The ErbB2–Dock7 Signaling Axis Mediates Excessive Cell Morphogenesis Induced by Autism Spectrum Disorder- and Intellectual Disability-Associated Sema5A p.Arg676Cys
Abstract
1. Introduction
2. Results
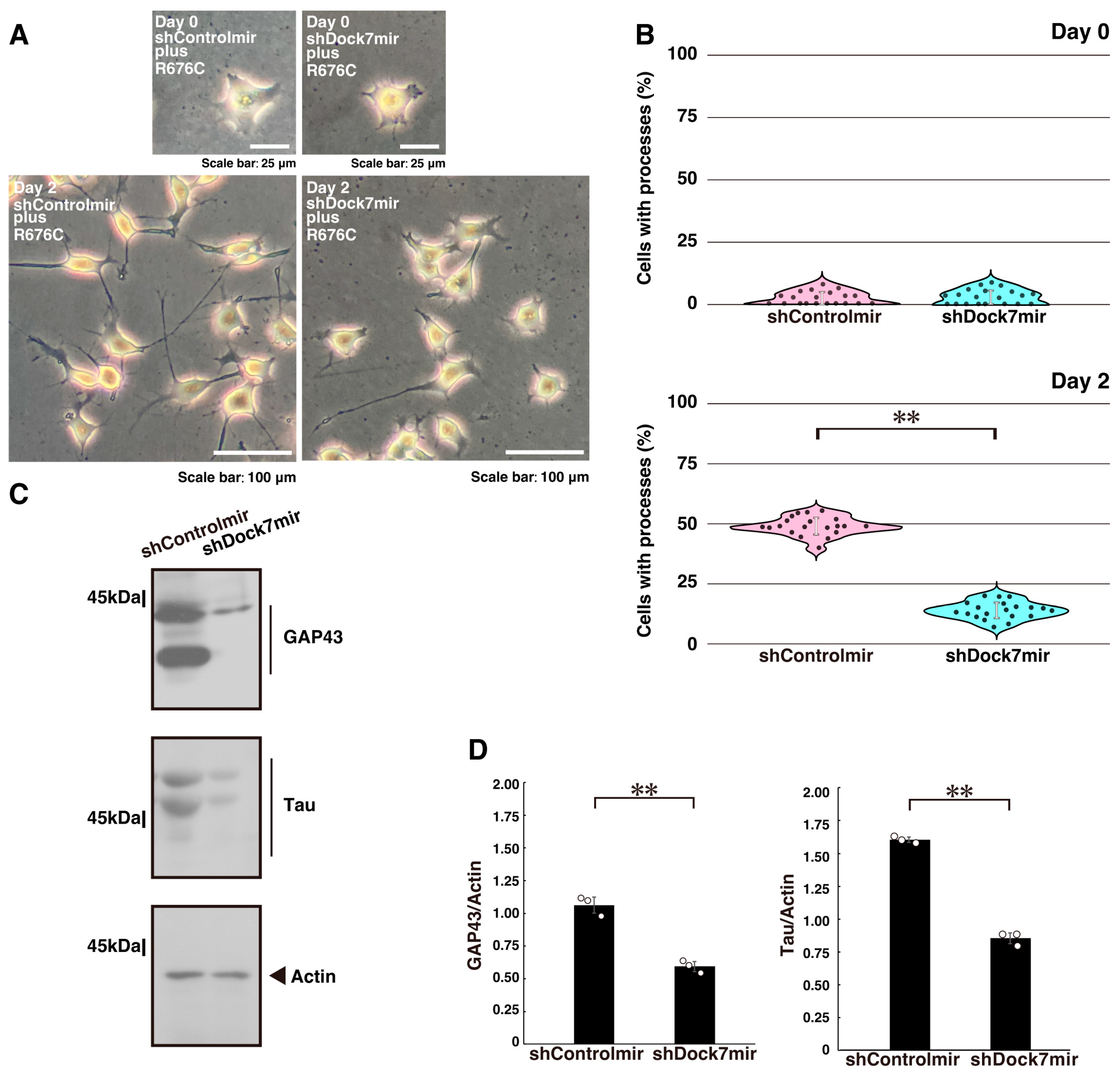
2.1. Knockdown of Dock7 Protein Decreases Mutated Sema5A-Induced Phenotypes
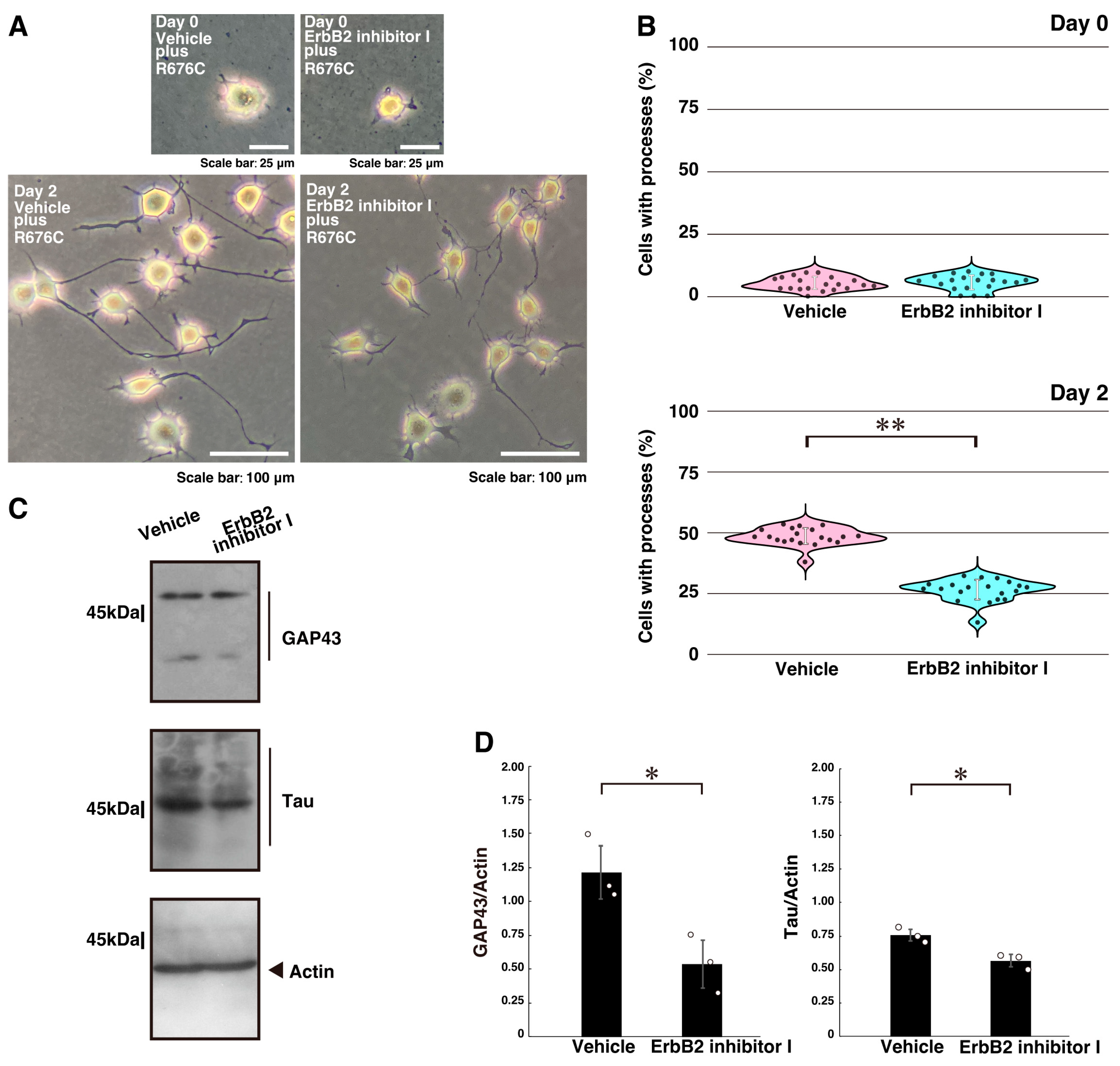
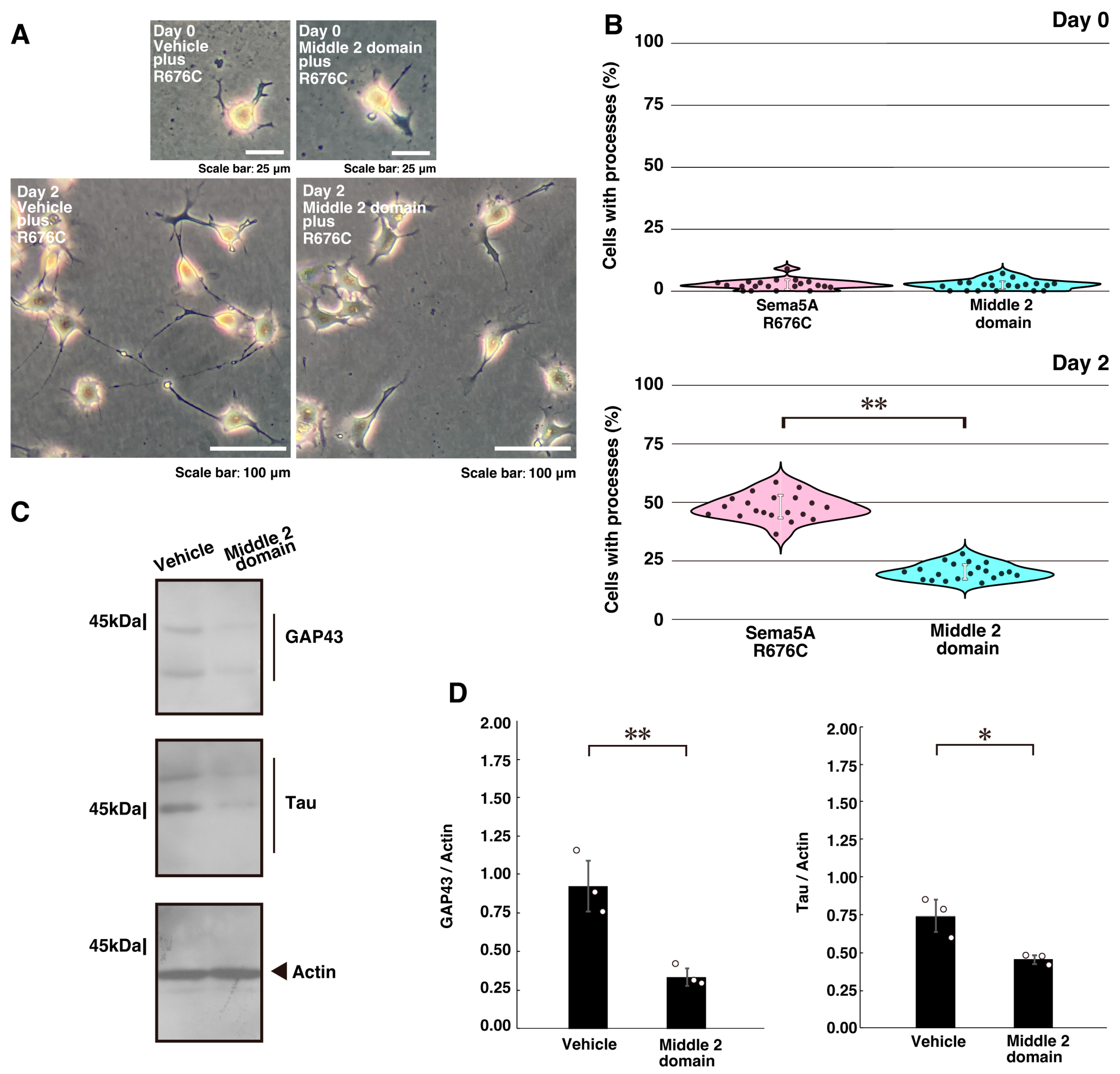
2.2. Inhibition of ErbB2 or Its Interaction with Dock7 Decreases Mutated Sema5A-Induced Phenotypes
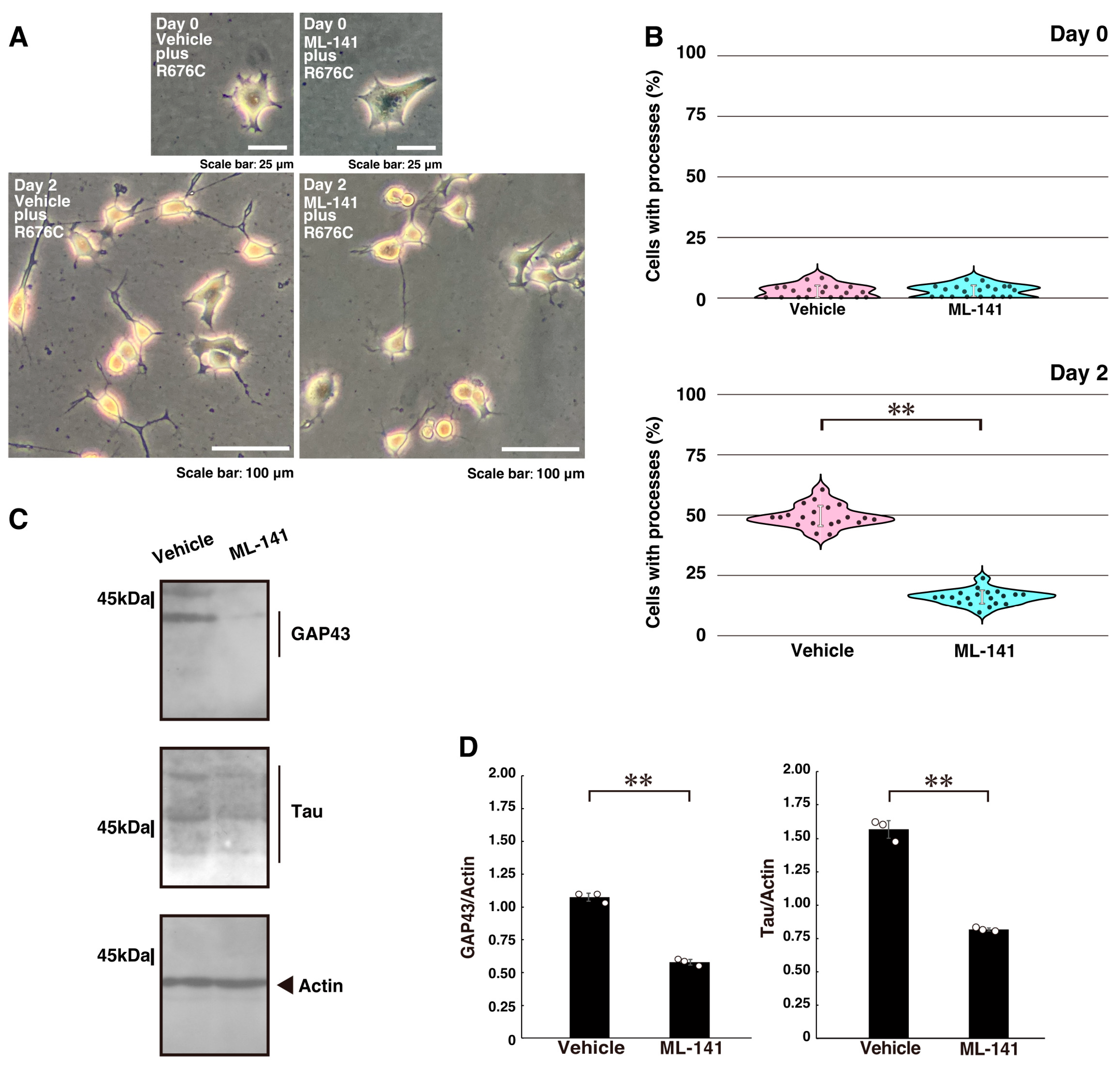
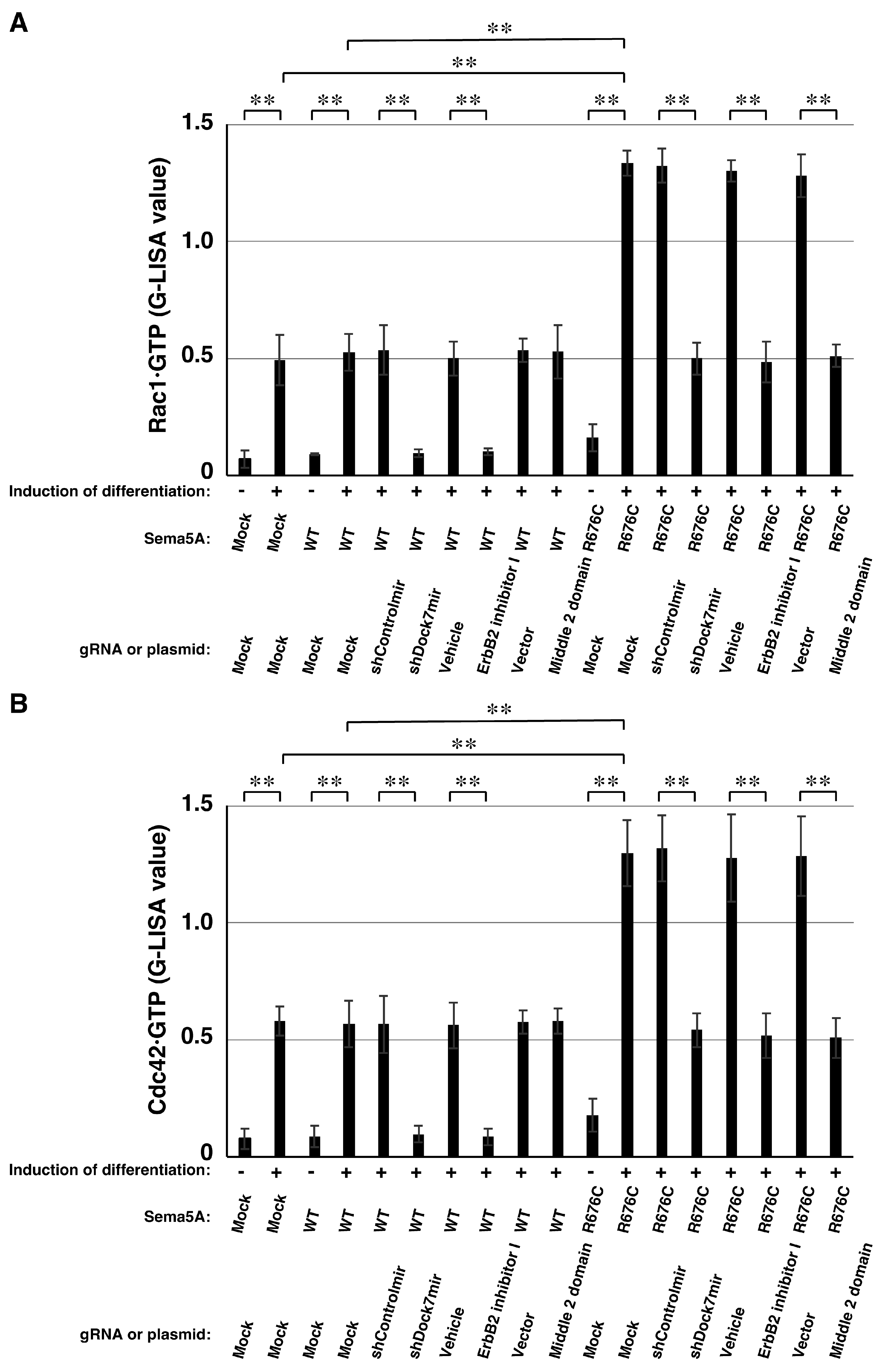
2.3. Downstream Effectors Rac1 and Cdc42 Contribute to Mutated Sema5A-Induced Phenotypes
3. Discussion
4. Materials and Methods
4.1. Key Antibodies and Plasmids
4.2. Primary Cell Cultures
4.3. Cell Line Cultures
4.4. Transfection of Plasmids
4.5. Microscopic Images
4.6. Polyacrylamide Gel Electrophoresis and Immunoblotting
4.7. Assay for GTP-Bound Rac1 or Cdc42
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernier, R.; Mao, A.; Yen, J. Psychopathology, families, and culture: Autism. Child Adolesc. Psychiatr. Clin. N. Am. 2010, 19, 855–867. [Google Scholar] [CrossRef]
- Mazza, M.; Pino, M.C.; Mariano, M.; Tempesta, D.; Ferrana, M.; Berardis, D.D.; Masedu, F.; Valenti, M. Affective and cognitive empathy in adolescents with autism spectrum disorder. Front. Hum. Neurosci. 2014, 8, 791. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.B.; Trinh, S.; Bernier, R.A. The state of research on the genetics of autism spectrum disorder: Methodological, clinical and conceptual progress. Curr. Opin. Psychol. 2019, 27, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.L.; Earl, R.; Fox, E.A.; Ma, R.; Haidar, G.; Pepper, M.; Berliner, L.; Wallace, A.; Bernier, R. Trauma and autism spectrum disorder: Review, proposed treatment adaptations and future directions. J. Child. Adolesc. Trauma 2019, 12, 529–547. [Google Scholar] [CrossRef] [PubMed]
- Modabbernia, A.; Velthorst, E.; Reichenberg, A. Environmental risk factors for autism: An evidence-based review of systematic reviews and meta-analyses. Mol. Autism 2017, 8, 13. [Google Scholar] [CrossRef]
- Varghese, M.; Keshav, N.; Jacot-Descombes, S.; Warda, T.; Wicinski, B.; Dickstein, D.L.; Harony-Nicolas, H.; De Rubeis, S.; Drapeau, E.; Buxbaum, J.D.; et al. Autism spectrum disorder: Neuropathology and animal models. Acta Neuropathol. 2017, 134, 537–566. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Wu, C.; Wang, J.; Sun, M. Autism spectrum disorder: Neurodevelopmental risk factors, biological mechanism, and precision therapy. Int. J. Mol. Sci. 2023, 24, 1819. [Google Scholar] [CrossRef]
- Ayoub, G. Autism spectrum disorder as a multifactorial disorder: The interplay of genetic factors and inflammation. Int. J. Mol. Sci. 2025, 26, 6483. [Google Scholar] [CrossRef]
- Bray, D. Surface movements during the growth of single explanted neurons. Proc. Natl. Acad. Sci. USA 1970, 65, 905–910. [Google Scholar] [CrossRef]
- Rigby, M.J.; Gomez, T.M.; Puglielli, L. Glial cell-axonal growth cone interactions in neurodevelopment and regeneration. Front. Neurosci. 2020, 14, 203. [Google Scholar] [CrossRef]
- Craig, A.M.; Banker, G. Neuronal polarity. Annu. Rev. Neurosci. 1994, 17, 267–310. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.S.; Dotti, C.G. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 2002, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Kaibuchi, K. Neuronal polarity: From extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Worzfeld, T.; Offermanns, S. Semaphorins and plexins as therapeutic targets. Nat. Rev. Drug Discov. 2014, 13, 603–621. [Google Scholar] [CrossRef]
- Lin, L.; Lesnick, T.G.; Maraganore, D.M.; Isacson, O. Axon guidance and synaptic maintenance: Preclinical markers for neurodegenerative disease and therapeutics. Trends Neurosci. 2009, 32, 142–149. [Google Scholar] [CrossRef]
- Limoni, G.; Niquille, M. Semaphorins and plexins in central nervous system patterning: The key to it all? Curr. Opin. Neurobiol. 2021, 66, 224–232. [Google Scholar] [CrossRef]
- Zang, Y.; Chaudhari, K.; Bashaw, G.J. New insights into the molecular mechanisms of axon guidance receptor regulation and signaling. Curr. Top Dev. Biol. 2021, 142, 147–196. [Google Scholar]
- Artigiani, S.; Conrotto, P.; Fazzari, P.; Gilestro, G.F.; Barberis, D.; Giordano, S.; Comoglio, P.M.; Tamagnone, L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004, 5, 710–714. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Vargas, M.E.; Wang, J.T.; Mandemakers, W.; Oster, S.F.; Sretavan, D.W.; Barres, B.A. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J. Neurosci. 2004, 24, 4989–4999. [Google Scholar] [CrossRef]
- Matsuoka, R.L.; Chivatakarn, O.; Badea, T.C.; Samuels, I.S.; Cahill, H.; Katayama, K.; Kumar, S.R.; Suto, F.; Chédotal, A.; Peachey, N.S.; et al. Class 5 transmembrane semaphorins control selective mammalian retinal lamination and function. Neuron 2011, 71, 460–473. [Google Scholar] [CrossRef]
- Nagy, G.N.; Zhao, X.F.; Karlsson, R.; Wang, K.; Duman, R.; Harlos, K.; El Omari, K.; Wagner, A.; Clausen, H.; Miller, R.L.; et al. Structure and function of Semaphorin-5A glycosaminoglycan interactions. Nat. Commun. 2024, 15, 2723. [Google Scholar] [CrossRef]
- Melin, M.; Carlsson, B.; Anckarsater, H.; Rastam, M.; Betancur, C.; Isaksson, A.; Gillberg, C.; Dahl, N. Constitutional downregulation of SEMA5A expression in autism. Neuropsychobiology 2006, 54, 64–69. [Google Scholar] [CrossRef]
- Zhang, B.; Willing, M.; Grange, D.K.; Shinawi, M.; Manwaring, L.; Vineyard, M.; Kulkarni, S.; Cottrell, C.E. Multigenerational autosomal dominant inheritance of 5p chromosomal deletions. Am. J. Med. Genet. A 2016, 170, 583–593. [Google Scholar] [CrossRef]
- Mosca-Boidron, A.L.; Gueneau, L.; Huguet, G.; Goldenberg, A.; Henry, C.; Gigot, N.; Pallesi-Pocachard, E.; Falace, A.; Duplomb, L.; Thevenon, J.; et al. A de novo microdeletion of SEMA5A in a boy with autism spectrum disorder and intellectual disability. Eur. J. Hum. Genet. 2016, 24, 838–843. [Google Scholar] [CrossRef]
- Ito, H.; Morishita, R.; Nagata, K.I. Autism spectrum disorder-associated genes and the development of dentate granule cells. Med. Mol. Morphol. 2017, 50, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Depienne, C.; Magnin, E.; Bouteiller, D.; Stevanin, G.; Saint-Martin, C.; Vidailhet, M.; Apartis, E.; Hirsch, E.; LeGuern, E.; Labauge, P.; et al. Familial cortical myoclonic tremor with epilepsy: The third locus (FCMTE3) maps to 5p. Neurology 2010, 74, 2000–2003. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Z.; Lin, Z.; Zhang, R.; Lu, Y.; Su, W.; Li, F.; Xu, X.; Tu, M.; Lou, Y.; et al. De novo germline mutations in SEMA5A associated with infantile spasms. Front. Genet. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Gunn, R.K.; Huentelman, M.J.; Brown, R.E. Are Sema5a mutant mice a good model of autism? A behavioral analysis of sensory systems, emotionality and cognition. Behav. Brain Res. 2011, 225, 142–150. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, S.H.; Song, J.; Mironova, Y.; Ming, G.L.; Kolodkin, A.L.; Giger, R.J. Semaphorin 5A inhibits synaptogenesis in early postnatal- and adult-born hippocampal dentate granule cells. Elife 2014, 3, e04390. [Google Scholar] [CrossRef][Green Version]
- Okabe, M.; Miyamoto, Y.; Ikoma, Y.; Takahashi, M.; Shirai, R.; Kukimoto-Niino, M.; Shirouzu, M.; Yamauchi, J. RhoG-binding domain of Elmo1 ameliorates excessive process elongation induced by autism spectrum disorder-associated Sema5A. Pathophysiology 2023, 30, 548–566. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M.; Sato, T.; Takahashi, M.; Honjo, A.; Okawa, M.; Ishida, M.; Kukimoto-Niino, M.; Shirouzu, M.; Miyamoto, Y.; Yamauchi, J. Autism spectrum disorder- and/or intellectual disability-associated semaphorin-5A exploits the mechanism by which Dock5 signalosome molecules control cell shape. Curr. Issues Mol. Biol. 2024, 46, 3092–3107. [Google Scholar] [CrossRef] [PubMed]
- Reddien, P.W.; Horvitz, H.R. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu. Rev. Cell Dev. Biol. 2004, 20, 193–221. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Yamauchi, J. Cellular signaling of Dock family proteins in neural function. Cell. Signal. 2010, 22, 175–182. [Google Scholar] [CrossRef]
- Citi, S.; Guerrera, D.; Spadaro, D.; Shah, J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases 2014, 5, e973760. [Google Scholar] [CrossRef]
- Kukimoto-Niino, M.; Ihara, K.; Murayama, K.; Shirouzu, M. Structural insights into the small GTPase specificity of the DOCK guanine nucleotide exchange factors. Curr. Opin. Struct. Biol. 2021, 71, 249–258. [Google Scholar] [CrossRef]
- Watabe-Uchida, M.; John, K.A.; Janas, J.A.; Newey, S.E.; Van Aelst, L. The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 2006, 51, 727–739. [Google Scholar] [CrossRef]
- Yamauchi, J.; Miyamoto, Y.; Chan, J.R.; Tanoue, A. ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration. J. Cell Biol. 2008, 181, 351–365. [Google Scholar] [CrossRef]
- Numakawa, T.; Yokomaku, D.; Kiyosue, K.; Adachi, N.; Matsumoto, T.; Numakawa, Y.; Taguchi, T.; Hatanaka, H.; Yamada, M. Basic fibroblast growth factor evokes a rapid glutamate release through activation of the MAPK pathway in cultured cortical neurons. J. Biol. Chem. 2002, 277, 28861–28869. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.E.; Ahn, S.G.; Lee, G.H. Psoralidin stimulates expression of immediate-early genes and synapse development in primary cortical neurons. Neurochem. Res. 2018, 43, 2460–2472, Correction in Neurochem. Res. 2019, 44, 509. [Google Scholar] [CrossRef]
- Amano, T.; Richelson, E.; Nirenberg, M. Neurotransmitter synthesis by neuroblastoma clones. Proc. Natl. Acad. Sci. USA 1972, 69, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Ishizaki, T.; Watanabe, N.; Uehata, M.; Kranenburg, O.; Moolenaar, W.H.; Matsumura, F.; Maekawa, M.; Bito, H.; Narumiya, S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 1998, 141, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Subramaniam, M.D.; Venkatesan, D.; Cho, S.G.; Ryding, M.; Meyer, M.; Vellingiri, B. Role of RhoA-ROCK signaling in Parkinson’s disease. Eur. J. Pharmacol. 2021, 894, 173815. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, Y.; Huang, Z.; Zou, Q.; Pu, Y.; Yu, C.; Cai, Z. Role of RhoA/ROCK signaling in Alzheimer’s disease. Behav. Brain Res. 2021, 414, 113481. [Google Scholar] [CrossRef]
- Mosaddeghzadeh, N.; Ahmadian, M.R. The RHO family GTPases: Mechanisms of regulation and signaling. Cells 2021, 10, 1831. [Google Scholar] [CrossRef]
- Takase, S.; Liao, J.; Liu, Y.; Tanaka, R.; Miyagawa, Y.; Sawahata, M.; Sobue, A.; Mizoguchi, H.; Nagai, T.; Kaibuchi, K.; et al. Antipsychotic-like effects of fasudil, a Rho-kinase inhibitor, in a pharmacologic animal model of schizophrenia. Eur. J. Pharmacol. 2022, 931, 175207. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, D.Y. HER2-targeted therapies beyond breast cancer-an update. Nat. Rev. Clin. Oncol. 2024, 21, 675–700. [Google Scholar] [CrossRef]
- Lemke, G. Neuregulin-1 and myelination. Sci. STKE 2006, 2006, pe11, Correction in Sci. STKE 2006, 2006, er5. [Google Scholar] [CrossRef]
- Fleck, D.; Garratt, A.N.; Haass, C.; Willem, M. BACE1 dependent neuregulin processing: Review. Curr. Alzheimer Res. 2012, 9, 178. [Google Scholar] [CrossRef]
- Kataria, H.; Alizadeh, A.; Karimi-Abdolrezaee, S. Neuregulin-1/ErbB network: An emerging modulator of nervous system injury and repair. Prog. Neurobiol. 2019, 180, 101643. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, K.; Qin, X.; Du, G.; Gao, L. The mechanisms of perineuronal net abnormalities in contributing aging and neurological diseases. Ageing Res. Rev. 2023, 92, 102092. [Google Scholar] [CrossRef]
- Lppolitov, D.; Lin, Y.H.; Spence, J.; Glogowska, A.; Thanasupawat, T.; Beiko, J.; Del Bigio, M.R.; Xu, X.; Wang, A.Q.; Williams, D.; et al. The brain-penetrant pan-erbb inhibitor poziotinib effectively targets her2+ breast cancer brain metastases. Cancer Res. 2025, 85, 1514–1529. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Elamin, Y.Y.; Vijayan, R.S.K.; Nilsson, M.B.; Hu, L.; He, J.; Zhang, F.; Pisegna, M.; Poteete, A.; Sun, H.; et al. Pan-Cancer Landscape and Analysis of ERBB2 Mutations Identifies Poziotinib as a Clinically Active Inhibitor and Enhancer of T-DM1 Activity. Cancer Cell 2022, 40, 754–767. [Google Scholar]
- Giordano, G.; Costa, L.G. Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. Methods Mol. Biol. 2011, 758, 13–27. [Google Scholar] [PubMed]
- Moretti, F.; Rolando, C.; Winker, M.; Ivanek, R.; Rodriguez, J.; Von Kriegsheim, A.; Taylor, V.; Bustin, M.; Pertz, O. Growth cone localization of the mRNA encoding the chromatin regulator HMGN5 modulates neurite outgrowth. Mol. Cell. Biol. 2015, 35, 2035–2050. [Google Scholar] [CrossRef]
- Ishida, M.; Ichikawa, R.; Ohbuchi, K.; Oizumi, H.; Miyamoto, Y.; Yamauchi, J. A tardive dyskinesia drug target VMAT-2 participates in neuronal process elongation. Sci. Rep. 2025, 15, 12049. [Google Scholar] [CrossRef]
| Reagents or Materials | Companies or Sources | Cat. Nos. | Lot. Nos. | Concentrations Used |
|---|---|---|---|---|
| Key antibodies | ||||
| Ant-Gap43 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) | sc-17790 | J0920 | Immunoblotting (IB), 1:10,000 |
| Anti-Tau | Santa Cruz Biotechnology | sc-121796 | J2524 | IB, 1:1000 |
| Anti-actin (also called pan-β type actin) | MBL (Tokyo, Japan) | M177-3 | 008 | IB, 1:10,000 |
| Anti-(pY1118)Dock7 | IBL (Tokyo, Japan) | 28079 | 002 | IB, 1:250 |
| Anti-Dock7 | IBL | 28057 | 002 | IB, 1:250 |
| Anti-hexa-histidine | MBL | D291-3 | 005 | IB, 1:5000 |
| Anti-rabbit IgG (goat) pre-absorbed HRP-conjugate | Nacalai Tesque (Kyoto, Japan) | 21858-24 | L3E2990 | IB, 1:10,000 |
| Anti-mouse IgG (goat) pre-absorbed HRP-conjugate | Nacalai Tesque | 21860-61 | L4B5968 | IB, 1:10,000 |
| Anti-IgG (H + L chain) (rabbit) pAb-HRP | MBL | 458 | 354 | IB, 1:10,000 |
| Anti-IgG (H + L chain) (mouse) pAb-HRP | MBL | 330 | 365 | IB, 1:10,000 |
| Recombinant DNAs | ||||
| pcDNA3.1(-), which is digested from pcDNA3.1(-)-shLuciferasemir | generated in this study | N/A | N/A | 1.25 micrograms of DNA per 6 cm dish |
| pcDNA3.1(-)-shLuciferase (control) mir | Genscript (Piscataway, NJ, USA; generated in this study) | J248X396G0-2 | W948408 | 1.25 micrograms of DNA per 6 cm dish |
| pcDNA3.1(-)-shDock7mir | Genscript (generated in this study) | J6826818G0-2 | W947171 | 1.25 micrograms of DNA per 6 cm dish |
| pCMV5 | N/A | N/A | N/A | 1.25 micrograms of DNA per 6 cm dish |
| pCMV5-FLAG-Dock7 middle 2 domain (corresponding to amino acids 1111 to 1431 of Dock7). | [38] | N/A | N/A | 1.25 micrograms of DNA per 6 cm dish |
| pEGFP-C1 (mamalian cell GFP expresssion plasmid) | Takara Bio (Kyoto, Japan) | not commercially available | N/A | 0.625 micrograms of DNA per 6 cm dish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, M.; Yako, H.; Suzuki, A.; Isa, R.; Miyamoto, Y.; Yamauchi, J. The ErbB2–Dock7 Signaling Axis Mediates Excessive Cell Morphogenesis Induced by Autism Spectrum Disorder- and Intellectual Disability-Associated Sema5A p.Arg676Cys. Int. J. Mol. Sci. 2025, 26, 10656. https://doi.org/10.3390/ijms262110656
Takahashi M, Yako H, Suzuki A, Isa R, Miyamoto Y, Yamauchi J. The ErbB2–Dock7 Signaling Axis Mediates Excessive Cell Morphogenesis Induced by Autism Spectrum Disorder- and Intellectual Disability-Associated Sema5A p.Arg676Cys. International Journal of Molecular Sciences. 2025; 26(21):10656. https://doi.org/10.3390/ijms262110656
Chicago/Turabian StyleTakahashi, Mikito, Hideji Yako, Ayaka Suzuki, Ryuma Isa, Yuki Miyamoto, and Junji Yamauchi. 2025. "The ErbB2–Dock7 Signaling Axis Mediates Excessive Cell Morphogenesis Induced by Autism Spectrum Disorder- and Intellectual Disability-Associated Sema5A p.Arg676Cys" International Journal of Molecular Sciences 26, no. 21: 10656. https://doi.org/10.3390/ijms262110656
APA StyleTakahashi, M., Yako, H., Suzuki, A., Isa, R., Miyamoto, Y., & Yamauchi, J. (2025). The ErbB2–Dock7 Signaling Axis Mediates Excessive Cell Morphogenesis Induced by Autism Spectrum Disorder- and Intellectual Disability-Associated Sema5A p.Arg676Cys. International Journal of Molecular Sciences, 26(21), 10656. https://doi.org/10.3390/ijms262110656








