Gαi2 Signaling Regulates Neonatal Respiratory Adaptation
Abstract
1. Introduction
2. Results
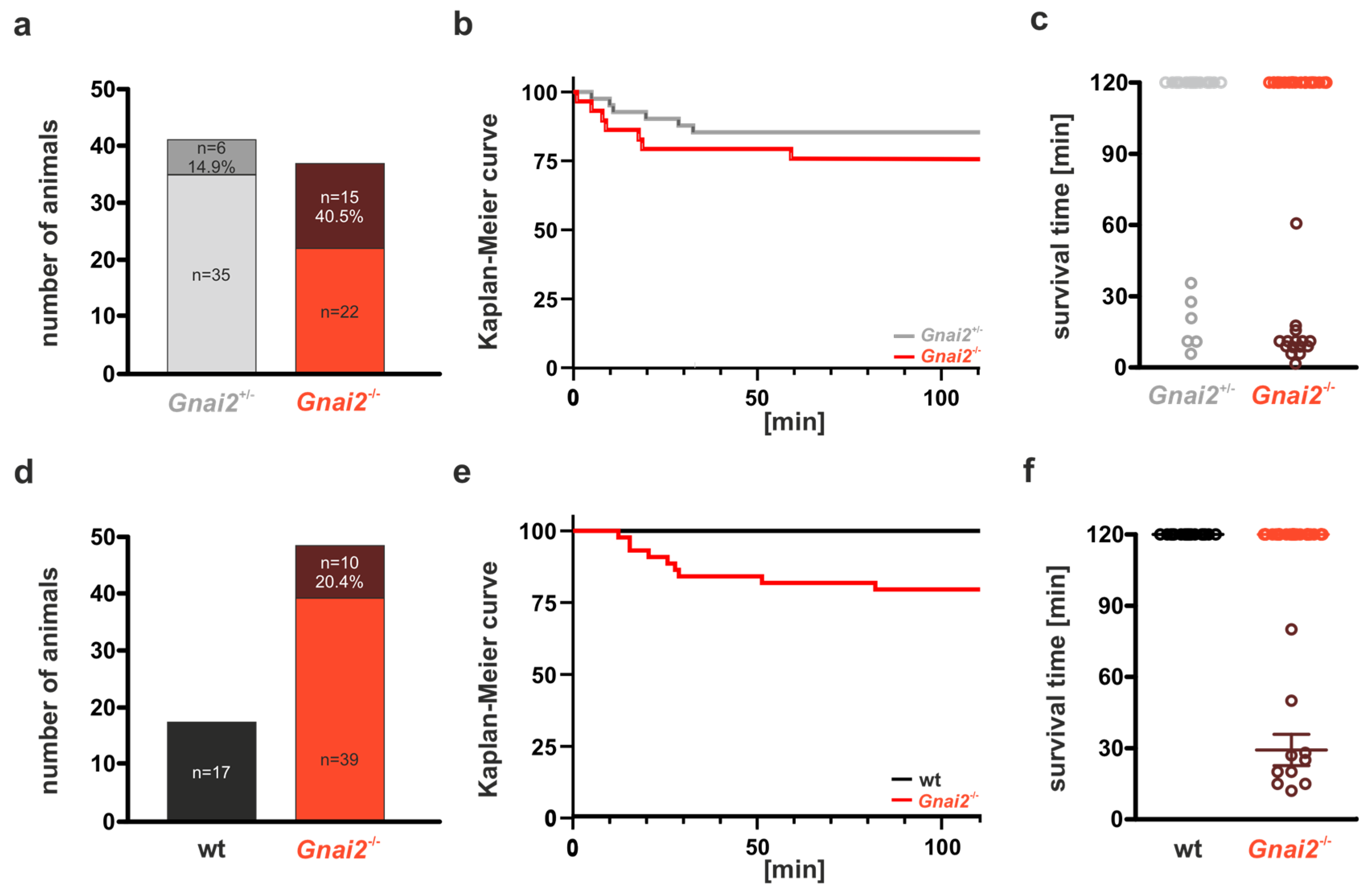
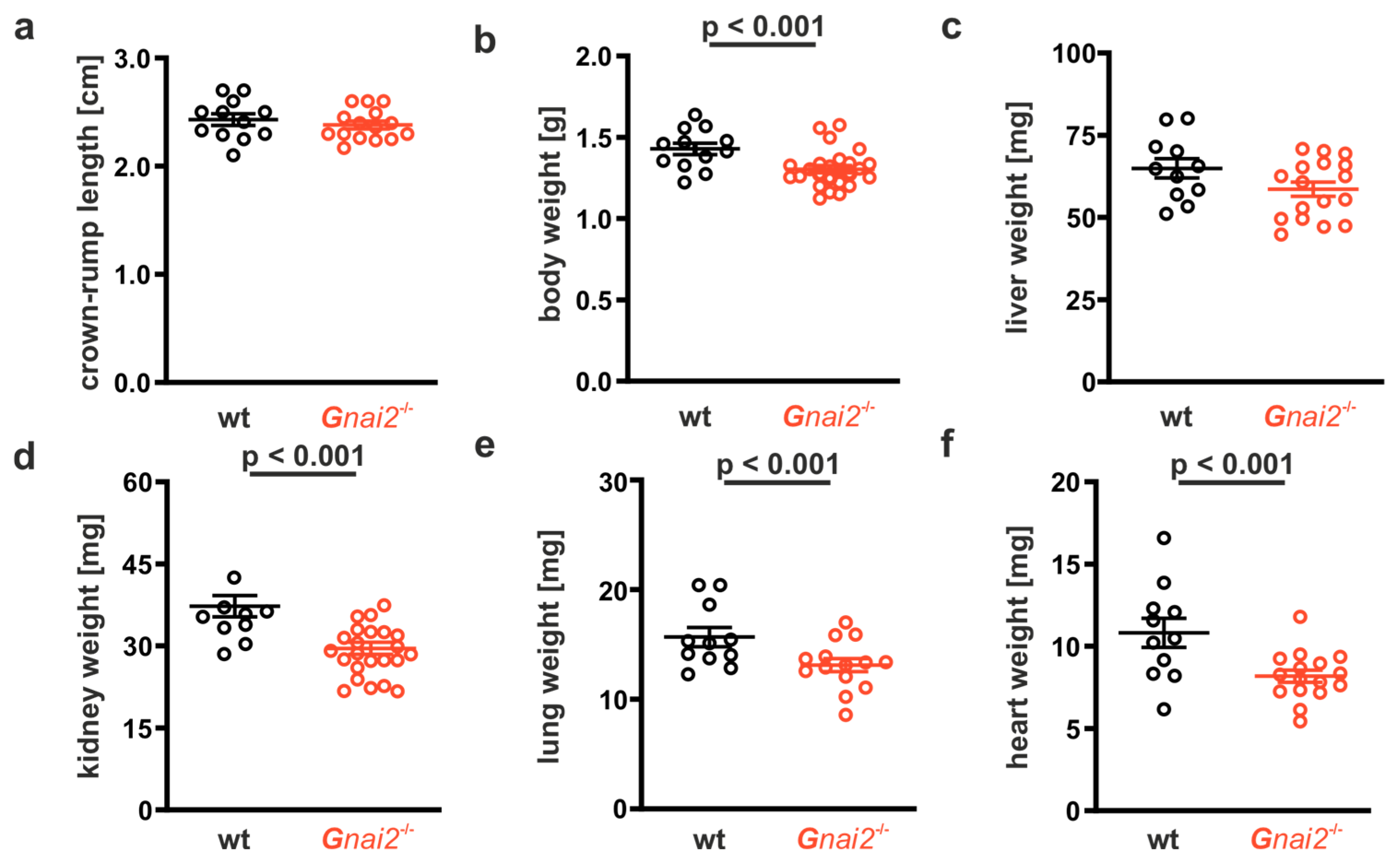
2.1. Gnai2-Deficient Mice Exhibit Early Perinatal Lethality
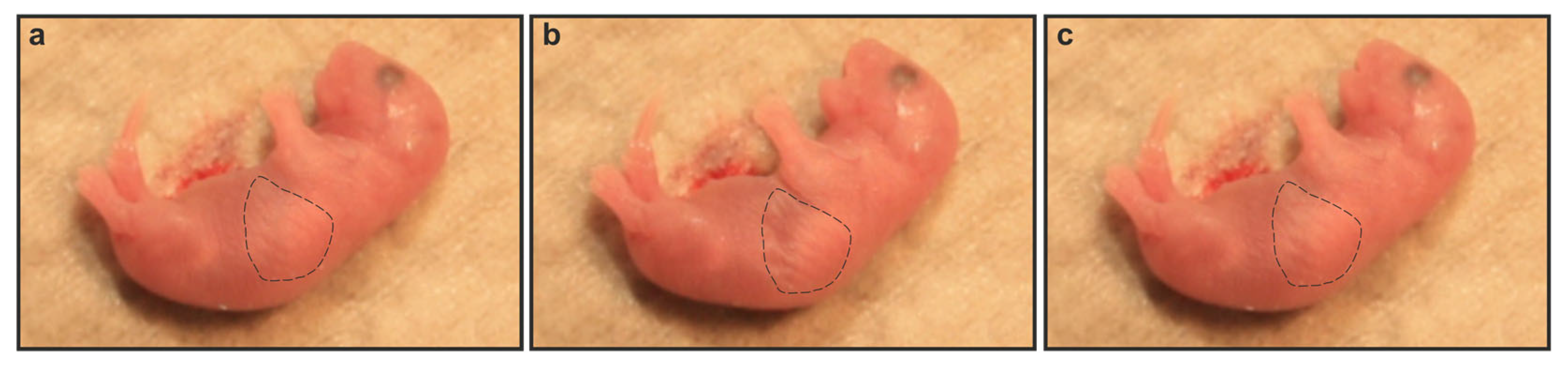
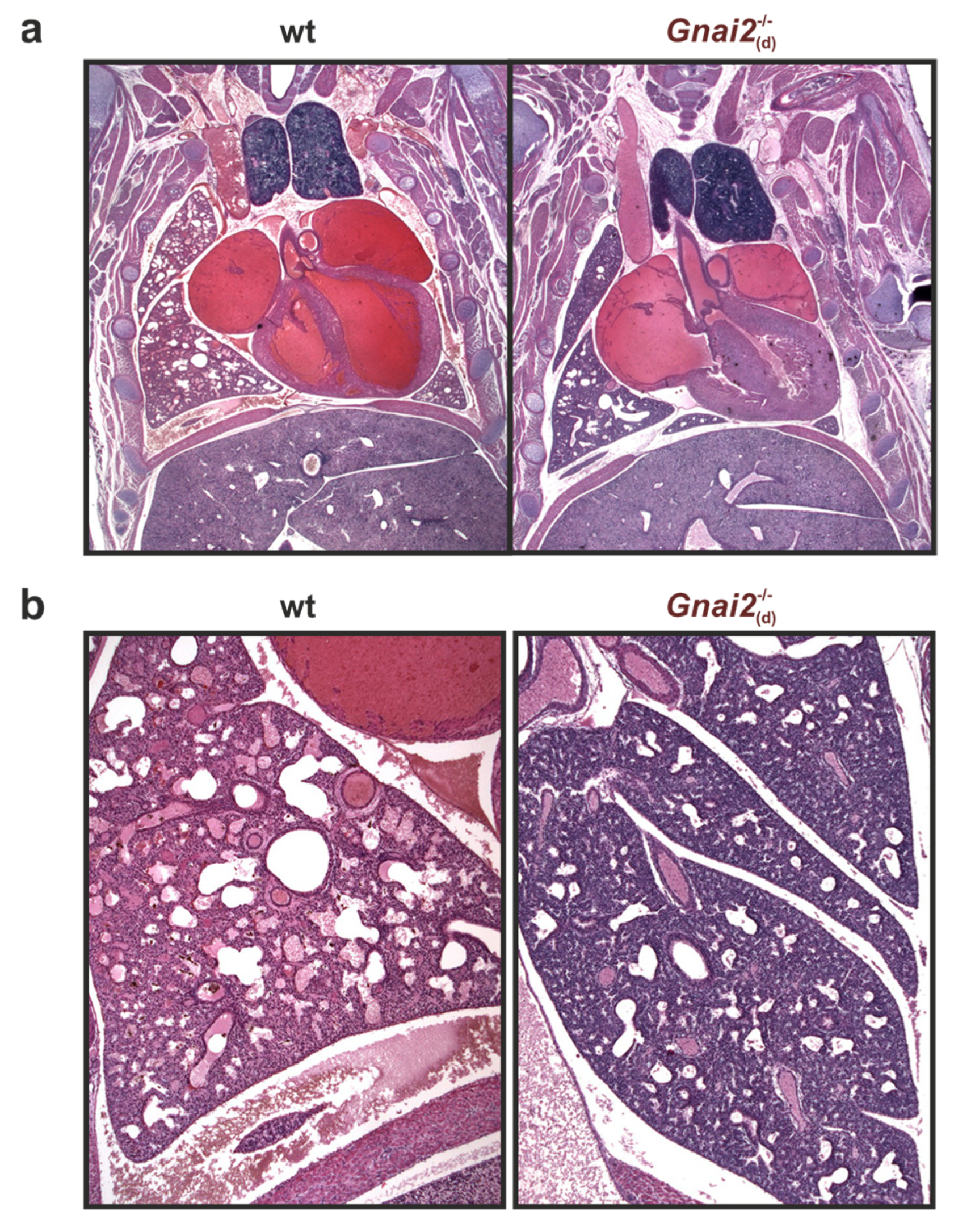
2.2. Gnai2-Deficiency Results in Lung Dysfunction in a Subset of Mice
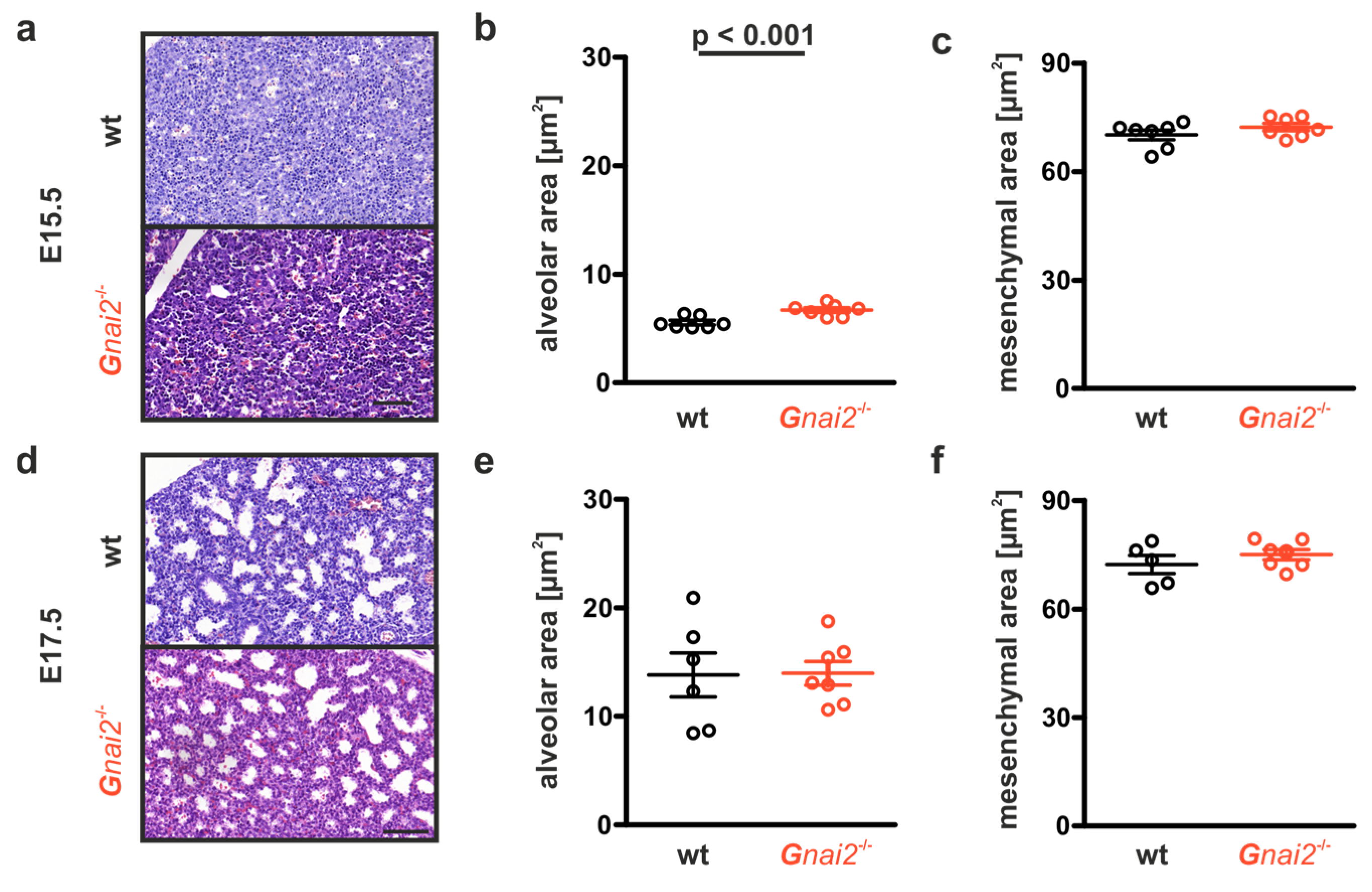
2.3. Gnai2-Deficiency Reduces Alveolar Surface Area in Deceased Newborn Mice
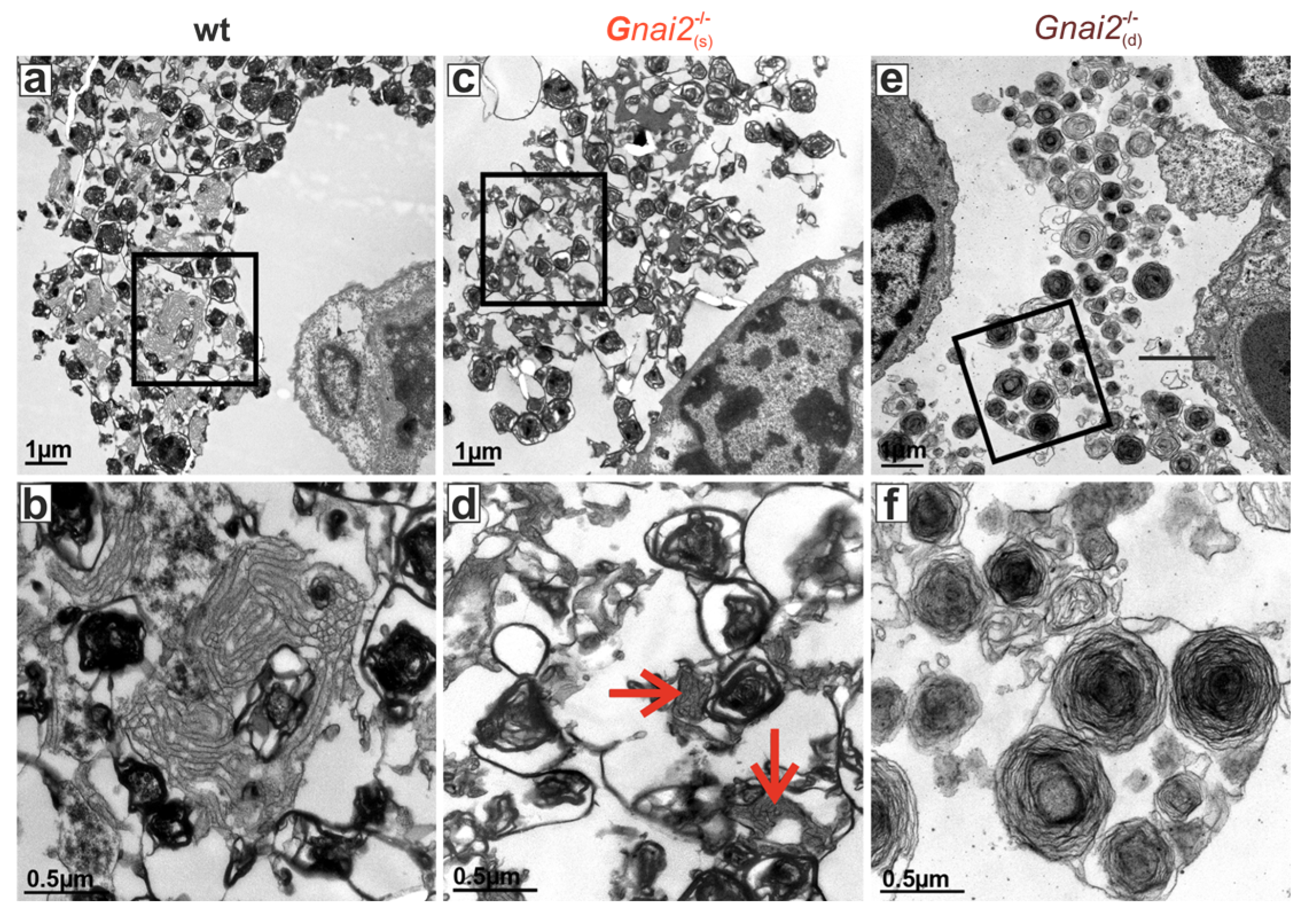
2.4. Secreted Alveolar Surfactant Fails to Properly Unfold in Deceased Newborn Gnai2-Deficiency Mice
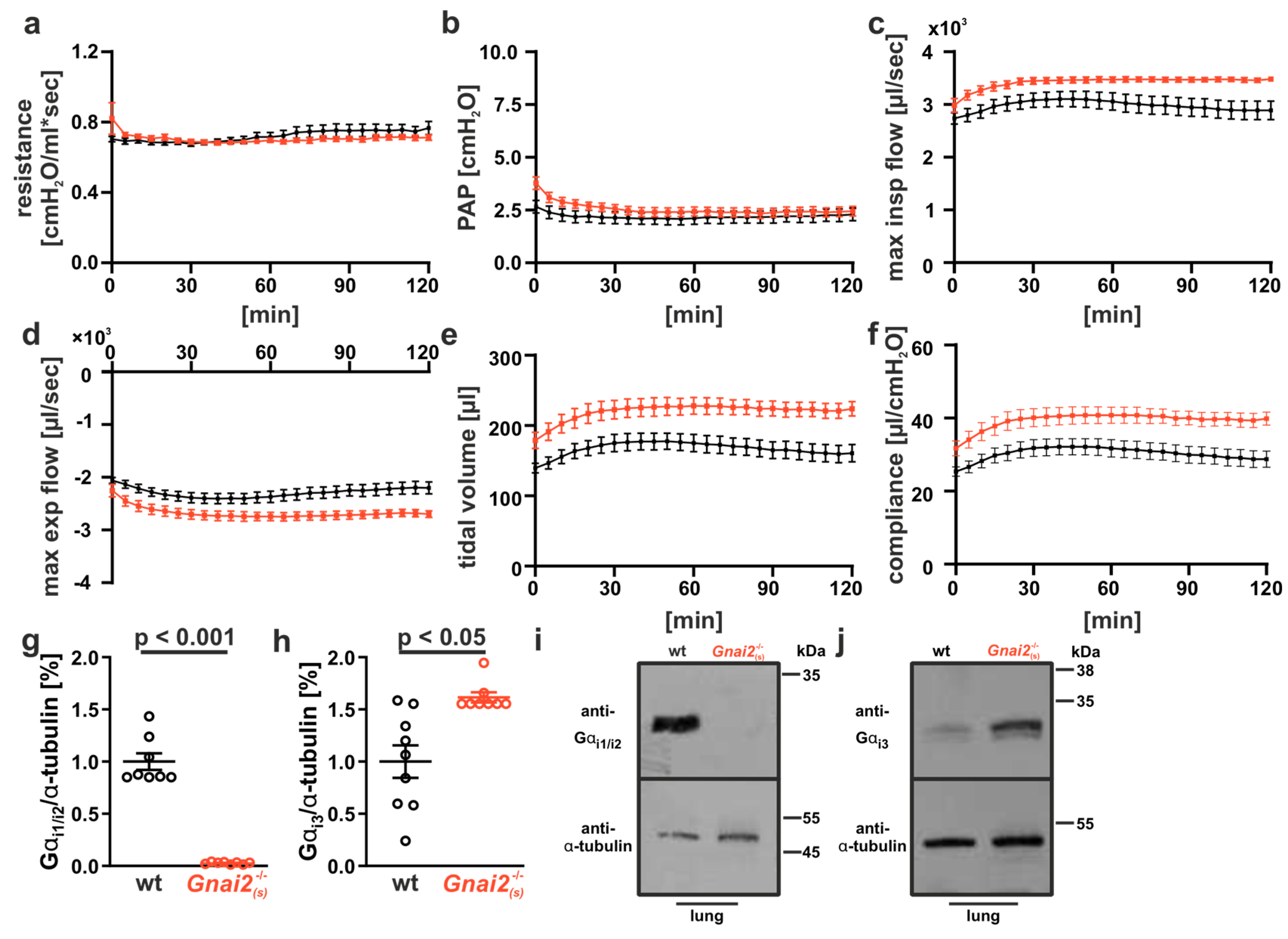
2.5. Improved Lung Function in Adult Gnai2-Deficient Mice
3. Discussion
3.1. Conclusions
3.2. Future Directions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | alveolar-type epithelial cells |
| GPCR | G-protein coupled receptors |
| HPS | Hermansky–Pudlak syndrome |
| RDS | Respiratory distress syndrome |
| TM | tubular myelin |
| wt | wild-type |
References
- Nürnberg, B.; Beer-Hammer, S.; Reisinger, E.; Leiss, V. Non-canonical G protein signaling. Pharmacol. Ther. 2024, 255, 108589. [Google Scholar] [CrossRef]
- Weis, W.I.; Kobilka, B.K. The molecular basis of g protein-coupled receptor activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, S.; Zhang, C.; Shen, J.M.; Liu, J. Gpcr heterodimers: Implications for biased signaling. Handb. Exp. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Zhou, J.C.; Li, A.; Long, Z.; Kahsai, A.W. Biased allosteric modulation in gpcr drug discovery. Handb. Exp. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Simon, M.I.; Strathmann, M.P.; Gautam, N. Diversity of G proteins in signal transduction. Science 1991, 252, 802–808. [Google Scholar] [CrossRef]
- Aydogdu, S.; Pepanian, A.; Rathod, D.C.; Schulz, F.; Imhof, D. Physiological functions and structural features of galpha12/13 proteins. Biomed. Pharmacother. 2025, 191, 118523. [Google Scholar] [CrossRef]
- Inoue, A.; Raimondi, F.; Kadji, F.M.N.; Singh, G.; Kishi, T.; Uwamizu, A.; Ono, Y.; Shinjo, Y.; Ishida, S.; Arang, N.; et al. Illuminating G-Protein-Coupling Selectivity of GPCRs. Cell 2019, 177, 1933–1947. [Google Scholar] [CrossRef]
- Qayum, S.; Petrin, D.; Tanny, J.C.; Hebert, T.E. Gbetagamma signaling: Lessons across the cellular multiverse. Annu. Rev. Pharmacol. Toxicol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, T.M.; Gilbert, D.J.; Olsen, A.S.; Chen, X.N.; Amatruda, T.T.; Korenberg, J.R.; Trask, B.J.; de Jong, P.; Reed, R.R.; Simon, M.I.; et al. Evolution of the mammalian G protein alpha subunit multigene family. Nat. Genet. 1992, 1, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gohla, A.; Klement, K.; Piekorz, R.P.; Pexa, K.; vom Dahl, S.; Spicher, K.; Dreval, V.; Haussinger, D.; Birnbaumer, L.; Nürnberg, B. An obligatory requirement for the heterotrimeric G protein Gi3 in the antiautophagic action of insulin in the liver. Proc. Natl. Acad. Sci. USA 2007, 104, 3003–3008. [Google Scholar] [CrossRef]
- Jantzen, H.M.; Milstone, D.S.; Gousset, L.; Conley, P.B.; Mortensen, R.M. Impaired activation of murine platelets lacking G alpha(i2). J. Clin. Investig. 2001, 108, 477–483. [Google Scholar] [CrossRef]
- Plummer, N.W.; Spicher, K.; Malphurs, J.; Akiyama, H.; Abramowitz, J.; Nürnberg, B.; Birnbaumer, L. Development of the mammalian axial skeleton requires signaling through the Gαi subfamily of heterotrimeric G proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 21366–21371. [Google Scholar] [CrossRef]
- Rudolph, U.; Finegold, M.J.; Rich, S.S.; Harriman, G.R.; Srinivasan, Y.; Brabet, P.; Boulay, G.; Bradley, A.; Birnbaumer, L. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nat. Genet. 1995, 10, 143–150. [Google Scholar] [CrossRef]
- Wettschureck, N.; Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 2005, 85, 1159–1204. [Google Scholar] [CrossRef]
- Beer-Hammer, S.; Lee, S.C.; Mauriac, S.A.; Leiss, V.; Groh, I.A.M.; Novakovic, A.; Piekorz, R.P.; Bucher, K.; Chen, C.; Ni, K.; et al. Gαi Proteins are Indispensable for Hearing. Cell Physiol. Biochem. 2018, 47, 1509–1532. [Google Scholar] [CrossRef]
- Devanathan, V.; Hagedorn, I.; Köhler, D.; Pexa, K.; Cherpokova, D.; Kraft, P.; Singh, M.; Rosenberger, P.; Stoll, G.; Birnbaumer, L.; et al. Platelet Gi protein Galphai2 is an essential mediator of thrombo-inflammatory organ damage in mice. Proc. Natl. Acad. Sci. USA 2015, 112, 6491–6496. [Google Scholar] [CrossRef]
- Ezan, J.; Lasvaux, L.; Gezer, A.; Novakovic, A.; May-Simera, H.; Belotti, E.; Lhoumeau, A.C.; Birnbaumer, L.; Beer-Hammer, S.; Borg, J.P.; et al. Primary cilium migration depends on G protein signalling control of subapical cytoskeleton. Nat. Cell Biol. 2013, 15, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Maass, M.; Dizayee, S.; Leiss, V.; Annala, S.; Koth, J.; Seemann, W.K.; Muller-Ehmsen, J.; Mohr, K.; Nurnberg, B.; et al. Lack of Galphai2 leads to dilative cardiomyopathy and increased mortality in beta1-adrenoceptor overexpressing mice. Cardiovasc. Res. 2015, 108, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Köhler, D.; Devanathan, V.; Bernardo de Oliveira Franz, C.; Eldh, T.; Novakovic, A.; Roth, J.M.; Granja, T.; Birnbaumer, L.; Rosenberger, P.; Beer-Hammer, S.; et al. Gαi2- and Gαi3-deficient mice display opposite severity of myocardial ischemia reperfusion injury. PLoS ONE 2014, 9, e98325. [Google Scholar] [CrossRef] [PubMed]
- Leiss, V.; Schönsiegel, A.; Gnad, T.; Kerner, J.; Kaur, J.; Sartorius, T.; Machann, J.; Schick, F.; Birnbaumer, L.; Haring, H.U.; et al. Lack of Galpha(i2) proteins in adipocytes attenuates diet-induced obesity. Mol. Metab. 2020, 40, 101029. [Google Scholar] [CrossRef]
- Wiege, K.; Le, D.D.; Syed, S.N.; Ali, S.R.; Novakovic, A.; Beer-Hammer, S.; Piekorz, R.P.; Schmidt, R.E.; Nürnberg, B.; Gessner, J.E. Defective macrophage migration in Gαi2- but not Gαi3-deficient mice. J. Immunol. 2012, 189, 980–987. [Google Scholar] [CrossRef]
- Bai, J.Y.; Li, Y.; Xue, G.H.; Li, K.R.; Zheng, Y.F.; Zhang, Z.Q.; Jiang, Q.; Liu, Y.Y.; Zhou, X.Z.; Cao, C. Requirement of galphai1 and galphai3 in interleukin-4-induced signaling, macrophage m2 polarization and allergic asthma response. Theranostics 2021, 11, 4894–4909. [Google Scholar] [CrossRef]
- Jarysta, A.; Tadenev, A.L.; Day, D.M.; Krawchuk, B.; Low, B.E.; Wiles, M.V.; Tarchini, B. Inhibitory g proteins play multiple roles to polarize sensory hair cell morphogenesis. Elife 2024, 12. [Google Scholar] [CrossRef]
- Katnahji, N.; Matthes, J. Opposite effects of galpha(i2) or galpha(i3) deficiency on reduced basal density and attenuated beta-adrenergic response of ventricular ca(2+) currents in myocytes of mice overexpressing the cardiac beta(1)-adrenoceptor. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 12543–12549. [Google Scholar] [CrossRef]
- van den Bos, E.; Ambrosy, B.; Horsthemke, M.; Walbaum, S.; Bachg, A.C.; Wettschureck, N.; Innamorati, G.; Wilkie, T.M.; Hanley, P.J. Knockout mouse models reveal the contributions of g protein subunits to complement c5a receptor-mediated chemotaxis. J. Biol. Chem. 2020, 295, 7726–7742. [Google Scholar] [CrossRef]
- Xie, H.; Ji, J.; Liu, Z.; Lu, N.; Wei, Y.; Zhou, A.; Liu, J.; Jiao, Q. Galphai1/3 signaling mediates il-5-induced eosinophil activation and type 2 inflammation in eosinophilic chronic rhinosinusitis. Front. Immunol. 2024, 15, 1460104. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Y.; Charbeneau, R.A.; Saunders, T.L.; Taylor, D.K.; Hankenson, K.D.; Russell, M.W.; D’Alecy, L.G.; Neubig, R.R. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol. Cell Biol. 2006, 26, 6870–6879. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Cowan, C.W.; Wensel, T.G. RGS9, a GTPase accelerator for phototransduction. Neuron 1998, 20, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.C.; Koschnitzky, J.E.; Youngquist, T.M.; Baertsch, N.A.; Smith, C.V.; Ramirez, J.M. Perinatal Breathing Patterns and Survival in Mice Born Prematurely and at Term. Front. Physiol. 2019, 10, 1113. [Google Scholar] [CrossRef] [PubMed]
- Whitsett, J.A.; Wert, S.E.; Weaver, T.E. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Annu. Rev. Med. 2010, 61, 105–119. [Google Scholar] [CrossRef]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef]
- Speier, R.; Cotten, C.M. Pulmonary Hypoplasia. In Manual of Neonatal Respiratory Care; Donn, S.M., Mammel, M.C., van Kaam, A.H.L.C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 699–703. [Google Scholar]
- Bartman, C.M.; Matveyenko, A.; Prakash, Y.S. It’s about time: Clocks in the developing lung. J. Clin. Investig. 2020, 130, 39–50. [Google Scholar] [CrossRef]
- Milad, N.; Morissette, M.C. Revisiting the role of pulmonary surfactant in chronic inflammatory lung diseases and environmental exposure. Eur. Respir. Rev. 2021, 30, 210077. [Google Scholar] [CrossRef]
- Possmayer, F.; Zuo, Y.Y.; Veldhuizen, R.A.W.; Petersen, N.O. Pulmonary Surfactant: A Mighty Thin Film. Chem. Rev. 2023, 123, 13209–13290. [Google Scholar] [CrossRef]
- Ustiyan, V.; Bolte, C.; Zhang, Y.; Han, L.; Xu, Y.; Yutzey, K.E.; Zorn, A.M.; Kalin, T.V.; Shannon, J.M.; Kalinichenko, V.V. FOXF1 transcription factor promotes lung morphogenesis by inducing cellular proliferation in fetal lung mesenchyme. Dev. Biol. 2018, 443, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, F.; Ornitz, D.M. Mesothelial- and epithelial-derived FGF9 have distinct functions in the regulation of lung development. Development 2011, 138, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Harfe, B.D.; Scherz, P.J.; Nissim, S.; Tian, H.; McMahon, A.P.; Tabin, C.J. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 2004, 118, 517–528. [Google Scholar] [CrossRef]
- Sosic, D.; Richardson, J.A.; Yu, K.; Ornitz, D.M.; Olson, E.N. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell 2003, 112, 169–180. [Google Scholar] [CrossRef]
- Harris, K.S.; Zhang, Z.; McManus, M.T.; Harfe, B.D.; Sun, X. Dicer function is essential for lung epithelium morphogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Bridges, J.P.; Safina, C.; Pirard, B.; Brown, K.; Filuta, A.; Panchanathan, R.; Bouhelal, R.; Reymann, N.; Patel, S.; Seuwen, K.; et al. Regulation of pulmonary surfactant by the adhesion GPCR GPR116/ADGRF5 requires a tethered agonist-mediated activation mechanism. elife 2022, 11, e69061. [Google Scholar] [CrossRef]
- Andreeva, A.V.; Kutuzov, M.A.; Voyno-Yasenetskaya, T.A. Regulation of surfactant secretion in alveolar type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2007, 293, L259–L271. [Google Scholar] [CrossRef]
- Pian, M.S.; Dobbs, L.G. Activation of G proteins may inhibit or stimulate surfactant secretion in rat alveolar type II cells. Am. J. Physiol. 1994, 266, L375–L381. [Google Scholar] [CrossRef] [PubMed]
- Pian, M.S.; Dobbs, L.G. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: Mediation by heterotrimeric G proteins. Am. J. Physiol. 1997, 273, L634–L639. [Google Scholar] [CrossRef] [PubMed]
- Dell’Angelica, E.C.; Shotelersuk, V.; Aguilar, R.C.; Gahl, W.A.; Bonifacino, J.S. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol. Cell 1999, 3, 11–21. [Google Scholar] [CrossRef]
- Anciuc-Crauciuc, M.; Cucerea, M.C.; Tripon, F.; Crauciuc, G.A.; Banescu, C.V. Descriptive and Functional Genomics in Neonatal Respiratory Distress Syndrome: From Lung Development to Targeted Therapies. Int. J. Mol. Sci. 2024, 25, 649. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Doetschman, T.; Gregg, R.G.; Maeda, N.; Hooper, M.L.; Melton, D.W.; Thompson, S.; Smithies, O. Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 1987, 330, 576–578. [Google Scholar] [CrossRef]
- Liao, J.; Aggarwal, V.S.; Nowotschin, S.; Bondarev, A.; Lipner, S.; Morrow, B.E. Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev. Biol. 2008, 316, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.H. Modifier genes in mice and humans. Nat. Rev. Genet. 2001, 2, 165–174. [Google Scholar] [CrossRef]
- Conlon, R.A.; Reaume, A.G.; Rossant, J. Notch1 is required for the coordinate segmentation of somites. Development 1995, 121, 1533–1545. [Google Scholar] [CrossRef]
- Ham, H.; Jing, H.; Lamborn, I.T.; Kober, M.M.; Koval, A.; Berchiche, Y.A.; Anderson, D.E.; Druey, K.M.; Mandl, J.N.; Isidor, B.; et al. Germline mutations in a G protein identify signaling cross-talk in T cells. Science 2024, 385, eadd8947. [Google Scholar] [CrossRef] [PubMed]
- Shrine, N.; Guyatt, A.L.; Erzurumluoglu, A.M.; Jackson, V.E.; Hobbs, B.D.; Melbourne, C.A.; Batini, C.; Fawcett, K.A.; Song, K.; Sakornsakolpat, P.; et al. New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries. Nat. Genet. 2019, 51, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Wiege, K.; Ali, S.R.; Gewecke, B.; Novakovic, A.; Konrad, F.M.; Pexa, K.; Beer-Hammer, S.; Reutershan, J.; Piekorz, R.P.; Schmidt, R.E.; et al. Gαi2 is the essential Gαi protein in immune complex-induced lung disease. J. Immunol. 2013, 190, 324–333. [Google Scholar] [CrossRef]
- Leiss, V.; Flockerzie, K.; Novakovic, A.; Rath, M.; Schönsiegel, A.; Birnbaumer, L.; Schürmann, A.; Harteneck, C.; Nürnberg, B. Insulin secretion stimulated by arginine and its metabolite ornithine depends on Gαi2. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E800–E812. [Google Scholar] [CrossRef] [PubMed]
- Mauriac, S.A.; Hien, Y.E.; Bird, J.E.; Carvalho, S.D.; Peyroutou, R.; Lee, S.C.; Moreau, M.M.; Blanc, J.M.; Geyser, A.; Medina, C.; et al. Defective Gpsm2/Gαi3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome. Nat. Commun. 2017, 8, 14907. [Google Scholar] [CrossRef]
- Bridges, J.P.; Ikegami, M.; Brilli, L.L.; Chen, X.; Mason, R.J.; Shannon, J.M. LPCAT1 regulates surfactant phospholipid synthesis and is required for transitioning to air breathing in mice. J. Clin. Investig. 2010, 120, 1736–1748. [Google Scholar] [CrossRef]
- Mays, L.E.; Ammon-Treiber, S.; Mothes, B.; Alkhaled, M.; Rottenberger, J.; Müller-Hermelink, E.S.; Grimm, M.; Mezger, M.; Beer-Hammer, S.; von Stebut, E.; et al. Modified Foxp3 mRNA protects against asthma through an IL-10-dependent mechanism. J. Clin. Invest. 2013, 123, 1216–1228. [Google Scholar] [CrossRef]

| Genotype | E14 | E18 | P5-10 | P21 | % Expected |
|---|---|---|---|---|---|
| Gnai2+/+ | 7 (15.9%) | 13 (22%) | 137 (26.9%) | 365 (31.1%) | 25 |
| Gnai2+/− | 29 (66%) | 34 (57.7%) | 316 (62.1%) | 695 (59.2%) | 50 |
| Gnai2−/− | 8 (18.1%) | 12 (20.3%) | 56 (11%) | 114 (9.7%) | 25 |
| 44 (6 litter) | 59 (8 litter) | 509 (92 litter) | 1174 (210 litter) | ||
| p value | n.s. | n.s. | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leiss, V.; Pexa, K.; Nowacki, A.; Bridges, J.P.; Duckworth-Mothes, B.; Ammon-Treiber, S.; Novakovic, A.; Zeyer, F.; Wolburg, H.; Fallier-Becker, P.; et al. Gαi2 Signaling Regulates Neonatal Respiratory Adaptation. Int. J. Mol. Sci. 2025, 26, 10655. https://doi.org/10.3390/ijms262110655
Leiss V, Pexa K, Nowacki A, Bridges JP, Duckworth-Mothes B, Ammon-Treiber S, Novakovic A, Zeyer F, Wolburg H, Fallier-Becker P, et al. Gαi2 Signaling Regulates Neonatal Respiratory Adaptation. International Journal of Molecular Sciences. 2025; 26(21):10655. https://doi.org/10.3390/ijms262110655
Chicago/Turabian StyleLeiss, Veronika, Katja Pexa, Andreas Nowacki, James P. Bridges, Benedikt Duckworth-Mothes, Susanne Ammon-Treiber, Ana Novakovic, Franziska Zeyer, Hartwig Wolburg, Petra Fallier-Becker, and et al. 2025. "Gαi2 Signaling Regulates Neonatal Respiratory Adaptation" International Journal of Molecular Sciences 26, no. 21: 10655. https://doi.org/10.3390/ijms262110655
APA StyleLeiss, V., Pexa, K., Nowacki, A., Bridges, J. P., Duckworth-Mothes, B., Ammon-Treiber, S., Novakovic, A., Zeyer, F., Wolburg, H., Fallier-Becker, P., Piekorz, R. P., Schwab, M., Quintanilla-Martínez, L., Beer-Hammer, S., & Nürnberg, B. (2025). Gαi2 Signaling Regulates Neonatal Respiratory Adaptation. International Journal of Molecular Sciences, 26(21), 10655. https://doi.org/10.3390/ijms262110655








