Myokine Levels in Relation to Bone Markers and Adipokines in Children with Prader–Willi Syndrome During Growth Hormone Therapy and Dietary Intervention
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biochemical Methods
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BALP | Bone alkaline phosphatase |
| BMC | Bone mineral content |
| BMD | Bone mineral density |
| BMI | Body mass index |
| FGF-2 | Fibroblast growth factor-2 |
| FLI | Free leptin index |
| GH | Growth hormone |
| Gla-OC | Carboxylated osteocalcin |
| HMW-adiponectin | High molecular weight-adiponectin |
| IGF-I | Insulin-like growth factor-I |
| IGFBP-2 | IGF-binding protein-2 |
| MSTN | Myostatin |
| OC | Osteocalcin |
| PWS | Prader–Willi syndrome |
| RANK | Receptor activator for nuclear factor kappa-B |
| sRANKL | Soluble receptor activator of nuclear factor kappa-B ligand |
| sOB-R | Soluble leptin receptor |
| TBLH-BMD | Total body less head-BMD |
| TRAcP 5b | Tartrate-resistant acid phosphatase 5b |
| VKDP | Vitamin K-dependent protein |
References
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi syndrome-clinical genetics, diagnosis and teatment approaches: An update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef]
- van Abswoude, D.H.; Pellikaan, K.; Rosenberg, A.G.W.; Davidse, K.; Coupaye, M.; Høybye, C.; Markovic, T.P.; Grugni, G.; Crinò, A.; Caixàs, A.; et al. Bone health in adults with Prader-Willi syndrome: Clinical recommendations based on a multicenter cohort study. J. Clin. Endocrinol. Metab. 2022, 108, 59–84, Correction in J. Clin. Endocrinol. Metab. 2023, 108, e30. [Google Scholar] [CrossRef]
- Reus, L.; Zwarts, M.; van Vlimmeren, L.A.; Willemsen, M.A.; Otten, B.J.; Nijhuis-van der Sanden, M.W. Motor problems in Prader-Willi syndrome: A systematic review on body composition and neuromuscular functioning. Neurosci. Biobehav. Rev. 2011, 35, 956–969. [Google Scholar] [CrossRef]
- Heksch, R.; Kamboj, M.; Anglin, K.; Obrynba, K. Review of Prader-Willi syndrome: The endocrine approach. Transl. Pediatr. 2017, 6, 274–285. [Google Scholar] [CrossRef]
- Colaianni, G.; Storlino, G.; Sanesi, L.; Colucci, S.; Grano, M. Myokines and osteokines in the pathogenesis of muscle and bone diseases. Curr. Osteoporos. Rep. 2020, 18, 401–407. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Gries, K.J.; Zysik, V.S.; Jobe, T.K.; Griffin, N.; Leeds, B.P.; Lowery, J.W. Muscle-derived factors influencing bone metabolism. Semin. Cell Dev. Biol. 2022, 123, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. J. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signalling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Divieti Pajevic, P.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J. Biol. Chem. 2017, 292, 11021–11033. [Google Scholar] [CrossRef] [PubMed]
- Amor, M.; Itariu, B.K.; Moreno-Viedma, V.; Keindl, M.; Jürets, A.; Prager, G.; Langer, F.; Grablowitz, V.; Zeyda, M.; Stulnig, T.M. Serum myostatin is upregulated in obesity and correlates with insulin resistance in humans. Exp. Clin. Endocrinol. Diabetes 2019, 127, 550–556. [Google Scholar]
- Carvalho, L.P.; Basso-Vanelli, R.P.; Di Thommazo-Luporini, L.; Mendes, R.G.; Oliveira-Junior, M.C.; Vieira, R.P.; Bonjorno-Junior, J.C.; Oliveira, C.R.; Luporini, R.; Borghi-Silva, A. Myostatin and adipokines: The role of the metabolically unhealthy obese phenotype in muscle function and aerobic capacity in young adults. Cytokine 2018, 107, 118–124. [Google Scholar] [CrossRef]
- Coffin, J.D.; Homer-Bouthiette, C.; Hurley, M.M. Fibroblast growth factor 2 and its receptors in bone biology and disease. J. Endocr. Soc. 2018, 2, 657–671. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.; Lv, Q.; Zhang, Y.; Gao, G.; Lu, S. Advances in the research on myokine-driven regulation of bone metabolism. Heliyon 2023, 10, e22547. [Google Scholar] [CrossRef]
- Lebrasseur, N.; Achenbach, S.J.; Melton, L.J., 3rd; Amin, S.; Khosla, S. Skeletal muscle mass is associated with bone geometry and microstructure and serum insulin-like growth factor binding protein-2 levels in adult women and men. J. Bone Miner. Res. 2012, 27, 2159–2169. [Google Scholar] [CrossRef]
- Determe, W.; Hauge, S.C.; Demeuse, J.; Massonnet, P.; Grifnée, E.; Huyghebaert, L.; Dubrowski, T.; Schoumacher, M.; Peeters, S.; Le Goff, C.; et al. Osteocalcin: A bone protein with multiple endocrine functions. Clin. Chim. Acta 2025, 567, 120067. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yan, K.; Guan, Q.; Guo, Q.; Zhao, C. Mechanism and physical activities in bone-skeletal muscle crosstalk. Front. Endocrinol. 2024, 14, 1287972. [Google Scholar] [CrossRef] [PubMed]
- Chiu, V.J.; Tsai, L.P.; Wei, J.T.; Tzeng, I.S.; Wu, H.C. Motor performance in Prader-Willi syndrome patients and its potential influence on caregiver’s quality of life. PeerJ 2017, 5, e4097. [Google Scholar] [CrossRef]
- Casamitjana, L.; Blanco-Hinojo, L.; Giménez-Palop, O.; Pujol, J.; Martínez-Vilavella, G.; Esteba-Castillo, S.; Pareja, R.; Freijo, V.; Vigil, L.; Deus, J.; et al. One year of recombinant human growth hormone treatment in adults with Prader-Willi syndrome improves body composition, motor skills and brain functional activity in the cerebellum. J. Clin. Med. 2022, 11, 1831. [Google Scholar] [CrossRef]
- Mai, S.; Grugni, G.; Mele, C.; Vietti, R.; Vigna, L.; Sartorio, A.; Aimaretti, G.; Scacchi, M.; Marzullo, P. Irisin levels in genetic and essential obesity: Clues for a potential dual role. Sci. Rep. 2020, 10, 1020. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Grugni, G.; Fintini, D.; Convertino, A.; Pignataro, P.; Crinò, A.; Colucci, S.; Grano, M. The genetic background and vitamin D supplementation can affect irisin levels in Prader-Willi syndrome. J. Endocrinol. Investig. 2021, 44, 2261–2271. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Szamotulska, K.; Klemarczyk, W.; Chełchowska, M.; Strucińska, M.; Ambroszkiewicz, J. Circulating levels of nesfatin-1 and spexin in children with Prader-Willi Syndrome during growth hormone treatment and dietary intervention. Nutrients 2023, 15, 1240. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Chełchowska, M.; Szamotulska, K.; Klemarczyk, W.; Strucińska, M.; Ambroszkiewicz, J. Differences in bone metabolism between children with Prader-Willi syndrome during growth hormone treatment and healthy subjects: A pilot study. Int. J. Mol. Sci. 2024, 25, 9159. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Fintini, D.; Mele, C.; Convertino, A.; Bocchini, S.; Grugni, G.; Aimaretti, G.; Vietti, R.; Scacchi, M.; Crinò, A.; et al. Circulating irisin in children and adolescents with Prader-Willi syndrome: Relation with glucose metabolism. Front. Endocrinol. 2022, 13, 918467. [Google Scholar] [CrossRef]
- Hirsch, H.J.; Gross, I.; Pollak, Y.; Eldar-Geva, T.; Gross-Tsur, V. Irisin and the metabolic phenotype of adults with Prader-Willi syndrome. PLoS ONE 2015, 10, e0136864. [Google Scholar] [CrossRef]
- Irizarry, K.A.; Miller, M.; Freemark, M.; Haqq, A.M. Prader Willi syndrome: Genetics, metabolomics, hormonal function, and new approaches to therapy. Adv. Pediatr. 2016, 63, 47–77. [Google Scholar] [CrossRef]
- Goodman, C.A.; McNally, R.M.; Hoffmann, F.M.; Hornberger, T.A. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol. Endocrinol. 2013, 27, 1946–1957. [Google Scholar] [CrossRef]
- Castro-Gago, M.; Gómez-Lado, C.; Eiris-Puñal, J.; Carneiro, I.; Arce, V.M.; Devesa, J. Muscle myostatin expression in children with muscle diseases. J. Child Neurol. 2007, 22, 38–40. [Google Scholar] [CrossRef]
- Bergen, H.R., 3rd; Farr, J.N.; Vanderboom, P.M.; Atkinson, E.J.; White, T.A.; Singh, R.J.; Khosla, S.; LeBrasseur, N.K. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: Insights using a new mass spectrometry-based assay. Skelet. Muscle 2015, 5, 21. [Google Scholar] [CrossRef]
- Tanaka, M.; Masuda, S.; Yamakage, H.; Inoue, T.; Ohue-Kitano, R.; Yokota, S.; Kusakabe, T.; Wada, H.; Sanada, K.; Ishii, K.; et al. Role of serum myostatin in the association between hyperinsulinemia and muscle atrophy in Japanese obese patients. Diabetes Res. Clin. Pract. 2018, 142, 195–202. [Google Scholar] [CrossRef]
- Malvandi, A.M.; Gerosa, L.; Banfi, G.; Lombardi, G. The bone-muscle unit: From mechanical coupling to soluble factors-mediated signaling. Mol. Aspects Med. 2025, 103, 101367. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Q.; Du, P.; Chen, X.; Zhang, Y. Roles of vitamin K-dependent protein in biomineralization (Review). Int. J. Mol. Med. 2024, 53, 6. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Schulz, L.; Palmisano, B.; Singh, P.; Berger, J.M.; Yadav, V.K.; Mera, P.; Ellingsgaard, H.; Hidalgo, J.; Brüning, J.; et al. Muscle-derived interleukin 6 increases exercise capacity by signaling in osteoblasts. J. Clin. Investig. 2020, 130, 2888–2902. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Nie, Y.; Ma, Y.; Chen, Y.; Cheng, R.; Yin, W.; Hu, Y.; Xu, W.; Xu, L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci. Rep. 2016, 6, 18732, Correction in Sci. Rep. 2016, 6, 21053. [Google Scholar] [CrossRef] [PubMed]
- Zerlotin, R.; Oranger, A.; Pignataro, P.; Dicarlo, M.; Maselli, F.; Mori, G.; Colucci, S.C.; Grano, M.; Colaianni, G. Irisin and secondary osteoporosis in humans. Int. J. Mol. Sci. 2022, 23, 690. [Google Scholar] [CrossRef]
- Young, J.A.; Zhu, S.; List, E.O.; Duran-Ortiz, S.; Slama, Y.; Berryman, D.E. Musculoskeletal effects of altered GH action. Front. Physiol. 2022, 13, 867921. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, X.; Chen, X.; Wang, Z.; Zheng, S.; Cheng, Y.; Liu, S.; Hao, L. The role of insulin-like growth factor-1 in bone remodeling: A review. Int. J. Biol. Macromol. 2023, 238, 124125. [Google Scholar] [CrossRef]
- Talebizadeh, Z.; Butler, M.G. Insulin resistance and obesity-related factors in Prader-Willi syndrome: Comparison with obese subjects. Clin. Genet. 2005, 67, 230–239. [Google Scholar] [CrossRef]
- Irizarry, K.A.; Bain, J.; Butler, M.G.; Ilkayeva, O.; Muehlbauer, M.; Haqq, A.M.; Freemark, M. Metabolic profiling in Prader-Willi syndrome and nonsyndromic obesity: Sex differences and the role of growth hormone. Clin. Endocrinol. 2015, 83, 797–805. [Google Scholar] [CrossRef]
- McAlister, K.L.; Fisher, K.L.; Dumont-Driscoll, M.C.; Rubin, D.A. The relationship between metabolic syndrome, cytokines and physical activity in obese youth with and without Prader-Willi syndrome. J. Pediatr. Endocrinol. Metab. 2018, 31, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, D.; Moutel, S.; Coupaye, M.; Huvenne, H.; Faucher, P.; Pelloux, V.; Rouault, C.; Bastard, J.P.; Cagnard, N.; Dubern, B.; et al. Metabolic and adipose tissue signatures in adults with Prader-Willi syndrome: A model of extreme adiposity. J. Clin. Endocrinol. Metab. 2015, 100, 850–859. [Google Scholar] [CrossRef]
- Kennedy, L.; Bittel, D.C.; Kibiryeva, N.; Kalra, S.P.; Torto, R.; Butler, M.G. Circulating adiponectin levels, body composition and obesity-related variables in Prader-Willi syndrome: Comparison with obese subjects. Int. J. Obes. 2006, 30, 382–387. [Google Scholar] [CrossRef]
- Rodríguez, A.; Becerril, S.; Ezquerro, S.; Méndez-Giménez, L.; Frühbeck, G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol. 2017, 219, 362–381. [Google Scholar] [CrossRef]
- Assyov, Y.S.; Velikova, T.V.; Kamenov, Z.A. Myostatin and carbohydrate disturbances. Endocr. Res. 2017, 42, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, C.; Nakanishi, K.; Nishida, M.; Shinomiya, H.; Shinzawa, M.; Kanayama, D.; Yamamoto, R.; Kudo, T.; Nagatomo, I.; Yamauchi-Takihara, K. Myostatin as a plausible biomarker for early stage of sarcopenic obesity. Sci. Rep. 2024, 14, 28629. [Google Scholar] [CrossRef] [PubMed]
- Karampatsou, S.I.; Genitsaridi, S.M.; Michos, A.; Kourkouni, E.; Kourlaba, G.; Kassari, P.; Manios, Y.; Charmandari, E. The effect of a life-style intervention program of diet and exercise on irisin and FGF-21 concentrations in children and adolescents with overweight and obesity. Nutrients 2021, 13, 1274. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Ludovica Monaco, M.; Polito, R.; Schettino, P.; Grandone, A.; Perrone, L.; Miraglia Del Giudice, E.; Daniele, A. Adiponectin profile and Irisin expression in Italian obese children: Association with insulin-resistance. Cytokine 2017, 94, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Efstathiadou, Z.A.; Makras, P.; Perakakis, N.; Kountouras, J.; Mantzoros, C.S. Irisin in metabolic diseases. Endocrine 2018, 59, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Dieta 5.0 Software for Individual and Group Nutrition Assessment and Diet Planning; National Food and Nutrition Institute: Warsaw, Poland, 2015. [Google Scholar]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Światek-Leśniak, A.; Litwin, M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 year of age. Stand. Med. 2015, 12, 119–135. [Google Scholar]
- Catli, G.; Anik, A.; Tuhan, H.Ü.; Kume, T.; Bober, E.; Abaci, A. The relation of leptin and soluble leptin receptor levels with metabolic and clinical parameters in obese and healthy children. Peptides 2014, 56, 72–76. [Google Scholar] [CrossRef]
- Pratapwar, M.P.; Sheth, H.J.; Ravi, A.K.; Block, M.L.; Korber, K.A.; Kepsel, A.; Leimanis-Laurens, M.; Comstock, S.S. Use of biomarkers in nutrition intervention studies of children: A scoping review. Nutrients 2024, 16, 3584. [Google Scholar] [CrossRef]
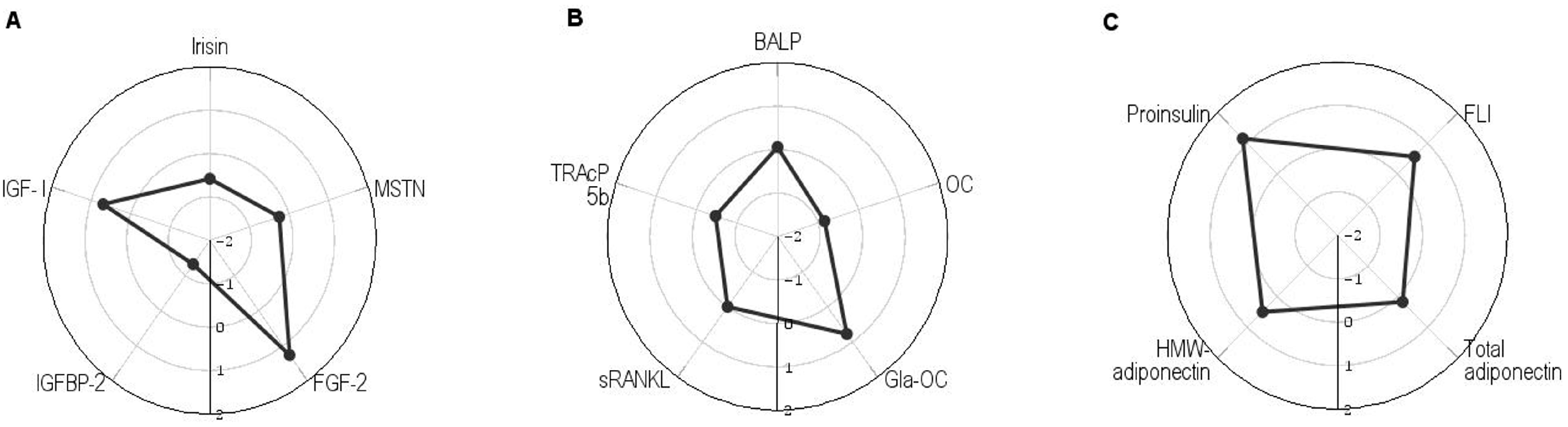
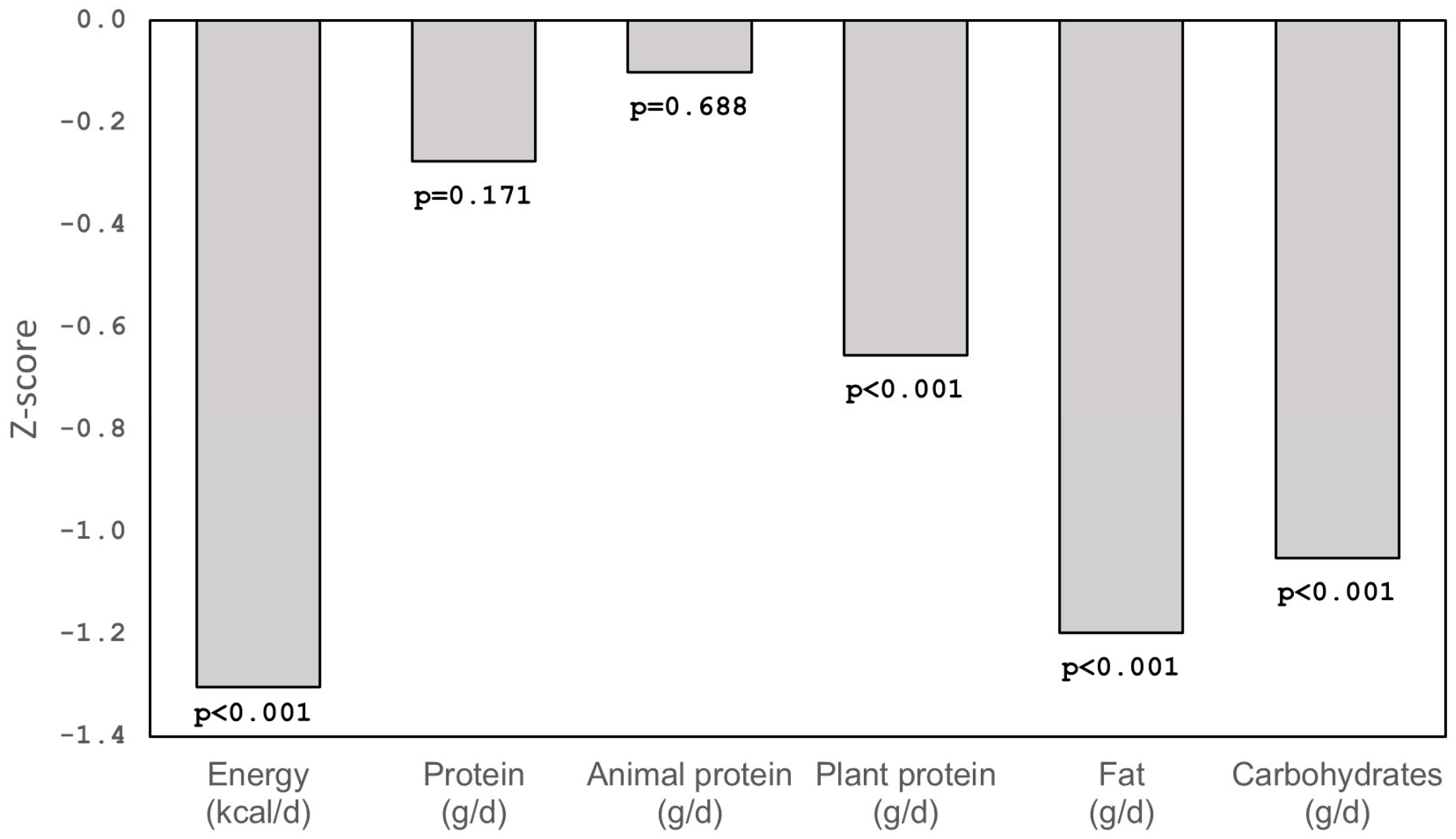
| Children with PWS n = 26 | Healthy Children n = 26 | p-Values | |
|---|---|---|---|
| Age (years) | 6.6 ± 3.3 | 7.6 ± 3.3 | 0.190 |
| Girls/boys | 15/11 | 15/11 | |
| Height (cm) | 116.1 ± 23.6 | 125.0 ± 19.1 | 0.131 |
| Weight (kg) | 21.5 ± 9.5 | 25.7 ± 8.7 | 0.072 |
| BMI (kg/m2) | 15.0 (14.2–15.9) | 16.1 (15.0–16.8) | 0.033 |
| BMI Z-score | −0.56 ± 0.68 | −0.35 ± 0.38 | 0.126 |
| Irisin (µg/mL) | 3.24 ± 1.46 | 4.06 ± 1.42 | 0.031 |
| MSTN (ng/mL) | 1.74 (1.42–2.09) | 2.07 (1.57–2.48) | 0.115 |
| FGF-2 (pg/mL) | 44.4 (9.3–125.3) | 26.8 (17.3–49.5) | 0.459 |
| IGFBP-2 (ng/mL) | 254.7 ± 132.4 | 358.8 ± 78.8 | <0.001 |
| IGF-I (ng/mL) | 297.7 ± 150.6 | 217.5 ± 115.3 | 0.035 |
| BALP (U/L) | 125.5 (97.9–148.1) | 120.3 (95.1–143.5) | 0.519 |
| OC (ng/mL) | 56.5 ± 22.6 | 87.8 ± 37.3 | <0.001 |
| Gla-OC (ng/mL) | 37.7 ± 16.8 | 29.0 ± 11.3 | 0.068 |
| Periostin (ng/mL) | 93.9 ± 38.3 | 60.6 ± 17.9 | <0.001 |
| sRANKL (ng/mL) | 825 (324–1695) | 926 (582–1478) | 0.778 |
| TRAcP 5b (U/L) | 10.3 ± 2.8 | 11.6 ± 2.8 | 0.106 |
| FLI | 0.10 (0.05–0.17) | 0.03 (0.02–0.09) | 0.008 |
| Total adiponectin (µg/mL) | 11.6 (9.5–13.8) | 9.9 (8.3–12.4) | 0.263 |
| HMW-adiponectin (µg/mL) | 6.13 (4.52–7.05) | 3.97 (3.53–5.34) | 0.009 |
| Proinsulin (pmol/L) | 1.95 (1.56–2.90) | 1.35 (0.91–2.24) | 0.025 |
| Correlation Coefficient | Irisin | MSTN | FGF-2 | IGF-I | IGFBP-2 | |
|---|---|---|---|---|---|---|
| Height | bivariate (p) partial * (p) | −0.043 (0.835) 0.064 (0.759) | 0.282 (0.162) −0.230 (0.268) | 0.485 (0.012) 0.289 (0.161) | 0.810 (<0.001) 0.169 (0.418) | −0.731 (<0.001) −0.590 (0.002) |
| Weight | bivariate (p) partial * (p) | −0.011 (0.956) 0.129 (0.539) | 0.297 (0.141) −0.088 (0.675) | 0.400 (0.043) −0.010 (0.962) | 0.770 (<0.001) 0.060 (0.775) | −0.645 (<0.001) −0.195 (0.350) |
| BMI | bivariate (p) partial * (p) | 0.160 (0.436) 0.182 (0.384) | 0.079 S (0.701) 0.031 S (0.883) | −0.192 S (0.348) −0.299 S (0.147) | 0.127 S (0.535) 0.013 S (0.952) | −0.083 S (0.685) −0.195 S (0.350) |
| BMI Z-score | bivariate (p) partial * (p) | 0.136 (0.507) 0.137 (0.515) | 0.007 (0.973) 0.006 (0.976) | −0.216 (0.289) −0.241 (0.245) | −0.124 (0.547) −0.217 (0.298) | 0.077 (0.707) 0.103 (0.624) |
| BALP | bivariate (p) partial * (p) | 0.427 (0.029) 0.447 (0.025) | 0.006 (0.978) −0.064 (0.763) | −0.020 (0.923) −0.112 (0.595) | 0.270 (0.183) 0.211 (0.312) | 0.001 (0.994) 0.155 (0.461) |
| OC | bivariate (p) partial * (p) | −0.157 (0.444) −0.145 (0.490) | 0.049 (0.812) −0.075 (0.721) | 0.041 (0.841) −0.118 (0.573) | 0.521 (0.006) 0.461 (0.020) | −0.075 (0.715) 0.183 (0.382) |
| Gla-OC | bivariate (p) partial * (p) | −0.414 (0.035) −0.411 (0.041) | 0.088 (0.669) 0.013 (0.951) | 0.444 (0.023) 0.398 (0.049) | 0.424 (0.031) 0.436 (0.030) | −0.092 (0.657) 0.061 (0.771) |
| Periostin | bivariate (p) partial * (p) | −0.529 (0.005) −0.541 (0.005) | 0.007 (0.971) 0.052 (0.805) | 0.239 (0.239) 0.324 (0.114) | −0.083 (0.685) 0.020 (0.923) | 0.352 (0.078) 0.362 (0.076) |
| sRANKL | bivariate (p) partial * (p) | 0.308 (0.126) −0.349 (0.088) | −0.020 S (0.925) 0.196 S (0.349) | −0.380 (0.324) −0.150 (0.473) | −0.521 (0.006) −0.007 (0.973) | 0.516 (0.007) 0.189 (0.366) |
| TRAcP 5b | bivariate (p) partial * (p) | 0.038 (0.852) 0.057 (0.787) | 0.410 (0.038) 0.351 (0.085) | 0.201 (0.324) 0.101 (0.632) | 0.277 (0.170) 0.113 (0.591) | −0.092 (0.656) 0.101 (0.631) |
| Correlation Coefficient | Irisin | MSTN | FGF-2 | IGF-I | IGFBP-2 | |
|---|---|---|---|---|---|---|
| FLI | bivariate (p) partial * (p) | −0.062 (0.762) −0.033 (0.877) | 0.403 (0.041) 0.261 (0.207) | 0.001 (0.998) −0.344 (0.093) | 0.418 S (0.034) −0.205 S (0.327) | −0.521 S (0.006) −0.124 S (0.555) |
| Total adiponectin | bivariate (p) partial * (p) | −0.540 S (0.004) −0.539 S (0.005) | 0.309 (0.125) 0.295 (0.152) | 0.116 (0.572) 0.083 (0.692) | 0.140 (0.496) 0.107 (0.610) | 0.170 (0.408) 0.299 (0.147) |
| HMW- adiponectin | bivariate (p) partial * (p) | −0.482 S (0.013) −0.484 S (0.014) | 0.392 S (0.048) 0.417 S (0.038) | 0.258 S (0.203) 0.296 S (0.150) | 0.140 S (0.496) 0.223 S (0.285) | 0.352 (0.078) 0.407 (0.043) |
| Proinsulin | bivariate (p) partial * (p) | 0.169 S (0.409) 0.196 S (0.347) | 0.677 S (<0.001) 0.638 S (0.001) | 0.401 S (0.042) 0.292 S (0.156) | 0.504 (0.009) 0.350 (0.086) | −0.225 (0.269) 0.030 (0.888) |
| Children with PWS n = 18 | Healthy Children n = 18 | p-Values | |
|---|---|---|---|
| Fat mass (kg) | 4.93 (4.17–6.61) | 4.39 (2.6–4.98) | 0.092 |
| Lean mass (kg) | 17.7 ± 5.6 | 21.0 ± 4.7 | 0.047 |
| Fat mass/lean mass | 0.31 (0.25–0.41) | 0.18 (0.15–0.22) | <0.001 |
| TBLH-BMC (kg) | 0.51 ± 0.15 | 0.49 ± 0.13 | 0.932 |
| TBLH-BMD Z-score | −0.71 ± 0.70 | −0.24 ± 0.52 | 0.040 |
| Correlation Coefficient | Irisin | MSTN | FGF-2 | IGF-I | IGFBP-2 | |
|---|---|---|---|---|---|---|
| Fat mass | bivariate (p) partial * (p) | −0.100 (0.693) −0.079 (0.764) | 0.029 (0.910) −0.241 (0.351) | 0.215 S (0.392) −0.230 S (0.374) | 0.560 S (0.016) 0.113 S (0.666) | −0.485 (0.042) −0.170 (0.513) |
| Lean mass | bivariate (p) partial * (p) | −0.206 (0.413) −0.373 (0.141) | 0.105 (0.678) −0.576 (0.015) | 0.454 (0.058) 0.161 (0.536) | 0.762 (<0.001) 0.076 (0.771) | −0.559 (0.016) 0.072 (0.784) |
| Fat/lean mass | bivariate (p) partial * (p) | −0.003 (0.990) 0.005 (0.985) | −0.004 (0.989) −0.054 (0.837) | −0.160 (0.527) −0.243 (0.348) | 0.021 (0.933) −0.149 (0.568) | −0.213 (0.396) −0.168 (0.519) |
| TBLH-BMC | bivariate (p) partial * (p) | −0.300 (0.226) −0.456 (0.066) | 0.071 S (0.779) −0.212 S (0.413) | 0.397 S (0.102) 0.032 S (0.902) | 0.741 (<0.001) 0.189 (0.467) | −0.627 (0.005) −0.227 (0.381) |
| TBLH-BMD Z-score | bivariate (p) partial * (p) | −0.130 (0.607) −0.121 (0.645) | 0.202 (0.421) 0.147 (0.573) | 0.230 (0.358) 0.166 (0.523) | 0.503 (0.033) 0.607 (0.010) | −0.011 (0.964) 0.145 (0.579) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, J.; Chełchowska, M.; Szamotulska, K.; Strucińska, M.; Klemarczyk, W.; Ambroszkiewicz, J. Myokine Levels in Relation to Bone Markers and Adipokines in Children with Prader–Willi Syndrome During Growth Hormone Therapy and Dietary Intervention. Int. J. Mol. Sci. 2025, 26, 10822. https://doi.org/10.3390/ijms262210822
Gajewska J, Chełchowska M, Szamotulska K, Strucińska M, Klemarczyk W, Ambroszkiewicz J. Myokine Levels in Relation to Bone Markers and Adipokines in Children with Prader–Willi Syndrome During Growth Hormone Therapy and Dietary Intervention. International Journal of Molecular Sciences. 2025; 26(22):10822. https://doi.org/10.3390/ijms262210822
Chicago/Turabian StyleGajewska, Joanna, Magdalena Chełchowska, Katarzyna Szamotulska, Małgorzata Strucińska, Witold Klemarczyk, and Jadwiga Ambroszkiewicz. 2025. "Myokine Levels in Relation to Bone Markers and Adipokines in Children with Prader–Willi Syndrome During Growth Hormone Therapy and Dietary Intervention" International Journal of Molecular Sciences 26, no. 22: 10822. https://doi.org/10.3390/ijms262210822
APA StyleGajewska, J., Chełchowska, M., Szamotulska, K., Strucińska, M., Klemarczyk, W., & Ambroszkiewicz, J. (2025). Myokine Levels in Relation to Bone Markers and Adipokines in Children with Prader–Willi Syndrome During Growth Hormone Therapy and Dietary Intervention. International Journal of Molecular Sciences, 26(22), 10822. https://doi.org/10.3390/ijms262210822









