Abstract
Ovarian cancer (OC) is usually diagnosed at an advanced stage, contributing to its high mortality rate. The presence of concurrent bacterial infections in these patients is a common clinical observation, and the mechanisms by which this coinfection influences tumor progression are still not fully understood. This study investigates the role of polydisperse extracellular vesicles (PEVs) secreted by OC cells in response to bacterial components, aiming to elucidate a potential communication pathway between OC and the bacterial microenvironment. We stimulated a human OC cell line in vitro with a fraction of E. coli. Our results show that this bacterial stimulation significantly increases the secretion of PEVs by cancer cells. A subsequent proteomic analysis of these PEVs revealed an enrichment of proteins, including filamin A, filamin B, alpha-enolase, and heat shock cognate 71 kDa protein. In addition, the PEVs displayed protease activity (on fibronectin and gelatin) and phosphatase activity against para-nitrophenyl phosphate, indicating their capacity to alter cellular signaling. This represents a novel mechanism through which bacterial coinfection may influence the biological behavior of OC if bacteria interact with tumor cells, potentially contributing to their aggressiveness and the challenges associated with their treatment. Our work highlights the importance of studying the interplay between the tumor and its associated microbiota to better understand ovarian cancer progression and identify new therapeutic targets.
1. Introduction
Ovarian cancer (OC) is one of the most lethal gynecological cancers, often diagnosed at advanced stages due to its asymptomatic nature in the early stages [1]. Despite therapeutic advances in treatment, the five-year survival rate remains low, highlighting the urgent need for continued research into the molecular mechanisms and interactions that drive its progression [2].
The interplay between microorganisms and carcinogenesis has become a focus of current research, as creating bacteria can amplify carcinogenic effects and create a microenvironment that favors cell transformation [3]. These microorganisms may accelerate cancer progression by promoting angiogenesis and metastasis. For instance, in colorectal cancer, the bacterium Fusobacterium nucleatum has been associated with more aggressive disease and a poorer response to chemotherapy [4,5]. Additionally, bacteria can modulate the tumor microenvironment (TME) by suppressing the antitumor immune response, thereby facilitating tumor escape [6].
Bacteria such as Mycoplasma hominis, Chlamydia trachomatis, and Escherichia coli (E. coli) have been reported in OC, implying their possible implication in chronic inflammation and co-carcinogenesis [7,8]. Among biomarkers of risk for ovarian cancer [9], bacteria have been suggested that could be influencing tumor development, such as Escherichia coli [3,10,11,12]. Among the bacterial species in the microbiome of vaginal and cervical samples that have been reported as prominent or enriched in patients with OC are those belonging to the genera Dialister (Gram-negative), Prevotella (Gram-negative), Corynebacterium (Gram-positive), and Peptoniphilus (Gram-positive), and the enriched specific taxa in low- and high-grade patients compared to the benign cohort include Streptococcus infantis, Fusobacterium nucleatum, Varibaculum cambiense, Escherichia coli, Faecalibacterium prausnitzii, and Bacteroides fragilis [13]. Gram-negative bacteria, including E. coli, are among the most common pathogens found in cancer patients [14,15,16], including those with OC [17]. Notably, E. coli has been shown to modulate tumor progression across various tissues [3,10,11,12,17]. Among the toxins produced by some strains of E. coli, colibactin has been linked to colorectal cancer and DNA damage [10,18]. Recently, OmpF and OmpC from E. coli have been associated with the ability to form toxic fibrillar aggregates with amyloid properties that could contribute to cancer pathogenesis [19].
Structural components of Gram-negative bacteria, such as lipopolysaccharide (LPS), contribute to chronic inflammation and carcinogenesis [20], as well as other outer membrane proteins, such as OipA from Helicobacter pylori (H. pylori) [21]. CagA is another toxin produced by H. pylori in the gastric epithelial cells, which has a key role in gastric cancer, causing dysregulation of signaling pathways, cell proliferation, adhesion, and migration [6].
It is currently known that pathogens can interact with target cells without the need for direct contact, through the secretion of biomolecules and extracellular vesicles (EVs) that can travel through biological fluids, making it possible for them to exert an effect on distant organs and cells [22]. Gram-negative bacteria have the ability to use their own EVs called ‘outer membrane vesicles’ to carry components of the bacterial outer membrane such as LPS and OMPs, delivering the cargo to tumor cells and immune cells (carrying out interactions between receptors and ligands, PAMPs-PRRs) in the microenvironment, affecting their proliferation, migration and metastatic capacity [23].
Intercellular communication mediated by EVs has emerged as a key player in cancer pathogenesis, metastasis, and chemotherapy resistance [24,25]. EVs are heterogeneous nanostructures that are commonly spherical in shape, with at least one lipid bilayer delimiting them. They are subdivided based on size and biogenesis into (i) exosomes, (ii) microvesicles, and (iii) apoptotic bodies. Polydisperse extracellular vesicles (PEVs) are so-called based on their sizes [26,27].
Biogenesis of EVs is distinguished by their subcellular origin: exosomes (40–150 nm) originate in the endosomal system through the release of intraluminal vesicles from multivesicular bodies; microvesicles or ectosomes (100–1000 nm) bud directly from the plasma membrane; and apoptotic bodies (800–5000 nm) originate from cells undergoing apoptosis [24,28]. There are also subtypes, such as large oncosomes (0.5–10 µm) [29,30], which are a subtype of large microvesicles released by tumor cells through plasma membrane shedding and are key in cancer progression [31,32].
EVs are released by both healthy cells and tumor cells [24], carrying a wide variety of molecules, such as proteins, lipids, and nucleic acids, which facilitate the crosstalk between parental and recipient cells [31,33,34]. The EVs secreted by tumors act as cell–cell mediators [24]. Proteomic analysis, such as that of pancreatic EV samples, has identified that cancer EVs contain a distinctive protein profile, where tumor-promoting candidates such as LAMA5 (laminin subunit alpha 5), SDCBP (Syndecan Binding Protein) and TENA (Tenascin-A) were found to be overexpressed [25]. Among other molecules that have been identified as cargo in EVs involved with cancer are integrin B1 that mediates EV uptake and RNA delivery [35], fibronectin and the induction of its assembly [35,36], osteopontin involved in the signaling process [37], cytokines that modulate metabolism and tumor microenvironment [33], and Hsp70, which is commonly found in small EVs [34].
The tumor microenvironment commonly contains a high concentration of PEVs, and tumor cells have been observed to be able to secrete a higher amount of EVs compared to healthy cells; for example, more PEVs are secreted from pancreatic cancer organoids versus healthy pancreas organoids [31]. Furthermore, it has been observed that more aggressive tumor cells secrete a significantly greater quantity and variety of PEVs than less aggressive cells [24,29,30,38]. This increased secretion of PEVs is therefore considered to be a key mechanism by which cancer cells transmit their malignant characteristics and remodel the environment to their advantage [32,33].
We have previously reported that a fraction of E. coli bacteria is able to induce macrophages to release abundant PEVs, using the LMW-PTP (ACP1) protein as a marker for them and monitoring them using confocal microscopy when large PEVs are present [26]. Therefore, we have used the induction strategy, and this study focuses on investigating whether a fraction of E. coli is capable of inducing PEV secretion in SKOV-3 ovarian cancer cells, which could be reflected in the abundance of PEVs, and could even lead to the secretion of those PEVs that allow their monitoring by confocal microscopy. We have also tested the biological activity of PEVs in terms of key functions such as proteolytic activity and phosphatase enzyme activity. Our findings could offer a new perspective on the pathogenesis of this disease and the role of bacterial infections as stimulants to cancer cells with phenotypes related to greater aggressiveness, and also provide a possible alternative to obtain in vitro the varieties of PEVs that these cancer cells could be producing in vivo.
2. Results
2.1. Electrophoretic Profile of SKOV-3 Extracellular Vesicles Induced by an E. coli Fraction
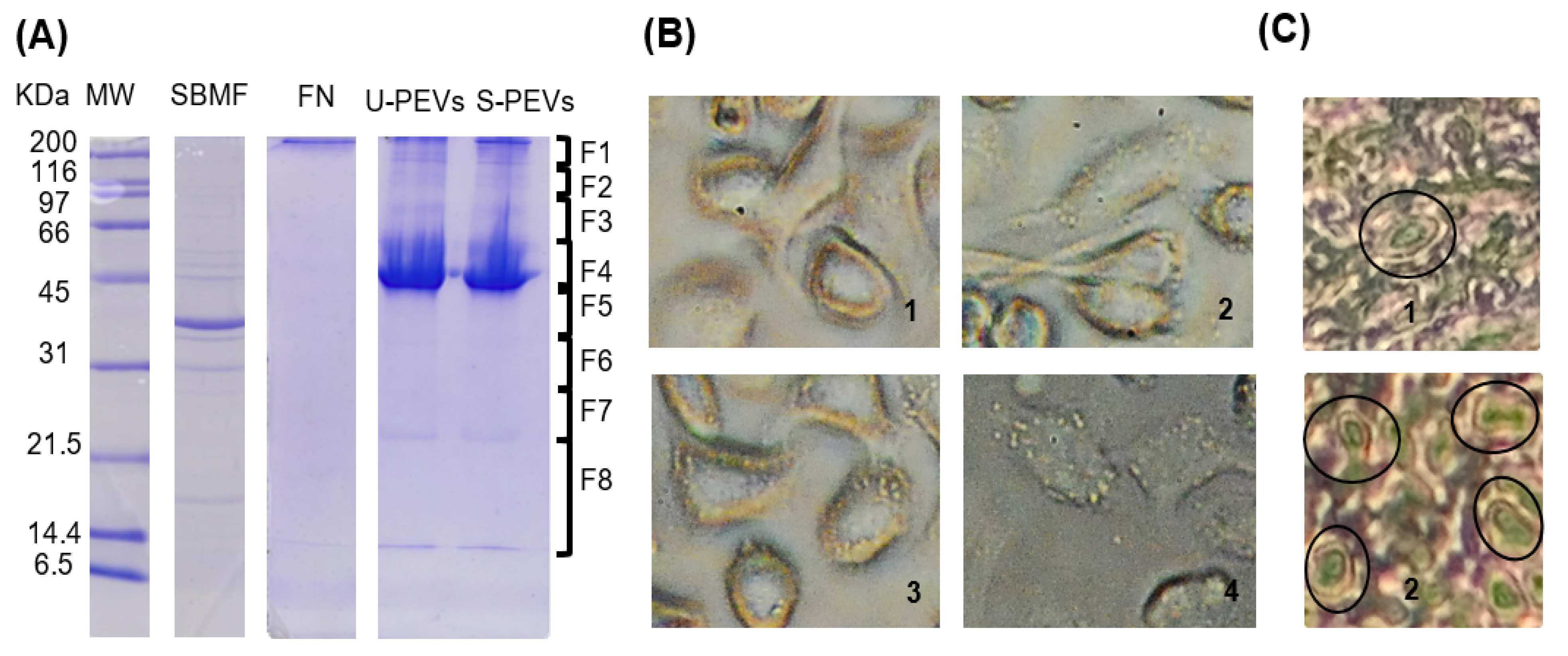
An electrophoretic analysis was performed to characterize the extracts and vesicles used in the study. In the SDS-PAGE gel (Figure 1A), column 2 shows the bacterial extract with the expected profile, which, according to the description of this fraction reported previously, is mainly composed of E. coli outer membrane proteins, which is why we named the fraction “SBMF,” and it is soluble in an SDS solution (Supplementary Materials, Table S1). The most intense band of SBMF corresponds to the 39.3 kD OmpF protein. Other proteins were also identified, such as OmpA (37.2 kD), OmpC (53.7 kD), ATP synthase subunits (atpD of 50.3 kD, atpA of 55.2 kD), maltoporin LamB (49.9 kD), several lipoproteins, and other outer membrane proteins (OmpX, Lpp, RcsF) [26]. The presence of these proteins suggests that the fraction corresponds mainly to the bacterial outer membrane, containing lipopolysaccharide (LPS) and bacterial lipids. Column 3 exhibited human fibronectin, identified as a single band above 200 kDa, which was used as an agent to stably distribute and adhere the bacterial fraction to cell culture surfaces. Column 4 showed the profile of polydisperse extracellular vesicles (PEVs) obtained from unstimulated cells, with a main band near 45 kDa, and other bands above 45 kDa and even above 200 kDa, as well as between 14 and 21.5 kDa. Column 5, with vesicles from stimulated cells, revealed a similar profile, but with more intense bands.
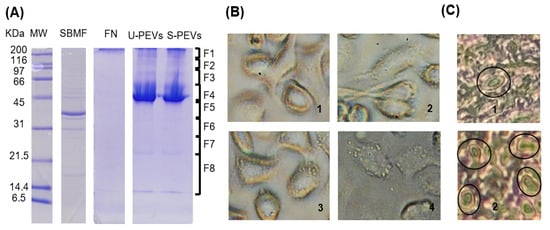
Figure 1.
A fraction of Gram-negative E. coli bacteria induces increased secretion of PEVs from SKOV-3 ovarian cancer cells. (A) The 12% SDS-PAGE results of protein profiles of U-PEVs (20 µg of unstimulated PEVs) and S-PEVs (20 µg of PEVs from stimulated cultures). SBMF (SDS-soluble E. coli membrane fraction) was placed as a substrate together with FN (human fibronectin) on the surfaces of cell cultures as a stimulant. MW: molecular weight. (B) Appearance of SKOV-3 cells after 2 h of unstimulated (1) or stimulated (2–4) culture. Multiple large EV-like particles are observed on the surface of cells in 2–4. (C) Histological sections of healthy ovary (1) and ovarian cancer (2) showing regions with several cells with large EV-like membrane protrusions (ovals), whereas these were rarely found in healthy ovary tissue.
Using an inverted light microscope (Figure 1B), the morphology of SKOV-3 cells was observed. Unstimulated cells (Figure 1(B1)) showed no vesicles, as is commonly observable by this technique. In contrast, stimulated cells (Figure 1(B2–4)) displayed a large number of extracellular vesicles anchored to their surface. Cell morphology varied, ranging from elongated cells (Figure 1(B2)), suggesting migration, to more oval and plump cells (Figure 1(B3)).
Histological analysis of ovaries (Figure 1C) revealed the presence of large extracellular vesicle-like protrusions. After searching, cells with these structures were rarely identified in the healthy ovary section (Figure 1(C1)), while in the cancerous ovary section, regions were found where multiple cells displayed these protuberances (Figure 1(C2)).
2.2. Localization and Quantification of LMW-PTP
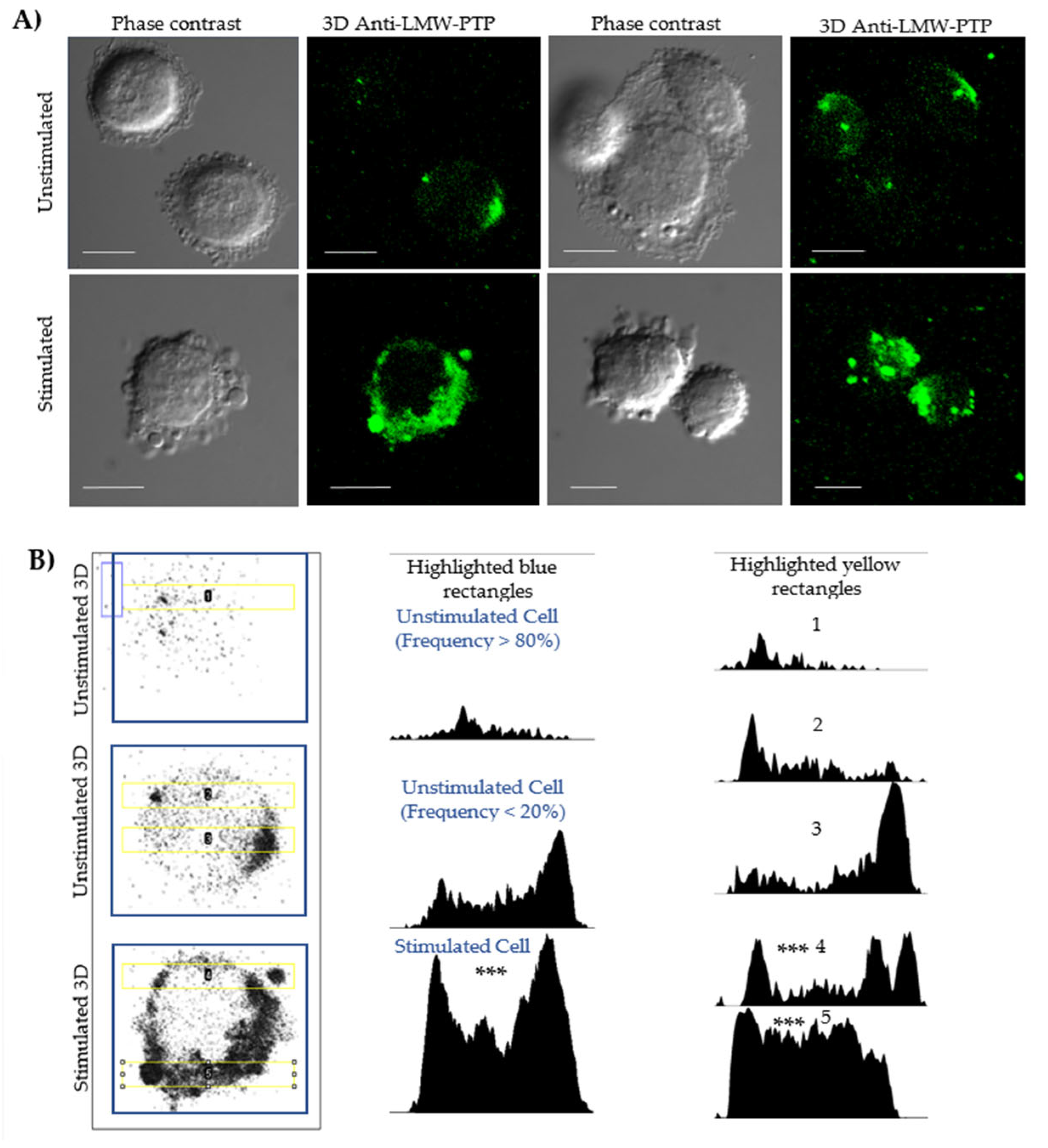
Fluorescence microscopy and densitometry analyses evaluated the localization and any increase in protein related to cells and PEVs (Figure 2, Supplementary Materials, Table S2). An antibody against LMW-PTP was used because previous studies have shown that this same bacterial fraction induces the secretion of PEVs enriched with this phosphatase in macrophages. In our model, unstimulated cells showed a distribution of LMW-PTP in the cytoplasm, close to the membrane (Figure 2B, column 1, row 1), with a polarization that is sometimes more visible in cells (Figure 2B, column 1, row 2). Densitometry of unstimulated whole cells (Figure 2B, column 2, row 1) showed that they commonly have a low LMW-PTP intensity.
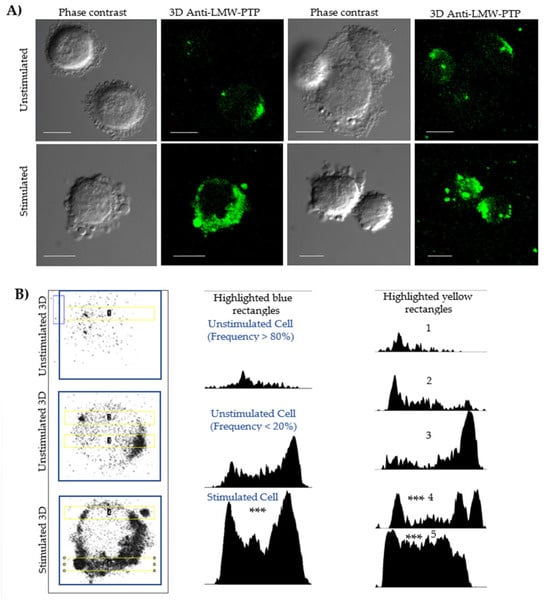
Figure 2.
Confocal microscopy and densitometry of the increase in LMW-PTP expression during stimulation of the secretion of PEVs in SKOV-3 ovarian cancer cells. (A) SKOV-3 cells were stimulated or not stimulated with SDS-SBMF-FN extract from E. coli bacteria, and after 2 h, they were analyzed by confocal microscopy. The observation of multiple large EVs increased in stimulated cells, some of which contained LMW-PTP (green—ITC); LMW-PTP labeling was also polarized in the plasma membranes and cytoplasm. Bar scale 10 µm. (B) Images of SKOV-3 cells in the left column in grayscale with 3D recognition of LMW-PTP; central column: densitometry corresponding to the amount of LMW-PTP analyzed at the cell scale (blue box); right column: densitometry of a 20 µm long rectangular region (yellow) that takes as many particles similar to large EVs and a portion with LMW-PTP labeling from the cells. It was analyzed with one-way ANOVA and the post hoc Bonferroni test, representing the difference between stimulated vs. frequent appearance of unstimulated cells (n = 4); *** p < 0.001 as significant.
In stimulated SKOV-3 cells (Figure 2A, row 2 Stimulated), a significant increase in the number of PEVs was observed. Although not all vesicles showed labeling, the intensity of LMW-PTP was significantly increased in specific populations, which was reflected in a higher signal intensity in the densitometry of stimulated cells (Figure 2B, column 2, row 3). Densitometry analyses in a 20 µm long strip (Figure 2B, column 3) validated that the peaks of highest LMW-PTP labeling intensity were coincident with the location of PEVs and the cell periphery and revealed a statistically significantly different pattern comparing the most frequent form of unstimulated cells with stimulated cells secreting PEVs.
2.3. Enzymatic Activity of EV
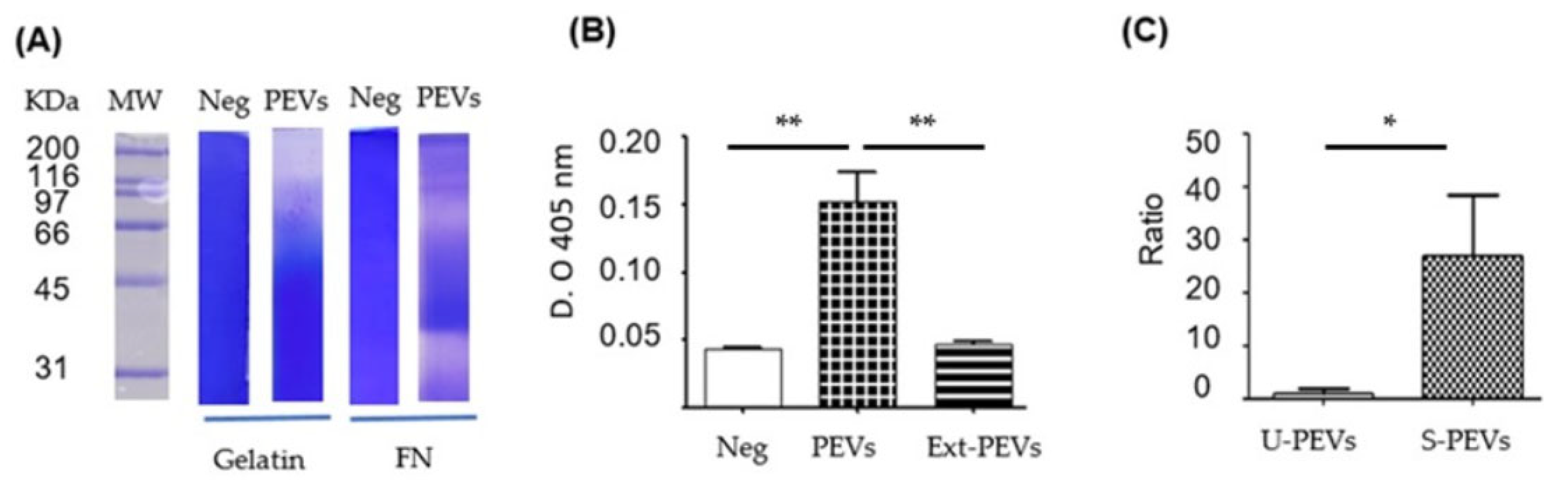
The activity of the vesicles was demonstrated using enzymatic assays (Figure 3). Zymography (Figure 3A) showed proteolytic activity against gelatin (column 3) and fibronectin (column 5). With gelatin as a substrate, intense proteolytic activity was shown at weights above 116 kDa and subtle activity between 50 and 116 kDa. With FN as a substrate, intense proteolytic activity was shown in different regions from around 31 kDa to above 200 kDa, mainly close to 31–35 and 66 kDa. The phosphatase activity assay (Figure 3B) revealed that the PEVs extract (column 3) showed no activity, similar to the negative control without PEVs, but where intact PEVs were placed, there was greater catalytic activity, indicating the possibility of phosphatase degradation in the total extracts of these PEVs.
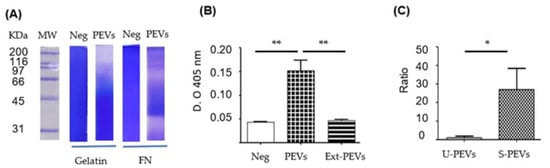
Figure 3.
Biological activity in SKOV-3 PEVs induced with FN-SBMF E. coli. (A) Zymograms of gelatin or FN as substrate, where the total extract of SKOV-3 PEVs induced with SDS-SBMF was run. (Neg) control without load. (B) Absolute phosphatase activity at 2 h with 1.6 µM pNPP; PEVs were pre-activated with DTT. (Neg) control without sample, (PEVs) unlysed PEVs, (Ext-PEVs) PEV extract by sonication. (C) PEV secretion ratio taken as Ratio 1 to the secretion detected with EVAnalyzer in unstimulated cells. U-PEVs: unstimulated; S-PEVs: stimulated. n = 3 (B) and n = 6 (C) were used for one-way Anova with Bonferroni’s post hoc test, * p < 0.05, ** p < 0.01.
2.4. Proteomic Profile of EVs
Mass spectrometry analysis identified the proteins present in PEVs obtained under induction with the membrane fraction of E. coli Gram-negative bacteria. Key proteins such as filamin A-B, alpha-enolase, heat shock cognate 71 kDa protein, tubulin, actin, ezrin, heat shock 70 kDa protein 1A/1B, pyruvate kinase PKM, vimentin, albumin, elonfation factor 1-alpha 1, peptidyl-prolyl cis trans isomerase A, annexin A1–5, heat shock protein HSP 90, phosphoglycerate kinase 1, spliceosome RNA helicase DDX39B, moesin, clathrin, peroxiredoxin 1, glyceraldehyde-3-phosphate dehydrogenase, fibronectin, alpha-2-macroglobulin, protein disulfide-isomerase A3, L-lactate dehydrogenase A chain, and radixin were found, among others with lower ‘Unused’ identification (Supplementary Materials, Table S3). The presence of LMW-PTP phosphatase was confirmed, although at a low concentration that resulted in a low ‘Unused’ value, which may be related to the degradation observed above.
3. Discussion
The role of PEVs in intercellular communication and cancer progression is a growing area of research. Traditionally, attention on EVs has focused on the smallest ones originating in multivesicular bodies and released by the endosomal pathway, known as exosomes (30–150 nm) [34,39], followed by EVs released by evagination of the plasma membrane, also known as microvesicles or ectosomes, whose dimensions can vary greatly, from a few tens of nm to several micrometers [28]. In recent years, it has been shown that cancer cells can release both small and large EVs of several micrometers in size, with characteristics specific to cancer cells, which is why they have also been given the names oncosomes and large oncosomes, due to the role of these populations of PEVs in each group within cancer biology [28,30]. In addition, these samples of oncosomes, mainly large oncosomes or PEVs, can provide markers for liquid biopsies [27,29].
As mentioned in the introduction, some microorganisms can trigger cancer or aggravate it if it is already present when they infect people. These include bacteria, among which species have been found that can enhance carcinogenic effects and create a microenvironment more favorable to cell transformation [3], increasing cancer progression and metastasis, as is the case with Fusobacterium nucleatum in colorectal cancer [4,5,40], Helicobacter pylori in gastric cancer [6], or increasing inflammatory damage as with Mycoplasma hominis and Chlamydia trachomatis in ovarian cancer [7]. Escherichia coli is among the bacterial species in the microbiome that have been suggested to influence cancer development in various tissues [10,12], being among the most prevalent Gram-negative bacteria in cancer patients [14,15,16]. Among the molecules and outer membrane proteins of Gram-negative bacteria that aggravate cancer or favor cancer cells in the microenvironment are lipopolysaccharide (LPS) [20] and outer membrane proteins such as OipA [21], OmpF, OmpC [19].
Our results demonstrate that the fraction we used from the outer membrane of Gram-negative E. coli bacteria contains a mixture of molecules that act as a potent inducer of EV secretion in SKOV-3 ovarian cancer cells. We previously described and reported this fraction as a strong inducer of the secretion of a wide variety of polydisperse EVs (PEVs) secreted by macrophages, which contain various porins, among other proteins, and are suggested to contain lipopolysaccharide (LPS) and lipids [26]. It has been documented that LPS is capable of inducing an increase in small EVs (exosomes) from human cells [37]. Likewise, subcellular fractions such as vesicles secreted by bacteria (called ‘outer membrane vesicles’) from Porphyromonas gingivalis [41] and Helicobacter pylori [42], which contain porins and LPS, are capable of inducing the general secretion of EVs into host cells. Furthermore, in our case, human fibronectin (FN), which was used to stabilize the bacterial stimulus, may have contributed to the response by acting as an adhesion substrate that would facilitate the interaction between SKOV-3 cells and the bacterial fraction of E. coli, helping to observe stimulated cells secreting PEVs at low doses. Then, in the membrane fraction that we have used for the stimulation of cells to secrete EVs, there are components that separately and in combination have been reported as inducers of EV secretion, which suggests that we have obtained a stimulant with components that could be acting with synergistic effects, and in turn suggests that these bacterial components could stimulate robust secretion of EVs to various types of tumor cells, a characteristic that is associated with aggressive cancer, which opens the possibility to take it for future studies.
A relevant finding of our study is the presence of LMW-PTP phosphatase in some EV populations, which increase in quantity after cell stimulation. This is particularly relevant because the previous study with the same bacterial fraction showed that it induces the production of LMW-PTP-enriched EVs in macrophages and increases the expression of this protein in both the cytoplasm and plasma membrane [26]. The detection of LMW-PTP in some populations or subpopulations of SKOV-3 EVs suggests that the induction of phosphatase and its packaging into vesicles is a conserved response mechanism in different cell types for some EV populations, not limited to immune system cells. This reasoning is supported by the report of the identification of LMW-PTPs in small EVs from colorectal cancer cells [43].
Analysis of the enzymatic activity of EVs revealed a crucial aspect the loss of phosphatase activity when lysing EVs. This phenomenon, coupled with the low ‘Unused’ LMW-PTP in mass spectrometry, suggests that proteases, also encapsulated in EVs, degrade both LMW-PTPs and other enzymes once the vesicular membrane is broken or when the integrity of the EVs is lost. Therefore, these EVs are not simply a transport vehicle, but a protective compartment that maintains an order that allows enzymes susceptible to degradation to be maintained in the extracellular microenvironment. The self-degradation of proteins in total extracts from some EV populations by their own proteases is an event that has been documented [44].
The detailed proteomic profile of EVs shows the presence of proteins associated with the cytoskeleton and vesicular biogenesis, such as actin, vimentin, annexins, and clathrin, supporting the idea that a portion of the mixture of EV-like particles are formed by the evagination of ectosomes or microvesicles from the plasma membrane. The identification of glycolytic enzymes (such as pyruvate kinase and alpha-enolase) and heat shock proteins (HSP70/90) in EVs suggests a possible state of metabolic stress in SKOV-3 cells, as has been observed in other cancer cells [45]. The presence of fibronectin in EVs could be explained by the internalization of substrate fibronectin by SKOV-3 cells, which loaded the glycoprotein into EVs, or by the fact that once secreted, EVs captured it from the cell culture surface where it was located together with the stimulus. The uptake and incorporation of proteins from the extracellular medium into EVs have been previously documented [35,36]. Additionally, the detection of albumin in EVs can be explained by the fact that the cells were previously cultured in a medium with fetal bovine serum, internalized and stored albumin, and subsequently released a portion of it by encapsulating it in EVs during stimulation in a serum-free medium.
The co-presence of proteases and phosphatases in EVs suggests a complex bioactive machinery whose purpose appears to be both the modification of the tumor microenvironment and the modification of the basal state of the host cells with which they come into contact. In addition to LMW-PTPs, other enzymes could be contributing to the phosphatase activity observed on para-nitrophenol phosphate, such as alkaline phosphatase.
Our findings, derived from the in vitro model we used, are significantly reinforced by observations of histological sections of ovarian tissue using an inverted microscope, where we distinguished the presence of cells with protrusions similar in appearance to the large EVs we observed anchored to the surface of stimulated SKOV-3 cells. This was detected rarely and with difficulty in healthy ovaries, while it was easier to find several cells with these characteristics in samples of ovarian cancer diagnosed as serous papillary cystadenocarcinoma, suggesting that this phenomenon of large EV secretion (large oncosomes) occurs naturally in vivo, increasing in tumor tissue. This parallelism is vital, as it indicates that vesicles are not merely a cell culture artifact, but could play a role in the biology of ovarian cancer, possibly in tumor progression and dissemination. To date, few studies have focused on evaluating or reporting the possibility of observing large oncosomes in histological sections. One such study corresponds to prostate cancer, in which these large EVs were only observed in tumor tissue sections, and not in healthy tissue [38], and in lung tumor tissue [30].
This study provided a strategy for studying a wide variety of EVs in vitro, from small to very large, and thus we use the term PEVs. Although our study was limited to an ovarian cancer cell line, it opens the possibility of investigating the secretion behavior of PEVs by other ovarian cancer lines and other cancer types, using the bacterial fraction as an inducer, and thus conducting research studies in a context related to patients suffering from cancer and bacterial coinfections. In our working group, we will continue to investigate the induction of EV secretion from cancer cells in response to bacterial components, taking advantage of the bacterial fraction we have been using as a substrate, focusing on a larger number of cell lines for ovarian cancer and other types of cancer such as leukemia. We will also focus on further investigating the biological activity of EVs emanating from cancer cells, as well as their potential uses with prospects that benefit future patients.
4. Materials and Methods
4.1. Human Samples for Assays
All samples were taken during the hospital stay. The study was approved by the Ethics Committee of the Hospital Regional de Alta Especialidad de Ixtapaluca, “IMSS-Bienestar” (NR-017-2025).
4.2. Cell Culture
The SKOV-3 (ATCC-HTB-77) reference cell line was used. In brief, cell culture was performed in McCoy’s 5A medium (Corning, 10-050-CVR) at 37 °C and 5% CO2. Furthermore, for continuous maintenance of the medium, it was supplemented with 10% fetal bovine serum (FBS) (Corning, 35-010-CV) and 1% penicillin/streptomycin (PAA, P11-010), as described previously [46]. BFS was not used during polydisperse extracellular vesicle (PEV) secretion stimulations.
4.3. Extraction of Escherichia coli Fraction
Briefly, Gram-negative E. coli strain BL21(DE3) pLysS bacteria were cultured in 300 mL of Luria–Bertani (LB) medium, and a soluble bacterial fraction was obtained in 15 mL of 10 mM Tris -HCl pH 8.0, 10 mM MgCl2, and 2% SDS (fraction called SDS-SBMF). As described in the method modified by Sierra-López et al. 2025 [26], which was dialyzed in a cold room (temperature below 15 °C) using 15,000-Dalton membranes and 4 L of milliQ per sample, ending dialysis after 72 h.
4.4. Standardization of SKOV-3 PEV (Short and Large) Secretion and Isolation
In brief, glass coverslips or plastic surfaces coated with a mixture of 0.250 µg of human fibronectin (FN) and 20 ng of dialyzed SDS-SBMF protein per cm2 were used. The surfaces were prepared and the FN obtained as indicated by Sierra-López et al. 2025 [26]. No stimulant was placed as a negative control. SKOV-3 cells were deposited at a ratio of 4 × 104/cm2 together with sufficient McCoy’s 5A medium with L-glutamine (Corning, 10–50-CV, USA) to coat the cultures without FBS. The cells were monitored under an inverted microscope, and after 120 min of interaction, the supernatant with the EVs was collected or fixed with 4% paraformaldehyde in PBS for 45 min for confocal microscopy assays. For SDS-PAGE, zymogram, and enzyme activity assays, EVs were isolated from cultures in 10 cm diameter Petri dishes containing 15 mL of initial medium, collecting 14 mL of supernatant after 2 h of interaction; the contaminating cells were then discarded together with 500 µL of contiguous supernatant by centrifuging at 120× g for 5 min in 15 mL tubes. The VE mix was precipitated with 0.2 mM ZnSO4 at 16,000× g for 20 min using 1.5 mL conical tubes (Centrifuge Eppendorf 5415C, EE.UU.).
4.5. SDS-PAGE and Western Blotting
The samples were resolved on 12% SDS-PAGE [47] and visualized by Coomassie blue or electrophoretically transferred to nitrocellulose membranes at 80 V. A total of 8 µL of β-mercaptoethanol, SDS-PAGE loading buffer, and cOmpleteTM protease inhibitor (Roche, Sigma-Aldrich) was added to each sample, which was then immediately boiled in a water bath for 8 min, followed by the addition of 8 µL of β-mercaptoethanol and basification using a 1M NaOH stock solution, as indicated in Sierra-López et al. 2021 [48]. Nitrocellulose Western blots (WBs) were incubated with mouse anti-human-LMW-PTP polyclonal antibodies at a dilution of 1:1000 (obtained from Sierra-López et al. 2025 [26]). Goat anti-mouse IgG (H + L) alkaline phosphatase conjugate (Invitrogen, Camarillo, CA, USA) at a dilution of 1:4000 in TBST was used as the secondary antibody. WBs were developed with the NBT/BCIP kit (Invitrogen, Camarillo, CA, USA).
4.6. Confocal Microscopy
SKOV-3 cells, either unstimulated or stimulated for 120 min with FN-SDS-SBMF on glass coverslips, were fixed with 4% p-formaldehyde in PBS for 45 min at 37 °C; they were gently washed with PBS and processed at room temperature. Permeabilization was performed with 0.0015% SDS and 0.0006% Triton X-100 in PBS for 8 min as indicated in Sierra-López et al. 2025 to reduce EV decoupling [26]. Samples were blocked with 5% BFS, washed with PBS, and incubated with anti-human-LMW-PTPs (1:300) in PBS for 1 h. They were then washed and incubated with FITC-conjugated anti-mouse as a secondary antibody (Zymed 1:300) for 1 h. Samples were preserved using Vectashield Antifade Reagent (Vector, Newark, CA, USA), examined with a Carl Zeiss LMS 700 confocal microscope, and processed with ZEN Blue 3.5 (Zeiss, White Plains, NY, USA). Green color was used to represent LMW-PTP loaded in EVs in the analysis with ImageJ2 (version 2.16.0) EVAnalyzer plugin (ImageJ/Fiji).
4.7. Liquid Chromatography and MALDI-MS/MS
Extracts from stimulated SKOV-3 cell EVs were resolved on 12% SDS-PAGE, and fragments between 10 and 200 kDa were processed at the Genomics, Proteomics, and Metabolomic Service of LaNSE (CINVESTAV IPN), according to the modified Shevchenko protocol [49], the method modified by Barrera-Rojas et al. 2018 [50] and as worked on by Sierra-López et al. 2025 [26]. The results of MS/MS spectra were compared using Protein Pilot v.2.0.1 (ABSciex, Framingham, MA, USA) and the Paragon algorithm [51] against Homo sapiens (Uniprot, 67,493 protein sequences database). The detection threshold was 1.3 to ensure 95% confidence. The identified proteins were grouped using the ProGroup algorithm to minimize redundancy. The profile and protein composition of the E. coli SDS-SBMF bacteria fraction were published in Sierra-López et al. 2025 [26].
4.8. Proteolytic Activity
Zymograms were performed to analyze the proteolytic activity of the EV mix using gelatin and fibronectin as substrates. EVs were prepared as previously described, avoiding heat denaturation and without β-ME, and were suspended with the standard loading buffer (glycerol, 0.5 M Tris-HCl, pH 6.8, SDS). Subsequently, 10 µg of EVs was separated by electrophoresis on standard 8% SDS–polyacrylamide gels (distilled water; acrylamide/bisacrylamide; 1.5 M Tris-HCl, pH 8.8; SDS; ammonium persulfate; and TEMED) copolymerized with 0.4% pork skin gelatin (Sigma G2500, Sigma-Aldrich, St. Louis, MO, USA) or 100 µg human fibronectin, and electrophoretic migration was performed at 4 °C without a reducing agent. The gels were washed with 1% Triton X-100 for 1 h, then incubated overnight at 37 °C in a developing buffer (50 mM Tris-HCl, 10 mM CaCl2, pH 7.0). The zymograms were stained with Coomassie blue; unstained areas indicated proteolytic activity.
4.9. Phosphatase Activity of Polydisperse Extracellular Vesicles (PEVs) of SKOV-3
The endpoint assay for phosphatase activity of SKOV-3 PVEs was performed using p-nitrophenyl phosphate (pNPP) (Sigma) as a substrate. A mixture was prepared with 60 µL of 200 mM sodium acetate pH 6.0 and 25 µL of 100 mM dithiothreitol (DTT). Approximately 5 µg of PEVs from samples obtained from stimulated supernatants without using ZnSO4 (to avoid interference) was added. MilliQ water was added to a total volume of 120 µL, followed by incubation for 10 min at room temperature. The mixture was then incubated with 2 µL of 100 mM pNPP and mixed by vortexing for 1 min. The mixture was then incubated at 37 °C. A total of 100 µL of reaction solution was taken after 2 h, and the reaction was stopped with 10 µL of 2 M NaOH to read the absorbance at 405 nm in 96-well plates. The PEV samples were added without breaking them or in the extract after 1 min of sonication in an ice bath. We detected that LMW-PTP was degraded in PEV extracts, so the phosphatase enzyme activity of the unlysed PEVs was tested.
4.10. Statistical Analysis
Confocal analysis of PEV secretion by SKOV-3 cells was performed using Fiji and the EV Analyzer plugin (version 8.1.3 beta). Function: PEV count; identical thresholds: 10; threshold method: ‘Li’; min circularity: 0.5; filter type: PEV-GFP (to FITC channel); particle size range: 1–999,999. Data was analyzed using GraphPad Prism 5 Software: One-way Anova Bonferroni post-test.
5. Conclusions
In summary, our findings suggest that the outer membrane fraction of the Gram-negative bacteria E. coli is a potent inductor of polydisperse extracellular vesicle (PEV) secretion in the SKOV-3 ovarian cancer cell line, a mechanism potentially amplified by the synergistic effect of bacterial components like LPS and porins. The PEVs function as protective compartments that encapsulate and maintain a complex bioactive machinery of proteases and phosphatases (including LMW-PTP), which, upon release, actively modify the tumor microenvironment by degrading the extracellular matrix (e.g., fibronectin). This modification correlates with an elongated and potentially more migratory cellular phenotype. The detection of structures resembling large PEVs (large oncosomes) in ovarian cancer tissue, in contrast to healthy tissue, critically validates the in vivo relevance of this phenomenon. However, it must be noted that these findings are currently limited to the SKOV-3 cell line. Collectively, these data suggest that bacterial coinfections can exacerbate cancer progression and dissemination by robustly stimulating PEV secretion, underscoring the importance of this communication axis in tumor biology and opening avenues for investigating other cancer types.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262110653/s1.
Author Contributions
Conceived and designed the experiments: F.S.-L., J.C.F.-H., M.S.-M., J.L.R.-E., P.T.-R. and G.A.-A. Performed the experiments: F.S.-L., J.C.F.-H., M.S.-M., L.B.-P., V.I.H.-R. and S.B.-H. Analyzed the data: F.S.-L., J.L.R.-E., M.S.-M., P.T.-R., V.I.-V., G.A.-A., S.B.-H., J.C.B.-A. and D.M.-E. Contributed reagents/material/analysis tools: J.L.R.-E., P.T.-R., M.S.-M., F.S.-L. and G.A.-A. Wrote the paper: F.S.-L., M.S.-M., J.L.R.-E. and J.C.F.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Consejo Nacional de Humanidades Ciencias y Tecnologías (CONAHCyT), Mexico (Grant 104119), to J.L.R.-E. This was part of the doctoral thesis work of F.S.-L. At CINVESTAV-IPN, for which he was the beneficiary of a Ph.D. fellowship from CONAHCyT, México (#362402).
Institutional Review Board Statement
The research protocol for obtaining anti-LMW-PTP immune sera from mice was approved by the Internal Committee for the Care and Use of Laboratory Animals (CICUAL) and Biosafety, Genetically modified organism and Pathogens Committee of the Centro de Investigación y de Estudios Avanzados (CINVESTAV) with registration number 0216-16; approval date: 17 November 2016. The study in humans was approved by the Research, Biosafety and Ethics Committee of the Hospital Regional de Alta Especialidad de Ixtapaluca. IMSS-Bienestar approval with registration number NR-017-2025.
Informed Consent Statement
All participants provided written informed consent prior to enrollment in the study. The study in humans was approved by the Research, Biosafety and Ethics Committee of the Hospital Regional de Alta Especialidad de Ixtapaluca. IMSS-Bienestar approval with registration number NR-017-2025.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Thanks to Emmanuel Ríos Castro for assistance in the protein detection using MALDI Ekspot and MALDI-TOF/TOF 4800 plus mass spectrometry (MS) in the UGPM LaNSE CINVESTAV. Thanks to Magdalena Miranda Sánchez for her technical assistance in confocal microscopy and Juan Carlos Osorio Trujillo for his assistance with cell line maintenance.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| EVs | Extracellular vesicles |
| PEVs | Polydisperse extracellular vesicles |
| LPS | Lipopolysaccharide |
| SKOV-3 | Human ovarian cancer cell line |
| SBMF-SDS | SDS-soluble bacterial membrane fraction |
| LMW-PTP | Low-molecular-weight protein tyrosine phosphatase |
| MALDI-MS/MS | Matrix-assisted laser desorption/ionization mass spectrometry |
| OC | Ovarian cancer |
| FN | Fibronectin |
| Omp | Outer membrane protein |
References
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Francescone, R.; Hou, V.; Grivennikov, S.I. Microbiome, inflammation, and cancer. Cancer J. 2014, 20, 181–189. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Li, R.; Shen, J.; Xu, Y. Fusobacterium nucleatum and Colorectal Cancer. Infect. Drug Resist. 2022, 15, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Wizenty, J.; Sigal, M. Helicobacter pylori, microbiota and gastric cancer—Principles of microorganism-driven carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 296–313. [Google Scholar] [CrossRef]
- Hosseininasab-Nodoushan, S.A.; Ghazvini, K.; Jamialahmadi, T.; Keikha, M.; Sahebkar, A. Association of Chlamydia and Mycoplasma infections with susceptibility to ovarian cancer: A systematic review and meta-analysis. Semin. Cancer Biol. 2022, 86, 923–928. [Google Scholar] [CrossRef]
- Koushik, A.; Grundy, A.; Abrahamowicz, M.; Arseneau, J.; Gilbert, L.; Gotlieb, W.H.; Lacaille, J.; Mes-Masson, A.M.; Parent, M.E.; Provencher, D.M.; et al. Hormonal and reproductive factors and the risk of ovarian cancer. Cancer Causes Control. 2017, 28, 393–403. [Google Scholar] [CrossRef]
- Chalif, J.; Wang, H.; Spakowicz, D.; Quick, A.; Arthur, E.K.; O’Malley, D.; Chambers, L.M. The microbiome and gynecologic cancer: Cellular mechanisms and clinical applications. Int. J. Gynecol. Cancer 2024, 34, 317–327. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, E.; Muhlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B.; et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.P.; Redinbo, M.R.; Bultman, S.J. The role of the microbiome in cancer development and therapy. CA Cancer J. Clin. 2017, 67, 326–344. [Google Scholar] [CrossRef]
- Davoody, S.; Tayebi, Z.; Sharif-Zak, M.; Azizmohammad Looha, M.; Mortezaei Ferizhandy, N.; Sadeghi Mofrad, S.; Houri, H. Assessing the role of Escherichia coli and Klebsiella pneumoniae in colorectal cancer oncogene expression: Insights from microbial colonization phenotypes. Mol. Biol. Rep. 2025, 52, 828. [Google Scholar] [CrossRef]
- Asangba, A.E.J. Chen, K.M. Goergen, M.C. Larson, A.L. Oberg, J. Casarin, F. Multinu, S.H. Kaufmann, A. Mariani, N. Chia; et al. Diagnostic and prognostic potential of the microbiome in ovarian cancer treatment response. Sci. Rep. 2023, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Guddati, A.K. Infections in Hospitalized Cancer Patients. World J. Oncol. 2021, 12, 195–205. [Google Scholar] [CrossRef]
- Murshed, I.A.S.; Zhao, L.; Zhang, W.; Yin, Y.; Li, Y.; Peng, Y.; Chen, H.; Wu, X. Bloodstream infections in pediatric hematology/oncology patients: A single-center study in Wuhan. Front. Cell Infect. Microbiol. 2024, 14, 1480952. [Google Scholar] [CrossRef]
- Daga, A.P.; Koga, V.L.; Soncini, J.G.M.; de Matos, C.M.; Perugini, M.R.E.; Pelisson, M.; Kobayashi, R.K.T.; Vespero, E.C. Escherichia coli Bloodstream Infections in Patients at a University Hospital: Virulence Factors and Clinical Characteristics. Front. Cell Infect. Microbiol. 2019, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mo, J.; Huang, W.; Bao, Y.; Luo, X.; Yuan, L. The ovarian cancer-associated microbiome contributes to the tumor’s inflammatory microenvironment. Front. Cell. Infect. Microbiol. 2024, 14, 1440742. [Google Scholar] [CrossRef] [PubMed]
- Severino, A.; Ianiro, G. Early-Life Colibactin Exposure Is Associated With Early-Onset Colorectal Cancer Occurrence. Gastroenterology 2025, 169, 1305. [Google Scholar] [CrossRef]
- Belousov, M.V.; Kosolapova, A.O.; Fayoud, H.; Sulatsky, M.I.; Sulatskaya, A.I.; Romanenko, M.N.; Bobylev, A.G.; Antonets, K.S.; Nizhnikov, A.A. OmpC and OmpF Outer Membrane Proteins of Escherichia coli and Salmonella enterica Form Bona Fide Amyloids. Int. J. Mol. Sci. 2023, 24, 15522. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Chen, Z.; Chen, K.; Liao, F.T.; Chung, C.E.; Liu, X.; Lin, Y.C.; Keohavong, P.; Leikauf, G.D.; Di, Y.P. Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Res. 2021, 81, 144–157. [Google Scholar] [CrossRef]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Asano-Inami, E.; Yokoi, A.; Yoshida, K.; Taki, K.; Kitagawa, M.; Suzuki, K.; Uekusa, R.; Nagao, Y.; Yoshikawa, N.; Niimi, K.; et al. Proteomic Profiling of Bacterial Extracellular Vesicles for Exploring Ovarian Cancer Biomarkers. J. Extracell. Biol. 2025, 4, e70073. [Google Scholar] [CrossRef]
- Bhanu, P.; Godwin, A.K.; Umar, S.; Mahoney, D.E. Bacterial Extracellular Vesicles in Oncology: Molecular Mechanisms and Future Clinical Applications. Cancers 2025, 17, 1774. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef]
- Buenafe, A.C.; Dorrell, C.; Reddy, A.P.; Klimek, J.; Marks, D.L. Proteomic analysis distinguishes extracellular vesicles produced by cancerous versus healthy pancreatic organoids. Sci. Rep. 2022, 12, 3556. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Lopez, F.; Iglesias-Vazquez, V.; Baylon-Pacheco, L.; Rios-Castro, E.; Osorio-Trujillo, J.C.; Lagunes-Guillen, A.; Chavez-Munguia, B.; Hernandez, S.B.; Acosta-Altamirano, G.; Talamas-Rohana, P.; et al. A Fraction of Escherichia coli Bacteria Induces an Increase in the Secretion of Extracellular Vesicle Polydispersity in Macrophages: Possible Involvement of Secreted EVs in the Diagnosis of COVID-19 with Bacterial Coinfections. Int. J. Mol. Sci. 2025, 26, 3741. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, M.; Zucal, C.; Modelska, A.; Pesce, I.; Scarduelli, G.; Potrich, C.; Lunelli, L.; Pederzolli, C.; Pavan, P.; la Marca, G.; et al. Ultrasensitive detection of cancer biomarkers by nickel-based isolation of polydisperse extracellular vesicles from blood. EBioMedicine 2019, 43, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: Emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef]
- Pezzicoli, G.; Tucci, M.; Lovero, D.; Silvestris, F.; Porta, C.; Mannavola, F. Large Extracellular Vesicles—A New Frontier of Liquid Biopsy in Oncology. Int. J. Mol. Sci. 2020, 21, 6543. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; You, S.; Spinelli, C.; Morley, S.; Zandian, M.; Aspuria, P.J.; Cavallini, L.; Ciardiello, C.; Reis Sobreiro, M.; Morello, M.; et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015, 6, 11327–11341. [Google Scholar] [CrossRef]
- Lopez, K.; Lai, S.W.T.; Lopez Gonzalez, E.J.; Davila, R.G.; Shuck, S.C. Extracellular vesicles: A dive into their role in the tumor microenvironment and cancer progression. Front. Cell Dev. Biol. 2023, 11, 1154576. [Google Scholar] [CrossRef]
- Majood, M.; Rawat, S.; Mohanty, S. Delineating the role of extracellular vesicles in cancer metastasis: A comprehensive review. Front. Immunol. 2022, 13, 966661. [Google Scholar] [CrossRef]
- Lucchetti, D.; Ricciardi Tenore, C.; Colella, F.; Sgambato, A. Extracellular Vesicles and Cancer: A Focus on Metabolism, Cytokines, and Immunity. Cancers 2020, 12, 171. [Google Scholar] [CrossRef]
- Javeed, N.; Mukhopadhyay, D. Exosomes and their role in the micro-/macro-environment: A comprehensive review. J. Biomed. Res. 2017, 31, 386–394. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; de Voogt, W.S.; Tognoli, M.L.; van der Werff, L.; Gitz-Francois, J.J.; Seinen, C.W.; Schiffelers, R.M.; de Jong, O.G.; Vader, P. Integrin beta 1 and fibronectin mediate extracellular vesicle uptake and functional RNA delivery. J. Biol. Chem. 2025, 301, 108305. [Google Scholar] [CrossRef]
- Sung, B.H.; Emmanuel, M.; Gari, M.K.; Guerrero, J.F.; Virumbrales-Munoz, M.; Inman, D.; Krystofiak, E.; Rapraeger, A.C.; Ponik, S.M.; Weaver, A.M. Exosomes are specialized vehicles to induce fibronectin assembly. bioRxiv 2025. [Google Scholar] [CrossRef]
- Bai, G.; Matsuba, T.; Niki, T.; Hattori, T. Stimulation of THP-1 Macrophages with LPS Increased the Production of Osteopontin-Encapsulating Exosome. Int. J. Mol. Sci. 2020, 21, 8490. [Google Scholar] [CrossRef]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, L.; Zou, X.; Wang, J.; Zhong, J.; Zhong, T. Extracellular vesicles in type 2 diabetes mellitus: Key roles in pathogenesis, complications, and therapy. J. Extracell. Vesicles 2019, 8, 1625677. [Google Scholar] [CrossRef]
- Duizer, C.; Salomons, M.; van Gogh, M.; Grave, S.; Schaafsma, F.A.; Stok, M.J.; Sijbranda, M.; Kumarasamy Sivasamy, R.; Willems, R.J.L.; de Zoete, M.R. Fusobacterium nucleatum upregulates the immune inhibitory receptor PD-L1 in colorectal cancer cells via the activation of ALPK1. Gut Microbes 2025, 17, 2458203. [Google Scholar] [CrossRef]
- Elsayed, R.; Elashiry, M.; Liu, Y.; El-Awady, A.; Hamrick, M.; Cutler, C.W. Porphyromonas gingivalis Provokes Exosome Secretion and Paracrine Immune Senescence in Bystander Dendritic Cells. Front. Cell Infect. Microbiol. 2021, 11, 669989. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Mao, L.; Yao, Y. Helicobacter pylori outer membrane vesicles and infected cell exosomes: New players in host immune modulation and pathogenesis. Front. Immunol. 2024, 15, 1512935. [Google Scholar] [CrossRef]
- Clerici, S.P.; Peppelenbosch, M.; Fuhler, G.; Consonni, S.R.; Ferreira-Halder, C.V. Colorectal Cancer Cell-Derived Small Extracellular Vesicles Educate Human Fibroblasts to Stimulate Migratory Capacity. Front. Cell Dev. Biol. 2021, 9, 696373. [Google Scholar] [CrossRef]
- Fochtman, D.; Marczak, L.; Pietrowska, M.; Wojakowska, A. Challenges of MS-based small extracellular vesicles proteomics. J. Extracell. Vesicles 2024, 13, e70020. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Alberto-Aguilar, D.R.; Hernández-Ramírez, V.I.; Osorio-Trujillo, J.C.; Gallardo-Rincón, D.; Toledo-Leyva, A.; Talamás-Rohana, P. PHD finger protein 20-like protein 1 (PHF20L1) in ovarian cancer: From its overexpression in tissue to its upregulation by the ascites microenvironment. Cancer Cell Int. 2022, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Lopez, F.; Baylon-Pacheco, L.; Vanegas-Villa, S.C.; Rosales-Encina, J.L. Characterization of low molecular weight protein tyrosine phosphatases of Entamoeba histolytica. Biochimie 2021, 180, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Barrera-Rojas, J.; de la Vara, L.G.; Rios-Castro, E.; Leyva-Castillo, L.E.; Gomez-Lojero, C. The distribution of divinyl chlorophylls a and b and the presence of ferredoxin-NADP(+) reductase in Prochlorococcus marinus MIT9313 thylakoid membranes. Heliyon 2018, 4, e01100. [Google Scholar] [CrossRef]
- Shilov, I.V.; Seymour, S.L.; Patel, A.A.; Loboda, A.; Tang, W.H.; Keating, S.P.; Hunter, C.L.; Nuwaysir, L.M.; Schaeffer, D.A. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell Proteom. 2007, 6, 1638–1655. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).