Clinical Applications of Bovine Colostrum in GastrointestinaI Disorders: Mechanisms, Evidence, and Therapeutic Potential
Abstract
1. Introduction
2. Key Bioactive Components Relevant to GI Health
2.1. Lactoferrin
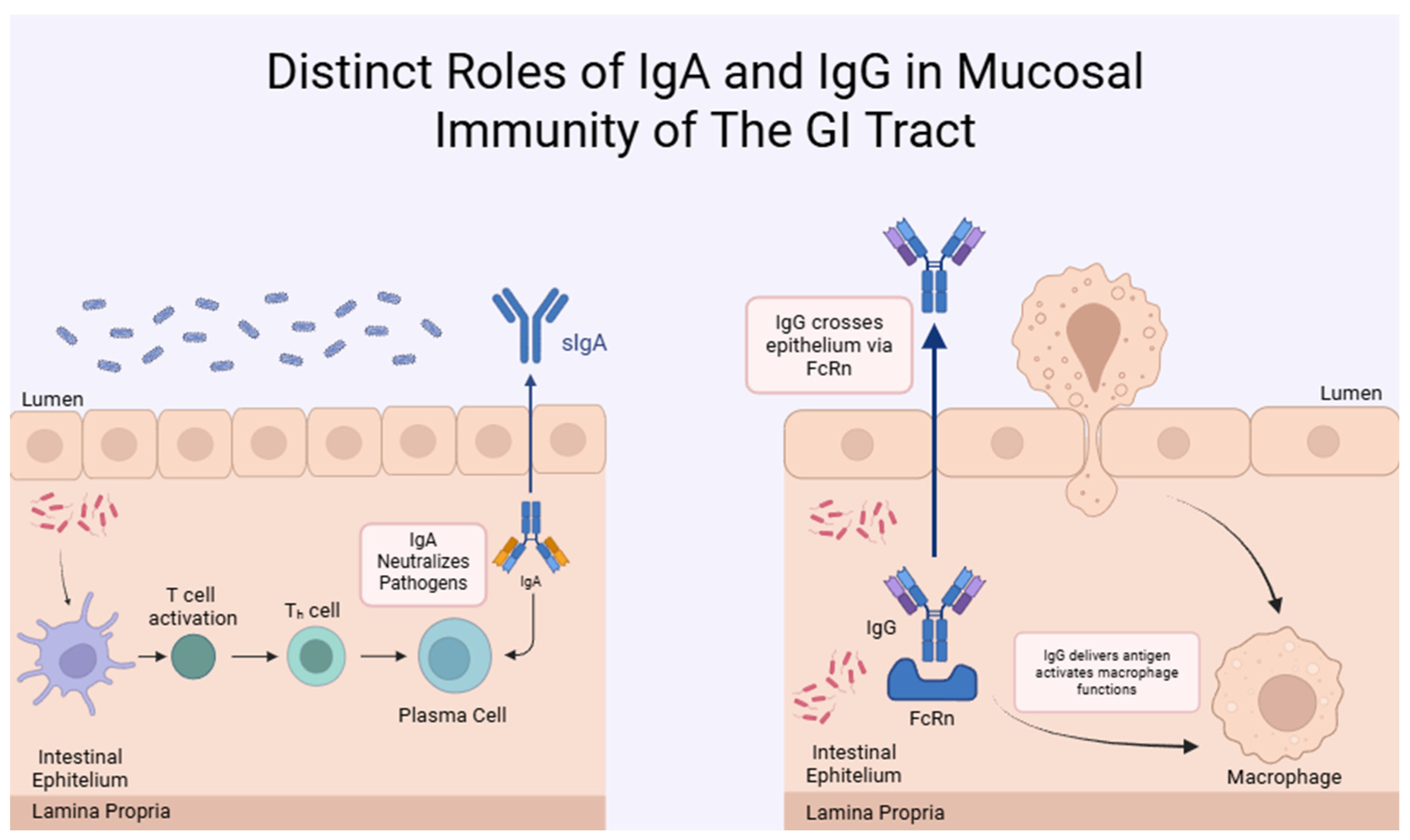
2.2. Immunoglobulin G (IgG)
2.3. Growth Factors
2.4. Oligosaccharides
2.5. Bioactive Peptides
3. Mechanisms of Action in the GI Tract
3.1. Mucosal Barrier Protection
3.2. Immunomodulation
- Immunoglobulins (Igs)
- Growth Factors
- LF
3.3. Anti-Pathogenic Effects
3.4. Microbiota Modulation
4. Evidence from Preclinical and Clinical Studies
4.1. Infectious Diarrhea
4.2. Inflammatory Bowel Disease (IBD)
4.3. Irritable Bowel Syndrome (IBS)
4.4. NSAID-Induced Enteropathy
4.5. Necrotizing Enterocolitis (NEC) in Preterm Infants
5. Formulations and Administration Routes
6. Regulatory and Safety Considerations
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Mazumder, A.; Das, S.; Kanda, A.; Prabhakar, V.; Mishra, R.; Chaitanya, M. Bovine Colostrum: Unveiling Its Potent Health Benefits and Emerging Trends in Optimizing Well-Being. Nat. Prod. J. 2025, 15, E130624231023. [Google Scholar] [CrossRef]
- Duan, H.; Sun, Q.; Chen, C.; Wang, R.; Yan, W. A Review: The Effect of Bovine Colostrum on Immunity in People of All Ages. Nutrients 2024, 16, 2007. [Google Scholar] [CrossRef] [PubMed]
- Sangild, P.T.; Vonderohe, C.; Melendez Hebib, V.; Burrin, D.G. Potential Benefits of Bovine Colostrum in Pediatric Nutrition and Health. Nutrients 2021, 13, 2551. [Google Scholar] [CrossRef] [PubMed]
- Guberti, M.; Botti, S.; Capuzzo, M.T.; Nardozi, S.; Fusco, A.; Cera, A.; Dugo, L.; Piredda, M.; De Marinis, M.G. Bovine Colostrum Applications in Sick and Healthy People: A Systematic Review. Nutrients 2021, 13, 2194. [Google Scholar] [CrossRef]
- Grigalevičiūtė, R.; Matusevičius, P.; Plančiūnienė, R.; Stankevičius, R.; Radzevičiūtė-Valčiukė, E.; Balevičiūtė, A.; Želvys, A.; Zinkevičienė, A.; Zigmantaitė, V.; Kučinskas, A.; et al. Understanding the Immunomodulatory Effects of Bovine Colostrum: Insights into IL-6/IL-10 Axis-Mediated Inflammatory Control. Vet. Sci. 2023, 10, 519. [Google Scholar] [CrossRef]
- Hajihashemi, P.; Haghighatdoost, F.; Kassaian, N.; Rahim Khorasani, M.; Hoveida, L.; Nili, H.; Tamizifar, B.; Adibi, P. Therapeutics Effects of Bovine Colostrum Applications on Gastrointestinal Diseases: A Systematic Review. Syst. Rev. 2024, 13, 76. [Google Scholar] [CrossRef]
- Gomes, R.D.S.; Anaya, K.; Galdino, A.B.S.; Oliveira, J.P.F.; Gama, M.A.S.; Medeiros, C.A.C.X.; Gavioli, E.C.; Porto, A.L.F.; Rangel, A.H.N. Bovine Colostrum: A Source of Bioactive Compounds for Prevention and Treatment of Gastrointestinal Disorders. NFS J. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Oswal, D.; Angolkar, M.; Mahantashetti, N.S.; Dhagavkar, P.; Haritay, S.; Godbole, M. Effect of Bovine Colostrum Supplementation on Gut Health of Children: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 1018–1027. [Google Scholar] [CrossRef]
- Yu, W.; Lin, Y.; Lu, Y.; Wang, Y.; Zhang, D.; Quan, H.; Luo, Y.; Zhang, Y.; Jiang, Z.; Chen, J.; et al. Lactoferrin Promotes Intestinal Stem Cell-Mediated Epithelial Regeneration by Activating Wnt Signaling. Food Front. 2024, 5, 2290–2304. [Google Scholar] [CrossRef]
- Bagwe-Parab, S.; Yadav, P.; Kaur, G.; Tuli, H.S.; Buttar, H.S. Therapeutic Applications of Human and Bovine Colostrum in the Treatment of Gastrointestinal Diseases and Distinctive Cancer Types: The Current Evidence. Front. Pharmacol. 2020, 11, 1100. [Google Scholar] [CrossRef]
- Ramani, A.; Taherabbas, S.; Manik, S. Bovine Colostrum as a Promising Nutraceutical: A Systematic Review. Sustain. Food Technol. 2024, 2, 531–547. [Google Scholar] [CrossRef]
- Ghosh, S.; Iacucci, M. Diverse Immune Effects of Bovine Colostrum and Benefits in Human Health and Disease. Nutrients 2021, 13, 3798. [Google Scholar] [CrossRef] [PubMed]
- Bodammer, P.; Maletzki, C.; Waitz, G.; Emmrich, J. Prophylactic Application of Bovine Colostrum Ameliorates Murine Colitis via Induction of Immunoregulatory Cells. J. Nutr. 2011, 141, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Costa, A.; Visentin, G.; Marchi, M.D. Mid-Infrared Spectroscopy for Large-Scale Phenotyping of Bovine Colostrum Gross Composition and Immunoglobulin Concentration. J. Dairy Sci. 2023, 106, 6388–6401. [Google Scholar] [CrossRef]
- Silva, F.G.; Silva, S.R.; Pereira, A.M.F.; Cerqueira, J.L.; Conceição, C. A Comprehensive Review of Bovine Colostrum Components and Selected Aspects Regarding Their Impact on Neonatal Calf Physiology. Animals 2024, 14, 1130. [Google Scholar] [CrossRef]
- Desloire, S.; Valiente Moro, C.; Chauve, C.; Zenner, L. Comparison of Four Methods of Extracting DNA from D. gallinae (Acari: Dermanyssidae). Vet. Res. 2006, 37, 725–732. [Google Scholar] [CrossRef]
- Yagi, H.; Suzuki, S.; Noji, T.; Nagashima, K.; Kuroume, T. Epidermal Growth Factor in Cow’s Milk and Milk Formulas. Acta Paediatr. Scand. 1986, 75, 233–235. [Google Scholar] [CrossRef]
- Elfstrand, L.; Lindmark-Månsson, H.; Paulsson, M.; Nyberg, L.; Åkesson, B. Immunoglobulins, Growth Factors and Growth Hormone in Bovine Colostrum and the Effects of Processing. Int. Dairy J. 2002, 12, 879–887. [Google Scholar] [CrossRef]
- Playford, R.J.; Cattell, M.; Marchbank, T. Marked Variability in Bioactivity between Commercially Available Bovine Colostrum for Human Use; Implications for Clinical Trials. PLoS ONE 2020, 15, e0234719, Correction in PLoS ONE 2020, 15, e0240392. https://doi.org/10.1371/journal.pone.0240392. [Google Scholar] [CrossRef]
- Elizondo-Salazar, J.A.; Jayarao, B.M.; Heinrichs, A.J. Effect of Heat Treatment of Bovine Colostrum on Bacterial Counts, Viscosity, and Immunoglobulin G Concentration. J. Dairy Sci. 2010, 93, 961–967. [Google Scholar] [CrossRef]
- Costa, A.; Franzoi, M.; Visentin, G.; Goi, A.; De Marchi, M.; Penasa, M. The Concentrations of Immunoglobulins in Bovine Colostrum Determined by the Gold Standard Method Are Genetically Correlated with Their Near-Infrared Prediction. Genet. Sel. Evol. 2021, 53, 87. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Goi, A.; Penasa, M.; Nardino, G.; Posenato, L.; De Marchi, M. Variation of Immunoglobulins G, A, and M and Bovine Serum Albumin Concentration in Holstein Cow Colostrum. Animal 2021, 15, 100299. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.I.; Jayarao, B.M.; Heinrichs, A.J. A Survey of Bovine Colostrum Composition and Colostrum Management Practices on Pennsylvania Dairy Farms. J. Dairy Sci. 2007, 90, 4108–4116. [Google Scholar] [CrossRef]
- Trajkovska, B.; Kochoski, L.; Dimitrovska, G.; Hajrulai-Musliu, Z.; Uzunov, R.; Petkov, V.; Badgujar, P.C. Changes in the Lactoferrin Concentration in the Bovine Colostrum During Postpartum Period. Maced. Vet. Rev. 2022, 45, 177. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Bunyatratchata, A.; Weinborn, V.; Barile, D. Bioactive Oligosaccharides in Colostrum and Other Liquid Feeds for Calf’s Early Life Nutrition: A Qualitative and Quantitative Investigation. Int. Dairy J. 2021, 121, 105100. [Google Scholar] [CrossRef]
- Robinson, R.C. Structures and Metabolic Properties of Bovine Milk Oligosaccharides and Their Potential in the Development of Novel Therapeutics. Front. Nutr. 2019, 6, 50. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. Colostrum and Lactoferrin Protect against Side Effects of Therapy with Antibiotics, Anti-Inflammatory Drugs and Steroids, and Psychophysical Stress: A Comprehensive Review. Biomedicines 2023, 11, 1015. [Google Scholar] [CrossRef]
- Patil, A.S.; Puri, R.; Wakure, B.S. A Revıew On Lactoferrın Prıncıple Constıtuent of Bovıne Colostrum: In-Covıd19. Int. J. Curr. Pharm. Res. 2022, 14, 1–8. [Google Scholar] [CrossRef]
- Conesa, C.; Bellés, A.; Grasa, L.; Sánchez, L. The Role of Lactoferrin in Intestinal Health. Pharmaceutics 2023, 15, 1569. [Google Scholar] [CrossRef]
- Ahmann, J.; Steinhoff-Wagner, J.; Büscher, W. Determining Immunoglobulin Content of Bovine Colostrum and Factors Affecting the Outcome: A Review. Animals 2021, 11, 3587. [Google Scholar] [CrossRef]
- Lopez, A.J.; Heinrichs, A.J. Invited Review: The Importance of Colostrum in the Newborn Dairy Calf. J. Dairy Sci. 2022, 105, 2733–2749. [Google Scholar] [CrossRef]
- Mero, A.; Miikkulainen, H.; Riski, J.; Pakkanen, R.; Aalto, J.; Takala, T. Effects of Bovine Colostrum Supplementation on Serum IGF-I, IgG, Hormone, and Saliva IgA during Training. J. Appl. Physiol. 1997, 83, 1144–1151. [Google Scholar] [CrossRef]
- Główka, N.; Durkalec-Michalski, K.; Woźniewicz, M. Immunological Outcomes of Bovine Colostrum Supplementation in Trained and Physically Active People: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1023. [Google Scholar] [CrossRef] [PubMed]
- Poonia, A. Shiva; Bioactive Compounds, Nutritional Profile and Health Benefits of Colostrum: A Review. Food Prod. Process. Nutr. 2022, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Dziewiecka, H.; Buttar, H.S.; Kasperska, A.; Ostapiuk-Karolczuk, J.; Domagalska, M.; Cichoń, J.; Skarpańska-Stejnborn, A. A Systematic Review of the Influence of Bovine Colostrum Supplementation on Leaky Gut Syndrome in Athletes: Diagnostic Biomarkers and Future Directions. Nutrients 2022, 14, 2512. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Tlustos, A.J.; Hertogs, K.; van Niekerk, J.K.; Nagorske, M.; Haines, D.M.; Steele, M.A. Oligosaccharide Concentrations in Colostrum, Transition Milk, and Mature Milk of Primi- and Multiparous Holstein Cows during the First Week of Lactation. J. Dairy Sci. 2020, 103, 3683–3695. [Google Scholar] [CrossRef]
- Carter, M.M.; Demis, D.; Perelman, D.; Onge, M.S.; Petlura, C.; Cunanan, K.; Mathi, K.; Maecker, H.T.; Chow, J.M.; Robinson, J.L.; et al. A Human Milk Oligosaccharide Alters the Microbiome, Circulating Hormones, and Metabolites in a Randomized Controlled Trial of Older Adults. Cell Rep. Med. 2025, 6, 102256. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, J.; Regenstein, J.M.; Liu, D.; Huang, Y.; Qiao, Y.; Zhou, P. α-Lactalbumin: Functional Properties and Potential Health Benefits. Food Biosci. 2024, 60, 104371. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Hui, Y.; Nguyen, D.N.; Ahnfeldt, A.M.; Burrin, D.G.; Hartmann, B.; Heckmann, A.B.; Sangild, P.T.; Thymann, T.; Bering, S.B. Alpha-Lactalbumin Enriched Whey Protein Concentrate to Improve Gut, Immunity and Brain Development in Preterm Pigs. Nutrients 2020, 12, 245. [Google Scholar] [CrossRef]
- Wusigale; Liang, L.; Luo, Y. Casein and Pectin: Structures, Interactions, and Applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar] [CrossRef]
- Guantario, B.; Giribaldi, M.; Devirgiliis, C.; Finamore, A.; Colombino, E.; Capucchio, M.T.; Evangelista, R.; Motta, V.; Zinno, P.; Cirrincione, S.; et al. A Comprehensive Evaluation of the Impact of Bovine Milk Containing Different Beta-Casein Profiles on Gut Health of Ageing Mice. Nutrients 2020, 12, 2147. [Google Scholar] [CrossRef] [PubMed]
- Chatterton, D.E.W.; Smithers, G.; Roupas, P.; Brodkorb, A. Bioactivity of β-Lactoglobulin and α-Lactalbumin—Technological Implications for Processing. Int. Dairy J. 2006, 16, 1229–1240. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, R.; Yan, Y.; Wu, Y.; Meng, X.; Yang, A.; Wu, Z.; Shi, L.; Li, X.; Chen, H. Digestive Stability and Transport Ability Changes of β-Lactoglobulin–Catechin Complexes by M Cell Model in Vitro. Front. Nutr. 2022, 9, 955135. [Google Scholar] [CrossRef] [PubMed]
- Chandwe, K.; Kelly, P. Colostrum Therapy for Human Gastrointestinal Health and Disease. Nutrients 2021, 13, 1956. [Google Scholar] [CrossRef]
- Aidos, L.; Pallaoro, M.; Mirra, G.; Serra, V.; Castrica, M.; Agradi, S.; Curone, G.; Vigo, D.; Riva, F.; Balzaretti, C.M.; et al. Intestine Health and Barrier Function in Fattening Rabbits Fed Bovine Colostrum. Vet. Sci. 2023, 10, 657. [Google Scholar] [CrossRef]
- Tang, X.; Liu, H.; Yang, S.; Li, Z.; Zhong, J.; Fang, R. Epidermal Growth Factor and Intestinal Barrier Function. Mediat. Inflamm. 2016, 2016, 1927348. [Google Scholar] [CrossRef]
- Samak, G.; Aggarwal, S.; Rao, R.K. ERK Is Involved in EGF-Mediated Protection of Tight Junctions, but Not Adherens Junctions, in Acetaldehyde-Treated Caco-2 Cell Monolayers. Am. J. Physiol.-Gastrointest. Liver Physiol. 2011, 301, G50–G59. [Google Scholar] [CrossRef]
- Marincola Smith, P.; Choksi, Y.A.; Markham, N.O.; Hanna, D.N.; Zi, J.; Weaver, C.J.; Hamaamen, J.A.; Lewis, K.B.; Yang, J.; Liu, Q.; et al. Colon Epithelial Cell TGFβ Signaling Modulates the Expression of Tight Junction Proteins and Barrier Function in Mice. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 320, G936–G957. [Google Scholar] [CrossRef]
- Gao, Y.-N.; Li, S.-L.; Yang, X.; Wang, J.-Q.; Zheng, N. The Protective Effects of Lactoferrin on Aflatoxin M1-Induced Compromised Intestinal Integrity. Int. J. Mol. Sci. 2022, 23, 289. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.-Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin Attenuates Intestinal Barrier Dysfunction and Inflammation by Modulating the MAPK Pathway and Gut Microbes in Mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef]
- Burton, R.E.; Kim, S.; Patel, R.; Hartman, D.S.; Tracey, D.E.; Fox, B.S. Structural Features of Bovine Colostral Immunoglobulin That Confer Proteolytic Stability in a Simulated Intestinal Fluid. J. Biol. Chem. 2020, 295, 12317–12327. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Björnsson, B.T.; Valdés, J.A.; Einarsdottir, I.E.; Lorca, B.; Alvarez, M.; Molina, A. IGF-I/PI3K/Akt and IGF-I/MAPK/ERK Pathways in Vivo in Skeletal Muscle Are Regulated by Nutrition and Contribute to Somatic Growth in the Fine Flounder. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, R1532–R1542. [Google Scholar] [CrossRef]
- Bæk, O.; Rasmussen, M.B.; Gerts, T.; Aunsholt, L.; Zachariassen, G.; Sangild, P.; Nguyen, D.N. Insulin-like Growth Factor 1 Associated with Altered Immune Responses in Preterm Infants and Pigs. Pediatr. Res. 2024, 95, 120–128. [Google Scholar] [CrossRef]
- Oh, J.-S.; Hwang, S.-U.; Noh, K.-E.; Lee, J.-H.; Choi, S.-Y.; Nam, J.-H.; Song, M.-S.; Jung, N.-C.; Song, J.-Y.; Seo, H.G.; et al. Synthetic TGF-β Signaling Agonist-Treated Dendritic Cells Induce Tolerogenicity and Antirheumatic Effects. Curr. Issues Mol. Biol. 2022, 44, 3809–3821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Chen, N.; Ashaolu, T.J. Prebiotic and Modulatory Evidence of Lactoferrin on Gut Health and Function. J. Funct. Foods 2023, 108, 105741. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Olin, A.B.; Nguyen, D.N. Fortification with Bovine Colostrum Enhances Antibacterial Activity of Human Milk. JPEN J. Parenter. Enteral. Nutr. 2021, 45, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Gruden, Š.; Poklar Ulrih, N. Diverse Mechanisms of Antimicrobial Activities of Lactoferrins, Lactoferricins, and Other Lactoferrin-Derived Peptides. Int. J. Mol. Sci. 2021, 22, 11264. [Google Scholar] [CrossRef]
- Sabancılar, İ.; Toprak, G.; Temiz, H. Determination of antibacterial efficacy of lactoferrin glycoprotein obtained from cow colostrum. Int. Arch. Med. Res. 2023, 15, 27–34. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gómez, C.; Ramírez-Texcalco, L.S.; Torres-López, E.; Ramírez-Mondragón, R.; Hernández-Ramírez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor. Emerg. Microbes Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, R.; Rosa, L.; Cutone, A.; Lepanto, M.S.; Franchitto, A.; Onori, P.; Gaudio, E.; Valenti, P. Viral Hepatitis and Iron Dysregulation: Molecular Pathways and the Role of Lactoferrin. Molecules 2020, 25, 1997. [Google Scholar] [CrossRef]
- Atyeo, C.; Alter, G. The Multifaceted Roles of Breast Milk Antibodies. Cell 2021, 184, 1486–1499. [Google Scholar] [CrossRef]
- Sawa, T.; Kinoshita, M.; Inoue, K.; Ohara, J.; Moriyama, K. Immunoglobulin for Treating Bacterial Infections: One More Mechanism of Action. Antibodies 2019, 8, 52. [Google Scholar] [CrossRef]
- Huus, K.E.; Petersen, C.; Finlay, B.B. Diversity and Dynamism of IgA−microbiota Interactions. Nat. Rev. Immunol. 2021, 21, 514–525. [Google Scholar] [CrossRef]
- Nöltner, C.; Bulashevska, A.; Hübscher, K.; Haberstroh, H.; Grimbacher, B.; Proietti, M. Fecal Immunoglobulin Levels as a Modifier of the Gut Microbiome in Patients with Common Variable Immunodeficiency. J. Clin. Immunol. 2023, 43, 1208–1220. [Google Scholar] [CrossRef]
- Leonardi, L.; Dib, S.; Costanzi, E.; Brecchia, G.; Traina, G. Antioxidant Activity of Bovine Colostrum in the Colon of a Mouse Model of TNBS-Induced Colitis. Antioxidants 2025, 14, 232. [Google Scholar] [CrossRef]
- Yu, P.; Satyaraj, E. Effect of Bovine Colostrum on Canine Immune Health. Animals 2025, 15, 185. [Google Scholar] [CrossRef]
- Sugiharto, S.; Poulsen, A.-S.R.; Canibe, N.; Lauridsen, C. Effect of Bovine Colostrum Feeding in Comparison with Milk Replacer and Natural Feeding on the Immune Responses and Colonisation of Enterotoxigenic Escherichia coli in the Intestinal Tissue of Piglets. Br. J. Nutr. 2015, 113, 923–934. [Google Scholar] [CrossRef]
- Morrin, S.T.; McCarthy, G.; Kennedy, D.; Marotta, M.; Irwin, J.A.; Hickey, R.M. Immunoglobulin G from Bovine Milk Primes Intestinal Epithelial Cells for Increased Colonization of Bifidobacteria. AMB Express 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kawase, H.; Kimura, K.; Watanabe, Y.; Ohtani, M.; Arai, I.; Urashima, T. Concentrations of Sialyloligosaccharides in Bovine Colostrum and Milk during the Prepartum and Early Lactation. J. Dairy Sci. 2003, 86, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Gallo, V.; Arienzo, A.; Tomassetti, F.; Antonini, G. Milk Bioactive Compounds and Gut Microbiota Modulation: The Role of Whey Proteins and Milk Oligosaccharides. Foods 2024, 13, 907. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, L.-Q.; Liu, F.; Wu, J.-Y. Human Milk Oligosaccharides and Infant Gut Microbiota: Molecular Structures, Utilization Strategies and Immune Function. Carbohydr. Polym. 2022, 276, 118738. [Google Scholar] [CrossRef]
- Gormley, A.; Garavito-Duarte, Y.; Kim, S.W. The Role of Milk Oligosaccharides in Enhancing Intestinal Microbiota, Intestinal Integrity, and Immune Function in Pigs: A Comparative Review. Biology 2024, 13, 663. [Google Scholar] [CrossRef]
- Pang, J.; Liu, Y.; Kang, L.; Ye, H.; Zang, J.; Wang, J.; Han, D. Bifidobacterium Animalis Promotes the Growth of Weaning Piglets by Improving Intestinal Development, Enhancing Antioxidant Capacity, and Modulating Gut Microbiota. Appl. Environ. Microbiol. 2022, 88, e01296-22. [Google Scholar] [CrossRef]
- Carter, H.S.M.; Renaud, D.L.; Steele, M.A.; Fischer-Tlustos, A.J.; Costa, J.H.C. A Narrative Review on the Unexplored Potential of Colostrum as a Preventative Treatment and Therapy for Diarrhea in Neonatal Dairy Calves. Animals 2021, 11, 2221. [Google Scholar] [CrossRef]
- Carter, H.S.M.; Steele, M.A.; Costa, J.H.C.; Renaud, D.L. Evaluating the Effectiveness of Colostrum as a Therapy for Diarrhea in Preweaned Calves. J. Dairy Sci. 2022, 105, 9982–9994. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.-W.; Jiang, J.-J.; Song, Q.-K. Bovine Colostrum and Product Intervention Associated with Relief of Childhood Infectious Diarrhea. Sci. Rep. 2019, 9, 3093. [Google Scholar] [CrossRef]
- Brunauer, M.; Roch, F.-F.; Conrady, B. Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium spp.: A Meta-Analysis. Animals 2021, 11, 1014. [Google Scholar] [CrossRef]
- Chen, K.; Chen, H.; Luo, J.; Zeng, C.; Dong, X.; Zhou, M.; Liu, C. The Prophylactic Effect of Bovine Colostrum on Respiratory Infection and Diarrhea in Formula-Fed Infants: A Randomized Trial. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Ashraf, H.; Mahalanabis, D.; Mitra, A.; Tzipori, S.; Fuchs, G. Hyperimmune Bovine Colostrum in the Treatment of Shigellosis in Children: A Double-Blind, Randomized, Controlled Trial. Acta Paediatr. 2001, 90, 1373–1378. [Google Scholar] [CrossRef]
- Playford, R.J.; Choudhry, N.; Kelly, P.; Marchbank, T. Effects of Bovine Colostrum with or without Egg on In Vitro Bacterial-Induced Intestinal Damage with Relevance for SIBO and Infectious Diarrhea. Nutrients 2021, 13, 1024. [Google Scholar] [CrossRef]
- Tawfeek, H.I.; Najim, N.H.; Al-Mashikhi, S. Efficacy of an Infant Formula Containing Anti-Escherichia coli Colostral Antibodies from Hyperimmunized Cows in Preventing Diarrhea in Infants and Children: A Field Trial. Int. J. Infect. Dis. 2003, 7, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Islam, D.; Ruamsap, N.; Imerbsin, R.; Khanijou, P.; Gonwong, S.; Wegner, M.D.; McVeigh, A.; Poly, F.M.; Crawford, J.M.; Swierczewski, B.E.; et al. Bioactivity and Efficacy of a Hyperimmune Bovine Colostrum Product- Travelan, against Shigellosis in a Non-Human Primate Model (Macaca mulatta). PLoS ONE 2023, 18, e0294021. [Google Scholar] [CrossRef] [PubMed]
- Debelo, M.; Abdela, H.; Tesfaye, A.; Tiruneh, A.; Mekonnen, G.; Asefa, Z.; Moje, N. Prevalence of Bovine Rotavirus and Coronavirus in Neonatal Calves in Dairy Farms of Addis Ababa, Ethiopia: Preliminary Study. Biomed. Res. Int. 2021, 2021, 5778455. [Google Scholar] [CrossRef] [PubMed]
- Lefkaditis, M.; Mpairamoglou, R.; Sossidou, A.; Spanoudis, K.; Tsakiroglou, M.; Györke, A. Importance of Colostrum IgG Antibodies Level for Prevention of Infection with Cryptosporidium parvum in Neonatal Dairy Calves. Prev. Vet. Med. 2020, 176, 104904. [Google Scholar] [CrossRef]
- Koohi, O.; Shahriarirad, R.; Erfani, A.; Nekouei, F.; Seifbehzad, S.; Ebrahimi, A.; Tanideh, N.; Hosseinzadeh, M.; Nadimi, E.; Ashkani-Esfahani, S. Evaluation of oral and topical bovine colostrum compared to mesalamine in the treatment of animal model of acetic acid-induced ulcerative colitis. Ann. Gastroenterol. 2023, 36, 300. [Google Scholar] [CrossRef]
- Khan, Z.; Macdonald, C.; Wicks, A.C.; Holt, M.P.; Floyd, D.; Ghosh, S.; Wright, N.A.; Playford, R.J. Use of the ‘Nutriceutical’, Bovine Colostrum, for the Treatment of Distal Colitis: Results from an Initial Study. Aliment. Pharmacol. Ther. 2002, 16, 1917–1922. [Google Scholar] [CrossRef]
- Menchetti, L.; Curone, G.; Filipescu, I.E.; Barbato, O.; Leonardi, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Riva, F.; Casano, A.B.; et al. The Prophylactic Use of Bovine Colostrum in a Murine Model of TNBS-Induced Colitis. Animals 2020, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Filipescu, I.E.; Leonardi, L.; Menchetti, L.; Guelfi, G.; Traina, G.; Casagrande-Proietti, P.; Piro, F.; Quattrone, A.; Barbato, O.; Brecchia, G. Preventive Effects of Bovine Colostrum Supplementation in TNBS-Induced Colitis in Mice. PLoS ONE 2018, 13, e0202929. [Google Scholar] [CrossRef] [PubMed]
- Spalinger, M.R.; Atrott, K.; Baebler, K.; Schwarzfischer, M.; Melhem, H.; Peres, D.R.; Lalazar, G.; Rogler, G.; Scharl, M.; Frey-Wagner, I. Administration of the Hyper-Immune Bovine Colostrum Extract IMM-124E Ameliorates Experimental Murine Colitis. J. Crohns Colitis 2019, 13, 785–797. [Google Scholar] [CrossRef]
- Yan, B.; Li, H.; Zhao, J.; Wang, R.; Chen, C.; Chen, W.; Yang, B. Dose-Response Effect of Bovine Colostrum against DSS-Induced Colitis in Mice. Food Biosci. 2025, 68, 106530. [Google Scholar] [CrossRef]
- Chae, A.; Aitchison, A.; Day, A.S.; Keenan, J.I. Bovine Colostrum Demonstrates Anti-Inflammatory and Antibacterial Activity in in Vitro Models of Intestinal Inflammation and Infection. J. Funct. Foods 2017, 28, 293–298. [Google Scholar] [CrossRef]
- Wilson, D.; Evans, M.; Weaver, E.; Shaw, A.L.; Klein, G.L. Evaluation of Serum-Derived Bovine Immunoglobulin Protein Isolate in Subjects with Diarrhea-Predominant Irritable Bowel Syndrome. Clin. Med. Insights Gastroenterol. 2013, 6, CGast.S13200. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Park, S.J.; Cheon, J.H. Effect of Colostrum on the Symptoms and Mucosal Permeability in Patients with Irritable Bowel Syndrome: A Randomized Placebo-Controlled Study. Intest. Res. 2014, 12, 80–82. [Google Scholar] [CrossRef]
- Velikova, T.; Tumangelova-Yuzeir, K.; Georgieva, R.; Ivanova-Todorova, E.; Karaivanova, E.; Nakov, V.; Nakov, R.; Kyurkchiev, D. Lactobacilli Supplemented with Larch Arabinogalactan and Colostrum Stimulates an Immune Response towards Peripheral NK Activation and Gut Tolerance. Nutrients 2020, 12, 1706. [Google Scholar] [CrossRef]
- Playford, R.J.; Garbowsky, M.; Marchbank, T. Pasteurized Chicken Egg Powder Stimulates Proliferation and Migration of AGS, RIE1, and Caco-2 Cells and Reduces NSAID-Induced Injury in Mice and Colitis in Rats. J. Nutr. 2020, 150, 1434–1442. [Google Scholar] [CrossRef]
- Zhang, S.; Lü, B.; Chao, G.; Chen, F.; Chen, M.; Chen, H. The effects of milk and milk products on non-steroidal anti-inflammatory drug-induced intestinal damage in rats. Zhonghua Nei Ke Za Zhi 2011, 50, 771–775. [Google Scholar] [PubMed]
- Kim, J.W.; Jeon, W.K.; Yun, J.W.; Park, D.I.; Cho, Y.K.; Sung, I.K.; Sohn, C.I.; Kim, B.I.; Yeom, J.S.; Park, H.S.; et al. Protective Effects of Bovine Colostrum on Non-Steroidal Anti-Inflammatory Drug Induced Intestinal Damage in Rats. Asia Pac. J. Clin. Nutr. 2005, 14, 103–107. [Google Scholar]
- Cho, M.; Bu, Y.; Park, J.-W.; Rahman, H.; Ko, S.-J. Efficacy of Complementary Medicine for Nonsteroidal Anti-Inflammatory Drug-Induced Small Intestinal Injuries: A Narrative Review. Medicine 2021, 100, e28005. [Google Scholar] [CrossRef]
- Emery, H.; Butt, T.M.; Coates, C.J. Nutraceutical Intervention Protects against Bacterial and Chemical-Induced Gastrotoxicity in a Non-Mammalian Model, Galleria mellonella. Food Chem. Toxicol. 2021, 154, 112354. [Google Scholar] [CrossRef]
- Fornai, M.; Pellegrini, C.; Benvenuti, L.; Tirotta, E.; Gentile, D.; Natale, G.; Ryskalin, L.; Colucci, R.; Piccoli, E.; Ghelardi, E.; et al. Protective Effects of the Combination Bifidobacterium longum plus Lactoferrin against NSAID-Induced Enteropathy. Nutrition 2020, 70, 110583. [Google Scholar] [CrossRef]
- Mir, R.; Singh, N.; Vikram, G.; Kumar, R.P.; Sinha, M.; Bhushan, A.; Kaur, P.; Srinivasan, A.; Sharma, S.; Singh, T.P. The Structural Basis for the Prevention of Nonsteroidal Antiinflammatory Drug-Induced Gastrointestinal Tract Damage by the C-Lobe of Bovine Colostrum Lactoferrin. Biophys. J. 2009, 97, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Macdonald, C.E.; Calnan, D.P.; Floyd, D.N.; Podas, T.; Johnson, W.; Wicks, A.C.; Bashir, O.; Marchbank, T. Co-Administration of the Health Food Supplement, Bovine Colostrum, Reduces the Acute Non-Steroidal Anti-Inflammatory Drug-Induced Increase in Intestinal Permeability. Clin. Sci. 2001, 100, 627–633. [Google Scholar] [CrossRef]
- OuYang, X.; Yang, C.-Y.; Xiu, W.-L.; Hu, Y.-H.; Mei, S.-S.; Lin, Q. Oropharyngeal Administration of Colostrum for Preventing Necrotizing Enterocolitis and Late-Onset Sepsis in Preterm Infants with Gestational Age ≤ 32 Weeks: A Pilot Single-Center Randomized Controlled Trial. Int. Breastfeed. J. 2021, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sangild, P.T.; Peng, Y.; Li, Y.; Bering, S.B.; Pan, X. Supplementary Bovine Colostrum Feedings to Formula-Fed Preterm Pigs Improve Gut Function and Reduce Necrotizing Enterocolitis. J. Pediatr. Gastroenterol. Nutr. 2021, 73, e39–e46. [Google Scholar] [CrossRef]
- Sharma, D.; Kaur, A.; Farahbakhsh, N.; Agarwal, S. Role of Oropharyngeal Administration of Colostrum in Very Low Birth Weight Infants for Reducing Necrotizing Enterocolitis: A Randomized Controlled Trial. Am. J. Perinatol. 2019, 37, 716–721. [Google Scholar] [CrossRef]
- Aggarwal, R.; Plakkal, N.; Bhat, V. Does Oropharyngeal Administration of Colostrum Reduce Morbidity and Mortality in Very Preterm Infants? A Randomised Parallel-Group Controlled Trial. J. Paediatr. Child Health 2021, 57, 1467–1472. [Google Scholar] [CrossRef]
- Li, Y.; Pan, X.; Nguyen, D.N.; Ren, S.; Moodley, A.; Sangild, P.T. Bovine Colostrum Before or After Formula Feeding Improves Systemic Immune Protection and Gut Function in Newborn Preterm Pigs. Front. Immunol. 2020, 10, 3062. [Google Scholar] [CrossRef]
- Balachandran, B.; Dutta, S.; Singh, R.; Prasad, R.; Kumar, P. Bovine Colostrum in Prevention of Necrotizing Enterocolitis and Sepsis in Very Low Birth Weight Neonates: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. J. Trop. Pediatr. 2017, 63, 10–17. [Google Scholar] [CrossRef]
- Møller, H.K.; Thymann, T.; Fink, L.N.; Frokiaer, H.; Kvistgaard, A.S.; Sangild, P.T. Bovine Colostrum Is Superior to Enriched Formulas in Stimulating Intestinal Function and Necrotising Enterocolitis Resistance in Preterm Pigs. Br. J. Nutr. 2011, 105, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Szymańska, P.; Fichna, J. Supplementation of Bovine Colostrum in Inflammatory Bowel Disease: Benefits and Contraindications. Adv. Nutr. 2021, 12, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J. Effects of Chicken Egg Powder, Bovine Colostrum, and Combination Therapy for the Treatment of Gastrointestinal Disorders. Nutrients 2024, 16, 3684. [Google Scholar] [CrossRef] [PubMed]
- Otto, W.; Najnigier, B.; Stelmasiak, T.; Robins-Browne, R.M. Randomized Control Trials Using a Tablet Formulation of Hyperimmune Bovine Colostrum to Prevent Diarrhea Caused by Enterotoxigenic Escherichia coli in Volunteers. Scand. J. Gastroenterol. 2011, 46, 862–868. [Google Scholar] [CrossRef]
- Hałasa, M.; Skonieczna-Żydecka, K.; Machaliński, B.; Bühner, L.; Baśkiewicz-Hałasa, M. Six Weeks of Supplementation with Bovine Colostrum Effectively Reduces URTIs Symptoms Frequency and Gravity for Up to 20 Weeks in Pre-School Children. Nutrients 2023, 15, 3626. [Google Scholar] [CrossRef]
- Bovıne Colostrum: Overview, Uses, Side Effects, Precautions, Interactions, Dosing and Reviews. Available online: https://www.webmd.com/vitamins/ai/ingredientmono-785/bovine-colostrum (accessed on 23 October 2025).
- Sanctuary, M.R.; Kain, J.N.; Chen, S.Y.; Kalanetra, K.; Lemay, D.G.; Rose, D.R.; Yang, H.T.; Tancredi, D.J.; German, J.B.; Slupsky, C.M.; et al. Pilot Study of Probiotic/Colostrum Supplementation on Gut Function in Children with Autism and Gastrointestinal Symptoms. PLoS ONE 2019, 14, e0210064. [Google Scholar] [CrossRef]
- Pantheryx. PanTheryx Bovine Colostrum Recognized as GRAS; PanTheryx: Boulder, CO, USA, 2019. [Google Scholar]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Bovine Milk Proteins and Protein Derivatives as Used in Cosmetics. Int. J. Toxicol. 2022, 41, 43S–56S. [Google Scholar] [CrossRef]
- Davis, P.F.; Greenhill, N.S.; Rowan, A.M.; Schollum, L.M. The Safety of New Zealand Bovine Colostrum: Nutritional and Physiological Evaluation in Rats. Food Chem. Toxicol. 2007, 45, 229–236. [Google Scholar] [CrossRef]
- Linehan, K.; Ross, R.P.; Stanton, C. Bovine Colostrum for Veterinary and Human Health Applications: A Critical Review. Annu. Rev. Food Sci. Technol. 2023, 14, 387–410. [Google Scholar] [CrossRef]
- Bagwe, S.; Tharappel, L.J.P.; Kaur, G.; Buttar, H.S. Bovine Colostrum: An Emerging Nutraceutical. J. Complement. Integr. Med. 2015, 12, 175–185. [Google Scholar] [CrossRef]

| Growth Factors | Concentration in BC (ng/L) | Functions | References |
|---|---|---|---|
| EGF | 324.2 | EGF stimulates cell proliferation, initiates signal transduction via binding to an EGF receptor and promotes DNA repair and replication. | [16,17] |
| TGF-β2 | 1130.43 | TGF-β1 elevates cell proliferation, modulates cell migration (colostrum effect on cell migration decreased with the addition of TGF-β neutralizing antibody). Moreover, it is essential for mucosal immunity | [18,19] |
| IGF-1 | 267.97 | IGF-1 contributes to cell proliferation in GI cells and is tolerant to 40–60 °C heat conditions. IGF-1 is involved in GI tissue repair, increased permeability, and cell repair mechanisms. | [19] |
| TGF-α | 200 | TGF-α contributes to the repair of the intestinal mucosa, has an intestinal affinity similar to EGFR, and has an intestinal barrier function | [18,19] |
| Immunoglobulins | Concentration in BC (g/L) | Functions | References |
| IgG1 | 44.96 | IgG1 is the major immunoglobulin isotype and plays a major role in the gaining of passive immunity, neutralizing enteric pathogens. | [20] |
| IgG2 | 2444 | IgG2 is essential for the immune responses and passive immunity transfer. | [20] |
| IgA | 1–5 | IgA has properties such as regulation of gut microbiota, mucosal immune balance, and bacterial infection protection. | [21,22] |
| IgM | 5.07 | IgM plays a key role in mucosal immunity, immune response, and neutralizing pathogens | [22,23] |
| Total IgG | 30–87 | ||
| Lactoferrin (LF) | Concentration in BC (g/L) | Functions | References |
| Total LF | 1–5 | LFs are iron binding proteins. These proteins have several beneficial effects on an organism’s health; they have antibacterial, antifungal, antiviral, antiparasitic, anticancer, and immunomodulatory properties. Proliferation of cells called osteoblasts is considered to have positive effects on Parkinson’s disease through reducing oxidative damage, affecting the blood-brain barrier | [24,25] |
| Glycans | Concentration in BC (g/L) | Functions | References |
| 3′ sialyl lactose | 0.867 g/L | They have prebiotic functions; they are involved in the growth of beneficial bifidobacterium. 3′-Siallyactose, 6′-siallyactose are involved in nervous system development, myelization, and learning processes. Glycans also have a key function in the regulation of the gut microbiota. | [26,27] |
| 6′ sialyl lactose | 0.136 g/L | ||
| 6′ siayllactosamine | 0.220 g/L | ||
| disialyllactose (DSL) | 0.283 g/L | ||
| Total Glycans |
| Disorder | Study Design and Sample Size | Dose Duration | Population Outcome | References |
|---|---|---|---|---|
| Diarrhea in preweaning dairy calves | Randomized controlled trial with 3 treatment arms (CON, STC, LTC) n = 108 preweaning calves) | 8 feedings over 4 days: 2.5 L of 50:50 milk replacer and colostrum replacer (65 g/L each) | LTC group had faster diarrhea resolution and 98 g/day higher weight gain over 56 days compared to control | [79] |
| Childhood infectious diarrhea | Meta-analysis of 5 RCTs | Not specified | BC reduces stool frequency by 1.42/day, diarrhea occurrence by 71%, and pathogen positivity (OR = 0.29) | [80] |
| Calf diarrhea (BRV, BCoV, ETEC, Crypto) | Meta-analysis (41 studies, 94 sub-studies) | Not specified | Highest pooled prevalence: BRV-Crypto (6.69%); diagnostic method influenced detection | [81] |
| Infant diarrhea and RTIs | Multi-center, randomized, blank-controlled intervention trial, n = 192 term infants, (96 intervention, 96 control group) | 1 sachet/day 3 months | BC reduced diarrhea incidence (RR = 0.25), duration, appetite loss, and respiratory symptoms | [82] |
| Shigellosis (children, S. dysenteriae type 1) | RCT: HBC vs. BC + antibiotic, n = 69 children (34 HBC group, 35 control group) | 100 mL HBC × 3/day for 3 days | No significant difference in symptoms; stool culture positivity: HBC 6% ve BC 14% | [83] |
| Infectious diarrhea and SIBO (in vitro) | Caco-2 cell monolayer study, In vitro study, no animal or human subject | Not applicable (in vitro study) | BC ± egg protected barrier function, reduced apoptosis, preserved tight junctions | [84] |
| EPEC diarrhea (infants | Double-blind, randomized field trial, n = 125 infants (107 with complete data) | Supplemented for 7 days | BC Ig-supplemented formula lowered diarrhea incidence and duration; better weight gain | [85] |
| Shigellosis (S. flexneri macaque model) | In vivo challenge model with Travelan®, n = 12 (8 Travelan® group, 4 placebo group) | Travelan® orally, twice daily for 6 days | 75% protection post-challenge in HBC group | [86] |
| Neonatal calf diarrhea (BRV, BCoV) | Cross-sectional observational study, n = 110 neonatal calves (<30 days old) from 57 daily herds | Colostrum timing within 12–24 h of birth (feeding timing analyzed) | BRV: 3.64%, BCoV:0.91%; BRV associated with sex and feeding time | [87] |
| Cryptosporidiosis (neonatal calves) | Observational (IgG quantification); n = 50 dam-calf pairs (50 dams and their newborn calves) | Colostrum collected <12 h after birth; IgG 570–4070 mg/dL | Higher anti-C. parvum IgG in colostrum associated with reduced infection (r = −0.425) | [88] |
| Ulcerative colitis (acetic acid-induced) | In vitro (rat mode, 4 groups); n = 37 Sprague-Dawley rats | 300 mg/kg BC (oral or rectal), 7 days | Reduced weight loss, increased SOD levels, decreased CRP, WBC and histopathological damage | [89] |
| Distal colitis | RCT, double-blind, n = 14 patients with mild to moderate distal colitis (colostrum group 8, placebo group 6) | 100 mL of 10% BC enema twice daily for 4 weeks | Symptom score decreased by −2.9 in colostrum group versus +0.5 in placebo, histological improvement in 5 of 8 patients with colostrum | [90] |
| TNBS-induced colitis | In vivo study; n = 24 mice (BC group = 12, control group = 12) | 7 days BC pre-treatment before TNBS | Body weight loss was reduced; expression levels of TLR4, IL-1β, IL-8 and IL-10 were lower; beneficial bacteria population were higher in colostrum group | [91] |
| TNBS-induced colitis | In vivo study; n = 24 mice (BC group = 12, control group = 12) | 300 mg/kg BC for 21 days before TNBS | Body weight loss and histological damage were reduced; TLR4, IL-1β, IL-8 and IL-10 expression was lower; microbiota changes were prevented | [92] |
| DSS-induced colitis | In vivo study; n = not explicitly stated mouse model | 200 mg/kg BC daily for 2 weeks | Colitis severity was reduced based on body weight and colon length; inflammation was reduced; changes in immune cell populations were observed | [13] |
| DSS and T cell transfer colitis | In vivo study; n = not explicitly stated, mouse model | 100 mg/kg IMM-124E (colostrum-based) daily by oral gavage | Mucosal damage was less severe, with reduced effector, T cells and increased regulatory T cells; systemic LPS exposure was decreased | [93] |
| DSS induced colitis (dose-response) | In vivo study; n = not explicitly specified, DSS induced mixed colitis model in mice treated with 4 different BC dose levels | 100–200 mg/kg BC daily | Disease activity index and histological damage were reduced; tight junction proteins and microbiota diversity were improved; Akkermansia increased, Escherichia-Shigella decreased | [94] |
| Inflammatory cell stimulation | In vitro study using human intestinal epithelial cell lines (Caco-2 and HT29 cells) | Dose-dependent concentrations of colostrum | IL-8 levels decreased after TNF-α or AIEC stimulation; bacterial adherence to cells was reduced; direct antimicrobial effect observed | [95] |
| Diarrhea-predominant IBS (IBS-D) | Randomized, double-blind, placebo-controlled clinical trials, 30 patients with diarrhea-predominant IBS (SBI group = 15, placebo group = 15) | 5 g/day or 10 g/day serum-derived bovine Ig (SBI) for 6 weeks | 10 g/day group showed reductions in abdominal pain, loose stools, bloating, flatulence, urgency, and overall symptoms; 5 g/day group showed reductions in the symptoms | [96] |
| IBS (mixed types) | Prospective, double-blind randomized, placebo-controlled clinical trial, n = 18 patients with IBS (BC group = 9, placebo group = 9) | 15 mL/day oral BC (MuKoBa™) for 4 weeks + 4-week follow up | No significant differences in symptom scores, quality of life, or endotoxin levels between groups; slight improvement trends observed in BC group | [97] |
| IBS | Single-center, blinded trial n = 40 participants (20 patients with IBS, 20 healthy individuals | 21-day supplementation with combined product (Lactobacillus spp., larch arabinogalactan, and colostrum) | Clinical improvement in 65–75% of IBS patients, complete resolution in 5/20; decrease in pro-inflammatory markers and increase in IL-10 and IL-17A; changes not statistically significant in IBS group | [98] |
| Indomethacin-induced intestinal injury | In vitro (Caco-2, AGS, RIE-2), In vivo = 4 groups of adult mice In vivo = 3 groups of Sprague-Dawley rats | 20 mg/kg/day for 7–9 (oral); 1 mg/mL in vitro | Colostrum and egg increased cell proliferation and migration; reduced villus shortening and colonic damage; combination more effective than either alone | [99] |
| Diclofenac-induced small intestinal damage | In vivo; n = 80 male Sprague-Dawley rats, divided into 5 groups | Diclofenac (15 mg/kg once); 10% colostrum orally for 5 days prior | Colostrum group had lower lesion scores and mucosal damage higher villus height and EGF expression vs. diclofenac; milk and yogurt showed no significant effect | [100] |
| Diclofenac-induced small intestinal injury | In vivo; n = 4 animal groups (number per group not specified) | Dİclofenac (100 mg/kg once); colostrum for 5 days before | Colostrum reduced intestinal permeability, enteric bacterial overgrowth, protein loss, and villus damage compared to diclofenac alone; milk was not effective | [101] |
| NSAID-induced small intestinal injury | Systematic review (22 studies: 3 clinical, 19 experimental) | Various models and regimens (review data) | Colostrum listed among CAMs that reduce permeability; bacteria, cytokines, and improve repair; mechanisms include prostaglandin increase, oxidative stress reduction | [102] |
| Indomethacin-induced gut leakiness | In vivo, n = Galleria melonella larvae (exact number not specified) divided into 4 groups | 10% (w/w) colostrum in feed | Colostrum-fed insects showed resistance to indomethacin-induced gut leakiness; better survival compared to standard diet | [103] |
| Diclofenac-induced enteropathy | In vivo (rat); n = 40-week-old male rats (exact number not specified) divided into 4 groups inflammation, TLR, oxidative stress | LF 100 mg/kg, twice daily, 14 days | LF and Bifidobacterium reduced MPO, MDA, NF-κB p65, calprotectin; LF preserved hemoglobin, combination gave additional benefit | [104] |
| NSAID-induced gastric/intestinal injury | In vivo (mouse); structural binding studies, n = Mouse models treated with four NSAIDs; exact number of groups not specified | C-lobe of LF; 4 NSAID tested | Co-administration of LF C-lobe prevented 47–70% of NSAID-induced injury; x-ray structure revealed NSAID binding site on C-lobe | [105] |
| Indomethacin-induced gut permeability | Randomized crossover trial in humans, n = 7 healthy male volunteers (randomized crossover trial) and 15 patients on long term NSAID therapy | 125 mL colostrum three times daily for 5–7 days | In volunteers, colostrum prevented 3-fold permeability increase caused by indomethacin; no effect in patients on long-term NSAIDs | [106] |
| NEC and late-onset sepsis | Pilot, single-center, parallel RCT, n = 252 preterm infants (OAC group = 127, control group = 125) with gestational age <32 weeks | 0.4 mL maternal colostrum oropharyngeallyally every 3 h for 10 days | The incidence of NEC was lower in the OAC group (2.36% vs. 10.40%; 4.72% vs. 13.60%) | [107] |
| NEC and intestinal immaturity | Preclinical, randomized piglet study, n = 68 preterm piglets (90% gestation), divided into 4 groups | 8 daily bolus feedings with 0%, 25%, 50%, or 75% BC for 5 days | BC75 reduced severe NEC-like lesions (27%, vs. 79% in BC100), improved gut permeability | [108] |
| NEC in very-low-birth-weight infants | RCT with 117 infants (<1250 g or <30 weeks) | 0.2 mL maternal colostrum every 2 h for 72 h | There was no significant reduction in the incidence of NEC (0% vs. 7.1%) but hospital stay was shorter (34.2 vs. 41.5 days | [109] |
| NEC, LOS, and death in very preterm infants | RCT with 260 infants (26–31 weeks GA) | 0.2 human milk or placebo every 3 h until oral feeds started | The composite outcome (death, NEC, LOS) was not significantly different between groups (33.6%, 29.7%). | [110] |
| NEC, diarrhea, and intestinal inflammation | Preclinical, randomized piglet study, n = 74 preterm piglets divided into six feeding groups | BC or F for 5 days, then maintained or switched for 4 more days | BC feeding decreased NEC (27% vs. 64%), diarrhea (16% vs. 49%), and improved immunity and intestinal function | [111] |
| NEC and sepsis in VLBW infants | Randomized, double-blind, placebo-controlled pilot trial, n = 86 very-low-birth-weight infants (BC group = 43, placebo group = 43) | BC or placebo 4× daily until day 21, discharge, or death | No clinical benefit was detected; trends towards increased IL-6 and NEC features in colostrum group | [112] |
| NEC and impaired gut function | Preclinical piglet study with control and enriched formula compounds, n = 47 preterm piglets delivered by caesarean section | BC, SL, Gang, or OPN enriched formulas fed over 1.5 days after 2 days TPN | All intestinal parameters significantly improved in pigs fed BC vs. formula; SL and Gang were ineffective | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakülah, Y.S.; Yalçıntaş, Y.M.; Bechelany, M.; Karav, S. Clinical Applications of Bovine Colostrum in GastrointestinaI Disorders: Mechanisms, Evidence, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 10673. https://doi.org/10.3390/ijms262110673
Karakülah YS, Yalçıntaş YM, Bechelany M, Karav S. Clinical Applications of Bovine Colostrum in GastrointestinaI Disorders: Mechanisms, Evidence, and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(21):10673. https://doi.org/10.3390/ijms262110673
Chicago/Turabian StyleKarakülah, Yusuf Serhat, Yalçın Mert Yalçıntaş, Mikhael Bechelany, and Sercan Karav. 2025. "Clinical Applications of Bovine Colostrum in GastrointestinaI Disorders: Mechanisms, Evidence, and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 21: 10673. https://doi.org/10.3390/ijms262110673
APA StyleKarakülah, Y. S., Yalçıntaş, Y. M., Bechelany, M., & Karav, S. (2025). Clinical Applications of Bovine Colostrum in GastrointestinaI Disorders: Mechanisms, Evidence, and Therapeutic Potential. International Journal of Molecular Sciences, 26(21), 10673. https://doi.org/10.3390/ijms262110673







