Abstract
Non-small cell lung cancer (NSCLC) remains a leading cause of cancer mortality, with therapeutic resistance posing the primary barrier to durable outcomes. Beyond genetic and epigenetic alterations, amino acid transporter-driven metabolic reprogramming—mediated by LAT1 (SLC7A5), ASCT2 (SLC1A5), and xCT (SLC7A11)—supports tumor proliferation, redox homeostasis, and immune escape. Their preferential expression in NSCLC highlights their potential as therapeutic targets and predictive biomarkers. In parallel, α-particle therapy has gained attention for its capacity to eradicate resistant clones through densely clustered, irreparable DNA double-strand breaks. Astatine-211 (211At) combines a clinically relevant half-life, high linear energy transfer, and predictable decay scheme, positioning it as a unique candidate among α-emitters. Preclinical studies of 211At-labeled transporter ligands, particularly LAT1-targeted conjugates, demonstrate potent tumor suppression and synergy with targeted therapy, chemotherapy, radiotherapy, immunotherapy, and ferroptosis inducers. Advances in radiochemistry, delivery systems (antibodies, peptides, and nanocarriers), and PET tracers such as [18F]FAMT and [18F]FSPG collectively support a theranostic framework for patient stratification and adaptive dosing. By linking transporter biology with α-particle delivery, 211At-based theranostics offer a mechanistically orthogonal strategy to overcome resistance and heterogeneity in NSCLC. Successful translation will depend on precise dosimetry, scaffold stabilization, and biomarker-guided trial design, enabling progression toward first-in-human studies and future integration into multimodal NSCLC therapy.
1. Introduction
Lung cancer remains the leading cause of cancer-related mortality worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for ~85% of cases [2] and continues to show limited long-term survival despite advances in surgery, chemotherapy, radiotherapy, targeted agents, and immunotherapy [3]. Therapeutic resistance—driven by both genetic and non-genetic mechanisms—remains the primary barrier to achieving durable benefits [4,5]. Among these mechanisms, metabolic reprogramming is a hallmark of adaptation under therapeutic pressure [6]. Amino acid transporters such as LAT1 (SLC7A5) and ASCT2 (SLC1A5) sustain nutrient influx, engage proliferative signaling, and contribute to resistance [7,8], while exhibiting preferential tumor expression that renders them actionable targets; however, no LAT1- or ASCT2-directed agents have progressed to late-phase trials [9,10,11].
Targeted α-particle therapy deposits large amounts of energy within a very short path length, generating densely ionizing tracks that induce complex and primarily irreparable DNA double-strand breaks, while maintaining cytotoxicity largely independent of oxygen tension [12]. Astatine-211 (211At) exhibits favorable radiophysical and radiochemical characteristics—including high linear energy transfer, short tissue penetration range, and a simple decay scheme yielding one α-particle and ending in stable 207Pb—that collectively support reliable radiopharmaceutical design and centralized clinical distribution [13]. Owing to these features, 211At is regarded as a particularly promising radionuclide for clinical translation. While prior reviews have summarized NSCLC resistance mechanisms [4,14] or radionuclide therapies as independent topics [14,15], no review has comprehensively integrated amino acid transporter biology with 211At-based α-particle therapeutics. This review seeks to bridge this gap and propose a translational framework for overcoming therapeutic resistance in NSCLC.
2. Therapeutic Approaches and Mechanisms of Drug Resistance in NSCLC
2.1. Therapeutic Approaches
Management of NSCLC is increasingly biomarker-driven [16] and differs substantially across disease stages [17]. Surgical resection remains the only curative modality for stage I–II disease and for selected stage IIIA patients, typically combined with perioperative chemotherapy or immunotherapy [18]. For medically inoperable early-stage NSCLC, stereotactic body radiotherapy (SBRT) provides a practical, non-invasive alternative with excellent local control rates [19,20]. In unresectable stage III disease, concurrent chemoradiation followed by consolidation durvalumab represents the current standard of care for eligible patients [21]. In the metastatic setting, first-line therapy is guided by biomarkers. Patients with oncogenic drivers such as EGFR, ALK, ROS1, or KRASG12C derive substantial benefit from targeted agents [22]. By contrast, immune checkpoint inhibitors (ICIs), either alone or combined with platinum-based chemotherapy, are recommended in tumors without actionable oncogenic drivers [23]. PD-L1 expression serves as a predictive biomarker of response to ICI monotherapy, particularly in tumors with high PD-L1 expression [16,24]. However, in oncogene-driven NSCLC, ICIs generally provide limited benefit [25,26]. Overall, although targeted therapy and immunotherapy have reshaped the therapeutic landscape, durable disease control and long-term survival remain unmet needs for many patients [27].
2.2. Resistance Mechanisms
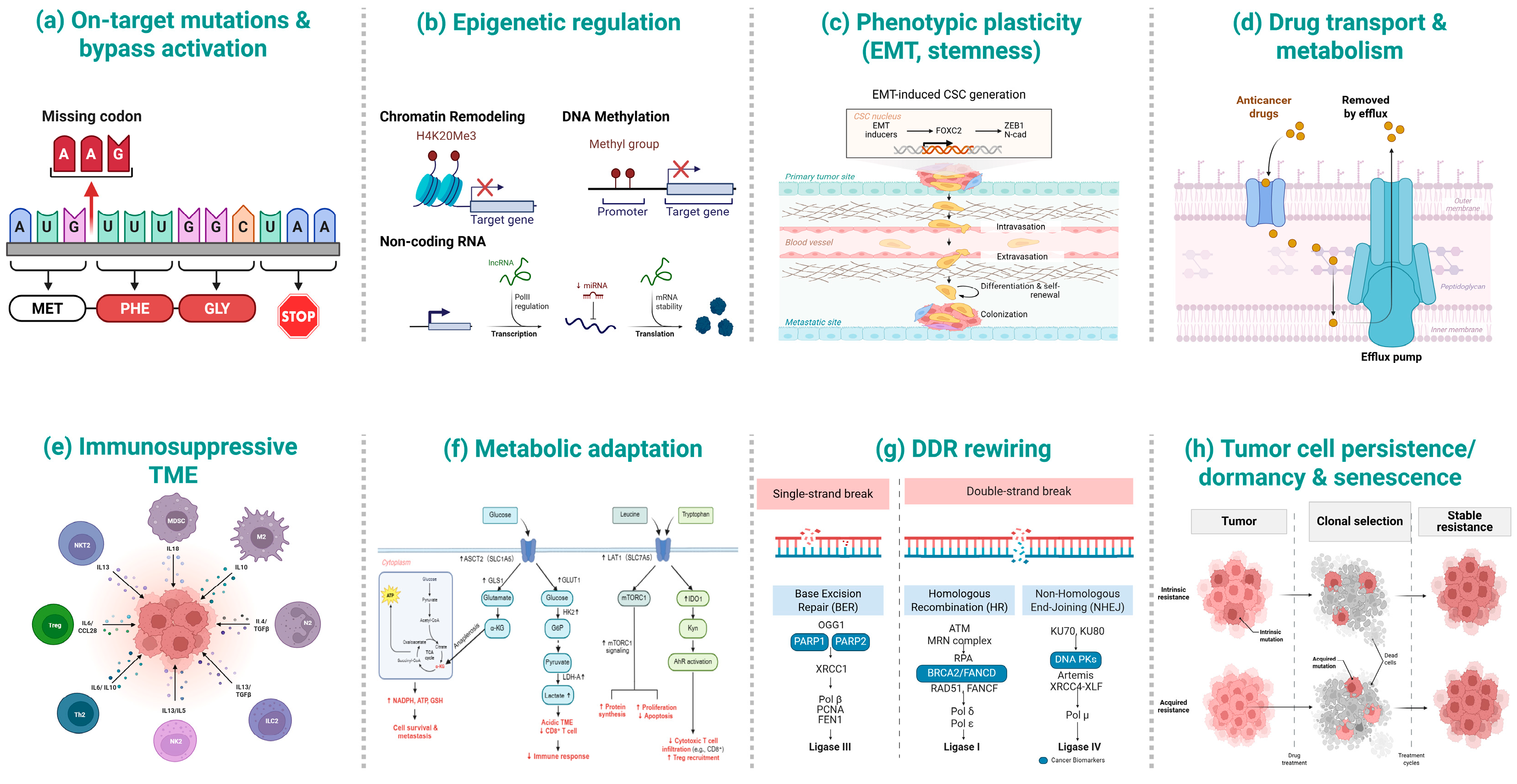
Therapeutic resistance in NSCLC is highly heterogeneous [28] and often involves multiple concurrent processes [29], with strong context dependence by inhibitor class, tumor genotype, and residual disease status [30]. Broadly, mechanisms span genetic, epigenetic, metabolic, and microenvironmental processes [31,32,33]. Representative resistance mechanisms—including on-target/bypass mutations, epigenetic remodeling, EMT/stemness, drug efflux and metabolism, immunosuppressive TME, metabolic adaptation, DNA damage response (DDR) rewiring, and tumor persistence/dormancy—are summarized in Table 1 and schematically illustrated in Figure 1.

Table 1.
Representative resistance mechanisms in NSCLC, with key molecular alterations, functional consequences, and therapeutic implications.
Figure 1.
Schematic overview of representative resistance mechanisms in NSCLC. (a) On-target mutations & bypass activation; (b) Epigenetic regulation (chromatin remodeling, DNA methylation, non-coding RNAs); (c) Phenotypic plasticity (EMT, stemness); (d) Drug transport & metabolism (efflux pumps and drug handling); (e) Immunosuppressive tumor microenvironment (TME) (myeloid, lymphoid and stromal components around the tumor); (f) Metabolic adaptation (LAT1/ASCT2/xCT-centered nutrient and redox pathways); (g) DNA-damage response (DDR) rewiring (BER, HR, NHEJ and checkpoint signaling); (h) Tumor cell persistence/dormancy & senescence (clonal selection and stable resistance).
2.3. Modality-Specific Resistance Examples
For tyrosine kinase inhibitors, on-target mutations such as EGFR T790M/C797S [73] and bypass activation of MET–ERBB3–PI3K/AKT are prototypical routes that compromise drug binding or reactivate downstream signaling [36]. For immune checkpoint inhibitors, resistance commonly involves defective antigen presentation (e.g., beta-2 microglobulin (B2M) or human leukocyte antigen class I (HLA-I) loss [74]) and T-cell exclusion/exhaustion driven by suppressive myeloid cells and Tregs [75]; adaptive upregulation of alternative checkpoints (TIM-3, LAG-3, TIGIT, VISTA) in non-inflamed or metabolically reprogrammed TMEs further limits benefit [75,76]. For chemotherapy and radiotherapy, DNA-damage response activation [66] and hypoxia-induced HIF-1 signaling are dominant drivers [77]. Other adaptations—including small cell transformation [78] and EMT [79], epigenetically sustained drug-tolerant persisters [69,80]—also contribute to treatment failure.
2.4. Shared Adaptive Programs Across Modalities
Although resistance manifests differently across therapeutic modalities, several convergent adaptive programs emerge as key drivers of treatment failure. These shared adaptations include (i) metabolic adaptation, such as enhanced glutamine and cystine flux via LAT1, ASCT2, and xCT, which sustain redox balance, biosynthesis, and immune evasion; (ii) epigenetic plasticity that maintains reversible drug-tolerant states; (iii) alterations in drug transport and metabolism involving ABC transporters and CYP/UGT enzymes, which modify intratumoral drug exposure; and (iv) persistence or dormancy programs that facilitate relapse from minimal residual disease. Collectively, these adaptations underlie the limited durability of current treatments. Biomarker-guided next-generation inhibitors, rational therapeutic combinations, and optimized sequencing can overcome specific resistance routes, such as MET-amplified resistance to EGFR TKIs. Nevertheless, tumors frequently retain shared metabolic vulnerabilities [81]. In particular, metabolic plasticity mediated by amino acid transporters such as LAT1, ASCT2, and xCT constitutes a tractable vulnerability across various treatment contexts, as it supports redox control, biosynthesis, and immune evasion [60,82]. This adaptation directly links resistance biology to therapeutic metabolism and to radiolabeled theranostic strategies.
3. Amino Acid Transporters in NSCLC: Structure, Function, and Therapeutic Targeting
Beyond genetic and epigenetic mechanisms, therapeutic resistance in NSCLC is also sustained by metabolic adaptations. Among these, amino acid transporters play a crucial role in sustaining tumor growth and maintaining redox balance, while also contributing to immune evasion and treatment resistance, thereby positioning them as critical mediators between tumor metabolism and therapeutic outcomes.
3.1. Overview and Structural Features
Amino acid transporters of the SLC superfamily orchestrate cellular nutrient flux to meet the anabolic and redox demands of cancer cells, thereby representing potentially tractable therapeutic targets in oncology [83]. In NSCLC, three transporters—LAT1 (SLC7A5), ASCT2 (SLC1A5), and xCT (SLC7A11)—have been most extensively studied for their roles in nutrient uptake, mTORC1 activation, and redox control. LAT1 is a nonglycosylated light chain that forms a disulfide-linked heterodimer with the glycosylated heavy chain 4F2hc (SLC3A2), which mediates Na-independent uptake of large neutral amino acids such as leucine and phenylalanine [84]. ASCT2 functions as a Na+-dependent exchanger with a substrate preference for glutamine. N-linked glycosylation at Asn163 and Asn212 ensures its membrane stability [85]. This transporter supports the “glutamine addiction” phenotype of NSCLC and is commonly overexpressed in tumors compared with adjacent lung tissue [85,86]. xCT, the light chain of system Xc−, heterodimerizes with 4F2hc to mediate equimolar cystine–glutamate exchange [87,88]. Imported cystine is reduced intracellularly to cysteine, the rate-limiting precursor of glutathione, thereby sustaining redox homeostasis and therapy resistance [88]. The stability of xCT is reinforced by CD44 variant isoforms [89] and NRF2-dependent transcription under oxidative stress [90]. High SLC7A11 expression correlates with poor prognosis [91] and synthetic lethality in KRAS-mutant lung adenocarcinoma models [92]. Collectively, LAT1, ASCT2, and xCT constitute a metabolic network in which glutamine efflux via ASCT2 fuels LAT1-mediated leucine uptake to activate mTORC1. In contrast, xCT-mediated cystine uptake maintains glutathione-based antioxidant defense, underscoring these transporters as central metabolic nodes in NSCLC. The key structural and functional features of these transporters are summarized in Table 2.

Table 2.
Amino acid transporters in NSCLC, with structural characteristics, transport substrates, functional roles, and clinical relevance.
3.2. Roles in Tumor Metabolism and the Microenvironment
These transporters not only ensure nutrient availability but also coordinate oncogenic signaling with stress adaptation in NSCLC. Leucine uptake via the LAT1 4F2hc complex plays a central role in mTORC1 activation. Binding of leucine to Sestrin2 disrupts its inhibitory interaction with GATOR2 [102], thereby enabling Rag GTPase-dependent lysosomal recruitment of mTORC1 [103]. Glutaminolysis further provides α-ketoglutarate, which reinforces Rag-mediated activation [104]. In colorectal cancer models, oncogenic KRAS amplifies this circuit by upregulating LAT1, ASCT2, and SNAT2 [105]. LAT1 inhibition with BCH reduces mTOR phosphorylation and synergizes with gefitinib to reduce NSCLC cell viability [106]. ASCT2 mediates glutamine import, which is essential for anaplerosis, maintaining redox balance, and promoting proliferation. Imported glutamine can feed exchange cycles that support leucine influx and sustain mTORC1 activation [107]. In the tumor microenvironment (TME), glutamine depletion restricts T-cell proliferation and cytokine production [108,109]. Pharmacologic inhibition with V-9302 synergizes with EGFR-TKIs [97], promoting apoptosis, suppressing autophagy, and enhancing the activity of CD8+ T cells [110]. xCT maintains glutathione biosynthesis by exchanging extracellular cystine for intracellular glutamate [87,88]. This glutathione pool buffers therapy-induced oxidative stress [111]. KEAP1/NRF2 mutations drive constitutive SLC7A11 expression [112], while the induction of ATF4 further enhances stress responses [60]. Blockade of xCT with sulfasalazine depletes GSH, radiosensitizes tumors [113], and augments cisplatin efficacy [114]. At the downstream node, GPX4 detoxifies lipid peroxides, thereby suppressing ferroptosis [115]. In NSCLC models, perturbing redox–ferroptosis defenses increases ferroptosis susceptibility (with links to GPX4 downregulation) [116], and, more broadly across drug-resistant solid tumors, inhibition of the System Xc−/GSH/GPX4 axis restores ferroptosis sensitivity [117].
3.3. Immune and Expression Profiles in NSCLC
Beyond intrinsic metabolism, amino acid transporters play critical roles in modulating antitumor immunity. Tumor-derived kynurenine is imported into T cells via the LAT1 (SLC7A5) transporter, thereby activating AHR signaling [118] to drive regulatory T-cell differentiation [119]. Effector T cells depend on LAT1 for clonal expansion [120], whereas Tregs compensate through methionine uptake via SLC43A2 [121]. Under metabolic stress, glutamine deprivation can induce PD-L1 via EGFR–ERK–c-Jun signaling (shown in renal cancer models), suggesting a metabolism–immune checkpoint link that may extend to other contexts [122], thereby suppressing antitumor immunity. IDO1 expression within tertiary lymphoid structures correlates with Treg infiltration [123]. In resected lung adenocarcinomas, IDO1 upregulation is associated with reduced T-cell density [124]. Collectively, LAT1, ASCT2, and xCT coordinate metabolic and immune adaptations that promote tumor growth and immune evasion. These findings underscore the dual role of amino acid transporters as therapeutic targets and biomarkers of immunotherapy response.
In parallel with their immunomodulatory functions, transporter expression in NSCLC is subtype-specific and carries prognostic significance. LAT1 is overexpressed in squamous cell carcinoma (91%) and large-cell carcinoma (67%), but is less frequent in adenocarcinoma (29%), and is absent in normal bronchial and alveolar epithelium [125]. ASCT2 is expressed in ~66% of tumors and correlates with advanced stage, lymphovascular invasion, and poor prognosis, particularly in adenocarcinoma [98]. Approximately 12% of adenocarcinomas coexpress LAT1 and ASCT2, a profile associated with a poor outcome [126]. In NSCLC, xCT is frequently overexpressed, promoting tumor progression [101]. Across cancers, elevated SLC7A11 maintains redox balance and suppresses ferroptosis [60] and has been linked to poor survival in glioma [127]. Clinically, LAT1 positivity predicts worse overall survival (5-year OS 51.8% vs. 87.8%) [125].
Mechanistically, oncogenic and microenvironmental cues further reinforce transporter activity [128]. Hypoxia drives HIF-2α-dependent regulation of LAT1 [129], NRF2 binding at the SLC7A11 promoter augments cystine uptake [130], and EZH2-dependent H3K27 trimethylation shapes amino acid metabolic programs, implicating the LAT1–mTORC1 axis [131]. ASCT2 requires N-linked glycosylation for membrane stability [132], whereas LAT1 transport activity is dependent on membrane cholesterol [133]. Functionally, glutamine imported via ASCT2 can be exchanged for leucine via LAT1 to activate mTORC1 [134], while xCT-mediated cystine uptake sustains glutathione synthesis [100,134]. KEAP1/NRF2-driven xCT activity promotes a resistance phenotype, as measured by [18F]FSPG PET [112]. Moreover, KEAP1 knockout increases resistance to KRAS G12C inhibitors [135], highlighting transporter-linked therapeutic adaptation.
Overall, LAT1, ASCT2, and xCT are overexpressed through oncogenic, hypoxic, and epigenetic programs, support metabolic and redox demands, and predict poor outcomes, thereby establishing them as both biomarkers and therapeutic targets in NSCLC. Their selective overexpression and cell-surface accessibility further create molecular gateways for precision delivery. Transporter-directed ligands can be engineered to carry α-emitters such as 211At, thereby linking metabolic targeting to radiotheranostic strategies and bridging fundamental transporter biology with the radiochemical and translational frameworks discussed in the following sections.
4. Physical and Radiobiological Basis of 211At Therapy
4.1. Physical & Radiobiological Properties
211At is produced via the 209Bi(α,2n)211At nuclear reaction on bismuth targets using α particles of approximately 28–29 MeV. Its ~7.2 h half-life accommodates synthesis, quality control, and distribution while limiting prolonged exposure [13]. After 48 h, less than 1% of the initial activity remains [136]. During its decay, 211At yields two α-particle energies of approximately 5.87 and 7.45 MeV [137]. α-particles deliver high linear energy transfer (LET, ~50–230 keV/μm) and induce densely clustered DNA double-strand breaks that are difficult to repair [12]. In soft tissue, their track range is ~50–80 μm (~5–8 cell diameters), thereby restricting cytotoxicity to targeted cells [138]. The oxygen-enhancement ratio is ~1.0 [139], indicating relative hypoxia independence compared with photons or β-particles.
4.2. Comparison with Other Therapeutic Radionuclides
Among α-emitters, actinium-225 (225Ac; t1/2 = 9.92 d) yields five α emissions but suffers from daughter redistribution and dosimetry complexity [140,141]. In contrast, bismuth-213 (213Bi; t1/2 ≈ 45.6 min) requires rapid pharmacokinetics and on-site preparation, thereby limiting broader applicability [142]. By comparison, β-emitters such as iodine-131 (131I; t1/2 = 8.05 d) provide long-range cross-fire but at the cost of higher collateral exposure [143,144]. Lutetium-177 (177Lu; t1/2 = 6.65 d) offers medium-range β emission with favorable imaging capabilities [145,146]. 211At uniquely generates short-range α tracks (~50–80 μm) capable of eradicating micrometastases and minimal residual disease [147], although effective delivery requires homogeneous target expression and efficient tumor penetration [148,149]. Clinical proof of concept for systemic α-therapy has been established by radium-223 in mCRPC [150], positioning 211At as a promising candidate due to its balanced half-life, potency, and logistics.
4.3. Radiolabeling Strategies of 211At
The weak C–At bond (~49 kcal·mol−1) and in vivo deactivation necessitate the development of robust labeling strategies [151]. Electrophilic astatination of aryl stannanes remains the gold standard [13], whereas boronic acid derivatives provide tin-free alternatives [152]. Diaryliodonium salts exhibit high regioselectivity and functional group tolerance [153]. Controlling reaction conditions—using mild temperature and short reaction times—has been reported to mitigate radiolysis and improve stability [151]. Several design principles can be distilled: (i) employ mild, rapid electrophilic conditions compatible with the 7.2 h half-life; (ii) favor para (and, to a lesser extent, ortho) aromatic substitution to improve labeling performance and stability [13]; and (iii) leverage the side-chain tolerance observed for LAT1-privileged amino acid mimetics, allowing incorporation of para-aryl astatine or small prosthetic groups without abolishing transport [13,154]. These guidelines explain why para-aryl astatination of LAT1-privileged amino acid mimetics consistently yields high tumor-to-blood ratios [154,155].
4.4. Targeting Amino Acid Transporters with 211At
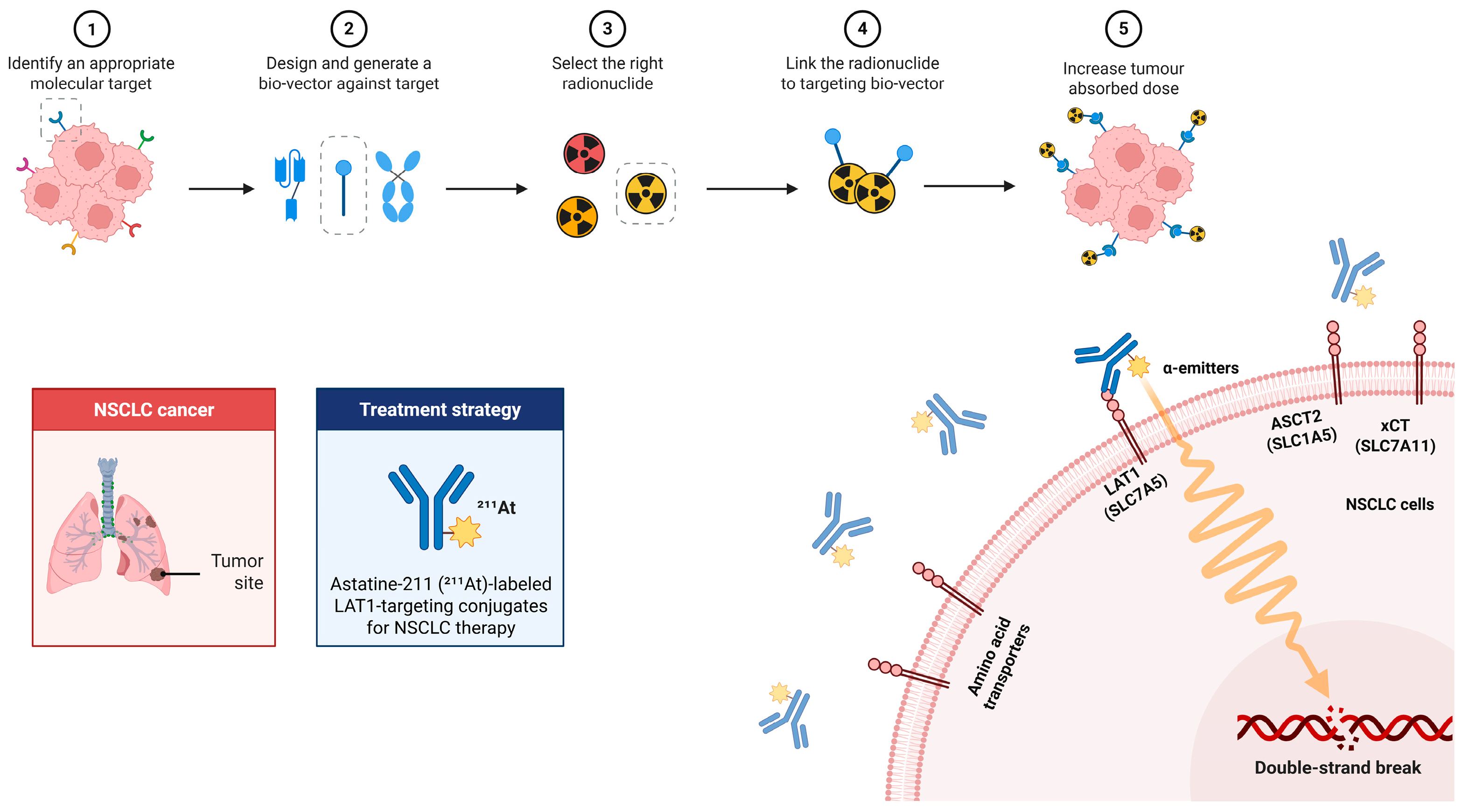
The central rationale is to integrate the unique radiophysical properties of 211At with the metabolic dependencies of NSCLC. A schematic overview of this therapeutic concept is provided in Figure 2, which illustrates the workflow of 211At-labeled LAT1-targeted therapy, including target identification, vector design (antibody/peptide/small molecule), radiolabeling, tumor accumulation, and the induction of clustered DNA double-strand breaks by short-range, high-LET α-particles.
Figure 2.
Workflow and mechanism of 211At-labeled LAT1-targeted therapy in NSCLC. Workflow (top row, steps 1–5): (1) Identify a molecular target (LAT1 in NSCLC). (2) Design a targeting bio-vector (e.g., antibody/scFv, peptide, small molecule); gray dashed icons indicate alternative formats not selected. (3) Select the radionuclide (preferred α-emitter 211At; other candidates shown as alternatives). (4) Link the radionuclide to the bio-vector to form the targeting conjugate. (5) Increase tumor absorbed dose via specific uptake at the tumor site. Mechanism (bottom right): LAT1 (SLC7A5) on the NSCLC-cell membrane mediates targeting of the 211At-labeled conjugate; emitted α-particles (wavy track) deposit high-LET energy over a short range, causing DNA double-strand breaks. ASCT2 (SLC1A5) and xCT (SLC7A11) are shown as related transporters for contextual comparison.
LAT1 (SLC7A5) is widely regarded as a leading amino acid transporter target, whereas ASCT2 (SLC1A5) and xCT (SLC7A11) are often discussed as complementary nodes that help support mTORC1 activity and redox balance. From a chemical standpoint, aryl C–At linkages obtained via electrophilic astatination—using, for example, destannylation, astatodeboronation, or diaryliodonium precursors—represent established labeling approaches, and are compatible with amino acid–mimetic scaffolds and late-stage modifications.
Two LAT1-targeted chemotypes currently define the 211At radiopharmaceutical landscape. 2-[211At]AAMP, a phenylalanine analogue, exhibits LAT1-dependent uptake, α-radiation-induced cytotoxicity, and survival benefit in tumor-bearing models [155]. In parallel, 211At-AAMT, an α-methyl-L-tyrosine derivative, shows dose-dependent activity, ranging from growth suppression at 0.4 MBq/mouse in PANC-1 xenografts to complete inhibition of B16F10 lung metastases at 1 MBq/mouse [63]. Structural studies indicate that LAT1 favors bulky neutral amino acids and tolerates para-substituted aromatic rings and α-methyl handles without loss of recognition [156]. Efficient labeling under mild electrophilic conditions [151] is required to match the ~7.2 h half-life of 211At [13]. Pharmacologic modulation of renal clearance offers an additional strategy: probenecid pretreatment prolongs circulation [157] and increases tumor uptake [154] of [211At]AAMP, thereby enhancing efficacy [58]. Together, these studies establish design rules—including LAT1-privileged scaffolds, para-aryl astatination, α-methyl stabilization, and circulation modulation—that consistently yield high tumor-to-blood ratios and reproducible in vivo efficacy in LAT1+ models.
ASCT2-mediated glutamine uptake and LAT1-facilitated essential amino acid transport are linked to mTORC1 activation [134]. LAT1 inhibitors such as JPH203 suppress amino acid signaling and global protein translation [158], although off-target effects on SNAT2 and LAT1 complicate selectivity [159]. Dual inhibition of ASCT2 and LAT1 has therefore been proposed to more effectively collapse the glutamine–leucine–mTOR axis [160].
In parallel, xCT (SLC7A11) sustains cystine uptake for glutathione biosynthesis and redox defense [100]. NSCLC can exhibit dysregulated KEAP1–NRF2 signaling, which drives xCT activation [100], and [18F]FSPG PET enables noninvasive readouts of NRF2-linked system Xc− activity [112]. Pharmacologic blockade of system Xc− depletes glutathione, increases lipid peroxidation, and radiosensitizes tumors in vivo [100,161,162]. In addition, erastin and sorafenib trigger ferroptosis through xCT inhibition [163,164]. Thus, ASCT2 and xCT represent rational co-targets that can be layered onto LAT1-directed α-radiopharmaceuticals, producing synthetic lethality through the combined deprivation of amino acids and the delivery of DNA damage.
4.5. Applications in Other Malignancies
Beyond NSCLC, amino acid transporter-targeted 211At radiopharmaceuticals have demonstrated potent and selective antitumor efficacy across multiple malignancies, highlighting their broad translational promise. In pancreatic cancer (PANC-1) xenografts, intravenous 211At-AAMT (0.4 MBq/mouse) significantly suppressed tumor growth, whereas BCH pretreatment reduced tumor uptake, confirming LAT1-mediated delivery. In the same preclinical study, a melanoma (B16F10) experimental lung-metastasis model treated with 211At-AAMT (0.1–1 MBq/mouse) showed markedly fewer pulmonary nodules without appreciable weight loss, indicating strong therapeutic efficacy with minimal systemic toxicity [63]. In ovarian cancer (SKOV3) xenografts, the LAT1-targeting analog 2-[211At]AAMP achieved selective uptake and significantly improved survival at tolerated activities [155]. Across glioma models—C6, U-87MG, and GL261—211At-labeled phenylalanine analogs produced dose-dependent tumor suppression at 0.1–1 MBq/mouse, with BCH competitively reducing cellular uptake, consistent with system-L/LAT1 transport [165]. In orthotopic GL261 glioblastoma, intratumoral 211At-anti-syndecan-1 yielded robust intracranial control and markedly prolonged survival, illustrating durable responses in brain tumors [166]. Extending beyond epithelial malignancies, bone and soft-tissue sarcomas also responded to LAT1-targeted 211At-AAMT-O-Me-L in preclinical studies, supporting its applicability to mesenchymal tumors [167]. Collectively, these cross-cancer findings verify mechanism-driven uptake, dose-dependent efficacy, and favorable tolerability, providing a strong biological foundation for adapting LAT1/ASCT2/xCT-targeted α-radiotherapeutics to NSCLC and guiding the translational strategies discussed below.
4.6. Delivery Platforms for Enhanced Retention
While small-molecule transporter ligands enable rapid tumor penetration and efficient uptake, their clinical translation is limited by their radiochemical fragility, rapid clearance, and restricted structural diversity. To address these limitations, biological and nanotechnology platforms, such as monoclonal antibodies (mAbs), antibody–drug conjugates (ADCs), engineered peptides, and nanocarriers, are being developed as complementary systems for 211At-based transporter-targeted therapies. These modalities extend beyond substrate analogues by enabling multivalent binding, prolonged circulation, and modular payload integration, thereby broadening the therapeutic landscape for resistant NSCLC.
4.6.1. Antibodies and ADCs
CD98hc (SLC3A2), which interacts with integrins and regulates adhesion-associated signaling [168], forms a disulfide-linked heterodimer with LAT1 [169]. Anti-LAT1 antibodies bind extracellular epitopes, induce internalization, and inhibit amino acid uptake, typically leading to antibody-dependent cellular cytotoxicity (ADCC) and tumor suppression in xenograft models. These effects suppress tumor growth in xenograft models [170]. CD98hc antibodies, such as IGN523, exhibit potent ADCC and achieve tumor control comparable to carboplatin in NSCLC xenografts [171]. ADC formats extend this principle. For example, CD98hc–DM1 and CD98hc–MMAE conjugates exploit antigen-mediated endocytosis and lysosomal release, producing mitotic catastrophe or DNA damage [171,172,173,174]. Payloads such as auristatins (MMAE) disrupt microtubules [175], whereas pyrrolobenzodiazepine (PBD) dimers generate lethal DNA cross-links [176]. For antibody-based targeted delivery of cytotoxic payloads, improving the therapeutic index through target/payload/linker optimization is a central design goal [177]. At the same time, tumor penetration and payload-related toxicities remain important considerations [178].
4.6.2. Peptides
Peptides offer intermediate pharmacokinetics, balancing target specificity with short circulation times [179] that are compatible with the 211At half-life. Cyclization, peptidomimetic engineering, and multivalency strategies further improve stability and avidity. 211At-labeled RGD peptides demonstrate high tumor uptake, with reduced blood radioactivity that improves tumor-over-blood contrast, and reproducible antitumor efficacy [180,181]. Although LAT1-selective peptides were not evaluated in these studies, these studies confirm that short-lived α-emitters can be effectively deployed using peptide scaffolds.
4.6.3. Nanocarriers
Table 3 provides a mechanism-oriented synopsis of amino acid transporter-directed strategies and 211At conjugates in NSCLC, with representative agents and key preclinical readouts, where indicated. Early translational data are also noted.

Table 3.
Therapeutic strategies targeting amino acid transporters and 211At radiopharmaceuticals in NSCLC.
Nanocarriers address the challenges of rapid clearance and deactivation by enhancing circulation and tumor accumulation. LAT1-targeted liposomes (100–135 nm, L-DOPA-functionalized) demonstrate transporter-mediated uptake and improved brain tumor penetration [191]. PLGA nanoparticles decorated with glutamate–polyoxyethylene stearate recycle LAT1 and enhance tumor uptake, leading to improved in vivo efficacy [192]. Glutamine-conjugated PLGA nanoparticles similarly increase tumor accumulation and enable tumor scintigraphy [193]. 211At can also be rapidly loaded onto gold nanoparticles (~5 min, high yield), achieving systemic antitumor activity [194]. PEGylation and tumor-penetrating peptides (e.g., iRGD) further enhance pharmacokinetics and intratumoral penetration [195,196]. Hybrid PET-theranostic nanoplatforms can aid patient selection, dosimetry, and therapy monitoring, although scalability and regulatory barriers remain [197,198].
4.7. Toward Clinical Translation
Across α-therapy applications beyond NSCLC, variability in response depth and durability indicates recurrent constraints relevant to 211At transporter-targeted strategies. Intratumoral heterogeneity of radiopharmaceutical uptake [199] and spatially uneven target/transporter expression [200] can generate micro-dosimetric cold spots, compromising tumor control. Meanwhile, activation of ATM/BRCA1-linked DNA-damage responses can mitigate radiation cytotoxicity [201] and regional hypoxia/perfusion deficits [202], further limiting the effectiveness of α-particle lethality and dose deposition. Metabolic compensation via ASCT2/xCT may restore amino acid flux when glutamine transport is perturbed [203], and pharmacokinetic liabilities—including in vivo deactivation and short half-life-related constraints—reduce target exposure [204]. Within this context, optimization of dosimetry and pharmacokinetics, structured mitigation of hematologic and thyroidal toxicity, and biomarker-guided selection using transporter PET, together with GMP-ready automation, are central considerations for advancing 211At in NSCLC.
4.7.1. Dosimetry and Pharmacokinetics
211At α-tracks traverse on the order of several tens of micrometers in soft tissue [138], underscoring the importance of microdistribution and tumor cell geometry beyond organ-averaged dosimetry [205]. Bone marrow is a key organ for systemic dose considerations [205], whereas in vivo deactivation increases exposure to the thyroid and stomach. Prophylactic administration of potassium iodide or perchlorate effectively reduces thyroid and gastric uptake [206]. Preclinical studies show that hematologic depression is typically transient, with blood counts recovering by approximately 28 days after sub-myeloablative dosing [207]. Scaffold stability further influences dosimetry. Aryl–astatine conjugates can exhibit variable in vivo deastatination, particularly upon internalization [13], whereas SAGMB prosthetics are designed to improve bond integrity, with halogen-bond interactions implicated relative to classical aryl–astatine motifs [208]. Clinically, intraperitoneal administration of 211At-MX35 F(ab′)2 was well tolerated [209], supporting the safety of localized α-radioimmunotherapy. By contrast, LAT1-directed small molecules remain at the preclinical stage, with no large-animal data available [210], defining a key translational gap. Beyond intraperitoneal applications, systemic α-therapy has clinical proof-of-concept with 223Ra in mCRPC [150], while 225Ac-PSMA is under clinical evaluation [211], providing a translational precedent for 211At.
4.7.2. Safety and Toxicity Mitigation
In systemic 211At therapy, normal-organ exposure is substantial and thyroid uptake of free astatine requires blocking [15]. Scaffold stabilization strategies, including neighboring-group substitution and hydrophilic linker engineering, reduce radiolysis and minimize off-target uptake [212]. Physiologically based pharmacokinetic modeling shows delivery-limited and heterogeneous tumor uptake, supporting the rationale for patient-specific dosimetry [213]. Together, these insights provide a framework for rational safety management in transporter-targeted 211At therapies.
4.7.3. Patient Selection and Trial Design
LAT1- and xCT-targeted PET tracers are emerging as dynamic biomarkers that capture transporter activity and provide non-invasive readouts of pathway activation that may support therapy monitoring. LAT1-targeted imaging with [18F]FAMT has been evaluated as a functional biomarker for monitoring LAT1 activity and prognosis in NSCLC [58,214]. Likewise, [18F]FSPG PET non-invasively quantifies xCT activity and KEAP1/NRF2-driven metabolic adaptation [112], underscoring the potential of transporter-based imaging to inform patient selection and patient-specific dosimetry in future 211At trials.
Building on these functional insights, several tracers have entered clinical validation as tools for stratification. [18F]FAMT correlates with LAT1 expression and may aid identification of LAT1+ NSCLC suitable for therapy planning, pending prospective validation [215]. [18F]FBPA PET reflects LAT1 expression in lung and mediastinal tumors [216]. [18F]FSPG PET quantifies redox metabolism and NRF2 activity [112], providing a complementary approach for selecting patients most likely to benefit from transporter-targeted α-therapy.
Early-phase protocols should incorporate theranostic, patient-specific dosimetry, using isotopic surrogates or trace therapeutic administrations to individualize activity [217]. GMP-compliant automation is advancing. The COSMiC-Mini dry-distillation system produces GMP-compliant, sterile [211At]NaAt with complete quality control, with total production achieved within ~3 h from target setup [218]. The same platform supports the automated synthesis of [211At]MABG with high radiochemical purity in ~28 min [219]. Such closed, GMP-compliant platforms not only ensure radiochemical reproducibility and sterility but also meet regulatory expectations, thereby lowering the barrier for multi-center clinical trials.
Collectively, translational studies emphasize that the success of 211At-based transporter-targeted theranostics will hinge on precise dosimetry, scaffold stabilization, patient stratification with companion imaging, and integration with established NSCLC treatments. With optimized chemistry and biologic delivery systems, transporter-guided 211At therapy is poised to advance toward first-in-human evaluation.
5. Integration with Existing NSCLC Therapies
5.1. Integration with Targeted Agents
Pharmacological blockade of LAT1 with JPH203 suppresses mTORC1 signaling [220]. In preclinical NSCLC models, LAT1 inhibition enhanced the effect of the EGFR-TKI gefitinib when using the competitive substrate BCH [106]. This was combined with JPH203-mediated radiosensitization, rather than EGFR-TKI synergy [182], suggesting a rational combination strategy with transporter-targeted α-therapy. However, resistance to kinase inhibitors inevitably emerges, typically within 8–14 months for first- and second-generation EGFR TKIs [221] and through diverse adaptations to KRASG12C inhibitors [222]. In this context, transporter-targeted 211At ligands offer a mechanistically orthogonal approach [223], as α-particles induce clustered DNA double-strand breaks that are poorly repaired [224]. Their cytotoxicity is largely oxygen-independent (OER ≈ 1), enabling efficacy in hypoxic niches where resistant clones persist [139]. Preclinical studies with LAT1-directed ligands such as 211At-AAMT confirm robust tumor uptake and suppression in xenograft models [63]. Collectively, these features support the rationale for exploring transporter-targeted 211At conjugates as complementary partners to EGFR and KRAS inhibitors, particularly for compound-resistant mutations with limited treatment options.
5.2. Therapeutic Synergy with Radiotherapy and Chemotherapy
Radiotherapy remains a central component of NSCLC management, particularly in cases of locally advanced disease [225]. Its efficacy, however, is constrained in hypoxic tumors, where the OER for low-LET photons approaches 2.5–3 [77]. By contrast, high-LET α-particles show markedly reduced oxygen dependence (with OER values approaching unity), enabling effective killing in hypoxic niches [139]. Targeting amino acid transporters further amplifies this synergy: in KEAP1/NRF2-activated contexts, SLC7A11 (xCT) dependency can be exploited [161], pharmacologic xCT inhibition with sulfasalazine depletes glutathione, elevates ROS, and enhances cisplatin cytotoxicity in preclinical models [226]. LAT1 inhibition (e.g., JPH203) sensitizes NSCLC cells to irradiation by downregulating mTOR signaling and promoting radiation-induced senescence [182]. Taken together, these mechanisms—reduced hypoxia sensitivity with α-radiation, heightened redox stress, and altered damage responses—provide a mechanistic rationale for integrating transporter-targeted α-therapy (e.g., 211At agents) with radiotherapy or platinum chemotherapy to broaden the therapeutic index and suppress resistant subpopulations.
5.3. Synergy with Immunotherapy
Metabolic interventions provide a unique bridge between α-particle therapy and immune checkpoint blockade. Inhibition of glutamine uptake with V-9302 enhances antitumor immunity in TNBC models [109], illustrating how transporter blockade remodels the tumor–immune interface. α-particle irradiation itself can stimulate immunogenic cell death (ICD), with DAMP exposure such as HMGB1 and calreticulin [227]. For example, 223Ra treatment increases the exposure of HMGB1 and calreticulin, promoting dendritic cell maturation and T-cell priming [228]. At the transporter–immune axis, LAT1 inhibition reduces PD-L1 expression in NSCLC cells [220], while xCT blockade disrupts glutathione homeostasis and supports combination with checkpoint blockade [229]. Preclinical evidence shows that α-therapy plus PD-L1 antibodies achieves superior tumor control compared with either modality alone [230]. Importantly, treatment sequencing remains unresolved: concurrent administration outperformed sequential regimens in peptide-based TRT models [231]. This synergy may be particularly impactful in oncogene-driven NSCLC, where ICIs alone have shown limited benefit [25,26], positioning transporter-targeted α-therapy as a mechanistically orthogonal partner to immunotherapy.
5.4. Synergies with Ferroptosis and DNA Damage
211At α-particles induce clustered, irreparable DNA double-strand breaks with minimal oxygen dependence [188,224]. When combined with the inhibition of ASCT2 and xCT, glutamine- and cystine-dependent metabolic circuits are disrupted, resulting in the depletion of NAD(P)H and glutathione, and amplifying ferroptotic cell death [100,232]. This effect is particularly relevant in KEAP1/NRF2-altered tumors, where NRF2-driven antioxidant programs confer resistance to both ferroptosis and radiation [233]. Parallel inhibition of mTOR signaling with LAT1 blockade (e.g., JPH203) further sensitizes tumors to irradiation [182]. These effects are mechanistically linked to the transporter-mediated metabolic adaptations outlined in Section 3.2, highlighting the potential of integrated targeting strategies.
5.5. Theranostic Framework
211At-labeled transporter ligands could serve within a unified theranostic framework that links patient selection, individualized treatment planning, and longitudinal monitoring [234]. LAT1-targeted PET tracers (e.g., 18F-FAMT, 18F-FBPA) allow noninvasive assessment of LAT1 activity and can aid treatment planning (e.g., BNCT selection using quantitative uptake) [58]. Similarly, [18F]FSPG PET captures xCT/NRF2 activity, and heterogeneous retention in NSCLC tumors offers a complementary biomarker for therapeutic stratification [112,235]. Preclinical PET studies further reveal that intra- and inter-tumoral uptake heterogeneity often contrasts with homogeneous ex vivo receptor expression, underscoring the predictive value of functional imaging for therapies requiring uniform target engagement [236]. Clinically, for NSCLC with KEAP1/NFE2L2 mutations, the benefit from immune checkpoint inhibitors remains uncertain, with conflicting data [237]. In EGFR-mutant resistance models, NRF2 inhibition restores vulnerability and supports a rationale for combinations with EGFR-TKIs [238]. These insights highlight transporter-based imaging as a companion biomarker to guide adaptive trial designs that integrate imaging–dosimetry feedback. Lessons from early-phase α-therapy trials, such as 225Ac-PSMA in prostate cancer, confirm the feasibility of this paradigm [211]. Collectively, transporter-targeted 211At theranostics may evolve from experimental probes into integral components of multimodal NSCLC management, with priorities including the development of standardized biomarkers, dosing algorithms, and the conduct of harmonized multi-center trials.
6. Conclusions and Future Perspectives
NSCLC remains a significant cause of cancer-related mortality, mainly because therapeutic resistance persists despite multimodal advances. Amino acid transporters—particularly LAT1, ASCT2, and xCT—serve as critical regulators of tumor metabolism and immune evasion, offering rational entry points for therapeutic intervention and molecular imaging. Among α-emitters, 211At combines an optimal half-life, high LET, and short-range energy deposition, which enable precise, mechanism-driven targeting. By exploiting these properties, 211At-labeled ligands directed at amino acid transporters provide an orthogonal strategy to overcome resistance and intratumoral heterogeneity—two enduring barriers to durable response in NSCLC. These advantages define the rationale for integrating 211At into translational frameworks that link radiochemistry, tumor metabolism, and clinical theranostics.
Looking ahead, critical priorities include biomarker-driven patient selection—for example, LAT1- and xCT-targeted PET imaging and multi-omics profiling; optimization of delivery platforms, including small molecules, antibodies, peptides, and nanocarriers; and integration into adaptive theranostic frameworks that enable longitudinal monitoring and individualized dosing. Rational combinations with EGFR and KRAS inhibitors, immunotherapy, radiotherapy, and ferroptosis inducers will be essential to maximize therapeutic efficacy. Early-phase clinical translation should leverage lessons from 223Ra and 225Ac α-therapy trials, supported by GMP-compliant automation and international cooperative networks.
With continued progress in radiochemistry, tumor biology, and translational infrastructure, 211At-based transporter targeting is expected to evolve from experimental innovation toward clinical implementation, representing a promising component of next-generation multimodal NSCLC therapy that may ultimately yield durable survival benefits for patients worldwide.
Author Contributions
Conceptualization, K.K.-N. and S.F.; writing—original draft preparation, S.F.; writing—review and editing, K.K.-N., K.H. and H.Y.; visualization, K.H.; supervision, Y.S.; project administration, K.K.-N.; funding acquisition, K.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP25K11007 and JP21K07619.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We thank H. Egawa and A. Saito-Kawakami for their great supports.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 131I | Iodine-131 |
| 177Lu | Lutetium-177 |
| 18F | Fluorine-18 |
| 18F-FAMT | 18F-L-3-fluoro-α-methyl-tyrosine |
| 18F-FBPA | 18F-fluoro-boronophenylalanine |
| [18F]FSPG | (4S)-4-(3-fluoropropyl)-L-glutamate |
| 211At | Astatine-211 |
| 213Bi | Bismuth-213 |
| 223Ra | Radium-223 |
| 225Ac | Actinium-225 |
| AA | Amino acid |
| AAMP | 2-[211At]AAMP (LAT1-targeted analogue) |
| AAMT | 211At-labeled α-methyl-L-tyrosine |
| ABCB1 | ATP-binding cassette subfamily B member 1 (P-glycoprotein) |
| ABCG2 | ATP-binding cassette subfamily G member 2 (BCRP) |
| ADC | Antibody–drug conjugate |
| ADCC | Antibody-dependent cellular cytotoxicity |
| AHR | Aryl hydrocarbon receptor |
| AKT | Protein kinase B |
| ALK | Anaplastic lymphoma kinase |
| ASCT2 | Alanine-serine-cysteine transporter 2 (SLC1A5) |
| ATF4 | Activating transcription factor 4 |
| ATR | Ataxia telangiectasia and Rad3-related protein |
| B2M | Beta-2-microglobulin |
| BCH | 2-Aminobicyclo[2.2.1]heptane-2-carboxylic acid |
| BenSer | Benzylserine |
| CD98hc/4F2hc | Heavy chain of CD98 (SLC3A2) |
| CDC | Complement-dependent cytotoxicity |
| CHK1 | Checkpoint kinase 1 |
| CSC | Cancer stem cell |
| CYP | Cytochrome P450 |
| DDR | DNA damage response |
| DM1 | Maytansinoid DM1 |
| DNA | Deoxyribonucleic acid |
| DSB(s) | Double-strand break(s) |
| DTP | Drug-tolerant persister(s) |
| EBRT | External-beam radiotherapy |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial–mesenchymal transition |
| ERBB3 | Erb-B2 receptor tyrosine kinase 3 (HER3) |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| F(ab′)2 | Antibody fragment F(ab′)2 |
| FIN(s) | Ferroptosis inducer(s) |
| GPX4 | Glutathione peroxidase 4 |
| GMP | Good Manufacturing Practice |
| GSH | Glutathione |
| GATOR2 | GAP activity toward Rags complex 2 |
| HIF-1 | Hypoxia-inducible factor 1 |
| HIF-2α | Hypoxia-inducible factor 2 alpha |
| HLA-I | Human leukocyte antigen class I |
| HMGB1 | High mobility group box 1 |
| HR | Homologous recombination |
| ICI(s) | Immune checkpoint inhibitor(s) |
| ICD | Immunogenic cell death |
| IDO1 | Indoleamine 2,3-dioxygenase 1 |
| IEDDA | Inverse electron-demand Diels–Alder reaction |
| iRGD | Internalizing the RGD peptide |
| JPH203 | Nanvuranlat (LAT1 inhibitor) |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| KRASG12C | KRAS p.G12C mutation |
| LAG-3 | Lymphocyte-activation gene 3 |
| LAT1 | L-type amino acid transporter 1 (SLC7A5) |
| LET | Linear energy transfer |
| MABG | meta-Astatobenzylguanidine |
| MAPK | Mitogen-activated protein kinase |
| mAb | Monoclonal antibody |
| mCRPC | Metastatic castration-resistant prostate cancer |
| MeV | Mega-electron volt |
| MDSC(s) | Myeloid-derived suppressor cell(s) |
| MMAE | Monomethyl auristatin E |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | mTOR complex 1 |
| NaAt | Sodium astatide ([211At]NaAt) |
| NAD(P)H | Nicotinamide adenine dinucleotide (phosphate), reduced form |
| NFE2L2 | Nuclear factor erythroid-derived 2-like 2 |
| NHEJ | Non-homologous end-joining |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| OER | Oxygen-enhancement ratio |
| OS | Overall survival |
| PD-L1 | Programmed death-ligand 1 |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| PI3K | Phosphoinositide 3-kinase |
| PK | Pharmacokinetics |
| PLGA | Poly(lactic-co-glycolic acid) |
| PBD | Pyrrolobenzodiazepine |
| PRIT | Pretargeted radioimmunotherapy |
| PSMA | Prostate-specific membrane antigen |
| Rag GTPases | Ras-related GTP-binding proteins A–D |
| RGD | Arg-Gly-Asp peptide |
| ROS | Reactive oxygen species |
| ROS1 | c-ros oncogene 1 (receptor tyrosine kinase) |
| RT | Radiotherapy |
| SAB | N-succinimidyl astatobenzoate |
| SAGMB | Guanidinomethyl-substituted succinimidyl astatobenzoate |
| SBRT | Stereotactic body radiotherapy |
| SLC1A5 | Solute carrier family one member 5 (ASCT2) |
| SLC3A2 | Solute carrier family three member 2 (CD98hc/4F2hc) |
| SLC7A5 | Solute carrier family seven member 5 (LAT1) |
| SLC7A11 | Solute carrier family seven member 11 (xCT) |
| SLC38A2 (SNAT2) | Sodium-coupled neutral amino acid transporter 2 |
| SLC43A2 | Solute carrier family 43 member 2 |
| SNAT2 | See SLC38A2 |
| TAZ | WW domain-containing transcription regulator 1 |
| TIGIT | T cell immunoreceptor with Ig and ITIM domains |
| TIM-3 | T cell immunoglobulin and mucin domain 3 |
| TKI(s) | Tyrosine kinase inhibitor(s) |
| TME | Tumor microenvironment |
| Treg(s) | Regulatory T cell(s) |
| TRT | Targeted radionuclide therapy |
| V-9302 | Small-molecule glutamine transport inhibitor |
| VISTA | V-domain Ig suppressor of T-cell activation |
| WEE1 | WEE1 G2 checkpoint kinase |
| xCT | Cystine/glutamate antiporter (SLC7A11) |
| YAP | Yes-associated protein |
References
- Zhou, J.; Xu, Y.; Liu, J.; Feng, L.; Yu, J.; Chen, D. Global burden of lung cancer in 2022 and projections to 2050: Incidence and mortality estimates from GLOBOCAN. Cancer Epidemiol. 2024, 93, 102693. [Google Scholar] [CrossRef]
- Tang, F.H.; Wong, H.Y.T.; Tsang, P.S.W.; Yau, M.; Tam, S.Y.; Law, L.; Yau, K.; Wong, J.; Farah, F.H.M.; Wong, J. Recent advancements in lung cancer research: A narrative review. Transl. Lung Cancer Res. 2025, 14, 975–990. [Google Scholar] [CrossRef]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Liu, X.; Wang, Y.; Zheng, D.; Meng, Q.; Jiang, L.; Yang, S.; Zhang, S.; Zhang, X.; Liu, Y.; et al. Mechanisms of resistance to targeted therapy and immunotherapy in non-small cell lung cancer: Promising strategies to overcome challenges. Front. Immunol. 2024, 15, 1366260. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Y.; Huang, Y.; Wu, J.; Bao, W.; Xue, C.; Li, X.; Dong, S.; Dong, Z.; Hu, S. Advances in molecular pathology and therapy of non-small cell lung cancer. Signal Transduct. Target. Ther. 2025, 10, 186. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wang, W.; Li, X.; Sun, X.; Zhao, Y.; Wang, Q.; Li, Y.; Hu, F.; Ren, H. Metabolic reprogramming and therapeutic resistance in primary and metastatic breast cancer. Mol. Cancer 2024, 23, 261. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zhang, F.; Xu, F.; Yang, C. Metabolic reprogramming and therapeutic targeting in non-small cell lung cancer: Emerging insights beyond the Warburg effect. Front. Oncol. 2025, 15, 1564226. [Google Scholar] [CrossRef]
- Furuse, J.; Ikeda, M.; Ueno, M.; Furukawa, M.; Morizane, C.; Takehara, T.; Nishina, T.; Todaka, A.; Okano, N.; Hara, K.; et al. A phase II placebo-controlled study of the effect and safety of nanvuranlat in patients with advanced biliary tract cancers previously treated by systemic chemotherapy. Clin. Cancer Res. 2024, 30, 3990–3995. [Google Scholar] [CrossRef]
- Morozova, V.; Pellegata, D.; Singer, S.; Charles, R.-P.; Müller, J.; Altmann, K.-H.; Gertsch, J. Pharmacodynamic analyses of LAT1 inhibitors in vitro and in vivo by targeted metabolomics reveal target-independent effects. Biomed. Pharmacother. 2025, 190, 118402. [Google Scholar] [CrossRef]
- Maris, M.; Salles, G.; Kim, W.S.; Kim, T.M.; Lyons, R.M.; Arellano, M.; Karmali, R.; Schiller, G.; Cull, E.; Abboud, C.N.; et al. ASCT2-targeting antibody-drug conjugate MEDI7247 in adult patients with relapsed/refractory hematological malignancies: A first-in-human, phase 1 study. Target. Oncol. 2024, 19, 321–332. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, J.; Qin, S.; Luan, X.; Zhang, H.; Yang, M.; Jin, Y.; Yang, G.; Yu, F. Targeted alpha therapy: A comprehensive analysis of the biological effects from “local-regional-systemic” dimensions. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 2031–2048. [Google Scholar] [CrossRef]
- Vanermen, M.; Ligeour, M.; Oliveira, M.-C.; Gestin, J.-F.; Elvas, F.; Navarro, L.; Guérard, F. Astatine-211 radiolabelling chemistry: From basics to advanced biological applications. EJNMMI Radiopharm. Chem. 2024, 9, 69. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Johnson, G.B.; Halfdanarson, T.R.; Sartor, O. Targeted alpha-particle therapy: A review of current trials. Int. J. Mol. Sci. 2023, 24, 11626. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, P.; Bäck, T.; Bergmark, K.; Hallqvist, A.; Johansson, M.; Aneheim, E.; Lindegren, S.; Timperanza, C.; Smerud, K.; Palm, S. Astatine-211-based radionuclide therapy: Current clinical trial landscape. Front. Med. 2023, 9, 1076210. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Zhou, Y.; Wang, J.; Wu, L. Immune checkpoint inhibitor-based therapy as the first-line treatment for advanced non-small cell lung cancer: Efficacy, challenges, and future perspectives. Thorac. Cancer 2025, 16, e70113. [Google Scholar] [CrossRef]
- Su, P.-L.; Furuya, N.; Asrar, A.; Rolfo, C.; Li, Z.; Carbone, D.P.; He, K. Recent advances in therapeutic strategies for non-small cell lung cancer. J. Hematol. Oncol. 2025, 18, 35. [Google Scholar] [CrossRef]
- Mirsky, M.M.; Myers, K.E.; Abul-Khoudoud, S.O.; Lee, J.Y.; Bruno, D.S. Systemic therapy for operable NSCLC: A review of the literature and discussion of future directions. J. Clin. Med. 2025, 14, 4127. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Hasan, S.; Horne, Z.D.; Colonias, A.; Wegner, R.E. Stereotactic body radiation therapy in early-stage NSCLC: Historical review, contemporary evidence, and future implications. Lung Cancer Manag. 2019, 8, LMT09. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.N.; Owen, D. Advances in stereotactic body radiation therapy for lung cancer. Cancer J. 2024, 30, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Burcher, K.; Karukonda, P.; Kelsey, C.; Mullikin, T.; Antonia, S.J.; Oduah, E.I. A critical review of immunomodulation in the management of inoperable stage III NSCLC. Cancers 2025, 17, 1829. [Google Scholar] [CrossRef]
- Gálffy, G.; Morócz, É.; Korompay, R.; Hécz, R.; Bujdosó, R.; Puskás, R.; Lovas, T.; Gáspár, E.; Yahya, K.; Király, P.; et al. Targeted therapeutic options in early and metastatic NSCLC: Overview. Pathol. Oncol. Res. 2024, 30, 1611715. [Google Scholar] [CrossRef]
- Ahluwalia, V.S.; Parikh, R.B. Chemoimmunotherapy vs immunotherapy monotherapy receipt in advanced non-small cell lung cancer. JAMA Netw. Open 2025, 8, e2459380. [Google Scholar] [CrossRef]
- Tian, T.; Li, Y.; Li, J.; Xu, H.; Fan, H.; Zhu, J.; Wang, Y.; Peng, F.; Gong, Y.; Du, Y.; et al. Immunotherapy for patients with advanced non-small cell lung cancer harboring oncogenic driver alterations other than EGFR: A multicenter real-world analysis. Transl. Lung Cancer Res. 2024, 13, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Attili, I.; Passaro, A.; Corvaja, C.; Aliaga, P.T.; Signore, E.D.; Spitaleri, G.; de Marinis, F. Immune checkpoint inhibitors in EGFR-mutant non-small cell lung cancer: A systematic review. Cancer Treat. Rev. 2023, 119, 102602. [Google Scholar] [CrossRef] [PubMed]
- Lisberg, A.; Cummings, A.; Goldman, J.W.; Bornazyan, K.; Reese, N.; Wang, T.; Coluzzi, P.; Ledezma, B.; Mendenhall, M.; Hunt, J.; et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1-positive, tyrosine kinase inhibitor-naïve patients with advanced NSCLC. J. Thorac. Oncol. 2018, 13, 1138–1145. [Google Scholar] [CrossRef]
- Addeo, A.; Passaro, A.; Malapelle, U.; Banna, G.L.; Subbiah, V.; Friedlaender, A. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat. Rev. 2021, 96, 102179. [Google Scholar] [CrossRef]
- Liu, L.; Soler, J.; Reckamp, K.L.; Sankar, K. Emerging targets in non-small cell lung cancer. Int. J. Mol. Sci. 2024, 25, 10046. [Google Scholar] [CrossRef] [PubMed]
- Vander Velde, R.; Yoon, N.; Marusyk, V.; Durmaz, A.; Dhawan, A.; Miroshnychenko, D.; Lozano-Peral, D.; Desai, B.; Balynska, O.; Poleszhuk, J.; et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor-specific selective pressures. Nat. Commun. 2020, 11, 2393. [Google Scholar] [CrossRef]
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef]
- Liu, J.; Cai, Y.; Liu, J.; Chen, D.; Wu, X. Immunotherapy resistance and therapeutic strategies in PD-L1 high-expression non-small cell lung cancer. Onco Targets Ther. 2025, 18, 953–966. [Google Scholar] [CrossRef]
- Xie, J.; Gao, Y.; Xu, W.; Zhu, J. Mechanisms of resistance to ALK inhibitors and corresponding treatment strategies in lung cancer. Int. J. Gen. Med. 2025, 18, 2151–2171. [Google Scholar] [CrossRef]
- Yu, J.; Kong, X.; Feng, Y. Tumor microenvironment-driven resistance to immunotherapy in non-small cell lung cancer: Strategies for cold-to-hot tumor transformation. Cancer Drug Resist. 2025, 8, 14. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Hatcher, J.M.; Bahcall, M.; Choi, H.G.; Gao, Y.; Sim, T.; George, R.; Jänne, P.A.; Gray, N.S. Discovery of inhibitors that overcome the G1202R anaplastic lymphoma kinase resistance mutation. J. Med. Chem. 2015, 58, 9296–9308. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wang, K.; Sun, S.-Y. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J. Hematol. Oncol. 2019, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Yang, J.C.-H.; Yu, H.; Kim, S.-W.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Mann, H.; Thress, K.S.; et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 2020, 31, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Huang, Y.; Zhao, Q. Epigenetic alterations and inflammation as emerging use for the advancement of treatment in non-small cell lung cancer. Front. Immunol. 2022, 13, 878740. [Google Scholar] [CrossRef]
- Lin, S.; Ruan, H.; Qin, L.; Zhao, C.; Gu, M.; Wang, Z.; Liu, B.; Wang, H.; Wang, J. Acquired resistance to EGFR-TKIs in NSCLC mediates epigenetic downregulation of MUC17 by facilitating NF-κB activity via UHRF1/DNMT1 complex. Int. J. Biol. Sci. 2023, 19, 832–851. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Liu, S.; Yang, W.; Chen, H.; Guo, N.; Sun, W.; Zhao, Y.; Ren, Y.; Jia, L.; et al. EZH2/G9a interact to mediate drug resistance in non-small-cell lung cancer by regulating the SMAD4/ERK/c-Myc signaling axis. Cell Rep. 2024, 43, 113714. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Qian, G.; Zong, D.; Fan, S.; Owonikoko, T.K.; Ramalingam, S.S.; Sun, S.-Y. Overcoming acquired resistance of epidermal growth factor receptor-mutant non-small cell lung cancer cells to osimertinib by combining osimertinib with the histone deacetylase inhibitor panobinostat (LBH589). Cancer 2020, 126, 2024–2033. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ren, S.; Li, X.; Wang, Y.; Garfield, D.; Zhou, S.; Chen, X.; Su, C.; Chen, M.; Kuang, P.; et al. MiR-21 overexpression is associated with acquired resistance of EGFR-TKI in non-small cell lung cancer. Lung Cancer 2014, 83, 146–153. [Google Scholar] [CrossRef]
- Ji, Y.; Xiao, C.; Fan, T.; Deng, Z.; Wang, D.; Cai, W.; Li, J.; Liao, T.; Li, C.; He, J. The epigenetic hallmarks of immune cells in cancer. Mol. Cancer 2025, 24, 66. [Google Scholar] [CrossRef]
- Mahfoudhi, E.; Ricordel, C.; Lecuyer, G.; Mouric, C.; Lena, H.; Pedeux, R. Preclinical models for acquired resistance to third-generation EGFR inhibitors in NSCLC: Functional studies and drug combinations used to overcome resistance. Front. Oncol. 2022, 12, 853501. [Google Scholar] [CrossRef]
- Liang, H.; Xu, Y.; Zhao, J.; Chen, M.; Wang, M. Hippo pathway in non-small cell lung cancer: Mechanisms, potential targets, and biomarkers. Cancer Gene Ther. 2024, 31, 652–666. [Google Scholar] [CrossRef]
- Liaghat, M.; Ferdousmakan, S.; Mortazavi, S.H.; Yahyazadeh, S.; Irani, A.; Banihashemi, S.; Seyedi Asl, F.S.; Akbari, A.; Farzam, F.; Aziziyan, F.; et al. The impact of epithelial–mesenchymal transition induced by metabolic processes and intracellular signaling pathways on chemoresistance, metastasis, and recurrence in solid tumors. Cell Commun. Signal. 2024, 22, 575. [Google Scholar] [CrossRef]
- Yan, T.; Shi, J. Angiogenesis and EMT regulators in the tumor microenvironment in lung cancer and immunotherapy. Front. Immunol. 2024, 15, 1509195. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, L.; Sun, Q. Clinically relevant ABC transporter for anti-cancer drug resistance. Front. Pharmacol. 2021, 12, 648407. [Google Scholar] [CrossRef]
- Nelson, R.S.; Seligson, N.D.; Bottiglieri, S.; Carballido, E.; Del Cueto, A.; Imanirad, I.; Levine, R.; Parker, A.S.; Swain, S.M.; Tillman, E.M.; et al. UGT1A1-guided cancer therapy: Review of the evidence and considerations for clinical implementation. Cancers 2021, 13, 1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Wang, Y.; Jin, W.; Zhang, Z.; Jin, L.; Qian, J.; Zheng, L. CYP3A4 and CYP3A5: The crucial roles in clinical drug metabolism and the significant implications of genetic polymorphisms. PeerJ 2024, 12, e18636. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Vaz, R.J. Validating the use of rational modification of compounds to reduce P-gp efflux. Arch. Pharmacol. Ther. 2024, 6, 34–39. [Google Scholar] [CrossRef]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming cancer drug resistance with nanoparticle strategies for key protein inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef]
- van der Kleij, M.B.A.; Guchelaar, N.A.D.; Meertens, M.; Westerdijk, K.; Giraud, E.L.; Bleckman, R.F.; Groenland, S.L.; van Eerden, R.A.G.; Imholz, A.L.T.; Vulink, A.J.E.; et al. Reasons for non-feasibility of therapeutic drug monitoring of oral targeted therapies in oncology: An analysis of the closed cohorts of a multicentre prospective study. Br. J. Cancer 2024, 131, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, W.; Zhang, P.; Guo, F.; Liu, M. Current trends in sensitizing immune checkpoint inhibitors for cancer treatment. Mol. Cancer 2024, 23, 279. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, T.; Liu, Y.; Yu, G.; Li, C.; Jiang, Z. Regulatory T cells in immune checkpoint blockade antitumor therapy. Mol. Cancer 2024, 23, 251. [Google Scholar] [CrossRef]
- Kurt, F.G.O.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing immunotherapy response in melanoma: Myeloid-derived suppressor cells as a therapeutic target. J. Clin. Investig. 2023, 133, e170762. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef] [PubMed]
- Achmad, A.; Hanaoka, H.; Holik, H.A.; Endo, K.; Tsushima, Y.; Kartamihardja, A.H.S. LAT1-specific PET radiotracers: Development and clinical experiences of a new class of cancer-specific radiopharmaceuticals. Theranostics 2025, 15, 1864–1878. [Google Scholar] [CrossRef]
- Cormerais, Y.; Massard, P.A.; Vucetic, M.; Giuliano, S.; Tambutté, E.; Durivault, J.; Vial, V.; Endou, H.; Wempe, M.F.; Parks, S.K.; et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). J. Biol. Chem. 2018, 293, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Wang, B.; Pei, J.; Xu, S.; Liu, J.; Yu, J. A glutamine tug-of-war between cancer and immune cells: Recent advances in unraveling the ongoing battle. J. Exp. Clin. Cancer Res. 2024, 43, 74. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Leekha, A.; Nandy, S.; Kulkarni, R.; Martinez-Paniagua, M.; Sefat, K.M.S.R.; Willson, R.C.; Varadarajan, N. Enzymatic depletion of circulating glutamine is immunosuppressive in cancers. iScience 2024, 27, 109817. [Google Scholar] [CrossRef] [PubMed]
- Kaneda-Nakashima, K.; Zhang, Z.; Manabe, Y.; Shimoyama, A.; Kabayama, K.; Watabe, T.; Kanai, Y.; Ooe, K.; Toyoshima, A.; Shirakami, Y.; et al. α-Emitting cancer therapy using 211At-AAMT targeting LAT1. Cancer Sci. 2021, 112, 1132–1140. [Google Scholar] [CrossRef]
- Gong, X.; Zhou, Y.; Deng, Y. Targeting DNA damage response-mediated resistance in non-small cell lung cancer: From mechanistic insights to drug development. Curr. Oncol. 2025, 32, 367. [Google Scholar] [CrossRef]
- da Costa, A.A.B.A.; Chowdhury, D.; Shapiro, G.I.; D’Andrea, A.D.; Konstantinopoulos, P.A. Targeting replication stress in cancer therapy. Nat. Rev. Drug Discov. 2023, 22, 38–58. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, J.A. Targeting replication stress response pathways to enhance genotoxic chemo- and radiotherapy. Molecules 2022, 27, 4736. [Google Scholar] [CrossRef]
- Gralewska, P.; Gajek, A.; Marczak, A.; Rogalska, A. Participation of the ATR/CHK1 pathway in replicative stress targeted therapy of high-grade ovarian cancer. J. Hematol. Oncol. 2020, 13, 39. [Google Scholar] [CrossRef]
- Lohberger, B.; Glänzer, D.; Eck, N.; Stasny, K.; Falkner, A.; Leithner, A.; Georg, D. The ATR inhibitor VE-821 enhances the radiosensitivity and suppresses DNA repair mechanisms of human chondrosarcoma cells. Int. J. Mol. Sci. 2023, 24, 2315. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, A.; Tang, F.; Duan, M.; Li, B. Drug-induced tolerant persisters in tumor: Mechanism, vulnerability and perspective implication for clinical treatment. Mol. Cancer 2025, 24, 150. [Google Scholar] [CrossRef]
- Prasanna, P.G.; Citrin, D.E.; Hildesheim, J.; Ahmed, M.M.; Venkatachalam, S.; Riscuta, G.; Xi, D.; Zheng, G.; van Deursen, J.; Goronzy, J.; et al. Therapy-induced senescence: Opportunities to improve anticancer therapy. J. Natl. Cancer Inst. 2021, 113, 1285–1298. [Google Scholar] [CrossRef]
- Barnieh, F.M.; Morton, J.; Olanrewaju, O.; El-Khamisy, S.F. Decoding the adaptive survival mechanisms of breast cancer dormancy. Oncogene 2025, 44, 3759–3773. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Greenberg, E.F.; Faber, A.C.; Harada, H.; Gewirtz, D.A. A critical appraisal of the utility of targeting therapy-induced senescence for cancer treatment. Cancer Res. 2025, 85, 1755–1768. [Google Scholar] [CrossRef]
- Li, Y.; Mao, T.; Wang, J.; Zheng, H.; Hu, Z.; Cao, P.; Yang, S.; Zhu, L.; Guo, S.; Zhao, X.; et al. Toward the next generation EGFR inhibitors: An overview of osimertinib resistance mediated by EGFR mutations in non-small cell lung cancer. Cell Commun. Signal. 2023, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Li, S.; Zhao, Y.; Cheng, K. Mechanisms of drug resistance to immune checkpoint inhibitors in non-small cell lung cancer. Front. Immunol. 2023, 14, 1127071. [Google Scholar] [CrossRef]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: Updated insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with tumor hypoxia for radiotherapy optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef]
- Catania, C.; Liu, S.V.; Garassino, M.; Delmonte, A.; Scotti, V.; Cappuzzo, F.; Genova, C.; Russo, A.; Russano, M.; Bennati, C.; et al. Correlation between treatments and outcomes of patients with EGFR-mutated non-small-cell lung cancer that transitioned into small-cell lung cancer: An international retrospective study. ESMO Open 2025, 10, 105326. [Google Scholar] [CrossRef]
- Lailler, C.; Didelot, A.; Garinet, S.; Berthou, H.; Sroussi, M.; de Reyniès, A.; Dedhar, S.; Martin-Lannerée, S.; Fabre, E.; Le Pimpec-Barthes, F.; et al. PrPC controls epithelial-to-mesenchymal transition in EGFR-mutated NSCLC: Implications for TKI resistance and patient follow-up. Oncogene 2024, 43, 2781–2794. [Google Scholar] [CrossRef]
- Rumde, P.H.; Burns, T.F. A path to persistence after EGFR inhibition. Cancer Res. 2024, 84, 1188–1190. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M.; Jiang, C.-H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Jakobsen, S.; Nielsen, C.U. Exploring amino acid transporters as therapeutic targets for cancer: An examination of inhibitor structures, selectivity issues, and discovery approaches. Pharmaceutics 2024, 16, 197. [Google Scholar] [CrossRef]
- Hushmandi, K.; Einollahi, B.; Saadat, S.H.; Lee, E.H.C.; Farani, M.R.; Okina, E.; Huh, Y.S.; Nabavi, N.; Salimimoghadam, S.; Kumar, A.P. Amino acid transporters within the solute carrier superfamily: Underappreciated proteins and novel opportunities for cancer therapy. Mol. Metab. 2024, 84, 101952. [Google Scholar] [CrossRef]
- Kahlhofer, J.; Teis, D. The human LAT1–4F2hc (SLC7A5–SLC3A2) transporter complex: Physiological and pathophysiological implications. Basic Clin. Pharmacol. Toxicol. 2023, 133, 459–472. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Console, L.; Losso, M.A.; Indiveri, C. The human SLC1A5 (ASCT2) amino acid transporter: From function to structure and role in cell biology. Front. Cell Dev. Biol. 2018, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Dong, Z.; Wang, J. Circ_0000808 promotes the development of non-small cell lung cancer by regulating glutamine metabolism via the miR-1827/SLC1A5 axis. World J. Surg. Oncol. 2022, 20, 329. [Google Scholar] [CrossRef]
- Parker, J.L.; Deme, J.C.; Kolokouris, D.; Kuteyi, G.; Biggin, P.C.; Lea, S.M.; Newstead, S. Molecular basis for redox control by the human cystine/glutamate antiporter system Xc−. Nat. Commun. 2021, 12, 7147. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, Z.; Sun, R.; Guo, S.; Gao, F.; Cao, B.; Aa, J. The role of SLC7A11 in cancer: Friend or foe? Cancers 2022, 14, 3059. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nagano, O.; Yae, T.; Tamada, M.; Motohara, T.; Oshima, H.; Oshima, M.; Ikeda, T.; Asaba, R.; Yagi, H.; et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system Xc− and thereby promotes tumor growth. Cancer Cell 2011, 19, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Cao, J.; Wu, C.; Tang, L.; Bian, W.; Chen, Y.; Yu, L.; Wu, Y.; Li, S.; et al. Targeting epigenetic and post-translational modifications of NRF2: Key regulatory factors in disease treatment. Cell Death Discov. 2025, 11, 189. [Google Scholar] [CrossRef]
- Wang, J.; Hao, S.; Song, G.; Wang, Y.; Hao, Q. The prognostic and clinicopathological significance of SLC7A11 in human cancers: A systematic review and meta-analysis. PeerJ 2023, 11, e14931. [Google Scholar] [CrossRef]
- Hu, K.; Li, K.; Lv, J.; Feng, J.; Chen, J.; Wu, H.; Cheng, F.; Jiang, W.; Wang, J.; Pei, H.; et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J. Clin. Investig. 2020, 130, 1752–1766. [Google Scholar] [CrossRef]
- Wu, D.; Yan, R.; Song, S.; Swansiger, A.K.; Li, Y.; Prell, J.S.; Zhou, Q.; Robinson, C.V. The complete assembly of human LAT1-4F2hc complex provides insights into its regulation, function and localisation. Nat. Commun. 2024, 15, 3711. [Google Scholar] [CrossRef]
- Wang, Q.; Holst, J. L-type amino acid transport and cancer: Targeting the mTORC1 pathway to inhibit neoplasia. Am. J. Cancer Res. 2015, 5, 1281–1294. [Google Scholar] [PubMed]
- Imai, H.; Kaira, K.; Oriuchi, N.; Yanagitani, N.; Sunaga, N.; Ishizuka, T.; Kanai, Y.; Endou, H.; Nakajima, T.; Mori, M. L-type amino acid transporter 1 expression is a prognostic marker in patients with surgically resected stage I non-small cell lung cancer. Histopathology 2009, 54, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Scalise, M.; Mazza, T.; Pappacoda, G.; Pochini, L.; Cosco, J.; Rovella, F.; Indiveri, C. The human SLC1A5 neutral amino acid transporter catalyzes a pH-dependent glutamate/glutamine antiport, as well. Front. Cell Dev. Biol. 2020, 8, 603. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Pang, J.; Zhang, Y.; Zhang, H.; Wu, H.; Fan, F.; Liu, H. Restricting glutamine uptake enhances NSCLC sensitivity to third-generation EGFR-TKI almonertinib. Front. Pharmacol. 2021, 12, 671328. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kaira, K.; Tomizawa, Y.; Sunaga, N.; Kawashima, O.; Oriuchi, N.; Tominaga, H.; Nagamori, S.; Kanai, Y.; Yamada, M.; et al. ASC amino acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br. J. Cancer 2014, 110, 2030–2039. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadée, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The role of cystine/glutamate antiporter SLC7A11/xCT in the pathophysiology of cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Ji, X.; Qian, J.; Rahman, S.M.J.; Siska, P.J.; Zou, Y.; Harris, B.K.; Hoeksema, M.D.; Trenary, I.A.; Chen, H.; Eisenberg, R.; et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 2018, 37, 5007–5019. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Lama-Sherpa, T.D.; Jeong, M.-H.; Jewell, J.L. Regulation of mTORC1 by the Rag GTPases. Biochem. Soc. Trans. 2023, 51, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Durán, R.V.; Oppliger, W.; Robitaille, A.M.; Heiserich, L.; Skendaj, R.; Gottlieb, E.; Hall, M.N. Glutaminolysis activates Rag-mTORC1 signaling. Mol. Cell 2012, 47, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Zlobec, I.; Nydegger, D.T.; Pujol-Giménez, J.; Bhardwaj, R.; Shirasawa, S.; Tsunoda, T.; Hediger, M.A. Oncogenic KRAS mutations enhance amino acid uptake by colorectal cancer cells via the Hippo signaling effector YAP1. Mol. Oncol. 2021, 15, 2782–2800. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kaira, K.; Oriuchi, N.; Shimizu, K.; Tominaga, H.; Yanagitani, N.; Sunaga, N.; Ishizuka, T.; Nagamori, S.; Promchan, K.; et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer. Res. 2010, 30, 4819–4828. [Google Scholar]
- Zhang, Z.; Liu, R.; Shuai, Y.; Huang, Y.; Jin, R.; Wang, X.; Luo, J. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br. J. Cancer 2020, 122, 82–93. [Google Scholar] [CrossRef]
- Wang, W.; Guo, M.-N.; Li, N.; Pang, D.-Q.; Wu, J.-H. Glutamine deprivation impairs function of infiltrating CD8+ T cells in hepatocellular carcinoma by inducing mitochondrial damage and apoptosis. World J. Gastrointest. Oncol. 2022, 14, 1124–1140. [Google Scholar] [CrossRef]
- Edwards, D.N.; Ngwa, V.M.; Raybuck, A.L.; Wang, S.; Hwang, Y.; Kim, L.C.; Cho, S.H.; Paik, Y.; Wang, Q.; Zhang, S.; et al. Selective glutamine metabolism inhibition in tumor cells improves antitumor T lymphocyte activity in triple-negative breast cancer. J. Clin. Investig. 2021, 131, e140100. [Google Scholar] [CrossRef]
- Li, Q.; Zhong, X.; Yao, W.; Yu, J.; Wang, C.; Li, Z.; Lai, S.; Qu, F.; Fu, X.; Huang, X.; et al. Inhibitor of Glutamine Metabolism V9302 Promotes ROS-Induced Autophagic Degradation of B7H3 to Enhance Antitumor Immunity. J. Biol. Chem. 2022, 298, 101753. [Google Scholar] [CrossRef]
- Liu, J.; Xia, X.; Huang, P. xCT: A critical molecule that links cancer metabolism to redox signaling. Mol. Ther. 2020, 28, 2358–2366. [Google Scholar] [CrossRef]
- Greenwood, H.E.; Barber, A.R.; Edwards, R.S.; Tyrrell, W.E.; George, M.E.; dos Santos, S.N.; Baark, F.; Tanc, M.; Khalil, E.; Falzone, A.; et al. Imaging NRF2 activation in non-small cell lung cancer with positron emission tomography. Nat. Commun. 2024, 15, 10484. [Google Scholar] [CrossRef]
- Nagane, M.; Kanai, E.; Shibata, Y.; Shimizu, T.; Yoshioka, C.; Maruo, T.; Yamashita, T. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS ONE 2018, 13, e0195151. [Google Scholar] [CrossRef] [PubMed]
- Sendo, K.; Seino, M.; Ohta, T.; Nagase, S. Impact of the glutathione synthesis pathway on sulfasalazine-treated endometrial cancer. Oncotarget 2022, 13, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhong, R.; Wei, T.; Jin, Y.; He, C.; Li, H.; Cheng, Y. Mechanism of targeting the mTOR pathway to regulate ferroptosis in NSCLC with different EGFR mutations. Oncol. Lett. 2024, 28, 1–9. [Google Scholar] [CrossRef]
- Li, F.-J.; Long, H.-Z.; Zhou, Z.-W.; Luo, H.-Y.; Xu, S.-G.; Gao, L.-C. System Xc−/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front. Pharmacol. 2022, 13, 910292. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Neyens, D.; Ramsay, G.; Taylor, P.M.; Cantrell, D.A. Single cell analysis of kynurenine and system L amino acid transport in T cells. Nat. Commun. 2018, 9, 1981. [Google Scholar] [CrossRef]
- Solvay, M.; Holfelder, P.; Klaessens, S.; Pilotte, L.; Stroobant, V.; Lamy, J.; Naulaerts, S.; Spillier, Q.; Frédérick, R.; De Plaen, E.; et al. Tryptophan depletion sensitizes the AHR pathway by increasing AHR expression and GCN2/LAT1-mediated kynurenine uptake, and potentiates induction of regulatory T lymphocytes. J. Immunother. Cancer 2023, 11, e006728. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.-B.; Taylor, P.M.; Cantrell, D.A. Control of amino acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef]
- Saini, N.; Naaz, A.; Metur, S.P.; Gahlot, P.; Walvekar, A.; Dutta, A.; Davathamizhan, U.; Sarin, A.; Laxman, S. Methionine uptake via the SLC43A2 transporter is essential for regulatory T-cell survival. Life Sci. Alliance 2022, 5, e202201663. [Google Scholar] [CrossRef]
- Ma, G.; Liang, Y.; Chen, Y.; Wang, L.; Li, D.; Liang, Z.; Wang, X.; Tian, D.; Yang, X.; Niu, H. Glutamine deprivation induces PD-L1 expression via activation of EGFR/ERK/c-Jun signaling in renal cancer. Mol. Cancer Res. 2020, 18, 324–339. [Google Scholar] [CrossRef]
- Bessede, A.; Peyraud, F.; Le Moulec, S.; Cousin, S.; Cabart, M.; Chomy, F.; Rey, C.; Lara, O.; Odin, O.; Nafia, I.; et al. Upregulation of indoleamine 2,3-dioxygenase 1 in tumor cells and tertiary lymphoid structures is a hallmark of inflamed non-small cell lung cancer. Clin. Cancer Res. 2023, 29, 4883–4893. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Yang, X.; Zhang, X.; Xie, J.; Li, S.; Liu, H.; Guo, J.; He, L.; Chen, W.; et al. T lymphocyte infiltration in association with IDO1 expression in resected lung adenocarcinoma and normal adjacent lung tissues. Biomed. Res. Int. 2022, 2022, 2381018. [Google Scholar] [CrossRef] [PubMed]
- Kaira, K.; Oriuchi, N.; Imai, H.; Shimizu, K.; Yanagitani, N.; Sunaga, N.; Hisada, T.; Tanaka, S.; Ishizuka, T.; Kanai, Y.; et al. Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I–III nonsmall cell lung cancer. Br. J. Cancer 2008, 98, 742–748. [Google Scholar] [CrossRef]
- Yazawa, T.; Shimizu, K.; Kaira, K.; Nagashima, T.; Ohtaki, Y.; Atsumi, J.; Obayashi, K.; Nagamori, S.; Kanai, Y.; Oyama, T.; et al. Clinical significance of coexpression of L-type amino acid transporter 1 (LAT1) and ASC amino acid transporter 2 (ASCT2) in lung adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1126–1139. [Google Scholar]
- Robert, S.M.; Buckingham, S.C.; Campbell, S.L.; Robel, S.; Holt, K.T.; Ogunrinu-Babarinde, T.; Warren, P.P.; White, D.M.; Reid, M.A.; Eschbacher, J.M.; et al. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Sci. Transl. Med. 2015, 7, 289ra86. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, metabolism, and cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Onishi, Y.; Hiraiwa, M.; Kamada, H.; Iezaki, T.; Yamada, T.; Kaneda, K.; Hinoi, E. Hypoxia affects Slc7a5 expression through HIF-2α in differentiated neuronal cells. FEBS Open Bio 2019, 9, 241–247. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, X.; Chen, D.; Liu, Y.; Li, Z.; Duan, S.; Zhang, Z.; Jiang, X.; Stockwell, B.R.; Gu, W. GAS41 modulates ferroptosis by anchoring NRF2 on chromatin. Nat. Commun. 2024, 15, 2531. [Google Scholar] [CrossRef]
- Gu, Z.; Liu, Y.; Cai, F.; Patrick, M.; Zmajkovic, J.; Cao, H.; Zhang, Y.; Tasdogan, A.; Chen, M.; Qi, L.; et al. Loss of EZH2 reprograms BCAA metabolism to drive leukemic transformation. Cancer Discov. 2019, 9, 1228–1247. [Google Scholar] [CrossRef]
- Console, L.; Scalise, M.; Tarmakova, Z.; Coe, I.R.; Indiveri, C. N-linked glycosylation of human SLC1A5 (ASCT2) transporter is critical for trafficking to membrane. Biochim. Biophys. Acta Mol. Cell Res. 2015, 1853, 1636–1645. [Google Scholar] [CrossRef]
- Dickens, D.; Chiduza, G.N.; Wright, G.S.; Pirmohamed, M.; Antonyuk, S.V.; Hasnain, S.S. Modulation of LAT1 (SLC7A5) transporter activity and stability by membrane cholesterol. Sci. Rep. 2017, 7, 43580. [Google Scholar] [CrossRef] [PubMed]
- Lieu, E.L.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Liu, C.; Zheng, S.; Shi, H.; Wei, F.; Jiang, W.; Dong, Y.; Xu, H.; Yin, E.; Sun, N.; et al. KEAP1 mutations as key crucial prognostic biomarkers for resistance to KRAS-G12C inhibitors. J. Transl. Med. 2025, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Roncali, L.; Hindré, F.; Samarut, E.; Lacoeuille, F.; Rousseau, A.; Lemée, J.-M.; Garcion, E.; Chérel, M. Current landscape and future directions of targeted alpha therapy for glioblastoma treatment. Theranostics 2025, 15, 4861–4889. [Google Scholar] [CrossRef]
- Li, F.; Yang, Y.; Liao, J.; Liu, N. Recent progress of astatine-211 in endoradiotherapy: Great advances from fundamental properties to targeted radiopharmaceuticals. Chin. Chem. Lett. 2022, 33, 3325–3338. [Google Scholar] [CrossRef]
- Eriksson, S.E.; Bäck, T.; Elgström, E.; Jensen, H.; Nilsson, R.; Lindegren, S.; Tennvall, J. Successful radioimmunotherapy of established syngeneic rat colon carcinoma with 211At-mAb. EJNMMI Res. 2013, 3, 23. [Google Scholar] [CrossRef]
- Guerra Liberal, F.D.C.; Moreira, H.; Redmond, K.M.; O’Sullivan, J.M.; Alshehri, A.H.D.; Wright, T.C.; Dunne, V.L.; Campfield, C.; Biggart, S.; McMahon, S.J.; et al. Differential responses to 223Ra and alpha-particles exposure in prostate cancer driven by mitotic catastrophe. Front. Oncol. 2022, 12, 877302. [Google Scholar] [CrossRef]
- Tronchin, S.; Forster, J.; Hickson, K.; Bezak, E. Modeling the effect of daughter migration on dosimetry estimates for unlabeled actinium-225. Med. Phys. 2024, 51, 5032–5044. [Google Scholar] [CrossRef]
- Hooijman, E.L.; Radchenko, V.; Ling, S.W.; Konijnenberg, M.; Brabander, T.; Koolen, S.L.W.; de Blois, E. Implementing Ac-225 labelled radiopharmaceuticals: Practical considerations and (pre-)clinical perspectives. EJNMMI Radiopharm. Chem. 2024, 9, 9. [Google Scholar] [CrossRef]
- Ahenkorah, S.; Cassells, I.; Deroose, C.M.; Cardinaels, T.; Burgoyne, A.R.; Bormans, G.; Ooms, M.; Cleeren, F. Bismuth-213 for targeted radionuclide therapy: From atom to bedside. Pharmaceutics 2021, 13, 599. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for targeted therapy: Physical properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef]
- Piccardo, A.; Ugolini, M.; Altrinetti, V.; Bottoni, G.; Lupi, I.; Tonacchera, M.; Vitti, P.; Trimboli, P. Radioiodine therapy of Graves’ disease. Q. J. Nucl. Med. Mol. Imaging 2021, 65, 132–137. [Google Scholar] [CrossRef]
- Meyer, C.; Stuparu, A.; Lueckerath, K.; Calais, J.; Czernin, J.; Slavik, R.; Dahlbom, M. Tandem isotope therapy with 225Ac- and 177Lu-PSMA-617 in a murine model of prostate cancer. J. Nucl. Med. 2023, 64, 1772–1778. [Google Scholar] [CrossRef] [PubMed]
- Sjögreen Gleisner, K.; Chouin, N.; Minguez Gabina, P.; Cicone, F.; Gnesin, S.; Stokke, C.; Konijnenberg, M.; Cremonesi, M.; Verburg, F.A.; Bernhardt, P.; et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1778–1809. [Google Scholar] [CrossRef] [PubMed]
- Palangka, C.R.A.P.; Mahendra, I.; Ritawidya, R.; Kondo, N.; Nakajima, T. Alpha particle emitter radiolabeled antibodies in cancer therapy: Current status, challenges, and future prospects. Pharmaceuticals 2025, 18, 1316. [Google Scholar] [CrossRef]
- Miederer, M.; Benešová-Schäfer, M.; Mamat, C.; Kästner, D.; Pretze, M.; Michler, E.; Brogsitter, C.; Kotzerke, J.; Kopka, K.; Scheinberg, D.A.; et al. Alpha-emitting radionuclides: Current status and future perspectives. Pharmaceuticals 2024, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Bolcaen, J.; Kleynhans, J.; Nair, S.; Verhoeven, J.; Goethals, I.; Sathekge, M.; Vandevoorde, C.; Ebenhan, T. A perspective on the radiopharmaceutical requirements for imaging and therapy of glioblastoma. Theranostics 2021, 11, 7911–7947. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.D.; Zhang, Y.; Korn, M.; Constantinovici, N.; McKay, R.R. Real-world utilization patterns and survival in men with metastatic prostate cancer treated with radium-223 in the United States. Prostate Cancer Prostatic Dis. 2025, 1–8. [Google Scholar] [CrossRef]
- Gao, J.; Li, M.; Yin, J.; Liu, M.; Wang, H.; Du, J.; Li, J. The different strategies for the radiolabeling of [211At]-astatinated radiopharmaceuticals. Pharmaceutics 2024, 16, 738. [Google Scholar] [CrossRef]
- Shirakami, Y.; Watabe, T.; Obata, H.; Kaneda, K.; Ooe, K.; Liu, Y.; Teramoto, T.; Toyoshima, A.; Shinohara, A.; Shimosegawa, E.; et al. Synthesis of [211At]4-astato-L-phenylalanine by dihydroxyboryl-astatine substitution reaction in aqueous solution. Sci. Rep. 2021, 11, 12982. [Google Scholar] [CrossRef]
- Guérard, F.; Lee, Y.-S.; Baidoo, K.; Gestin, J.-F.; Brechbiel, M.W. Unexpected behavior of the heaviest halogen astatine in the nucleophilic substitution of aryliodonium salts. Chem. Eur. J. 2016, 22, 12332–12339. [Google Scholar] [CrossRef]
- Ohshima, Y.; Suzuki, H.; Hanaoka, H.; Sasaki, I.; Watanabe, S.; Haba, H.; Arano, Y.; Tsushima, Y.; Ishioka, N.S. Preclinical evaluation of new α-radionuclide therapy targeting LAT1: 2-[211At]astato-α-methyl-L-phenylalanine in tumor-bearing model. Nucl. Med. Biol. 2020, 90–91, 15–22. [Google Scholar] [CrossRef]
- Hanaoka, H.; Ohshima, Y.; Suzuki, H.; Sasaki, I.; Watabe, T.; Ooe, K.; Watanabe, S.; Ishioka, N.S. Enhancing the therapeutic effect of 2-211At-astato-α-methyl-L-phenylalanine with probenecid loading. Cancers 2021, 13, 5514. [Google Scholar] [CrossRef]
- Lee, Y.; Jin, C.; Ohgaki, R.; Xu, M.; Ogasawara, S.; Warshamanage, R.; Yamashita, K.; Murshudov, G.; Nureki, O.; Murata, T.; et al. Structural basis of anticancer drug recognition and amino acid transport by LAT1. Nat. Commun. 2025, 16, 1635. [Google Scholar] [CrossRef]
- Kanai, A.; Hanaoka, H.; Yamaguchi, A.; Mahendra, I.; Palangka, C.; Ohshima, Y.; Higuchi, T.; Tsushima, Y. Enhancing the accumulation level of 3-[18F]fluoro-L-α-methyltyrosine in tumors by preloading probenecid. Nucl. Med. Biol. 2022, 104–105, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, K.; Ohgaki, R.; Okanishi, H.; Okuda, S.; Xu, M.; Endou, H.; Kanai, Y. Pharmacologic inhibition of LAT1 predominantly suppresses transport of large neutral amino acids and downregulates global translation in cancer cells. J. Cell. Mol. Med. 2022, 26, 5246–5256. [Google Scholar] [CrossRef]
- Bröer, A.; Fairweather, S.; Bröer, S. Disruption of amino acid homeostasis by novel ASCT2 inhibitors involves multiple targets. Front. Pharmacol. 2018, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Minami, Y.; Yoshimoto, S.; Hayashi, N.; Yamasaki, A.; Ueda, S.; Masuko, K.; Masuko, T. Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Med. 2019, 9, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Olszewski, K.; Zhang, Y.; Kondiparthi, L.; Liu, X.; Lei, G.; Das, M.; Fang, B.; Poyurovsky, M.V.; Gan, B. KEAP1 deficiency drives glucose dependency and sensitizes lung cancer cells and tumors to GLUT inhibition. iScience 2021, 24, 102649. [Google Scholar] [CrossRef]
- Cobler, L.; Zhang, H.; Suri, P.; Park, C.; Timmerman, L.A. xCT inhibition sensitizes tumors to γ-radiation via glutathione reduction. Oncotarget 2018, 9, 32280–32297. [Google Scholar] [CrossRef]
- Gan, B. How erastin assassinates cells by ferroptosis revealed. Protein Cell 2023, 14, 84–86. [Google Scholar] [CrossRef]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Liu, Y.; Ooe, K.; Teramoto, T.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Kanai, Y.; et al. Targeted alpha therapy using astatine (211At)-labeled phenylalanine: A preclinical study in glioma bearing mice. Oncotarget 2020, 11, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Roncali, L.; Marionneau-Lambot, S.; Roy, C.; Eychenne, R.; Gouard, S.; Avril, S.; Chouin, N.; Riou, J.; Allard, M.; Rousseau, A.; et al. Brain intratumoural astatine-211 radiotherapy targeting syndecan-1 leads to durable glioblastoma remission and immune memory in female mice. EBioMedicine 2024, 105, 105202. [Google Scholar] [CrossRef]
- Takami, H.; Imura, Y.; Outani, H.; Nakai, S.; Inoue, A.; Kotani, Y.; Okada, S.; Kaneda-Nakashima, K. LAT1-targeted alpha therapy using 211At-AAMT for bone and soft tissue sarcomas. Int. J. Mol. Sci. 2025, 26, 8599. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Dubrovska, A. CD98 heavy chain as a prognostic biomarker and target for cancer treatment. Front. Oncol. 2023, 13, 1251100. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Wiriyasermkul, P.; Jin, C.; Quan, L.; Ohgaki, R.; Okuda, S.; Kusakizako, T.; Nishizawa, T.; Oda, K.; Ishitani, R.; et al. Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Nat. Struct. Mol. Biol. 2019, 26, 510–517. [Google Scholar] [CrossRef]
- Hayashi, N.; Yamasaki, A.; Ueda, S.; Okazaki, S.; Ohno, Y.; Tanaka, T.; Endo, Y.; Tomioka, Y.; Masuko, K.; Masuko, T.; et al. Oncogenic transformation of NIH/3T3 cells by the overexpression of L-type amino acid transporter 1, a promising anti-cancer target. Oncotarget 2021, 12, 1256–1270. [Google Scholar] [CrossRef]
- Hayes, G.M.; Chinn, L.; Cantor, J.M.; Cairns, B.; Levashova, Z.; Tran, H.; Velilla, T.; Duey, D.; Lippincott, J.; Zachwieja, J.; et al. Antitumor activity of an anti-CD98 antibody. Int. J. Cancer 2015, 137, 710–720. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Li, M.; Yang, N.; Lu, H.; Zhang, Z.; Dong, Y.; Chen, Y.; Zhu, Z.; Tong, A.; et al. A novel SLC3A2-targeting antibody-drug conjugate exerts potent antitumor efficacy in head and neck squamous cell cancer. Transl. Oncol. 2024, 45, 101981. [Google Scholar] [CrossRef]
- Montero, J.C.; Calvo-Jiménez, E.; del Carmen, S.; Abad, M.; Ocaña, A.; Pandiella, A. Surfaceome analyses uncover CD98hc as an antibody drug-conjugate target in triple negative breast cancer. J. Exp. Clin. Cancer Res. 2022, 41, 106. [Google Scholar] [CrossRef]
- Montero, J.C.; del Carmen, S.; Abad, M.; Sayagués, J.M.; Barbáchano, A.; Fernández-Barral, A.; Muñoz, A.; Pandiella, A. An amino acid transporter subunit as an antibody–drug conjugate target in colorectal cancer. J. Exp. Clin. Cancer Res. 2023, 42, 200. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, D.V.; Allevato, M.M.; Camargo, M.F.; Lesperance, J.; Quraishi, M.A.; Aguilera, J.; Franiak-Pietryga, I.; Scanderbeg, D.J.; Wang, Z.; Molinolo, A.A.; et al. Monomethyl auristatin antibody and peptide drug conjugates for trimodal cancer chemo-radio-immunotherapy. Nat. Commun. 2022, 13, 3869. [Google Scholar] [CrossRef]
- Hartley, J.A. Antibody-drug conjugates (ADCs) delivering pyrrolobenzodiazepine (PBD) dimers for cancer therapy. Expert Opin. Biol. Ther. 2021, 21, 931–943. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Gangwar, S.; Betts, A. Therapeutic index improvement of antibody-drug conjugates. MAbs 2023, 15, 2230618. [Google Scholar] [CrossRef]
- Metrangolo, V.; Engelholm, L.H. Antibody–drug conjugates: The dynamic evolution from conventional to next-generation constructs. Cancers 2024, 16, 447. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gurung, S.; Lee, H.-S.; Gunassekaran, G.R.; Lee, S.-M.; Yoon, J.-W.; Lee, Y.-K.; Lee, B. Peptides as multifunctional players in cancer therapy. Exp. Mol. Med. 2023, 55, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Echigo, H.; Munekane, M.; Fuchigami, T.; Washiyama, K.; Mishiro, K.; Wakabayashi, H.; Takahashi, K.; Kinuya, S.; Ogawa, K. Optimizing the pharmacokinetics of an 211At-labeled RGD peptide with an albumin-binding moiety via the administration of an albumin-binding inhibitor. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 2663–2671. [Google Scholar] [CrossRef]
- Echigo, H.; Mishiro, K.; Fuchigami, T.; Shiba, K.; Kinuya, S.; Ogawa, K. Synthesis and evaluation of a dimeric RGD peptide as a preliminary study for radiotheranostics with radiohalogens. Molecules 2021, 26, 6107. [Google Scholar] [CrossRef]
- Bo, T.; Kobayashi, S.; Inanami, O.; Fujii, J.; Nakajima, O.; Ito, T.; Yasui, H. LAT1 inhibitor JPH203 sensitizes cancer cells to radiation by enhancing radiation-induced cellular senescence. Transl. Oncol. 2021, 14, 101212. [Google Scholar] [CrossRef]
- van Geldermalsen, M.; Quek, L.-E.; Turner, N.; Freidman, N.; Pang, A.; Guan, Y.F.; Krycer, J.R.; Ryan, R.; Wang, Q.; Holst, J. Benzylserine inhibits breast cancer cell growth by disrupting intracellular amino acid homeostasis and triggering amino acid response pathways. BMC Cancer 2018, 18, 689. [Google Scholar] [CrossRef]
- Schulte, M.L.; Fu, A.; Zhao, P.; Li, J.; Geng, L.; Smith, S.T.; Kondo, J.; Coffey, R.J.; Johnson, M.O.; Rathmell, J.C.; et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 2018, 24, 194–202. [Google Scholar] [CrossRef]
- Pillai, R.; LeBoeuf, S.E.; Hao, Y.; New, C.; Blum, J.L.E.; Rashidfarrokhi, A.; Huang, S.M.; Bahamon, C.; Wu, W.L.; Karadal-Ferrena, B.; et al. Glutamine antagonist DRP-104 suppresses tumor growth and enhances response to checkpoint blockade in KEAP1 mutant lung cancer. Sci. Adv. 2024, 10, eadm9859. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Suda, K.; Masuko, K.; Yagi, H.; Hashimoto, Y.; Masuko, T. Production and characterization of highly tumor-specific rat monoclonal antibodies recognizing the extracellular domain of human L-type amino acid transporter 1. Cancer Sci. 2008, 99, 1000–1007. [Google Scholar] [CrossRef]
- Ueda, S.; Hayashi, H.; Miyamoto, T.; Abe, S.; Hirai, K.; Matsukura, K.; Yagi, H.; Hara, Y.; Yoshida, K.; Okazaki, S.; et al. Anti-tumor effects of mAb against L-type amino acid transporter 1 (LAT1) bound to human and monkey LAT1 with dual avidity modes. Cancer Sci. 2019, 110, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Byun, J.-K.; Choi, Y.-K.; Park, K.-G. Targeting glutamine metabolism as a therapeutic strategy for cancer. Exp. Mol. Med. 2023, 55, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Herth, M.M.; Hvass, L.; Poulie, C.B.M.; Müller, M.; García-Vázquez, R.; Gustavsson, T.; Shalgunov, V.; Clausen, A.S.; Jørgensen, J.T.; Hansson, E.; et al. An 211At-labeled tetrazine for pretargeted therapy. J. Med. Chem. 2025, 68, 4410–4425. [Google Scholar] [CrossRef]
- Timperanza, C.; Jensen, H.; Bäck, T.; Lindegren, S.; Aneheim, E. Pretargeted alpha therapy of disseminated cancer combining click chemistry and astatine-211. Pharmaceuticals 2023, 16, 595. [Google Scholar] [CrossRef]
- Bhunia, S.; Vangala, V.; Bhattacharya, D.; Ravuri, H.G.; Kuncha, M.; Chakravarty, S.; Sistla, R.; Chaudhuri, A. Large amino acid transporter 1 selective liposomes of L-DOPA functionalized amphiphile for combating glioblastoma. Mol. Pharm. 2017, 14, 3834–3847. [Google Scholar] [CrossRef]
- Li, L.; Di, X.; Wu, M.; Sun, Z.; Zhong, L.; Wang, Y.; Fu, Q.; Kan, Q.; Sun, J.; He, Z. Targeting tumor highly-expressed LAT1 transporter with amino acid-modified nanoparticles: Toward a novel active targeting strategy in breast cancer therapy. Nanomedicine 2017, 13, 987–998. [Google Scholar] [CrossRef] [PubMed]
- De, K.; Prasad, P.; Sinha, S.; Mukhopadhyay, S.; Roy, S.S. Synthesis, characterization, and biological evaluation of radiolabeled glutamine conjugated polymeric nanoparticles: A simple approach for tumor imaging. ACS Appl. Bio Mater. 2023, 6, 2172–2183. [Google Scholar] [CrossRef]
- Huang, X.; Kaneda-Nakashima, K.; Kadonaga, Y.; Kabayama, K.; Shimoyama, A.; Ooe, K.; Kato, H.; Toyoshima, A.; Shinohara, A.; Haba, H.; et al. Astatine-211-labeled gold nanoparticles for targeted alpha-particle therapy via intravenous injection. Pharmaceutics 2022, 14, 2705. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor-penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef]
- Makharadze, D.; Valle, L.J.D.; Katsarava, R.; Puiggalí, J. The art of PEGylation: From simple polymer to sophisticated drug delivery system. Int. J. Mol. Sci. 2025, 26, 3102. [Google Scholar] [CrossRef]
- Salgueiro, M.J.; Zubillaga, M. Theranostic nanoplatforms in nuclear medicine: Current advances, emerging trends, and perspectives for personalized oncology. J. Nanotheranostics 2025, 6, 27. [Google Scholar] [CrossRef]
- Chakravarty, R.; Hong, H.; Cai, W. Positron emission tomography image-guided drug delivery: Current status and future perspectives. Mol. Pharm. 2014, 11, 3777–3797. [Google Scholar] [CrossRef] [PubMed]
- Mellhammar, E.; Dahlbom, M.; Vilhelmsson-Timmermand, O.; Strand, S.-E. Tumor Control Probability and Small-Scale Monte Carlo Dosimetry: Effects of Heterogenous Intratumoral Activity Distribution in Radiopharmaceutical Therapy. J. Nucl. Med. 2023, 64, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, C.; Wang, X.; Wu, D. PSMA Targeted Therapy: From Molecular Mechanisms to Clinical Breakthroughs in Castration-Resistant Prostate Cancer. Eur. J. Med. Chem. 2025, 296, 117829. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Wu, J.; Ranjan, K.; Cui, X.; Wang, X.; Zhang, D.; Zhu, S. Key Molecular DNA Damage Responses of Human Cells to Radiation. Front. Cell Dev. Biol. 2024, 12, 1422520. [Google Scholar] [CrossRef] [PubMed]
- Rama, E.; May, J.-N.; Rix, A.; Lammers, T.; Kiessling, F. Image-guided strategies to improve drug delivery to tumors beyond using the enhanced permeability and retention (EPR) effect. Biochem. Biophys. Res. Commun. 2025, 778, 152346. [Google Scholar] [CrossRef] [PubMed]
- Kahya, U.; Lukiyanchuk, V.; Gorodetska, I.; Weigel, M.M.; Köseer, A.S.; Alkan, B.; Savic, D.; Linge, A.; Löck, S.; Peitzsch, M.; et al. Disruption of Glutamine Transport Uncouples the NUPR1 Stress-Adaptation Program and Induces Prostate Cancer Radiosensitivity. Cell Commun. Signal. 2025, 23, 351. [Google Scholar] [CrossRef]
- Müller, M.; Pedersen, N.B.; Shalgunov, V.; Jensen, A.I.; Battisti, U.M.; Herth, M.M. Astatine-211—Towards in vivo stable astatine-211 labeled radiopharmaceuticals and their (pre)clinical applications. Med. Res. Rev. 2025; in press. [Google Scholar] [CrossRef]
- Oriuchi, N.; Aoki, M.; Ukon, N.; Washiyama, K.; Tan, C.; Shimoyama, S.; Nishijima, K.; Takahashi, K.; Ito, H.; Ikezoe, T.; et al. Possibility of cancer-stem-cell-targeted radioimmunotherapy for acute myelogenous leukemia using 211At-CXCR4 monoclonal antibody. Sci. Rep. 2020, 10, 6810. [Google Scholar] [CrossRef]
- Larsen, R.H.; Slade, S.; Zalutsky, M.R. Blocking [211At]astatide accumulation in normal tissues: Preliminary evaluation of seven potential compounds. Nucl. Med. Biol. 1998, 25, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Nagatsu, K.; Minegishi, K.; Ishioka, N.S.; Ito, H.; Yoshinaga, K.; Higashi, T. Preclinical evaluation of the acute radiotoxicity of the α-emitting molecular-targeted therapeutic agent 211At-MABG for the treatment of malignant pheochromocytoma in normal mice. Transl. Oncol. 2019, 12, 879–888. [Google Scholar] [CrossRef]
- Yssartier, T.; Liu, L.; Pardoue, S.; Le Questel, J.-Y.; Guérard, F.; Montavon, G.; Galland, N. In vivo stability of 211At-radiopharmaceuticals: On the impact of halogen bond formation. RSC Med. Chem. 2024, 15, 223–233. [Google Scholar] [CrossRef]
- Andersson, H.; Cederkrantz, E.; Bäck, T.; Divgi, C.; Elgqvist, J.; Himmelman, J.; Horvath, G.; Jacobsson, L.; Jensen, H.; Lindegren, S.; et al. Intraperitoneal α-particle radioimmunotherapy of ovarian cancer patients: Pharmacokinetics and dosimetry of 211At-MX35 F(ab′)2—A phase I study. J. Nucl. Med. 2009, 50, 1153–1160. [Google Scholar] [CrossRef]
- Kaizuka, Y.; Suzuki, H.; Watabe, T.; Ooe, K.; Toyoshima, A.; Takahashi, K.; Sawada, K.; Iimori, T.; Masuda, Y.; Uno, T.; et al. Neopentyl glycol-based radiohalogen-labeled amino acid derivatives for cancer radiotheranostics. EJNMMI Radiopharm. Chem. 2024, 9, 17. [Google Scholar] [CrossRef]
- Ling, S.W.; van der Veldt, A.A.M.; Konijnenberg, M.; Segbers, M.; Hooijman, E.; Bruchertseifer, F.; Morgenstern, A.; de Blois, E.; Brabander, T. Evaluation of the tolerability and safety of [225Ac]Ac-PSMA-I&T in patients with metastatic prostate cancer: A phase I dose escalation study. BMC Cancer 2024, 24, 146. [Google Scholar] [CrossRef]
- Zhao, T.; Liang, S.H. Enhancing the stability of 211At radiopharmaceuticals: Insights from ortho-substituent strategies. ACS Med. Chem. Lett. 2025, 16, 504–507. [Google Scholar] [CrossRef]
- Thurber, G.M.; Weissleder, R. A systems approach for tumor pharmacokinetics. PLoS ONE 2011, 6, e24696. [Google Scholar] [CrossRef]
- Wei, L.; Tominaga, H.; Ohgaki, R.; Wiriyasermkul, P.; Hagiwara, K.; Okuda, S.; Kaira, K.; Oriuchi, N.; Nagamori, S.; Kanai, Y. Specific transport of 3-fluoro-L-α-methyl-tyrosine by LAT1 explains its specificity to malignant tumors in imaging. Cancer Sci. 2016, 107, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Gu, X.; Tong, K.; Chu, F.; Ye, P.; Kaneda-Nakashima, K.; Hou, W.; Li, Y.; Wei, L. L-3-[18F]-fluoro-α-methyl tyrosine as a PET tracer for tumor diagnosis: A systematic review from mechanisms to clinical applications. Int. J. Mol. Sci. 2025, 26, 5848. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Ose, N.; Naka, S.; Fukui, E.; Kimura, T.; Kanou, T.; Funaki, S.; Sasaki, H.; Kamiya, T.; Kurimoto, K.; et al. Evaluation of LAT1 expression in patients with lung cancer and mediastinal tumors: 18F-FBPA PET study with immunohistological comparison. Clin. Nucl. Med. 2023, 48, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Bednarz, B. Theranostics and patient-specific dosimetry. Semin. Radiat. Oncol. 2023, 33, 317–326. [Google Scholar] [CrossRef]
- Naka, S.; Ooe, K.; Shirakami, Y.; Kurimoto, K.; Sakai, T.; Takahashi, K.; Toyoshima, A.; Wang, Y.; Haba, H.; Kato, H.; et al. Production of [211At]NaAt solution under GMP compliance for investigator-initiated clinical trial. EJNMMI Radiopharm. Chem. 2024, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Joho, T.; Sasaki, S.; Mochizuki, K.; Hasegawa, N.; Ukon, N.; Nishijima, K.; Washiyama, K.; Tanaka, H.; Higashi, T.; et al. Production of 211At and automated radiosynthesis of [211At]MABG via electrophilic astatodesilylation. EJNMMI Radiopharm. Chem. 2025, 10, 52. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Li, Y.-L.; Shen, H.-T.; Chien, P.-J.; Sheu, G.-T.; Wang, B.-Y.; Chang, W.-W. L-type amino acid transporter 1 regulates cancer stemness and the expression of programmed cell death 1 ligand 1 in lung cancer cells. Int. J. Mol. Sci. 2021, 22, 10955. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, H.; Wang, Y.; Yang, J.; Lin, C.; Shi, X.; Zou, Y.; Chen, J.; Jia, X.; Su, L. The advance of the third-generation EGFR-TKI in the treatment of non-small cell lung cancer: A review. Oncol. Rep. 2024, 51, 16. [Google Scholar] [CrossRef]
- Awad, M.M.; Liu, S.; Rybkin, I.I.; Arbour, K.C.; Dilly, J.; Zhu, V.W.; Johnson, M.L.; Heist, R.S.; Patil, T.; Riely, G.J.; et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 2021, 384, 2382–2393. [Google Scholar] [CrossRef]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-emitters for radiotherapy: From basic radiochemistry to clinical studies—Part 1. J. Nucl. Med. 2018, 59, 878–884. [Google Scholar] [CrossRef]
- Mladenova, V.; Mladenov, E.; Stuschke, M.; Iliakis, G. DNA damage clustering after ionizing radiation and consequences in the processing of chromatin breaks. Molecules 2022, 27, 1540. [Google Scholar] [CrossRef] [PubMed]
- Lüdeking, M.; Stemwedel, K.; Ramachandran, D.; Grosche, S.; Christiansen, H.; Merten, R.; Henkenberens, C.; Bogdanova, N.V. Efficiency of moderately hypofractionated radiotherapy in NSCLC cell model. Front. Oncol. 2024, 14, 1293745. [Google Scholar] [CrossRef]
- Ogihara, K.; Kikuchi, E.; Okazaki, S.; Hagiwara, M.; Takeda, T.; Matsumoto, K.; Kosaka, T.; Mikami, S.; Saya, H.; Oya, M. Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 2019, 110, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Kleinendorst, S.C.; Oosterwijk, E.; Bussink, J.; Westdorp, H.; Konijnenberg, M.W.; Heskamp, S. Combining targeted radionuclide therapy and immune checkpoint inhibition for cancer treatment. Clin. Cancer Res. 2022, 28, 3652–3657. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, H.; Lou, J.; Zhang, J.; Zuo, C.; Zhu, M.; Zhang, X.; Yin, Y.; Zhang, Y.; Qin, S.; et al. Alpha-emitter radium-223 induces STING-dependent pyroptosis to trigger robust antitumor immunity. Small 2024, 20, e2307448. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.; Liu, G.; Bi, C.; Wang, X.; Zhou, Q.; Jin, H. SLC7A11/xCT in cancer: Biological functions and therapeutic implications. Am. J. Cancer Res. 2020, 10, 3106–3126. [Google Scholar]
- Ertveldt, T.; Krasniqi, A.; Ceuppens, H.; Puttemans, J.; Dekempeneer, Y.; De Jonghe, K.; de Mey, W.; Lecocq, Q.; De Vlaeminck, Y.; Awad, R.M.; et al. Targeted α-therapy using 225Ac radiolabeled single-domain antibodies induces antigen-specific immune responses and instills immunomodulation both systemically and at the tumor microenvironment. J. Nucl. Med. 2023, 64, 751–758. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, L.; Fu, K.; Lin, Q.; Wen, X.; Jacobson, O.; Sun, L.; Wu, H.; Zhang, X.; Guo, Z.; et al. Integrin αvβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergistically enhances anti-tumor efficacy. Theranostics 2019, 9, 7948–7960. [Google Scholar] [CrossRef]
- Kim, J.-W.; Kim, M.-J.; Han, T.-H.; Lee, J.-Y.; Kim, S.; Kim, H.; Oh, K.-J.; Kim, W.K.; Han, B.-S.; Bae, K.-H.; et al. FSP1 confers ferroptosis resistance in KEAP1-mutant non-small cell lung carcinoma in NRF2-dependent and -independent manner. Cell Death Dis. 2023, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Lei, G.; Zhang, Y.; Yan, Y.; Mao, C.; Kondiparthi, L.; Shi, J.; Liu, X.; Horbath, A.; Das, M.; et al. A targetable CoQ–FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1-inactive lung cancers. Nat. Commun. 2022, 13, 2206. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Krol, V.; Bansal, A.; Andersson, J.D.; Wuest, F.; Pandey, M.K. Aspects and prospects of preclinical theranostic radiopharmaceutical development. Theranostics 2024, 14, 6446–6470. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, A.R.; Koglin, N.; Mittra, E.S.; Han, S.; Cook, G.J.R.; Witney, T.H. Clinical [18F]FSPG positron emission tomography imaging reveals heterogeneity in tumor-associated system xC− activity. Cancers 2024, 16, 1437. [Google Scholar] [CrossRef]
- Cheng, Q.; Wållberg, H.; Grafström, J.; Lu, L.; Thorell, J.-O.; Hägg Olofsson, M.; Linder, S.; Johansson, K.; Tegnebratt, T.; Arnér, E.S.J.; et al. Preclinical PET imaging of EGFR levels: Pairing a targeting with a non-targeting Sel-tagged affibody-based tracer to estimate the specific uptake. EJNMMI Res. 2016, 6, 58. [Google Scholar] [CrossRef]
- Hellyer, J.A.; Padda, S.K.; Diehn, M.; Wakelee, H.A. Clinical implications of KEAP1–NFE2L2 mutations in NSCLC. J. Thorac. Oncol. 2021, 16, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Kang, H.-M.; Na, K.; Kim, J.; Kim, T.-W.; Jung, J.; Lim, H.; Seo, H.; Lee, S.H. KEAP1–NRF2 pathway as a novel therapeutic target for EGFR-mutant non-small cell lung cancer. Tuberc. Respir. Dis. 2025, 88, 138–149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).