ability to accelerate diabetic wound healing; induction of re-epithelialisation, neovascularisation, and collagen deposition; stimulation of HaCaT cell migration (in vitro); therapeutic efficacy in the treatment of chronic diabetic wounds. |
| Kondo S., 2012 A [42] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (2 μg/cm2) | in vitro/in vivo Sprague–Dawley rat, surgical wound; Sprague–Dawley rat, burn wound | reduction in wound size; re-epithelialisation and formation of granulation tissue associated with angiogenesis; increased secretion of VEGF and HGF by fibroblasts |
| Kondo S., 2012 B [48] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (2 μg/cm2) | in vivo type II diabetic db/db mice, burn wound | positive effect on burn wound healing; reduction in wound surface area; angiogenesis, re-epithelialisation, and granulation tissue formation |
| Yu A., 2013 [49] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF bFGF (1 μg/cm2) | in vitro | increased secretion of VEGF and HGF; modulation of fibroblast response and promotion of angiogenesis |
| Yu A., 2015 [50] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (1 μg/cm2) | clinical trial human patients with burns, chronic ulcers, and traumatic skin defects | confirmed therapeutic efficacy of the formulation; granulation tissue formation and rapid re-epithelialisation within the wound |
| Matsumoto Y., 2010 [51] | collagen–hyaluronic acid sponge containing arginine (Arg) polydisperse: HMW-HA: n.d./LMW-HA (autoclave at 120 °C for 1 h): n.d. Freeze-drying of HMW-HA with EX 810 (chemical crosslinking), blending with autoclaved LMW-HA containing Arg; immersion, incubation at 4 °C, freezing | EGF: 100 μg Arg: 0.5 g | in vitro/in vivo Sprague–Dawley rat, surgical wound; Sprague–Dawley rat, burn wound | accelerated re-epithelialisation; inflammatory response beneficial to healing processes |
| Niiyama H., 2014 [52] | collagen–hyaluronic acid sponge containing vitamin C derivative (VC) polydisperse: HMW-HA: n.d./LMW-HA (autoclave at 120 °C for 1 h): n.d. HMW-HA and LMW-HA blended with heat-denatured collagen; poured into trays, refrigerated 4 °C, frozen at −85 °C, freeze-dried; UV irradiation of collagen for crosslinking | EGF (1 μg/cm2) VC (2.5 mg/cm2) | in vitro/in vivo type II diabetic db/db mice, dorsal wound | in vitro production of VEGF and HGF; stimulation of granulation tissue formation associated with angiogenesis and collagen deposition in vivo |
| Sawa M., 2013 [33] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (1 μg/cm2) | in vitro | stimulation of fibroblasts to release VEGF and HGF |
| Mineo A., 2013 [53] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (1 μg/cm2) | in vitro/in vivo Sprague–Dawley rat, burn wound | stimulation of fibroblasts to release VEGF and HGF; promotion of angiogenesis, and formation of a vascularised wound bed |
| Kuroyanagi M., 2014 [54] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/LMW-HA (autoclave at 120 °C for 1 h): n.d. Physical blending + freeze-drying + UV crosslinking of collagen | EGF (1 μg/cm2) | in vitro | stimulation of fibroblasts to release VEGF and HGF |
| Iijima E., 2014 [55] | collagen–hyaluronic acid sponge polydisperse: HMW-HA 2000 kDa/partially hydrolyzed LMW-HA 150 kDa Physical blending + freeze-drying + UV crosslinking (collagen or EX810) | EGF (0.1, 0.2, 0.5 μg/cm2) | in vitro | dose-dependent stimulation of fibroblasts to release VEGF and HGF |
| Thönes S., 2019 [56] | hyaluronate–collagen dressing supplemented with acrylated sulfated hyaluronan (sHA); native HA 1100 kDa; LMW-HA and sulfated oligosaccharides–depending on degree of polymerization (dp4, dp6) Physical blending + freeze-drying + UV crosslinking of collagen | heparin-binding EGF-like growth factor (HB-EGF) (10 µg/mL) | in vitro | ensuring the bioactivity of the growth factor; stimulation of keratinocytes and fibroblasts; effective wound healing |
| Wang Y., 2022 [57] | collagen–hyaluronic acid composite HA: 150–250 kDa Physical blending + freeze-drying + EDC/NHS crosslinking | EGF | in vitro/in vivo type II diabetic Sprague-Dawley rats | confirmed healing efficacy in chronic diabetic wounds; prolonged release; combination of antioxidant properties and inflammation modulation |
| Liu Y., 2014 [58] | alginate–hyaluronic acid (from 1:1 to 1:5,) hydrogel Mw HA: n.d. Physical blending + chemical crosslinking (using ADH, EDC, and Ca2+) | rh-EGF (100.0 μg/mL) | in vitro | extended release, biocompatible, non-toxic |
| Ali M., 2023 A [59] | alginate–hyaluronic acid (80:20) composite beads, heparin crosslink Mw HA: n.d. Physical blending + ionic crosslinking with Ca2+ | VEGF (150 ng/mL) Heparin (Hep) 5IU | in vitro/in vivo Sprague-Dawley rats, full-thickness skin wounds | extended release; 70% wound closure two weeks after implantation, stimulation of vascularisation, production of collagen type-1 (Col-1) and fibronectin (FN) |
| Ali M., 2023 B [60] | alginate–hyaluronic acid (80:20) composite beads, heparin crosslink Mw HA: n.d. Physical blending + ionic crosslinking with Ca2+ | EGF (100.0, 150.0 ng/mL) Heparin (Hep) 5IU | in vitro/in vivo Sprague-Dawley rats, full-thickness skin wounds | prolonged release; high expression of FLK-1 and ICAM-1 in rbMSC, 69% and 77% reduction in wound area |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostróżka-Cieślik, A.; Tanwar, A.; Michalak, M. Hyaluronan-Based Hybrid Systems as Growth Factor Carriers in the Treatment of Chronic Wounds. Int. J. Mol. Sci. 2025, 26, 10871. https://doi.org/10.3390/ijms262210871
Ostróżka-Cieślik A, Tanwar A, Michalak M. Hyaluronan-Based Hybrid Systems as Growth Factor Carriers in the Treatment of Chronic Wounds. International Journal of Molecular Sciences. 2025; 26(22):10871. https://doi.org/10.3390/ijms262210871
Chicago/Turabian StyleOstróżka-Cieślik, Aneta, Archana Tanwar, and Monika Michalak. 2025. "Hyaluronan-Based Hybrid Systems as Growth Factor Carriers in the Treatment of Chronic Wounds" International Journal of Molecular Sciences 26, no. 22: 10871. https://doi.org/10.3390/ijms262210871
APA StyleOstróżka-Cieślik, A., Tanwar, A., & Michalak, M. (2025). Hyaluronan-Based Hybrid Systems as Growth Factor Carriers in the Treatment of Chronic Wounds. International Journal of Molecular Sciences, 26(22), 10871. https://doi.org/10.3390/ijms262210871







