The Current State of Research in the Field of Photosensitizers and Photoactivators for Photodynamic/Photothermal Cancer Therapy: A Review
Abstract
1. Introduction
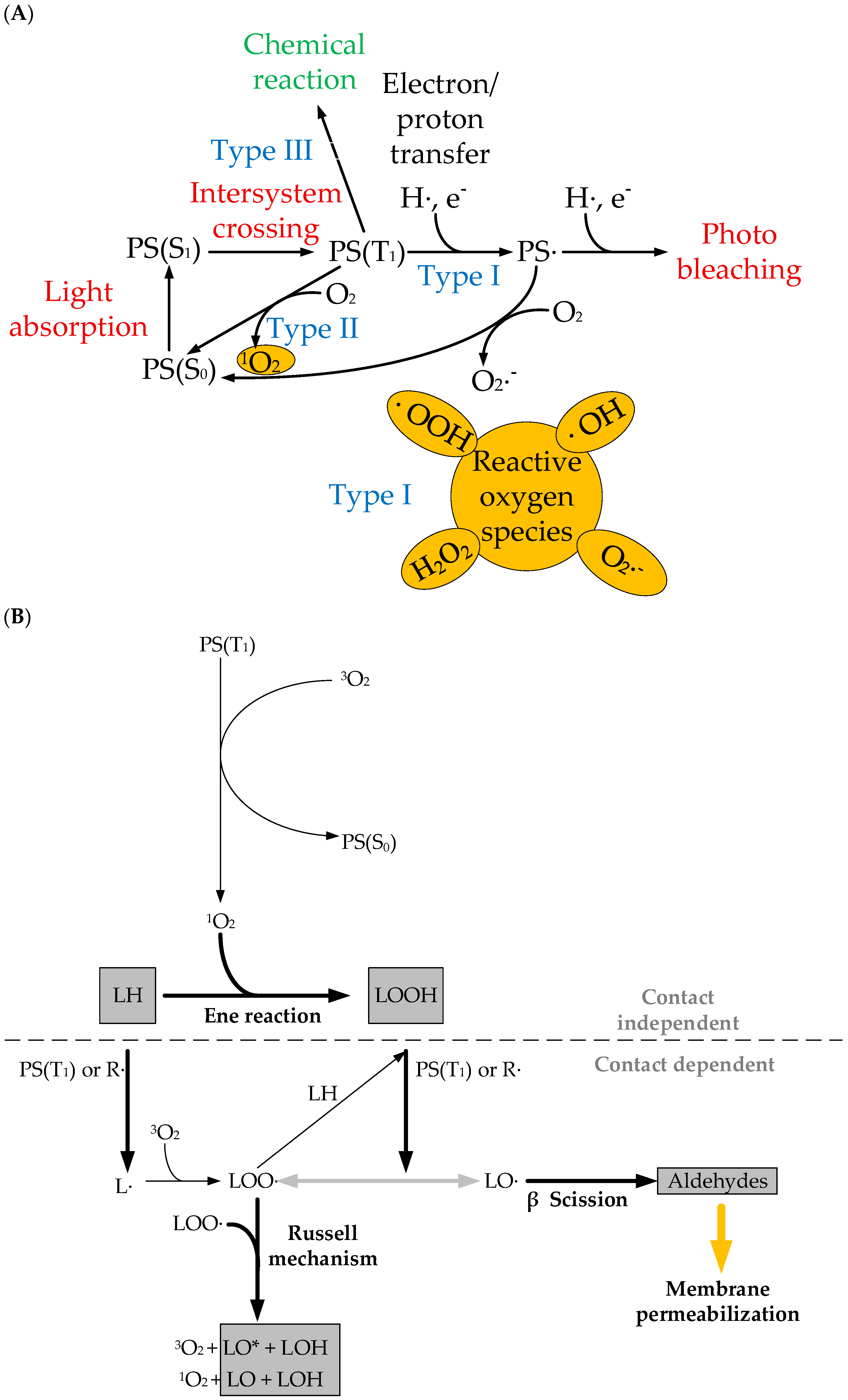
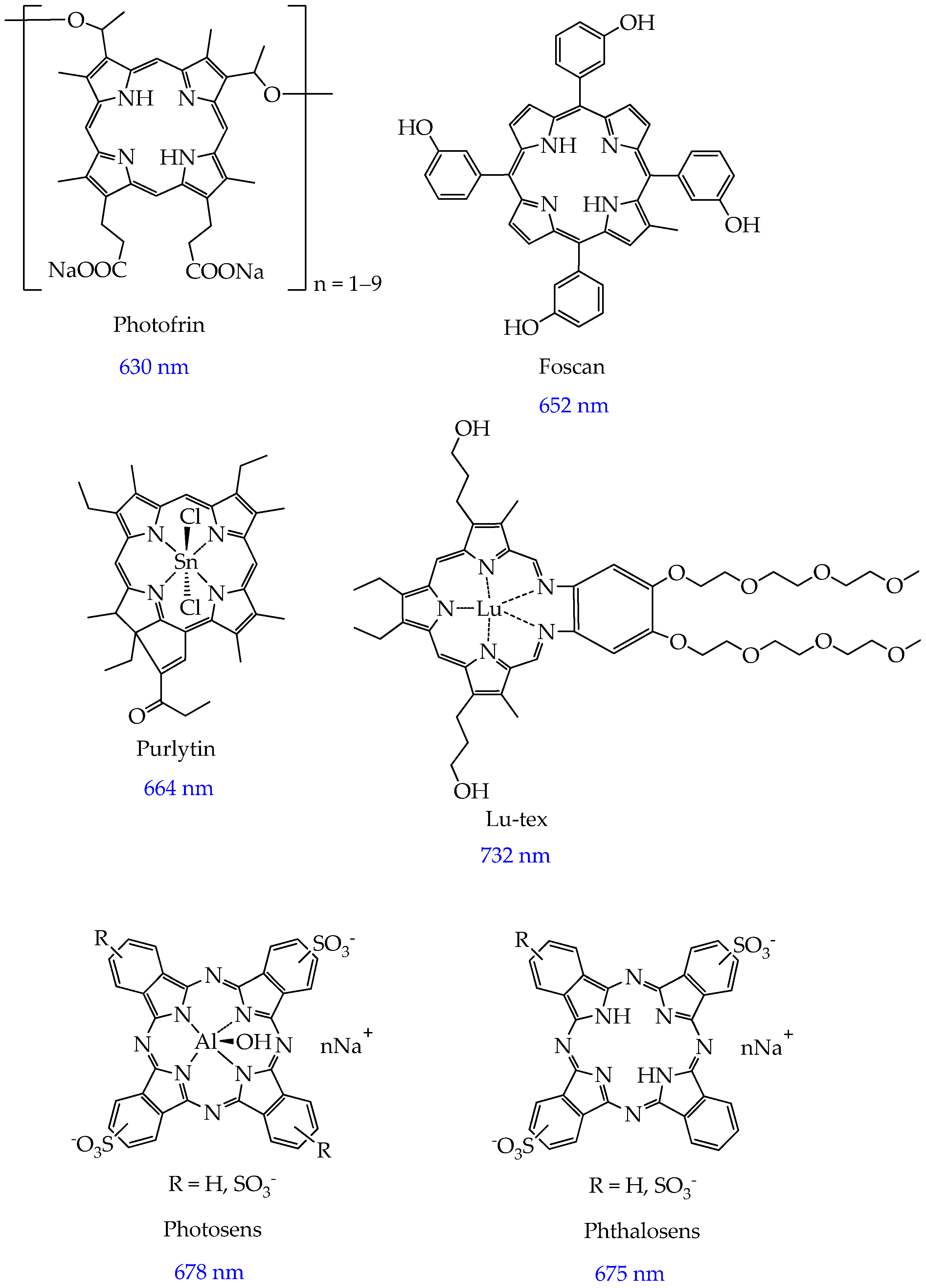
2. Photosensitizers Based on Porphyrin Derivatives and Metal Complexes
| Name | Λ Absorption, nm | Quantum Yield of Singlet Oxygen, % | Cell Line | Phototoxicity, μM | Dark Toxicity, IC50, μM | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|
| 5,10,15,20-tetrakis(N-ethylpyridinium-3-yl)porphyrin chloride (H2P) | 520 (green light) | 16.2 | MDA- MB-231 | 0.21 ± 0.13 | >1000 | Apoptosis | [70] |
| Metallocomplex H2P and Sn(IV), SnP | 17.3 | 0.77 ± 0.25 | >1000 | ||||
| AuP | <1 | 25.24 ± 12.11 | >1000 | ||||
| ZnP | 41 | 0.22 ± 0.16 | >1000 | ||||
| Tetrakis (1-methylpyridinium-4-yl) p-toluenesulfonate porphyrin (TMPyP) | 690 | 61 | MDA- MB-231 | 60.1 ± 4.81 | Cell viability above 90% | Apoptosis | [71] |
| T47D | 24.48 ± 1.99 | ||||||
| Sn (IV) complex of 5-(9-butyl-9H-carbazol-3-yl)-10,15,20-tris(4-(2-(2-methoxyethoxy)ethoxy)phenyl) porphyrin | 433–610 | 43 | A549 | 1.36 | >50 | Localization in ER, probably autophagy and apoptosis | [72] |
| Sn (IV) complex of N,N-diphenyl-4-(10,15,20-tris(4-(2-(2-methoxyethoxy)ethoxy)phenyl)porphyrin-5-yl)aniline | 430–612 | 17 | 0.76 | ||||
| In (III) complex of D(+) glucose-substituted tetrakis-(4- -ethylthiophenyl) porphyrin | 415 | 63 | MDA- MB-231 | Cell viability 55.2% | Cell viability 100% | Not researched | [73] |
| Ga (III) complex of D(+) glucose-substituted tetrakis-(4- -ethylthiophenyl) porphyrin | 63 | Cell viability 50.7% |
3. Photoactivators Based on Non-Porphyrin Complexes
4. Photosensitizers Based on BODIPY Derivatives
5. Photosensitizers Based on Squaraines
6. Nanosized Carriers of Photosensitizers
6.1. Organic Nanoparticles (NPs)
6.2. Inorganic Nanoparticles
6.3. Liposomes
6.4. Extracellular Vesicles
6.4.1. Microvesicles
6.4.2. Exosomes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bax | Protein, apoptosis regulator |
| BODIPY | 4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene |
| CDBTN | 131-Hexamethylenediaminyl-desthiobiotinylchlorin e6 dimethyl ester |
| Ce6 | Chlorin e6 |
| DCF-DA | 2′,7′-Dichlorodihydrofluorescein diacetate |
| DNA | Deoxyribonucleic acid |
| DMSO | Dimethylsulfoxide |
| EMA | European Medicines Agency |
| ER | Endoplasmic reticulum |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| JC-1 | Dye is a commonly used tool, used for studying mitochondrial membrane potential |
| HIF-1α | Hypoxia-induced factor 1-alpha |
| ICG | Indocyanine green |
| MNZ | Metronidazole |
| MSNs | Mesoporous silica nanoparticles |
| NIR | Near infrared |
| NPs | Nanoparticles |
| PARP-1 | Poly [ADP-ribose] polymerase 1 |
| PEG | Polyethylene glycol |
| PDT | Photodynamic therapy |
| PLGA | Poly(lactic-co-glycolic) acid |
| POEGMA | Poly(oligo(ethylene glycol) methyl ether methacrylate) |
| PPI | Poly(propylene)imine |
| PTT | Photothermal therapy |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| SQs | Squaraines |
| TEM | Transmission electron microscopy |
| 1O2 | Singlet oxygen |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Alexandru, I.; Davidescu, L.; Motofelea, A.C.; Ciocarlie, T.; Motofelea, N.; Costachescu, D.; Marc, M.S.; Suppini, N.; Șovrea, A.S.; Coșeriu, R.-L.; et al. Emerging Nanomedicine Approaches in Targeted Lung Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 11235. [Google Scholar] [CrossRef] [PubMed]
- Morise, Z.; Katsuno, H.; Kikuchi, K.; Endo, T.; Matsuo, K.; Asano, Y.; Horiguchi, A. Laparoscopic Repeat Liver Resection—Selecting the Best Approach for Repeat Liver Resection. Cancers 2023, 15, 421. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Ma, Y.; Zheng, J.; Xu, K.; Chang, Y.; Ye, Z.; Ling, Y.; Wang, L. Novel Type I/II Carbazole/Benzindole Photosensitizers Achieve Chemo-Photodynamic Synergistic Therapy for Suppressing Solid Tumors and Drug-Resistant Bacterial Infections. Molecules 2025, 30, 2560. [Google Scholar] [CrossRef]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past Is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef]
- Kim, T.E.; Chang, J.-E. Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials. Pharmaceutics 2023, 15, 2257. [Google Scholar] [CrossRef]
- Wang, K.; Yu, B.; Pathak, J.L. An Update in Clinical Utilization of Photodynamic Therapy for Lung Cancer. J. Cancer 2021, 12, 1154–1160. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Bartusik-Aebisher, D. The Latest Look at PDT and Immune Checkpoints. Curr. Issues Mol. Biol. 2024, 46, 7239–7257. [Google Scholar] [CrossRef] [PubMed]
- Grin, M.; Suvorov, N.; Ostroverkhov, P.; Pogorilyy, V.; Kirin, N.; Popov, A.; Sazonova, A.; Filonenko, E. Advantages of Combined Photodynamic Therapy in the Treatment of Oncological Diseases. Biophys. Rev. 2022, 14, 941–963. [Google Scholar] [CrossRef]
- Dos Santos, A.F.; De Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef]
- Alvarez, N.; Sevilla, A. Current Advances in Photodynamic Therapy (PDT) and the Future Potential of PDT-Combinatorial Cancer Therapies. Int. J. Mol. Sci. 2024, 25, 1023. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, K.; Kang, M.; Yan, D.; Niu, N.; Yan, S.; Sun, P.; Zhang, L.; Sun, L.; Wang, D.; et al. A New Era of Cancer Phototherapy: Mechanisms and Applications. Chem. Soc. Rev. 2024, 53, 12014–12042. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef]
- Bacellar, I.O.L.; Oliveira, M.C.; Dantas, L.S.; Costa, E.B.; Junqueira, H.C.; Martins, W.K.; Durantini, A.M.; Cosa, G.; Di Mascio, P.; Wainwright, M.; et al. Photosensitized Membrane Permeabilization Requires Contact-Dependent Reactions between Photosensitizer and Lipids. J. Am. Chem. Soc. 2018, 140, 9606–9615. [Google Scholar] [CrossRef] [PubMed]
- Chiaviello, A.; Postiglione, I.; Palumbo, G. Targets and Mechanisms of Photodynamic Therapy in Lung Cancer Cells: A Brief Overview. Cancers 2011, 3, 1014–1041. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, X.; Liu, R.; Zhao, D.; Guo, C.; Zhu, A.; Wen, M.; Liu, Z.; Qu, G.; Meng, H. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Guo, S.; Song, Z.; Ji, D.-K.; Reina, G.; Fauny, J.-D.; Nishina, Y.; Ménard-Moyon, C.; Bianco, A. Combined Photothermal and Photodynamic Therapy for Cancer Treatment Using a Multifunctional Graphene Oxide. Pharmaceutics 2022, 14, 1365. [Google Scholar] [CrossRef]
- Xenodochidis, C.; Hristova-Panusheva, K.; Kamenska, T.; Santhosh, P.B.; Petrov, T.; Stoychev, L.; Genova, J.; Krasteva, N. Graphene Oxide Nanoparticles for Photothermal Treatment of Hepatocellular Carcinoma Using Low-Intensity Femtosecond Laser Irradiation. Molecules 2024, 29, 5650. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, J.; Fang, L.; Zhao, J.; Yu, F.; Wang, B. Recent Advancements of Porphyrin-Based Supramolecular Nanomaterials for Phototherapy. Coord. Chem. Rev. 2026, 547, 217121. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally Enhanced Photodynamic Therapy Delivered by Nano-Graphene Oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef]
- Dąbrowski, J.M. Reactive Oxygen Species in Photodynamic Therapy: Mechanisms of Their Generation and Potentiation. Adv. Inorg. Chem. 2017, 70, 343–394. [Google Scholar]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical Development of Photodynamic Agents and Therapeutic Applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Bellnier, D.A. Tissue Localization of Photosensitizers and the Mechanism of Photodynamic Tissue Destruction. Ciba Found. Symp. 1989, 146, 112–125, discussion in 125–130. [Google Scholar] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Photodynamic Therapy and the Development of Metal-Based Photosensitisers. Met.-Based Drugs 2008, 2008, 276109. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Atif, M.; Fakhar-e-Alam, M.; Zaidi, S.S.Z.; Suleman, R. Study of the Efficacy of Photofrin®-Mediated PDT on Human Hepatocellular Carcinoma (HepG2) Cell Line. Laser Phys. 2011, 21, 1135–1144. [Google Scholar] [CrossRef]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-Tetra(m-hydroxyphenyl)Chlorin)—A Second-generation Photosensitizer †,‡. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef]
- Müller, S.; Walt, H.; Dobler-Girdziunaite, D.; Fiedler, D.; Haller, U. Enhanced Photodynamic Effects Using Fractionated Laser Light. J. Photochem. Photobiol. B Biol. 1998, 42, 67–70. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Kaplan, M.J.; Somers, R.G.; Greenberg, R.H.; Ackler, J. Photodynamic Therapy in the Management of Metastatic Cutaneous Adenocarcinomas: Case Reports from Phase 1/2 Studies Using Tin Ethyl Etiopurpurin (SnET2). J. Surg. Oncol. 1998, 67, 121–125. [Google Scholar] [CrossRef]
- Kostenich, G.; Orenstein, A.; Roitman, L.; Malik, Z.; Ehrenberg, B. In Vivo Photodynamic Therapy with the New Near-IR Absorbing Water Soluble Photosensitizer Lutetium Texaphyrin and a High Intensity Pulsed Light Delivery System. J. Photochem. Photobiol. B Biol. 1997, 39, 36–42. [Google Scholar] [CrossRef]
- Young, S.W.; Woodburn, K.W.; Wright, M.; Mody, T.D.; Fan, Q.; Sessler, J.L.; DOW, W.C.; Miller, R.A. Lutetium Texaphyrin (PCI-0123): A Near-Infrared, Water-Soluble Photosensitizer. Photochem. Photobiol. 1996, 63, 892–897. [Google Scholar] [CrossRef]
- Brilkina, A.A.; Dubasova, L.V.; Sergeeva, E.A.; Pospelov, A.J.; Shilyagina, N.Y.; Shakhova, N.M.; Balalaeva, I.V. Photobiological Properties of Phthalocyanine Photosensitizers Photosens, Holosens and Phthalosens: A Comparative in Vitro Analysis. J. Photochem. Photobiol. B Biol. 2019, 191, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique Diagnostic and Therapeutic Roles of Porphyrins and Phthalocyanines in Photodynamic Therapy, Imaging and Theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, H.; Liu, Y.; Zhang, L.; Feng, G.; Tang, B.Z. Recent Progress in Type I Aggregation-Induced Emission Photosensitizers for Photodynamic Therapy. Molecules 2022, 28, 332. [Google Scholar] [CrossRef]
- Rybkin, A.Y.; Kurmaz, S.V.; Urakova, E.A.; Filatova, N.V.; Sizov, L.R.; Kozlov, A.V.; Koifman, M.O.; Goryachev, N.S. Nanoparticles of N-Vinylpyrrolidone Amphiphilic Copolymers and Pheophorbide a as Promising Photosensitizers for Photodynamic Therapy: Design, Properties and In Vitro Phototoxic Activity. Pharmaceutics 2023, 15, 273. [Google Scholar] [CrossRef]
- Dantas, K.C.F.; Rosário, J.d.S.; Silva-Caldeira, P.P. Polymeric Nanosystems Applied for Metal-Based Drugs and Photosensitizers Delivery: The State of the Art and Recent Advancements. Pharmaceutics 2022, 14, 1506. [Google Scholar] [CrossRef]
- Yu, X.-T.; Sui, S.-Y.; He, Y.-X.; Yu, C.-H.; Peng, Q. Nanomaterials-Based Photosensitizers and Delivery Systems for Photodynamic Cancer Therapy. Biomater. Adv. 2022, 135, 212725. [Google Scholar] [CrossRef]
- Yudaev, P.; Tupikov, A.; Chistyakov, E. Organocyclophosphazenes and Materials Based on Them for Pharmaceuticals and Biomedicine. Biomolecules 2025, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Pröll, S.; Wilhelm, B.; Robert, B.; Scheer, H. Myoglobin with Modified Tetrapyrrole Chromophores: Binding Specificity and Photochemistry. Biochim. Biophys. Acta Bioenerg. 2006, 1757, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.P.; Assunção, M.A.; Bessa, L.J.; Alves, R.C. Microbiological Impact of Antimicrobial Photodynamic Therapy in Non-Surgical Periodontal Treatment. Pharmaceutics 2025, 17, 1070. [Google Scholar] [CrossRef]
- Wiench, R.; Fiegler-Rudol, J.; Zięba, N.; Misiołek, M. Laser Interventions for Intraoral Halitosis: A Systematic Review of Randomized Controlled Trials. Pharmaceutics 2025, 17, 1046. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Saad, M.A.; Przygórzewska, A.; Aebisher, D. Recent Advances in Nanoparticle and Nanocomposite-Based Photodynamic Therapy for Cervical Cancer: A Review. Cancers 2025, 17, 2572. [Google Scholar] [CrossRef]
- Chornovolenko, K.; Koczorowski, T. Phthalocyanines Conjugated with Small Biologically Active Compounds for the Advanced Photodynamic Therapy: A Review. Molecules 2025, 30, 3297. [Google Scholar] [CrossRef]
- Amendola, G.; Di Luca, M.; Sgarbossa, A. Natural Biomolecules and Light: Antimicrobial Photodynamic Strategies in the Fight Against Antibiotic Resistance. Int. J. Mol. Sci. 2025, 26, 7993. [Google Scholar] [CrossRef]
- Ailioaie, L.M.; Ailioaie, C.; Litscher, G. Fighting Cancer with Photodynamic Therapy and Nanotechnologies: Current Challenges and Future Directions. Int. J. Mol. Sci. 2025, 26, 2969. [Google Scholar] [CrossRef]
- Aziz, B.; Aziz, I.; Khurshid, A.; Raoufi, E.; Esfahani, F.N.; Jalilian, Z.; Mozafari, M.R.; Taghavi, E.; Ikram, M. An Overview of Potential Natural Photosensitizers in Cancer Photodynamic Therapy. Biomedicines 2023, 11, 224. [Google Scholar] [CrossRef]
- Tian, Z.; Li, H.; Liu, Z.; Yang, L.; Zhang, C.; He, J.; Ai, W.; Liu, Y. Enhanced Photodynamic Therapy by Improved Light Energy Capture Efficiency of Porphyrin Photosensitizers. Curr. Treat. Options Oncol. 2023, 24, 1274–1292. [Google Scholar] [CrossRef]
- Zach, P.W.; Freunberger, S.A.; Klimant, I.; Borisov, S.M. Electron-Deficient Near-Infrared Pt(II) and Pd(II) Benzoporphyrins with Dual Phosphorescence and Unusually Efficient Thermally Activated Delayed Fluorescence: First Demonstration of Simultaneous Oxygen and Temperature Sensing with a Single Emitter. ACS Appl. Mater. Interfaces 2017, 9, 38008–38023. [Google Scholar] [CrossRef] [PubMed]
- Žárská, L.; Malá, Z.; Langová, K.; Malina, L.; Binder, S.; Bajgar, R.; Kolářová, H. The Effect of Two Porphyrine Photosensitizers TMPyP and ZnTPPS4 for Application in Photodynamic Therapy of Cancer Cells in Vitro. Photodiagnosis Photodyn. Ther. 2021, 34, 102224. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, D.A.; Cadima-Couto, I.; Buga, C.C.; Arnaut, Z.A.; Schaberle, F.A.; Arnaut, L.G.; Castanho, M.A.R.B.; Cruz-Oliveira, C. Repurposing Anti-Cancer Porphyrin Derivative Drugs to Target SARS-CoV-2 Envelope. Biomed. Pharmacother. 2024, 176, 116768. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, R.; Ahmed, S.; Bhat, A.R.; Bajju, G.D.; Sheikh, H.N. Investigation of Antimicrobial Potential of Nanocomposites Based on Functionalization of Graphene Oxide with Zinc Porphyrin Complexes. Inorganica Chim. Acta 2024, 569, 122155. [Google Scholar] [CrossRef]
- Cao, N.; Jiang, Y.; Song, Z.-B.; Chen, D.; Wu, D.; Chen, Z.-L.; Yan, Y.-J. Synthesis and Evaluation of Novel Meso-Substitutedphenyl Dithieno[3,2-b]Thiophene-Fused BODIPY Derivatives as Efficient Photosensitizers for Photodynamic Therapy. Eur. J. Med. Chem. 2024, 264, 116012. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, H.; Chen, Z.; Dong, X.; Zhao, W.; Shi, Y.; Zhu, Q. Discovery of an Amino Acid-Modified Near-Infrared Aza-BODIPY Photosensitizer as an Immune Initiator for Potent Photodynamic Therapy in Melanoma. J. Med. Chem. 2022, 65, 3616–3631. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, H.-Y.; Yan, Y.-J.; Romanishkin, I.D.; Meerovich, G.A.; Reshetov, I.V.; Zhou, X.-P.; Chen, Z.-L. The Synthesis, Photophysical and Biological Properties of 5,10,15,20-Tetra(4-Substituted Phenyl)Tetrabenzoporphyrin Derivatives. Eur. J. Med. Chem. 2025, 291, 117612. [Google Scholar] [CrossRef]
- Pisarek, S.; Maximova, K.; Gryko, D. Strategies toward the Synthesis of Amphiphilic Porphyrins. Tetrahedron 2014, 70, 6685–6715. [Google Scholar] [CrossRef]
- Mehraban, N.; Freeman, H. Developments in PDT Sensitizers for Increased Selectivity and Singlet Oxygen Production. Materials 2015, 8, 4421–4456. [Google Scholar] [CrossRef]
- Malatesti, N.; Munitic, I.; Jurak, I. Porphyrin-Based Cationic Amphiphilic Photosensitisers as Potential Anticancer, Antimicrobial and Immunosuppressive Agents. Biophys. Rev. 2017, 9, 149–168. [Google Scholar] [CrossRef]
- Luciano, M.; Brückner, C. Modifications of Porphyrins and Hydroporphyrins for Their Solubilization in Aqueous Media. Molecules 2017, 22, 980. [Google Scholar] [CrossRef]
- Lei, W.; Xie, J.; Hou, Y.; Jiang, G.; Zhang, H.; Wang, P.; Wang, X.; Zhang, B. Mitochondria-Targeting Properties and Photodynamic Activities of Porphyrin Derivatives Bearing Cationic Pendant. J. Photochem. Photobiol. B Biol. 2010, 98, 167–171. [Google Scholar] [CrossRef]
- Odeh, A.M.; Craik, J.D.; Ezzeddine, R.; Tovmasyan, A.; Batinic-Haberle, I.; Benov, L.T. Targeting Mitochondria by Zn(II)N-Alkylpyridylporphyrins: The Impact of Compound Sub-Mitochondrial Partition on Cell Respiration and Overall Photodynamic Efficacy. PLoS ONE 2014, 9, e108238. [Google Scholar] [CrossRef]
- Ezzeddine, R.; Al-Banaw, A.; Tovmasyan, A.; Craik, J.D.; Batinic-Haberle, I.; Benov, L.T. Effect of Molecular Characteristics on Cellular Uptake, Subcellular Localization, and Phototoxicity of Zn(II) N-Alkylpyridylporphyrins. J. Biol. Chem. 2013, 288, 36579–36588. [Google Scholar] [CrossRef] [PubMed]
- Mušković, M.; Lončarić, M.; Ratkaj, I.; Malatesti, N. Impact of the Hydrophilic-Lipophilic Balance of Free-Base and Zn(II) Tricationic Pyridiniumporphyrins and Irradiation Wavelength in PDT against the Melanoma Cell Lines. Eur. J. Med. Chem. 2025, 282, 117063. [Google Scholar] [CrossRef] [PubMed]
- Mušković, M.; Lončarić, M.; Ratkaj, I.; Malatesti, N. (Oxidopyridyl)Porphyrins of Different Lipophilicity: Photophysical Properties, ROS Production and Phototoxicity on Melanoma Cells Under CoCl2-Induced Hypoxia. Antioxidants 2025, 14, 992. [Google Scholar] [CrossRef] [PubMed]
- Balázs, B.; Stoean (Vasile), B.; Molnár, É.; Fischer-Fodor, E.; Bălăcescu, O.; Borlan, R.; Focsan, M.; Grozav, A.; Achimaş-Cadariu, P.; Gál, E.; et al. Meso-Substituted AB3 -Type Phenothiazinyl Porphyrins and Their Indium and Zinc Complexes Photosensitising Properties, Cytotoxicity and Phototoxicity on Ovarian Cancer Cells. RSC Med. Chem. 2025, 16, 747–766. [Google Scholar] [CrossRef]
- Rutkowski, B.N.; Isaac-Lam, M.F. Photodynamic Evaluation of Synthesized Chlorin-Desthiobiotin Conjugate with Chemotherapeutic Drugs in Triple-Negative Breast Cancer Cells In Vitro and in Hydra Organisms In Vivo. Int. J. Mol. Sci. 2025, 26, 5357. [Google Scholar] [CrossRef]
- McAnlis, H.E.; Stewart, J.R.; Burton, S.T.; Steinke, S.J.; Turro, C.; Bonvallet, P.A. Design, Synthesis, and Photophysical Properties of Hybrid Porphyrin-Natural Product Compounds. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 343, 126512. [Google Scholar] [CrossRef]
- Vieira, N.I.D.; Fonseca-Pinheiro, P.; Bento, L.P.; de Oliveira Silva, M.; de Souza, C.E.M.; de Lima, R.G.; Diniz, R.; deFreitas-Silva, G.; de Souza-Fagundes, E.M.; da Silva Martins, D.C. Water-Soluble Porphyrins Derived from 5,10,15,20-Tetrakis(N-Ethylpyridinium-3-Yl)Porphyrin: Metal Center Effect on the Photodynamic Activity against Aggressive Triple-Negative Breast Cancer Lineage. Dye. Pigment. 2026, 245, 113158. [Google Scholar] [CrossRef]
- Mokhtar, A.; Mohamed, T.; Eigza, A.O.; El-Khouly, M.E. Water-Soluble Porphyrin-Mediated Enhanced Photodynamic and Chemotherapy Employing Doxorubicin for Breast Cancer. Lasers Med. Sci. 2025, 40, 241. [Google Scholar] [CrossRef]
- Gupta, I.; Bishnoi, R.; Manav, N.; Jain, A.; Chavda, J. Endoplasmic Reticulum Targeting Tin Porphyrins with Oligoethyleneglycol Chains: Synthesis, DFT Studies and Anti-Cancer Activities. J. Mol. Struct. 2025, 1345, 143121. [Google Scholar] [CrossRef]
- Vukeya, T.S.; Matshitse, R.; Mack, J.; Openda, Y.; Prinsloo, E.; Nyokong, T. Effects of Central Metals on Sugar Decorated Tetrakis-(4-Methylthiophenyl) Porphyrin Considered for Use in Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2025, 54, 104658. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Tang, Z.; Karges, J.; Shu, J.; Xiong, K.; Jin, C.; Chen, Y.; Gasser, G.; Ji, L.; Chao, H. An Iridium(iii)-Based Photosensitizer Disrupting the Mitochondrial Respiratory Chain Induces Ferritinophagy-Mediated Immunogenic Cell Death. Chem. Sci. 2024, 15, 6752–6762. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guan, R.; Xie, L.; Liao, X.; Xiong, K.; Rees, T.W.; Chen, Y.; Ji, L.; Chao, H. An ER-Targeting Iridium(III) Complex That Induces Immunogenic Cell Death in Non-Small-Cell Lung Cancer. Angew. Chemie Int. Ed. 2021, 60, 4657–4665. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.; Wei, F.; Karges, J.; Ke, L.; Xiong, K.; Liao, X.; Gasser, G.; Ji, L.; Chao, H. Photodecaging of a Mitochondria-Localized Iridium(III) Endoperoxide Complex for Two-Photon Photoactivated Therapy under Hypoxia. J. Am. Chem. Soc. 2022, 144, 4091–4101. [Google Scholar] [CrossRef]
- Lie, Q.; Jiang, H.; Lu, X.; Chen, Z.; Liang, J.; Zhang, Y.; Chao, H. Photo-Activated Ferrocene-Iridium(III) Prodrug Induces Immunogenic Cell Death in Melanoma Stem Cells. J. Med. Chem. 2025, 68, 8894–8906. [Google Scholar] [CrossRef]
- Shi, H.; Kasparkova, J.; Ponte, F.; Kostrhunova, H.; Clarkson, G.J.; Sicilia, E.; Brabec, V.; Sadler, P.J. Excited-State Cis and Trans Pt(IV) Diamine Anticancer Complexes. Inorg. Chem. 2025, 64, 11301–11311. [Google Scholar] [CrossRef]
- Shi, H.; Clarkson, G.J.; Sadler, P.J. Tuning the Phototherapeutic Activity of Pt(iv) Complexes for Bladder Cancer via Modification of Trans N -Heterocyclic Ligands. Inorg. Chem. Front. 2024, 11, 7898–7909. [Google Scholar] [CrossRef]
- McFarland, S.A.; Mandel, A.; Dumoulin-White, R.; Gasser, G. Metal-Based Photosensitizers for Photodynamic Therapy: The Future of Multimodal Oncology? Curr. Opin. Chem. Biol. 2020, 56, 23–27. [Google Scholar] [CrossRef]
- Monro, S.; Colón, K.L.; Yin, H.; Roque, J.; Konda, P.; Gujar, S.; Thummel, R.P.; Lilge, L.; Cameron, C.G.; McFarland, S.A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119, 797–828. [Google Scholar] [CrossRef]
- Kulkarni, G.S.; Lilge, L.; Nesbitt, M.; Dumoulin-White, R.J.; Mandel, A.; Jewett, M.A.S. A Phase 1b Clinical Study of Intravesical Photodynamic Therapy in Patients with Bacillus Calmette-Guérin–Unresponsive Non–Muscle-Invasive Bladder Cancer. Eur. Urol. Open Sci. 2022, 41, 105–111. [Google Scholar] [CrossRef]
- Liang, B.-F.; Jiang, S.; Zhi, Y.-S.; Pan, Z.-Y.; Su, X.-Q.; Gong, Q.; He, Z.-D.; Yao, D.-H.; He, L.; Li, C.-Y. Photoactivation of the CGAS-STING Pathway and Pyroptosis by an Endoplasmic Reticulum-Targeting Ruthenium(ii) Complex for Cancer Immunotherapy. Inorg. Chem. Front. 2025, 12, 2294–2302. [Google Scholar] [CrossRef]
- de la Torre-Rubio, E.; Arias-Pérez, M.; Gude, L.; Cuenca, T.; García-Iriepa, C.; Royo, E. Exploring the Impact of Phenanthroline-Based Glycoconjugated Ru(II) Polypyridyl Photosensitizers on Metastasis-Related Processes. Chem. A Eur. J. 2025, 31, e202501105. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.-L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef]
- Sanz-Villafruela, J.; Bermejo-Casadesus, C.; Zafon, E.; Martínez-Alonso, M.; Durá, G.; Heras, A.; Soriano-Díaz, I.; Giussani, A.; Ortí, E.; Tebar, F.; et al. Insights into the Anticancer Photodynamic Activity of Ir(III) and Ru(II) Polypyridyl Complexes Bearing β-Carboline Ligands. Eur. J. Med. Chem. 2024, 276, 116618. [Google Scholar] [CrossRef] [PubMed]
- Manduca, N.; Maccafeo, E.; De Maria, R.; Sistigu, A.; Musella, M. 3D Cancer Models: One Step Closer to in Vitro Human Studies. Front. Immunol. 2023, 14, 1175503. [Google Scholar] [CrossRef] [PubMed]
- Massoud, J.; Pinon, A.; Gallardo-Villagrán, M.; Paulus, L.; Ouk, C.; Carrion, C.; Antoun, S.; Diab-Assaf, M.; Therrien, B.; Liagre, B. A Combination of Ruthenium Complexes and Photosensitizers to Treat Colorectal Cancer. Inorganics 2023, 11, 451. [Google Scholar] [CrossRef]
- Ghaddar, S.; Pinon, A.; Gallardo-Villagran, M.; Massoud, J.; Ouk, C.; Carrion, C.; Diab-Assaf, M.; Therrien, B.; Liagre, B. Photodynamic Therapy against Colorectal Cancer Using Porphin-Loaded Arene Ruthenium Cages. Int. J. Mol. Sci. 2024, 25, 10847. [Google Scholar] [CrossRef]
- Yogo, T.; Urano, Y.; Ishitsuka, Y.; Maniwa, F.; Nagano, T. Highly Efficient and Photostable Photosensitizer Based on BODIPY Chromophore. J. Am. Chem. Soc. 2005, 127, 12162–12163. [Google Scholar] [CrossRef]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY Dyes in Photodynamic Therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Tatar, B.; Say, B.; Demirsoy, Z.; Boyacı, A.İ.; Gülseren, G.; Cakmak, Y. Design and Synthesis of Hydrazone-Substituted BODIPY Derivatives for Photodynamic Therapy. J. Photochem. Photobiol. A Chem. 2025, 469, 116592. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Zhou, S.; Gu, P.; Zhu, X.; Wang, C.; Zhang, Q. Evolution of BODIPY as Triplet Photosensitizers from Homogeneous to Heterogeneous: The Strategies of Functionalization to Various Forms and Their Recent Applications. Coord. Chem. Rev. 2023, 482, 215074. [Google Scholar] [CrossRef]
- Upadhyay, A.; Kundu, P.; Ramu, V.; Kondaiah, P.; Chakravarty, A.R. BODIPY-Tagged Platinum(II) Curcumin Complexes for Endoplasmic Reticulum-Targeted Red Light PDT. Inorg. Chem. 2022, 61, 1335–1348. [Google Scholar] [CrossRef]
- Liu, C.; Ji, X.; Yu, Z.; Zhang, S.; Zhang, R.; Zhao, W.; Dong, X. Discovery of Subcellular-Targeted Aza-BODIPY Photosensitizers for Efficient Photodynamic Antitumor Therapy. J. Med. Chem. 2023, 66, 7205–7220. [Google Scholar] [CrossRef]
- Bongo, A.M.; Shim, J.; Cho, S.; Lee, J.; Kim, H.-J. Lipophilic Quaternary Ammonium-Functionalized BODIPY Photosensitizers for Mitochondrial-Targeted Photodynamic Therapy and Fluorescence Cell Imaging. J. Photochem. Photobiol. A Chem. 2025, 468, 116469. [Google Scholar] [CrossRef]
- Gibbs, J.H.; Zhou, Z.; Kessel, D.; Fronczek, F.R.; Pakhomova, S.; Vicente, M.G.H. Synthesis, Spectroscopic, and in Vitro Investigations of 2,6-Diiodo-BODIPYs with PDT and Bioimaging Applications. J. Photochem. Photobiol. B Biol. 2015, 145, 35–47. [Google Scholar] [CrossRef]
- Wang, Z.; Hong, X.; Zong, S.; Tang, C.; Cui, Y.; Zheng, Q. BODIPY-Doped Silica Nanoparticles with Reduced Dye Leakage and Enhanced Singlet Oxygen Generation. Sci. Rep. 2015, 5, 12602. [Google Scholar] [CrossRef]
- Gorman, A.; Killoran, J.; O’Shea, C.; Kenna, T.; Gallagher, W.M.; O’Shea, D.F. In Vitro Demonstration of the Heavy-Atom Effect for Photodynamic Therapy. J. Am. Chem. Soc. 2004, 126, 10619–10631. [Google Scholar] [CrossRef]
- Wrochna, K.; Natkowski, D.R.; Blacha-Grzechnik, A.; Pluczyk-Malek, S.; Armstrong, C.B.; Mastroeni, P.J.; Black, D.J.; Pal, R.; Durka, K.; Marek-Urban, P.H.; et al. Heavy-Atom Free Bodipy-Borafluorene Photosensitizer Decorated with Coumarin Antenna Selectively Staining Endoplasmic Reticulum for Application in PDT. Chem. A Eur. J. 2025, 31, e01949. [Google Scholar] [CrossRef]
- Ksenofontova, K.V.; Ksenofontov, A.A.; Kerner, A.A.; Molchanov, E.E.; Gessel, T.V.; Galembikova, A.R.; Krestova, A.N.; Borisovskaya, E.P.; Khodov, I.A.; Boichuk, S.V. Spectral Properties and Anticancer Activity of Novel Cisplatin-BODIPY Conjugates. Opt. Mater. 2025, 159, 116680. [Google Scholar] [CrossRef]
- Wang, L.; Mei, A.; Li, N.; Ruan, X.; Sun, X.; Cai, Y.; Shao, J.; Dong, X. Aza-BODIPY Dye with Unexpected Bromination and High Singlet Oxygen Quantum Yield for Photoacoustic Imaging-Guided Synergetic Photodynamic/Photothermal Therapy. Chin. Chem. Lett. 2024, 35, 108974. [Google Scholar] [CrossRef]
- Dong, L.; Li, H.; Tian, Y.; Niu, L.-Y. Novel Phenothiazine-Based Amphiphilic Aza-BODIPYs for High-Efficient Photothermal Therapy. Dye. Pigment. 2023, 217, 111369. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Gao, H.; Tang, C.; Feng, Z.; Lin, L.; Che, S.; Luo, C.; Ding, D.; Zheng, D.; et al. Phototheranostic Nanoparticles with Aggregation-Induced Emission as a Four-Modal Imaging Platform for Image-Guided Photothermal Therapy and Ferroptosis of Tumor Cells. Biomaterials 2022, 289, 121779. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.; Wang, Z.; Hou, Y.; Zhao, J.; Elmali, A.; Escudero, D.; Karatay, A. Thienyl/Phenyl Bay-Substituted Perylenebisimides: Intersystem Crossing and Application as Heavy Atom-Free Triplet Photosensitizers. Dye. Pigment. 2021, 184, 108708. [Google Scholar] [CrossRef]
- Xiao, X.; Mu, T.; Sukhanov, A.A.; Zhou, Y.; Yu, P.; Yu, F.; Elmali, A.; Zhao, J.; Karatay, A.; Voronkova, V.K. The Effect of Thionation of the Carbonyl Group on the Photophysics of Compact Spiro Rhodamine-Naphthalimide Electron Donor–Acceptor Dyads: Intersystem Crossing, Charge Separation, and Electron Spin Dynamics. Phys. Chem. Chem. Phys. 2023, 25, 31667–31682. [Google Scholar] [CrossRef]
- Wang, P.; Dong, C.; Huang, Y.; Qin, Z.; Wu, Y.; Zhang, Y.; Zhang, B.; Shen, Y.; Zhao, J.; Yan, D.; et al. Design and Application of Multi-Rotor Aza-BODIPY for Enhanced Phototherapeutic Efficacy. Dye. Pigment. 2025, 243, 113098. [Google Scholar] [CrossRef]
- Zhang, X.; Sukhanov, A.A.; Liu, X.; Taddei, M.; Zhao, J.; Harriman, A.; Voronkova, V.K.; Wan, Y.; Dick, B.; Di Donato, M. Origin of Intersystem Crossing in Highly Distorted Organic Molecules: A Case Study with Red Light-Absorbing N,N,O,O-Boron-Chelated Bodipys. Chem. Sci. 2023, 14, 5014–5027. [Google Scholar] [CrossRef]
- Hu, M.; Dong, X.; Zhao, W. Novel Boron-Modified Aza-BODIPY Photosensitizers for Low-Dose Light-Dependent Anti-Cancer Photodynamic Therapy. Eur. J. Med. Chem. 2025, 297, 117934. [Google Scholar] [CrossRef]
- Niu, Y.; Ma, Q.; Li, Y.; Shen, Y.; Li, S.; Du, J.; Jiang, X.-D.; Qin, G. NIR-II Emitting B,O-Chelated Twisted Dye Aza-BODIPY for Phototherapy. Dye. Pigment. 2025, 240, 112855. [Google Scholar] [CrossRef]
- Paul, S.; Kundu, P.; Kondaiah, P.; Chakravarty, A.R. BODIPY-Ruthenium(II) Bis-Terpyridine Complexes for Cellular Imaging and Type-I/-II Photodynamic Therapy. Inorg. Chem. 2021, 60, 16178–16193. [Google Scholar] [CrossRef]
- Barretta, P.; Ponte, F.; Scoditti, S.; Vigna, V.; Mazzone, G.; Sicilia, E. Computational Analysis of the Behavior of BODIPY Decorated Monofunctional Platinum(II) Complexes in the Dark and under Light Irradiation. J. Phys. Chem. A 2022, 126, 7159–7167. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Li, Z. Recent Progress of Squaraine-Based Fluorescent Materials and Their Biomedical Applications. Symmetry 2022, 14, 966. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, X.; Kim, B.; Yao, S.; Bondar, M.V.; Belfield, K.D. Bovine Serum Albumin Nanoparticles with Fluorogenic Near-IR-Emitting Squaraine Dyes. ACS Appl. Mater. Interfaces 2013, 5, 8710–8717. [Google Scholar] [CrossRef] [PubMed]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine Dyes: A Mine of Molecular Materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Shafeekh, K.M.; Soumya, M.S.; Rahim, M.A.; Abraham, A.; Das, S. Synthesis and Characterization of Near-Infrared Absorbing Water Soluble Squaraines and Study of Their Photodynamic Effects in DLA Live Cells. Photochem. Photobiol. 2014, 90, 585–595. [Google Scholar] [CrossRef]
- Beverina, L.; Salice, P. Squaraine Compounds: Tailored Design and Synthesis towards a Variety of Material Science Applications. European J. Org. Chem. 2010, 2010, 1207–1225. [Google Scholar] [CrossRef]
- Ajayaghosh, A. Chemistry of Squaraine-Derived Materials: Near-IR Dyes, Low Band Gap Systems, and Cation Sensors. Acc. Chem. Res. 2005, 38, 449–459. [Google Scholar] [CrossRef]
- Park, Y.; Park, M.H.; Hyun, H. Tumor-Targeted Squaraine Dye for Near-Infrared Fluorescence-Guided Photodynamic Therapy. Int. J. Mol. Sci. 2024, 25, 3428. [Google Scholar] [CrossRef]
- Li, J.-H.; You, P.-D.; Lu, F.; Huang, J.-T.; Fu, J.-L.; Tang, H.-Y.; Zhou, C.-Q. Single Aromatics Sulfonamide Substituted Dibenzothiazole Squaraines for Tumor NIR Imaging and Efficient Photodynamic Therapy at Low Drug Dose. J. Photochem. Photobiol. B Biol. 2023, 240, 112653. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Pontremoli, C.; De Toni, S.; Bondar, K.; Galliano, S.; Paja, H.; Civalleri, B.; Fiorio Pla, A.; Barolo, C.; Bonino, F.; et al. Hafnium-Based Metal–Organic Framework Nanosystems Entrapping Squaraines for Efficient NIR-Responsive Photodynamic Therapy. ACS Appl. Mater. Interfaces 2025, 17, 524–536. [Google Scholar] [CrossRef]
- Gu, Z.; Tian, X.; Guang, S.; Wei, G.; Mao, Y.; Xu, H. POSS Engineering of Squaraine Nanoparticle with High Photothermal Conversion Efficiency for Photothermal Therapy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123576. [Google Scholar] [CrossRef]
- Arun, K.T.; Epe, B.; Ramaiah, D. Aggregation Behavior of Halogenated Squaraine Dyes in Buffer, Electrolytes, Organized Media, and DNA. J. Phys. Chem. B 2002, 106, 11622–11627. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Deng, W.; Wu, S.; Sun, P.; Liu, Y. Water-Soluble Polymer Brush-Substituted Squaraine NIR-II Dye for Efficient Photothermal Therapy. J. Mater. Chem. B 2023, 11, 4389–4395. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In Vivo Photodynamic Therapy Using Upconversion Nanoparticles as Remote-Controlled Nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef]
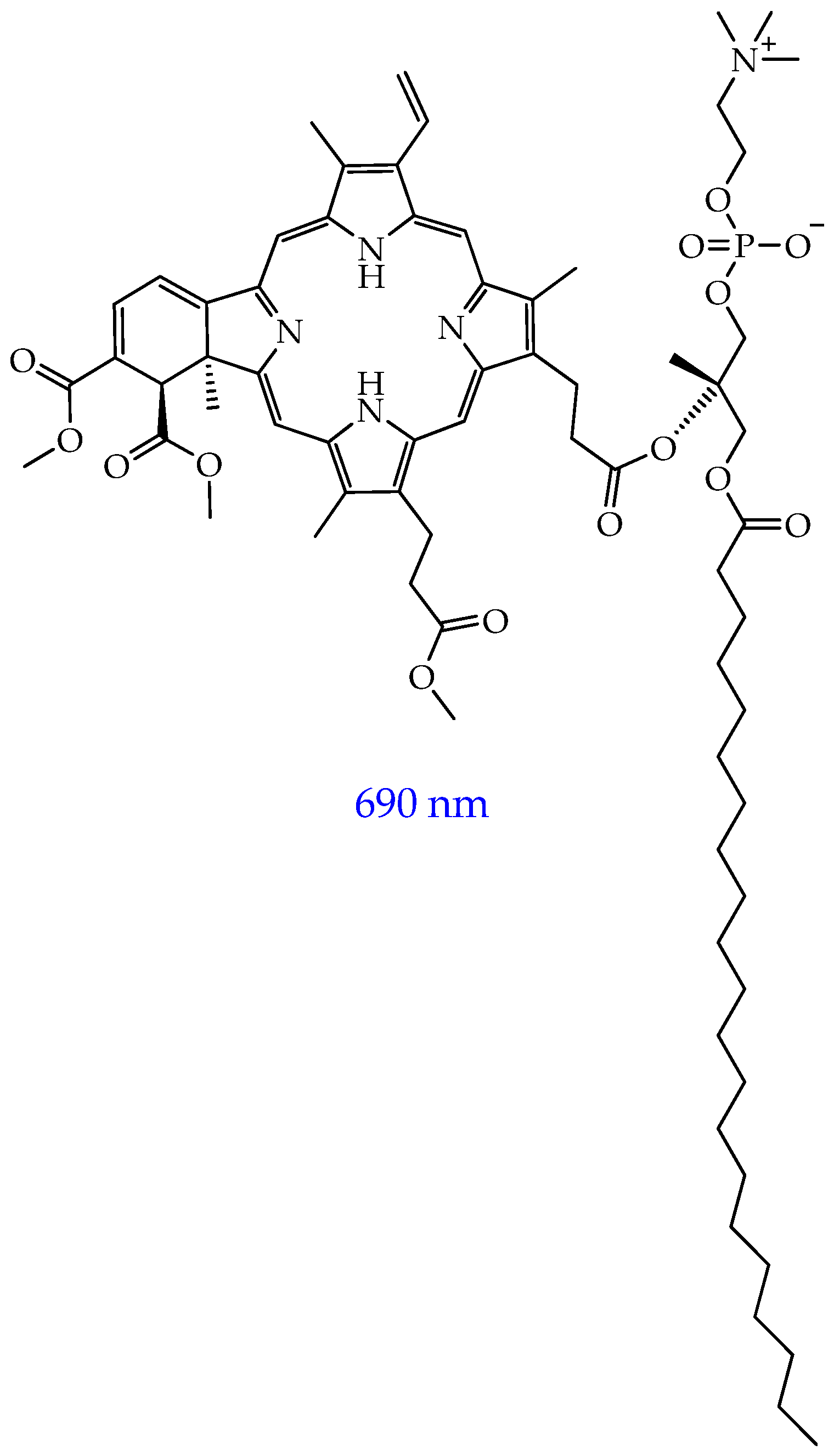
- Chkair, R.; Couvez, J.; Brégier, F.; Diab-Assaf, M.; Sol, V.; Blanchard-Desce, M.; Liagre, B.; Chemin, G. Activity of Hydrophilic, Biocompatible, Fluorescent, Organic Nanoparticles Functionalized with Purpurin-18 in Photodynamic Therapy for Colorectal Cancer. Nanomaterials 2024, 14, 1557. [Google Scholar] [CrossRef]
- Comincini, S.; Manai, F.; Sorrenti, M.; Perteghella, S.; D’Amato, C.; Miele, D.; Catenacci, L.; Bonferoni, M.C. Development of Berberine-Loaded Nanoparticles for Astrocytoma Cells Administration and Photodynamic Therapy Stimulation. Pharmaceutics 2023, 15, 1078. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Bai, H.; Zhang, J.; Wang, L.; Li, P.; Ge, Y.; Yang, H.; Wang, H.; Peng, B.; Hu, W.; et al. Albumins Constrainting the Conformation of Mitochondria-Targeted Photosensitizers for Tumor-Specific Photodynamic Therapy. Biomaterials 2025, 315, 122914. [Google Scholar] [CrossRef] [PubMed]
- Caverzan, M.D.; Morales Vasconsuelo, A.B.; Cerchia, L.; Palacios, R.E.; Chesta, C.A.; Ibarra, L.E. Preclinical Toxicological Characterization of Porphyrin-Doped Conjugated Polymer Nanoparticles for Photodynamic Therapy. Pharmaceutics 2025, 17, 593. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New Photodynamic Therapy with Next-Generation Photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef]
- Lee, Y.-E.K.; Kopelman, R. Polymeric Nanoparticles for Photodynamic Therapy. Methods Mol. Biol. 2011, 726, 151–178. [Google Scholar]
- Wen, J.; Yan, H.; Xia, P.; Xu, Y.; Li, H.; Sun, S. Mesoporous Silica Nanoparticles-Assisted Ruthenium(II) Complexes for Live Cell Staining. Sci. China Chem. 2017, 60, 799–805. [Google Scholar] [CrossRef]
- Zhao, T.; Nguyen, N.-T.; Xie, Y.; Sun, X.; Li, Q.; Li, X. Inorganic Nanocrystals Functionalized Mesoporous Silica Nanoparticles: Fabrication and Enhanced Bio-Applications. Front. Chem. 2017, 5, 118. [Google Scholar] [CrossRef] [PubMed]
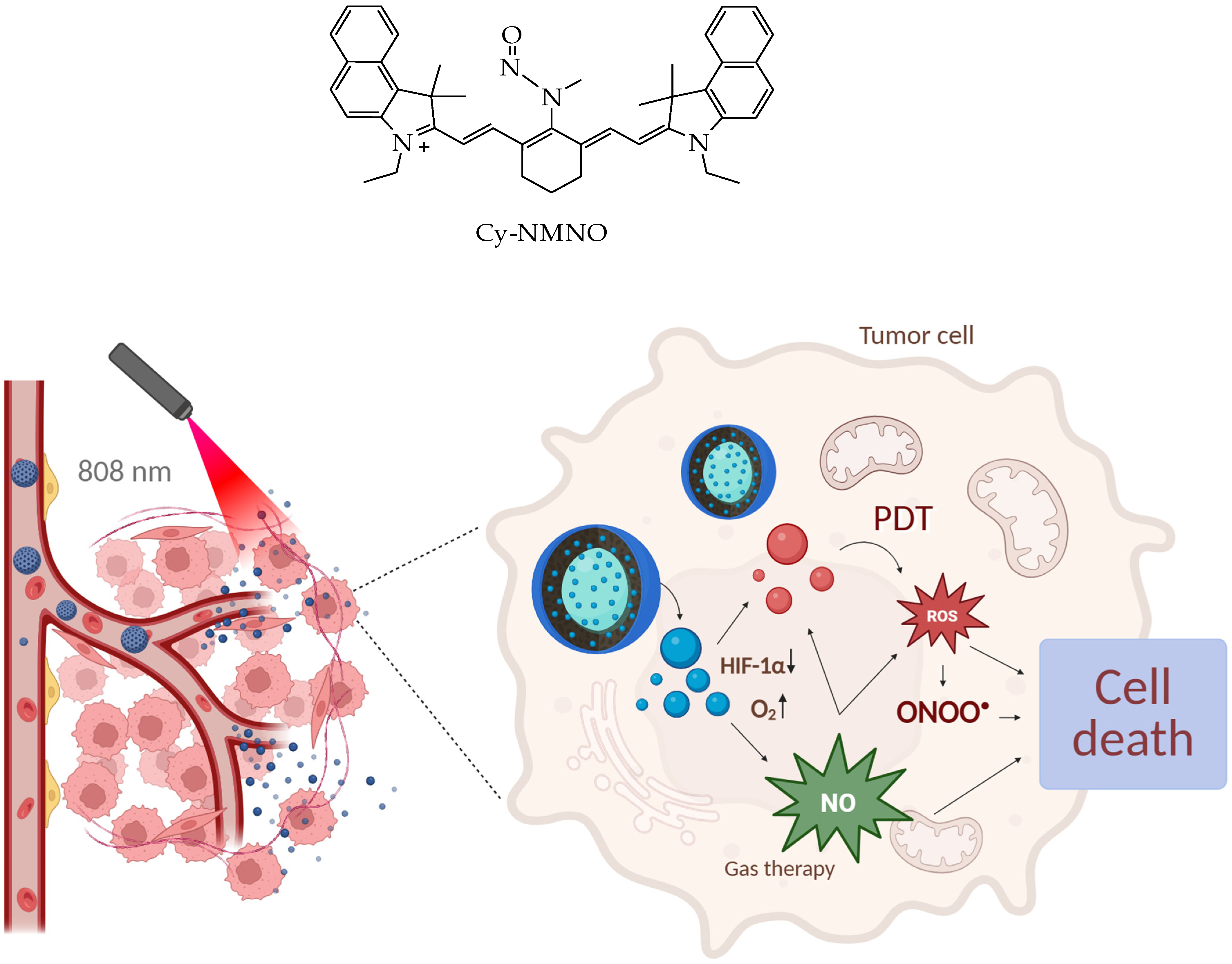
- Fu, L.; Huang, Y.; Shan, X.; Sun, X.; Wang, X.; Wang, X.; Chen, L.; Yu, S. NIR-Activatable Nitric Oxide Generator Based on Nanoparticles Loaded Small-Molecule Photosensitizers for Synergetic Photodynamic/Gas Therapy. J. Nanobiotechnology 2024, 22, 595. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Duan, X.; Cai, Y.; Zhang, F.; Jiang, S.; Han, S.; Shen, J.; Shuai, X. Multifunctional Nanoregulator Reshapes Immune Microenvironment and Enhances Immune Memory for Tumor Immunotherapy. Adv. Sci. 2019, 6, 1900037. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-Inducible Factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef]
- Semenza, G.L. Targeting HIF-1 for Cancer Therapy. Nat. Rev. Cancer 2003, 3, 721–732. [Google Scholar] [CrossRef]
- Xing, C.; Deng, J.; Fu, W.; Li, J.; Xu, L.; Sun, R.; Wang, D.; Li, C.; Liang, K.; Gao, M.; et al. Interfacially Super-Assembled Benzimidazole Derivative-Based Mesoporous Silica Nanoprobe for Sensitive Copper (II) Detection and Biosensing in Living Cells. Chem. A Eur. J. 2022, 28, e202103642. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.-G.; Li, C.; Hou, X.-L.; Cheng, K.; Zhang, B.; Zhang, R.-Y.; Li, D.-Y.; Liu, S.-J.; Liu, B.; et al. A PH/Ultrasound Dual-Response Biomimetic Nanoplatform for Nitric Oxide Gas-Sonodynamic Combined Therapy and Repeated Ultrasound for Relieving Hypoxia. Biomaterials 2020, 230, 119636. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, S.; Shen, Z.; Jin, Q.; Fu, G.; Ji, J. Nitric Oxide as an All-Rounder for Enhanced Photodynamic Therapy: Hypoxia Relief, Glutathione Depletion and Reactive Nitrogen Species Generation. Biomaterials 2018, 187, 55–65. [Google Scholar] [CrossRef]
- Khan, F.H.; Dervan, E.; Bhattacharyya, D.D.; McAuliffe, J.D.; Miranda, K.M.; Glynn, S.A. The Role of Nitric Oxide in Cancer: Master Regulator or NOt? Int. J. Mol. Sci. 2020, 21, 9393. [Google Scholar] [CrossRef]
- Bakay, E.; Pamukçu, A.; Sen Karaman, D.; Topaloğlu, N. Apoptosis-Inducing Photodynamic Therapy via Dual-Wavelength Responsive ICG/Ce6 Incorporated Mesoporous Silica Nanoparticles in Prostate Cancer Cells. Photochem. Photobiol. Sci. 2025, 24, 1243–1264. [Google Scholar] [CrossRef]
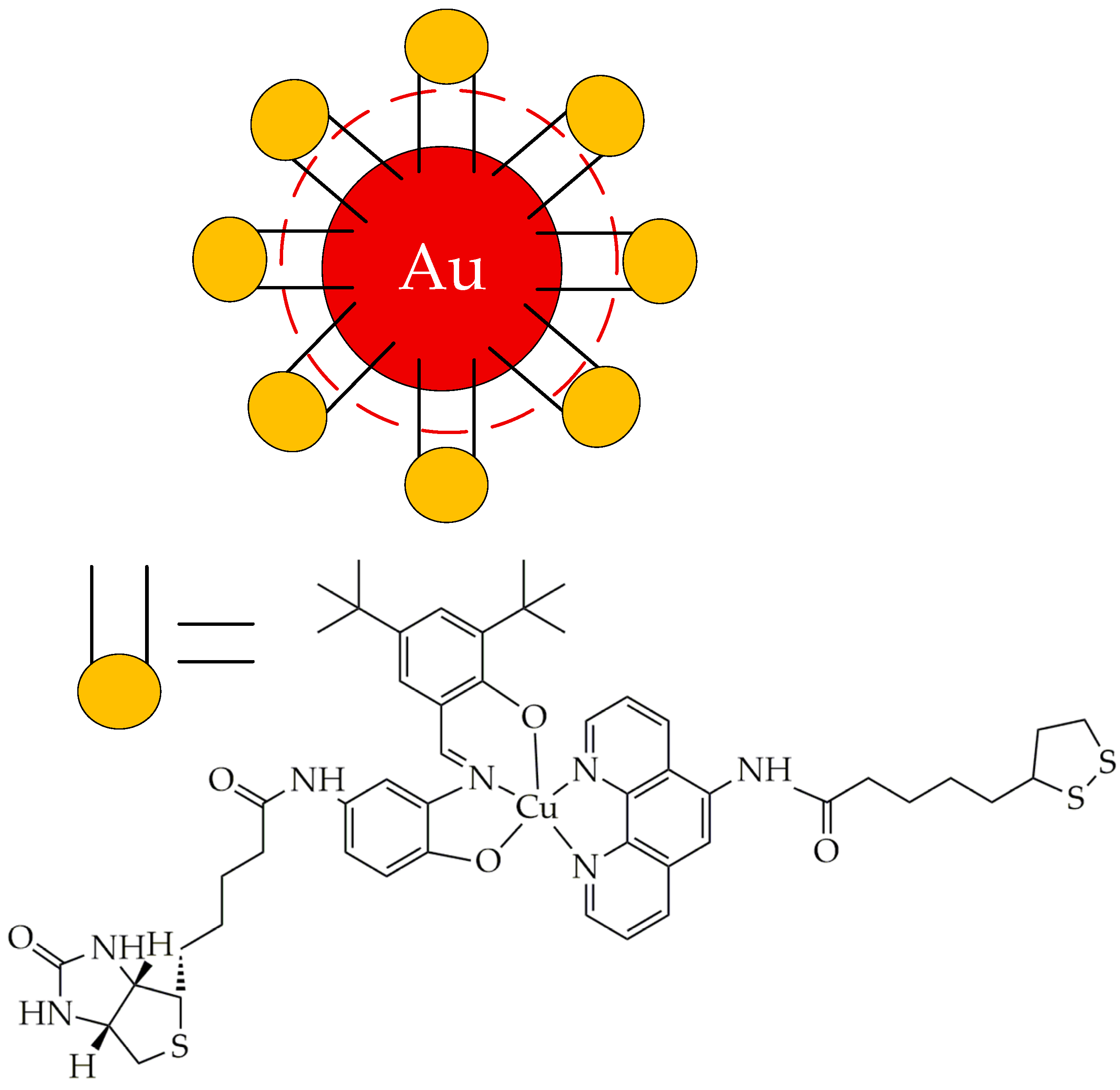
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall Gold Nanoparticles in Cancer Diagnosis and Therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Quintana, C.; Cifuentes, M.P.; Humphrey, M.G. Transition Metal Complex/Gold Nanoparticle Hybrid Materials. Chem. Soc. Rev. 2020, 49, 2316–2341. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic Nanoshells: From Probe Design to Imaging and Treatment of Cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold Nanocages: From Synthesis to Theranostic Applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef]
- Musib, D.; Upadhyay, A.; Pal, M.; Raza, M.K.; Saha, I.; Kunwar, A.; Roy, M. Red Light-Activable Biotinylated Copper(II) Complex-Functionalized Gold Nanocomposite (Biotin-Cu@AuNP) towards Targeted Photodynamic Therapy. J. Inorg. Biochem. 2023, 243, 112183. [Google Scholar] [CrossRef]
- Dasri, T.; Chingsungnoen, A. Surface Plasmon Resonance Enhanced Light Absorption and Wavelength Tuneable in Gold-Coated Iron Oxide Spherical Nanoparticle. J. Magn. Magn. Mater. 2018, 456, 368–371. [Google Scholar] [CrossRef]
- Wolińska, A.; Drozd, M.; Buchalska, B.; Dybko, A.; Grabowska-Jadach, I. Au@Fe3O4@PEG Nanocubes as Photoactive Agents in Photothermal Therapy: An in Vitro Study. Biomed. Pharmacother. 2025, 187, 118051. [Google Scholar] [CrossRef]
- Balfourier, A.; Kolosnjaj-Tabi, J.; Luciani, N.; Carn, F.; Gazeau, F. Gold-Based Therapy: From Past to Present. Proc. Natl. Acad. Sci. USA 2020, 117, 22639–22648. [Google Scholar] [CrossRef]
- Higbee-Dempsey, E.M.; Amirshaghaghi, A.; Case, M.J.; Bouché, M.; Kim, J.; Cormode, D.P.; Tsourkas, A. Biodegradable Gold Nanoclusters with Improved Excretion Due to PH-Triggered Hydrophobic-to-Hydrophilic Transition. J. Am. Chem. Soc. 2020, 142, 7783–7794. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, Y.; Chen, Z.; Geng, Y.; Xie, X.; Shen, X.; Li, T.; Li, S.; Wu, C.; Liu, Y. Chemo-Photodynamic Combined Gene Therapy and Dual-Modal Cancer Imaging Achieved by PH-Responsive Alginate/Chitosan Multilayer-Modified Magnetic Mesoporous Silica Nanocomposites. Biomater. Sci. 2017, 5, 1001–1013, Erratum in Biomater. Sci. 2022, 11, 341. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Nie, W.; He, C.; Zhou, X.; Chen, L.; Qiu, K.; Wang, W.; Yin, Z. Effect of PH-Responsive Alginate/Chitosan Multilayers Coating on Delivery Efficiency, Cellular Uptake and Biodistribution of Mesoporous Silica Nanoparticles Based Nanocarriers. ACS Appl. Mater. Interfaces 2014, 6, 8447–8460. [Google Scholar] [CrossRef] [PubMed]
- Derycke, A. Liposomes for Photodynamic Therapy. Adv. Drug Deliv. Rev. 2004, 56, 17–30. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Azzazy, H.M.E.-S.; Schaefer, J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. [Google Scholar] [CrossRef]
- de Jonge, M.J.A.; Slingerland, M.; Loos, W.J.; Wiemer, E.A.C.; Burger, H.; Mathijssen, R.H.J.; Kroep, J.R.; den Hollander, M.A.G.; van der Biessen, D.; Lam, M.-H.; et al. Early Cessation of the Clinical Development of LiPlaCis, a Liposomal Cisplatin Formulation. Eur. J. Cancer 2010, 46, 3016–3021. [Google Scholar] [CrossRef]
- Araújo, J.G.C.; Mota, L.d.G.; Leite, E.A.; Maroni, L.d.C.; Wainstein, A.J.A.; Coelho, L.G.V.; Savassi-Rocha, P.R.; Pereira, M.T.; de Carvalho, A.T.; Cardoso, V.N.; et al. Biodistribution and Antitumoral Effect of Long-Circulating and PH-Sensitive Liposomal Cisplatin Administered in Ehrlich Tumor-Bearing Mice. Exp. Biol. Med. 2011, 236, 808–815. [Google Scholar] [CrossRef]
- Dunne, M.; Dou, Y.N.; Drake, D.M.; Spence, T.; Gontijo, S.M.L.; Wells, P.G.; Allen, C. Hyperthermia-Mediated Drug Delivery Induces Biological Effects at the Tumor and Molecular Levels That Improve Cisplatin Efficacy in Triple Negative Breast Cancer. J. Control. Release 2018, 282, 35–45. [Google Scholar] [CrossRef]
- Dou, Y.; Hynynen, K.; Allen, C. To Heat or Not to Heat: Challenges with Clinical Translation of Thermosensitive Liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef]
- Tak, W.Y.; Lin, S.-M.; Wang, Y.; Zheng, J.; Vecchione, A.; Park, S.Y.; Chen, M.H.; Wong, S.; Xu, R.; Peng, C.-Y.; et al. Phase III HEAT Study Adding Lyso-Thermosensitive Liposomal Doxorubicin to Radiofrequency Ablation in Patients with Unresectable Hepatocellular Carcinoma Lesions. Clin. Cancer Res. 2018, 24, 73–83. [Google Scholar] [CrossRef]
- Mellish, K.J.; Brown, S.B. Verteporfin: A Milestone in Opthalmology and Photodynamic Therapy. Expert Opin. Pharmacother. 2001, 2, 351–361. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Telnova, V.; Vodovozova, E.; Alekseeva, A.; Boldyrev, I.; Fedosov, I.; Dubrovsky, A.; Khorovodov, A.; et al. Intranasal Delivery of Liposomes to Glioblastoma by Photostimulation of the Lymphatic System. Pharmaceutics 2022, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.P.; Runyan, J.; Birch, G.; Ward, J.A.; Sen, S.E.; Feller, S.E.; Wassall, S.R. The Effect of Trans Unsaturation on Molecular Organization in a Phospholipid Membrane. Biophys. J. 2009, 96, 608a. [Google Scholar] [CrossRef]
- Siriwardane, D.A.; Jiang, W.; Mudalige, T. Profiling In-Vitro Release of Verteporfin from VISUDYNE® Liposomal Formulation and Investigating Verteporfin Binding to Human Serum Albumin. Int. J. Pharm. 2023, 646, 123449. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, K.; Yamamoto, M.; Suzuki, S.; Sanomachi, T.; Togashi, K.; Seino, S.; Kitanaka, C.; Okada, M. Verteporfin Inhibits Oxidative Phosphorylation and Induces Cell Death Specifically in Glioma Stem Cells. FEBS J. 2020, 287, 2023–2036. [Google Scholar] [CrossRef]
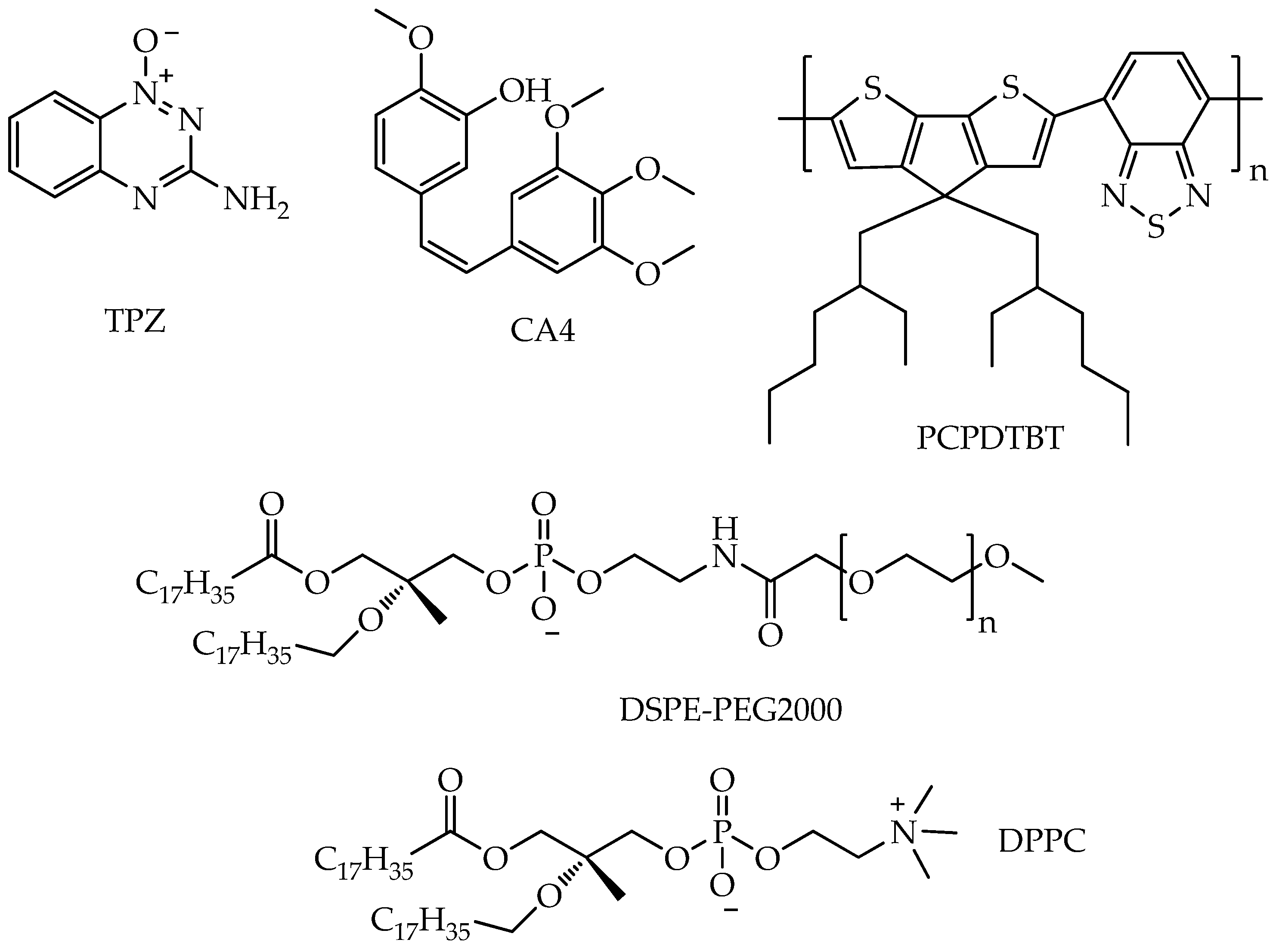
- Yu, N.; Zhou, J.; Xu, H.; Wang, F.; Wang, X.; Tang, L.; Li, J.; Wang, X.; Lu, X. Near-Infrared Photoactivatable Three-in-One Nanoagents to Aggravate Hypoxia and Enable Amplified Photo-Chemotherapy. Biomater. Adv. 2024, 163, 213962. [Google Scholar] [CrossRef]
- Bhandari, C.; Moffat, A.; Shah, N.; Khan, A.; Quaye, M.; Fakhry, J.; Soma, S.; Nguyen, A.; Eroy, M.; Malkoochi, A.; et al. PD-L1 Immune Checkpoint Targeted Photoactivable Liposomes (ITPALs) Prime the Stroma of Pancreatic Tumors and Promote Self-Delivery. Adv. Healthc. Mater. 2024, 13, 2304340. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, J.-E.; Lee, J.-S.; Park, J.; Oh, C.; Lee, S.; Yu, J.; Zhang, W.; Im, H.-J. Development of Indocyanine Green/Methyl-β-Cyclodextrin Complex-Loaded Liposomes for Enhanced Photothermal Cancer Therapy. ACS Appl. Mater. Interfaces 2024, 16, 32945–32956. [Google Scholar] [CrossRef]
- Zenga, J.; Awan, M.; Hadi Razeghi Kondelaji, M.; Hansen, C.; Shafiee, S.; Frei, A.; Foeckler, J.; Kuehn, R.; Bruening, J.; Massey, B.; et al. Photoactivated HPPH-Liposomal Therapy for the Treatment of HPV-Negative Head and Neck Cancer. Oral Oncol. 2023, 144, 106487. [Google Scholar] [CrossRef]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating Cell Lines as Tumour Models by Comparison of Genomic Profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Le Clainche, T.; Abdelhamid, A.G.A.; Carigga Gutierrez, N.M.; Jourdain, M.-A.; Leo, S.; Sancey, L.; Hurbin, A.; Coll, J.-L.; Elena-Herrmann, B.; Broekgaarden, M. Photodynamic Drug Delivery for Cancer Therapy: Designing Liposomes for Light-Controlled Release and Enhanced Drug Efficacy. Eur. J. Pharm. Sci. 2025, 213, 107221. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Hill, A.F. Therapeutically Harnessing Extracellular Vesicles. Nat. Rev. Drug Discov. 2022, 21, 379–399. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fu, S.; Xiao, H.; Du, J.; Cheng, F.; Wan, S.; Zhu, H.; Li, D.; Peng, F.; Ding, X.; et al. Advances in Extracellular Vesicle Nanotechnology for Precision Theranostics. Adv. Sci. 2023, 10, 2204814. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular Vesicles in Cancer Nanomedicine. Semin. Cancer Biol. 2021, 69, 212–225. [Google Scholar] [CrossRef]
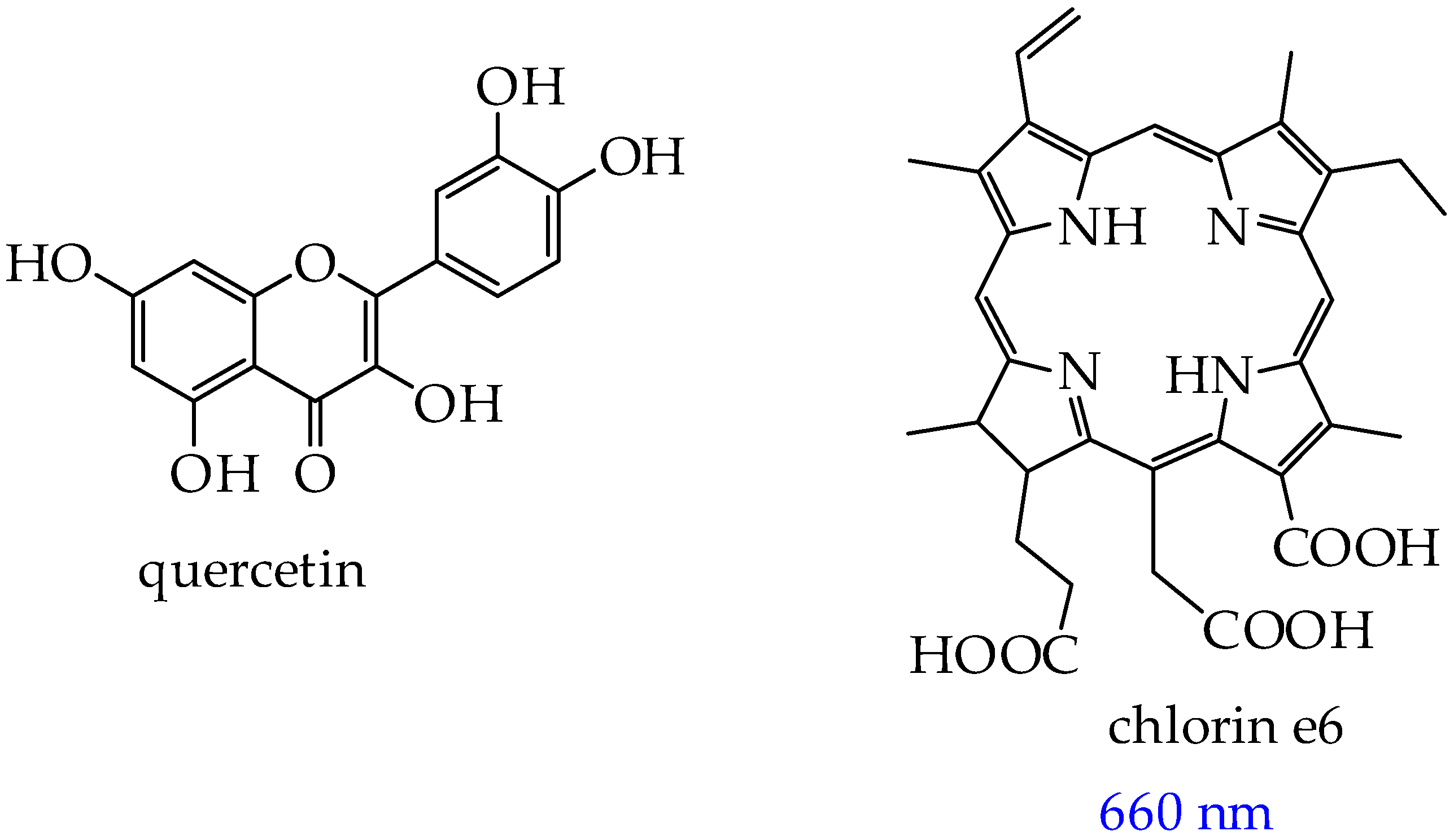
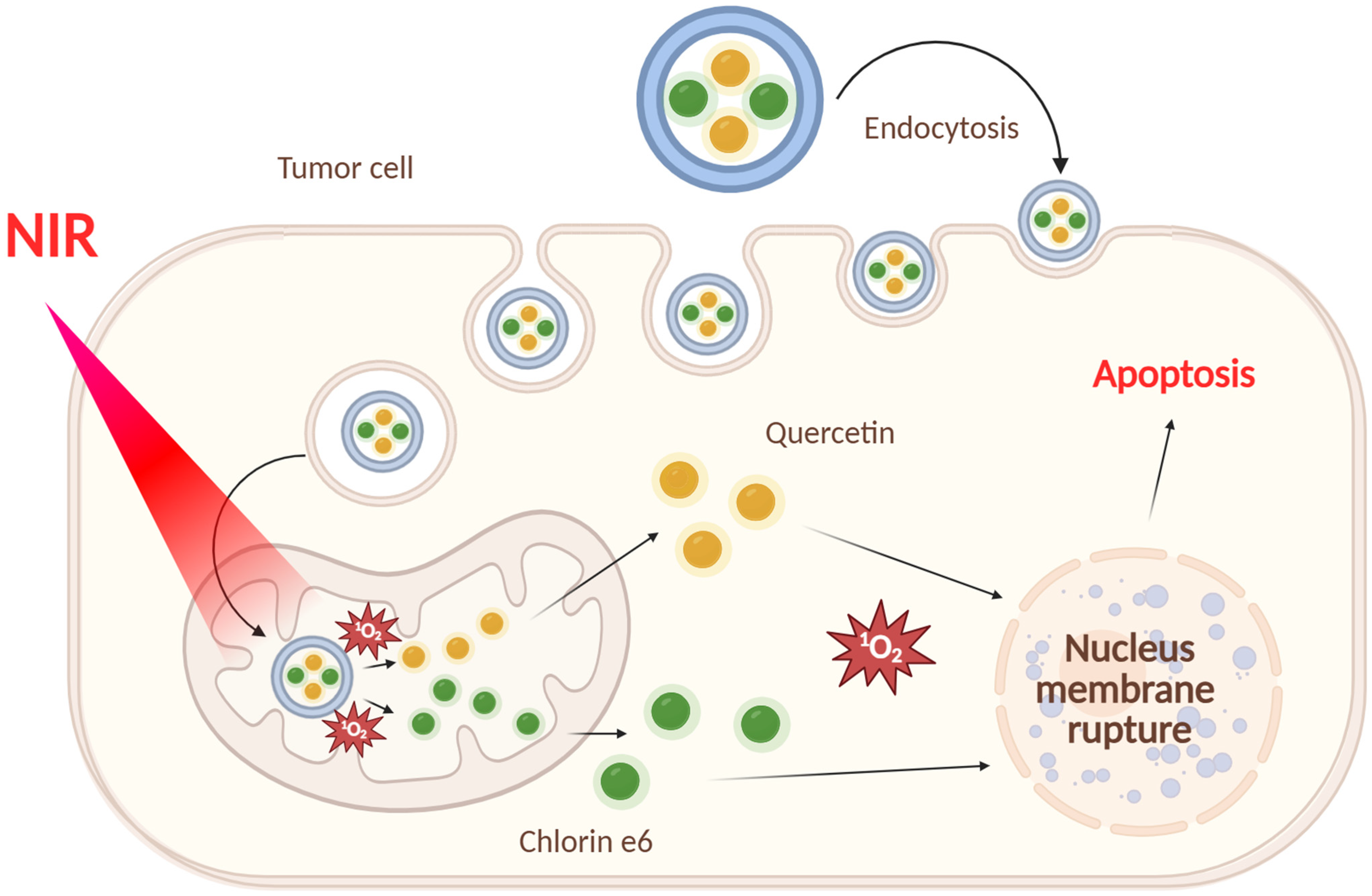
- Liu, Q.; Jiang, D.; Zhang, S.; Ru, Y.; Li, J.; Guo, P.; Jiao, W.; Miao, J.; Sun, L.; Chen, M.; et al. Light-Activated Photosensitizer/Quercetin Co-Loaded Extracellular Vesicles for Precise Oral Squamous Cell Carcinoma Therapy. Int. J. Pharm. 2025, 671, 125224. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent Advances in Extracellular Vesicles for Therapeutic Cargo Delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
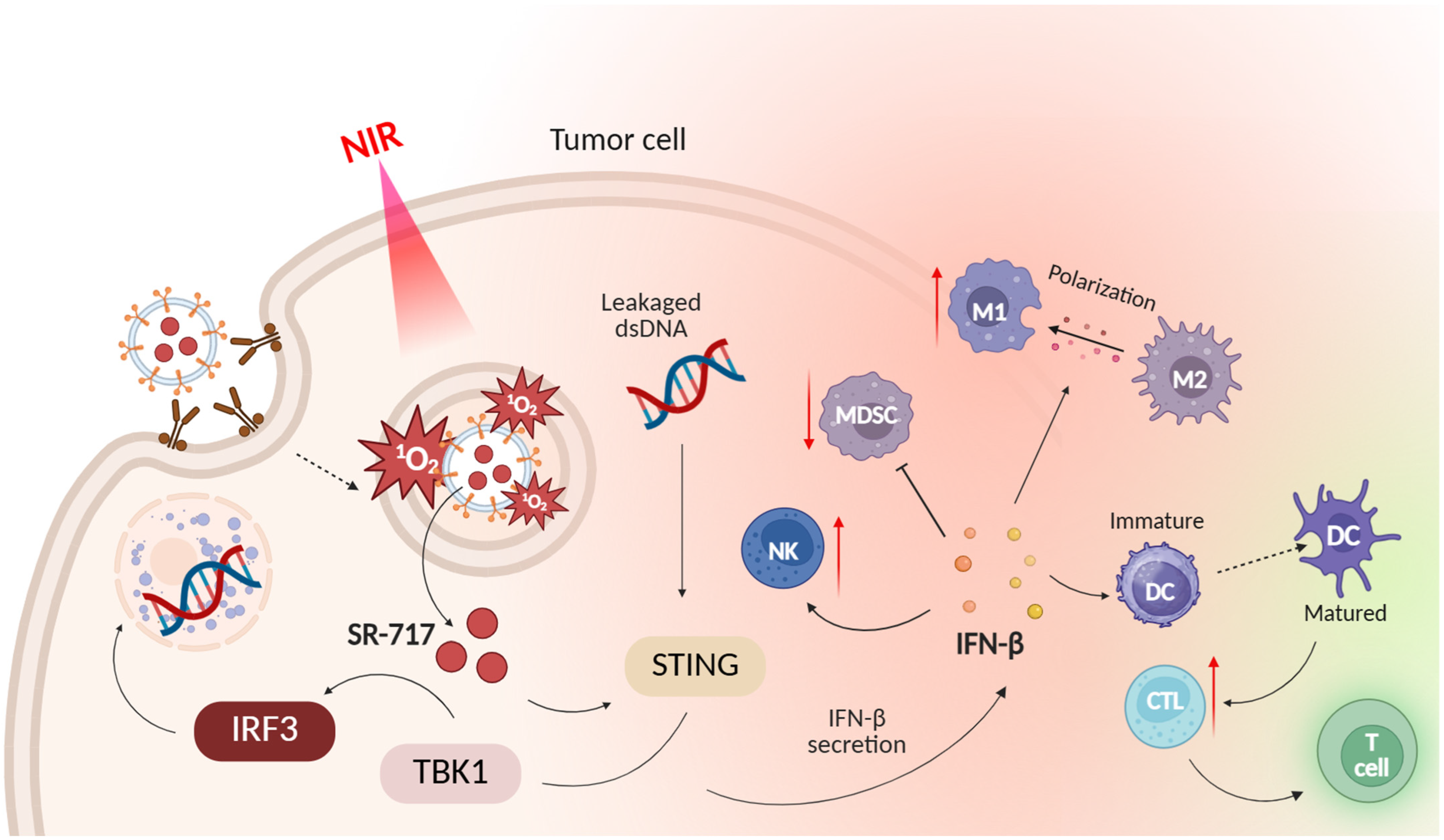
- Nguyen, V.; Nguyen Cao, T.G.; Jeong, H.; Truong Hoang, Q.; Pham, B.T.T.; Bang, J.; Koh, C.W.; Kang, J.H.; Lee, J.H.; Wu, X.; et al. Tumor-Targeted Exosome-Based Heavy Atom-Free Nanosensitizers With Long-Lived Excited States for Safe and Effective Sono-Photodynamic Therapy of Solid Tumors. Adv. Healthc. Mater. 2025, 14, 2570106. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Yang, Z.; Kang, L.; Ma, Z.; Luo, J.; Fan, Z.; Tian, X.; Deng, Y.; Ke, H.; et al. J-Aggregated Indocyanine Green-Loaded Exosomes Enable Photoactivatable Cytoplasmic Delivery of STING Agonist for Targeted Pancreatic Cancer Immunotherapy. Nano Today 2025, 62, 102727. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Xu, A.; Kang, L.; Ma, Z.; Liu, F.; Yang, T.; Sun, P.; Tang, Y. Synergistic Phototherapy-Molecular Targeted Therapy Combined with Tumor Exosome Nanoparticles for Oral Squamous Cell Carcinoma Treatment. Pharmaceutics 2023, 16, 33. [Google Scholar] [CrossRef]
- Guo, Z.; Li, G.; Shen, L.; Pan, J.; Dou, D.; Gong, Y.; Shi, W.; Sun, Y.; Zhang, Y.; Ma, K.; et al. Ginger-Derived Exosome-Like Nanoparticles Loaded With Indocyanine Green Enhances Phototherapy Efficacy for Breast Cancer. Int. J. Nanomed. 2025, 20, 1147–1169. [Google Scholar] [CrossRef]
- Ma, X.; Chen, N.; Zeng, P.; He, Y.; Zhang, T.; Lu, Y.; Li, Z.; Xu, J.; You, J.; Zheng, Y.; et al. Hypericum Perforatum-Derived Exosomes-Like Nanovesicles: A Novel Natural Photosensitizer for Effective Tumor Photodynamic Therapy. Int. J. Nanomed. 2025, 20, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-Lipid-PEG Hybrid Nanoparticles as Photosensitizer Carrier for Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2017, 173, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Yang, S.-B.; Park, S.J.; Kweon, S.; Ma, G.; Seo, M.; Kim, H.R.; Kang, T.-B.; Lim, J.-H.; Park, J. Cell-Penetrating Peptide Like Anti-Programmed Cell Death-Ligand 1 Peptide Conjugate-Based Self-Assembled Nanoparticles for Immunogenic Photodynamic Therapy. ACS Nano 2025, 19, 2870–2889. [Google Scholar] [CrossRef]
- Kachynski, A.V.; Pliss, A.; Kuzmin, A.N.; Ohulchanskyy, T.Y.; Baev, A.; Qu, J.; Prasad, P.N. Photodynamic Therapy by in Situ Nonlinear Photon Conversion. Nat. Photonics 2014, 8, 455–461. [Google Scholar] [CrossRef]

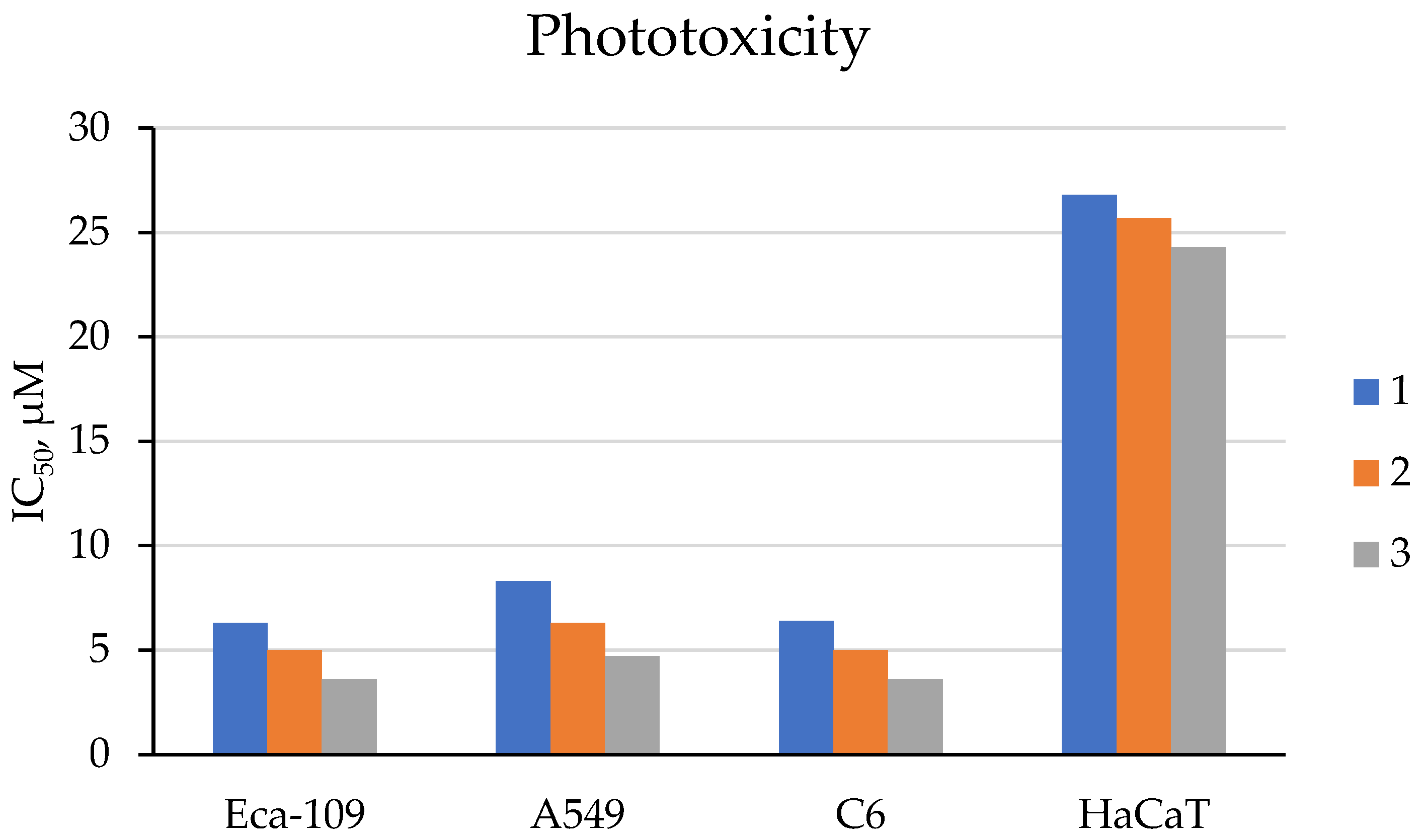

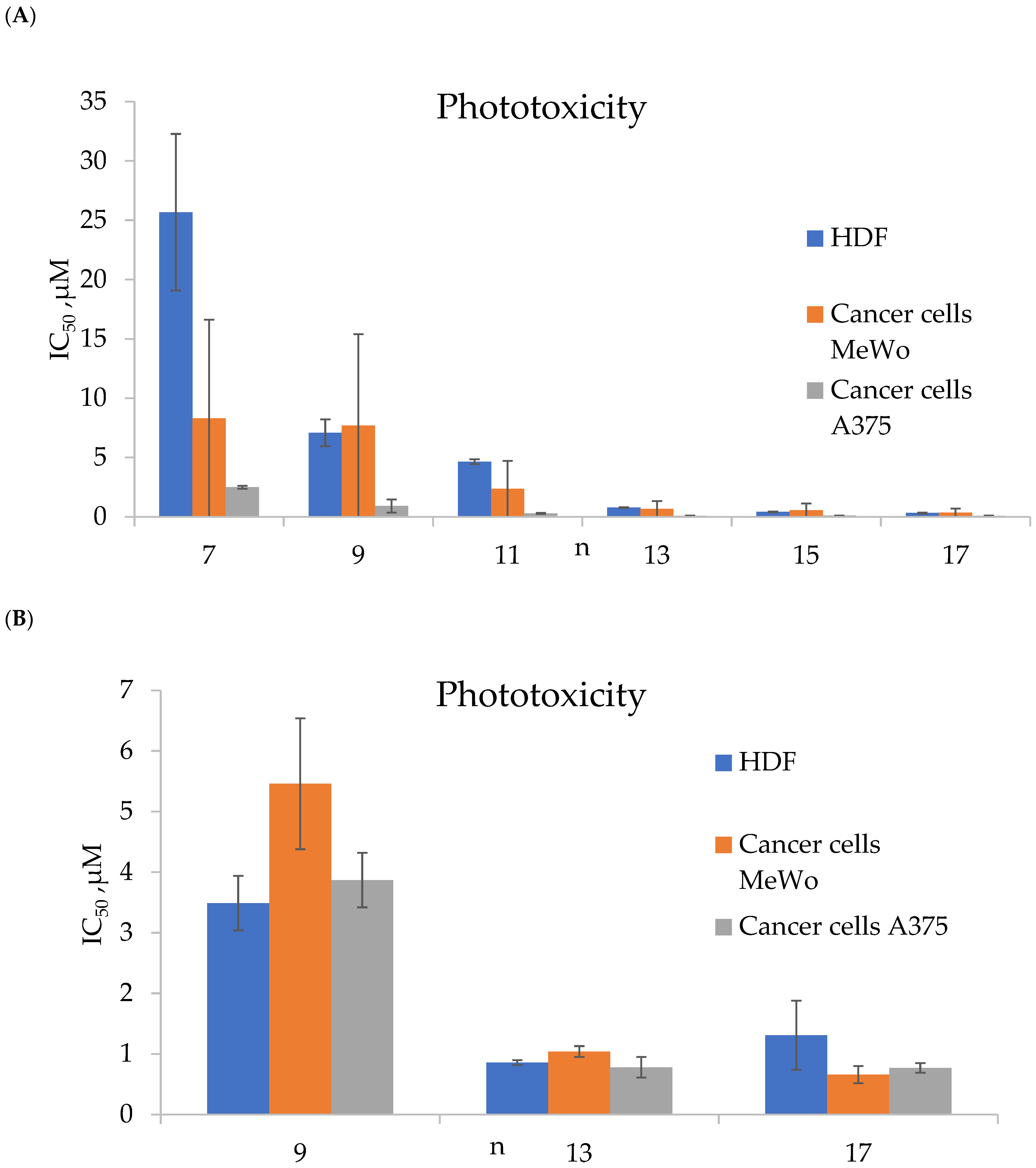
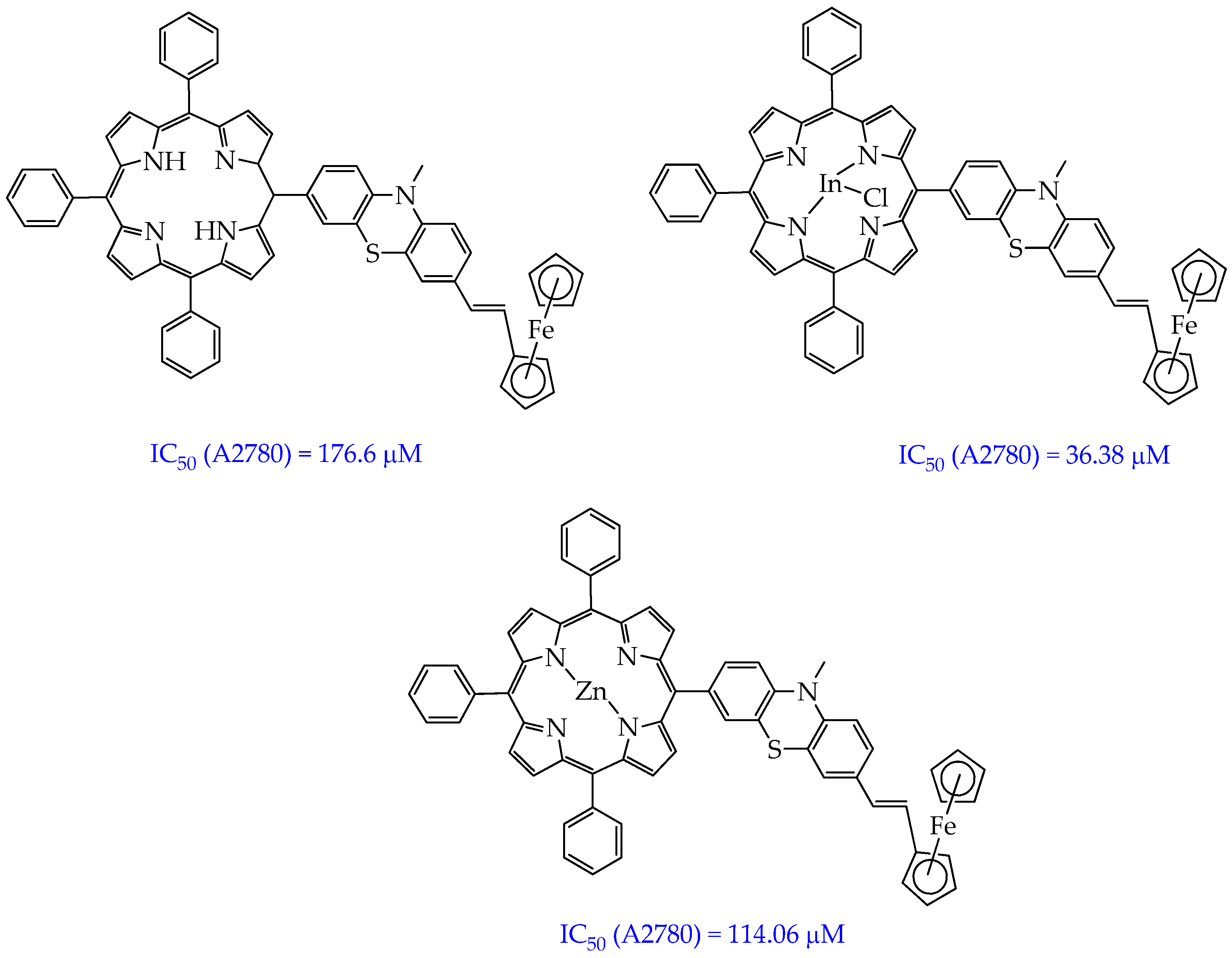
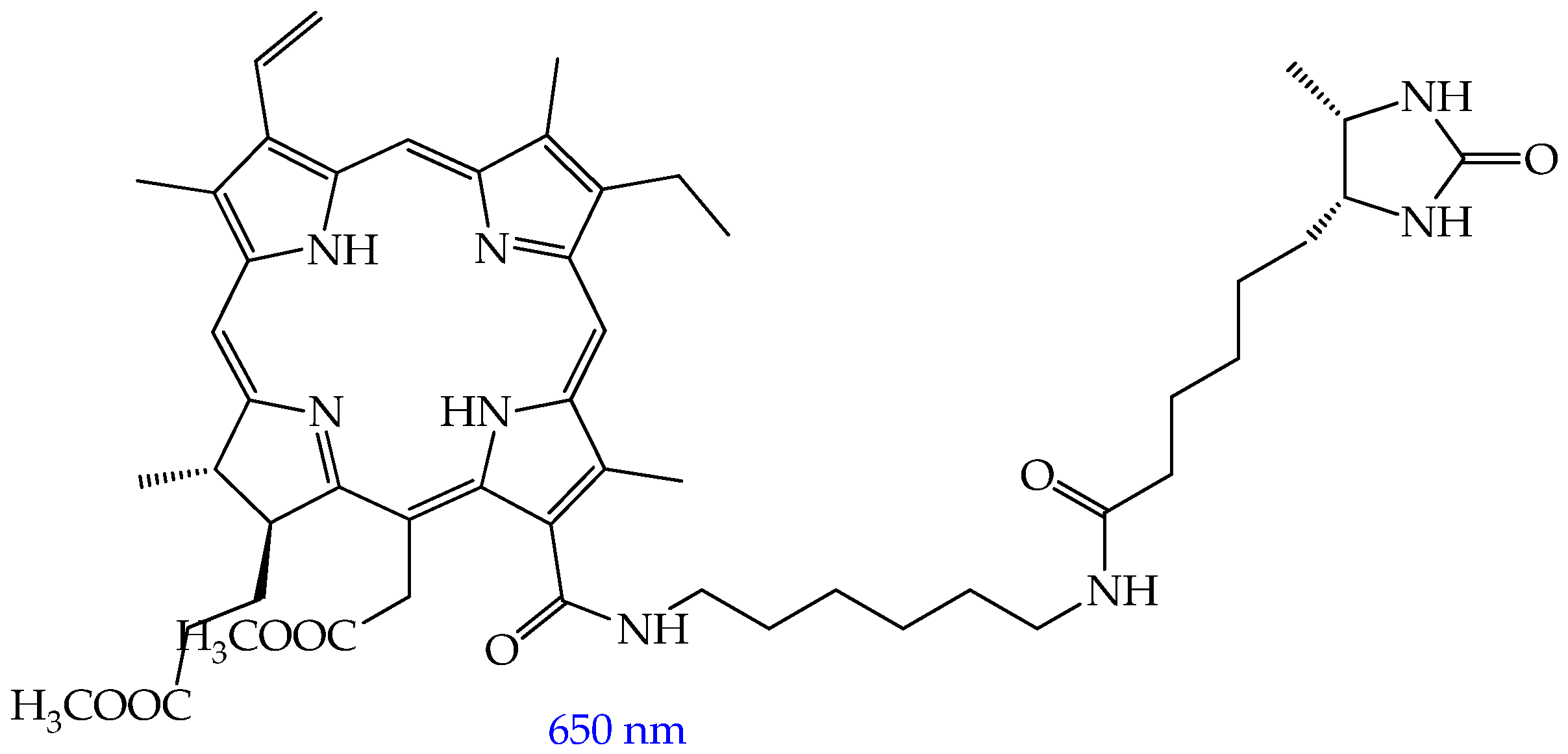




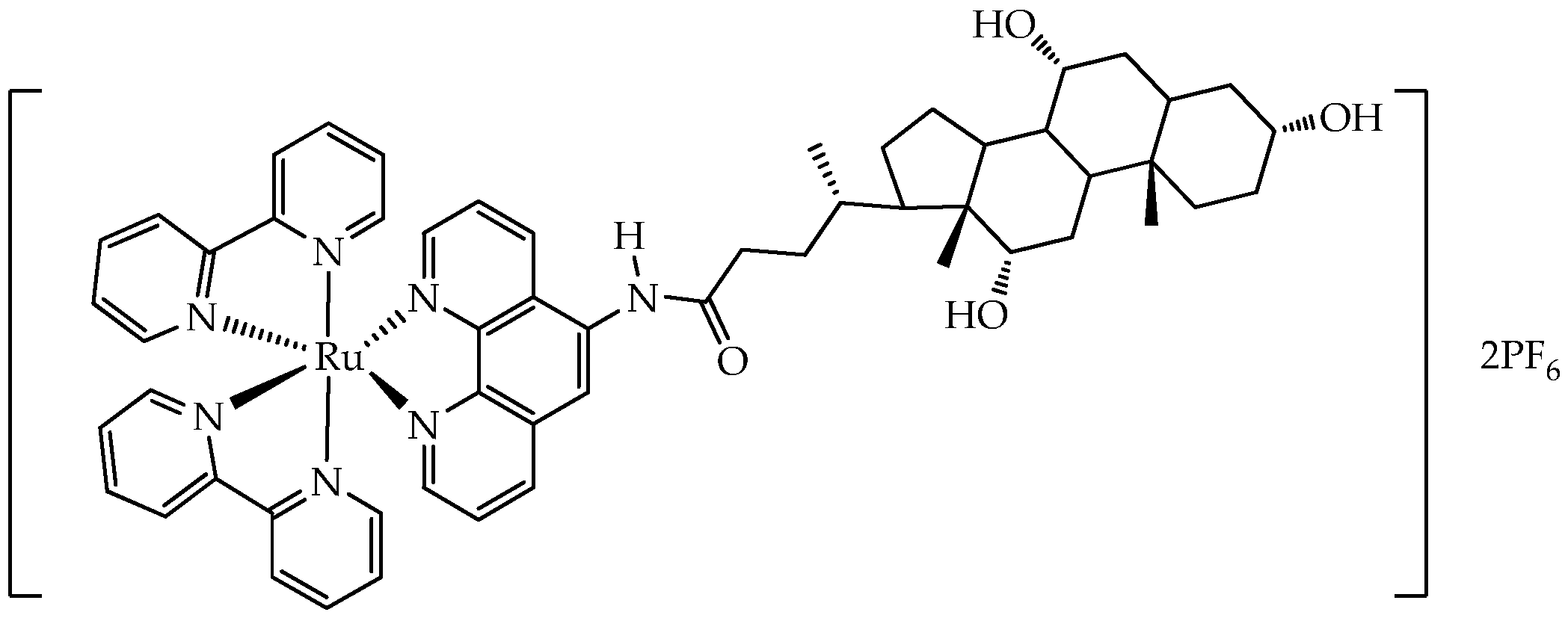
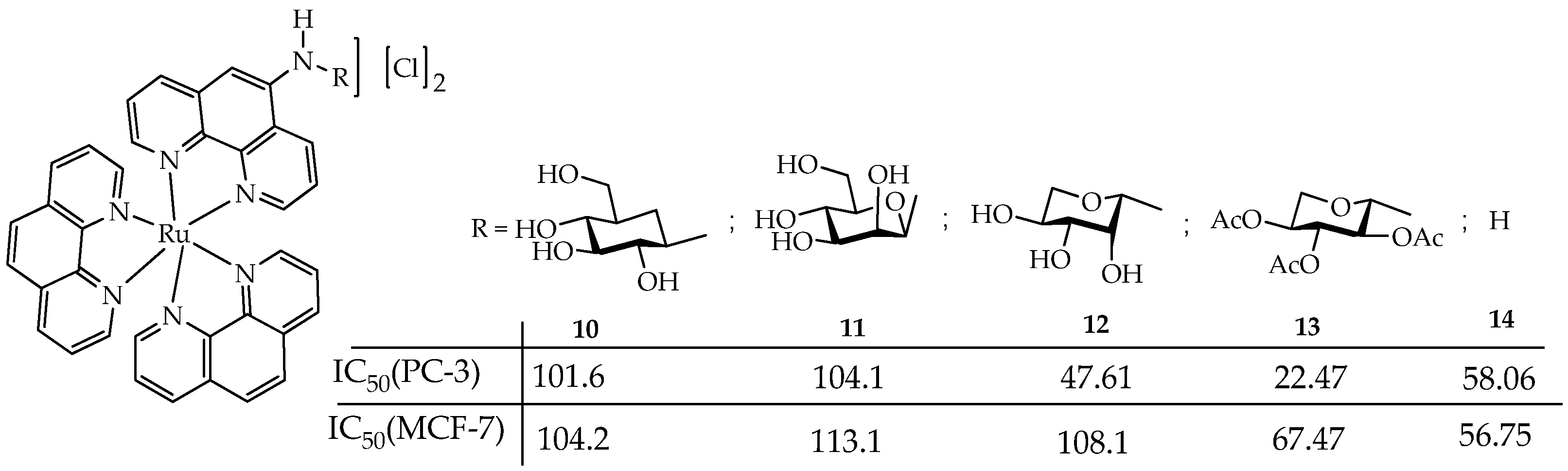
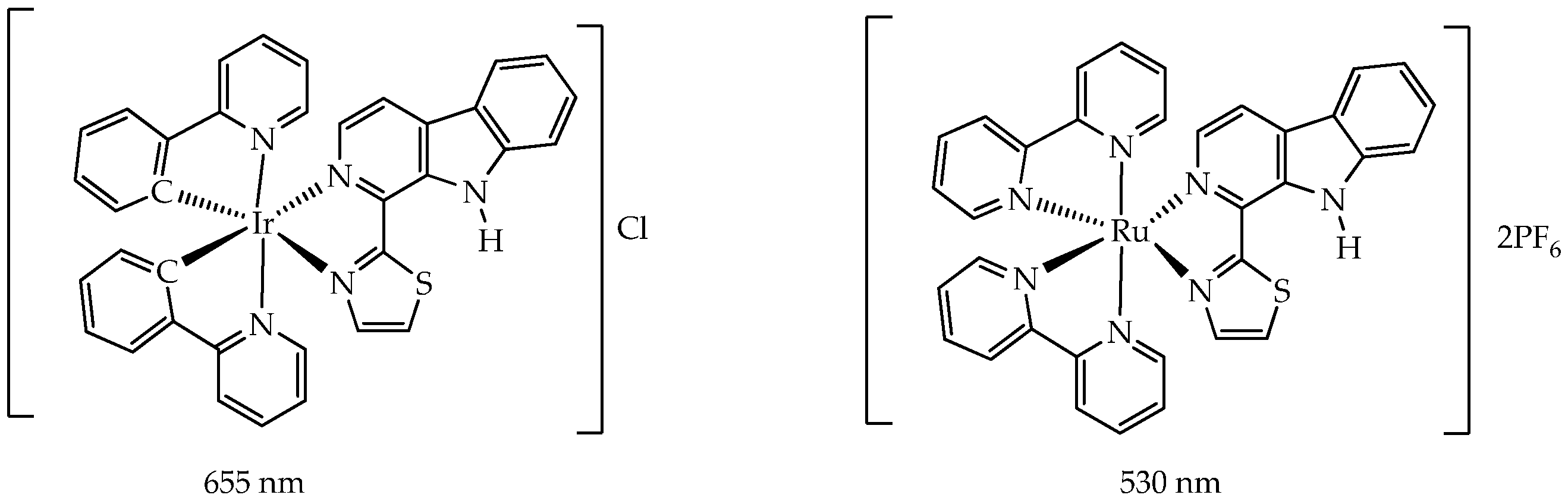
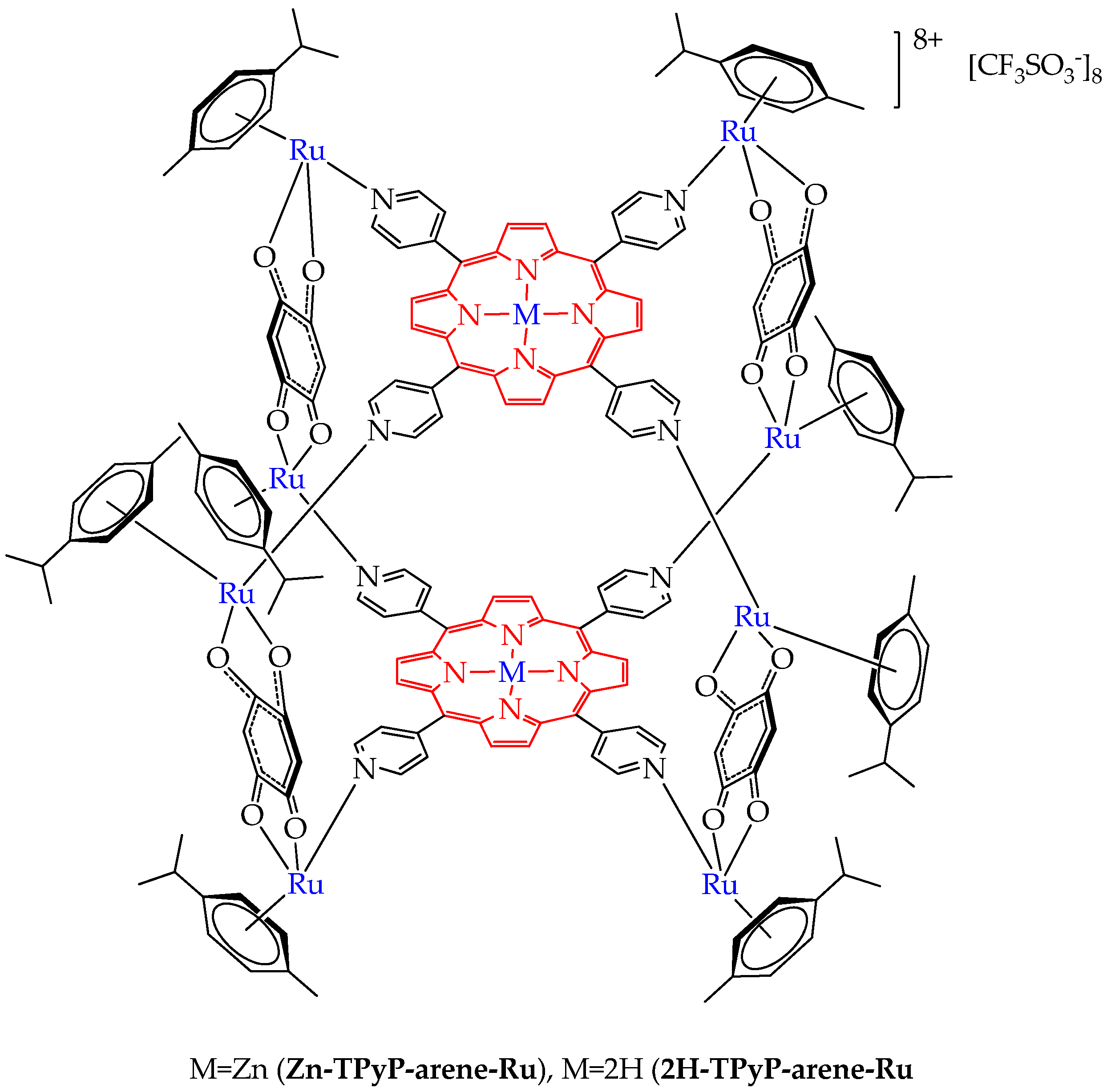
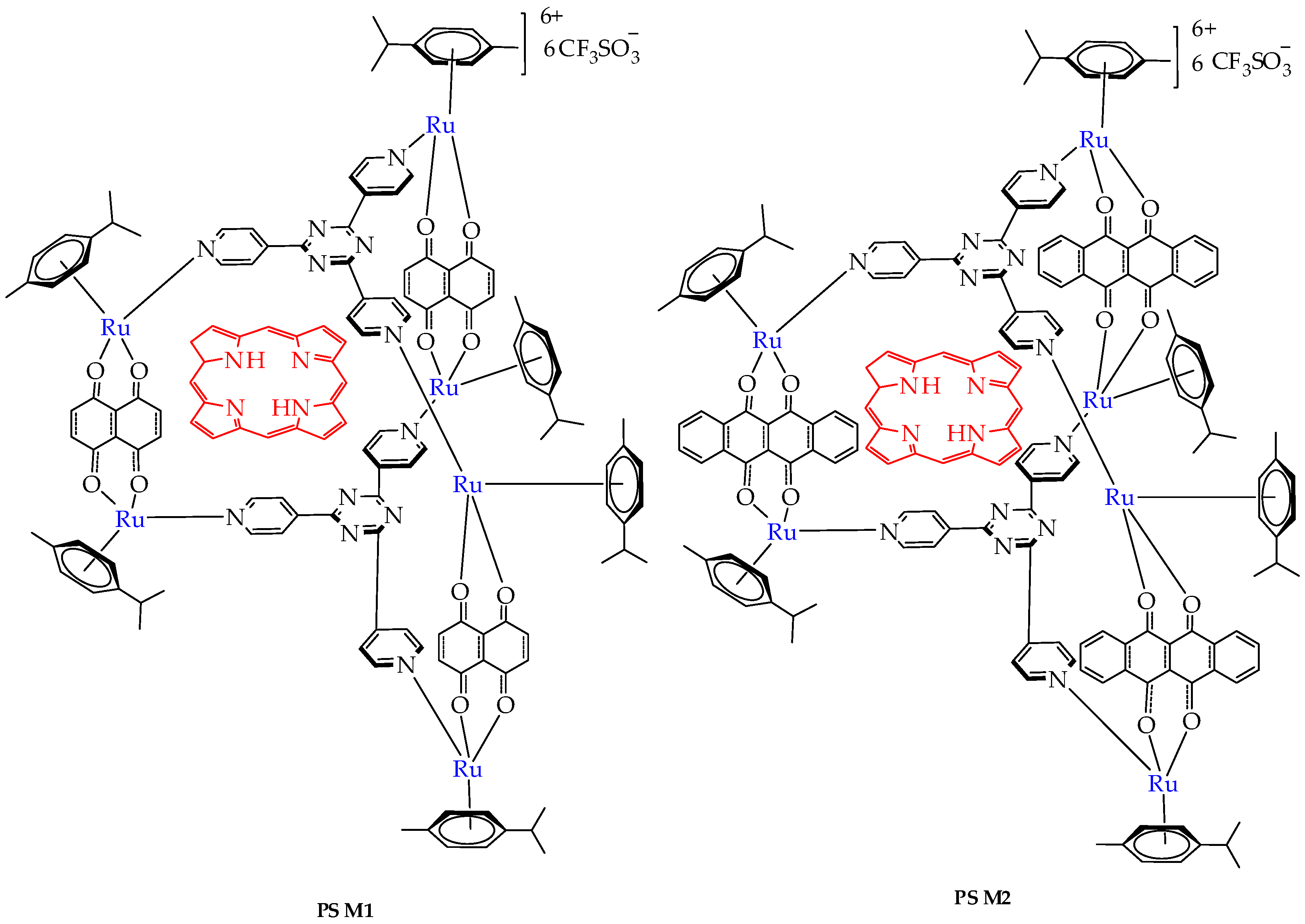
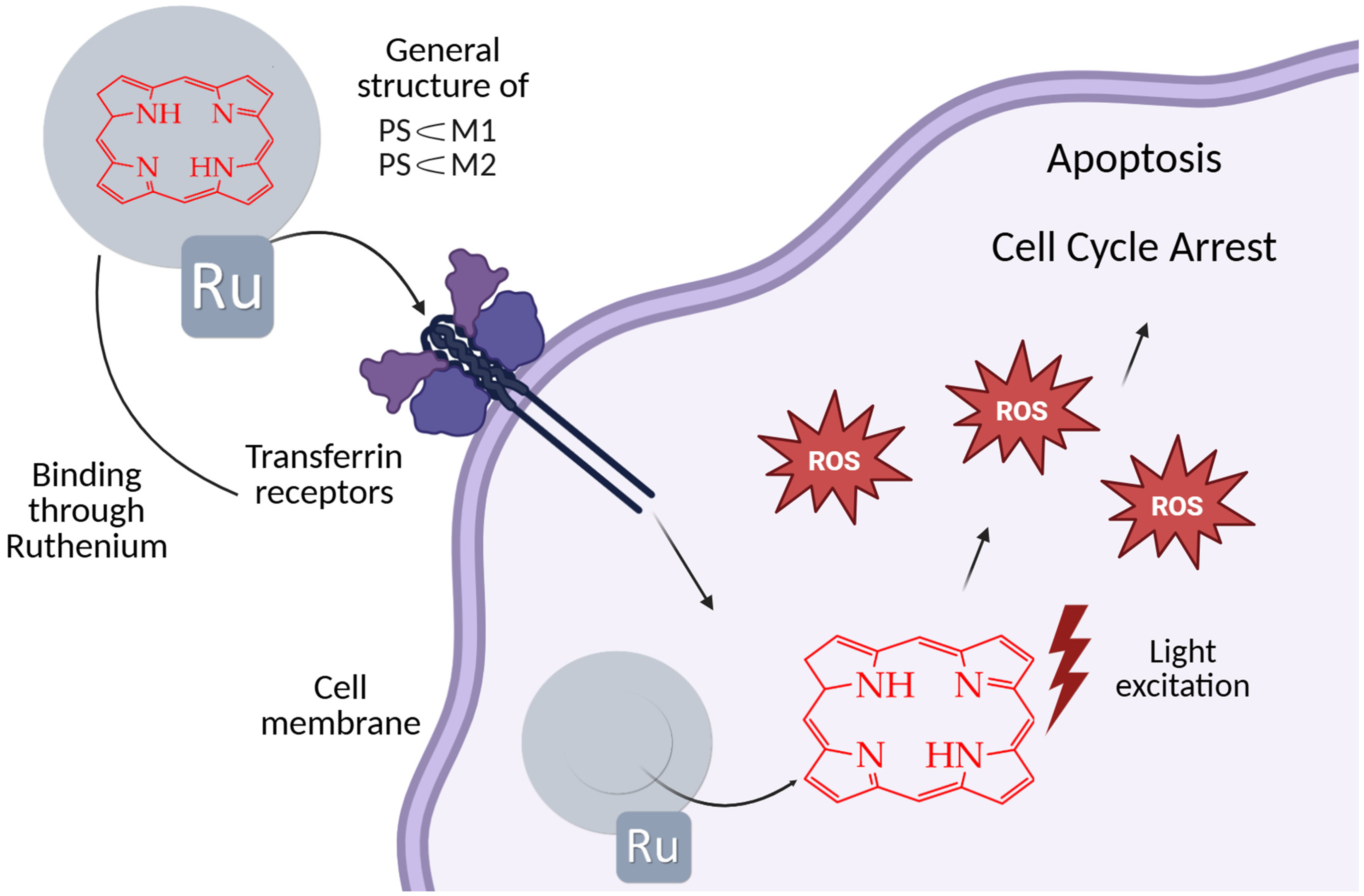
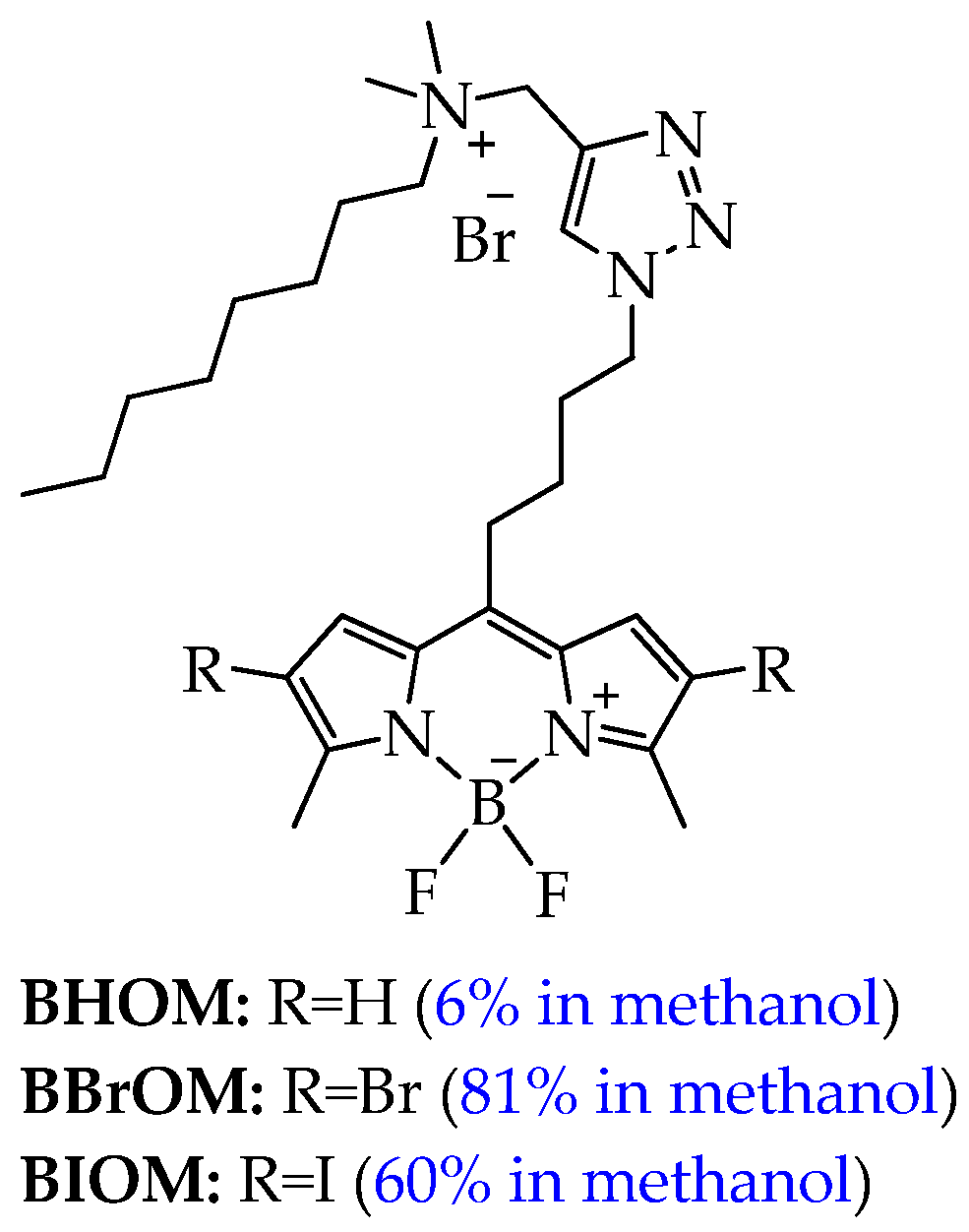
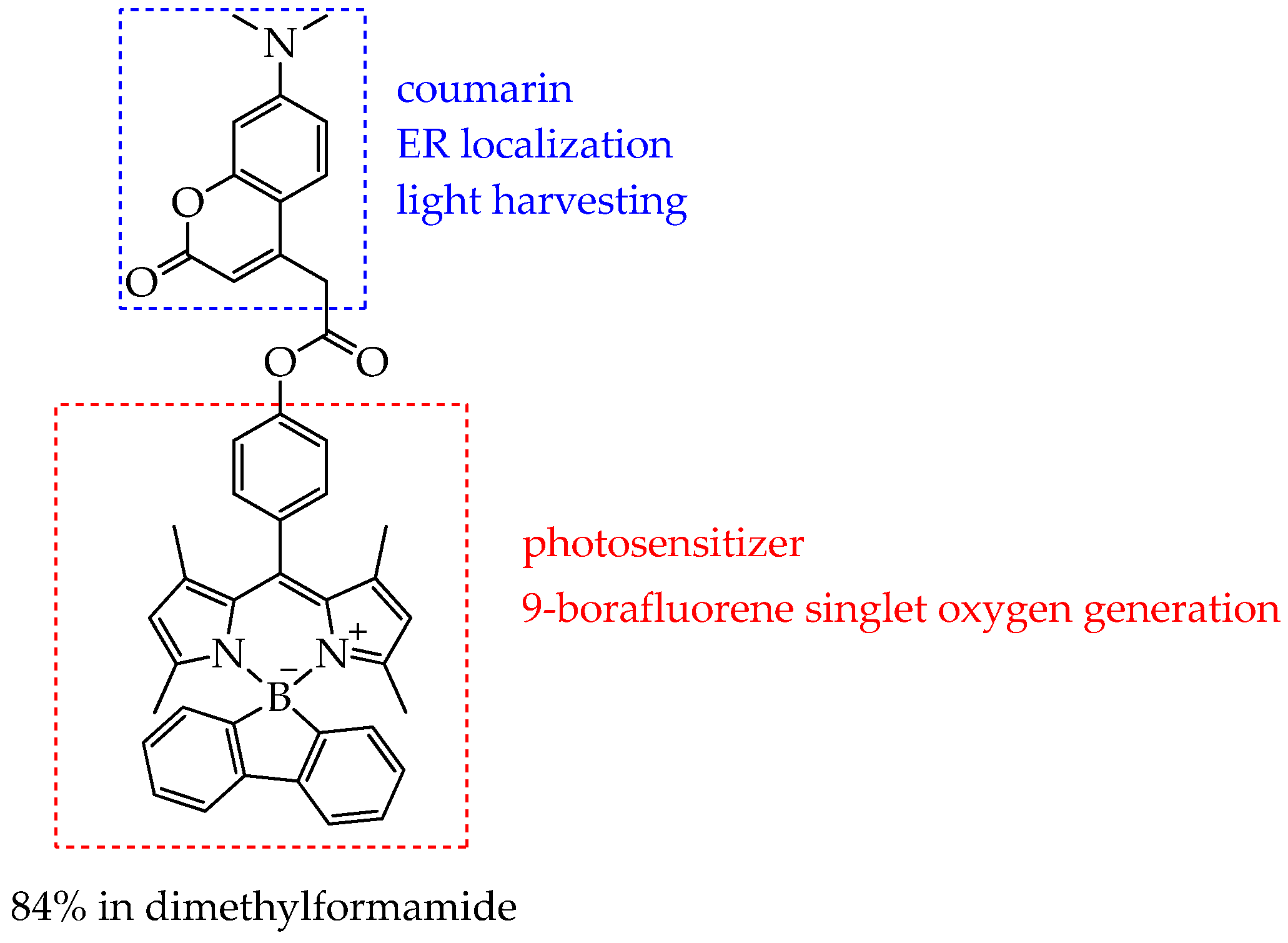
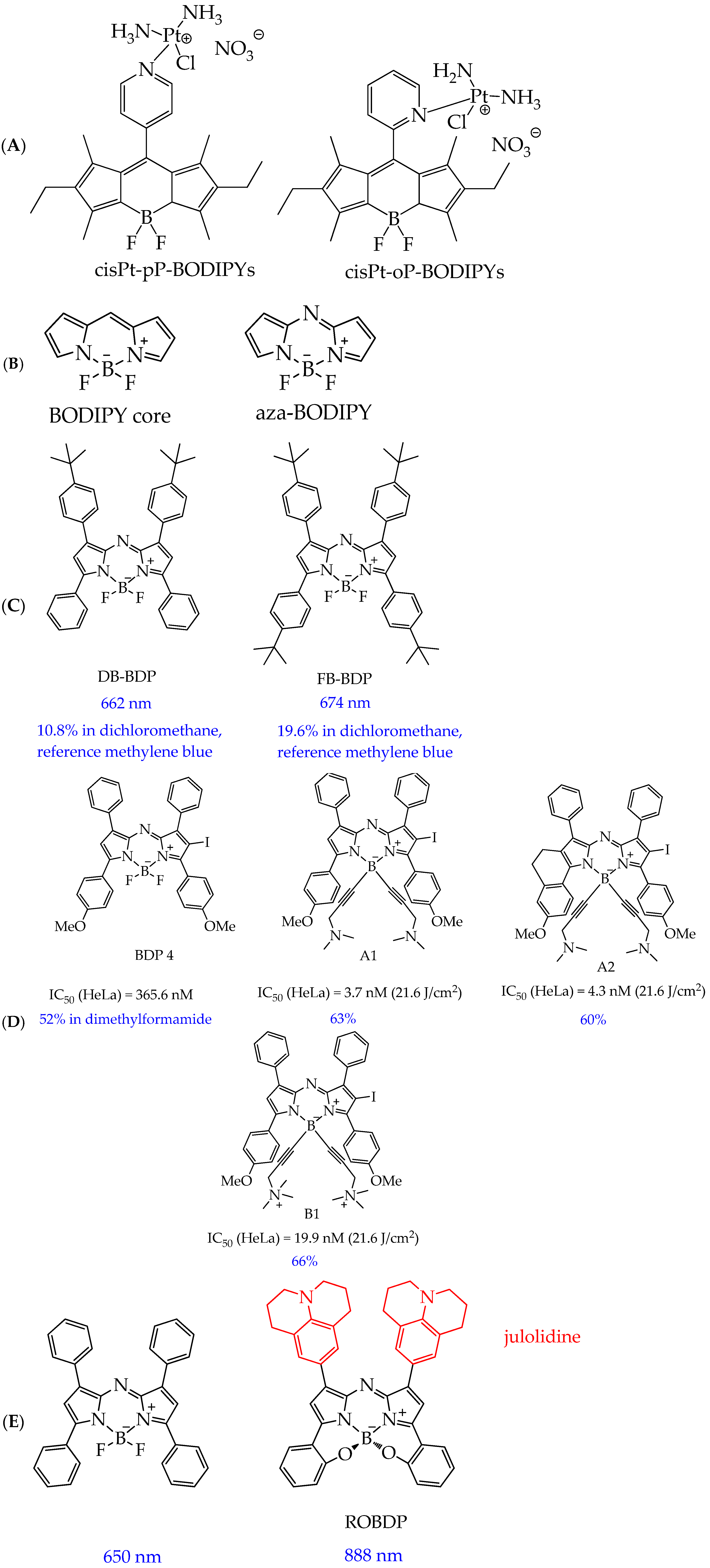
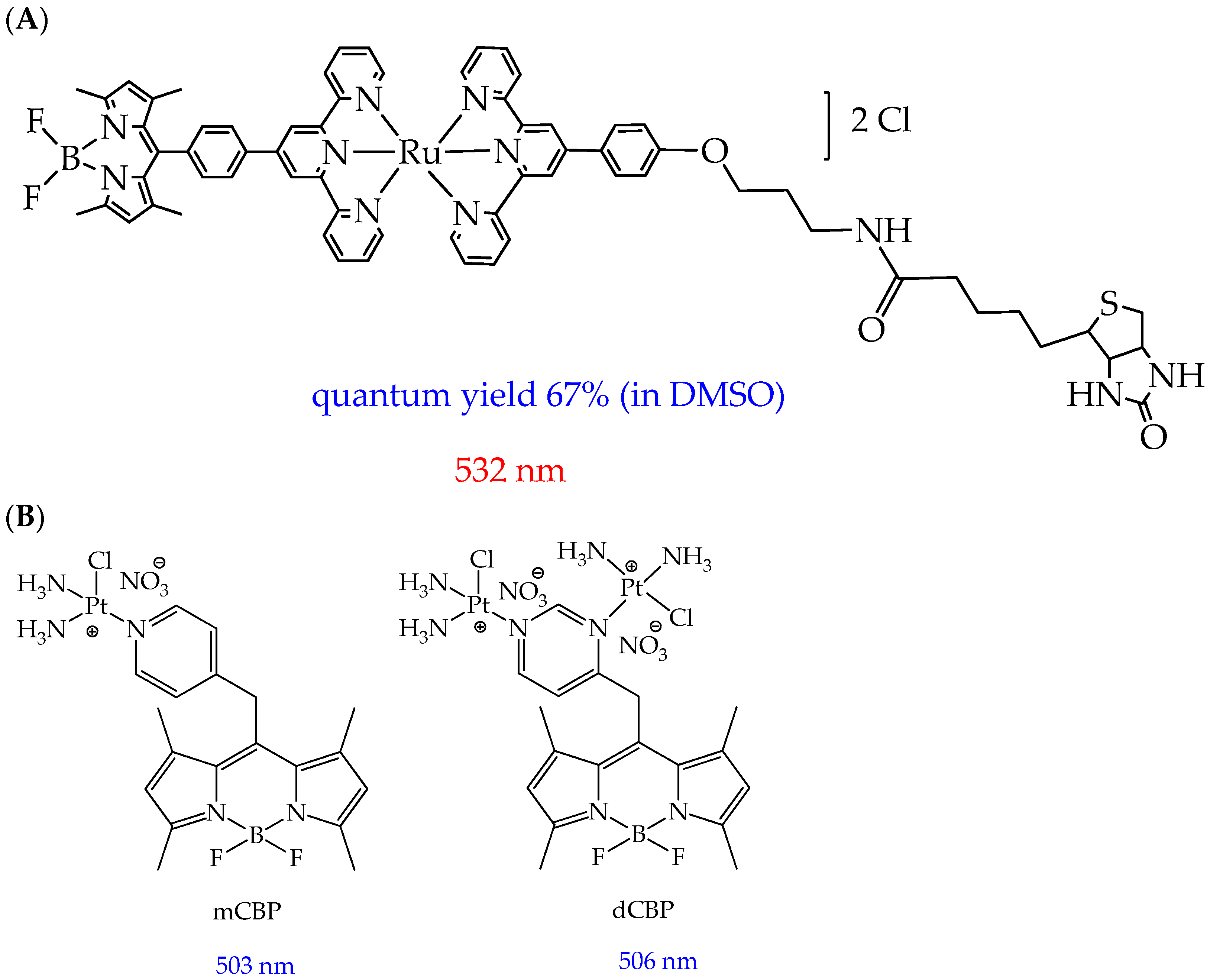
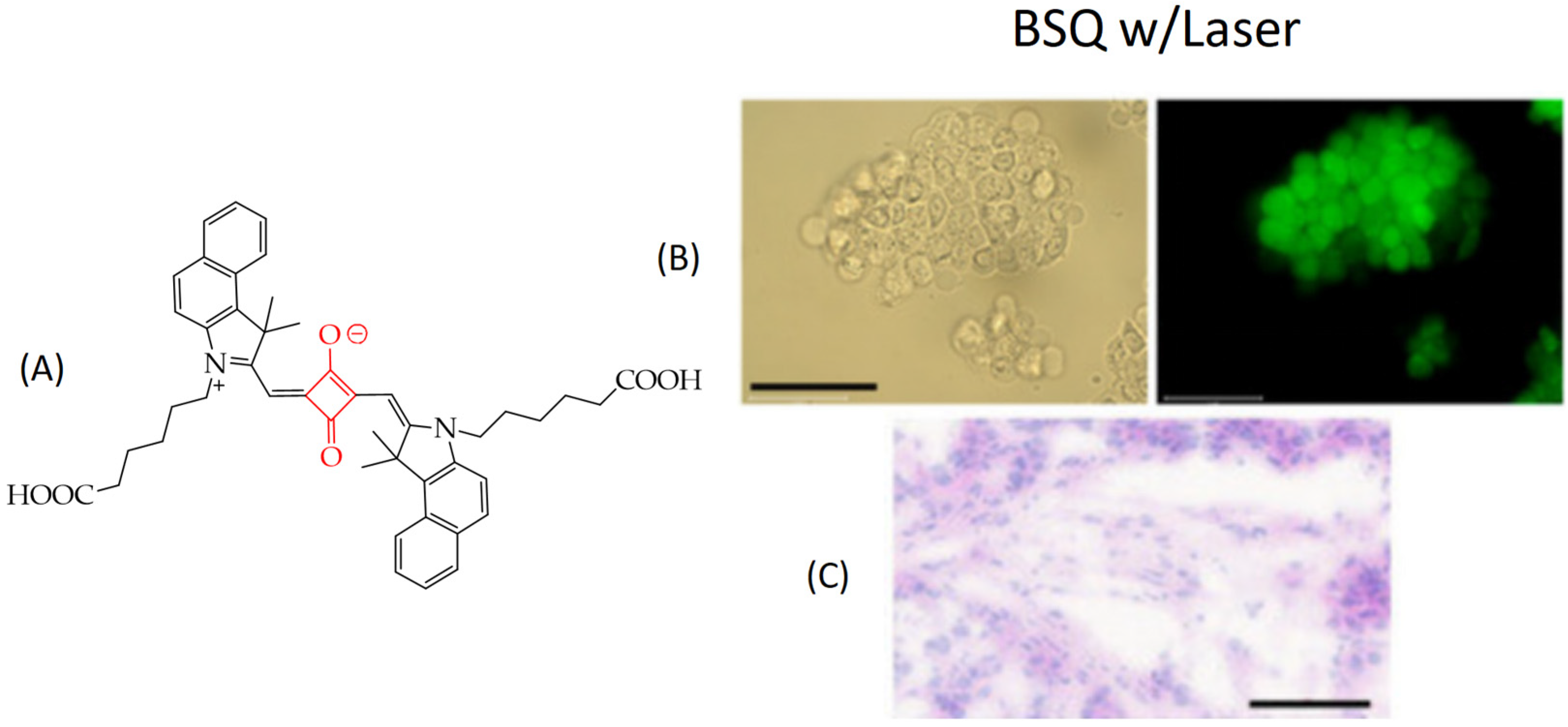
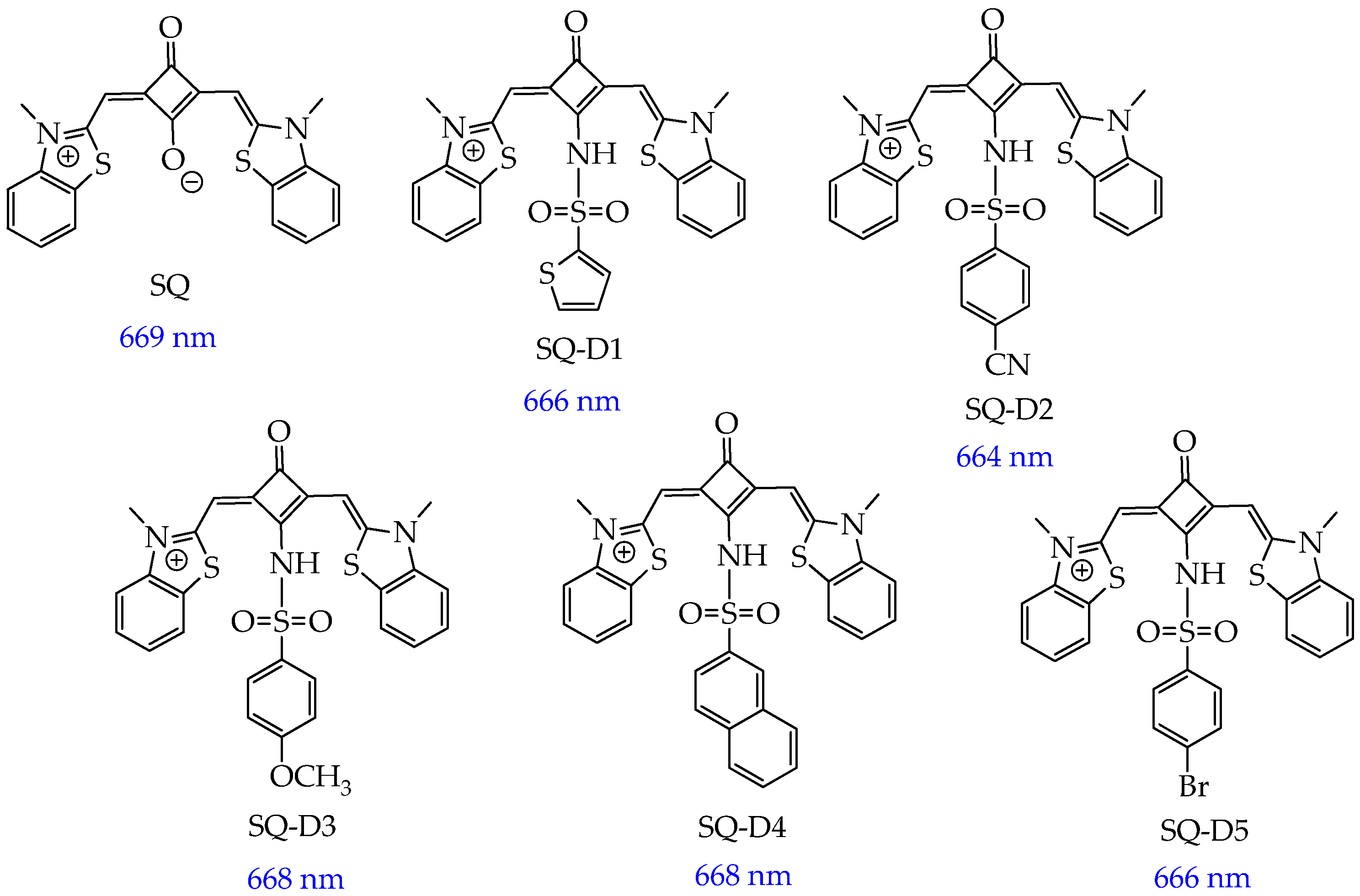
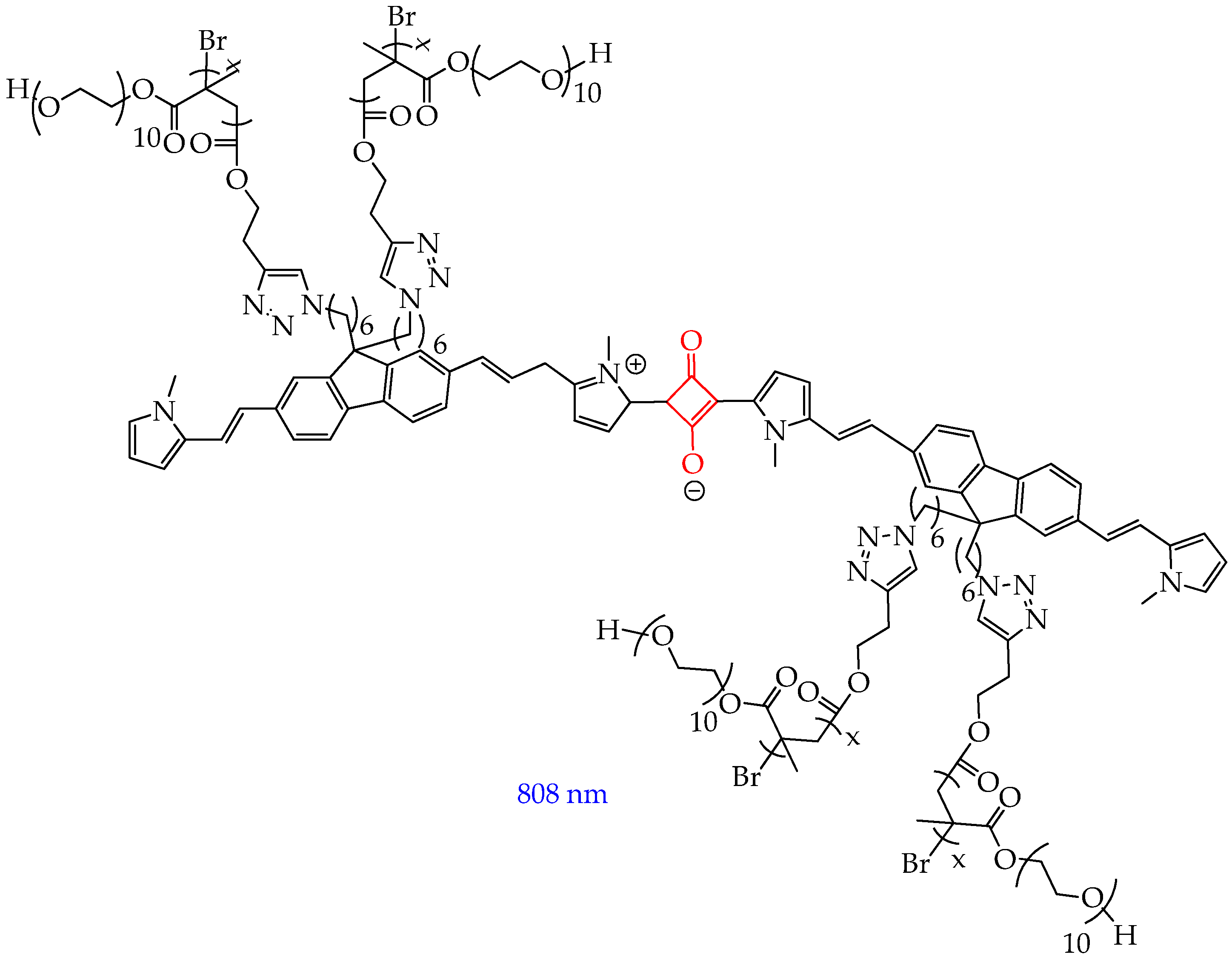

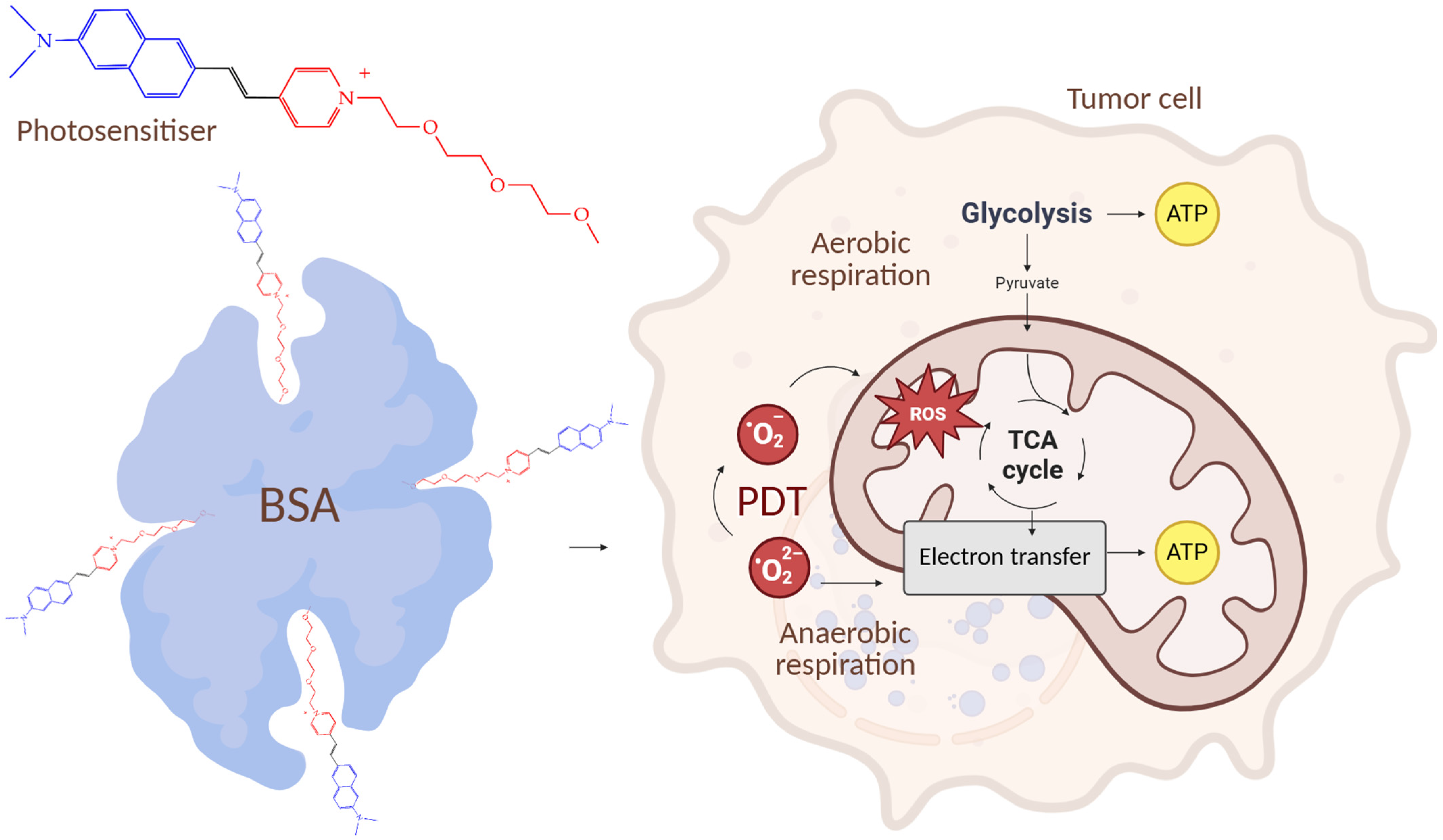

| Cells | Nanoparticles | |
|---|---|---|
| Fe3O4@Au@PEG-OH | Fe3O4@Au@PEG-NH2 | |
| A549 | 59 | 55 |
| A375 | 45 | 67 |
| MRC-5 | 19 | 45 |
| HaCaT | 29 | 54 |
| Photoactivation System | Number of Research Articles in Scopus 2024–2025 and Search Query | Advantages | Disadvantages | Cost of Starting Materials for the Synthesis of a Photoactivated System (Excluding Solvents, Equipment Costs, and Other Costs) or Matrix of Nanoparticles and Cells |
|---|---|---|---|---|
| Porphyrin and metal porphyrin derivatives | 658 (porphyrin photodynamic therapy) | Excellent phototoxicity against melanoma, lung, ovarian, and breast adenocarcinoma cells (IC50 less than 30 μM); selectivity against cancer cells compared to normal cells (IC50 more than 100 μM); and high singlet oxygen yield (more than 60%) | Absorption of light is not in the red region but in the green region for some derivatives, which leads to a low light penetration depth | Pyridine carboxaldehyde (about 200 USD/100 g), 4-acetamidobenzaldehyde (about 800 USD/100 g), pyrrole (about 80 USD/100 g), hydrochloric acid (about 6 USD/100 g), stearoyl chloride (about 260 USD/100 g), and methyl iodide (about 80 USD/100 g). Sum: 1426 USD. |
| Non-porphyrin metal complexes | 155 (metal complex photodynamic therapy except porphyrin, porphyrins) | High phototoxicity against cells of prostate cancer, melanoma, ovarian cancer, bladder cancer, breast cancer, and colorectal cancer (IC50 less than 30 μM); low dark cytoxicity (IC50 more 100 μM); water solubility of some complexes; possibility of use not only in normoxia but also in hypoxia | Low ROS yield (less than 60%) | Ferrocene carboxaldehyde (about 1000 USD/100 g), 2-acetylpyridine (140 USD/100 g), KOH (about 150 USD/100 g), IrCl3·3H2O (17,000 USD/100 g), and phenylisoquinoline (9500 USD/100 g). Sum: 27,790 USD. |
| BODIPY derivatives | 112 (BODIPY derivative photodynamic therapy) | Resistant to photobleaching, high singlet oxygen yield (more 60%), and phototoxicity against breast and ovarian cancer cells | Unpredictability of absorption and fluorescence spectra | 2,4-dimethylpyrrole, 5200 USD/100 g; 5-bromovaleryl chloride, 1720 USD/100 g; NaN3, 100 USD/100 g; N-iodosuccinimide, 547 USD/100 g; sodium ascorbate, 334 USD/100 g; and CuSO4·5H2O, 114 USD/100 g. Sum: 8015 USD. |
| Squaraines | 16 (squaraine photodynamic therapy) | Excellent photostability; simple structural tuning, which leads to appropriate photophysical and photochemical properties; low dark cytotoxicity; and phototoxicity against breast cancer cells and colon cancer cells (IC50 less than 30 μM) | Low solubility in water and the need for modification by hydrophilic fragments | Squaric acid, 830 USD/100 g; 1,1,2 trimethylbenz[e]indole, 480 USD/100 g; and 6-bromohexanoic acid, 267 USD/100 g. Sum: 1577 USD. |
| Polymeric nanoparticles | 71 (polymeric nanoparticle photodynamic therapy) | Biocompatibility and biodegradation | Aggregation of PSs in some cases (e.g., PLGA) and the effect of polymer molecular weight on phototoxicity; polymers with a lower molecular weight (more than 40 kDa) form larger nanoparticles that can accumulate in tumor tissue [185] | Diethylenetriamine, 44 USD/100 g; citric acid, 2930 USD/100 g; and ethylene diamine, 45 USD/100 g. Sum: 3019 USD. |
| Inorganic nanoparticles | 33 (inorganic nanoparticle photodynamic therapy) | Biocompatibility and ease of surface modification | Poor biodegradability, which can lead to long-term accumulation in organs, potentially causing chronic damage or dysfunction | Hexadecyl trimethyl ammonium bromide, 85 USD/100 g; NaOH, 4 USD/100 g; and tetraethyl orthosilicate, 25 USD/100 g. Sum: 114 USD. |
| Liposomes | 223 (liposome photodynamic therapy) | Water-soluble substances can be included in the aqueous space of liposomes, and fat-soluble substances in the lipid bilayer; increased efficiency of binding to the mitochondrial membrane; membranotropism; and biocompatibility | Possibility of oxidation and hydrolysis of phospholipids, which disrupts the structure of liposomes and leads to premature release of PSs and complicates release control, as well as the ability of liposomes to be quickly absorbed by the reticuloendothelial system; high cost of synthesis | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)], 160,000 USD/100 g, and 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, 80,000 USD/100 g. Sum: 240,000 USD/100 g. |
| Microvesicles | 18 (microvesicle cancer) | Biocompatibility and in vivo safety | Limited ability to penetrate tumor tissue; difficulty of obtaining pure samples uncontaminated by other cellular components, blood plasma proteins, and exogenous substances; disruption of structure during isolation using methods such as ultracentrifugation; heterogeneity in size and origin; and complex chemical composition | Mouse oral squamous carcinoma cell lines MOC2 (about 1000–2000 USD/1 vial). |
| Exosomes | 37 (exosome photodynamic therapy) | Natural biocompatibility, low immunogenicity, and stimulate an antitumor immune response | Difficulty of obtaining pure samples uncontaminated by other cellular components, blood plasma proteins, and exogenous substances; disruption of structure during isolation using methods such as ultracentrifugation; heterogeneity in size and origin; and complex chemical composition | Human oral squamous carcinoma cell lines SCC180, 1300 USD/1 vial. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yudaev, P.; Aleksandrova, Y.; Chugunova, E.; Neganova, M. The Current State of Research in the Field of Photosensitizers and Photoactivators for Photodynamic/Photothermal Cancer Therapy: A Review. Int. J. Mol. Sci. 2025, 26, 10733. https://doi.org/10.3390/ijms262110733
Yudaev P, Aleksandrova Y, Chugunova E, Neganova M. The Current State of Research in the Field of Photosensitizers and Photoactivators for Photodynamic/Photothermal Cancer Therapy: A Review. International Journal of Molecular Sciences. 2025; 26(21):10733. https://doi.org/10.3390/ijms262110733
Chicago/Turabian StyleYudaev, Pavel, Yulia Aleksandrova, Elena Chugunova, and Margarita Neganova. 2025. "The Current State of Research in the Field of Photosensitizers and Photoactivators for Photodynamic/Photothermal Cancer Therapy: A Review" International Journal of Molecular Sciences 26, no. 21: 10733. https://doi.org/10.3390/ijms262110733
APA StyleYudaev, P., Aleksandrova, Y., Chugunova, E., & Neganova, M. (2025). The Current State of Research in the Field of Photosensitizers and Photoactivators for Photodynamic/Photothermal Cancer Therapy: A Review. International Journal of Molecular Sciences, 26(21), 10733. https://doi.org/10.3390/ijms262110733








