Cytoprotective Compounds in the Primate Eye: Baseline Metabolomic Profiles of Macaca fascicularis Ocular Tissues
Abstract
1. Introduction
2. Results
2.1. Identification and Quantitation of Metabolites in 1H NMR Spectra
2.2. Identification and Quantitation of Molecular UV Filters by LC-MS and LC-OD
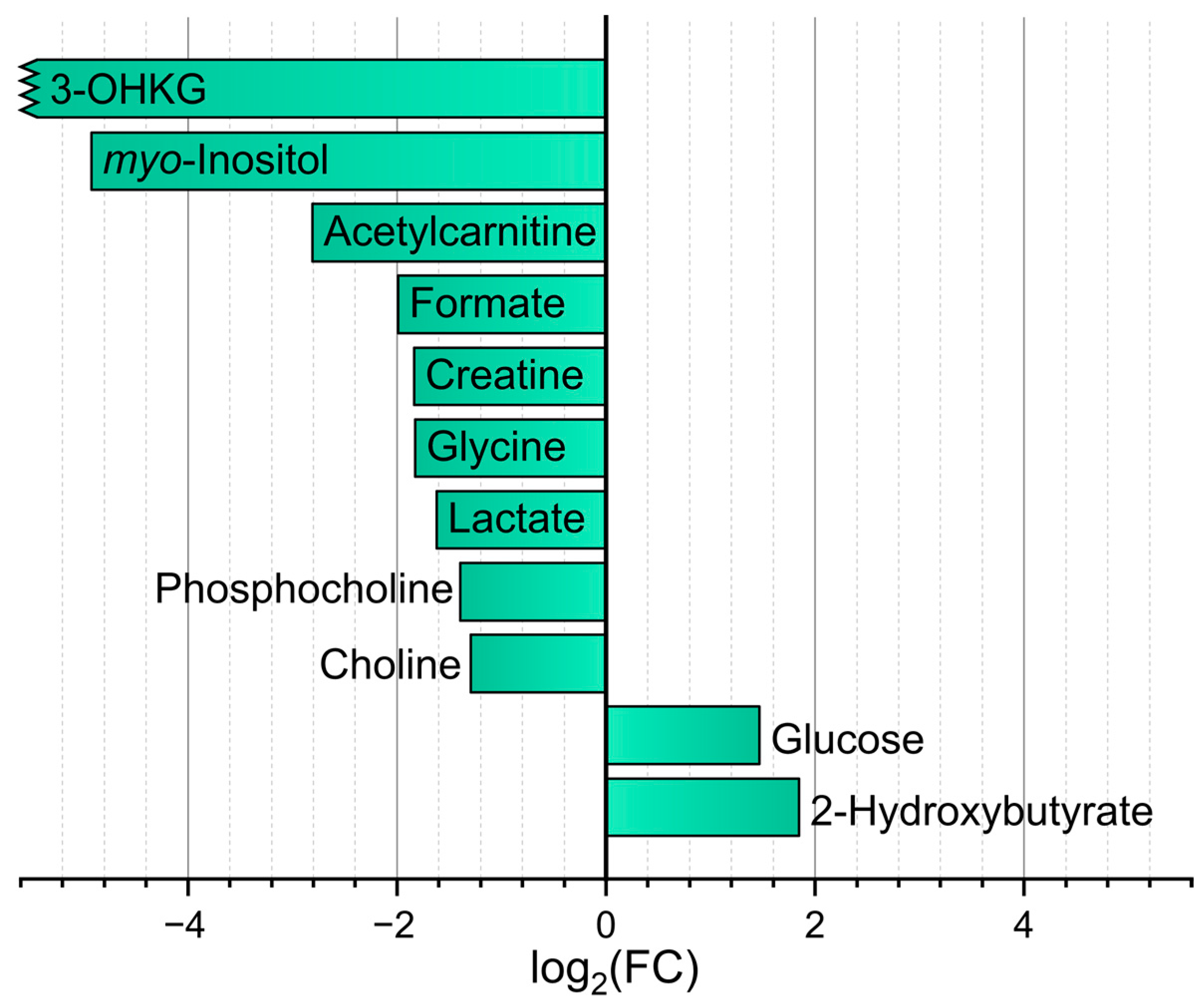
2.3. Comparison of In Vivo and Post-Mortem Blood Samples
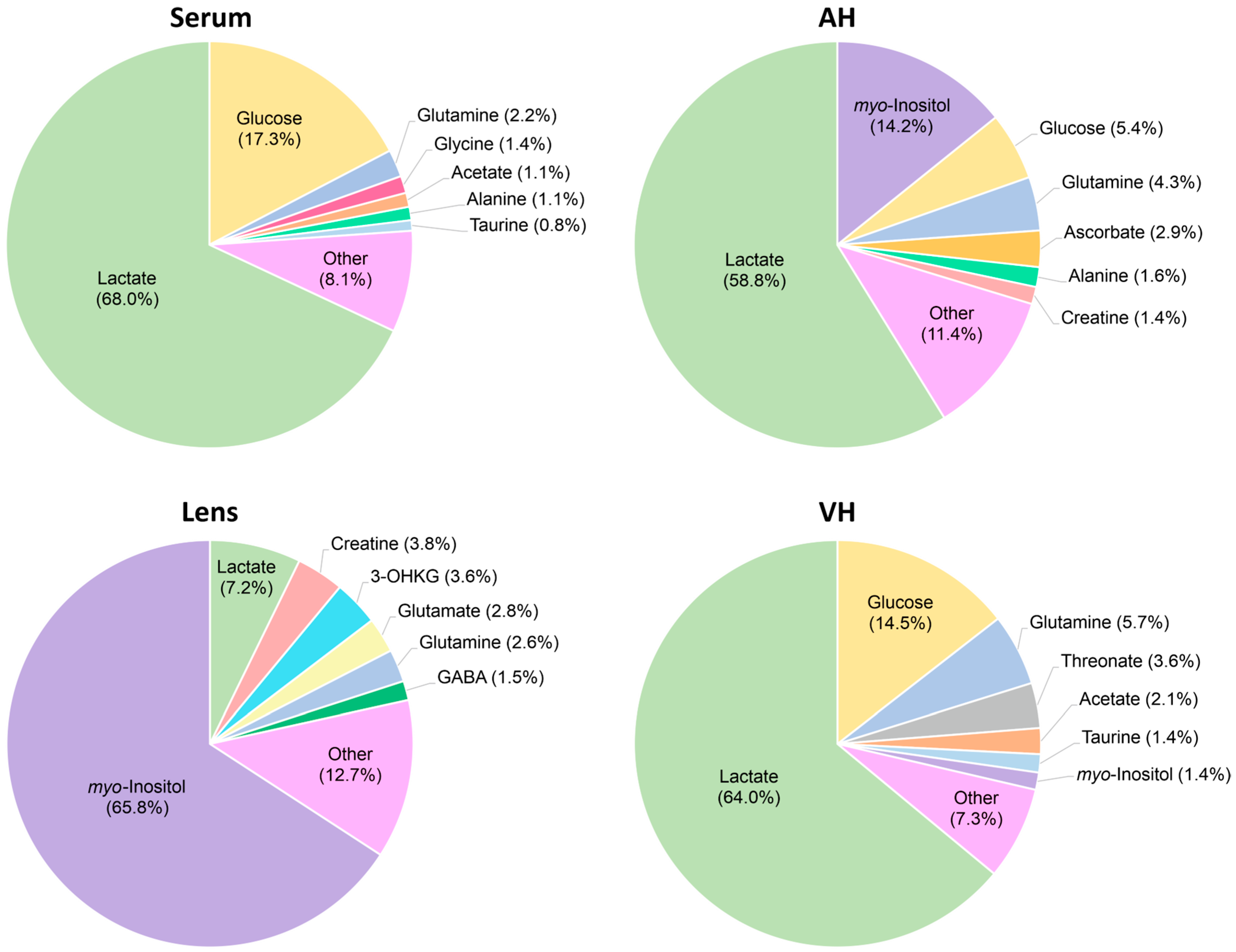
2.4. Serum Metabolomic Composition
2.5. AH Metabolomic Composition
2.6. Lens Metabolomic Composition
2.7. VH Metabolomic Composition
3. Discussion
3.1. Comparison of M. fascicularis Serum and AH Metabolomic Profiles
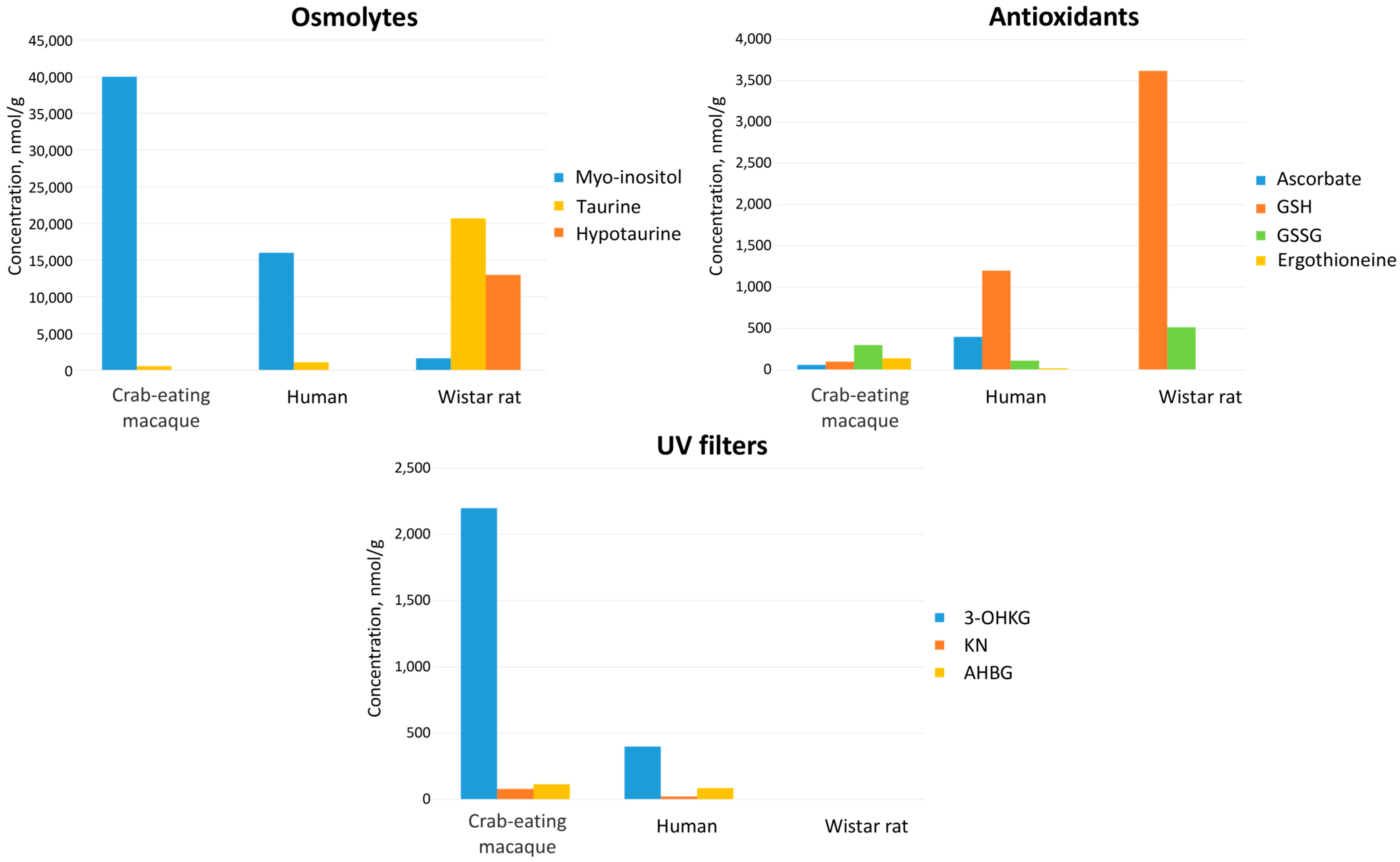
3.2. Comparison of Metabolomic Profiles of Human and M. fascicularis Tissues
3.2.1. Metabolomic Profiles of Serum
3.2.2. Metabolomic Profiles of AH
3.2.3. Metabolomic Profiles of Lens
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation
4.3. NMR Measurements
4.4. LC-MS and LC-OD Measurements
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (U)HPLC | (ultra)high performance liquid chromatography |
| 3-OHCKAG | 4-(2-amino-3-hydroxyphenyl)-4-oxocrotonic acid O-β-d-glucoside |
| 3-OHKDG | 3-hydroxykynurenine O-β-d-diglucoside |
| 3-OHKG | 3-hydroxykynurenine O-β-d-glucoside |
| 3-OHKN | 3-hydroxykynurenine |
| AH | aqueous humor |
| AHBDG | 4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid O-β-d-diglucoside |
| AHBG | 4-(2-amino-3-hydroxyphenyl)-4-oxobutanoic acid O-β-d-glucoside |
| AMP | adenosine monophosphate |
| DSS | sodium 4,4-dimethyl-4-silapentane-1-sulfonate |
| FC | fold change |
| FDR | false discovery rate |
| GABA | gamma-aminobutyric acid |
| GSH | glutathione |
| KN | kynurenine |
| LC-MS | liquid chromatography with mass spectrometric detection |
| LC-OD | liquid chromatography with optical detection |
| Me-3-OHKG | methylated 3-OHKG |
| NMR | nuclear magnetic resonance |
| PMI | post-mortem interval |
| SD | standard deviation |
| TCA | tricarboxylic acid cycle |
| UV | ultraviolet |
| VH | vitreous humor |
References
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in Metabolomics—A Review in Human Disease Diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef]
- Emwas, A.-H.M.; Salek, R.M.; Griffin, J.L.; Merzaban, J. NMR-Based Metabolomics in Human Disease Diagnosis: Applications, Limitations, and Recommendations. Metabolomics 2013, 9, 1048–1072. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Snyder, M.P. Integrative Omics for Health and Disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Suhre, K.; Arnold, M.; Bhagwat, A.M.; Cotton, R.J.; Engelke, R.; Raffler, J.; Sarwath, H.; Thareja, G.; Wahl, A.; DeLisle, R.K.; et al. Connecting Genetic Risk to Disease End Points through the Human Blood Plasma Proteome. Nat. Commun. 2017, 8, 14357, Erratum in Nat. Commun. 2017, 8, 15345. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2021, 50, D622–D631. [Google Scholar] [CrossRef]
- Kryczka, T.; Ehlers, N.; Nielsen, K.; Wylegala, E.; Dobrowolski, D.; Midelfart, A. Metabolic Profile of Keratoconic Cornea. Curr. Eye Res. 2013, 38, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Kryczka, T.; Wylęgała, E.; Dobrowolski, D.; Midelfart, A. NMR Spectroscopy of Human Eye Tissues: A New Insight into Ocular Biochemistry. Sci. World J. 2014, 2014, 546192. [Google Scholar] [CrossRef] [PubMed]
- Tsentalovich, Y.P.; Verkhovod, T.D.; Yanshole, V.V.; Kiryutin, A.S.; Yanshole, L.V.; Fursova, A.Z.; Stepakov, D.A.; Novoselov, V.P.; Sagdeev, R.Z. Metabolomic Composition of Normal Aged and Cataractous Human Lenses. Exp. Eye Res. 2015, 134, 15–23. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Yanshole, L.V.; Snytnikova, O.A.; Tsentalovich, Y.P. Quantitative Metabolomic Analysis of Changes in the Lens and Aqueous Humor under Development of Age-Related Nuclear Cataract. Metabolomics 2019, 15, 29. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Yanshole, L.V.; Iskakov, I.A.; Yanshole, V.V.; Chernykh, V.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Quantitative Metabolomic Analysis of the Human Cornea and Aqueous Humor. Metabolomics 2017, 13, 152. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Khlichkina, A.A.; Yanshole, L.V.; Yanshole, V.V.; Iskakov, I.A.; Egorova, E.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Metabolomics of the Human Aqueous Humor. Metabolomics 2017, 13, 5. [Google Scholar] [CrossRef]
- Pietrowska, K.; Dmuchowska, D.A.; Krasnicki, P.; Bujalska, A.; Samczuk, P.; Parfieniuk, E.; Kowalczyk, T.; Wojnar, M.; Mariak, Z.; Kretowski, A.; et al. An Exploratory LC-MS-Based Metabolomics Study Reveals Differences in Aqueous Humor Composition between Diabetic and Non-Diabetic Patients with Cataract. Electrophoresis 2018, 39, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.E.; Lamont, I.L. Biochemistry Changes That Occur after Death: Potential Markers for Determining Post-Mortem Interval. PLoS ONE 2013, 8, e82011. [Google Scholar] [CrossRef]
- Palmiere, C.; Mangin, P. Urea Nitrogen, Creatinine, and Uric Acid Levels in Postmortem Serum, Vitreous Humor, and Pericardial Fluid. Int. J. Leg. Med. 2015, 129, 301–305. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Snytnikova, O.A.; Yanshole, V.V.; Tsentalovich, Y.P.; Sagdeev, R.Z. Post-Mortem Changes in the Metabolomic Compositions of Rabbit Blood, Aqueous and Vitreous Humors. Metabolomics 2016, 12, 172. [Google Scholar] [CrossRef]
- Zelentsova, E.A.; Yanshole, L.V.; Melnikov, A.D.; Kudryavtsev, I.S.; Novoselov, V.P.; Tsentalovich, Y.P. Post-Mortem Changes in Metabolomic Profiles of Human Serum, Aqueous Humor and Vitreous Humor. Metabolomics 2020, 16, 80. [Google Scholar] [CrossRef]
- Locci, E.; Stocchero, M.; Noto, A.; Chighine, A.; Natali, L.; Napoli, P.E.; Caria, R.; De-Giorgio, F.; Nioi, M.; d’Aloja, E. A 1H NMR Metabolomic Approach for the Estimation of the Time since Death Using Aqueous Humour: An Animal Model. Metabolomics 2019, 15, 76. [Google Scholar] [CrossRef]
- Zigler, J.S.; Bodaness, R.S.; Gery, I.; Kinoshita, J.H. Effects of Lipid Peroxidation Products on the Rat Lens in Organ Culture: A Possible Mechanism of Cataract Initiation in Retinal Degenerative Disease. Arch. Biochem. Biophys. 1983, 225, 149–156. [Google Scholar] [CrossRef]
- Son, H.-Y.; Kim, H.; Kwon, Y.H. Taurine Prevents Oxidative Damage of High Glucose-Induced Cataractogenesis in Isolated Rat Lenses. J. Nutr. Sci. Vitaminol. 2007, 53, 324–330. [Google Scholar] [CrossRef]
- Yanshole, V.V.; Snytnikova, O.A.; Kiryutin, A.S.; Yanshole, L.V.; Sagdeev, R.Z.; Tsentalovich, Y.P. Metabolomics of the Rat Lens: A Combined LC-MS and NMR Study. Exp. Eye Res. 2014, 125, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Z.; Mullard, G.; Hollywood, K.A.; Dunn, W.B.; Bishop, P.N. Characterisation of the Metabolome of Ocular Tissues and Post-Mortem Changes in the Rat Retina. Exp. Eye Res. 2016, 149, 8–15. [Google Scholar] [CrossRef]
- Mustari, M.J. Nonhuman Primate Studies to Advance Vision Science and Prevent Blindness. ILAR J. 2017, 58, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.A.; Kaufman, P.L. Primate Glaucoma Models. J. Glaucoma 2005, 14, 311. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, C.L.; Fleuriet, J.; Walton, M.M.; Mustari, M.J.; McLoon, L.K. Adaptation of Slow Myofibers: The Effect of Sustained BDNF Treatment of Extraocular Muscles in Infant Nonhuman Primates. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3467–3483. [Google Scholar] [CrossRef]
- Lambert, W.S.; Carlson, B.J.; Ghose, P.; Vest, V.D.; Yao, V.; Calkins, D.J. Towards A Microbead Occlusion Model of Glaucoma for a Non-Human Primate. Sci. Rep. 2019, 9, 11572. [Google Scholar] [CrossRef]
- Liu, Z.; Liow, S.S.; Lai, S.L.; Alli-Shaik, A.; Holder, G.E.; Parikh, B.H.; Krishnakumar, S.; Li, Z.; Tan, M.J.; Gunaratne, J.; et al. Retinal-Detachment Repair and Vitreous-like-Body Reformation via a Thermogelling Polymer Endotamponade. Nat. Biomed. Eng. 2019, 3, 598–610. [Google Scholar] [CrossRef]
- Gaillard, E.R.; Zheng, L.; Merriam, J.C.; Dillon, J. Age-Related Changes in the Absorption Characteristics of the Primate Lens. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1454–1459. [Google Scholar]
- Gaillard, E.R.; Merriam, J.; Zheng, L.; Dillon, J. Transmission of Light to the Young Primate Retina: Possible Implications for the Formation of Lipofuscin. Photochem. Photobiol. 2011, 87, 18–21. [Google Scholar] [CrossRef]
- Barba, I.; Garcia-Ramírez, M.; Hernández, C.; Alonso, M.A.; Masmiquel, L.; García-Dorado, D.; Simó, R. Metabolic Fingerprints of Proliferative Diabetic Retinopathy: An 1H-NMR–Based Metabonomic Approach Using Vitreous Humor. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4416–4421. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. Quantitating Metabolites in Protein Precipitated Serum Using NMR Spectroscopy. Anal. Chem. 2014, 86, 5433–5440. [Google Scholar] [CrossRef] [PubMed]
- Gowda, N.G.A.; Gowda, Y.N.; Raftery, D. Expanding the Limits of Human Blood Metabolite Quantitation Using NMR Spectroscopy. Anal. Chem. 2015, 87, 706–715. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Khlichkina, A.A.; Sagdeev, R.Z.; Tsentalovich, Y.P. Evaluation of Sample Preparation Protocols for Quantitative NMR-Based Metabolomics. Metabolomics 2019, 15, 84. [Google Scholar] [CrossRef]
- Bova, L.M.; Sweeney, M.H.; Jamie, J.F.; Truscott, R.J. Major Changes in Human Ocular UV Protection with Age. Investig. Ophthalmol. Vis. Sci. 2001, 42, 200–205. [Google Scholar]
- Tsentalovich, Y.P.; Snytnikova, O.A.; Forbes, M.D.E.; Chernyak, E.I.; Morozov, S.V. Photochemical and Thermal Reactivity of Kynurenine. Exp. Eye Res. 2006, 83, 1439–1445. [Google Scholar] [CrossRef]
- Taylor, L.M.; Andrew Aquilina, J.; Jamie, J.F.; Truscott, R.J.W. UV Filter Instability: Consequences for the Human Lens. Exp. Eye Res. 2002, 75, 165–175. [Google Scholar] [CrossRef]
- Sherin, P.S.; Grilj, J.; Tsentalovich, Y.P.; Vauthey, E. Ultrafast Excited-State Dynamics of Kynurenine, a UV Filter of the Human Eye. J. Phys. Chem. B 2009, 113, 4953–4962. [Google Scholar] [CrossRef]
- Tsentalovich, Y.P.; Sherin, P.S.; Kopylova, L.V.; Cherepanov, I.V.; Grilj, J.; Vauthey, E. Photochemical Properties of UV Filter Molecules of the Human Eye. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7687–7696. [Google Scholar] [CrossRef] [PubMed]
- Tsentalovich, Y.P.; Snytnikova, O.A.; Sherin, P.S.; Forbes, M.D.E. Photochemistry of Kynurenine, a Tryptophan Metabolite: Properties of the Triplet State. J. Phys. Chem. A 2005, 109, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.E.; Lamont, I.L. Estimation of Post-Mortem Interval Using Biochemical Markers. Aust. J. Forensic Sci. 2014, 46, 8–26. [Google Scholar] [CrossRef]
- Donaldson, A.E.; Lamont, I.L. Metabolomics of Post-Mortem Blood: Identifying Potential Markers of Post-Mortem Interval. Metabolomics 2015, 11, 237–245. [Google Scholar] [CrossRef]
- Cotran, R.S.; Kumar, V.; Robbins, S.L. Cellular Injury and Cellular Death, 5th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1994. [Google Scholar]
- Marcantonio, J.; Duncan, G. Amino Acid Transport and Protein Synthesis in Human Normal and Cataractous Lenses. Curr. Eye Res. 1987, 6, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Lorentzen, K.A.; Kistler, J.; Donaldson, P.J. Molecular Identification and Characterisation of the Glycine Transporter (GLYT1) and the Glutamine/Glutamate Transporter (ASCT2) in the Rat Lens. Exp. Eye Res. 2006, 83, 447–455. [Google Scholar] [CrossRef]
- Knöpfel, E.B.; Vilches, C.; Camargo, S.M.R.; Errasti-Murugarren, E.; Stäubli, A.; Mayayo, C.; Munier, F.L.; Miroshnikova, N.; Poncet, N.; Junza, A.; et al. Dysfunctional LAT2 Amino Acid Transporter Is Associated With Cataract in Mouse and Humans. Front. Physiol. 2019, 10, 688. [Google Scholar] [CrossRef]
- Elmi, A.; Ventrella, D.; Laghi, L.; Carnevali, G.; Zhu, C.; Pertile, G.; Barone, F.; Benfenati, F.; Bacci, M.L. 1H NMR Spectroscopy Characterization of Porcine Vitreous Humor in Physiological and Photoreceptor Degeneration Conditions. Investig. Ophthalmol. Vis. Sci. 2019, 60, 741–747. [Google Scholar] [CrossRef]
- Hung, P.; Froenicke, L.; Lin, C.Y.; Lyons, L.A.; Miller, M.G.; Pinkerton, K.E.; VandeVoort, C.A. Effects of Environmental Tobacco Smoke in Vivo on Rhesus Monkey Semen Quality, Sperm Function, and Sperm Metabolism. Reprod. Toxicol. 2009, 27, 140–148. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hung, P.; VandeVoort, C.A.; Miller, M.G. 1H NMR to Investigate Metabolism and Energy Supply in Rhesus Macaque Sperm. Reprod. Toxicol. 2009, 28, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rezzi, S.; Martin, F.-P.J.; Shanmuganayagam, D.; Colman, R.J.; Nicholson, J.K.; Weindruch, R. Metabolic Shifts Due to Long-Term Caloric Restriction Revealed in Nonhuman Primates. Exp. Gerontol. 2009, 44, 356–362. [Google Scholar] [CrossRef]
- Rao, R.; Ennis, K.; Oz, G.; Lubach, G.R.; Georgieff, M.K.; Coe, C.L. Metabolomic Analysis of Cerebrospinal Fluid Indicates Iron Deficiency Compromises Cerebral Energy Metabolism in the Infant Monkey. Neurochem. Res. 2013, 38, 573–580. [Google Scholar] [CrossRef]
- O’Sullivan, A.; He, X.; McNiven, E.M.S.; Hinde, K.; Haggarty, N.W.; Lönnerdal, B.; Slupsky, C.M. Metabolomic Phenotyping Validates the Infant Rhesus Monkey as a Model of Human Infant Metabolism. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tan, W.; Li, X.; Li, B.; Gong, B.; Pyle, W.G.; Wu, J.; Li, L.; Luo, T.; Zou, Y.; et al. Aged Monkeys Fed a High-Fat/High-Sugar Diet Recapitulate Metabolic Disorders and Cardiac Contractile Dysfunction. J. Cardiovasc. Trans. Res. 2021, 14, 799–815. [Google Scholar] [CrossRef]
- Crook, A.; De Lima Leite, A.; Payne, T.; Bhinderwala, F.; Woods, J.; Singh, V.K.; Powers, R. Radiation Exposure Induces Cross-Species Temporal Metabolic Changes That Are Mitigated in Mice by Amifostine. Sci. Rep. 2021, 11, 14004. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Zhang, Z.; Taha, A.Y.; Capitanio, J.P.; Bauman, M.D.; Golub, M.S.; Van de Water, J.; VandeVoort, C.A.; Walker, C.K.; Slupsky, C.M. Impact of Maternal Obesity on the Gestational Metabolome and Infant Metabolome, Brain, and Behavioral Development in Rhesus Macaques. Metabolites 2022, 12, 764. [Google Scholar] [CrossRef]
- Civan, M.M.; Macknight, A.D.C. The Ins and Outs of Aqueous Humour Secretion. Exp. Eye Res. 2004, 78, 625–631. [Google Scholar] [CrossRef]
- DiMattio, J. A Comparative Study of Ascorbic Acid Entry into Aqueous and Vitreous Humors of the Rat and Guinea Pig. Investig. Ophthalmol. Vis. Sci. 1989, 30, 2320–2331. [Google Scholar]
- Delamere, N.A. Ascorbic Acid and the Eye. In Subcellular Biochemistry: Ascorbic Acid: Biochemistry and Biomedical Cell Biology; Harris, J.R., Ed.; Subcellular Biochemistry; Springer: Boston, MA, USA, 1996; pp. 313–329. ISBN 978-1-4613-0325-1. [Google Scholar]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the Ergothioneine Transporter. Proc. Natl. Acad. Sci. USA 2005, 102, 5256–5261. [Google Scholar] [CrossRef]
- Tang, R.M.Y.; Cheah, I.K.-M.; Yew, T.S.K.; Halliwell, B. Distribution and Accumulation of Dietary Ergothioneine and Its Metabolites in Mouse Tissues. Sci. Rep. 2018, 8, 1601. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A Diet-Derived Antioxidant with Therapeutic Potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Tang, R.M.Y.; Cheah, I.K. Diet-Derived Antioxidants: The Special Case of Ergothioneine. Annu. Rev. Food Sci. Technol. 2023, 14, 323–345. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, M.V.; Yanshole, L.V.; Tsentalovich, Y.P. Stability of Metabolomic Content during Sample Preparation: Blood and Brain Tissues. Metabolites 2022, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Berthoud, V.M.; Gao, J.; Minogue, P.J.; Jara, O.; Mathias, R.T.; Beyer, E.C. Connexin Mutants Compromise the Lens Circulation and Cause Cataracts through Biomineralization. Int. J. Mol. Sci. 2020, 21, 5822. [Google Scholar] [CrossRef]
- Wada, E.; Tsumita, T. Ageing and Compositional Changes of Rat Lens. Mech. Ageing Dev. 1984, 27, 287–294. [Google Scholar] [CrossRef]
- Heys, K.R.; Friedrich, M.G.; Truscott, R.J.W. Free and Bound Water in Normal and Cataractous Human Lenses. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1991. [Google Scholar] [CrossRef] [PubMed]
- Serebryany, E.; Chowdhury, S.; Woods, C.N.; Thorn, D.C.; Watson, N.E.; McClelland, A.A.; Klevit, R.E.; Shakhnovich, E.I. A Native Chemical Chaperone in the Human Eye Lens. eLife 2022, 11, e76923. [Google Scholar] [CrossRef]
- Kopylova, L.V.; Snytnikova, O.A.; Chernyak, E.I.; Morozov, S.V.; Tsentalovich, Y.P. UV Filter Decomposition. A Study of Reactions of 4-(2-Aminophenyl)-4-Oxocrotonic Acid with Amino Acids and Antioxidants Present in the Human Lens. Exp. Eye Res. 2007, 85, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Bova, L.M.; Wood, A.M.; Jamie, J.F.; Truscott, R.J. UV Filter Compounds in Human Lenses: The Origin of 4-(2-Amino-3-Hydroxyphenyl)-4-Oxobutanoic Acid O-Beta-D-Glucoside. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3237–3244. [Google Scholar]
- Kopylova, L.V.; Snytnikova, O.A.; Chernyak, E.I.; Morozov, S.V.; Forbes, M.D.E.; Tsentalovich, Y.P. Kinetics and Mechanism of Thermal Decomposition of Kynurenines and Biomolecular Conjugates: Ramifications for the Modification of Mammalian Eye Lens Proteins. Org. Biomol. Chem. 2009, 7, 2958–2966. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the Gap between Raw Spectra and Functional Insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]


| Metabolite | Serum In Vivo, µM | Serum Post-Mortem, µM | AH Post-Mortem, µM | Lens Post-Mortem, nmol/g | VH Post-Mortem, µM |
|---|---|---|---|---|---|
| Osmolytes | |||||
| Taurine | 109 ± 21 | 246 ± 25 | 108 ± 24 | 570 ± 160 | 190 ± 190 |
| myo-Inositol | 92 ± 11 | 110 ± 40 | 2900 ± 1100 | 40,000 ± 5000 | 180 ± 40 |
| Antioxidants | |||||
| Ascorbate | ND 1 | ND | 590 ± 150 | 63 ± 25 | 83 ± 9 |
| Ergothioneine | 0.7 ± 1.0 | 3.0 ± 2.9 | 46 ± 21 | 140 ± 60 | ND |
| GSH | ND | ND | ND | 83 ± 22 | ND |
| GSSG | ND | ND | ND | 320 ± 70 | ND |
| Molecular UV filters | |||||
| 3-OHKG | ND | ND | 160 ± 60 | 2200 ± 700 | ND |
| 3-OHCKAG 2 | ND | ND | ND | 1.8 ± 0.9 | ND |
| 3-OHKDG 2 | ND | ND | ND | 0.7 ± 0.4 | ND |
| 3-OHKN 2 | ND | ND | ND | 4.9 ± 1.1 | ND |
| AHBDG 2 | ND | ND | ND | 5.8± 2.8 | ND |
| AHBG 2 | ND | ND | ND | 109 ± 18 | ND |
| KN | ND | ND | ND | 80 ± 50 | ND |
| Me-3-OHKG 2 | ND | ND | ND | 9.4 ± 1.1 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fomenko, M.V.; Yanshole, L.V.; Yanshole, V.V.; Radomskaya, E.Y.; Bulgin, D.V.; Sagdeev, R.Z.; Tsentalovich, Y.P. Cytoprotective Compounds in the Primate Eye: Baseline Metabolomic Profiles of Macaca fascicularis Ocular Tissues. Int. J. Mol. Sci. 2025, 26, 10816. https://doi.org/10.3390/ijms262210816
Fomenko MV, Yanshole LV, Yanshole VV, Radomskaya EY, Bulgin DV, Sagdeev RZ, Tsentalovich YP. Cytoprotective Compounds in the Primate Eye: Baseline Metabolomic Profiles of Macaca fascicularis Ocular Tissues. International Journal of Molecular Sciences. 2025; 26(22):10816. https://doi.org/10.3390/ijms262210816
Chicago/Turabian StyleFomenko, Maxim V., Lyudmila V. Yanshole, Vadim V. Yanshole, Elena Y. Radomskaya, Dmitry V. Bulgin, Renad Z. Sagdeev, and Yuri P. Tsentalovich. 2025. "Cytoprotective Compounds in the Primate Eye: Baseline Metabolomic Profiles of Macaca fascicularis Ocular Tissues" International Journal of Molecular Sciences 26, no. 22: 10816. https://doi.org/10.3390/ijms262210816
APA StyleFomenko, M. V., Yanshole, L. V., Yanshole, V. V., Radomskaya, E. Y., Bulgin, D. V., Sagdeev, R. Z., & Tsentalovich, Y. P. (2025). Cytoprotective Compounds in the Primate Eye: Baseline Metabolomic Profiles of Macaca fascicularis Ocular Tissues. International Journal of Molecular Sciences, 26(22), 10816. https://doi.org/10.3390/ijms262210816







