The Therapeutic Potential of Galium verum for Psoriasis: A Combined Phytochemical, In Silico, and Experimental Approach
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content and HPLC Characterization of G. verum Extract
2.2. Anti-Oxidant Activity
2.3. In Silico Simulations
2.3.1. Blind Molecular Docking Studies
2.3.2. Focused Molecular Docking Studies
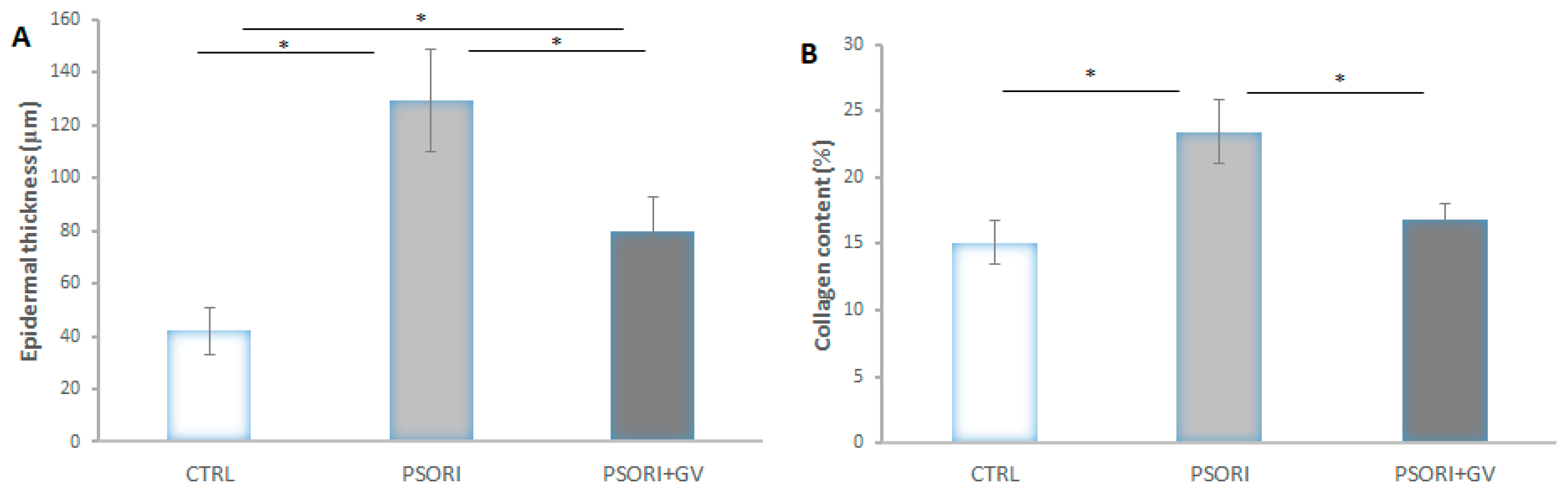
2.4. Effects of the Treatment of G. verum Extract on the Skin of Psoriatic Rats
2.5. Morphometric Analysis
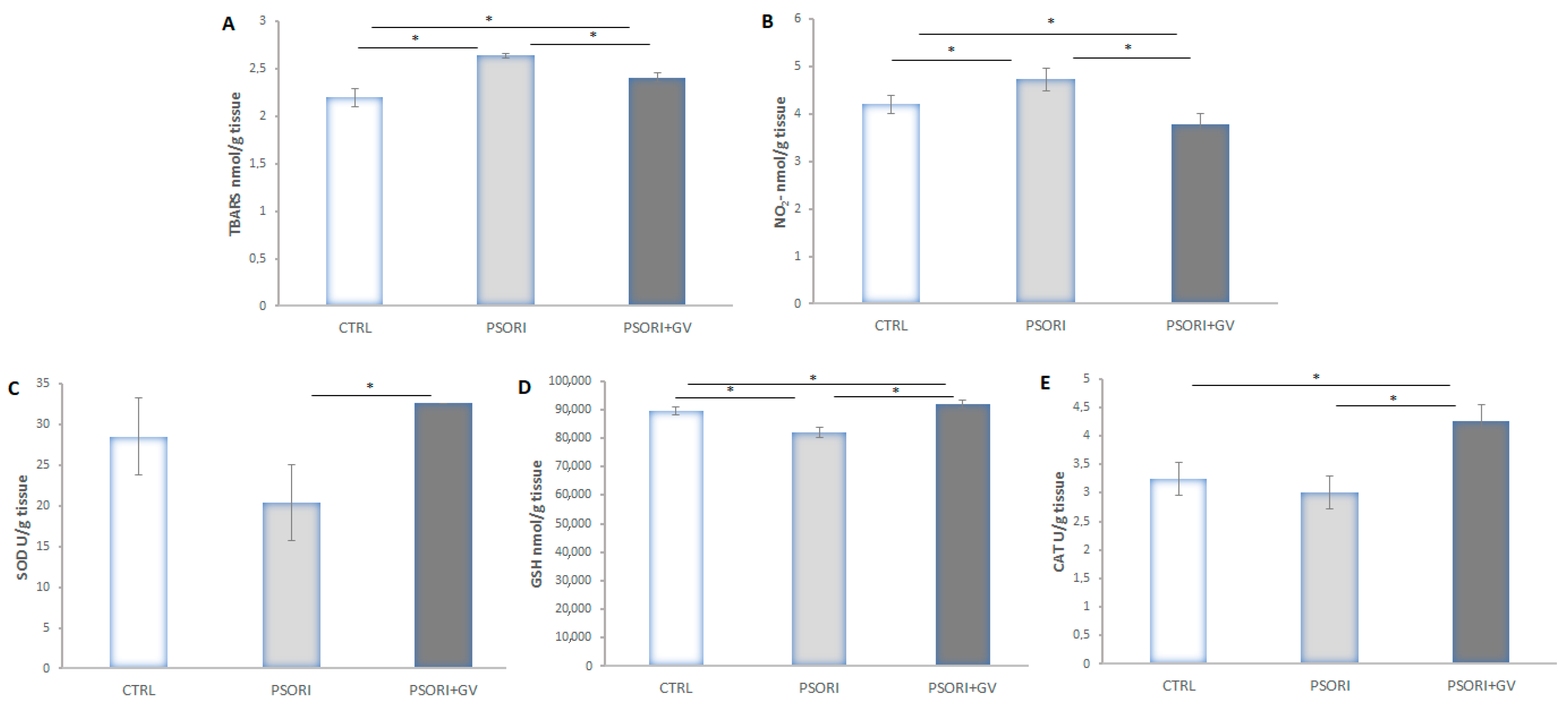
2.6. Tissue Redox State Analysis
3. Discussion
4. Materials and Methods
4.1. Extract Preparation and Characterization
4.1.1. HPLC Analysis of G. verum Extract
4.1.2. Total Phenolic Content
4.1.3. Antioxidative Activity
DPPH Radical Scavenging Assay
ABTS Radical Cation Decolorization Assay
FRAP Assay
4.2. Molecular Docking Protocol
4.3. In Vivo Experiment
4.3.1. Induction of Psoriasis and Treatment
4.3.2. Tissue Redox State
Lipid Peroxidation Index (TBARS)
Nitrite (NO2−) Determination
CAT Activity
SOD Activity
GSH Level
4.3.3. Histology and Morphometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of psoriasis: A review. J. Dermatol. 2021, 48, 722–731. [Google Scholar] [CrossRef]
- Langley, R.G.; Krueger, G.G.; Griffiths, C.E. Psoriasis: Epidemiology, clinical features, and quality of life. Ann. Rheum. Dis. 2005, 64 (Suppl. 2), ii18–ii23; discussion ii24–ii25. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, Y.; Zhang, J.; Zhang, H.; Ma, C.; Tang, X.; Wu, J.; Li, L.; Wei, J.; Chen, H.; et al. Anti-Angiogenic Efficacy of PSORI-CM02 and the Associated Mechanism in Psoriasis In Vitro and In Vivo. Front. Immunol. 2021, 12, 649591. [Google Scholar] [CrossRef]
- Corbic, M.; Jakovljevic, V.; Nikolic, M.; Jeremic, N.; Bradic, J.; Novakovic, J.; Kocovic, A.; Savic, M.; Tadic, V.; Zugic, A.; et al. Galium verum L. extract mitigates cardiovascular events in psoriasis rats. Mol. Cell. Biochem. 2025, 480, 3783–3798. [Google Scholar] [CrossRef]
- Dobrică, E.C.; Cozma, M.A.; Găman, M.A.; Voiculescu, V.M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef]
- Shakoei, S.; Nakhjavani, M.; Mirmiranpoor, H.; Motlagh, M.A.; Azizpour, A.; Abedini, R. The Serum Level of Oxidative Stress and Antioxidant Markers in Patients with Psoriasis: A Cross-sectional Study. J. Clin. Aesthet. Dermatol. 2021, 14, 38–41. [Google Scholar] [PubMed]
- Bilski, R.; Kupczyk, D.; Woźniak, A. Oxidative Imbalance in Psoriasis with an Emphasis on Psoriatic Arthritis: Therapeutic Antioxidant Targets. Molecules 2024, 29, 5460. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Bakshi, H.; Nagpal, M.; Singh, M.; Dhingra, G.A.; Aggarwal, G. Treatment of Psoriasis: A Comprehensive Review of Entire Therapies. Curr. Drug Saf. 2020, 15, 82–104. [Google Scholar] [CrossRef]
- Nowak-Perlak, M.; Szpadel, K.; Jabłońska, I.; Pizon, M.; Woźniak, M. Promising Strategies in Plant-Derived Treatments of Psoriasis-Update of In Vitro, In Vivo, and Clinical Trials Studies. Molecules 2022, 27, 591. [Google Scholar] [CrossRef]
- Talbott, W.; Duffy, N. Complementary and alternative medicine for psoriasis: What the dermatologist needs to know. Am. J. Clin. Dermatol. 2015, 16, 147–165. [Google Scholar] [CrossRef]
- Yargholi, A.; Shirbeigi, L.; Rahimi, R.; Mansouri, P.; Ayati, M.H. The effect of Melissa officinalis syrup on patients with mild to moderate psoriasis: A randomized, double-blind placebo-controlled clinical trial. BMC Res. Notes 2021, 14, 253. [Google Scholar] [CrossRef]
- Dabholkar, N.; Rapalli, V.K.; Singhvi, G. Potential herbal constituents for psoriasis treatment as protective and effective therapy. Phytother. Res. 2021, 35, 2429–2444. [Google Scholar] [CrossRef]
- Halim, S.A.; Khan, A.; Csuk, R.; Al-Rawahi, A.; Al-Harrasi, A. Diterpenoids and Triterpenoids From Frankincense Are Excellent Anti-psoriatic Agents: An in silico Approach. Front. Chem. 2020, 8, 486. [Google Scholar] [CrossRef]
- Alanzi, A.R.; Alsalhi, M.S.; Mothana, R.A.; Alqahtani, J.H.; Alqahtani, M.J. Insilico discovery of novel Phosphodiesterase 4 (PDE4) inhibitors for the treatment of psoriasis: Insights from computer aided drug design approaches. PLoS ONE 2024, 19, e0305934. [Google Scholar] [CrossRef]
- Ibezim, A.; Onah, E.; Dim, E.N.; Ntie-Kang, F. A computational multi-targeting approach for drug repositioning for psoriasis treatment. BMC Complement. Med. Ther. 2021, 21, 193. [Google Scholar] [CrossRef]
- Bradic, J.; Jeremic, N.; Petkovic, A.; Jeremic, J.; Zivkovic, V.; Srejovic, I.; Sretenovic, J.; Matic, S.; Jakovljevic, V.; Tomovic, M. Cardioprotective effects of Galium verum L. extract against myocardial ischemia-reperfusion injury. Arch. Physiol. Biochem. 2020, 126, 408–415. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Galium verum–A review. Indo Am. J. Pharm. Sci. 2018, 5, 2142–2149. [Google Scholar]
- Vuletic, M.; Jakovljevic, V.; Zivanovic, S.; Papic, M.; Papic, M.; Mladenovic, R.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Andjic, M.; et al. The Evaluation of Healing Properties of Galium verum-Based Oral Gel in Aphthous Stomatitis in Rats. Molecules 2022, 27, 4680. [Google Scholar] [CrossRef]
- Bradic, J.; Andjic, M.; Novakovic, J.; Kocovic, A.; Tomovic, M.; Petrovic, A.; Nikolic, M.; Mitrovic, S.; Jakovljevic, V.; Pecarski, D. Lady’s Bedstraw as a Powerful Antioxidant for Attenuation of Doxorubicin-Induced Cardiotoxicity. Antioxidants 2023, 12, 1277. [Google Scholar] [CrossRef]
- Farcas, A.D.; Mot, A.C.; Zagrean-Tuza, C.; Toma, V.; Cimpoiu, C.; Hosu, A.; Parvu, M.; Roman, I.; Silaghi-Dumitrescu, R. Chemo-mapping and biochemical-modulatory and antioxidant/prooxidant effect of Galium verum extract during acute restraint and dark stress in female rats. PLoS ONE 2018, 13, e0200022. [Google Scholar] [CrossRef] [PubMed]
- Laanet, P.-R.; Saar-Reismaa, P.; Jõul, P.; Bragina, O.; Vaher, M. Phytochemical Screening and Antioxidant Activity of Selected Estonian Galium Species. Molecules 2023, 28, 2867. [Google Scholar] [CrossRef] [PubMed]
- Lakic, N.S.; Mimica-Dukic, N.M.; Isak, J.M.; Bozin, B.N. Antioxidant properties of Galium verum L. (Rubiaceae) extracts. Centr. Eur. J. Biol. 2010, 5, 331–337. [Google Scholar]
- Turcov, D.; Barna, A.S.; Trifan, A.; Blaga, A.C.; Tanasă, A.M.; Suteu, D. Antioxidants from Galium verum as Ingredients for the Design of New Dermatocosmetic Products. Plants 2022, 11, 2454. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Chen, T.; Shao, M.; Yang, X.; Qi, Y.; Kong, S.; Jiang, L.; Yang, E. Unveiling the molecular mechanisms of Haitang-Xiaoyin Mixture in psoriasis treatment based on bioinformatics, network pharmacology, machine learning, and molecular docking verification. Comput. Biol. Chem. 2025, 115, 108352. [Google Scholar] [CrossRef]
- Sundarrajan, S.; Nandakumar, M.P.; Prabhu, D.; Jeyaraman, J.; Arumugam, M. Conformational insights into the inhibitory mechanism of phyto-compounds against Src kinase family members implicated in psoriasis. J. Biomol. Struct. Dyn. 2020, 38, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Dhanabal, S.P.; Muruganantham, N.; Basavaraj, K.H.; Wadhwani, A.; Shamasundar, N.M. Antipsoriatic activity of extracts and fractions obtained from Memecylon malabaricum leaves. J. Pharm. Pharmacol. 2012, 64, 1501–1509. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Y.; Zhai, H.; Wu, X.; Liu, A. Rutin alleviates psoriasis-related inflammation in keratinocytes by regulating the JAK2/STAT3 signaling. Skin. Res. Technol. 2024, 30, e70011. [Google Scholar] [CrossRef]
- Forsyth, T.; Kearney, P.C.; Kim, B.G.; Johnson, H.W.; Aay, N.; Arcalas, A.; Brown, D.S.; Chan, V.; Chen, J.; Du, H.; et al. SAR and in vivo evaluation of 4-aryl-2-aminoalkylpyrimidines as potent and selective Janus kinase 2 (JAK2) inhibitors. Bioorg. Med. Chem. Lett. 2012, 22, 7653–7658. [Google Scholar] [CrossRef]
- Argiriadi, M.A.; Ericsson, A.M.; Harris, C.M.; Banach, D.L.; Borhani, D.W.; Calderwood, D.J.; Demers, M.D.; Dimauro, J.; Dixon, R.W.; Hardman, J.; et al. 2,4-Diaminopyrimidine MK2 inhibitors. Part I: Observation of an unexpected inhibitor binding mode. Bioorg. Med. Chem. Lett. 2010, 20, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, H.; Xu, R.; Zhao, Y.; Chinnaswamy, K.; McEachern, D.; Chen, J.; Yang, C.Y.; Liu, Z.; Wang, M.; et al. A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo. Cancer Cell 2019, 36, 498–511.e17. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Liu, A.; Zhao, W.; Zhang, B.; Tu, Y.; Wang, Q.; Li, J. Cimifugin ameliorates imiquimod-induced psoriasis by inhibiting oxidative stress and inflammation via NF-κB/MAPK pathway. Biosci. Rep. 2020, 40, BSR20200471. [Google Scholar] [CrossRef]
- Lin, J.; Fang, Y.; Cao, Y.; Ma, L.; Tao, M.; Wang, X.; Li, Y.; Qing, L. Zerumbone attenuates the excessive proliferation of keratinocytes in psoriasis mice through regulating NLRP3/NF-κB pathway. Toxicol. Res. 2023, 12, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated suppression of NF-κB and MAPKs signaling pathways. Bioengineered 2021, 12, 183–195. [Google Scholar] [CrossRef]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef] [PubMed]
- Blagov, A.; Sukhorukov, V.; Guo, S.; Zhang, D.; Eremin, I.; Orekhov, A. The Role of Oxidative Stress in the Induction and Development of Psoriasis. Front. Biosci. (Landmark Ed.) 2023, 28, 118. [Google Scholar] [CrossRef]
- Bijelić, K.; Srdjenović Čonić, B.; Prpa, B.; Pilija, V.; Vukmirović, S.; Kladar, N. The Potential of Hemp Extracts to Modify the Course of Oxidative-Stress Related Conditions. Plants 2024, 13, 1630. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Acharya, K. Simplified methods for microtiter based analysis of in vitro antioxidant activity. Asian J. Pharm. 2017, 11, 327–335. [Google Scholar] [CrossRef]
- Ramírez-García, O.; Salinas-Moreno, Y.; Santillán-Fernández, A.; Sumaya-Martínez, M.T. Screening antioxidant capacity of Mexican maize (Zea mays L.) landraces with colored grain using ABTS, DPPH and FRAP methods. Cereal Res. Commun. 2022, 50, 1075–1083. [Google Scholar] [CrossRef]
- Johnson, J.B.; Mani, J.S.; Naiker, M. Microplate methods for measuring phenolic content and antioxidant capacity in chickpea: Impact of shaking. Eng. Proc. 2023, 48, 57. [Google Scholar] [CrossRef]
- Bolanos de la Torre, A.A.; Henderson, T.; Nigam, P.S.; Owusu-Apenten, R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to Manuka honey. Food Chem. 2015, 174, 119–123. [Google Scholar] [CrossRef]
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. Available online: https://www.pymol.org/pymol (accessed on 20 June 2025).
- Zhan, Y.P.; Chen, B.S. Drug Target Identification and Drug Repurposing in Psoriasis through Systems Biology Approach, DNN-Based DTI Model and Genome-Wide Microarray Data. Int. J. Mol. Sci. 2023, 24, 10033. [Google Scholar] [CrossRef]
- Potestio, L.; Tommasino, N.; Lauletta, G.; Martora, F.; Megna, M. Psoriasis and Molecular Target Therapies: Evidence of Efficacy in Preventing Cardiovascular Comorbidities. Dermatol. Ther. 2024, 14, 841–852. [Google Scholar] [CrossRef]
- Liu, S.; Dakin, L.A.; Xing, L.; Withka, J.M.; Sahasrabudhe, P.V.; Li, W.; Banker, M.E.; Balbo, P.; Shanker, S.; Chrunyk, B.A.; et al. Binding site elucidation and structure guided design of macrocyclic IL-17A antagonists. Sci. Rep. 2016, 6, 30859. [Google Scholar] [CrossRef]
- Bleicher, L.; de Moura, P.R.; Watanabe, L.; Colau, D.; Dumoutier, L.; Renauld, J.C.; Polikarpov, I. Crystal structure of the IL-22/IL-22R1 complex and its implications for the IL-22 signaling mechanism. FEBS Lett. 2008, 582, 2985–2992. [Google Scholar] [CrossRef]
- Bloch, Y.; Bouchareychas, L.; Merceron, R.; Składanowska, K.; Van den Bossche, L.; Detry, S.; Govindarajan, S.; Elewaut, D.; Haerynck, F.; Dullaers, M.; et al. Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rβ1. Immunity 2018, 48, 45–58.e6. [Google Scholar] [CrossRef]
- Cramer, P.; Larson, C.J.; Verdine, G.L.; Müller, C.W. Structure of the human NF-kappaB p52 homodimer-DNA complex at 2.1 A resolution. EMBO J. 1997, 16, 7078–7090. [Google Scholar] [CrossRef]
- Biovia, D.S.; Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N. Dassault Systèmes BIOVIA, Discovery Studio Visualizer, V. 17.2; Dassault Systèmes: San Diego, CA, USA, 2000. [Google Scholar]
- Milevic, A.; Simic, M.; Tomovic, M.; Rankovic, M.; Jakovljevic, V.; Bradic, J. The effects of methanol extract of Galium verum L on cardiac redox state in hypertensive rats. Braz. J. Pharm. Sci. 2022, 58, e191062. [Google Scholar] [CrossRef]
- Sretenovic, J.; Joksimovic Jovic, J.; Srejovic, I.; Zivkovic, V.; Mihajlovic, K.; Labudovic-Borovic, M.; Trifunovic, S.; Milosevic, V.; Lazic, D.; Bolevich, S.; et al. Morphometric analysis and redox state of the testicles in nandrolone decanoate and swimming treated adult male rats. Basic. Clin. Androl. 2021, 31, 17. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite and [15 N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
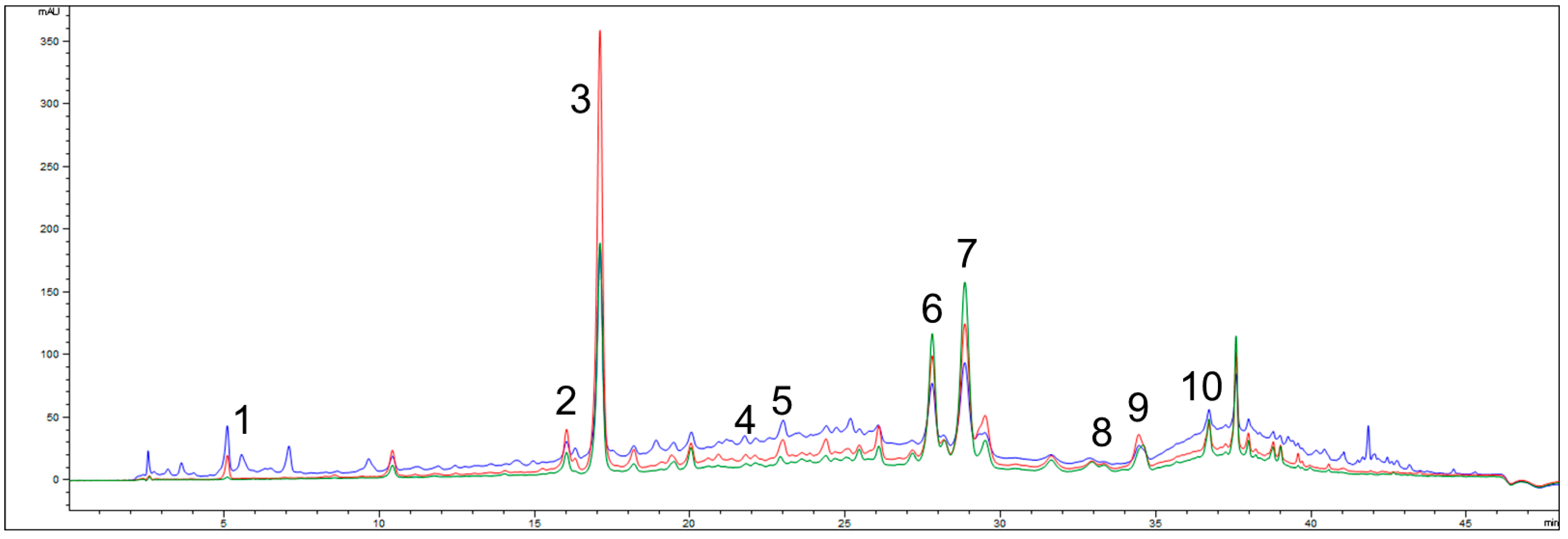
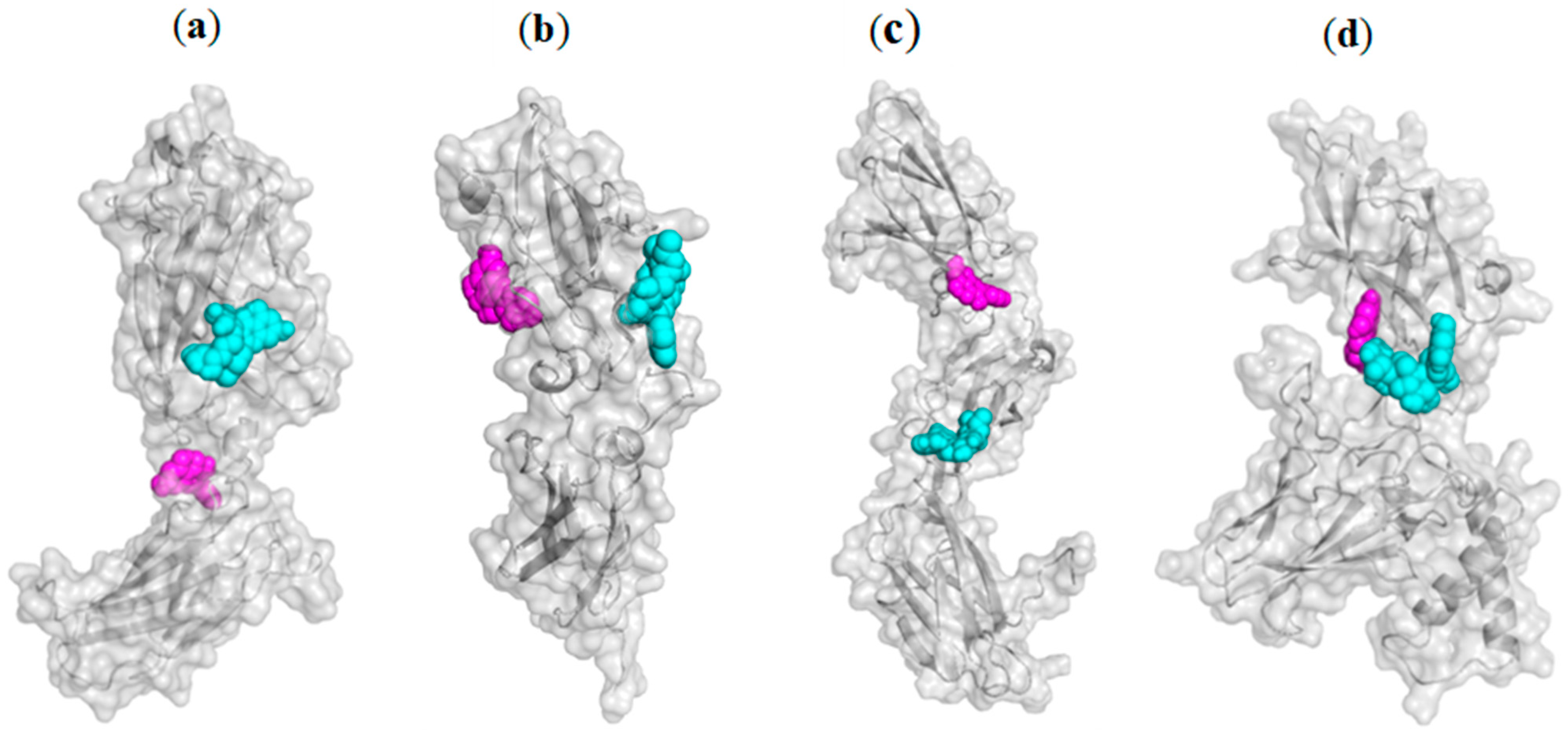

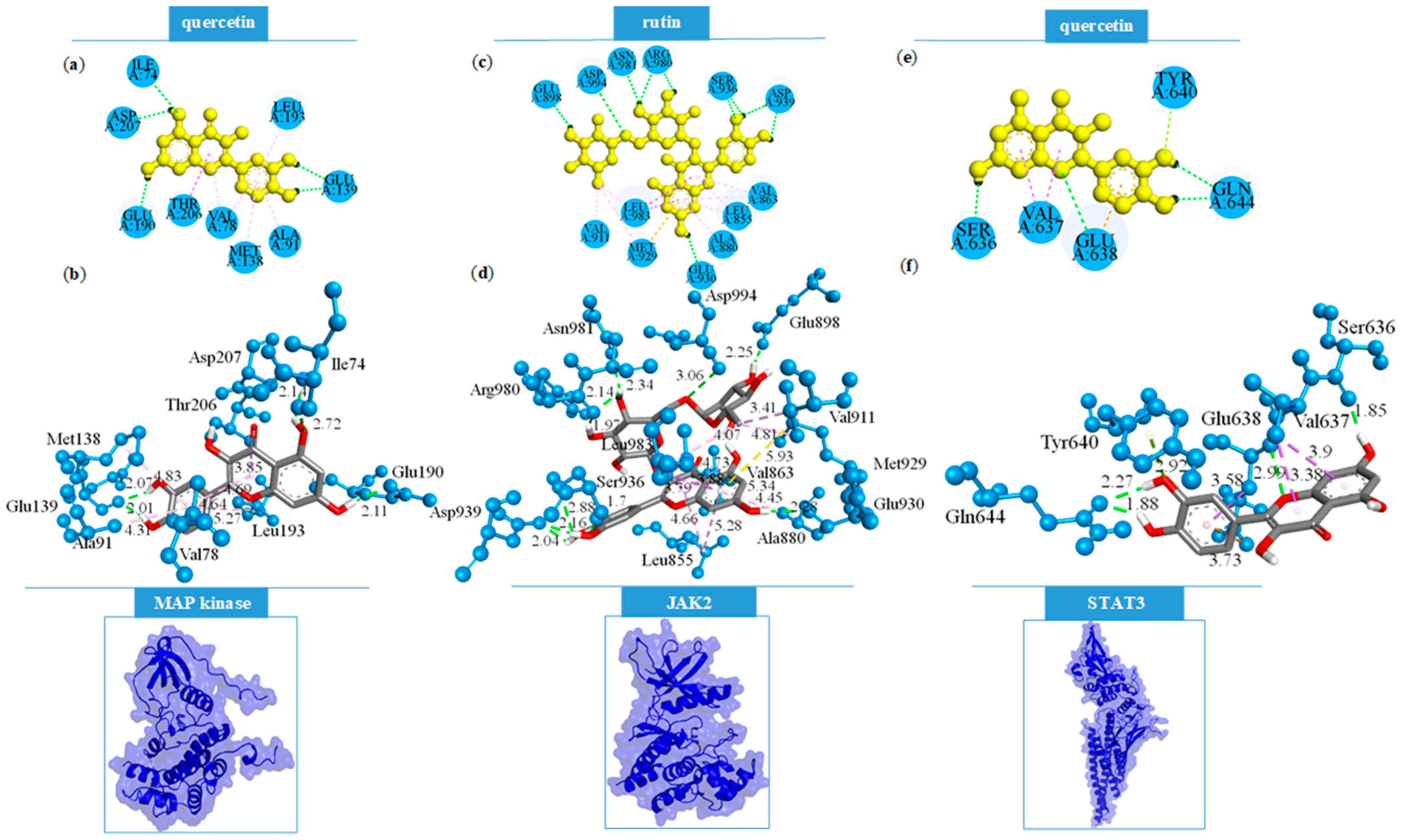

| Name of Compound | G. verum Extract |
|---|---|
| Rutin | 18.85 ± 1.51 |
| Quercetin | 6.60 ± 0.46 |
| Rosmarinic acid | 4.80 ± 0.29 |
| Ferulic acid | 3.43 ± 0.21 |
| Gallic acid | 1.03 ± 0.15 |
| Trans-cinnamic acid | 0.41 ± 0.04 |
| Quercitrin | 0.35 ± 0.02 |
| p-coumaric acid | 0.21 ± 0.02 |
| Chlorogenic acid | 0.09 ± 0.0 |
| Caffeic acid | 0.06 ± 0.0 |
| Investigated Samples and Standards | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) |
|---|---|---|
| GVE | 87.45 ± 6.95 a,b,c | 96.21 ± 6.25 a,b,c |
| AA | 9.15 ± 0.72 | 10.19 ± 0.99 |
| BHA | 11.29 ± 1.03 | 12.46 ± 1.07 |
| Trolox | 5.33 ± 0.18 | 7.36 ± 0.84 |
| Ligand–Protein Complex | ΔGbind (kJ/mol) | Kb (M−1) | ΔGIntermol. Energy (vdw+Hbond+desolv) (kJ/mol) | ΔGelec (kJ/mol) | ΔGFinal Intermol. Energy (kJ/mol) | ΔGtotal (kJ/mol) | ΔGtor (kJ/mol) | ΔGunb (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| Rutin–IL-17 receptor | −25.78 | 3.29 × 104 | −41.84 | −2.30 | −44.14 | −27.32 | 18.36 | −27.32 |
| Rutin–IL-22 receptor | −17.94 | 1.39 × 103 | −34.43 | −1.88 | −36.31 | −25.31 | 18.37 | −25.31 |
| Rutin–IL-23 receptor | −15.90 | 6.10 × 102 | −34.06 | −0.21 | −34.27 | −28.41 | 18.37 | −28.41 |
| Rutin–NF-κB | −22.85 | 1.00 × 104 | −39.25 | −1.97 | −41.22 | −22.68 | 18.37 | −22.68 |
| Quercetin–IL-17 receptor | −23.35 | 1.24 × 104 | −29.20 | −1.05 | −30.25 | −9.16 | 6.90 | −9.16 |
| Quercetin–IL-22 receptor | −24.73 | 1.37 × 103 | −30.12 | 1.51 | −31.63 | −9.07 | 6.90 | −9.07 |
| Quercetin–IL-23 receptor | −23.73 | 1.44 × 104 | −30.21 | −0.42 | −30.63 | −7.78 | 6.90 | −7.78 |
| Quercetin–NF-κB | −25.86 | 3.39 × 104 | −30.88 | −1.88 | −32.76 | −9.37 | 6.90 | −9.37 |
| Ligand | Protein | Interacting Residue * |
|---|---|---|
| Rutin | IL-17 receptor | Leu4 (π-alkyl), Ser24 (HBA × 2), Thr25 (HBA), Asn91 (HBA), Glu92 (HBA × 2), Arg93 (HBD × 2), Leu 94 (CHBD), Leu94 (π-alkyl × 2), Cys95 (HBD × 2), Cys95 (HBA) |
| IL-22 receptor | Tyr57 (HBA × 3), Glu62 (HBA), Val83 (π-alkyl × 2), Val83 (π-lone Pair), Glu90 (HBA), Tyr92 (HBA), Arg147 (HBA × 2) | |
| IL-23 receptor | Ile154 (HBA), Thr152 (π-donor HBD × 2), Leu151 (π-alkyl), Thr152 (π-lone Pair), Tyr153 (π-π stacked) Thr156 (HBA × 2), Ser176 (HBA) | |
| NF-κB | Gly50 (HBA × 2), Ser222 (HBA), Pro223 (HBA), Ser226 (HBD), Asn227 (HBD × 2), Lys229 (alkyl), Asp251 (π-anion), Lys252 (HBD), Lys252 (π-σ) | |
| Quercetin | IL-17 receptor | Ser168 (bump), Gly169 (HBA × 2), Trp172 (HBA), Cys259 (π-sulfur × 2), Asp262 (HBA × 2), Cys263 (π-alkyl), Cys263 (π-sulfur), Leu264 (HBD), Leu264 (π-σ), Leu264 (π-alkyl) |
| IL-22 receptor | His27 (π-π stacked), Lys29 (HBD), Lys29 (π-cation), Lys29 (π-σ), Lys29 (π-alkyl × 2), Lys108 (CHBD), Asp111 (π-anion), Arg112 (HBA × 2) | |
| IL-23 receptor | Glu137 (HBD), Glu137 (HBA), Ile219 (HBA), Pro220 (HBA), Ala223 (HBA), Trp307 (bump) | |
| NF-κB | Leu228 (HBD), Lys229 (π-alkyl × 2), Ile230 (HBD), Ile230 (HBA), Asp316 (HBA × 2), Val317 (π-lone Pair), Asp319 (π-anion × 2) |
| Ligand–Protein Complex | ΔGbind (kJ/mol) | Ki (M) | ΔGIntermol. Energy (vdw+Hbond+desolv) (kJ/mol) | ΔGelec (kJ/mol) | ΔGFinal Intermol. Energy (kJ/mol) | ΔGtotal (kJ/mol) | ΔGtor (kJ/mol) | ΔGunb (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| Rutin–MAPK2 | −30.67 | 4.26 × 10−6 | −47.40 | −1.63 | −49.04 | −23.68 | 18.37 | −23.68 |
| Rutin–JAK2 | −33.89 | 1.15 × 10−6 | −49.62 | −2.68 | −52.26 | −31.84 | 18.37 | −31.84 |
| Rutin–STAT3 | −21.92 | 1.43 × 10−4 | −37.66 | −2.64 | −40.29 | −22.64 | 18.37 | −22.64 |
| Quercetin–MAPK2 | −30.92 | 3.81 × 10−6 | −36.61 | −1.21 | −37.82 | −9.00 | 6.90 | −9.00 |
| Quercetin–JAK2 | −29.25 | 7.57 × 10−6 | −33.97 | −2.13 | −36.11 | −6.95 | 6.90 | −6.95 |
| Quercetin–STAT3 | −24.39 | 5.36 × 10−5 | −30.38 | −0.88 | −31.25 | −9.29 | 6.90 | −9.29 |
| Ligand | Protein | Interacting Residue * |
|---|---|---|
| Rutin | MAPK2 | Gly71 (π-σ), Gly73 (CHBD), Val78 (π-alkyl × 2), Lys93 (HBD), Lys93 (π-alkyl), Met138 (π-sulfur), Glu139 (HBA), Leu141 (CHBA), Glu190 (π-anion), Leu193 (π-alkyl), Thr206 (HBD) |
| JAK2 | Leu855 (π-alkyl × 2), Val863 (π-alkyl × 2), Ala880 (π-alkyl), Glu898 (HBA), Val911 (alkyl), Met929 (π-sulfur), Met929 (alkyl), Glu930 (HBA), Ser936 (HBD), Ser936 (HBA), Asp939 (HBA × 2), Arg980 (HBA × 2), Asn981 (HBA), Leu983 (π-σ × 2), Leu983 (alkyl), Asp994 (HBD) | |
| STAT3 | Val637 (π-σ), Glu638 (HBA), Glu638 (CHBA × 2), Glu638 (π-anion × 2), Pro639 (CHBD), Pro639 (alkyl), Gln644 (HBA), Tyr657 (π-π T-shaped) | |
| Quercetin | MAPK2 | Ile74 (HBD), Val78 (π-alkyl × 2), Ala91 (π-alkyl), Met138 (π-alkyl), Glu139 (HBA × 2), Glu190 (HBA), Leu193 (π-alkyl), Thr206 (π-σ), Asp207 (HBA) |
| JAK2 | Leu855 (π-σ), Leu855 (π-alkyl × 2), Val863 (π-alkyl), Ala880 (π-alkyl), Met929 (π-sulfur), Glu930 (HBA), Leu932 (HBD × 2), Leu932 (HBA), Asp939 (HBA × 2), Leu983 (π-σ), Leu983 (π-alkyl) | |
| STAT3 | Ser636 (HBA), Val637 (π-σ × 2), Glu638 (HBD), Glu638 (π-anion), Glu638 (π-σ), Tyr640 (π-lone pair), Gln644 (HBA × 2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalovic, B.; Jakovljevic, V.; Bolevic, S.; Andjic, M.; Bradic, J.; Kocovic, A.; Nikolic, M.; Nedeljkovic, N.; Milosavljevic, J.; Baljak, J.; et al. The Therapeutic Potential of Galium verum for Psoriasis: A Combined Phytochemical, In Silico, and Experimental Approach. Int. J. Mol. Sci. 2025, 26, 7290. https://doi.org/10.3390/ijms26157290
Daskalovic B, Jakovljevic V, Bolevic S, Andjic M, Bradic J, Kocovic A, Nikolic M, Nedeljkovic N, Milosavljevic J, Baljak J, et al. The Therapeutic Potential of Galium verum for Psoriasis: A Combined Phytochemical, In Silico, and Experimental Approach. International Journal of Molecular Sciences. 2025; 26(15):7290. https://doi.org/10.3390/ijms26157290
Chicago/Turabian StyleDaskalovic, Branislava, Vladimir Jakovljevic, Sergej Bolevic, Marijana Andjic, Jovana Bradic, Aleksandar Kocovic, Milos Nikolic, Nikola Nedeljkovic, Jovan Milosavljevic, Jovan Baljak, and et al. 2025. "The Therapeutic Potential of Galium verum for Psoriasis: A Combined Phytochemical, In Silico, and Experimental Approach" International Journal of Molecular Sciences 26, no. 15: 7290. https://doi.org/10.3390/ijms26157290
APA StyleDaskalovic, B., Jakovljevic, V., Bolevic, S., Andjic, M., Bradic, J., Kocovic, A., Nikolic, M., Nedeljkovic, N., Milosavljevic, J., Baljak, J., Krivokapic, M., Trifunovic, S., & Sretenovic, J. (2025). The Therapeutic Potential of Galium verum for Psoriasis: A Combined Phytochemical, In Silico, and Experimental Approach. International Journal of Molecular Sciences, 26(15), 7290. https://doi.org/10.3390/ijms26157290











