Significant Suppression of Multiple Sclerosis in the Mouse EAE Model Using the PrC-210 Aminothiol
Abstract
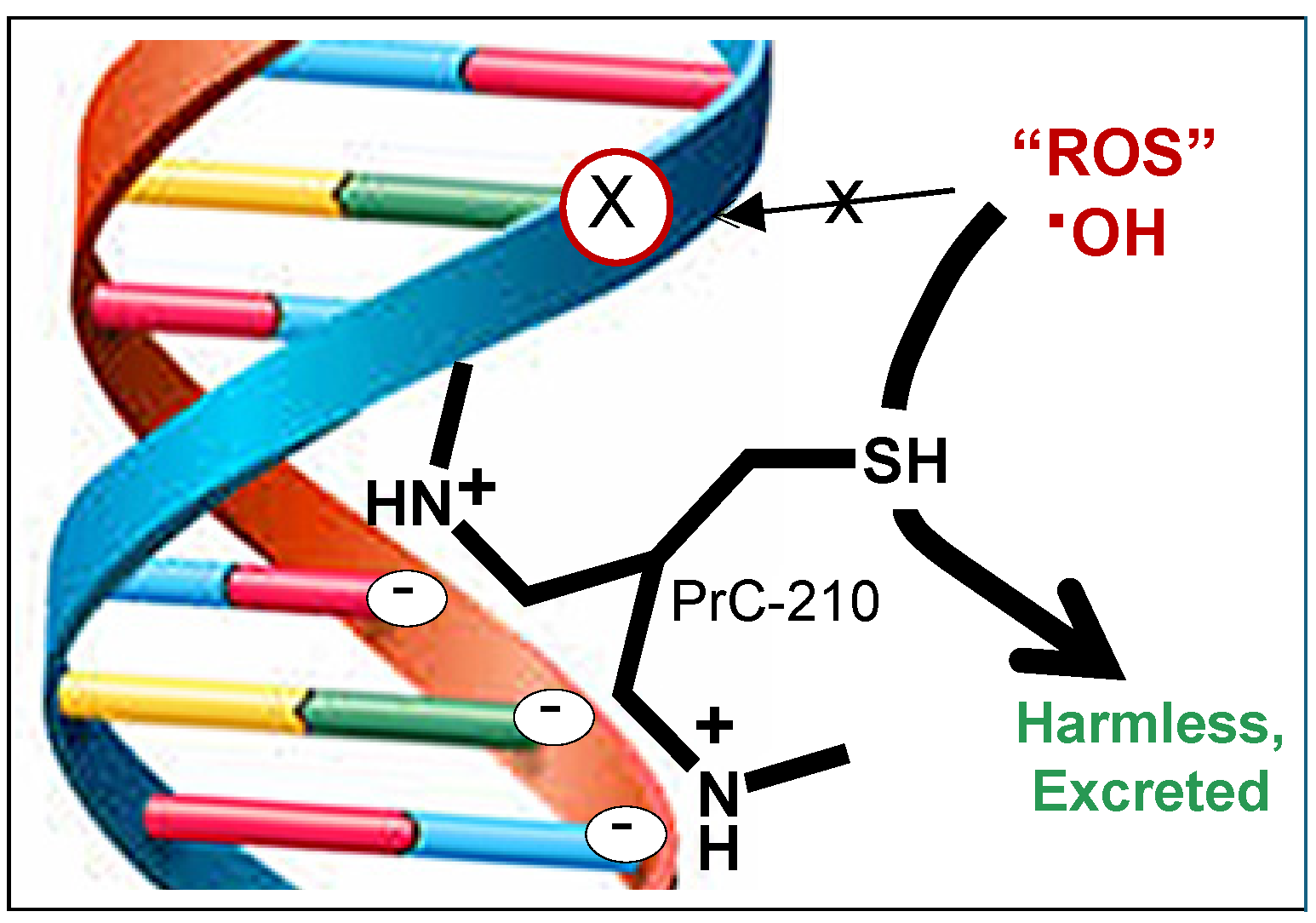
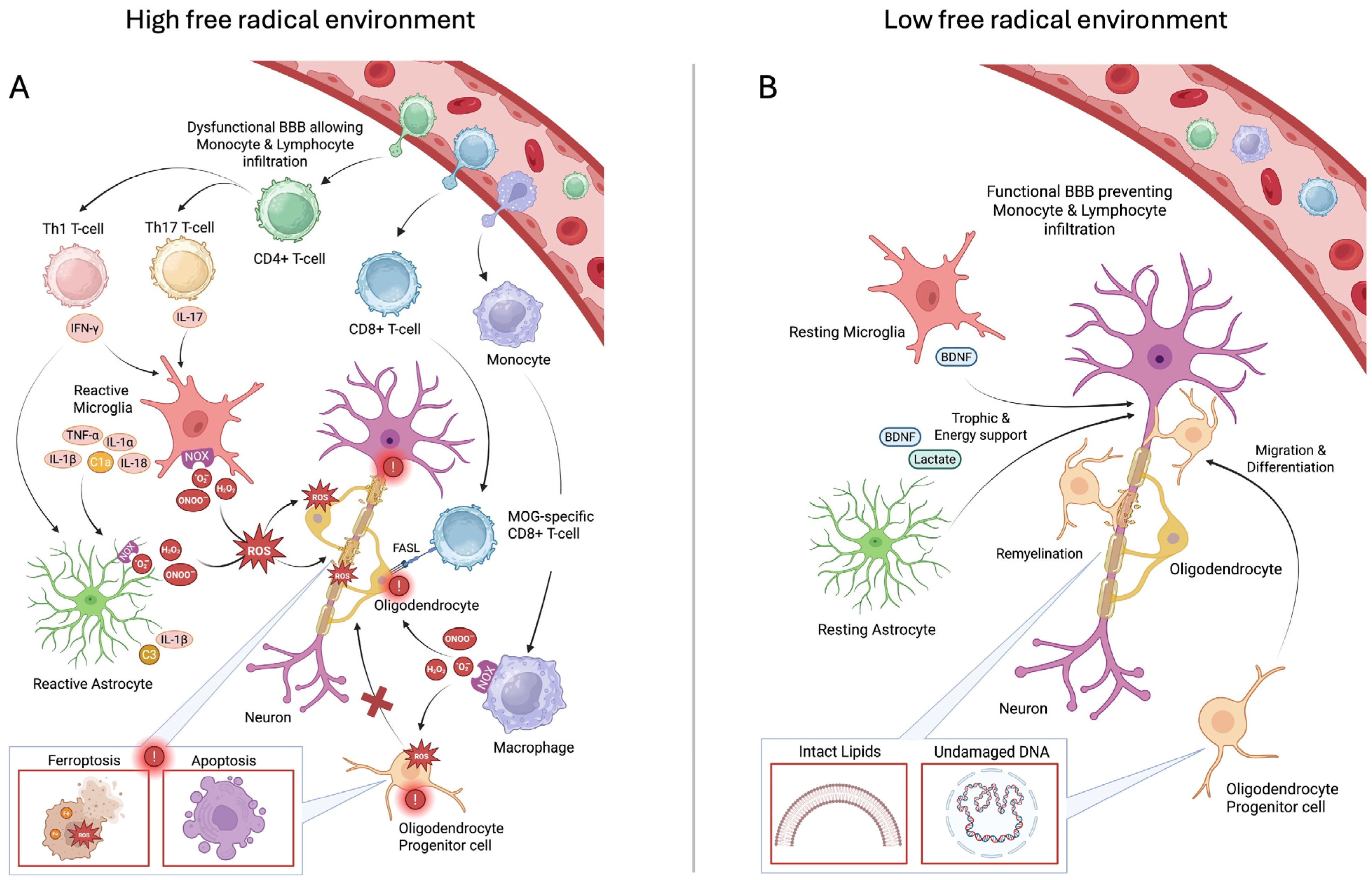
1. Introduction
2. Results
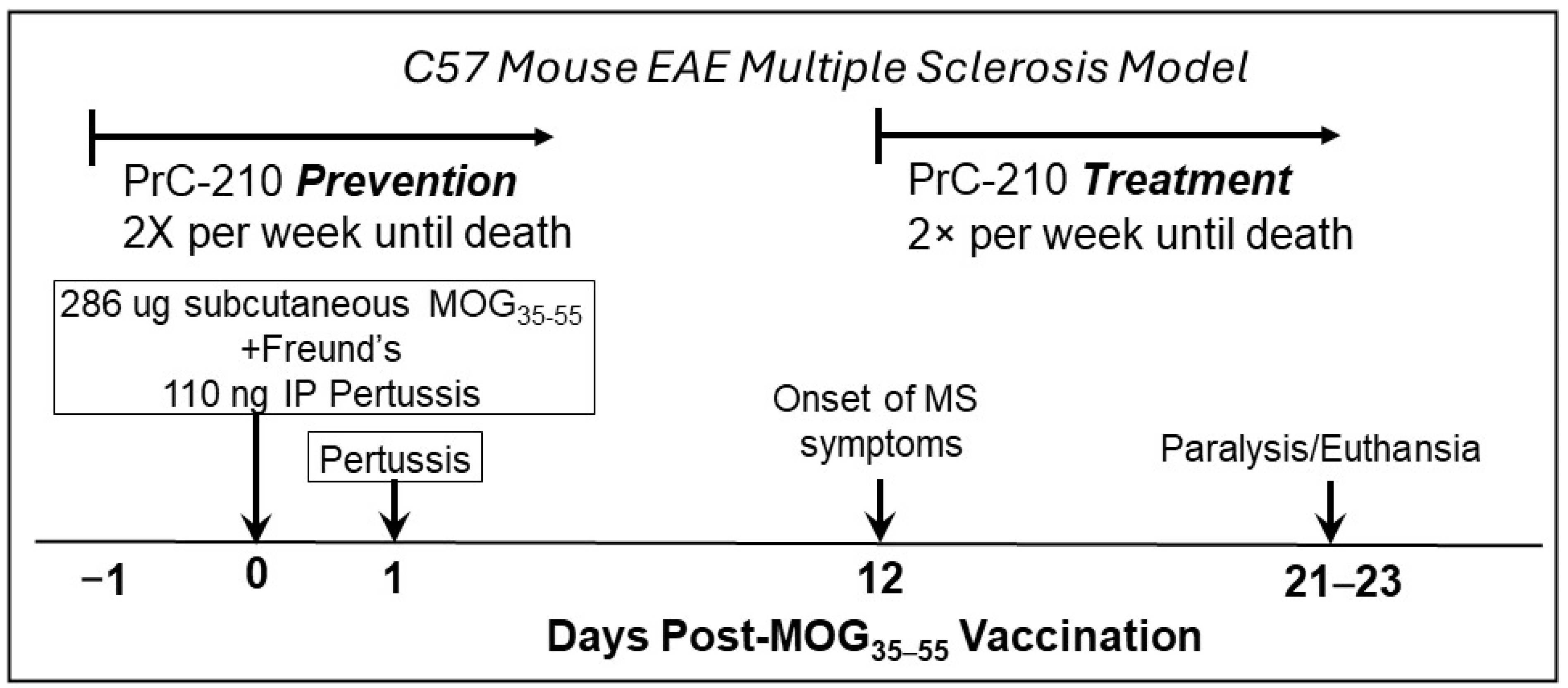
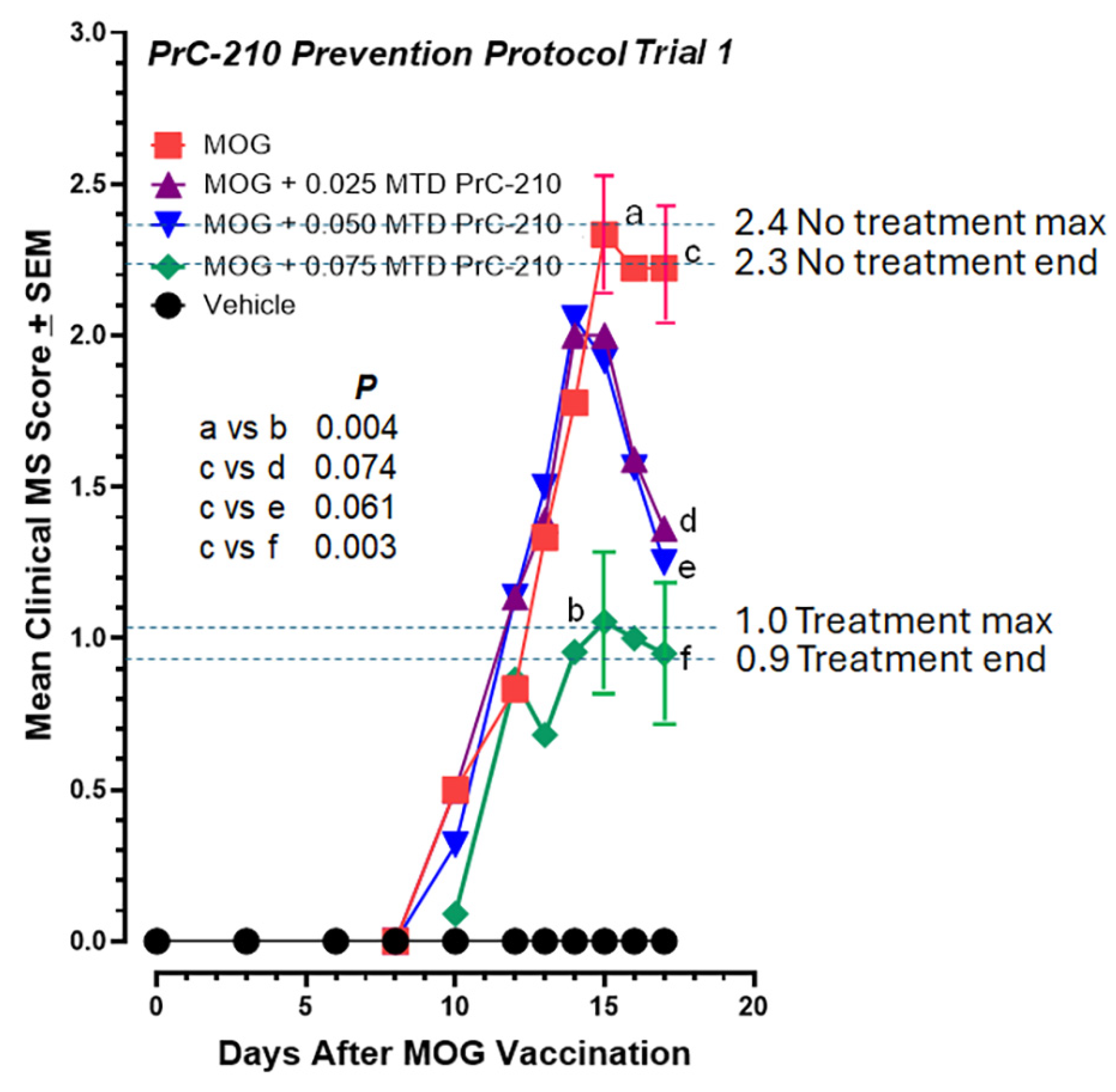
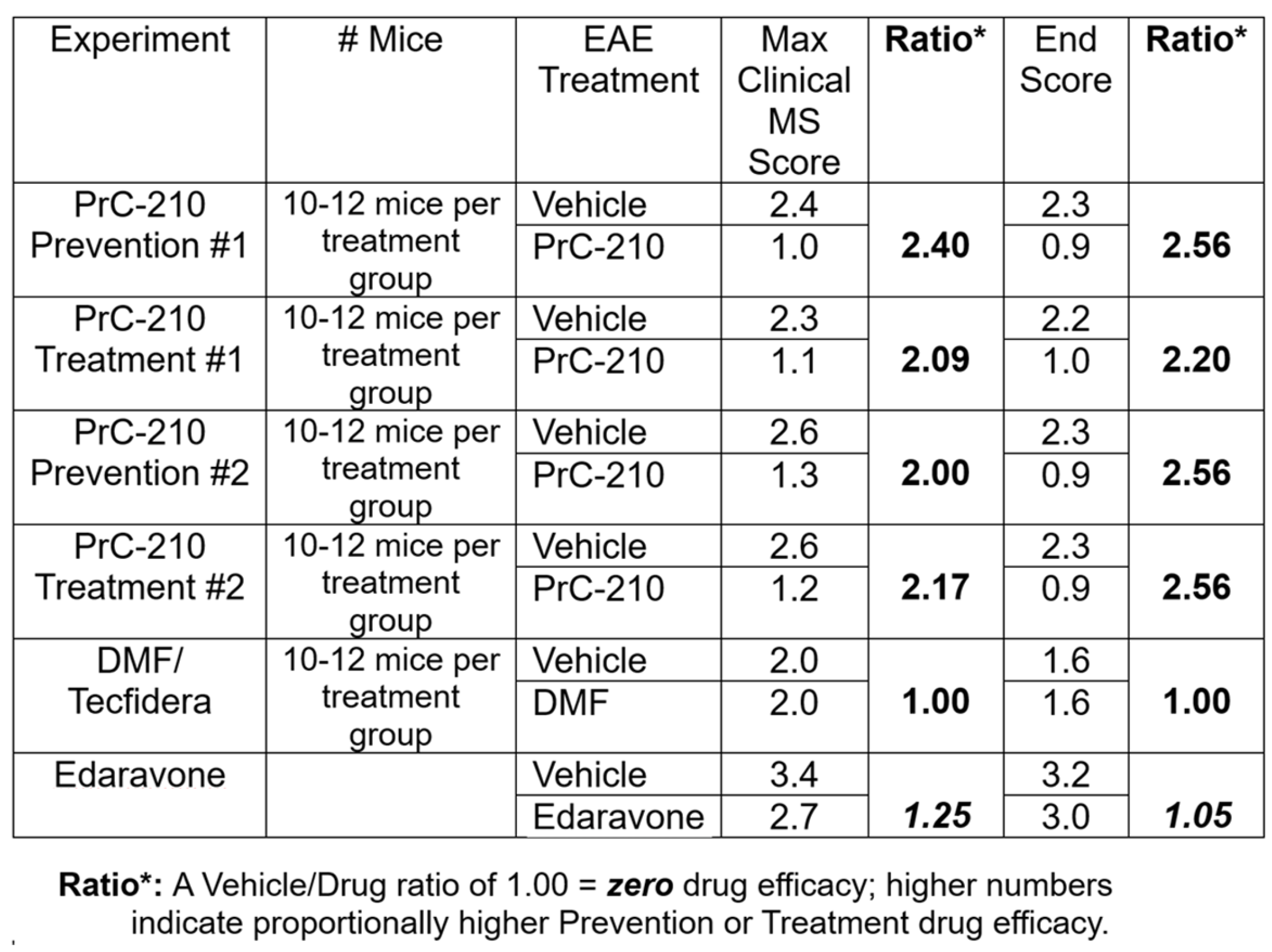
2.1. Incidence and Initial Prevention of EAE Paralysis
2.2. Initial Treatment Regimen to Suppress EAE Paralysis
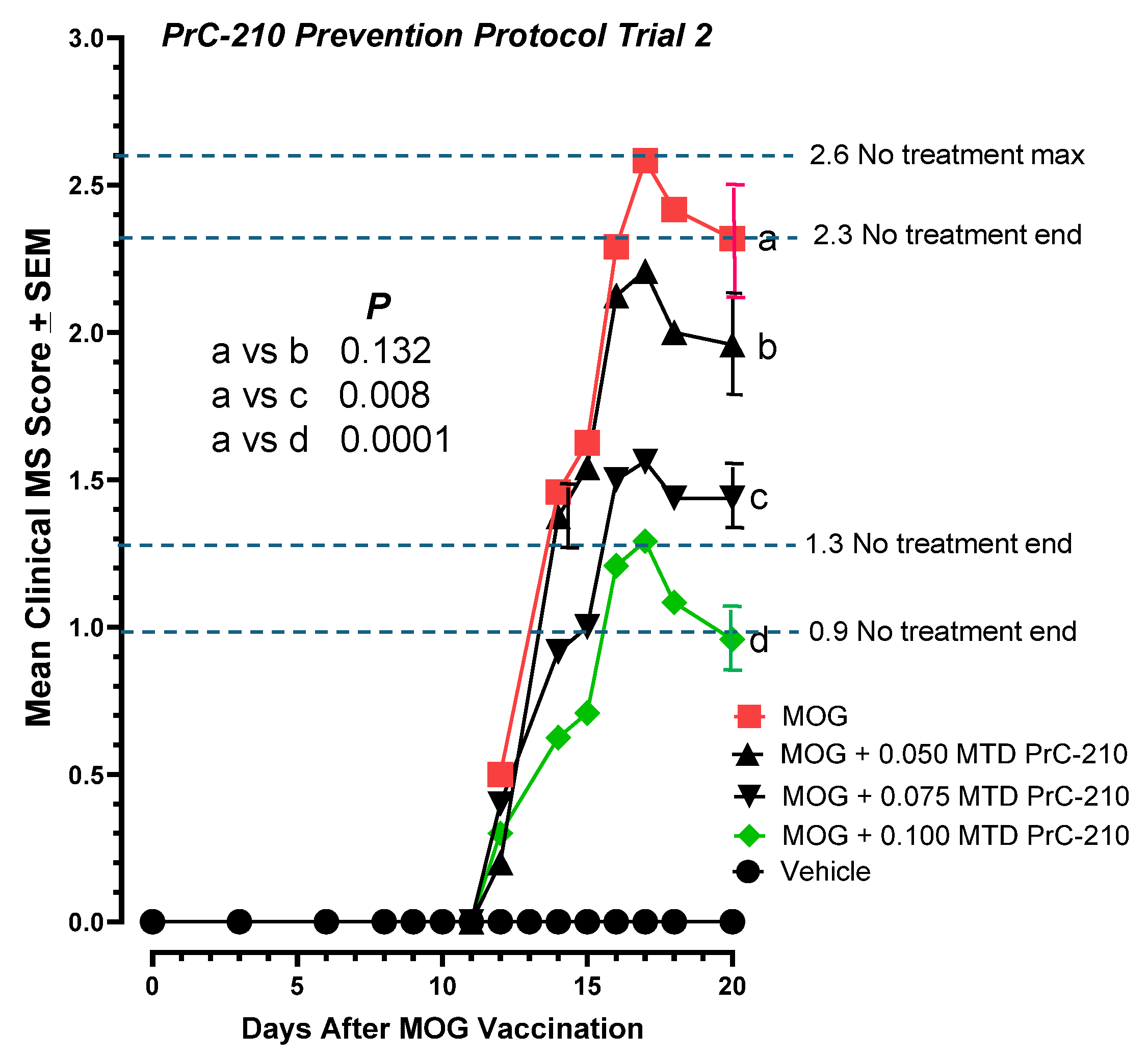
2.3. Higher-Dose PrC-210 Prevention Protocol to Suppress EAE Paralysis
2.4. Higher-Dose PrC-210 Treatment Protocol to Suppress EAE Paralysis
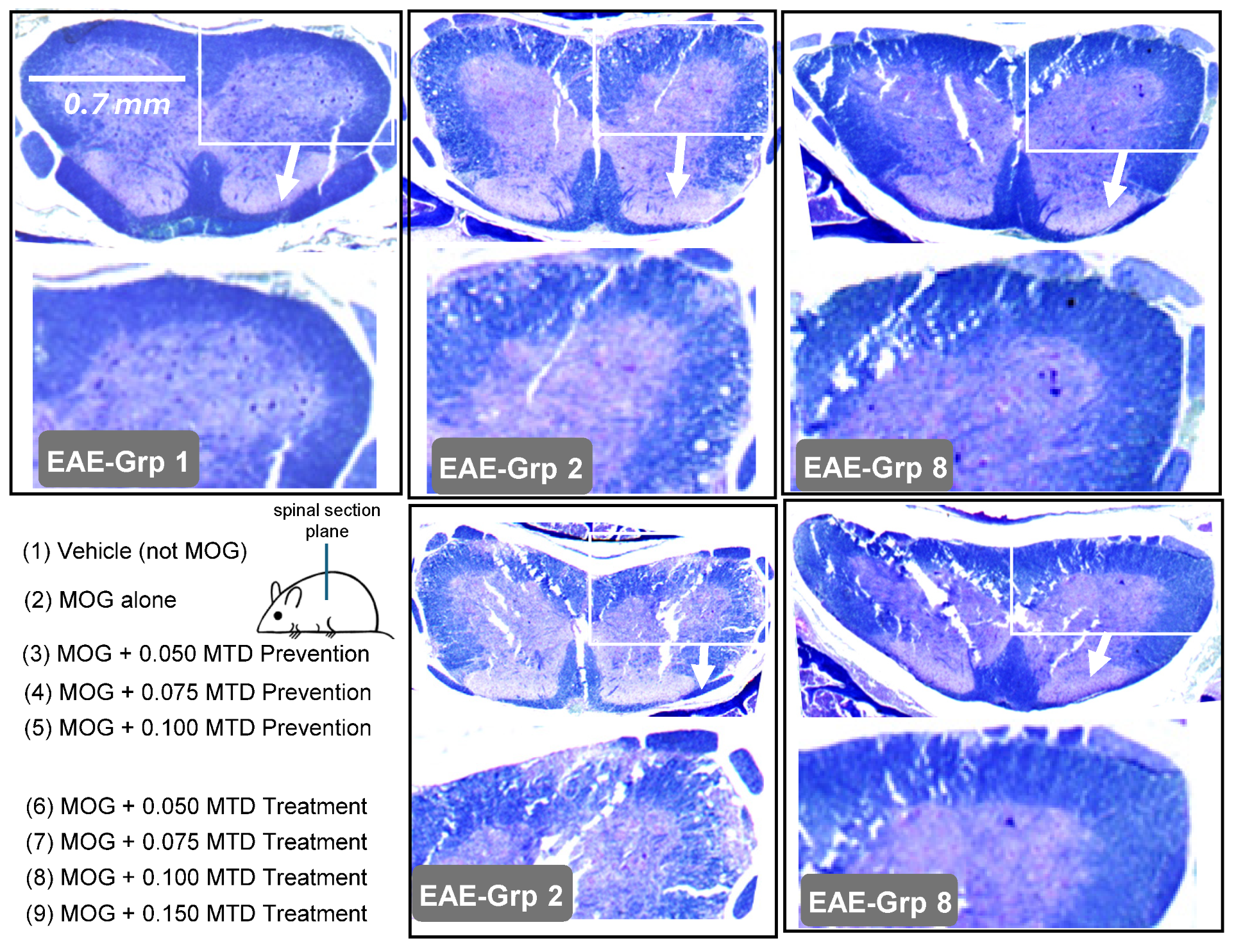
2.5. Spinal Histology Supports Paralysis Suppression Induced by PrC-210
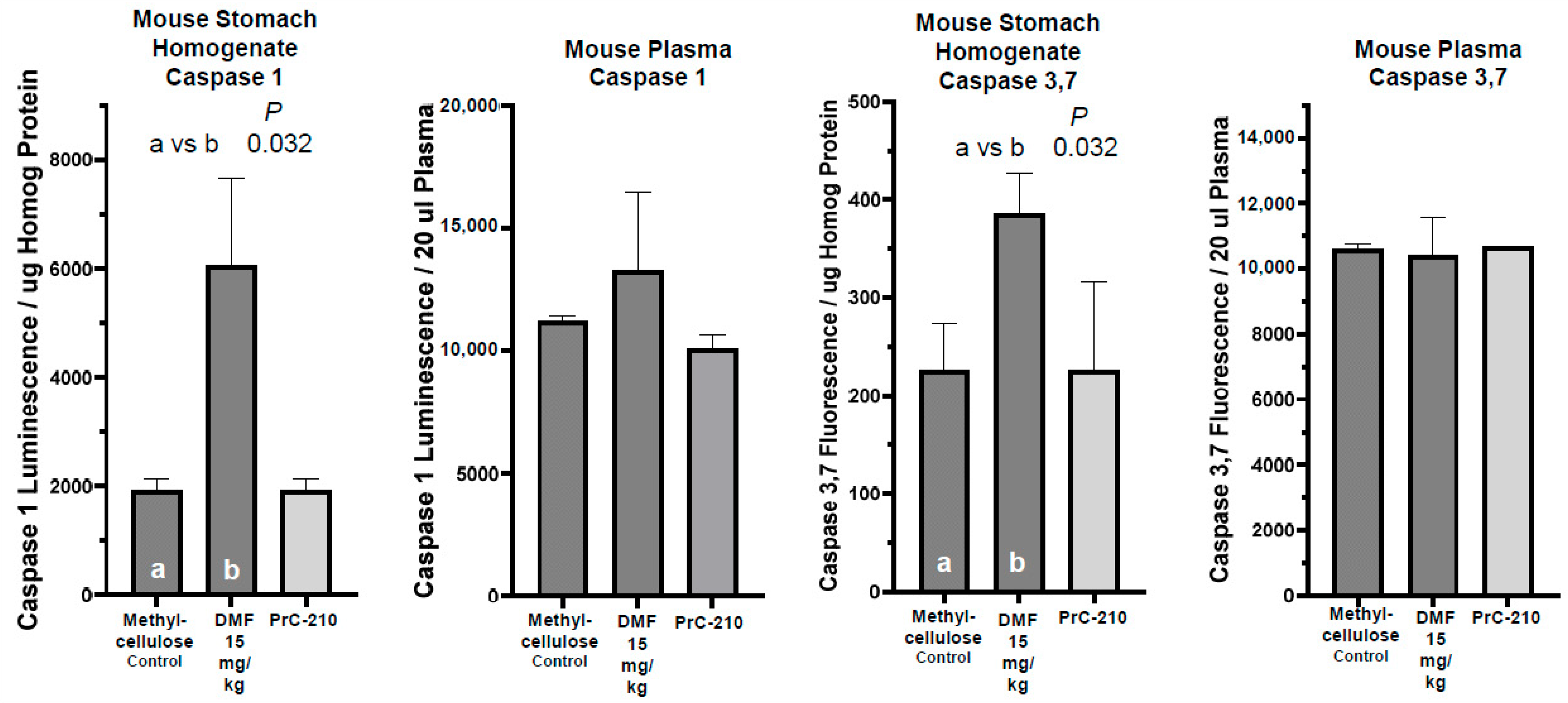
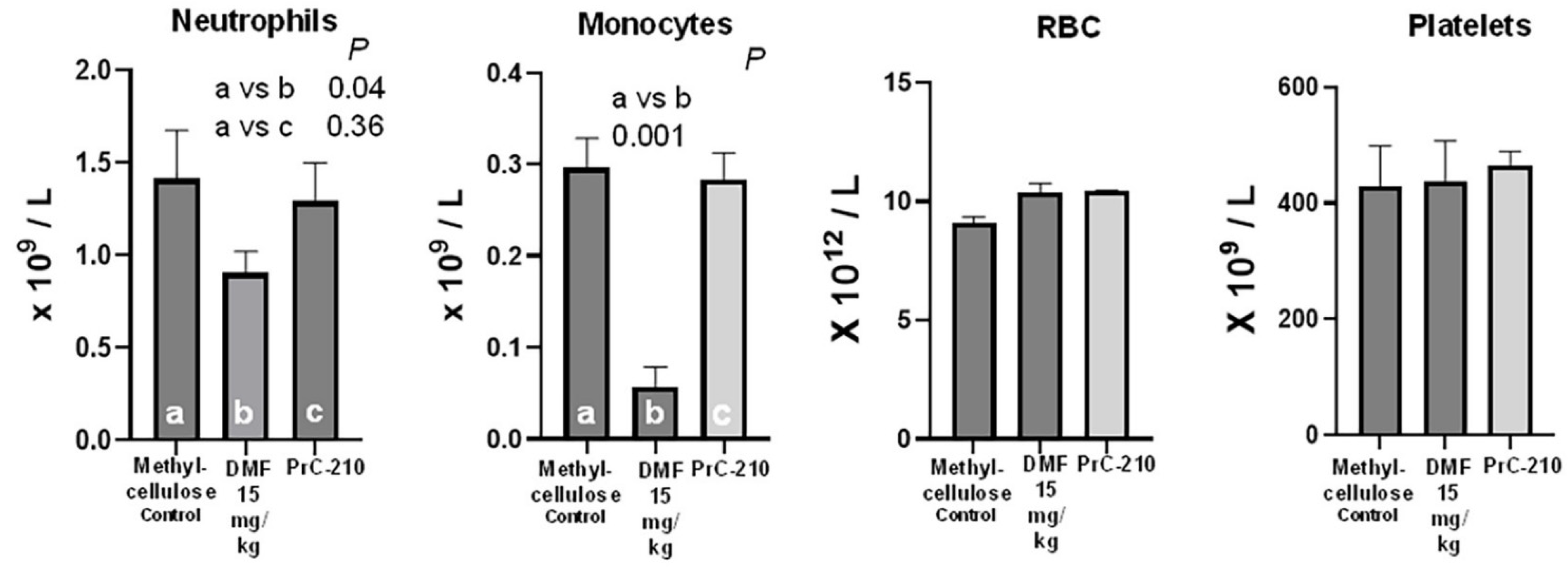
2.6. Oral DMF’s Toxicity Versus That of Oral Control or Oral PrC-210 Treatments
Stomach Tissue Toxicity
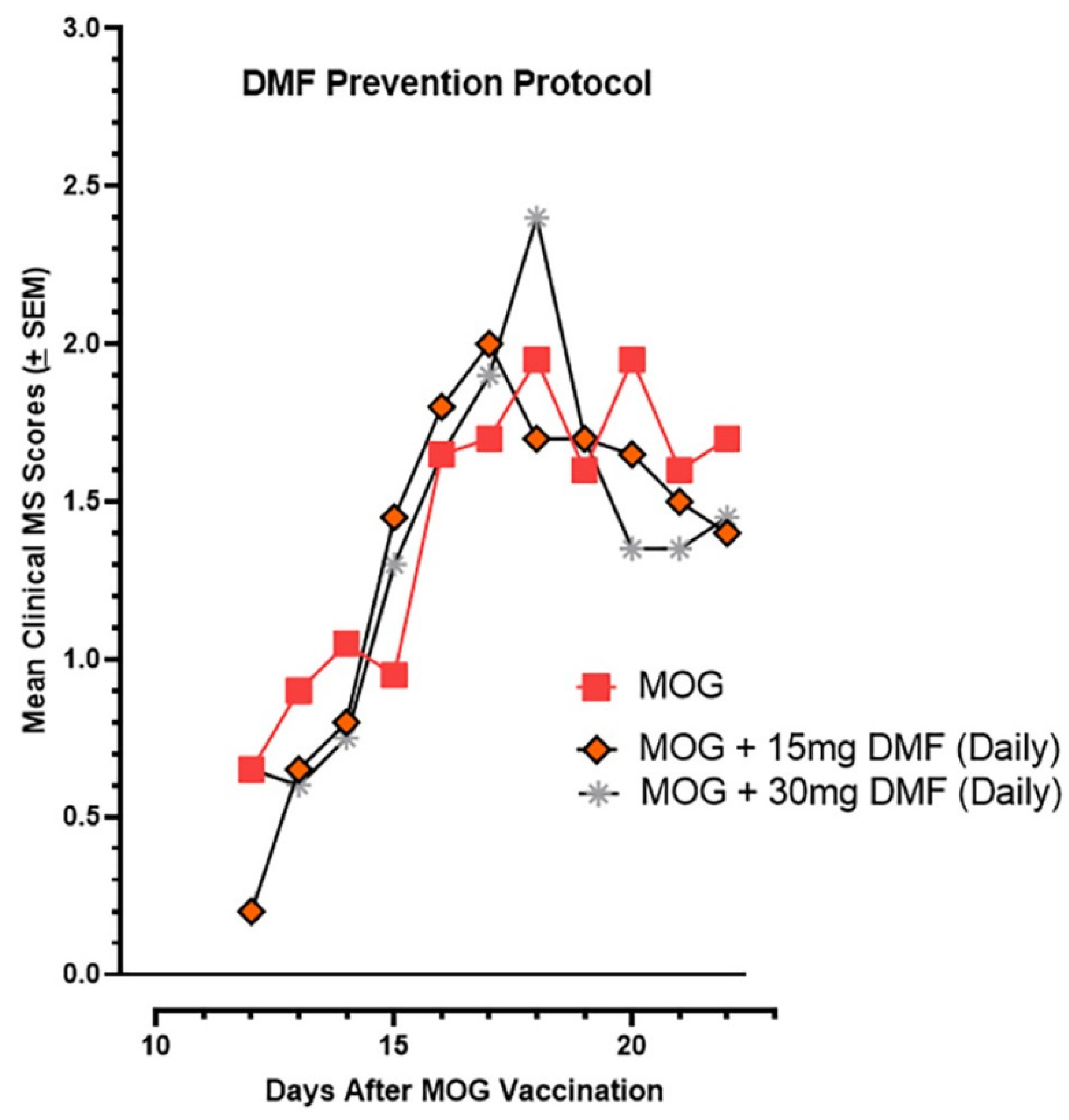
2.7. Oral DMF’s Efficacy in Suppressing EAE Paralysis
3. Discussion
4. Materials and Methods
4.1. Materials and PrC-210
4.2. Animal Studies
4.3. Histology
4.4. DMF Toxicity Analyses
4.4.1. Stomach Tissue Homogenization and Protein Determination
4.4.2. Caspase 1 Assay
4.4.3. Caspase 3, 7 Assay
4.4.4. Mouse Blood Complete Blood Count (CBC)
4.4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The Immunopathology of Multiple Sclerosis: An Overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Tavassolifar, M.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H.; van Horssen, J. Oxidative Stress and Its Impact on Neurons and Glia in Multiple Sclerosis Lesions. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2016, 1862, 506–510. [Google Scholar] [CrossRef] [PubMed]
- van Horssen, J.; Schreibelt, G.; Drexhage, J.; Hazes, T.; Dijkstra, C.; van der Valk, P.; de Vries, H. Severe Oxidative Damage in Multiple Sclerosis Lesions Coincides with Enhanced Antioxidant Enzyme Expression. Free Radic. Biol. Med. 2008, 45, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Bierhansl, L.; Hartung, H.-P.; Aktas, O.; Ruck, T.; Roden, M.; Meuth, S.G. Thinking Outside the Box: Non-Canonical Targets in Multiple Sclerosis. Nat. Rev. Drug Discov. 2022, 21, 578–600. [Google Scholar] [CrossRef]
- Garg, N.; Smith, T.W. An Update on Immunopathogenesis, Diagnosis, and Treatment of Multiple Sclerosis. Brain Behav. 2015, 5, e00362. [Google Scholar] [CrossRef]
- Ziabreva, I.; Campbell, G.; Rist, J.; Zambonin, J.; Rorbach, J.; Wydro, M.M.; Lassmann, H.; Franklin, R.J.M.; Mahad, D. Injury and Differentiation Following Inhibition of Mitochondrial Respiratory Chain Complex IV in Rat Oligodendrocytes. Glia 2010, 58, 1827. [Google Scholar] [CrossRef]
- Hollen, C.; Neilson, L.E.; Barajas, R.F., Jr.; Greenhouse, I.; Spain, R.I. Oxidative Stress in Multiple Sclerosis—Emerging imaging techniques. Front. Neurol. 2023, 13, 1025659. [Google Scholar] [CrossRef]
- Johnson, D.A.; Amirahmadi, S.; Ward, C.; Fabry, Z.; Johnson, J.A. The Absence of the Pro-antioxidant Transcription Factor Nrf2 Exacerbates Experimental Autoimmune Encephalomyelitis. Toxicol. Sci. 2009, 114, 237–246. [Google Scholar] [CrossRef]
- International Multiple Sclerosis Genetics Consortium. Multiple Sclerosis Genomic Map Implicates Peripheral Immune Cells and Microglia in Susceptibility. Science 2019, 365, eaav7188. [Google Scholar] [CrossRef]
- Trapp, B.D.; Peterson, J.; Ransohoff, R.M.; Rudick, R.; Mörk, S.; Bö, L. Axonal Transection in the Lesions of Multiple Sclerosis. N. Engl. J. Med. 1998, 338, 278–285. [Google Scholar] [CrossRef]
- Stys, P.K.; Zamponi, G.W.; van Minnen, J.; Geurts, J.J.G. Will the Real Multiple Sclerosis Please Stand Up? Nat. Rev. Neurosci. 2012, 13, 507–514, Erratum in Nat. Rev. Neurosci. 2012, 13, 597. [Google Scholar] [CrossRef]
- Ramos-González, E.J.; Bitzer-Quintero, O.K.; Ortiz, G.; Hernández-Cruz, J.J.; Ramírez-Jirano, L.J. Relationship Between Inflammation and Oxidative Stress and its Effect on Multiple Sclerosis. Neurología 2024, 39, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of Multiple Sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Schetters, S.T.T.; Gomez-Nicola, D.; Garcia-Vallejo, J.J.; Van Kooyk, Y. Neuroinflammation: Microglia and T Cells Get Ready to Tango. Front. Immunol. 2018, 8, 1905. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Mucke, L. Inflammation in Neurodegenerative Disease—A Double-Edged Sword. Neuron 2002, 35, 419–432. [Google Scholar] [CrossRef]
- Chastain, E.M.L.; Duncan, D.S.; Rodgers, J.M.; Miller, S.D. The Role of Antigen Presenting Cells in Multiple Sclerosis. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2011, 1812, 265–274. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Bitzer-Quintero, O.K.; Ramírez-Anguiano, A.C.; Flores-Alvarado, L.J.; Ramírez-Ramírez, V.; Macias-Islas, M.A.; Torres-Sánchez, E.D. Immunology and Oxidative Stress in Multiple Sclerosis: Clinical and Basic Approach. J. Immunol. Res. 2013, 2013, 708659. [Google Scholar] [CrossRef]
- Fischer, M.-T.; Sharma, R.; Lim, J.L.; Haider, L.; Frischer, J.M.; Drexhage, J.; Mahad, D.; Bradl, M.; Van Horssen, J.; Lassmann, H. NADPH Oxidase Expression in Active Multiple Sclerosis Lesions in Relation to Oxidative Tissue Damage and Mitochondrial Injury. Brain 2012, 135, 886–899. [Google Scholar] [CrossRef]
- Haider, L.; Fischer, M.T.; Frischer, J.M.; Bauer, J.; Höftberger, R.; Botond, G.; Esterbauer, H.; Binder, C.J.; Witztum, J.L.; Lassmann, H. Oxidative Damage in Multiple Sclerosis Lesions. Brain 2011, 134, 1914–1924. [Google Scholar] [CrossRef]
- Yong, V.W. Microglia in Multiple Sclerosis: Protectors Turn Destroyers. Neuron 2022, 110, 3534–3548. [Google Scholar] [CrossRef] [PubMed]
- Jermusek, F., Jr.; Benedict, C.; Dreischmeier, E.; Brand, M.; Uder, M.; Jeffery, J.J.; Ranallo, F.N.; Fahl, W.E. Significant Suppression of CT Radiation-Induced DNA Damage in Normal Human Cells by the PrC-210 Radioprotector. Radiat. Res. 2018, 190, 133–141. [Google Scholar] [CrossRef]
- Peebles, D.D.; Soref, C.M.; Copp, R.R.; Thunberg, A.L.; Fahl, W.E. ROS-Scavenger and Radioprotective Efficacy of the New PrC-210 Aminothiol. Radiat. Res. 2012, 178, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hacker, T.A.; Diarra, G.; Fahl, B.L.; Back, S.; Kaufmann, E.; Fahl, W.E. Significant Reduction of Ischemia-Reperfusion Cell Death in Mouse Myocardial Infarcts Using the Immediate-Acting PrC-210 ROS-Scavenger. Pharmacol. Res. Perspect. 2019, 7, e00500. [Google Scholar] [CrossRef] [PubMed]
- Goesch, T.R.; Wilson, N.A.; Zeng, W.; Verhoven, B.M.; Zhong, W.; Gitter, M.M.C.; Fahl, W.E. Suppression of Inflammation-Associated Kidney Damage Post-Transplant Using the New PrC-210 Free Radical Scavenger in Rats. Biomolecules 2021, 11, 1054. [Google Scholar] [CrossRef]
- Liermann-Wooldrik, K.T.; Chatterjee, A.; Kosmacek, E.A.; Myers, M.S.; Adebisi, O.; Monga-Wells, L.; Mei, L.; Takacs, M.P.; Dussault, P.H.; Draney, D.R.; et al. Identification of Potential Prophylactic Medical Countermeasures Against Acute Radiation Syndrome (ARS). Int. J. Mol. Sci. 2025, 26, 4055. [Google Scholar] [CrossRef]
- Soref, C.M.; Hacker, T.A.; Fahl, W.E. A New Orally Active, Aminothiol Radioprotector Free of Nausea and Hypotension Side Effects at its Highest Radioprotective Doses. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, e701–e707. [Google Scholar] [CrossRef]
- Hooke Laboratories. Mouse EAE Scoring. Available online: https://hookelabs.com/services/cro/eae/MouseEAEscoring.html (accessed on 26 March 2025).
- Schilling, S.; Goelz, S.; Linker, R.; Luehder, F.; Gold, R. Fumaric Acid Esters are Effective in Chronic Experimental Autoimuune Encephalomyelitis and Suppress Macrophage Infiltration. Clin. Exp. Immunol. 2006, 145, 101–107. [Google Scholar] [CrossRef]
- Koulinska, I.; Riester, K.; Chalkias, S.; Edwards, M.R. Effect of Bismuth Subsalicylate on Gastrointestinal Tolerability in Healthy Volunteers Receiving Oral Delayed-release Dimethyl Fumarate: PREVENT, a Randomized, Multicenter, Double-blind, Placebo-Controlled Study. Clin. Ther. 2018, 40, 2021–2030.e1. [Google Scholar] [CrossRef]
- Bajwa, N.A.; Bajwa, A.A.; Ghazala, S.; Masood, U. Dimethyl Fumarate-Associated Enterocolitis. Am. J. Ther. 2024, 31, e448–e450. [Google Scholar] [CrossRef]
- de Bruin, N.; Schmitz, K.; Schiffmann, S.; Tafferner, N.; Schmidt, M.; Jordan, H.; Häußler, A.; Tegeder, I.; Geisslinger, G.; Parnham, M. Multiple Rodent Models and Behavioral Measures Reveal Unexpected Responses to FTY720 and DMF in Experimental Autoimmuune Encephalomyelitis. Behav. Brain Res. 2016, 300, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.D.R.; Ferrari, B.B.; Pradella, F.; Carvalho, R.M.; Rivero, S.L.S.; Quintiliano, R.P.S.; Souza, M.A.; Brunetti, N.S.; Marques, A.M.; Santos, I.P.; et al. Dimethyl Fumarate Modulates the Regulatory T Cell Response in Lymph Nodes of Mice with Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2024, 15, 1391949. [Google Scholar] [CrossRef]
- Buckle, G.; Bandari, D.; Greenstein, J.; Gudesblatt, M.; Khatri, B.; Kita, M.; Repovic, P.; Riser, E.; Weinstock-Guttman, B.; Thrower, B.; et al. Effect of dimethyl fumarate on lymphocyte subsets in patients with relapsing multiple sclerosis. Mult. Scler. J. ETC 2020, 6, 2055217320918619. [Google Scholar] [CrossRef]
- Michaličková, D.; Öztürk, H.K.; Hroudová, J.; Ľupták, M.; Kučera, T.; Hrnčíř, T.; Canová, N.K.; Šíma, M.; Slanař, O. Edaravone Attenuates Disease Severity of Experimental Auto-Immune Encephalomyelitis and Increases Gene Expression of Nrf2 and HO-1. Physiol. Res. 2022, 71, 147–157. [Google Scholar] [CrossRef]
- van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical Changes in Multiple Sclerosis Pathogenesis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 141–150. [Google Scholar] [CrossRef]
- Copp, R.R.; Peebles, D.D.; Fahl, W.E. Synthesis and Growth Regulatory Activity of a Prototype Member of a New Family of Aminothiol Radioprotectors. Bioorg. Med. Chem. Lett. 2011, 21, 7426–7430. [Google Scholar] [CrossRef]
- Bath, N.M.; Fahl, W.E.; Redfield, R.R. Significant Reduction of Murine Renal Ischemia-Reperfusion Cell Death Using the Immediate-Acting PrC-210 Reactive Oxygen Species Scavenger. Transplant. Direct 2019, 5, e469. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahl, W.E.; Fahl, B.L.; Goesch, S.R.; Goesch, H.R.; Goesch, T.R. Significant Suppression of Multiple Sclerosis in the Mouse EAE Model Using the PrC-210 Aminothiol. Int. J. Mol. Sci. 2025, 26, 10597. https://doi.org/10.3390/ijms262110597
Fahl WE, Fahl BL, Goesch SR, Goesch HR, Goesch TR. Significant Suppression of Multiple Sclerosis in the Mouse EAE Model Using the PrC-210 Aminothiol. International Journal of Molecular Sciences. 2025; 26(21):10597. https://doi.org/10.3390/ijms262110597
Chicago/Turabian StyleFahl, William E., Bryan L. Fahl, Sarah R. Goesch, Hannah R. Goesch, and Torsten R. Goesch. 2025. "Significant Suppression of Multiple Sclerosis in the Mouse EAE Model Using the PrC-210 Aminothiol" International Journal of Molecular Sciences 26, no. 21: 10597. https://doi.org/10.3390/ijms262110597
APA StyleFahl, W. E., Fahl, B. L., Goesch, S. R., Goesch, H. R., & Goesch, T. R. (2025). Significant Suppression of Multiple Sclerosis in the Mouse EAE Model Using the PrC-210 Aminothiol. International Journal of Molecular Sciences, 26(21), 10597. https://doi.org/10.3390/ijms262110597





