The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential
Abstract
1. Introduction
2. TGR5: Structure, Expression, and Mechanism of Action
3. TGR5 Agonists
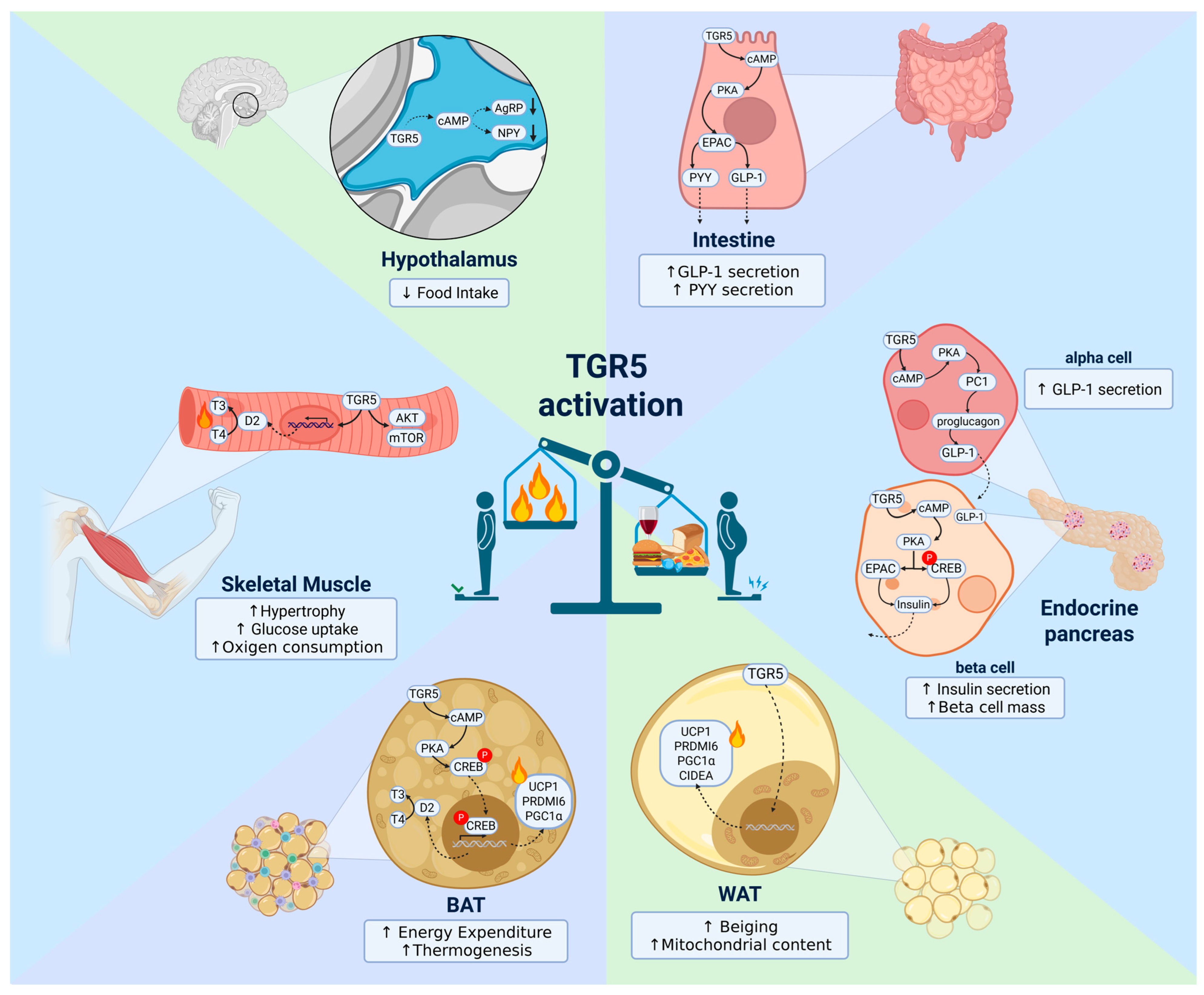
4. TGR5 in Energy Homeostasis Regulation
4.1. Hypothalamus
4.2. Brown Adipose Tissue (BAT)
4.3. White Adipose Tissue (WAT)
4.4. Skeletal Muscle
4.5. Intestine
4.6. Endocrine Pancreas
5. Clinical Approaches
6. Limitations, Challenges, and Controversies
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Shulpekova, Y.; Shirokova, E.; Zharkova, M.; Tkachenko, P.; Tikhonov, I.; Stepanov, A.; Sinitsyna, A.; Izotov, A.; Butkova, T.; Shulpekova, N.; et al. A Recent Ten-Year Perspective: Bile Acid Metabolism and Signaling. Molecules 2022, 27, 1983. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Fiamoncini, J.; Rist, M.J.; Frommherz, L.; Giesbertz, P.; Pfrang, B.; Kremer, W.; Huber, F.; Kastenmüller, G.; Skurk, T.; Hauner, H.; et al. Dynamics and determinants of human plasma bile acid profiles during dietary challenges. Front. Nutr. 2022, 9, 932937. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef]
- Xiang, D.; Yang, J.; Liu, L.; Yu, H.; Gong, X.; Liu, D. The regulation of tissue-specific farnesoid X receptor on genes and diseases involved in bile acid homeostasis. Biomed. Pharmacother. 2023, 168, 115606. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Carvalho, M.; Velloso, L.A.; Barbosa, H.C.L. Endocrine FGFs and their signaling in the brain: Relevance for energy homeostasis. Eur. J. Pharmacol. 2024, 963, 176248. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Carvalho, M.; Solon, C.; Sidarta-Oliveira, D.; Soares, G.M.; Marmentini, C.; Boschero, A.C.; Tseng, Y.H.; Velloso, L.A.; Barbosa, H.C.L. Central FGF19 signaling enhances energy homeostasis and adipose tissue thermogenesis through sympathetic activation in obese mice. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E524–E542. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef]
- Perino, A.; Demagny, H.; Velazquez-Villegas, L.; Schoonjans, K. Molecular Physiology of Bile Acid Signaling in Health, Disease, and Aging. Physiol. Rev. 2021, 101, 683–731. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J. Hepatol. 2011, 54, 1263–1272. [Google Scholar] [CrossRef]
- Fleishman, J.S.; Kumar, S. Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Miyamoto, Y.; Nakamura, T.; Tamai, Y.; Okada, H.; Sugiyama, E.; Itadani, H.; Tanaka, K. Identification of membrane-type receptor for bile acids (M-BAR). Biochem. Biophys. Res. Commun. 2002, 298, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G protein-coupled receptor responsive to bile acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Chen, W.D.; Wang, Y.D. TGR5, Not Only a Metabolic Regulator. Front. Physiol. 2016, 7, 646. [Google Scholar] [CrossRef]
- Maruyama, T.; Tanaka, K.; Suzuki, J.; Miyoshi, H.; Harada, N.; Nakamura, T.; Miyamoto, Y.; Kanatani, A.; Tamai, Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J. Endocrinol. 2006, 191, 197–205. [Google Scholar] [CrossRef]
- Baars, A.; Oosting, A.; Lohuis, M.; Koehorst, M.; El Aidy, S.; Hugenholtz, F.; Smidt, H.; Mischke, M.; Boekschoten, M.V.; Verkade, H.J.; et al. Sex differences in lipid metabolism are affected by presence of the gut microbiota. Sci. Rep. 2018, 8, 13426. [Google Scholar] [CrossRef]
- Vassileva, G.; Hu, W.; Hoos, L.; Tetzloff, G.; Yang, S.; Liu, L.; Kang, L.; Davis, H.R.; Hedrick, J.A.; Lan, H.; et al. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J. Endocrinol. 2010, 205, 225–232. [Google Scholar] [CrossRef]
- Vassileva, G.; Golovko, A.; Markowitz, L.; Abbondanzo, S.J.; Zeng, M.; Yang, S.; Hoos, L.; Tetzloff, G.; Levitan, D.; Murgolo, N.J.; et al. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem. J. 2006, 398, 423–430. [Google Scholar] [CrossRef]
- Wan, Y.Y.; Sheng, L. Regulation of bile acid receptor activity. Liver Res. 2018, 2, 180–185. [Google Scholar] [CrossRef]
- Gao, S.; Ji, X.F.; Li, F.; Sun, F.K.; Zhao, J.; Fan, Y.C.; Wang, K. Aberrant DNA methylation of G-protein-coupled bile acid receptor Gpbar1 predicts prognosis of acute-on-chronic hepatitis B liver failure. J. Viral Hepat. 2015, 22, 112–119. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuboyama, A.; Mita, M.; Murata, S.; Shimizu, M.; Inoue, J.; Mori, K.; Sato, R. The exercise-inducible bile acid receptor Tgr5 improves skeletal muscle function in mice. J. Biol. Chem. 2018, 293, 10322–10332. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Asgharpour, A.; Mirshahi, F.; Park, S.H.; Liu, S.; Imai, Y.; Nadler, J.L.; Grider, J.R.; Murthy, K.S.; Sanyal, A.J. Activation of Transmembrane Bile Acid Receptor TGR5 Modulates Pancreatic Islet α Cells to Promote Glucose Homeostasis. J. Biol. Chem. 2016, 291, 6626–6640. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Kumar, D.P.; Mahavadi, S.; Bhattacharya, S.; Zhou, R.; Corvera, C.U.; Bunnett, N.W.; Grider, J.R.; Murthy, K.S. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G527–G535. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Huang, B.Q.; Radtke, B.N.; Gajdos, G.B.; Splinter, P.L.; Masyuk, T.V.; Gradilone, S.A.; LaRusso, N.F. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1013–G1024. [Google Scholar] [CrossRef]
- Takahashi, M.; Li, Y.; Dillon, T.J.; Stork, P.J. Phosphorylation of Rap1 by cAMP-dependent Protein Kinase (PKA) Creates a Binding Site for KSR to Sustain ERK Activation by cAMP. J. Biol. Chem. 2017, 292, 1449–1461. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X receptor (FXR): Structures and ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- Lun, W.; Yan, Q.; Guo, X.; Zhou, M.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Mechanism of action of the bile acid receptor TGR5 in obesity. Acta Pharm. Sin. B 2024, 14, 468–491. [Google Scholar] [CrossRef]
- Pols, T.W.; Noriega, L.G.; Nomura, M.; Auwerx, J.; Schoonjans, K. The bile acid membrane receptor TGR5: A valuable metabolic target. Dig. Dis. 2011, 29, 37–44. [Google Scholar] [CrossRef]
- Keitel, V.; Häussinger, D. Perspective: TGR5 (Gpbar-1) in liver physiology and disease. Clin. Res. Hepatol. Gastroenterol. 2012, 36, 412–419. [Google Scholar] [CrossRef]
- Sato, H.; Macchiarulo, A.; Thomas, C.; Gioiello, A.; Une, M.; Hofmann, A.F.; Saladin, R.; Schoonjans, K.; Pellicciari, R.; Auwerx, J. Novel potent and selective bile acid derivatives as TGR5 agonists: Biological screening, structure-activity relationships, and molecular modeling studies. J. Med. Chem. 2008, 51, 1831–1841. [Google Scholar] [CrossRef]
- Pellicciari, R.; Gioiello, A.; Macchiarulo, A.; Thomas, C.; Rosatelli, E.; Natalini, B.; Sardella, R.; Pruzanski, M.; Roda, A.; Pastorini, E.; et al. Discovery of 6alpha-ethyl-23(S)-methylcholic acid (S-EMCA, INT-777) as a potent and selective agonist for the TGR5 receptor, a novel target for diabesity. J. Med. Chem. 2009, 52, 7958–7961. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Patil, A.; Aware, U.; Deshmukh, P.; Darji, B.; Sasane, S.; Sairam, K.V.; Priyadarsiny, P.; Giri, P.; Patel, H.; et al. Discovery of a Potent and Orally Efficacious TGR5 Receptor Agonist. ACS Med. Chem. Lett. 2016, 7, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Renga, B.; Cipriani, S.; Carino, A.; Simonetti, M.; Zampella, A.; Fiorucci, S. Reversal of Endothelial Dysfunction by GPBAR1 Agonism in Portal Hypertension Involves a AKT/FOXOA1 Dependent Regulation of H2S Generation and Endothelin-1. PLoS ONE 2015, 10, e0141082. [Google Scholar] [CrossRef]
- Hodge, R.J.; Lin, J.; Vasist Johnson, L.S.; Gould, E.P.; Bowers, G.D.; Nunez, D.J.; Team, S.-P. Safety, Pharmacokinetics, and Pharmacodynamic Effects of a Selective TGR5 Agonist, SB-756050, in Type 2 Diabetes. Clin. Pharmacol. Drug Dev. 2013, 2, 213–222. [Google Scholar] [CrossRef]
- Luo, Z.; Zhou, W.; Xie, T.; Xu, W.; Shi, C.; Xiao, Z.; Si, Y.; Ma, Y.; Ren, Q.; Di, L.; et al. The role of botanical triterpenoids and steroids in bile acid metabolism, transport, and signaling: Pharmacological and toxicological implications. Acta Pharm. Sin. B 2024, 14, 3385–3415. [Google Scholar] [CrossRef]
- Horiba, T.; Katsukawa, M.; Mita, M.; Sato, R. Dietary obacunone supplementation stimulates muscle hypertrophy, and suppresses hyperglycemia and obesity through the TGR5 and PPARγ pathway. Biochem. Biophys. Res. Commun. 2015, 463, 846–852. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Tran, L.T.; Park, S.; Kim, S.K.; Lee, J.S.; Kim, K.W.; Kwon, O. Hypothalamic control of energy expenditure and thermogenesis. Exp. Mol. Med. 2022, 54, 358–369. [Google Scholar] [CrossRef]
- Castellanos-Jankiewicz, A.; Guzmán-Quevedo, O.; Fénelon, V.S.; Zizzari, P.; Quarta, C.; Bellocchio, L.; Tailleux, A.; Charton, J.; Fernandois, D.; Henricsson, M.; et al. Hypothalamic bile acid-TGR5 signaling protects from obesity. Cell Metab. 2021, 33, 1483–1492.e1410. [Google Scholar] [CrossRef]
- Perino, A.; Velázquez-Villegas, L.A.; Bresciani, N.; Sun, Y.; Huang, Q.; Fénelon, V.S.; Castellanos-Jankiewicz, A.; Zizzari, P.; Bruschetta, G.; Jin, S.; et al. Central anorexigenic actions of bile acids are mediated by TGR5. Nat. Metab. 2021, 3, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, T.; Sato, A.; Nishimoto-Kusunose, S.; Yoshizawa, K.; Higashi, T. Further evidence for blood-to-brain influx of unconjugated bile acids by passive diffusion: Determination of their brain-to-serum concentration ratios in rats by LC/MS/MS. Steroids 2024, 204, 109397. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi, T.; Zanella, D.; Bhatt, M.; Di Iacovo, A.; Galli, A.; Bossi, E. Bile acid interactions with neurotransmitter transporters. Front. Cell. Neurosci. 2023, 17, 1161930. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Huang, X.; Wang, D.; Yu, D.; Hou, S.; Cui, H.; Song, L. Roles of bile acids signaling in neuromodulation under physiological and pathological conditions. Cell Biosci. 2023, 13, 106. [Google Scholar] [CrossRef]
- Higashi, T.; Watanabe, S.; Tomaru, K.; Yamazaki, W.; Yoshizawa, K.; Ogawa, S.; Nagao, H.; Minato, K.; Maekawa, M.; Mano, N. Unconjugated bile acids in rat brain: Analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids 2017, 125, 107–113. [Google Scholar] [CrossRef]
- Doignon, I.; Julien, B.; Serrière-Lanneau, V.; Garcin, I.; Alonso, G.; Nicou, A.; Monnet, F.; Gigou, M.; Humbert, L.; Rainteau, D.; et al. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J. Hepatol. 2011, 54, 481–488. [Google Scholar] [CrossRef]
- Li, X.Y.; Zhang, S.Y.; Hong, Y.Z.; Chen, Z.G.; Long, Y.; Yuan, D.H.; Zhao, J.J.; Tang, S.S.; Wang, H.; Hong, H. TGR5-mediated lateral hypothalamus-dCA3-dorsolateral septum circuit regulates depressive-like behavior in male mice. Neuron 2024, 112, 1795–1814.e10. [Google Scholar] [CrossRef]
- Zizzari, P.; Castellanos-Jankiewicz, A.; Yagoub, S.; Simon, V.; Clark, S.; Maître, M.; Dupuy, N.; Leste-Lasserre, T.; Gonzales, D.; Schoonjans, K.; et al. TGR5 receptors in SF1-expressing neurons of the ventromedial hypothalamus regulate glucose homeostasis. Mol. Metab. 2025, 91, 102071. [Google Scholar] [CrossRef]
- Vanden Brink, H.; Vandeputte, D.; Brito, I.L.; Ronnekleiv, O.K.; Roberson, M.S.; Lomniczi, A. Changes in the Bile Acid Pool and Timing of Female Puberty: Potential Novel Role of Hypothalamic TGR5. Endocrinology 2024, 165, bqae098. [Google Scholar] [CrossRef]
- Brüning, J.C.; Fenselau, H. Integrative neurocircuits that control metabolism and food intake. Science 2023, 381, eabl7398. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Solon, C.; Soares, G.M.; Engel, D.F.; Velloso, L.A.; Boschero, A.C.; Carneiro, E.M.; Barbosa, H.C.L. Energy homeostasis deregulation is attenuated by TUDCA treatment in streptozotocin-induced Alzheimer’s disease mice model. Sci. Rep. 2021, 11, 18114. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, X.; Chen, D.; Yu, B.; Zheng, P.; Luo, Y.; He, J.; Huang, Z. Oleanolic acid inhibits appetite through the TGR5/cAMP signaling pathway. J. Nutr. Biochem. 2025, 138, 109844. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, J.Y.; Lee, A.; Lu, Y.X.; Zhou, S.Y.; Owyang, C. Satiety induced by bile acids is mediated via vagal afferent pathways. JCI Insight 2020, 5, e132400. [Google Scholar] [CrossRef] [PubMed]
- Cypess, A.M.; Cannon, B.; Nedergaard, J.; Kazak, L.; Chang, D.C.; Krakoff, J.; Tseng, Y.H.; Schéele, C.; Boucher, J.; Petrovic, N.; et al. Emerging debates and resolutions in brown adipose tissue research. Cell Metab. 2025, 37, 12–33. [Google Scholar] [CrossRef]
- Zingaretti, M.C.; Crosta, F.; Vitali, A.; Guerrieri, M.; Frontini, A.; Cannon, B.; Nedergaard, J.; Cinti, S. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009, 23, 3113–3120. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Watanabe, M.; Morimoto, K.; Houten, S.M.; Kaneko-Iwasaki, N.; Sugizaki, T.; Horai, Y.; Mataki, C.; Sato, H.; Murahashi, K.; Arita, E.; et al. Bile acid binding resin improves metabolic control through the induction of energy expenditure. PLoS ONE 2012, 7, e38286. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Zouhar, P.; Flachs, P.; Bardova, K.; Janovska, P.; Gomes, A.P.; Duarte, F.V.; Varela, A.T.; Rolo, A.P.; Palmeira, C.M.; et al. Enhancement of brown fat thermogenesis using chenodeoxycholic acid in mice. Int. J. Obes. 2014, 38, 1027–1034. [Google Scholar] [CrossRef]
- Broeders, E.P.; Nascimento, E.B.; Havekes, B.; Brans, B.; Roumans, K.H.; Tailleux, A.; Schaart, G.; Kouach, M.; Charton, J.; Deprez, B.; et al. The Bile Acid Chenodeoxycholic Acid Increases Human Brown Adipose Tissue Activity. Cell Metab. 2015, 22, 418–426. [Google Scholar] [CrossRef]
- Watanabe, M.; Horai, Y.; Houten, S.M.; Morimoto, K.; Sugizaki, T.; Arita, E.; Mataki, C.; Sato, H.; Tanigawara, Y.; Schoonjans, K.; et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J. Biol. Chem. 2011, 286, 26913–26920. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.C.; Duan, G.Z.; Mao, W.; Liu, Q.; Zhang, Y.L.; Li, P.F. Taurochenodeoxycholic acid mediates cAMP-PKA-CREB signaling pathway. Chin. J. Nat. Med. 2020, 18, 898–906. [Google Scholar] [CrossRef] [PubMed]
- da-Silva, W.S.; Ribich, S.; Arrojo e Drigo, R.; Castillo, M.; Patti, M.E.; Bianco, A.C. The chemical chaperones tauroursodeoxycholic and 4-phenylbutyric acid accelerate thyroid hormone activation and energy expenditure. FEBS Lett. 2011, 585, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Zangerolamo, L.; Carvalho, M.; Barssotti, L.; Soares, G.M.; Marmentini, C.; Boschero, A.C.; Barbosa, H.C.L. The bile acid TUDCA reduces age-related hyperinsulinemia in mice. Sci. Rep. 2022, 12, 22273. [Google Scholar] [CrossRef]
- Carino, A.; Cipriani, S.; Marchianò, S.; Biagioli, M.; Scarpelli, P.; Zampella, A.; Monti, M.C.; Fiorucci, S. Gpbar1 agonism promotes a Pgc-1α-dependent browning of white adipose tissue and energy expenditure and reverses diet-induced steatohepatitis in mice. Sci. Rep. 2017, 7, 13689. [Google Scholar] [CrossRef]
- Xie, R.; Yan, S.; Zhou, X.; Gao, Y.; Qian, Y.; Hou, J.; Chen, Z.; Lai, K.; Gao, X.; Wei, S. Activation of METTL3 Promotes White Adipose Tissue Beiging and Combats Obesity. Diabetes 2023, 72, 1083–1094. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Svensson, P.A.; Olsson, M.; Andersson-Assarsson, J.C.; Taube, M.; Pereira, M.J.; Froguel, P.; Jacobson, P. The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem. Biophys. Res. Commun. 2013, 433, 563–566. [Google Scholar] [CrossRef]
- Wang, Y.N.; Tang, Y.; He, Z.; Ma, H.; Wang, L.; Liu, Y.; Yang, Q.; Pan, D.; Zhu, C.; Qian, S.; et al. Slit3 secreted from M2-like macrophages increases sympathetic activity and thermogenesis in adipose tissue. Nat. Metab. 2021, 3, 1536–1551. [Google Scholar] [CrossRef]
- Perino, A.; Pols, T.W.; Nomura, M.; Stein, S.; Pellicciari, R.; Schoonjans, K. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J. Clin. Investig. 2014, 124, 5424–5436. [Google Scholar] [CrossRef]
- Castillo, Í.; Argilés, J.M.; Rueda, R.; Ramírez, M.; Pedrosa, J.M.L. Skeletal muscle atrophy and dysfunction in obesity and type-2 diabetes mellitus: Myocellular mechanisms involved. Rev. Endocr. Metab. Disord. 2025. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, F.; Tan, W.; Zhao, W.; Li, Y.; Zhu, X.; Gao, P.; Shu, G.; Wang, S.; Jiang, Q.; et al. Lithocholic acid promotes skeletal muscle regeneration through the TGR5 receptor. Acta Biochim. Biophys. Sin. 2023, 55, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Tamai, Y.; Eguchi, A.; Shigefuku, R.; Kitamura, H.; Tempaku, M.; Sugimoto, R.; Kobayashi, Y.; Iwasa, M.; Takei, Y.; Nakagawa, H. Association of lithocholic acid with skeletal muscle hypertrophy through TGR5-IGF-1 and skeletal muscle mass in cultured mouse myotubes, chronic liver disease rats and humans. Elife 2022, 11, e80638. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.J.; Lang, M.J.; Johnson, J.M.; Watanabe, S.; McLaughlin, K.L.; Maschek, J.A.; Verkerke, A.R.P.; Siripoksup, P.; Chaix, A.; Cox, J.E.; et al. Weight loss increases skeletal muscle mitochondrial energy efficiency in obese mice. Life Metab. 2023, 2, load014. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P. Human brown adipose tissue is not enough to combat cardiometabolic diseases. J. Clin. Investig. 2023, 133, e175288. [Google Scholar] [CrossRef]
- Gereben, B.; Zavacki, A.M.; Ribich, S.; Kim, B.W.; Huang, S.A.; Simonides, W.S.; Zeöld, A.; Bianco, A.C. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 2008, 29, 898–938. [Google Scholar] [CrossRef]
- Baxter, J.D.; Webb, P. Metabolism: Bile acids heat things up. Nature 2006, 439, 402–403. [Google Scholar] [CrossRef]
- Huang, S.; Ma, S.; Ning, M.; Yang, W.; Ye, Y.; Zhang, L.; Shen, J.; Leng, Y. TGR5 agonist ameliorates insulin resistance in the skeletal muscles and improves glucose homeostasis in diabetic mice. Metabolism 2019, 99, 45–56. [Google Scholar] [CrossRef]
- Stepanov, V.; Stankov, K.; Mikov, M. The bile acid membrane receptor TGR5: A novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J. Recept. Signal Transduct. 2013, 33, 213–223. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, Y.; Kuboyama, A.; Oikawa, A.; Shimizu, M.; Yamauchi, Y.; Sato, R. Muscle-specific TGR5 overexpression improves glucose clearance in glucose-intolerant mice. J. Biol. Chem. 2021, 296, 100131. [Google Scholar] [CrossRef]
- Bany Bakar, R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Møller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Sousa, K.M.; Jin, L.; Dong, B.; Kim, B.W.; Ramirez, R.; Xiao, Z.; Gu, Y.; Yang, Q.; Wang, J.; et al. Vertical sleeve gastrectomy activates GPBAR-1/TGR5 to sustain weight loss, improve fatty liver, and remit insulin resistance in mice. Hepatology 2016, 64, 760–773. [Google Scholar] [CrossRef]
- Burchat, N.; Vidola, J.; Pfreundschuh, S.; Sharma, P.; Rizzolo, D.; Guo, G.L.; Sampath, H. Intestinal Stearoyl-CoA Desaturase-1 Regulates Energy Balance via Alterations in Bile Acid Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2024, 18, 101403. [Google Scholar] [CrossRef]
- Parker, H.E.; Wallis, K.; le Roux, C.W.; Wong, K.Y.; Reimann, F.; Gribble, F.M. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 2012, 165, 414–423. [Google Scholar] [CrossRef]
- Chao, J.; Coleman, R.A.; Keating, D.J.; Martin, A.M. Gut Microbiome Regulation of Gut Hormone Secretion. Endocrinology 2025, 166, bqaf004. [Google Scholar] [CrossRef]
- Bala, V.; Rajagopal, S.; Kumar, D.P.; Nalli, A.D.; Mahavadi, S.; Sanyal, A.J.; Grider, J.R.; Murthy, K.S. Release of GLP-1 and PYY in response to the activation of G protein-coupled bile acid receptor TGR5 is mediated by Epac/PLC-ε pathway and modulated by endogenous H2S. Front. Physiol. 2014, 5, 420. [Google Scholar] [CrossRef]
- Alonso, A.M.; Cork, S.C.; Phuah, P.; Hansen, B.; Norton, M.; Cheng, S.; Xu, X.; Suba, K.; Ma, Y.; Dowsett, G.K.; et al. The vagus nerve mediates the physiological but not pharmacological effects of PYY. Mol. Metab. 2024, 81, 101895. [Google Scholar] [CrossRef]
- Münzker, J.; Haase, N.; Till, A.; Sucher, R.; Haange, S.B.; Nemetschke, L.; Gnad, T.; Jäger, E.; Chen, J.; Riede, S.J.; et al. Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity. Microbiome 2022, 10, 96. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A.; Cipriani, S.; Francisci, D.; Marchianò, S.; Scarpelli, P.; Sorcini, D.; Zampella, A.; Fiorucci, S. The Bile Acid Receptor GPBAR1 Regulates the M1/M2 Phenotype of Intestinal Macrophages and Activation of GPBAR1 Rescues Mice from Murine Colitis. J. Immunol. 2017, 199, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Niu, K.M.; Liu, Y.; Lin, C.; Wu, X. The Gut Microbiota-Bile Acids-TGR5 Axis Mediates. Front. Microbiol. 2021, 12, 727681. [Google Scholar] [CrossRef] [PubMed]
- Arrojo e Drigo, R.; Ali, Y.; Diez, J.; Srinivasan, D.K.; Berggren, P.O.; Boehm, B.O. New insights into the architecture of the islet of Langerhans: A focused cross-species assessment. Diabetologia 2015, 58, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Hulett, N.A.; Scalzo, R.L.; Reusch, J.E.B. Glucose Uptake by Skeletal Muscle within the Contexts of Type 2 Diabetes and Exercise: An Integrated Approach. Nutrients 2022, 14, 647. [Google Scholar] [CrossRef]
- De la Cruz-Concepción, B.; Flores-Cortez, Y.A.; Barragán-Bonilla, M.I.; Mendoza-Bello, J.M.; Espinoza-Rojo, M. Insulin: A connection between pancreatic β cells and the hypothalamus. World J. Diabetes 2023, 14, 76–91. [Google Scholar] [CrossRef]
- Kumar, D.P.; Rajagopal, S.; Mahavadi, S.; Mirshahi, F.; Grider, J.R.; Murthy, K.S.; Sanyal, A.J. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells. Biochem. Biophys. Res. Commun. 2012, 427, 600–605. [Google Scholar] [CrossRef]
- Rosa, L.R.O.; Vettorazzi, J.F.; Zangerolamo, L.; Carneiro, E.M.; Barbosa, H.C.L. TUDCA receptors and their role on pancreatic beta cells. Prog. Biophys. Mol. Biol. 2021, 167, 26–31. [Google Scholar] [CrossRef]
- Whalley, N.M.; Pritchard, L.E.; Smith, D.M.; White, A. Processing of proglucagon to GLP-1 in pancreatic α-cells: Is this a paracrine mechanism enabling GLP-1 to act on β-cells? J. Endocrinol. 2011, 211, 99–106. [Google Scholar] [CrossRef]
- Vettorazzi, J.F.; Ribeiro, R.A.; Borck, P.C.; Branco, R.C.; Soriano, S.; Merino, B.; Boschero, A.C.; Nadal, A.; Quesada, I.; Carneiro, E.M. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 2016, 65, 54–63. [Google Scholar] [CrossRef]
- Makki, K.; Brolin, H.; Petersen, N.; Henricsson, M.; Christensen, D.P.; Khan, M.T.; Wahlström, A.; Bergh, P.O.; Tremaroli, V.; Schoonjans, K.; et al. 6α-hydroxylated bile acids mediate TGR5 signalling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 2023, 72, 314–324. [Google Scholar] [CrossRef]
- Adrian, T.E.; Gariballa, S.; Parekh, K.A.; Thomas, S.A.; Saadi, H.; Al Kaabi, J.; Nagelkerke, N.; Gedulin, B.; Young, A.A. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 2012, 55, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, N.; Yun, Y.; Zhang, C.; Guo, S.; Yin, J.; Zhao, T.; Ge, X.; Gu, M.; Xie, X.; Nan, F. Discovery of betulinic acid derivatives as gut-restricted TGR5 agonists: Balancing the potency and physicochemical properties. Bioorg. Chem. 2024, 144, 107132. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Une, N.; Nishizawa, M.; Suzuki, S.; Ito, H.; Horiuchi, T. Incretin secretion stimulated by ursodeoxycholic acid in healthy subjects. Springerplus 2013, 2, 20. [Google Scholar] [CrossRef]
- Kim, G.W.; Lin, J.E.; Valentino, M.A.; Colon-Gonzalez, F.; Waldman, S.A. Regulation of appetite to treat obesity. Expert Rev. Clin. Pharmacol. 2011, 4, 243–259. [Google Scholar] [CrossRef]
- Jordi, J.; Guggiana-Nilo, D.; Bolton, A.D.; Prabha, S.; Ballotti, K.; Herrera, K.; Rennekamp, A.J.; Peterson, R.T.; Lutz, T.A.; Engert, F. High-throughput screening for selective appetite modulators: A multibehavioral and translational drug discovery strategy. Sci. Adv. 2018, 4, eaav1966. [Google Scholar] [CrossRef]
- Ockenga, J.; Valentini, L.; Schuetz, T.; Wohlgemuth, F.; Glaeser, S.; Omar, A.; Kasim, E.; duPlessis, D.; Featherstone, K.; Davis, J.R.; et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J. Clin. Endocrinol. Metab. 2012, 97, 535–542. [Google Scholar] [CrossRef]
- Xun, Z.; Li, T.; Xue, X. The application strategy of liposomes in organ targeting therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2024, 16, e1955. [Google Scholar] [CrossRef]
- Desai, M.S.; Shabier, Z.; Taylor, M.; Lam, F.; Thevananther, S.; Kosters, A.; Karpen, S.J. Hypertrophic cardiomyopathy and dysregulation of cardiac energetics in a mouse model of biliary fibrosis. Hepatology 2010, 51, 2097–2107. [Google Scholar] [CrossRef]
- Eblimit, Z.; Thevananther, S.; Karpen, S.J.; Taegtmeyer, H.; Moore, D.D.; Adorini, L.; Penny, D.J.; Desai, M.S. TGR5 activation induces cytoprotective changes in the heart and improves myocardial adaptability to physiologic, inotropic, and pressure-induced stress in mice. Cardiovasc. Ther. 2018, 36, e12462. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, W.Q.; Fu, M.J.; Li, J.; Hu, C.H.; Chen, Y.; Zhou, M.M.; Gao, Z.J.; He, Y.L. Overview of bile acid signaling in the cardiovascular system. World J. Clin. Cases 2021, 9, 308–320. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Holmstrom, S.R.; Kir, S.; Umetani, M.; Schmidt, D.R.; Kliewer, S.A.; Mangelsdorf, D.J. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol. Endocrinol. 2011, 25, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Fogelson, K.A.; Dorrestein, P.C.; Zarrinpar, A.; Knight, R. The Gut Microbial Bile Acid Modulation and Its Relevance to Digestive Health and Diseases. Gastroenterology 2023, 164, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, J.; Ding, Y.; Pang, Y.; Jiang, C. Gut microbiota, immunity, and bile acid metabolism: Decoding metabolic disease interactions. Life Metab. 2023, 2, load032. [Google Scholar] [CrossRef]
- Wise, J.L.; Cummings, B.P. The 7-α-dehydroxylation pathway: An integral component of gut bacterial bile acid metabolism and potential therapeutic target. Front. Microbiol. 2022, 13, 1093420. [Google Scholar] [CrossRef]
- Pi, Y.; Wu, Y.; Zhang, X.; Lu, D.; Han, D.; Zhao, J.; Zheng, X.; Zhang, S.; Ye, H.; Lian, S.; et al. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]
- de Jonge, N.; Carlsen, B.; Christensen, M.H.; Pertoldi, C.; Nielsen, J.L. The Gut Microbiome of 54 Mammalian Species. Front. Microbiol. 2022, 13, 886252. [Google Scholar] [CrossRef]
- Nishida, A.H.; Ochman, H. Rates of gut microbiome divergence in mammals. Mol. Ecol. 2018, 27, 1884–1897. [Google Scholar] [CrossRef]
- Zheng, D.; Ge, K.; Qu, C.; Sun, T.; Wang, J.; Jia, W.; Zhao, A. Comparative profiling of serum, urine, and feces bile acids in humans, rats, and mice. Commun. Biol. 2024, 7, 641. [Google Scholar] [CrossRef]
- Duboc, H.; Taché, Y.; Hofmann, A.F. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig. Liver Dis. 2014, 46, 302–312. [Google Scholar] [CrossRef]
- Schieren, A.; Koch, S.; Pecht, T.; Simon, M.C. Impact of Physiological Fluctuations of Sex Hormones During the Menstrual Cycle on Glucose Metabolism and the Gut Microbiota. Exp. Clin. Endocrinol. Diabetes 2024, 132, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, C.; Reimúndez, A.; Viana, F.; Arce, V.M.; Señarís, R. Sex differences in thermoregulation in mammals: Implications for energy homeostasis. Front. Endocrinol. 2023, 14, 1093376. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Guo, Y.; Klaassen, C.D. Effect of Gender and Various Diets on Bile Acid Profile and Related Genes in Mice. Drug Metab. Dispos. 2021, 49, 62–71. [Google Scholar] [CrossRef] [PubMed]

| TGR5 Agonist | EC50 (μM) | Reference |
|---|---|---|
| LCA | 0.53 | [32] |
| DCA | 1.25 | [30] |
| CDCA | 6.71 | [30] |
| CA | 13.6 | [30] |
| UDCA | 36.4 | [30] |
| TLCA | 0.33 | [32] |
| TDCA | 0.79 | [30] |
| TCDCA | 1.92 | [30] |
| TCA | 4.95 | [30] |
| TUDCA | 30.0 | [30] |
| INT-777 | 0.82 | [33] |
| BAR501 | 1.0 | [34] |
| SB-756050 | 1.3 | [35] |
| Oleanolic acid | 2.25 | [36] |
| Obacunone/Liminoid | Not specified |
| TGR5 Agonist | Condition | Study Title | Clinical Trials Identifier: | Study Design, Interventions, and Location |
|---|---|---|---|---|
| SB756050 (GlaxoSmithKline) | Type 2 Diabetes Mellitus | A Study to Test How SB756050 Affects Subjects With Type 2 Diabetes Mellitus After 6 Days of Dosing | NCT00733577 | Study Type: Interventional Enrollment: 48 participants Phase: 1 Doses: 15 to 600 mg Duration: 6 days Placebo-Controlled Location: USA |
| SB756050 (GlaxoSmithKline) | Type 2 Diabetes Mellitus | First-Time-in-Humans Study to Assess Safety, Pharmacokinetics & Pharmacodynamics of SB756050 | NCT00607906 | Study Type: Interventional Enrollment: 36 participants Phase: 1 Doses: 5–100 mg Duration: single dose Placebo-Controlled Location: USA |
| Bile acid CDCA and oleanolic acid | Healthy Volunteers | Effect of Bile Acids on the Secretion of Satiation Peptides in Humans | NCT01674946 | Study Type: Interventional Enrollment: 12 participants Phase: 1 Doses: Oleanolic acid: 1–20 mM/L; CDCA: 5–15 mM/L Duration: single intraduodenal perfusion Placebo-Controlled Location: Switzerland |
| Bile acid CDCA | Type 2 Diabetes Mellitus | Effect of Bile Acids on GLP-1 Secretion | NCT01666223 | Study Type: Interventional Enrollment: 20 participants Phase: Not Applicable Doses: 1250 mg Duration: single intraduodenal perfusion Placebo-Controlled Location: Denmark |
| Bile acid CDCA | Metabolic Syndrome | Effects of FXR Activation on Hepatic Lipid and Glucose Metabolism | NCT00465751 | Study Type: Interventional Enrollment: 30 participants Phase: 1 Doses: 500 mg Duration: 3 months Placebo-Controlled Location: Switzerland |
| Bile acid UDCA | Type 2 Diabetes Mellitus | Efficacy of Ursodeoxycholic Acid (UDCA) in Patients With Type 2 Diabetes | NCT05416580 | Study Type: Interventional Enrollment: 60 participants Phase: 3 Doses: 1500 mg Duration: 8 weeks Placebo-Controlled Location: Bosnia and Herzegovina |
| Bile acid UDCA (Ursodiol) | Obesity and Type 2 Diabetes Mellitus | Ursodiol on Insulin Sensitivity, Gastric Emptying, and Body Weight With Type 2 Diabetes on Metformin | NCT02033876 | Study Type: Interventional Enrollment: 24 participants Phase: 2 Doses: 600 mg twice daily Duration: 2 weeks Placebo-Controlled Location: USA |
| Bile acid TUDCA | Obesity | Effect of Endoplasmic Reticulum Stress on Metabolic Function (TUDCA/PBA) | NCT00771901 | Study Type: Interventional Enrollment: 101 participants Phase: Not Applicable Doses: 1750 mg/day Duration: 4 weeks Placebo-Controlled Location: USA |
| Bile acid TUDCA | Type 2 Diabetes Mellitus | Effects of Dietary Supplement Tauroursodeoxycholic Acid on Vascular Function | NCT03331432 | Study Type: Interventional Enrollment: 8 participants Phase: Not Applicable Doses: 1750 mg/day Duration: 4 weeks Location: USA |
| Oleanolic acid (olive oil) | Metabolic Diseases | Prevention With Oleanolic Acid of Insulin Resistance (PREOLIA) | NCT05049304 | Study Type: Interventional Enrollment: 22 participants Phase: Not Applicable Doses: Functional olive oil enriched in Oleanolic acid Duration: single intake Location: Spain |
| Oleanolic acid (olive oil) | Type 2 Diabetes Mellitus | Oleanolic Acid as Therapeutic Adjuvant for Type 2 Diabetes Mellitus (OLTRAD STUDY) | NCT06030544 | Study Type: Interventional Enrollment: 100 participants Phase: 2 Doses: 55 mL/day of a functional olive oil enriched in Oleanolic acid Duration: 1 year Location: Spain |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangerolamo, L.; Carvalho, M.; Barbosa, H.C.L. The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 6547. https://doi.org/10.3390/ijms26146547
Zangerolamo L, Carvalho M, Barbosa HCL. The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(14):6547. https://doi.org/10.3390/ijms26146547
Chicago/Turabian StyleZangerolamo, Lucas, Marina Carvalho, and Helena C. L. Barbosa. 2025. "The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 14: 6547. https://doi.org/10.3390/ijms26146547
APA StyleZangerolamo, L., Carvalho, M., & Barbosa, H. C. L. (2025). The Critical Role of the Bile Acid Receptor TGR5 in Energy Homeostasis: Insights into Physiology and Therapeutic Potential. International Journal of Molecular Sciences, 26(14), 6547. https://doi.org/10.3390/ijms26146547








