Cannabinoid Receptor Type 2 Agonist JWH-133 Stimulates Antiviral Factors and Decreases Proviral, Inflammatory, and Neurotoxic Proteins in HIV-Infected Macrophage Secretome
Abstract
1. Introduction
2. Results
2.1. HIV Stimulates the Secretion of CATB by HIV-MDM, While JWH-133 Stimulates the Secretion of Proteins Related to Metabolism, Cell Organization, and Antiviral and Stress Responses
2.2. HIV Infection and JWH−133 Treatment in HIV-Infected MDMs Lead to a Unique Profile of Differentially Expressed Proteins in Supernatants
2.3. Proteomics Pathway Analysis of Differentially Expressed Proteins Reveals That HIV Infection Upregulated the Secretion of Inflammatory and Neurotoxic Pathways (e.g., NF-κB, MHC Class I Processing), Whereas JWH-133 Treatment Downregulated These Responses
3. Discussion
4. Materials and Methods
4.1. Macrophage Isolation, HIV-1ADA Infection, Treatments with JWH-133 Agonist, and Collection of Serum-Free Supernatants
4.2. Preparation of MDM Serum-Free Supernatants for Protein ID and TMT Labeling
4.3. Protein ID and TMT Labeling
4.4. Mass Spectrometry
4.5. Protein Identification and Quantitative Analyses
4.6. Bioinformatics and Statistical Analyses
4.7. Ingenuity Pathway Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AIDS, Crisis and the Power to Transform: UNAIDS Global AIDS Update 2025; UNAIDS: Geneva, Switzerland, 2025.
- Zenebe, Y.; Necho, M.; Yimam, W.; Akele, B. Worldwide Occurrence of HIV-Associated Neurocognitive Disorders and Its Associated Factors: A Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 814362. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, W.; Li, H.; Shi, Y.; Zhao, L.; Lu, Y.; Wei, X.; Li, H. Decoding HIV-associated neurocognitive disorders: A new perspective from multimodal connectomics. Front. Neurol. 2025, 16, 1467175. [Google Scholar] [CrossRef]
- Adhikary, K.; Banerjee, A.; Sarkar, R.; Banerjee, R.; Chowdhury, S.R.; Ganguly, K.; Karak, P. HIV-associated neurocognitive disorders (HAND): Optimal diagnosis, antiviral therapy, pharmacological treatment, management, and future scopes. J. Neurol. Sci. 2025, 470, 123410. [Google Scholar] [CrossRef]
- Freed, E.O. HIV-1 assembly, release and maturation. Nat. Rev. Microbiol. 2015, 13, 484–496. [Google Scholar] [CrossRef]
- Borrajo, A.; Ranazzi, A.; Pollicita, M.; Bellocchi, M.C.; Salpini, R.; Mauro, M.V.; Ceccherini-Silberstein, F.; Perno, C.F.; Svicher, V.; Aquaro, S. Different Patterns of HIV-1 Replication in MACROPHAGES is Led by Co-Receptor Usage. Medicina 2019, 55, 297. [Google Scholar] [CrossRef]
- Chan, P.; Li, X.; Li, F.; Emu, B.; Price, R.W.; Spudich, S. Longitudinal CNS and systemic T-lymphocyte and monocyte activation before and after antiretroviral therapy beginning in primary HIV infection. Front. Immunol. 2025, 16, 1531828. [Google Scholar] [CrossRef]
- Jia, F.; Brew, B.J. Neuropathogenesis of acute HIV: Mechanisms, biomarkers, and therapeutic approaches. Curr. Opin. HIV AIDS 2025, 20, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.S.; Bolton, D.L. The utility of nonhuman primate models for understanding acute HIV-1 infection. Curr. Opin. HIV AIDS 2025, 20, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, Y.A.; Narasipura, S.D.; Olivares, L.J.; Al-Harthi, L. CD4dimCD8brightT Cells Home to the Brain and Mediate HIV Neuroinvasion. J. Virol. 2022, 96, e0080422. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Franco, E.J.; Cantres-Rosario, Y.M.; Plaud-Valentin, M.; Romeu, R.; Rodríguez, Y.; Skolasky, R.; Meléndez, V.; Cadilla, C.L.; Melendez, L.M. Dysregulation of Macrophage-Secreted Cathepsin B Contributes to HIV-1-Linked Neuronal Apoptosis. PLoS ONE 2012, 7, e36571. [Google Scholar] [CrossRef]
- Zenón-Meléndez, C.N.; Carrión, K.C.; Rosario, Y.C.; Lima, A.R.; Meléndez, L.M. Inhibition of Cathepsin B and SAPC Secreted by HIV-Infected Macrophages Reverses Common and Unique Apoptosis Pathways. J. Proteome Res. 2022, 21, 301–312. [Google Scholar] [CrossRef]
- Zenón, F.; Cantres-Rosario, Y.; Adiga, R.; Gonzalez, M.; Rodriguez-Franco, E.; Langford, D.; Melendez, L.M. HIV-infected microglia mediate cathepsin B-induced neurotoxicity. J. Neurovirol. 2015, 21, 544–558. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosario-Rodríguez, L.J.; Colón, K.; Borges-Vélez, G.; Negrón, K.; Meléndez, L.M. Dimethyl Fumarate Prevents HIV-Induced Lysosomal Dysfunction and Cathepsin B Release from Macrophages. J. Neuroimmune Pharmacol. 2018, 13, 345–354. [Google Scholar] [CrossRef]
- Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrión, K.; Rodríguez-De Jesús, A.E.; Cartagena-Isern, L.J.; García-Requena, L.A.; Roche-Lima, A.; Meléndez, L.M. Quantitative Proteomics Reveal That CB2R Agonist JWH-133 Downregulates NF-κB Activation, Oxidative Stress, and Lysosomal Exocytosis from HIV-Infected Macrophages. Int. J. Mol. Sci. 2024, 25, 3246. [Google Scholar] [CrossRef]
- Rosario-Rodríguez, L.J.; Gerena, Y.; García-Requena, L.A.; Cartagena-Isern, L.J.; Cuadrado-Ruiz, J.C.; Borges-Vélez, G.; Meléndez, L.M. Cannabinoid receptor type 2 agonist JWH-133 decreases cathepsin B secretion and neurotoxicity from HIV-infected macrophages. Sci. Rep. 2022, 12, 233. [Google Scholar] [CrossRef]
- Cantres-Rosario, Y.M.; Hernandez, N.; Negron, K.; Perez-Laspiur, J.; Leszyk, J.; Shaffer, S.A.; Meléndez, L.M. Interacting partners of macrophage-secreted cathepsin B contribute to HIV-induced neuronal apoptosis. AIDS 2015, 29, 2081–2092. [Google Scholar] [CrossRef]
- Cantres-Rosario, Y.M.; Ortiz-Rodríguez, S.C.; Santos-Figueroa, A.G.; Plaud, M.; Negron, K.; Cotto, B.; Langford, D.; Melendez, L.M. HIV Infection Induces Extracellular Cathepsin B Uptake and Damage to Neurons. Sci. Rep. 2019, 9, 8006. [Google Scholar] [CrossRef] [PubMed]
- López, O.V.; Gorantla, S.; Segarra, A.C.; Norat, M.C.A.; Álvarez, M.; Skolasky, R.L.; Meléndez, L.M. Sigma-1 Receptor Antagonist (BD1047) Decreases Cathepsin B Secretion in HIV-Infected Macrophages Exposed to Cocaine. J. Neuroimmune Pharmacol. 2018, 14, 226–240. [Google Scholar] [CrossRef]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cabral, G.A.; Martin, B.R.; Peterson, P.K. WIN55,212-2-Mediated Inhibition of HIV-1 Expression in Microglial Cells: Involvement of Cannabinoid Receptors. J. Neuroimmune Pharmacol. 2006, 2, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.M.; Gupta, A.; Yewdall, A.W.; Dale, B.M.; Devi, L.A.; Chen, B.K. Cannabinoid Receptor 2-Mediated Attenuation of CXCR4-Tropic HIV Infection in Primary CD4+ T Cells. PLoS ONE 2012, 7, e33961. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Reichenbach, N.L.; Fan, S.; Rom, S.; Merkel, S.F.; Wang, X.; Ho, W.; Persidsky, Y. Attenuation of HIV-1 Rep-lication in Macrophages by Cannabinoid Receptor 2 Agonists. J. Leukoc. Biol. 2013, 93, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Appelberg, S.; Goldberger, B.A.; Klein, T.W.; Sleasman, J.W.; Goodenow, M.M. Δ9-Tetrahydrocannabinol Treatment During Human Monocyte Differentiation Reduces Macrophage Susceptibility to HIV-1 Infection. J. Neuroimmune Pharmacol. 2014, 9, 369–379. [Google Scholar] [CrossRef]
- Meanti, R.; Bresciani, E.; Rizzi, L.; Molteni, L.; Coco, S.; Omeljaniuk, R.J.; Torsello, A. Cannabinoid Receptor 2 (CB2R) as potential target for the pharmacological treatment of neurodegenerative diseases. Biomed. Pharmacother. 2025, 186, 118044. [Google Scholar] [CrossRef]
- O’rourke, M.B.; Town, S.E.L.; Dalla, P.V.; Bicknell, F.; Belic, N.K.; Violi, J.P.; Steele, J.R.; Padula, M.P. What is Normalization? The Strategies Employed in Top-Down and Bottom-Up Proteome Analysis Workflows. Proteomes 2019, 7, 29. [Google Scholar] [CrossRef]
- Tanyaratsrisakul, S.; Dy, A.B.C.; Polverino, F.; Numata, M.; Ledford, J.G. Myeloid-associated differentiation marker is associated with type 2 asthma and is upregulated by human rhinovirus infection. Front. Immunol. 2023, 14, 1237683. [Google Scholar] [CrossRef]
- Rodrigues, V.; Ruffin, N.; San-Roman, M.; Benaroch, P. Myeloid Cell Interaction with HIV: A Complex Relationship. Front. Immunol. 2017, 8, 1698. [Google Scholar] [CrossRef]
- Alvarez-Carbonell, D.; Garcia-Mesa, Y.; Milne, S.; Das, B.; Dobrowolski, C.; Rojas, R.; Karn, J. Toll-like receptor 3 activation selectively reverses HIV latency in microglial cells. Retrovirology 2017, 14, 9. [Google Scholar] [CrossRef]
- Biasin, M.; Sironi, M.; Saulle, I.; de Luca, M.; la Rosa, F.; Cagliani, R.; Forni, D.; Agliardi, C.; Caputo, S.L.; Mazzotta, F.; et al. Endoplasmic reticulum aminopeptidase 2 haplotypes play a role in modulating susceptibility to HIV infection. AIDS 2013, 27, 1697–1706. [Google Scholar] [CrossRef]
- Saulle, I.; Ibba, S.V.; Torretta, E.; Vittori, C.; Fenizia, C.; Piancone, F.; Minisci, D.; Lori, E.M.; Trabattoni, D.; Gelfi, C.; et al. Endoplasmic Reticulum Associated Aminopeptidase 2 (ERAP2) Is Released in the Secretome of Activated MDMs and Reduces in vitro HIV-1 Infection. Front. Immunol. 2019, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Miyagi, E.; Bouamr, F.; Farkašová, H.; Strebel, K. Mannose Receptor 1 Restricts HIV Particle Release from Infected Macrophages. Cell Rep. 2018, 22, 786–795. [Google Scholar] [CrossRef] [PubMed]
- de Morrée, A.; Flix, B.; Bagaric, I.; Wang, J.; Boogaard, M.v.D.; Moursel, L.G.; Frants, R.R.; Illa, I.; Gallardo, E.; Toes, R.; et al. Dysferlin Regulates Cell Adhesion in Human Monocytes. J. Biol. Chem. 2013, 288, 14147–14157. [Google Scholar] [CrossRef]
- Fan, T.-J.; Xie, C.; Li, L.; Jin, X.; Cui, J.; Wang, J.-H. HIV-1 Nef activates proviral DNA transcription by recruiting Src kinase to phosphorylate host protein Nef-associated factor 1 to compromise its viral restrictive function. J. Virol. 2025, 99, e0028025. [Google Scholar] [CrossRef]
- Barclay, R.A.; Mensah, G.A.; Cowen, M.; DeMarino, C.; Kim, Y.; Pinto, D.O.; Erickson, J.; Kashanchi, F. Extracellular Vesicle Activation of Latent HIV-1 Is Driven by EV-Associated c-Src and Cellular SRC-1 via the PI3K/AKT/mTOR Pathway. Viruses 2020, 12, 665. [Google Scholar] [CrossRef] [PubMed]
- Badieyan, S.; Lichon, D.; Keceli, S.K.; Andreas, M.P.; Gillies, J.P.; Peng, W.; Shi, J.; DeSantis, M.E.; Aiken, C.R.; Böcking, T.; et al. HIV-1 binds dynein directly to hijack microtubule transport machinery. Sci. Adv. 2025, 11, 6796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Cheung, C.Y.; Seo, S.-U.; Liu, H.; Pardeshi, L.; Wong, K.H.; Chow, L.M.C.; Chau, M.P.; Wang, Y.; Lee, A.R.; et al. RUVBL1/2 Complex Regulates Pro-Inflammatory Responses in Macrophages via Regulating Histone H3K4 Trimethylation. Front. Immunol. 2021, 12, 679184. [Google Scholar] [CrossRef]
- Mu, X.; Fu, Y.; Zhu, Y.; Wang, X.; Xuan, Y.; Shang, H.; Goff, S.P.; Gao, G. HIV-1 Exploits the Host Factor RuvB-like 2 to Balance Viral Protein Expression. Cell Host Microbe 2015, 18, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef]
- Fleming, J.M.; Ginsburg, E.; Oliver, S.D.; Goldsmith, P.; Vonderhaar, B.K. Hornerin, an S100 family protein, is functional in breast cells and aberrantly expressed in breast cancer. BMC Cancer 2012, 12, 266. [Google Scholar] [CrossRef]
- Cosenza-Nashat, M.A.; Bauman, A.; Zhao, M.; Morgello, S.; Suh, H.; Lee, S.C. Cannabinoid receptor expression in HIV encephalitis and HIV-associated neuropathologic comorbidities. Neuropathol. Appl. Neurobiol. 2011, 37, 464–483. [Google Scholar] [CrossRef]
- Benito, C.; Kim, W.-K.; Chavarría, I.; Hillard, C.J.; Mackie, K.; Tolón, R.M.; Williams, K.; Romero, J. A Glial Endogenous Cannabinoid System Is Upregulated in the Brains of Macaques with Simian Immunodeficiency Virus-Induced Encephalitis. J. Neurosci. 2005, 25, 2530–2536. [Google Scholar] [CrossRef]
- Benito, C.; Núñez, E.; Tolón, R.M.; Carrier, E.J.; Rábano, A.; Hillard, C.J.; Romero, J. Cannabinoid CB2Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’ s Disease Brains. J. Neurosci. 2003, 23, 11136–11141. [Google Scholar] [CrossRef]
- Shapiro, L.; Pott, G.B.; Ralston, A.H. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 2000, 15, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Münch, J.; Ständker, L.; Adermann, K.; Schulz, A.; Schindler, M.; Chinnadurai, R.; Pöhlmann, S.; Chaipan, C.; Biet, T.; Peters, T.; et al. Discovery and Optimization of a Natural HIV-1 Entry Inhibitor Targeting the gp41 Fusion Peptide. Cell 2007, 129, 263–275. [Google Scholar] [CrossRef]
- Zhou, X.; Shapiro, L.; Fellingham, G.; Willardson, B.M.; Burton, G.F. HIV Replication in CD4+ T Lymphocytes in the Presence and Absence of Follicular Dendritic Cells: Inhibition of Replication Mediated by α-1-Antitrypsin through Altered IκBα Ubiquitination. J. Immunol. 2011, 186, 3148–3155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, Z.; Zhang, J.; Adelsberger, J.W.; Yang, J.; Burton, G.F. Alpha-1-antitrypsin interacts with gp41 to block HIV-1 entry into CD4+ T lymphocytes. BMC Microbiol. 2016, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, A.V.; Münch, J.; Kirchhoff, F.; Brockmeyer, N.H. HIV infection in a patient with alpha-1 antitrypsin deficiency: A detrimental combination? AIDS 2007, 21, 2115–2116. [Google Scholar] [CrossRef]
- Bryan, C.L.; Beard, K.S.; Pott, G.B.; Rahkola, J.; Gardner, E.M.; Janoff, E.N.; Shapiro, L. HIV infection is associated with reduced serum alpha-1-antitrypsin concentrations. Clin. Investig. Med. 2010, 33, E384–E389. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Chan, M.-H.; Li, C.-H.; Yang, C.-J.; Tseng, Y.-W.; Tsai, H.-F.; Chiou, J.; Hsiao, M. Metabolic protein phosphoglycerate kinase 1 confers lung cancer migration by directly binding HIV Tat specific factor 1. Cell Death Discov. 2021, 7, 135. [Google Scholar] [CrossRef]
- Suga, S.; Tsurudome, M.; Ohgimoto, S.; Tabata, N.; Watanabe, N.; Nishio, M.; Kawano, M.; Komada, H.; Ito, Y. Identification of Fusion Regulatory Protein (FRP)-1/4F2 Related Molecules: Cytoskeletal Proteins are Associated with FRP-1 Molecules that Regulate Multinucleated Giant Cell Formation of Monocytes and HIV-Induced Cell Fusion. Cell Struct. Funct. 1995, 20, 473–483. [Google Scholar] [CrossRef]
- Luo, Q.; Jiang, L.; Chen, G.; Feng, Y.; Lv, Q.; Zhang, C.; Qu, S.; Zhu, H.; Zhou, B.; Xiao, X. Constitutive heat shock protein 70 interacts with α-enolase and protects cardiomyocytes against oxidative stress. Free. Radic. Res. 2011, 45, 1355–1365. [Google Scholar] [CrossRef]
- Ding, H.; Chen, Z.; Wu, K.; Huang, S.M.; Wu, W.L.; LeBoeuf, S.E.; Pillai, R.G.; Rabinowitz, J.D.; Papagiannakopoulos, T. Activation of the NRF2 antioxidant program sensitizes tumors to G6PD inhibition. Sci. Adv. 2021, 7, eabk1023. [Google Scholar] [CrossRef]
- Raposo, R.A.S.; Trudgian, D.C.; Thomas, B.; van Wilgenburg, B.; Cowley, S.A.; James, W. Protein Kinase C and NF-κB–Dependent CD4 Downregulation in Macrophages Induced by T Cell-Derived Soluble Factors: Consequences for HIV-1 Infection. J. Immunol. 2011, 187, 748–759. [Google Scholar] [CrossRef]
- Frey, B.F.; Jiang, J.; Sui, Y.; Boyd, L.F.; Yu, B.; Tatsuno, G.; Billeskov, R.; Solaymani-Mohammadi, S.; Berman, P.W.; Margulies, D.H.; et al. Effects of Cross-Presentation, Antigen Processing, and Peptide Binding in HIV Evasion of T Cell Immunity. J. Immunol. 2018, 200, 1853–1864. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Z.; Deng, J.; Song, Y. Cathepsin S: Molecular mechanisms in inflammatory and immunological processes. Front. Immunol. 2025, 16, 1600206. [Google Scholar] [CrossRef]
- Makhina, T.; Loers, G.; Schulze, C.; Ueberle, B.; Schachner, M.; Kleene, R. Extracellular GAPDH binds to L1 and enhances neurite outgrowth. Mol. Cell. Neurosci. 2009, 41, 206–218. [Google Scholar] [CrossRef]
- Harmon, B.; Campbell, N.; Ratner, L. Role of Abl Kinase and the Wave2 Signaling Complex in HIV-1 Entry at a Post-Hemifusion Step. PLoS Pathog. 2010, 6, e1000956. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Yoshii, H.; Kamiyama, H.; Tominaga, C.; Tanaka, Y.; Sato, H.; Yamamoto, N. Ezrin, Radixin, and Moesin (ERM) proteins function as pleiotropic regulators of human immunodeficiency virus type 1 infection. Virology 2008, 375, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, U.J.; Corbeil, J.; Mosier, D.E.; Theofilopoulos, A.N. Early modifications of host cell gene expression induced by HIV-1. AIDS 1998, 12, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Z.; Gasperino, S.; Zeichner, S.L. Nuclear Transit and HIV LTR Binding of NF-κB Subunits Held by IκB Proteins: Implications for HIV-1 Activation. Viruses 2019, 11, 1162. [Google Scholar] [CrossRef]
- Dickey, L.L.; Martins, L.J.; Planelles, V.; Hanley, T.M. HIV-1-induced type I IFNs promote viral latency in macrophages. J. Leukoc. Biol. 2022, 112, 1343–1356. [Google Scholar] [CrossRef]
- Younas, M.; Gimenez, S.; Lin, Y.-L.; Mettling, C.; Maiorano, D.; Reynes, J.; Pasero, P.; Rondard, P.; Psomas, C.K.; Corbeau, P. γ-Aminobutyric Acid-Induced Monocytic Reactive Oxygen Species Impair CD4 Restoration in Treated Adults With HIV-1. J. Infect. Dis. 2025, 231, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Morotti, M.; Zois, C.E.; El-Ansari, R.; Craze, M.L.; Rakha, E.A.; Fan, S.-J.; Valli, A.; Haider, S.; Goberdhan, D.C.I.; Green, A.R.; et al. Increased expression of glutamine transporter SNAT2/SLC38A2 promotes glutamine dependence and oxidative stress resistance, and is associated with worse prognosis in triple-negative breast cancer. Br. J. Cancer 2020, 124, 494–505. [Google Scholar] [CrossRef]
- Castellano, P.; Prevedel, L.; Valdebenito, S.; Eugenin, E.A. HIV infection and latency induce a unique metabolic signature in human macrophages. Sci. Rep. 2019, 9, 3941. [Google Scholar] [CrossRef]
- Saulle, I.; Marventano, I.; Saresella, M.; Vanetti, C.; Garziano, M.; Fenizia, C.; Trabattoni, D.; Clerici, M.; Biasin, M. ERAPs Reduce In Vitro HIV Infection by Activating Innate Immune Response. J. Immunol. 2021, 206, 1609–1617. [Google Scholar] [CrossRef]
- Obeagu, E.I. Neutrophils in HIV pathogenesis: Dual roles, clinical implications, and therapeutic frontiers. Ann. Med. Surg. 2025, 87, 5578–5587. [Google Scholar] [CrossRef] [PubMed]
- Aljawai, Y.; Richards, M.; Seaton, M.; Narasipura, S.; Al-Harthi, L. β-Catenin/TCF-4 Signaling Regulates Susceptibility of Macrophages and Resistance of Monocytes to HIV-1 Productive Infection. Curr. HIV Res. 2014, 12, 164–173. [Google Scholar] [CrossRef]
- Bouassa, R.-S.M.; Comeau, E.; Alexandrova, Y.; Pagliuzza, A.; Yero, A.; Samarani, S.; Needham, J.; Singer, J.; Lee, T.; Bobeuf, F.; et al. Effects of Oral Cannabinoids on Systemic Inflammation and Viral Reservoir Markers in People with HIV on Antiretroviral Therapy: Results of the CTN PT028 Pilot Clinical Trial. Cells 2023, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Borges, L.B.; Oliveira, F.R.M.B.; Silva, P.C.d.S.; Patricio, D.O.; Rosales, T.O.; Souza, N.F.; Spiller, F.; Mansur, D.S.; Assreuy, J.; et al. Cannabinoid CB2 receptor agonist reduces local and systemic inflammation associated with pneumonia-induced sepsis in mice. Eur. J. Pharmacol. 2023, 959, 176092. [Google Scholar] [CrossRef]
- Craft, R.M.; Wakley, A.A.; Tsutsui, K.T.; Laggart, J.D. Sex Differences in Cannabinoid 1 vs. Cannabinoid 2 Receptor-Selective Antagonism of Antinociception Produced by Δ9-Tetrahydrocannabinol and CP55,940 in the Rat. J. Pharmacol. Exp. Ther. 2012, 340, 787–800. [Google Scholar] [CrossRef]
- Krebs-Kraft, D.L.; Hill, M.N.; Hillard, C.J.; McCarthy, M.M. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc. Natl. Acad. Sci. USA 2010, 107, 20535–20540. [Google Scholar] [CrossRef]
- Argue, K.J.; VanRyzin, J.W.; Falvo, D.J.; Whitaker, A.R.; Yu, S.J.; McCarthy, M.M. Activation of Both CB1 and CB2 Endocannabinoid Receptors Is Critical for Masculinization of the Developing Medial Amygdala and Juvenile Social Play Behavior. eNeuro 2017, 4, ENEURO.0344-16.2017. [Google Scholar] [CrossRef]
- Piotrowska, Ż.; Niezgoda, M.; Łebkowski, W.; Filipek, A.; Domian, N.; Kasacka, I. Sex differences in distribution of cannabinoid receptors (CB1 and CB2), S100A6 and CacyBP/SIP in human ageing hearts. Biol. Sex Differ. 2018, 9, 50. [Google Scholar] [CrossRef]
- Trifilo, M.J.; Bergmann, C.C.; Kuziel, W.A.; Lane, T.E. CC Chemokine Ligand 3 (CCL3) Regulates CD8+-T-Cell Effector Function and Migration following Viral Infection. J. Virol. 2003, 77, 4004–4014. [Google Scholar] [CrossRef]
- Lopalco, L. CCR5: From Natural Resistance to a New Anti-HIV Strategy. Viruses 2010, 2, 574–600. [Google Scholar] [CrossRef]
- Borges-Vélez, G.; Rosado-Philippi, J.; Cantres-Rosario, Y.M.; Carrasquillo-Carrion, K.; Roche-Lima, A.; Pérez-Vargas, J.; González-Martínez, A.; Correa-Rivas, M.S.; Meléndez, L.M. Zika virus infection of the placenta alters extracellular matrix proteome. Histochem. J. 2021, 53, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Vélez-López, O.; Carrasquillo-Carrión, K.; Cantres-Rosario, Y.M.; Machín-Martínez, E.; Álvarez-Ríos, M.E.; Roche-Lima, A.; Tosado-Rodríguez, E.L.; Meléndez, L.M. Analysis of Sigma-1 Receptor Antagonist BD1047 Effect on Upregulating Proteins in HIV-1-Infected Macrophages Exposed to Cocaine Using Quantitative Proteomics. Biomedicines 2024, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Tripathi, G.; Sharma, N.; Bindal, V.; Yadav, M.; Mathew, B.; Sharma, S.; Gupta, E.; Maras, J.S.; Sarin, S.K. Protocol for global proteome, virome, and metaproteome profiling of respiratory specimen (VTM) in COVID-19 patient by LC-MS/MS-based analysis. STAR Protoc. 2021, 3, 101045. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Caterino, M.; Salvatori, I.; Manganelli, V.; Ferri, A.; Misasi, R.; Ruoppolo, M. Proteome data of neuroblastoma cells overexpressing Neuroglobin. Data Brief 2022, 41, 107843. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Bandeira, N.; Sharma, V.; Perez-Riverol, Y.; Carver, J.J.; Kundu, D.J.; García-Seisdedos, D.; Jarnuczak, A.F.; Hewapathirana, S.; Pullman, B.S.; et al. The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 2020, 48, D1145–D1152. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef] [PubMed]
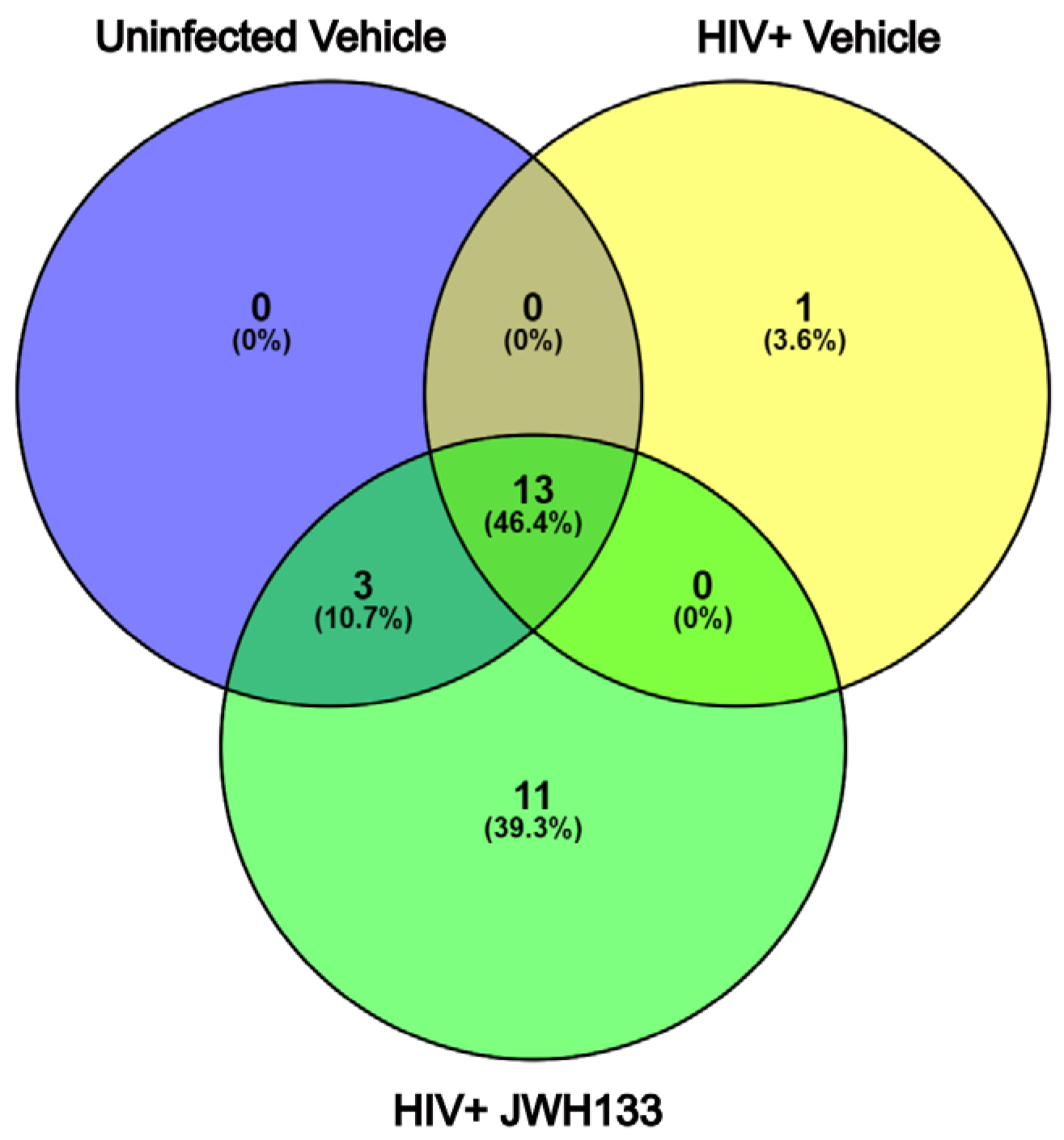
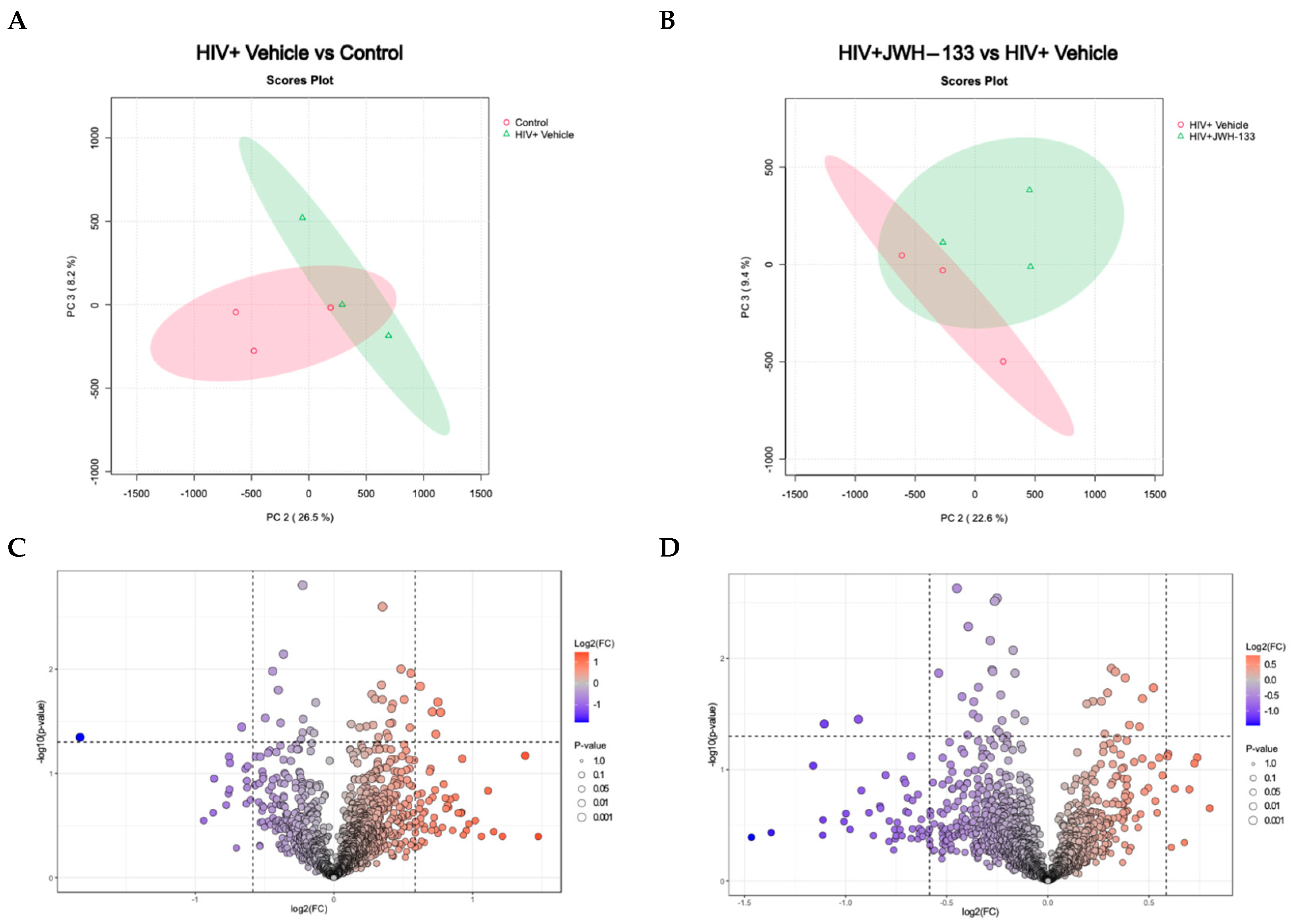

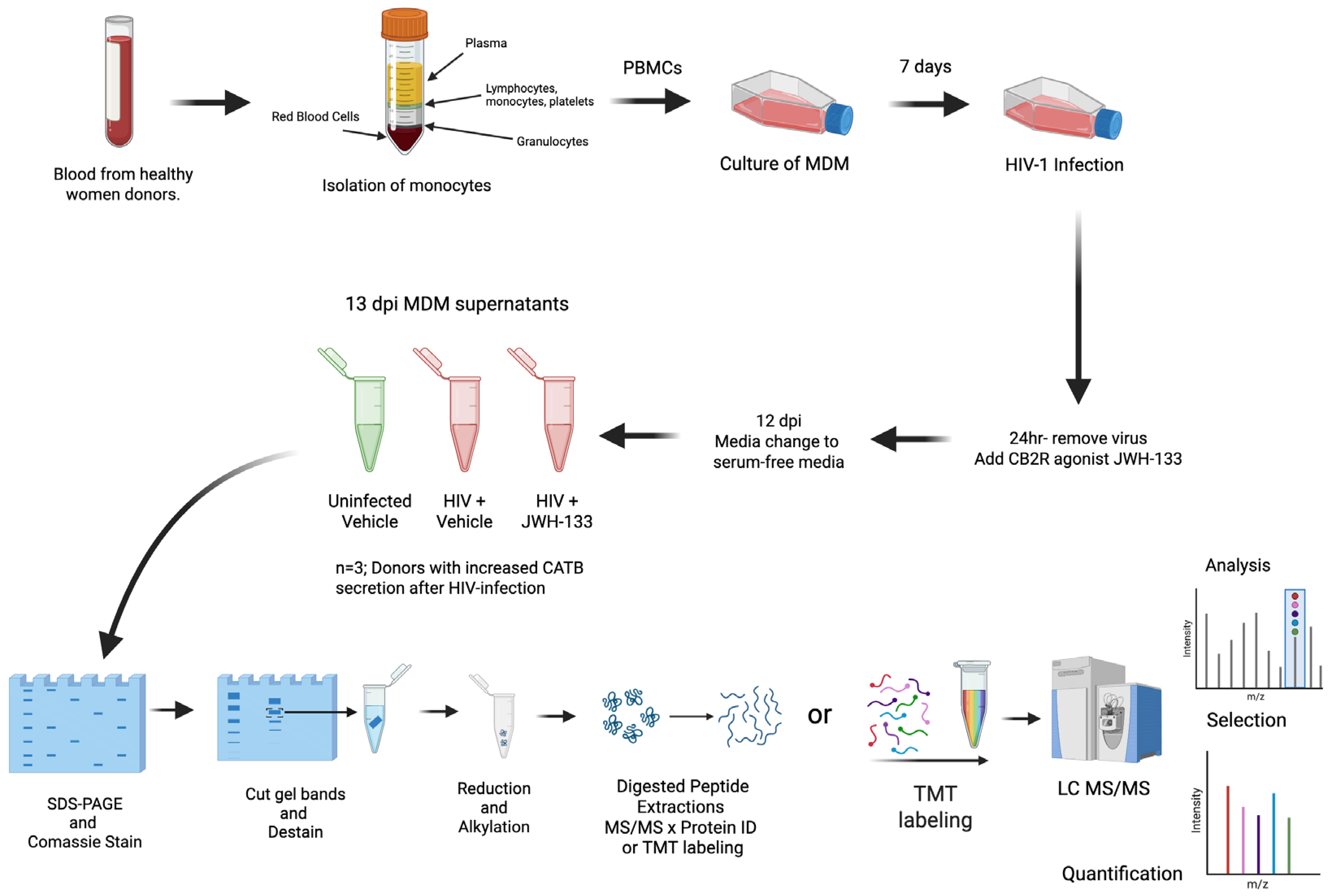
| Protein | Symbol | # Unique Peptides | Molecular Function |
|---|---|---|---|
| Cathepsin B | CTSB | 12 | Lysosomal enzyme that causes proteolysis. Extracellular CTSB secreted by macrophages plays an important role in neuronal death [11,12,13,14,15,16,17,18,19]. |
| Protein | Symbol | # Unique Peptides | Molecular Function |
|---|---|---|---|
| Phosphoglycerate kinase 1 | PGK1 | 7 | Enzyme that catalyzes the reversible transfer of phosphate groups. This is part of glycolysis, a critical energy-producing process. |
| Alpha-1-antitrypsin | SERPINA1 | 5 | Enzyme with many with anti-inflammatory and immunomodulatory properties. |
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 7 | Enzyme that participates in glycolysis, an energy-producing process. |
| Heat shock cognate 71 kDa protein | HSPA8 | 5 | Molecular chaperone implicated in several cell processes, including the protection of the proteome from stress. |
| Alpha-actinin-1 | ACTN1 | 6 | Cytoskeletal activity that helps to maintain the cell shape. |
| Galectin-3 | IGALS3 | 3 | Contributes to the regulation of innate and adaptive immunity. |
| Cathepsin S | CTSS | 5 | Lysosomal enzyme that causes proteolysis in the extracellular space at a neutral pH and plays a key role in neuron regeneration. |
| Moesin | MSN | 8 | Signal transduction activity or receptor binding; cytoskeletal activity; nucleic acid binding activity. |
| 6-Phosphogluconate dehydrogenase, decarboxylating | PGD | 7 | Produces NADPH, a major reducing protein required for lipid production and protecting the cell against oxidative stress. |
| Actin-related protein 3 | ACTR3 | 5 | Cytoskeletal protein, chromatin remodeling. |
| Comparison | Differentially More Abundant | Differentially Less Abundant | Total Differentially Abundant |
|---|---|---|---|
| HIV + Vehicle vs. Control | ↑ 5 | ↓ 2 | 7 |
| HIV + JWH-133 vs. HIV + Vehicle | 0 | ↓ 2 | 2 |
| Symbol | Entrez Gene Name | Function in HIV Infection of Macrophages | HIV vs. Uninfected | |
|---|---|---|---|---|
| Fold Change | p-Value | |||
| Q96S97 | Myeloid-associated differentiation marker (MYADM) | Transmembrane protein that increases with myeloid differentiation and is associated with inflammatory processes. Changes in myeloid differentiation marker levels were reported during inflammation in Rhinovirus asthma [26] and during HIV activation from latency, particularly in macrophages [27,28]. No direct evidence found linking MYADM to HIV infection of macrophages. | 1.54 | 1.46 × 10−2 |
| Q96QD8 | Sodium-coupled neutral amino acid symporter 2 (SNAT2 or SLC38A2) | Transports small neutral amino acids glutamine and glycine along with sodium ions into cells. Plays vital roles in glutamine–glutamate circulation, synthesis of proteins, and cell growth. Functions as a symporter, co-transports two substances in the same direction across a membrane. No direct evidence found linking SNAT2 to HIV infection of macrophages. | 1.68 | 2.07 × 10−2 |
| Q6P179 | Endoplasmic reticulum aminopeptidase 2 (ERAP2) | Located in the ER lumen and crucial for peptide trimming during antigen processing for major histocompatibility complex (MHC) class I presentation, which is essential in initiating an immune response to infected cells. ERAP2 is correlated with resistance to HIV-1 infection, possibly secondary to its effect on antigen processing and presentation [29]. Secreted ERAP2 from MDMs has been shown to reduce HIV-1 replication in cell cultures by activating monocytes and T cells [30]. | 1.64 | 2.56 × 10−2 |
| Q96IJ6 | Mannose-1-phosphate guanyl transferase alpha (GMPPA) | Enzyme that belongs to the family of transferases. They transfer phosphorus-containing nucleotide groups, participating in fructose and mannose metabolism. This process is relevant to HIV because mannose-containing glycans on the HIV envelope (gp120) are essential for binding to host cells, and the HIV mannose receptor 1 (hMRC1) interacts with these glycans, influencing both viral entry and release. While hMRC1 can act as an antiviral factor that restricts HIV particle release, the virus can counteract this by downregulating hMRC1 expression, and the interaction between hMRC1 and Env is often virus isolate-specific [31]. | 1.66 | 4.20 × 10−2 |
| O75923 | Dysferlin (DYSF) | Dysferlin is essential for muscle membrane repair and, when mutated, causes a group of genetic muscle diseases. It functions by mediating calcium-dependent vesicle fusion and recruitment to damaged muscle cell membranes. Studies have shown that dysferlin is present in monocytes and macrophages and that dysferlin-deficient macrophages exhibit altered behavior, such as increased phagocytosis [32]. No specific role in HIV-1 infection was found. | 1.70 | 2.59 × 10−2 |
| O75563 | Src kinase-associated phosphoprotein 2 (SKAP2) | Human adaptor protein involved in the Src signaling pathway, B cell and macrophage adhesion, and cytoskeletal organization. Acts as a substrate for Src family kinases (SFKs) and couples the B cell receptor to integrin activation, playing a role in immune cell function. Regulates actin polymerization, which is important for the movement and function of immune cells like T cells and macrophages. HIV-1 protein Nef recruits SFKs to promote viral transcription, and SFKs like Lck facilitate HIV-1 assembly [33]. Src kinase, in its phosphorylated form, is present in and secreted via extracellular vesicles (EVs) in HIV-infected cells. EV-associated c-Src plays a role in reactivating latent HIV-1 by initiating signaling cascades that promote viral gene expression and the remodeling of chromatin [34]. | −1.59 | 3.59 × 10−2 |
| Q5VUJ9 | Dynein regulatory complex protein 8 (DRC8 or EFCAB2) | Dynein regulatory complex protein 8 (DRC8) is a protein found in various organisms, including humans. A component of the nexin–dynein regulatory complex (N-DRC); a key regulator of ciliary/flagellar motility, which maintains the alignment and integrity of the distal axoneme and regulates microtubule sliding. HIV “hijacks” the dynein transport machinery for microtubule motility, establishing a new model of viral trafficking by directly co-opting host dynein motors [35]. | −3.56 | 4.50 × 10−2 |
| Symbol | Entrez Gene Name | Function in HIV Infection of Macrophages | HIV + Agonist vs. HIV | |
|---|---|---|---|---|
| Fold Change | p-Value | |||
| Q9Y265 | RuvB-like 1 (RVBL1) | RVBL1 is a component of several important host protein complexes involved in DNA repair and gene regulation. RVBL1/2 is indispensable for pro-inflammatory responses and the antimicrobial activity of macrophages. RUVBL1/2 inhibitors may have therapeutic potential in treating diseases caused by the aberrant activation of pro-inflammatory pathways [36]. RuvB-like 1 (RVBL1) and its homolog, RuvB-like 2 (RVBL2), interact with HIV-1 to regulate viral protein expression and replication. The viral envelope protein (Env) antagonizes RVBL2, which normally inhibits HIV-1 Gag expression. This interaction is crucial for HIV-1 to balance viral protein production, control viral RNA stability, and produce infectious virion particles. In HIV-1-positive patients, RVBL2 levels positively correlate with viral loads and disease progression status. These findings reveal a mechanism by which HIV-1 regulates its protein expression [37]. While HIV-1 can hijack exosomal machinery for the trafficking of viral RNA and proteins, infected cells can secrete exosomes containing viral components like the Env protein. The specific role of RVBL1 in this context has not been reported [38]. | −1.91 | 3.52 × 10−2 |
| Q86YZ3 | Hornerin (HRNR) | Hornerin is an S100 protein family member that plays a key role in calcium binding. Hornerin is involved in normal and pathological cell functions including gene transcription, inflammatory and immune responses, the regulation of protein phosphorylation, transcription factors, antimicrobial responses, Ca2+ homeostasis, and the dynamics of cytoskeleton constituents, as well as cell proliferation, differentiation, and death. Inhibitors of specific S100 proteins are currently being developed as therapeutics for diseases including diabetes mellitus, heart failure, neurological diseases, and several types of cancer [39]. It has been reported that biotinylated HRNR can be incorporated into HIV-Gag virus-like particles [39]. | −2.15 | 3.88 × 10−2 |
| Comparison | ID | Symbol | Entrez Gene Name | Location | Type(s) |
|---|---|---|---|---|---|
| HIV+ Vehicle vs. Control | O75923 | DYSF | Dysferlin | Plasma membrane | Other |
| Q5VUJ9 | EFCAB2 | EF-hand calcium binding domain 2 | Other | Other | |
| Q6P179 | ERAP2 | Endoplasmic reticulum aminopeptidase 2 | Cytoplasm | Peptidase | |
| Q96IJ6 | GMPPA | GDP-mannose pyrophosphorylase A | Cytoplasm | Enzyme | |
| Q96S97 | MYADM | Myeloid-associated differentiation marker | Nucleus | Other | |
| O75563 | SKAP2 | Src kinase-associated phosphoprotein 2 | Cytoplasm | Other | |
| Q96QD8 | SLC38A2 | Solute carrier family 38 member 2 | Plasma membrane | Transporter | |
| HIV+ JWH-133 vs. HIV + Vehicle | Q86YZ3 | HRNR | Hornerin | Cytoplasm | Other |
| Q9Y265 | RUVBL1 | RuvB-like AAA ATPase 1 | Nucleus | Transcription regulator |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosario-Rodríguez, L.J.; Cantres-Rosario, Y.M.; Rodríguez De Jesús, A.E.; Mera-Pérez, A.M.; Tosado-Rodríguez, E.L.; Roche Lima, A.; Meléndez, L.M. Cannabinoid Receptor Type 2 Agonist JWH-133 Stimulates Antiviral Factors and Decreases Proviral, Inflammatory, and Neurotoxic Proteins in HIV-Infected Macrophage Secretome. Int. J. Mol. Sci. 2025, 26, 10596. https://doi.org/10.3390/ijms262110596
Rosario-Rodríguez LJ, Cantres-Rosario YM, Rodríguez De Jesús AE, Mera-Pérez AM, Tosado-Rodríguez EL, Roche Lima A, Meléndez LM. Cannabinoid Receptor Type 2 Agonist JWH-133 Stimulates Antiviral Factors and Decreases Proviral, Inflammatory, and Neurotoxic Proteins in HIV-Infected Macrophage Secretome. International Journal of Molecular Sciences. 2025; 26(21):10596. https://doi.org/10.3390/ijms262110596
Chicago/Turabian StyleRosario-Rodríguez, Lester J., Yadira M. Cantres-Rosario, Ana E. Rodríguez De Jesús, Alana M. Mera-Pérez, Eduardo L. Tosado-Rodríguez, Abiel Roche Lima, and Loyda M. Meléndez. 2025. "Cannabinoid Receptor Type 2 Agonist JWH-133 Stimulates Antiviral Factors and Decreases Proviral, Inflammatory, and Neurotoxic Proteins in HIV-Infected Macrophage Secretome" International Journal of Molecular Sciences 26, no. 21: 10596. https://doi.org/10.3390/ijms262110596
APA StyleRosario-Rodríguez, L. J., Cantres-Rosario, Y. M., Rodríguez De Jesús, A. E., Mera-Pérez, A. M., Tosado-Rodríguez, E. L., Roche Lima, A., & Meléndez, L. M. (2025). Cannabinoid Receptor Type 2 Agonist JWH-133 Stimulates Antiviral Factors and Decreases Proviral, Inflammatory, and Neurotoxic Proteins in HIV-Infected Macrophage Secretome. International Journal of Molecular Sciences, 26(21), 10596. https://doi.org/10.3390/ijms262110596








