1. Introduction
Usmani–Riazzudin syndrome (USRIS, OMIM 619467) (
https://www.omim.org/entry/619467, accessed on 27 October 2025) is a rare autosomal neurodevelopmental disorder, described for the first time only a few years ago [
1]. Its dominant and recessive forms have been reported in a cohort of patients showing overlapping clinical features. In 2021, Usmani et al. identified and studied nine patients with de novo dominant missense, splice site, and frameshift variants and two patients with biallelic, recessive, and missense variants.
The clinical phenotype is characterized by common signs such as intellectual disability, speech delay, developmental delay, hypotonia, and behavioral problems (mainly aggressive behavior), variably associated with congenital anomalies, epilepsy, spasticity, autism, bone abnormalities, vertebral and limb defects, and variable facial features (eyes and ear shape anomalies). Recently, an additional de novo variant was identified in a patient with multisystemic involvement and specific facial features, including synophrys, convergents quint, hypotelorism, upslanted palpebral fissures, broad nasal tip, thick upper and lower lip vermilion, and abnormal teeth [
2], thus defining a more characteristic facial dysmorphism of the disease. The
AP1G1 gene is located on human chr16q22.2 and encodes the gamma-1 (γ) adaptor protein subunit of the AP-1 complex (AP1γ1, a protein of 822 amino acids). AP1γ1 is predicted to have four transmembrane domains, followed by a carboxyl-terminal alpha-adaptin C2 domain, which binds to clathrin and other receptors in coated vesicles [
3,
4]. Ubiquitous adaptor proteins (APs) are required for assembly, cargo sorting, and vesicular transport among endosomes, lysosomes, trans-Golgi networks (TGNs), and the plasma membrane [
5,
6,
7,
8]. It has been shown that AP-1 complexes not only are involved in these cellular functions in eukaryotic cells [
9,
10], but also mediate basolateral sorting in polarized epithelial cells [
11,
12,
13,
14], and transmembrane protein transport to somato-dendritic domains in neurons [
15,
16,
17]. Loss of AP-1 complex subunits in murine models has been previously associated with death or severe growth deficits [
11,
16,
18,
19,
20]. Notably, the AP1 complex plays a critical role in neural function, as evidenced by the fact that its dysregulation is a common hallmark of several neurological diseases [
21].
Rare diseases are characterized by a low prevalence, which often means that patients with such diseases are undiagnosed and do not have effective treatment options. Neurodevelopmental and neurological disorders comprise approximately 40% of rare diseases [
22], and in recent years, attempts have been made to identify the genes and mechanisms linked to these pathologies. This highlights the importance of using an animal model that can recapitulate the phenotype of rare diseases and, therefore, provides an excellent tool for elucidating the role of the genes involved, revealing mechanisms, and assessing therapeutic methods for rare diseases. Zebrafish (
Danio rerio), a vertebrate with transparent embryos that develop externally, has emerged as an excellent model for studying different biological processes involved in central nervous system pathologies owing to advancements in CRISPR/Cas9 genome editing and transgenesis technologies [
23]. Recently, our research group [
24] generated a zebrafish
ap1g1 gene knockout using CRISPR/Cas9 genome editing to better understand the functional role of
AP1G1 in adaptinopathies. Heterozygous females and males showed morphological alterations in the brain, gonads, and intestinal epithelium. Analysis of the mRNA expression profiles of marker proteins, along with altered tissue morphology, revealed dysregulated cadherin-mediated cell adhesion. These findings highlight the utility of the zebrafish model for studying the molecular mechanisms of adaptinopathies and developing potential treatment strategies.
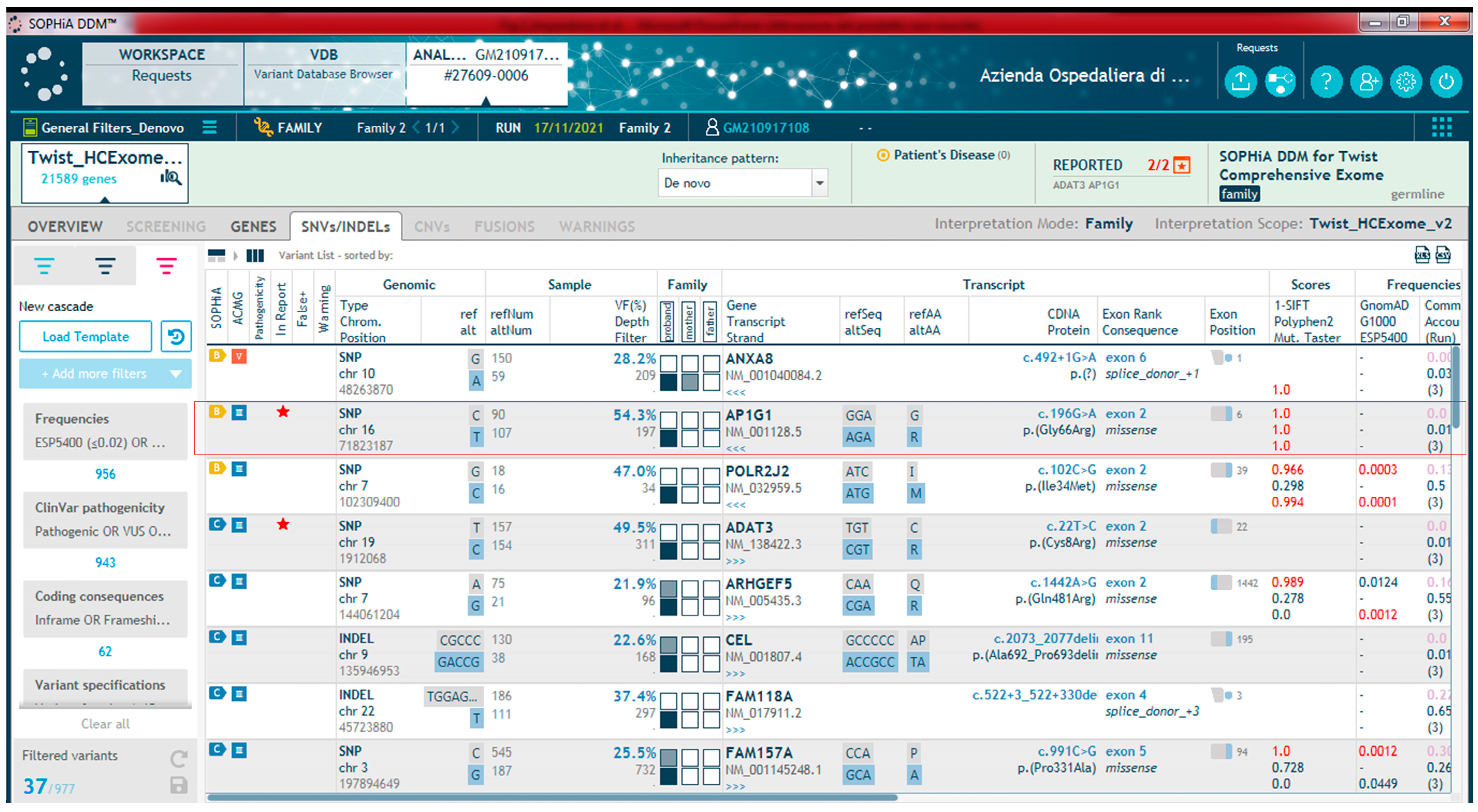
This study describes and characterizes another new missense variant (c.196G>A, p.Gly66Arg) in AP1G1 in a girl with intellectual disability and abnormal behavior, thus expanding the case history. The phenotype of the zebrafish model expressing the human mutated allele is consistent with the clinical features of the patient, supporting a possible dominant-negative effect of the new mutation.
3. Discussion
Ubiquitin adaptor protein complexes (AP) mediate selective intracellular vesicular trafficking and polarized localization of somato-dendritic proteins in neurons. AP complexes play a key role in the trafficking and correct localization of cargos in different cell types, particularly neurons. Notably, most patients with disorders related to defects in the subunits of AP complexes exhibit abnormalities in brain function and structure, likely due to the complex organization of neurons and glial cells, which have very long differentiated axons that increase their vulnerability to defects in intracellular transport. Disease-causing mutations have been found in all AP complexes, including AP-1 and AP-2, which are essential for vertebrate survival.
The lack of an AP subunit renders the entire complex non-functional [
6]; therefore, loss-of-function mutations in any subunit of a particular complex should have the same phenotype. A substantial part of our understanding regarding the function of AP-1 complex has been derived from studies employing
knockout and
knockdown techniques. However, recent evidence from animal models and human patients indicates that point mutations in ubiquitously expressed AP-1 subunits can lead to distinct diseases collectively known as
adaptinopathies. Mice homozygous for a null mutation of the AP-1 γ1 subunit die at day 3.5 post-fertilization, while heterozygous mice display a reduced growth rate exclusively during nursing and impaired T-cell development [
19]. In murine models, the loss of AP-1 complex subunits is associated with prenatal lethality or severe growth deficits, and disease-causing alleles of various subunits of AP complexes have been implicated in several human disorders, such as intellectual disabilities (IDs). In 2024, Zhang J. and his colleagues reported that pathogenetic variants in 13 genes encoding different subunits, of the 5 AP complexes, are associated with several neurodevelopmental disorders; the data suggested the important role of AP complexes in neurodevelopment and neuronal functions [
33].
The important and essential role of the AP-1 complex in correct development and cognitive function in humans has been demonstrated. In 2021, 11 unrelated patients were described with mild to severe intellectual disability, speech delay, and aggressive behavior in 100% of the patients, while other aspects (including autism, epilepsy, hypotonia, spasticity, and limb defects) were variably expressed in the cohort [
1]. In 2024, another missense variant that appeared to be correlated with specific facial features was identified [
2].
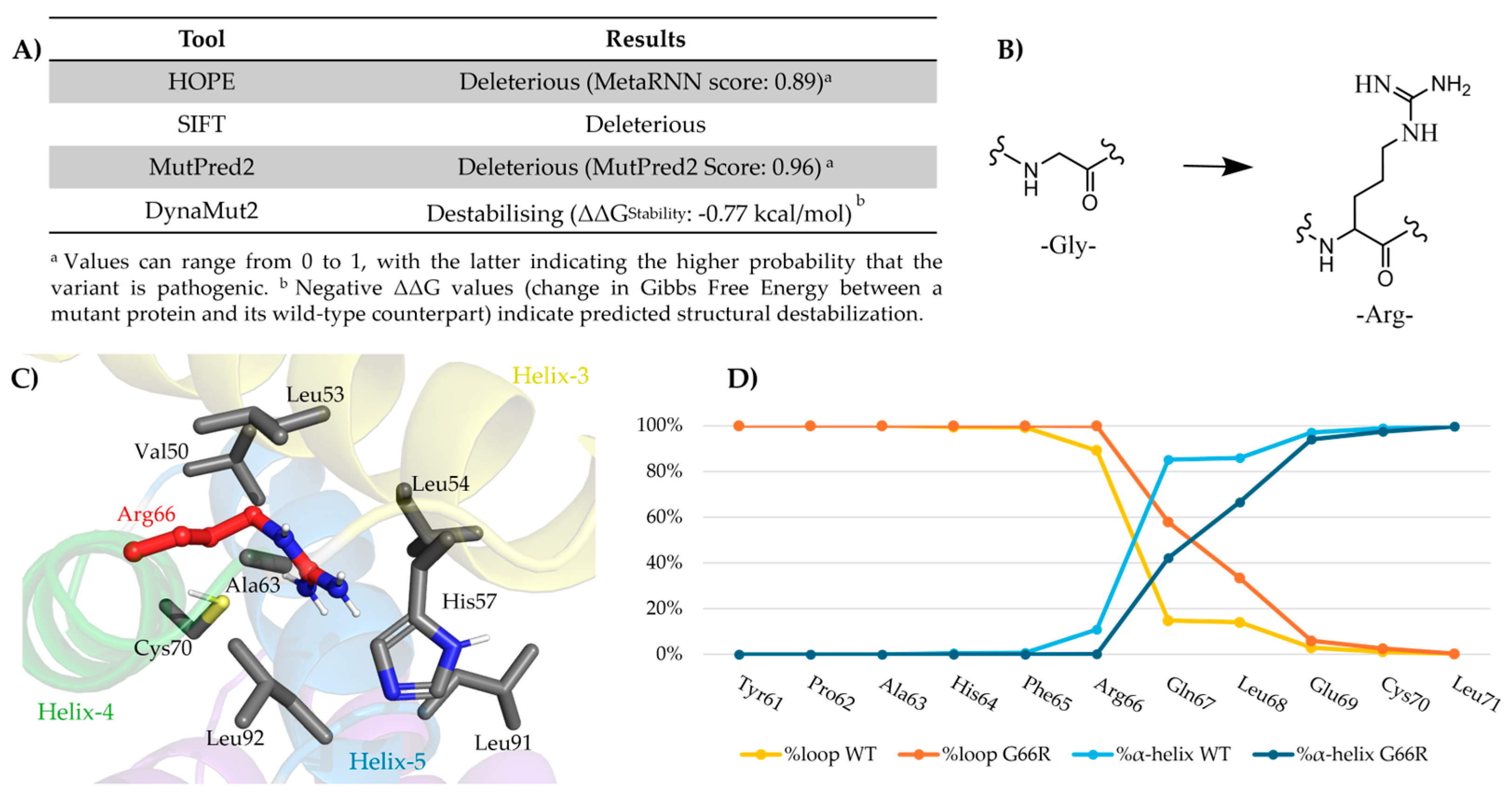
Our proband showed a novel missense variant in the amino-terminal region involving a glycine-conserved residue that was substituted with an arginine. The normal final folding of the protein results in compression due to the substantially different properties of the two amino acids, leading to a dysfunctional protein. In particular, the chemical structure of glycine is characterized by a neutral charge and apolarity with a high facilitation of local folding and movements, whereas arginine is a positive polar amino acid with a rigid local structure. These findings indicated that the variant was responsible for the girl’s phenotype.
To date, all reported pathogenic missense variants are located within the N-terminal region and involve highly conserved residues. All functional studies in correlation with the clinical phenotype of all patients suggested that these de novo heterozygous variants could have a toxic dominant-negative effect on biological mechanisms.
To further support this hypothesis, we performed a comparative analysis of clinical and zebrafish phenotypes across all reported N-terminal AP1G1 missense variants, including our novel p.Gly66Arg. Patients carrying already reported p.Arg15Gln, p.Arg35Trp, and p.Arg35Gln variants consistently presented with global developmental delay, intellectual disability, speech delay, and behavioral anomalies, including aggression and, variably, seizures, hypotonia, and autistic traits. Our proband exhibited a highly overlapping clinical profile, reinforcing the phenotypic convergence within this mutational cluster.
Functional studies in zebrafish further corroborate this interpretation.
Complementing rodent models, zebrafish have emerged as a valuable model organism for CNS research and related diseases. The suitability of zebrafish as an in vivo model for investigating rare neurodevelopmental disorders is further corroborated by the fact that approximately 80% of the human disease genes are conserved in
Danio rerio. This conservation of protein-coding sequences enables the functional analysis of individual mutations, thereby shedding light on pathogenic mechanisms and potentially guiding therapeutic exploration. Moreover, the external development of zebrafish embryos, combined with their optical transparency and the availability of transgenic lines expressing EGFP in distinct neuronal populations, enables direct real-time visualization of neuronal phenotype development and organization. This approach provides researchers with deeper insights into the mechanisms underlying neurodevelopmental diseases [
29]. It is well known that there are high anatomical similarities between zebrafish and the human nervous system, particularly in its main structures such as the hippocampus, diencephalon, tectum, and cerebellum [
34]. Additionally, zebrafish dopaminergic neurons located in the diencephalon are homologous to mammalian midbrain catecholaminergic neurons, which play a crucial role in motor behavior. The projection of dopaminergic neurons is closely related to the regulation of certain behaviors [
35]. Finally, with the help of genome engineering technologies, including DNA editing technology (CRISPR/Cas9), next-generation DNA sequencing, transgenesis, and overexpression of both wild-type and mutated mRNA, it is possible to replicate the pathogenic phenotype associated with mutations identified in human patients. To further investigate the pathogenic mechanism of the identified variant, we injected one-cell-stage embryos with both WT and MUT
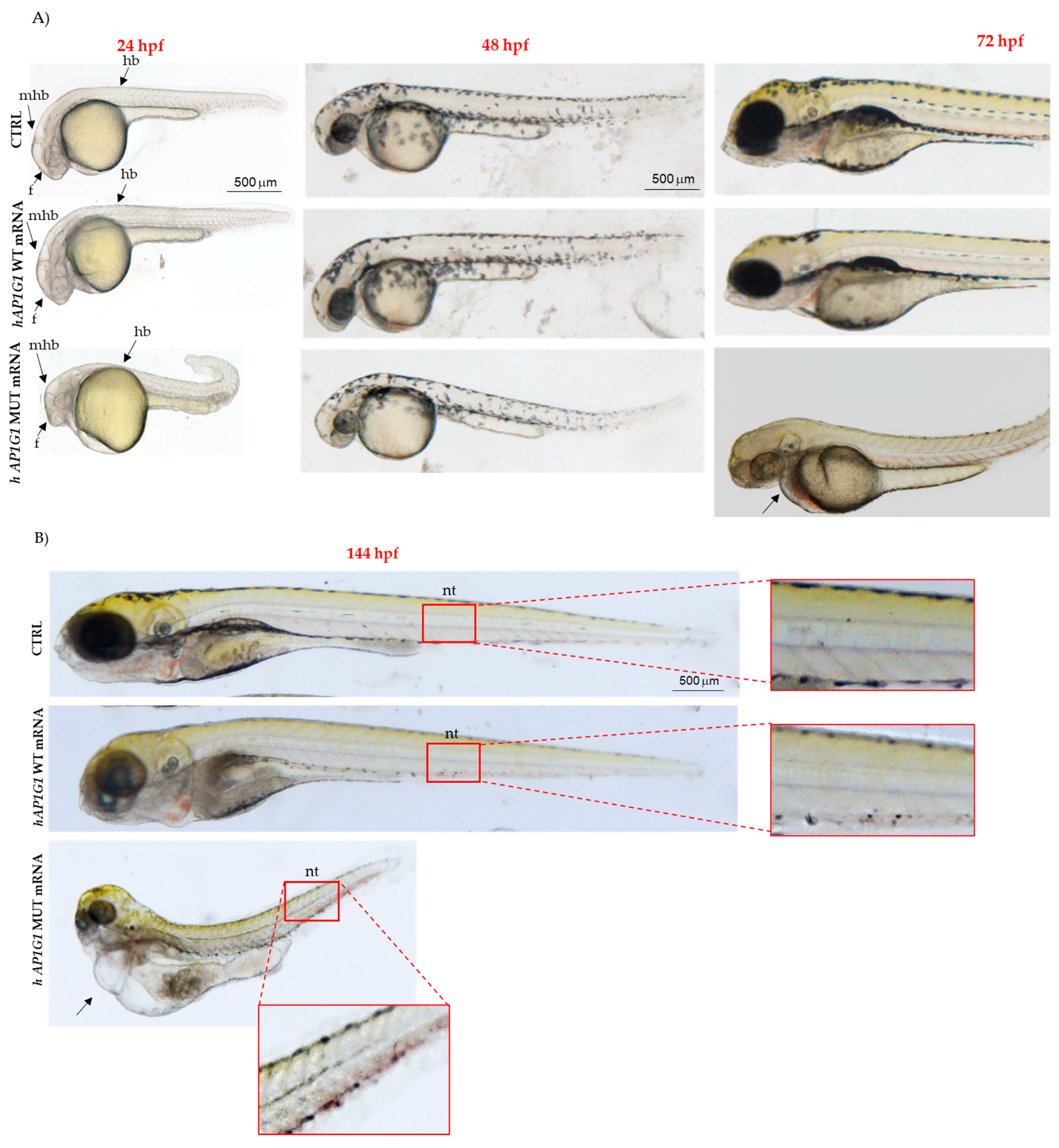
AP1G1 mRNAs. The most notable findings were morphological developmental abnormalities characterized by shorter body length, pericardial edema, abnormal body shape, notochord, and tail deformities, which led us to infer a strong effect of human mutant
AP1G1 mRNA expression. In particular, at 48 hpf, a strong neuronal phenotype, including brain abnormalities, was observed in embryos injected with human MUT mRNA. These morphological alterations were accompanied by significant behavioral deficits, as evidenced by decreased total swimming distance, average speed, and movement time.
Among vertebrate models, zebrafish provides a special opportunity to connect brain circuit activity with behavior. The first movements of the larva, characterized by spontaneous tail contractions, appear at 17 hpf [
30]; at this stage, only six types of spinal neurons are active, with a repeating segmental organization [
36]. Touch-evoked responses mediated by Rohon-Beard sensory neurons were detectable at 21 hpf. By 48–72 hpf, fish are able to swim in a coordinated manner and respond appropriately to different stimuli [
30]. Given the well-established link between neural activity and motor behavior, our study explored the potential role of
ap1g1 during crucial phases of embryonic neurodevelopment using simple motor and behavioral assays. In zebrafish, the hatching period typically occurs between 48 and 72 hpf and represents the transition point from a developing embryo to a free-living larva. This process is tightly regulated by many factors and is highly sensitive to the first motor movements in response to midbrain neurons. Embryos injected with human MUT
AP1G1 mRNA showed a strong reduction in hatching rate compared to embryos injected with human WT
AP1G1 mRNA. The spinal cord of zebrafish embryos harbors primary mechanosensory neurons that play a critical role in the early development of the somatosensory system. At 72 hpf, we examined whether human mRNA injection affected somatosensory function. To assess this, we performed a touch-evoked test by gently touching the tail with a pipette tip and recording the distance swum following the stimulus. Responses were classified based on the total distance swum using a threshold of 20 mm to distinguish between reduced (<20 mm) and normal or enhanced (>20 mm) escape behaviors. Control embryos and embryos injected with human WT
AP1G1 mRNA were able to travel a distance > 20 mm, in contrast to a large percentage of embryos injected with human MUT
AP1G1 mRNA, which did not respond to touch stimuli. These findings suggest that transient expression of human MUT
AP1G1 mRNA may affect spinal motor neuron function, impacting embryo locomotor behavior. Furthermore, we studied their swimming performance at 144 hpf after injection of both
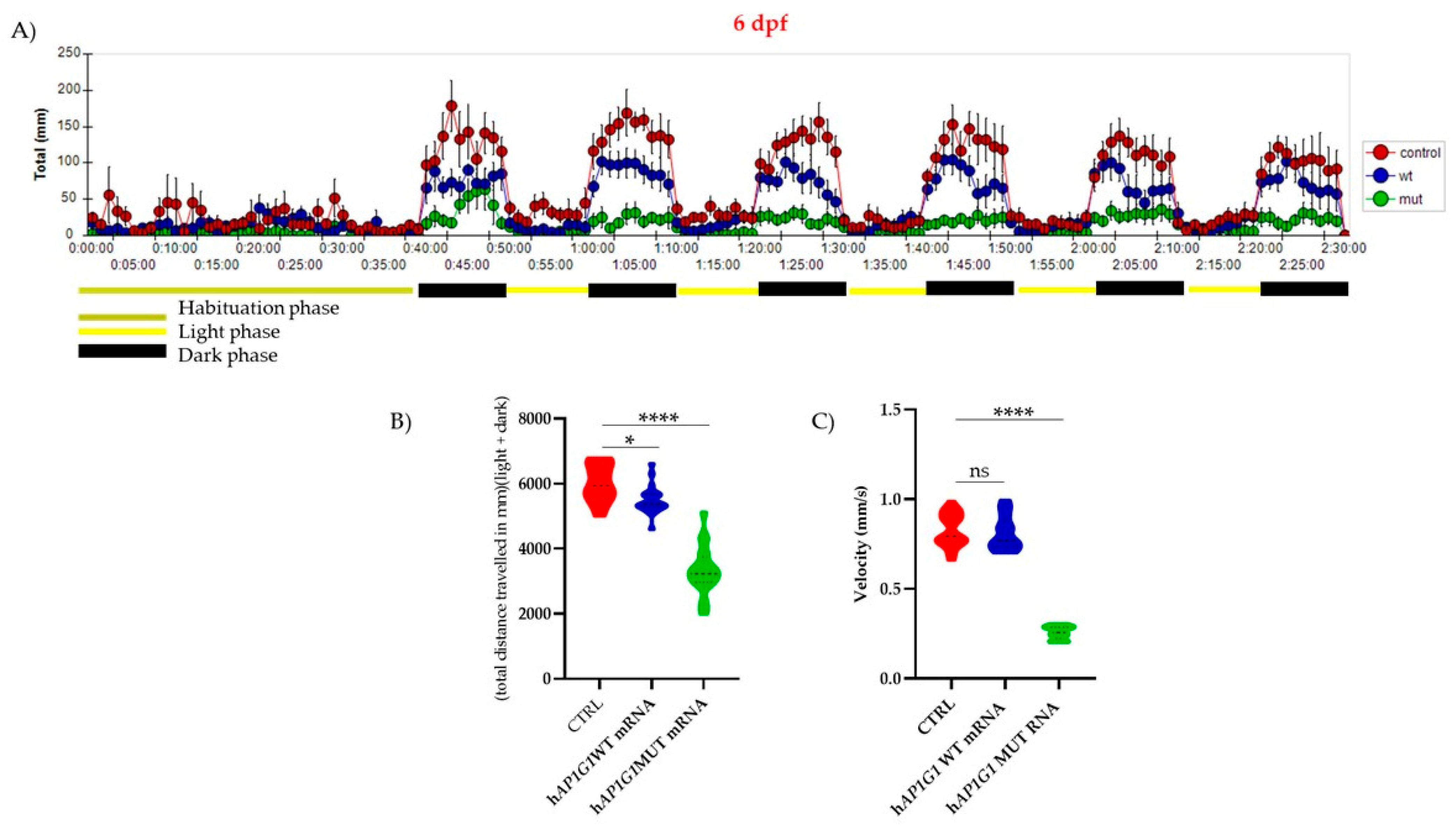
AP1G1 mRNAs, considering that at this stage the neurogenesis process is already complete. Using an animal motion-tracking system (
EthoVision XT), we quantified the total swimming distance under alternating light and dark conditions. Zebrafish larvae injected with WT
AP1G1 mRNA displayed normal locomotor activity, whereas larvae injected with MUT
AP1G1 exhibited a marked reduction in swum distance. Collectively, these findings indicate that the c.196G>A (p.Gly66Arg) mutation in
AP1G1 significantly alters the behavior of zebrafish embryos.
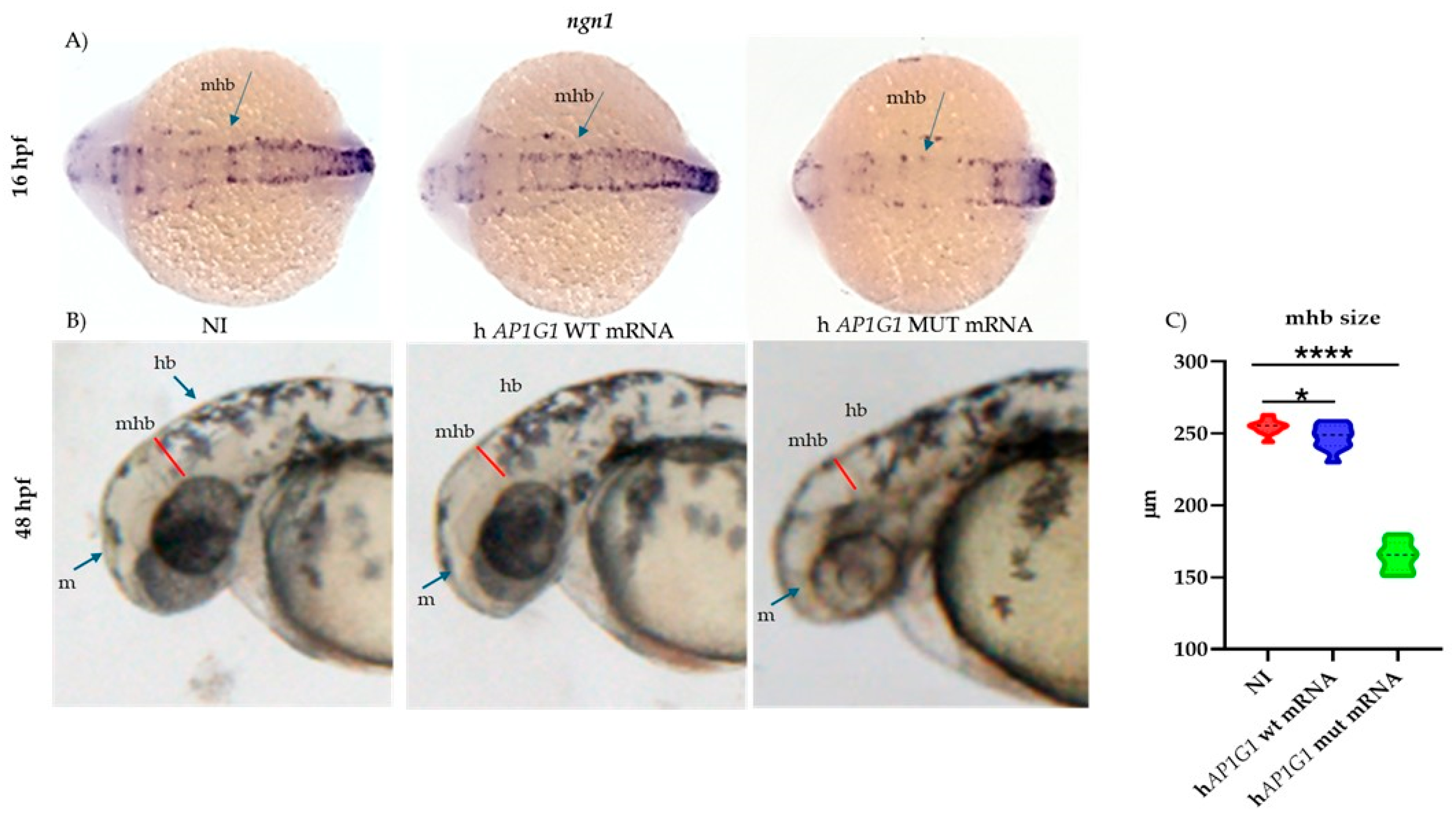
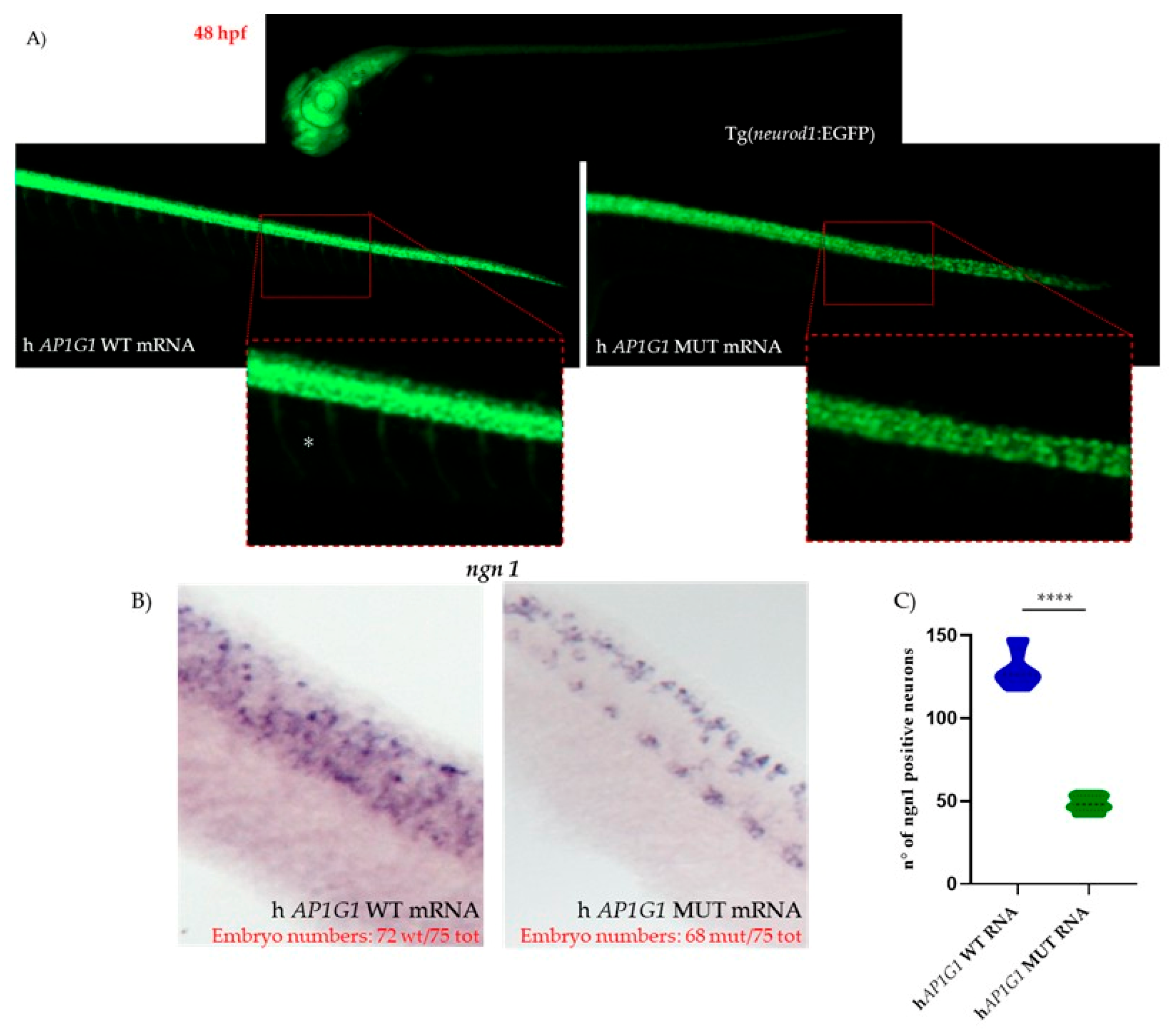
The midbrain–hindbrain boundary (MHB) serves as a critical organizing center during vertebrate brain development. This structure is composed of motor neurons that regulate eye, head, and body movements and plays an essential role in sensory-motor integration. Furthermore, the MHB acts as a key signaling hub required for patterning and neural differentiation—particularly of
ngn1-positive neurons—in the midbrain and anterior hindbrain [
37]. One of the most crucial genes involved in early embryonic neurodevelopment and neuronal commitment is
neurogenin 1 (
ngn1), a neural-specific basic helix-loop-helix (bHLH) transcription factor.
ngn1 is expressed at the onset of neurogenesis in zebrafish embryo neural plate domains that give rise to sensory neurons of the dorsal root ganglia and primary motor neurons that control embryo motility [
38]. Using the WISH technique, we observed that the injection of mutant mRNA caused the downregulation of
ngn1 mRNA expression at the early stages of development. This reduction may explain the initial phenotypic alterations observed, particularly delayed or impaired hatching rates.
ngn1 is also required for the development of sensory neurons in the zebrafish trunk. It is expressed early in the Rohon-Beard spinal sensory neurons, located within the spinal cord, and later in the dorsal root ganglia. These sensory neurons are considered touch-responsive neurons that induce locomotion following a tactile stimulus, a form of escape behavior [
39]. Following the injection of human MUT
AP1G1 mRNA,
ngn1 expression was lower in spinal cord neurons, where primitive sensory neurons reside, and in the main brain regions analyzed, such as the diencephalic area (where dopaminergic neurons are located), midbrain, and hindbrain, compared to embryos injected with human WT mRNA. Our findings suggest that mutations in the human
AP1G1 gene might also affect the somatosensory system; the observed locomotor impairments could be attributed not only to altered sensory neuron development but also to dysfunction in dopaminergic and motor neurons, as previously discussed. These findings are consistent with previously published results obtained from different in vivo models, in which the removal of one of the subunits belonging to different complexes reduced motor coordination or caused behavioral disturbances [
40].
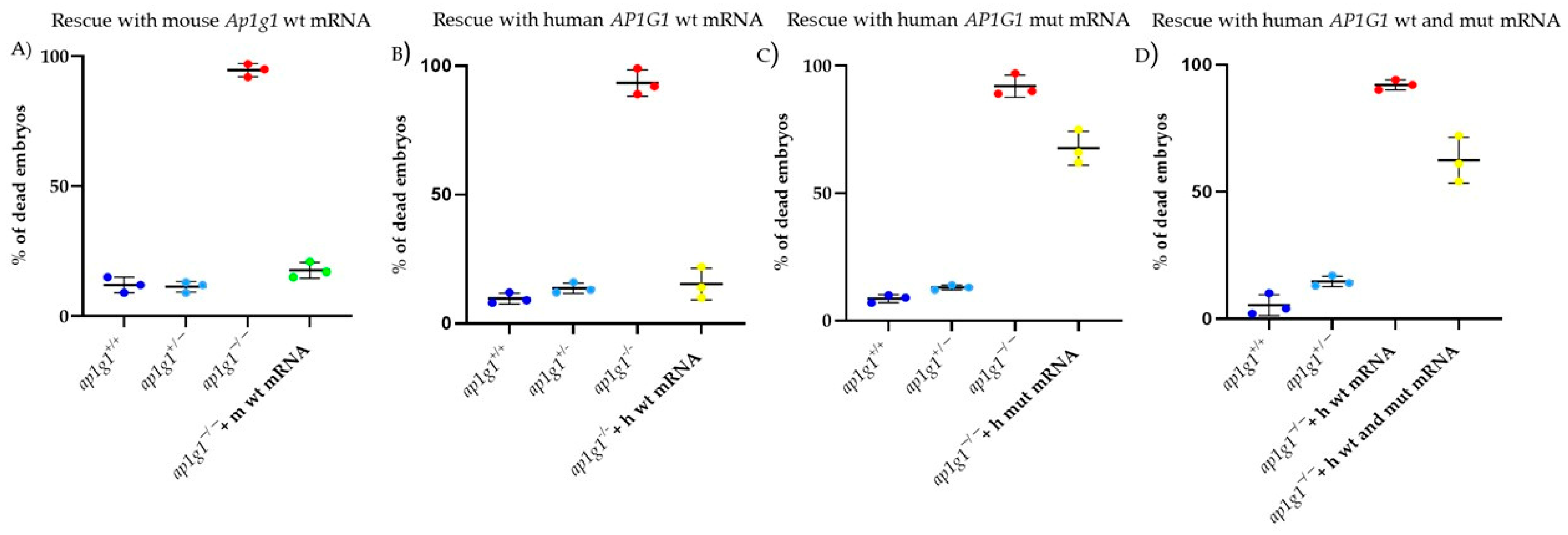
Finally, we were able to rescue the mortality rate of
ap1g1−/− knockout embryos (mutant line generated by our group [
24]) when we injected the human WT
AP1G1 mRNA; in contrast, embryos injected with human MUT
AP1G1 mRNA exhibited a significantly increased mortality rate. Co-injection of both mRNAs resulted in a slight increase in the survival rate, highlighting the dominant-negative effect exerted by the human MUT mRNA.
In conclusion, the use of zebrafish not only contributes to elucidating the mechanisms underlying neurodegenerative diseases but also holds promise for identifying genetic and molecular targets to develop effective therapeutic strategies in the context of adaptinopathies.
4. Materials and Methods
4.1. Samples and DNA Extraction
We conducted a “Whole-Exome Sequencing project” funded by the Italian Health Ministry (SG-2018-12368345 to V.I.). Patients were enrolled from a cohort of individuals showing intellectual disability and/or autism and/or congenital malformation syndromes who were referred to the Medical Genetics Unit of Perugia’s Hospital (Italy) for genetic counseling and remained undiagnosed after several negative genetic tests. Informed consent for the study was obtained, and genomic DNAs (gDNAs) of the proband and her parents were isolated from EDTA peripheral blood samples using a QIAamp DNA Blood Kit according to the manufacturer’s protocol (Qiagen, Hilden, Germany).
4.2. Case Presentation
The patient, currently a 12-year-old girl, was born to healthy, non-consanguineous parents following an uncomplicated pregnancy and delivery (
Figure S1). She exhibited motor and speech delays, achieved independent walking at 18 months, and pronounced fewer than 10 words at 36 months. At the age of 2 years, she experienced a single febrile seizure, and subsequent EEG and polysomnography results were normal. In addition, the brain MRI results were normal. She had self-harmed since the first year of life, with scratching injuries to the face and bite injuries to the fingers. Neuropsychiatric evaluation defined a mild to moderate form of intellectual disability (verbal IQ 53, performance 59, total 49), abnormal behavior including a tendency to aggression toward both peers and adults, hyperactivity, obsessive-compulsive behavior, and non-compliance. She presented with autonomic symptoms in situations of high stress, such as anxiety, cold hands, sweating, mydriasis, and stiffening of the facial features, which led to real crises. The patient was repeatedly treated with antibiotics (mainly macrolides) for recurrent upper respiratory tract infections. Interestingly, during this period, both parents and doctors reported a general but significant improvement in behavioral aspects. In particular, the patient appeared less aggressive and less hyperactive, even after the acute period, when antibiotic therapy continued. Based on clinical features, genetic tests were conducted on her genomic DNA, including sequencing of a panel of genes for intellectual disability, array-CGH, and FRAXA. All tests were negative. After obtaining written informed consent from the parents, exome sequencing (ES) was performed on the proband and her parents gDNA.
4.3. ES
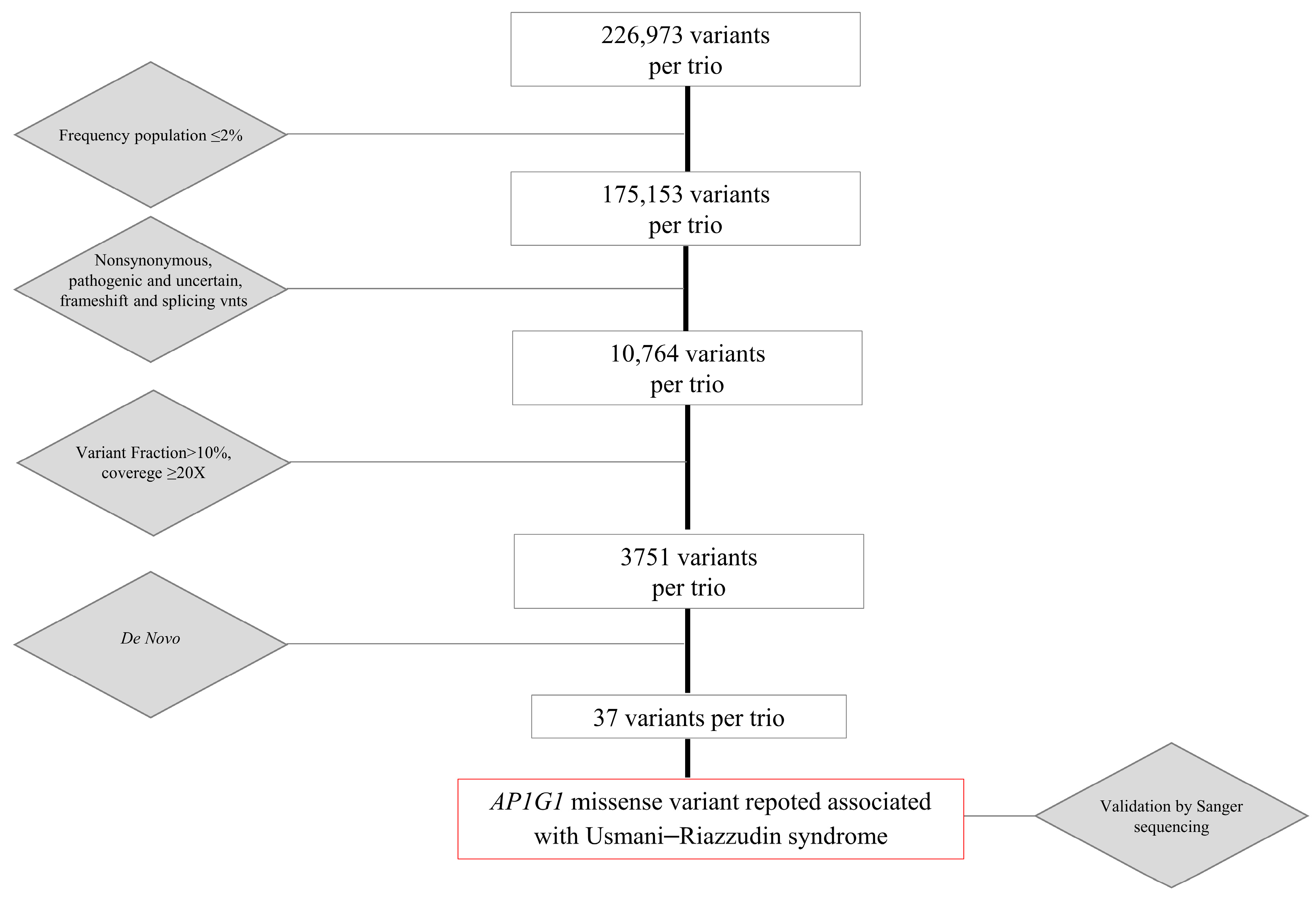
Exome sequencing was performed on the DNA samples of the proband and parent’s DNA samples using the Illumina Novaseq 6000 NGS platform (Illumina Inc., San Diego, CA, USA). Library preparation was performed using the BS Twist Human Core Exome (Twist Bioscience, South San Francisco, CA, USA), according to a specific protocol. Raw files (fastq) of each sample generated by the sequencing system were uploaded to the Sophia DDM platform v5.10.42 (Sophia Genetics, Rolle, Switzerland). The total amount of data already filtered (clean data), obtained by removing adapter contamination and low-quality sequences, was then mapped to the Human Reference Genome sequence (hg19) and then to the target regions. The analysis of these data is performed by the platform using both advanced bioinformatic tools and Sophia’s own patented software v5.10.42 that combine three specific algorithms for variant identification: (1) PEPPER for identification of SNVs and Indel; (2) MUSKAT for identification of CNVs; and (3) MOKA for variant annotation including different annotation tools (SIFT, Polyphen, MutationTaster, Mutation Assessor, FATHMM and GERP++ combined algorithm, LRT CADD, Varsome, Varsite and HOPE and Swiss Model; listed in section “web sources”). Variants were recalibrated using external datasets such as GnomAD (which includes 1000 Genomes and ExAC variants), ESP, COSMIC, and dbSNP (listed in section “web sources”). Variants were also filtered using custom-selected cutoffs to minimize false-positive detection. In particular, variants with allele frequencies < 2% and coverage ≥ 20× were retained. Variants that were reported to be benign or likely to be benign were excluded. The analysis of all remaining variants was initially conducted on trio data based on a “virtual gene panel” including potentially disease-causing genes correlated to the clinical signs presented by the patient. We then analyzed the data using a hereditary model approach by selecting all the de novo variants. The identified variants were validated using Sanger sequencing.
4.4. Sanger Sequencing
The c.196G>A (p.Gly66Arg) AP1G1 variant was validated using standard Sanger sequencing. A 409 bp region was PCR-amplified using the primers AP1G1-F (5′-acatctctatttaatgtctttc-3′) and AP1G1-R (5′-ctggaaaggcatgatattgct-3′). Sequencing was performed on an ABI Prism 3500 genetic analyzer (PE Applied Biosystems Inc., Foster City, CA, USA), and the data were analyzed using Sequencher software V.4.9 (Gene Codes Corporation, Ann Arbor, MI, USA).
4.5. Mutation Nomenclature
All mutations were described according to the Human Genome Variation Society (HGVS) [
41]. Nucleotide numbers were derived from the cDNA sequence of
AP1G1 (GenBank accession no. NM_001128).
4.6. Human Fibroblasts Cultures
Human fibroblasts from skin biopsies of the patient carrying the
AP1G1 variant and controls were obtained after obtaining informed consent and used at passage P6–10. A clinical description of the patients is reported in
Section 4.2. Control fibroblasts were obtained from adults. Ethical approval for skin biopsies was obtained from the reference hospital centers.
4.7. Immunofluorescence Assay
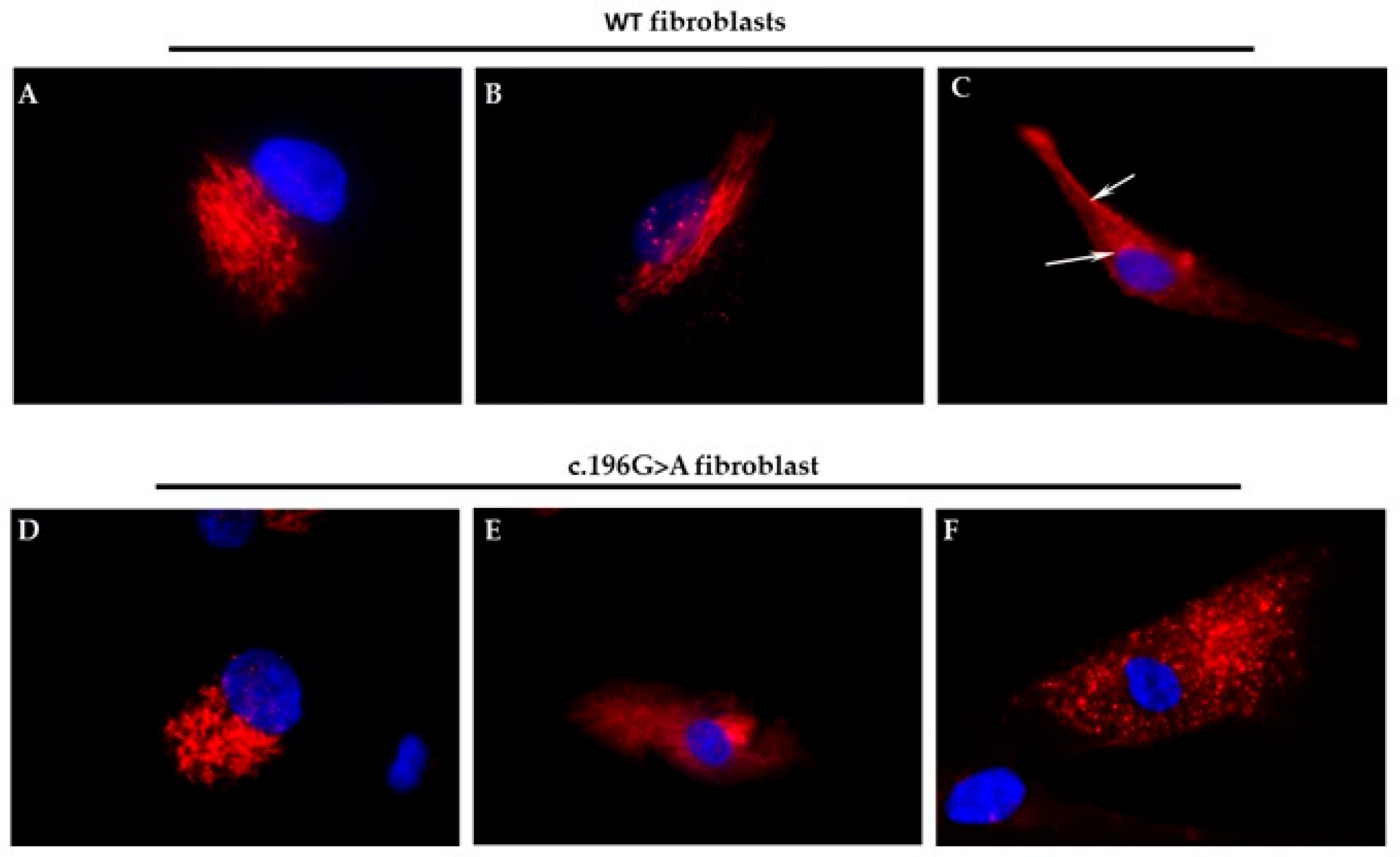
For immunofluorescent studies, 4 × 104 AP1G1 mutant and control fibroblasts were seeded and cultured for 24 h or 48 h on glass coverslips. Cells were extensively washed with PBS, fixed at 20 °C with cold methanol for 7 min, permeabilized with 0.1% Triton X-100 in PBS at room temperature for 5 min and washed three times with PBS. After overnight incubation with blocking buffer (3% BSA, 1% glycine in PBS), the cells were subjected to immunolabeling using polyclonal anti TGN46, anti G130, anti AP1G1 (1:100; ABclonal, Düsseldorf, Germany) and goat anti-rabbit TRITC (1:50; Sigma Aldrich, Saint Louis, MO, USA) antibodies with fluorescein-conjugated goat anti-mouse IgG (Sigma-Aldrich) (1:50 in PBS, containing 3% BSA). After washing three times with PBS containing 0.1% Tween 20 and twice with PBS alone, the fibroblasts were incubated with 4′,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich) (2 μg/mL) for 5 min and air-dried. The preparations were viewed under a DMRB Leica epimicroscope (Wetzlar, Germany) equipped with a digital camera.
4.8. In Vitro Synthesis of h WT AP1G1 and MUT AP1G1 mutantmRNAs
A total of 2 µg of recombinant plasmid pCS2+ containing the coding region of human AP1G1, both WT and mutant forms (Gly66Arg), was digested with EcoRI. The insert containing the specific AP1G1 cDNA was gel-purified and transcribed with SP6 RNA polymerase using the MEGAscript SP6 in vitro transcription kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions.
4.9. Zebrafish Maintenance and Eggs Collection
Zebrafish (
Danio rerio) embryos were collected from three different lines: AB wild-type line, transgenic line Tg (
neurod:EGFP), and CRISPR/Cas9-generated
ap1g1−/− Adult fish were maintained under standard laboratory conditions in a circulating water system (Techniplast ZebTec, Buguggiate, Varese, Italy) at 27+/−1 °C in a 14 h light and 10 h dark daily cycle. The housing system guaranteed fish water (0.1 g/L
−1 of Instant Ocean Sea Salts, 0.1 g/L
−1 of sodium bicarbonate, and 0.19g/L
−1 of calcium sulfate) at constant pH and conductivity values; ammonia, nitrite, and nitrate were kept below detection limits. Adult male and female animals were mated overnight in a breeding box. The next morning, freshly spawned eggs were collected, washed with fresh fish water, and maintained at 28 °C in 10 cm diameter Petri dishes containing fresh fish water until the onset of different embryonic stages, depending on the different experiments. Embryo staging was performed as described by Kimmel et al. (1995) [
42].
4.10. Microinjections
In vitro-synthesized mRNAs of either human WT or MUT
AP1G1 mRNAs were injected with the dye tracer phenol red in 1X Danieau buffer (pH 7.6) into 1- to 2-cell stage embryos, according to an established protocol while not injected embryos were used as controls (CTRL) [
43]. After microinjection, the embryos were incubated in fish water at 28 °C. To determine the optimal mRNA amount for further experiments, a dose–response curve was established. The following doses of each mRNA were injected: 10, 50, 100, and 250 pg/embryo. Survival rates and morphology were evaluated at 48 and 144 hpf. Data were obtained from four different experiments, with
n = 30 for each experimental point.
4.11. Whole-Mount In Situ Hybridization (Wish)
Whole-mount in situ hybridization (WISH) experiments were performed according to standard methods as previously described [
31]. All experiments were performed with embryos injected with the established optimal dose (50 pg/embryo) of either human WT or MUT
AP1G1 mRNAs at 16 and 48 hpf. Digoxigenin (DIG)-labeled antisense RNA probes for
neurogenin1 (
ngn1) and
neuronal differentiation 1 (
neurod1) genes were synthesized by in vitro transcription of linearized cDNA clones with T7 and SP6 RNA polymerases using DIG Labeling Mix (Roche, Basel, Switzerland) as previously described. Briefly, injected embryos at different developmental stages (hpf) were fixed overnight in 4% (
v/
v) paraformaldehyde (Sigma-Aldrich) at 4 °C, dehydrated through an ascending methanol (Sigma-Aldrich) series, and stored at −20 °C. After permeabilization with 10 µg/mL proteinase K (Roche), embryos were hybridized overnight at 68 °C. The following day, repeated washes were performed at high stringent temperature with saline-sodium citrate buffer (SSC) 2×/phosphate-buffered saline (PBS) 1× and SSC 0·2×/PBS 1X, then embryos were incubated with anti-DIG antibody (1:10.000) conjugated with alkaline-phosphatase (Roche) overnight at 4 °C. Staining was performed with nitroblue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl-phosphate (BCIP) (Roche) alkaline-phosphatase substrates according to the manufacturer’s instructions. Embryos were mounted in agarose-coated dishes, and WISH images were acquired using an AxioZoom V16 microscope equipped with a Zeiss Axiocam 506 color digital camera and processed using Zen 3.5 (Blue Version) software from Zeiss (Oberkochen, Germany). WISH experiments were performed in triplicate for both probes, and 30 embryos were analyzed for each experimental condition.
4.12. Microscopy
Bright-field images of embryos (anesthetized with tricaine 0.16 mg/mL embedded in 0.8% low-melting agarose and mounted on a depression slide) were captured using a Zeiss Axio Zoom V16 microscope equipped with a Zeiss Axiocam 506 color digital camera and processed using Zen 3.5 (Blue Version) software from Zeiss (Oberkochen, Germany). For light-sheet fluorescence microscopy analysis, embryos were first anesthetized using tricaine (0.02% in fish water) and subsequently embedded in a low-melting agarose matrix (Top Vision Low Melting Point Agarose, Thermo Fisher Scientific) at 0.5% in fish water. Images were acquired using a Zeiss Light Sheet microscope V1 supported by ZenPro software (Zen3.5 Blue version) using a 488 nm laser and 505–545 nm filter. Images from the same experiment were acquired using the same laser intensity and exposure time to obtain comparable data. After acquisition, 3D images were generated and manipulated using Arivis Vision 4D (Zeiss, Oberkochen, Germany). Three-dimensional reconstructions of EGFP-positive cells were manipulated to obtain images comparable to each other in terms of fluorescence intensities. Three-dimensional reconstructions were exported as single snapshots using the same compression settings.
4.13. Statistical Analysis
All graphs were plotted and analyzed using GraphPad Prism version 8 (GraphPad Software). Statistical significance was determined using one-way ANOVA with Tukey’s multiple comparison test. In all cases, p-values less than 0.05 were considered statistically significant (* = p < 0.05; ** = p < 0.005; *** = p < 0.001; **** = p < 0.0001). All data are presented as the mean ± standard error of the mean (SEM). Data from the behavioral experiments were analyzed using an unpaired t-test.
4.14. Locomotor Behavior Assessment
The injected embryos were maintained in fish water and incubated at 28 °C until 144 hpf. The light–dark locomotion test was performed as previously described [
44]. Briefly, for each treatment, 12 larvae at 144 hpf were collected in a 96-square well plate with one larva per well and a volume of 200 μL. The 96-square well plate was then placed in the observation chamber of the
Danio Vision Noldus system holder (Noldus, Wageningen, The Netherlands) in an isolated noise-free room. The larvae were allowed to adapt for 30 min before video recording. The system was then set up to track movements by recording the distance traveled in 2 min intervals over a 2 h observation period during 6 cycles of alternating light and dark 10 min periods. Data were analyzed using Noldus
Ethovision software (XT 13.0 Version). Movements were reported as the total distance (mm) traveled by the larvae and speed (mm/s), calculated under both light and dark stimuli. Each light–dark locomotion experiment was repeated three times for both injected embryos, with 12 embryos in each group and each experiment.
4.15. Touch-Evoked Test
Non-injected and injected embryos were grown until 96 hpf. At this stage, 20 embryos were injected with either WT or MUT AP1G1 mRNA and each of the three experiments was subjected to a touch-evoked test. Briefly, embryos were placed in Petri dishes beneath which a motility wheel was positioned, consisting of two concentric circles of increasing diameter (20 mm and >20 mm). Each embryo was initially placed at the center of a Petri dish, and the tail was gently touched with a smooth pipette tip. The touch response was observed and categorized as follows: (1) embryos that did not move, (2) embryos that swam < 20 mm, and (3) embryos that crossed the inner circle and swam > 20 mm. The percentages of each category (non-injected and injected) were calculated.