Genetic Stability and Photosystem II Functioning of In Vitro-Recovered Lamprocapnos spectabilis (L.) Fukuhara After ZnO + Ag Nanoparticles or Melatonin Exposure During Vitrification—Preliminary Study
Abstract
1. Introduction
2. Results
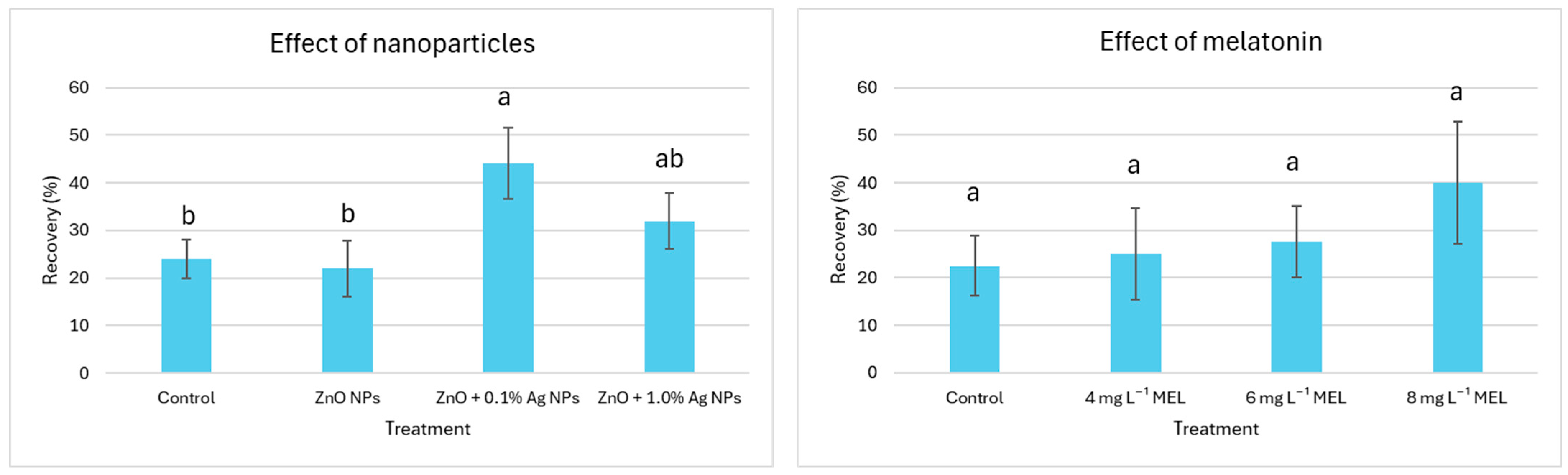
2.1. Effect of Nanoparticles and Melatonin on the Explant Recovery
2.2. Effect of Nanoparticles and Melatonin on Chlorophyll a Fluorescence
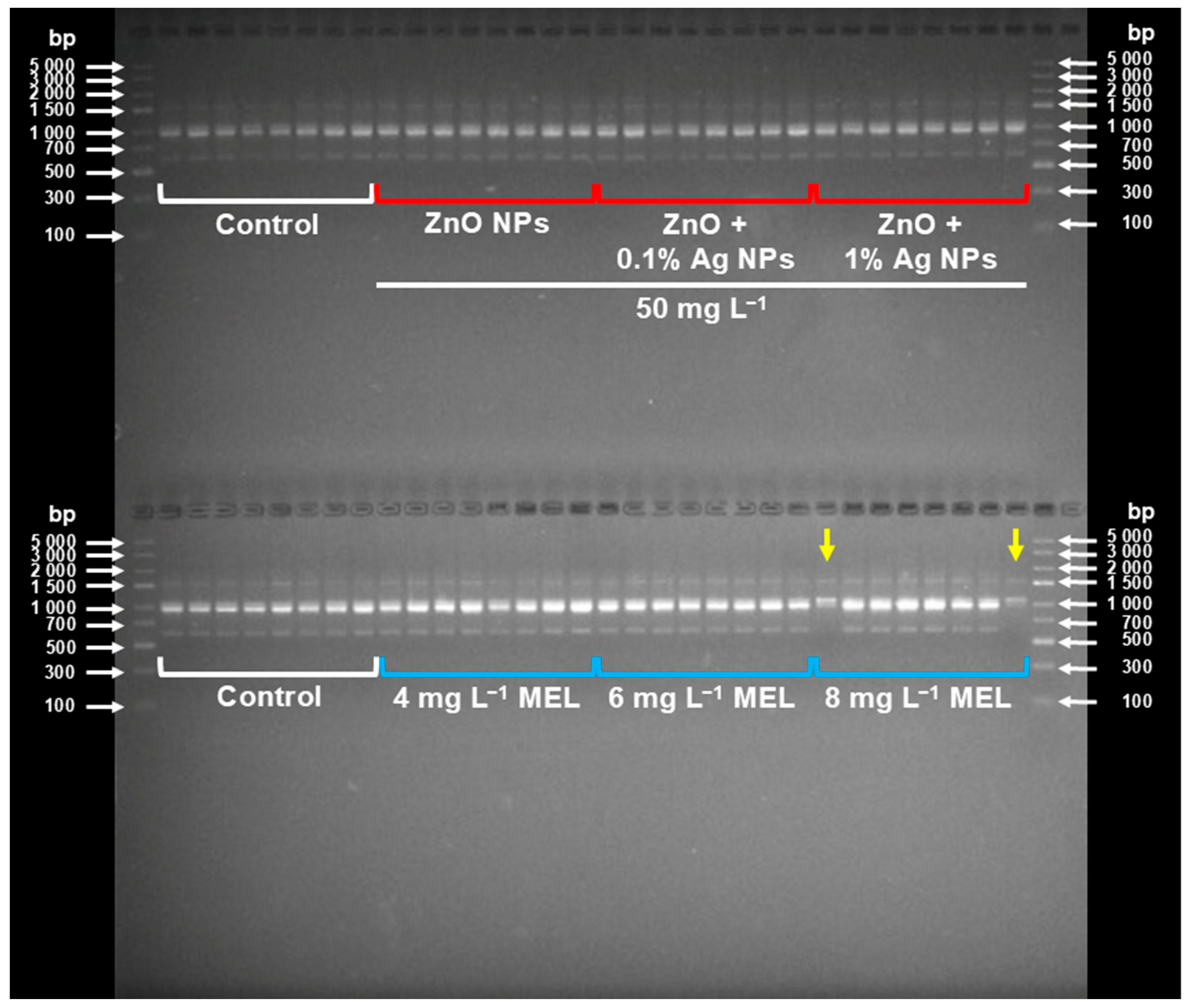
2.3. Effect of Nanoparticles and Melatonin on the Genetic Integrity of Plants
3. Discussion
3.1. Effect of Nanoparticles and Melatonin on Explant Recovery Post LN Storage
3.2. Effect of Nanoparticles and Melatonin on Plants’ Physiological Condition
3.3. Effect of Nanoparticles and Melatonin on Plants’ Genetic Stability
4. Materials and Methods
4.1. Preculture of Explants
4.2. Cryopreservation Experiment
4.2.1. Effect of NPs and Melatonin Added into the PVS
Nanoparticle-Supplemented PVS3
Melatonin-Supplemented PVS3
4.3. Encapsulation of Explants, Dehydration, and LN Storage
4.4. Rewarming and Plant Recovery
4.5. Chlorophyll a Fluorescence
4.6. Genetic Stability Evaluation
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ChF | Chlorophyll fluorescence |
| IAA | Indole-3-acetic acid |
| LN | Liquid nitrogen |
| MEL | Melatonin |
| MS | Murashige and Skoog |
| NPs | Nanoparticles |
| PCR | Polymerase chain reaction |
| PSII | Photosystem II |
| PGR | Plant growth regulator |
| PVS | Plant vitrification solution |
| ROS | Reactive oxygen species |
| SCoT | Start Codon Targeted Polymorphism |
| WS | Washing solution |
| ΦPT | Proportion of genetic variation among populations relative to total variation |
References
- Roberts, C.M.; Serek, M.; Andersen, A.S. Supplemental Irradiance and STS Improve the Display Life of Dicentra Species Forced as Flowering Potted Plants. Sci. Hortic. 1995, 62, 121–128. [Google Scholar] [CrossRef]
- Petruczynik, A.; Plech, T.; Tuzimski, T.; Misiurek, J.; Kaproń, B.; Misiurek, D.; Szultka-Młyńska, M.; Buszewski, B.; Waksmundzka-Hajnos, M. Determination of Selected Isoquinoline Alkaloids from Mahonia aquifolia; Meconopsis cambrica; Corydalis lutea; Dicentra spectabilis; Fumaria officinalis; Macleaya cordata Extracts by HPLC-DAD and Comparison of Their Cytotoxic Activity. Toxins 2019, 11, 575. [Google Scholar] [CrossRef]
- Kulus, D. In Vitro Morphogenesis, Cryopreservation and Induction of Variability in Bleeding Heart (Lamprocapnos spectabilis (L.) Fukuhara): A Review. Plant Cell Tissue Organ Cult. 2024, 158, 61. [Google Scholar] [CrossRef]
- Savov, S.; Marinova, B.; Teofanova, D.; Savov, M.; Odjakova, M.; Zagorchev, L. Parasitic Plants—Potential Vectors of Phytopathogens. Pathogens 2024, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus Latency and the Impact on Plants. Front. Microbiol. 2019, 10, 2764. [Google Scholar] [CrossRef]
- Lockhart, B.E.L. Dicentra, Epimedium, and Heuchera: New Perennial Ornamental Hosts of Tobacco Rattle Virus in the United States. Plant Dis. 2000, 84, 1344. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.R. Identification of Two Tobacco Rattle Virus Variants Associated with Line Pattern Disease of Bleeding Heart in Ohio. Plant Health Prog. 2013, 14, 26. [Google Scholar] [CrossRef]
- Lihnell, D.; Nilsson, B. Vorkommen von Tabakrattle-Virus an Dicentra spectabilis, in Schweden. J. Phytopathol. 1969, 65, 1–6. [Google Scholar] [CrossRef]
- Igori, D.; Shin, A.-Y.; Kim, S.-E.; Choi, E.K.; Hwang, U.S.; Kwon, S.; Moon, J.S. Complete Genome Sequence and Genome Characterization of a Novel Potyvirus from Lamprocapnos spectabilis. Arch. Virol. 2023, 168, 25. [Google Scholar] [CrossRef]
- Gadhave, K.R.; Gautam, S.; Rasmussen, D.A.; Srinivasan, R. Aphid Transmission of Potyvirus: The Largest Plant-Infecting RNA Virus Genus. Viruses 2020, 12, 773. [Google Scholar] [CrossRef]
- Namba, S. Molecular and Biological Properties of Phytoplasmas. Proc. Jpn. Acad. Ser. B 2019, 95, 401–418. [Google Scholar] [CrossRef]
- FAO. Climate Change and Food Security: Risks and Responses; FAO: Rome, Italy, 2016; p. 110. Available online: https://openknowledge.fao.org/handle/20.500.14283/i5188e (accessed on 4 November 2025).
- Rehman, M.U.; Rather, G.H.; Gull, Y.; Mir, M.R.; Mir, M.M.; Waida, U.I.; Hakeem, K.R. Effect of Climate Change on Horticultural Crops. In Crop Production and Global Environmental Issues, 1st ed.; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 211–239. [Google Scholar] [CrossRef]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of Extreme Weather Disasters on Global Crop Production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.; von Braun, J. Climate Change Impacts on Global Food Security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Rai, M.K.; Rathour, R.; Behera, S.; Kaushik, S.; Naik, S.K. Encapsulation Technology: An Assessment of Its Role in In Vitro Conservation of Medicinal and Threatened Plant Species. In Agricultural Biotechnology: Latest Research and Trends, 1st ed.; Srivastava, D.K., Thakur, A.K., Kumar, P., Eds.; Springer Nature: Singapore, 2021; pp. 103–128. [Google Scholar] [CrossRef]
- Engelmann, F. Use of Biotechnologies for the Conservation of Plant Biodiversity. In Vitro Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Sharma, N.; Gowthami, R.; Pandey, R. Synthetic Seeds: A Valuable Adjunct for Conservation of Medicinal Plants. In Synthetic Seeds, 1st ed.; Faisal, M., Alatar, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 181–216. [Google Scholar] [CrossRef]
- Sakai, A.; Engelmann, F. Vitrification, Encapsulation-Vitrification and Droplet-Vitrification: A Review. CryoLetters 2007, 28, 151–172. [Google Scholar]
- Kulus, D. Cryopreservation of Bleeding Heart (Lamprocapnos spectabilis (L.) Fukuhara) Shoot Tips Using Encapsulation-Dehydration. CryoLetters 2020, 41, 75–85. [Google Scholar]
- Kulus, D. Effect of Bead Composition, PVS Type, and Recovery Medium in Cryopreservation of Bleeding Heart ‘Valentine’—Preliminary Study. Agronomy 2020, 10, 891. [Google Scholar] [CrossRef]
- Adu-Gyamfi, R.; Wetten, A.; Marcelino Rodríguez López, C. Effect of Cryopreservation and Post-Cryopreservation Somatic Embryogenesis on the Epigenetic Fidelity of Cocoa (Theobroma cacao L.). PLoS ONE 2016, 11, e0158857. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-R.; Bi, W.; Shukla, M.R.; Ren, L.; Hamborg, Z.; Blystad, D.-R.; Saxena, P.K.; Wang, Q.-C. Epigenetic and Genetic Integrity, Metabolic Stability, and Field Performance of Cryopreserved Plants. Plants 2021, 10, 1889. [Google Scholar] [CrossRef]
- Popova, E.; Kulichenko, I.; Kim, H.-H. Critical Role of Regrowth Conditions in Post-Cryopreservation of In Vitro Plant Germplasm. Biology 2023, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Choi, Y.; Song, J.-Y.; Lee, J.-R.; Yoon, M.; Lee, Y.-Y. Genetic Stability Assessment of Six Cryopreserved Strawberry (Fragaria × ananassa Duch.) Accessions by Phenotypic and Molecular Studies. Biology 2022, 11, 1746. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a Fluorescence as a Tool to Monitor Physiological Status of Plants under Abiotic Stress Conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Swoczyna, T.; Kalaji, H.M.; Bussotti, F.; Mojski, J.; Pollastrini, M. Environmental Stress—What Can We Learn from Chlorophyll a Fluorescence Analysis in Woody Plants? A Review. Front. Plant Sci. 2022, 13, 1048582. [Google Scholar] [CrossRef]
- Hazrati, S.; Tahmasebi-Sarvestani, Z.; Modarres-Sanavy, S.A.M.; Mokhtassi-Bidgoli, A.; Nicola, S. Effects of Water Stress and Light Intensity on Chlorophyll Fluorescence Parameters and Pigments of Aloe vera L. Plant Physiol. Biochem. 2016, 106, 141–148. [Google Scholar] [CrossRef]
- Mazur, M.; Matoša Kočar, M.; Jambrović, A.; Sudarić, A.; Volenik, M.; Duvnjak, T.; Zdunić, Z. Crop-Specific Responses to Cold Stress and Priming: Insights from Chlorophyll Fluorescence and Spectral Reflectance Analysis in Maize and Soybean. Plants 2024, 13, 1204. [Google Scholar] [CrossRef]
- Peterson, A.; Kishchenko, O.; Kuhlmann, M.; Tschiersch, H.; Fuchs, J.; Tikhenko, N.; Schubert, I.; Nagel, M. Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants. Plants 2023, 12, 3302. [Google Scholar] [CrossRef] [PubMed]
- Kulus, D.; Tymoszuk, A.; Kulpińska, A.; Wojnarowicz, J.; Szałaj, U. Nanoparticle-Mediated Enhancement of Plant Cryopreservation: Cultivar-Specific Insights into Morphogenesis and Biochemical Responses in Lamprocapnos spectabilis (L.) Fukuhara ‘Gold Heart’ and ‘Valentine’. PLoS ONE 2024, 19, e0304586. [Google Scholar] [CrossRef]
- Kumari, A.; Gupta, A.K.; Sharma, S.; Jadon, V.S.; Sharma, V.; Chun, S.C.; Sivanesan, I. Nanoparticles as a Tool for Alleviating Plant Stress: Mechanisms, Implications, and Challenges. Plants 2024, 13, 1528. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Tripathi, A.; Kant, K. Gold Nanoparticles and Plant Pathogens: An Overview and Prospective for Biosensing in Forestry. Sensors 2022, 22, 1259. [Google Scholar] [CrossRef] [PubMed]
- Qari, S.H.; Hassan, M.U.; Chattha, M.U.; Mahmood, A.; Naqve, M.; Nawaz, M.; Barbanti, L.; Alahdal, M.A.; Aljabri, M. Melatonin Induced Cold Tolerance in Plants: Physiological and Molecular Responses. Front. Plant Sci. 2022, 13, 843071. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef]
- Sharma, P.; Thakur, N.; Mann, N.A.; Umar, A. Melatonin as Plant Growth Regulator in Sustainable Agriculture. Sci. Hortic. 2024, 323, 112421. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. Melatonin Enhances the Recovery of Cryopreserved Shoot Tips of American Elm (Ulmus americana L.). J. Pineal Res. 2013, 55, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qi, L.; Wang, W.; Saxena, P.K.; Liu, C. Melatonin Improves the Survival of Cryopreserved Callus of Rhodiola crenulata. J. Pineal Res. 2011, 50, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-W.; Jang, H. Application of Nanoparticles and Melatonin for Cryopreservation of Gametes and Embryos. Curr. Issues Mol. Biol. 2022, 44, 4028–4044. [Google Scholar] [CrossRef]
- Abbasi, Y.; Hajiaghalou, S.; Baniasadi, F.; Mahabadi, V.P.; Ghalamboran, M.R.; Fathi, R. Fe3O4 Magnetic Nanoparticles Improve the Vitrification of Mouse Immature Oocytes and Modulate the Pluripotent Genes Expression in Derived Pronuclear-Stage Embryos. Cryobiology 2021, 100, 81–89. [Google Scholar] [CrossRef]
- Iqbal, R.; Khan, T. Application of Exogenous Melatonin in Vitro and in Planta: A Review of Its Effects and Mechanisms of Action. Biotechnol. Lett. 2022, 44, 933–950. [Google Scholar] [CrossRef]
- Uchendu, E.E.; Shukla, M.R.; Reed, B.M.; Saxena, P.K. An Efficient Method for Cryopreservation of St John’s Wort and Tobacco: Role of Melatonin. Acta Hortic. 2014, 1039, 233–241. [Google Scholar] [CrossRef]
- Asadi, Z.; Safari-Faramani, R.; Aghaz, F. Effects of Adding Antioxidant Nanoparticles on Sperm Parameters of Non-Human Species after the Freezing and Thawing Process: A Systematic Review and Meta-Analysis. Anim. Reprod. Sci. 2023, 257, 107323. [Google Scholar] [CrossRef]
- Alfosea-Simón, F.J.; Burgos, L.; Alburquerque, N. Silver Nanoparticles Help Plants Grow, Alleviate Stresses, and Fight Against Pathogens. Plants 2025, 14, 428. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The Size-Dependent Effects of Silver Nanoparticles on Germination, Early Seedling Development and Polar Metabolite Profile of Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Kulus, D. Effect of Silver Nanoparticles on the In Vitro Regeneration, Biochemical, Genetic, and Phenotype Variation in Adventitious Shoots Produced from Leaf Explants in Chrysanthemum. Int. J. Mol. Sci. 2022, 23, 7406. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as Master Regulator in Plant Growth, Development and Stress Alleviator for Sustainable Agricultural Production: Current Status and Future Perspectives. Sustainability 2020, 13, 294. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural Changes of the Thylakoid Membrane Network Induced by High Light Stress in Plant Chloroplasts. Phil. Trans. R. Soc. B 2014, 369, 20130225. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to High Temperature Stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Silva, C.S.; Tonelli, F.M.P.; Delgado, V.M.S.; Lourenço, V.d.O.; Pinto, G.d.C.; Azevedo, L.S.; Lima, L.A.R.d.S.; Furtado, C.A.; Ferreira, D.R.C.; Tonelli, F.C.P.; et al. Nanoremediation and Antioxidant Potential of Biogenic Silver Nanoparticles Synthesized Using Leucena’s Leaves, Stem, and Fruits. Int. J. Mol. Sci. 2024, 25, 3993. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The Role of Melatonin in Plant Growth and Metabolism, and Its Interplay with Nitric Oxide and Auxin in Plants under Different Types of Abiotic Stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Xie, M.; Zhao, Z.; Yang, L.; Liu, J.; Hou, D. Estimation of Fv/Fm in Spring Wheat Using UAV-Based Multispectral and RGB Imagery with Multiple Machine Learning Methods. Agronomy 2023, 13, 1003. [Google Scholar] [CrossRef]
- Kurczyńska, E.; Godel-Jędrychowska, K.; Sala, K.; Milewska-Hendel, A. Nanoparticles—Plant Interaction: What We Know, Where We Are? Appl. Sci. 2021, 11, 5473. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A.; Kulpińska, A.; Dębska, B.; Michalska, A.; Nowakowska, J.; Wichrowska, D.; Wojnarowicz, J.; Szałaj, U. Nanoparticles in Plant Cryopreservation: Effects on Genetic Stability, Metabolic Profiles, and Structural Integrity in Bleeding Heart (Papaveraceae) Cultivars. Nanotechnol. Sci. Appl. 2025, 18, 35–56. [Google Scholar] [CrossRef]
- Osmani, Z.; Kulka, M. Form and Function: The Factors That Influence the Efficacy of Nanomaterials for Gene Transfer to Plants. Molecules 2025, 30, 446. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zheng, Y.; Pan, L.; Wang, W.; Li, Y.; Liu, Z.; Zhang, X. Nanodelivery of Nucleic Acids for Plant Genetic Engineering. Discover Nano 2025, 20, 31. [Google Scholar] [CrossRef]
- Abrica-González, P.; Gómez-Arroyo, S.; Sotelo-López, A.; Jazcilevich-Diamant, A.; Flores-Márquez, A.R.; Cortés-Eslava, J. DNA Damage Induced by ZnO and CuO Nanoparticles: A Comparative Study against Bulk Materials. Environ. Sci. Pollut. Res. 2025, 32, 18461–18477. [Google Scholar] [CrossRef]
- Al-Saleh, M.A.; Al-Harbi, H.F.; Al-Humaid, L.A.; Awad, M.A. An Assessment of the Cyto-Genotoxicity Effects of Green-Synthesized Silver Nanoparticles and ATCBRA Insecticide on the Root System of Vicia faba. Nanomaterials 2025, 15, 77. [Google Scholar] [CrossRef] [PubMed]
- Tymoszuk, A.; Kulus, D. Silver Nanoparticles Induce Genetic, Biochemical, and Phenotype Variation in Chrysanthemum. Plant Cell Tissue Organ Cult. 2020, 143, 331–344. [Google Scholar] [CrossRef]
- Musatov, S.A.; Anisimov, V.N.; André, V.; Vigreux, C.; Godard, T.; Gauduchon, P.; Sichel, F. Modulatory Effects of Melatonin on Genotoxic Response of Reference Mutagens in the Ames Test and the Comet Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 417, 75–84. [Google Scholar] [CrossRef]
- Gao, S.; Ma, W.; Lyu, X.; Cao, X.; Yao, Y. Melatonin May Increase Disease Resistance and Flavonoid Biosynthesis through Effects on DNA Methylation and Gene Expression in Grape Berries. BMC Plant Biol. 2020, 20, 231. [Google Scholar] [CrossRef]
- Kulus, D.; de Dieu Muhire, J.; Aksoy, B. Growth Regulation and Validation of Homogeneity in In Vitro-Derived Bleeding Heart by Molecular Markers and Spectral Analysis of Pigments. J. Plant Growth Regul. 2021, 40, 1521–1538. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kulus, D.; Tymoszuk, A.; Kulpińska, A.; Viehmannova, I.; Wojnarowicz, J.; Szałaj, U. Effect of Nanoparticles on the Ex-Vitro Performance of Cryopreservation-Derived Plant Material. PLoS ONE 2024, 19, e0310424. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Chudoba, T.; Koltsov, I.; Gierlotka, S.; Dworakowska, S.; Lojkowski, W. Size Control Mechanism of ZnO Nanoparticles Obtained in Microwave Solvothermal Synthesis. Nanotechnology 2018, 29, 065601. [Google Scholar] [CrossRef]
- Wojnarowicz, J.; Opalinska, A.; Chudoba, T.; Gierlotka, S.; Mukhovskyi, R.; Pietrzykowska, E.; Sobczak, K.; Lojkowski, W. Effect of Water Content in Ethylene Glycol Solvent on the Size of ZnO Nanoparticles Prepared Using Microwave Solvothermal Synthesis. J. Nanomater. 2016, 2016, 2789871. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Sławkowska, N.; Szałaj, U.; Kulus, D.; Antkowiak, M.; Wojnarowicz, J. Synthesis, Characteristics, and Effect of Zinc Oxide and Silver Nanoparticles on the In Vitro Regeneration and Biochemical Profile of Chrysanthemum Adventitious Shoots. Materials 2022, 15, 8192. [Google Scholar] [CrossRef]
- Tymoszuk, A.; Szałaj, U.; Wojnarowicz, J.; Kowalska, J.; Antkowiak, M.; Kulus, D. Zinc Oxide and Silver Effects on the Growth, Pigment Content and Genetic Stability of Chrysanthemums Propagated by the Node Culture Method. Folia Hortic. 2024, 36, 35–66. [Google Scholar] [CrossRef]
- Pokrowiecki, R.; Wojnarowicz, J.; Zareba, T.; Koltsov, I.; Lojkowski, W.; Tyski, S.; Mielczarek, A.; Zawadzki, P. Nanoparticles And Human Saliva: A Step Towards Drug Delivery Systems For Dental And Craniofacial Biomaterials. Int. J. Nanomed. 2019, 14, 9235–9257. [Google Scholar] [CrossRef]
- Roháček, K. Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Zlatev, Z.S.; Yordanov, I.T. Effects of Soil Drought on Photosynthesis and Chlorophyll Fluorescence in Bean Plants. Bulg. J. Plant Physiol. 2004, 30, 3–18. [Google Scholar]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) Polymorphism: A Simple, Novel DNA Marker Technique for Generating Gene-Targeted Markers in Plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research—An Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Fluorescence Parameters | ||||
|---|---|---|---|---|---|
| F0 | Fv | Fm | Fv/Fm | Fv/F0 | |
| Nanoparticles in PVS3 | |||||
| Control | 170.05 ± 11.59 b | 114.35 ± 19.35 b | 284.40 ± 26.08 c | 0.38 ± 0.03 c | 0.70 ± 0.09 c |
| ZnO NPs | 203.70 ± 9.64 a | 143.05 ± 11.43 b | 346.75 ± 19.69 bc | 0.41 ± 0.01 c | 0.70 ± 0.04 c |
| ZnO + 0.1% Ag NPs | 212.45 ± 13.94 a | 246.80 ± 23.87 a | 459.25 ± 36.07 a | 0.52 ± 0.01 b | 1.12 ± 0.07 b |
| ZnO + 1.0% Ag NPs | 137.40 ± 7.53 c | 280.20 ± 14.01 a | 417.60 ± 16.37 ab | 0.67 ± 0.02 a | 2.16 ± 0.18 a |
| Melatonin in PVS3 | |||||
| Control | 152.10 ± 7.82 b | 162.65 ± 22.51 b | 314.75 ± 22.51 b | 0.48 ± 0.03 b | 1.07 ± 0.15 b |
| 4 mg L−1 MEL | 188.55 ± 16.56 a | 200.20 ± 21.21 ab | 388.75 ± 21.21 ab | 0.50 ± 0.03 b | 1.18 ± 0.14 b |
| 6 mg L−1 MEL | 148.10 ± 8.34 b | 249.25 ± 19.81 a | 397.35 ± 19.81 a | 0.61 ± 0.02 a | 1.71 ± 0.14 a |
| 8 mg L−1 MEL | 153.20 ± 10.66 b | 194.00 ± 22.21 ab | 347.20 ± 22.21 ab | 0.53 ± 0.04 ab | 1.37 ± 0.16 ab |
| No. | Primer Sequence 5′➞3′ | No. of Bands | No. of Loci | Share of Polymorphic Loci [%] | No. (%) of Polymorphic Plants | No. of Genotypes | |||
|---|---|---|---|---|---|---|---|---|---|
| ∑ | Mono. | Poly. | Spec. | ||||||
| S1 | CAA CAA TGG CTA CCA CCG | 512 | 8 | 8 | 0 | 0 | 0 | 0 | 1 |
| S2 | CAA CAA TGG CTA CCA CCT | 64 | 1 | 1 | 0 | 0 | 0 | 0 | 1 |
| S3 | CAA CAA TGG CTA CCA CGT | 320 | 5 | 5 | 0 | 0 | 0 | 0 | 1 |
| S4 | ACG ACA TGG CGA CCA ACG | 192 | 3 | 3 | 0 | 0 | 0 | 0 | 1 |
| S5 | ACG ACA TGG CGA CCA TCG | 448 | 7 | 7 | 0 | 0 | 0 | 0 | 1 |
| S6 | ACC ATG GCT ACC ACC GTG | 254 | 5 | 2 | 3 | 0 | 60 | 2 (3%) | 2 |
| S7 | CCA TGG CTA CCA CCG CCA | 128 | 2 | 2 | 0 | 0 | 0 | 0 | 1 |
| S8 | CCA TGG CTA CCA CCG CAG | 576 | 9 | 9 | 0 | 0 | 0 | 0 | 1 |
| No. | Marker | Sequence (5′-3′) | %GC |
|---|---|---|---|
| S1 | SCoT3 | CAACAATGGCTACCACCG | 56 |
| S2 | SCoT4 | CAACAATGGCTACCACCT | 50 |
| S3 | SCoT8 | CAACAATGGCTACCACGT | 50 |
| S4 | SCoT12 | ACGACATGGCGACCAACG | 61 |
| S5 | SCoT13 | ACGACATGGCGACCATCG | 61 |
| S6 | SCoT25 | ACCATGGCTACCACCGGG | 67 |
| S7 | SCoT26 | ACCATGGCTACCACCGTC | 61 |
| S8 | SCoT28 | CCATGGCTACCACCGCCA | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulus, D.; Tymoszuk, A.; Cichorek, M. Genetic Stability and Photosystem II Functioning of In Vitro-Recovered Lamprocapnos spectabilis (L.) Fukuhara After ZnO + Ag Nanoparticles or Melatonin Exposure During Vitrification—Preliminary Study. Int. J. Mol. Sci. 2025, 26, 10817. https://doi.org/10.3390/ijms262210817
Kulus D, Tymoszuk A, Cichorek M. Genetic Stability and Photosystem II Functioning of In Vitro-Recovered Lamprocapnos spectabilis (L.) Fukuhara After ZnO + Ag Nanoparticles or Melatonin Exposure During Vitrification—Preliminary Study. International Journal of Molecular Sciences. 2025; 26(22):10817. https://doi.org/10.3390/ijms262210817
Chicago/Turabian StyleKulus, Dariusz, Alicja Tymoszuk, and Mateusz Cichorek. 2025. "Genetic Stability and Photosystem II Functioning of In Vitro-Recovered Lamprocapnos spectabilis (L.) Fukuhara After ZnO + Ag Nanoparticles or Melatonin Exposure During Vitrification—Preliminary Study" International Journal of Molecular Sciences 26, no. 22: 10817. https://doi.org/10.3390/ijms262210817
APA StyleKulus, D., Tymoszuk, A., & Cichorek, M. (2025). Genetic Stability and Photosystem II Functioning of In Vitro-Recovered Lamprocapnos spectabilis (L.) Fukuhara After ZnO + Ag Nanoparticles or Melatonin Exposure During Vitrification—Preliminary Study. International Journal of Molecular Sciences, 26(22), 10817. https://doi.org/10.3390/ijms262210817








