Bio-Active Peptides from Marine Sources: Mechanistic Insights into Immune Regulation, Microbiota Modulation, and Intestinal Barrier Protection
Abstract
1. Introduction
2. Sources and Extraction of Bioactive Compounds
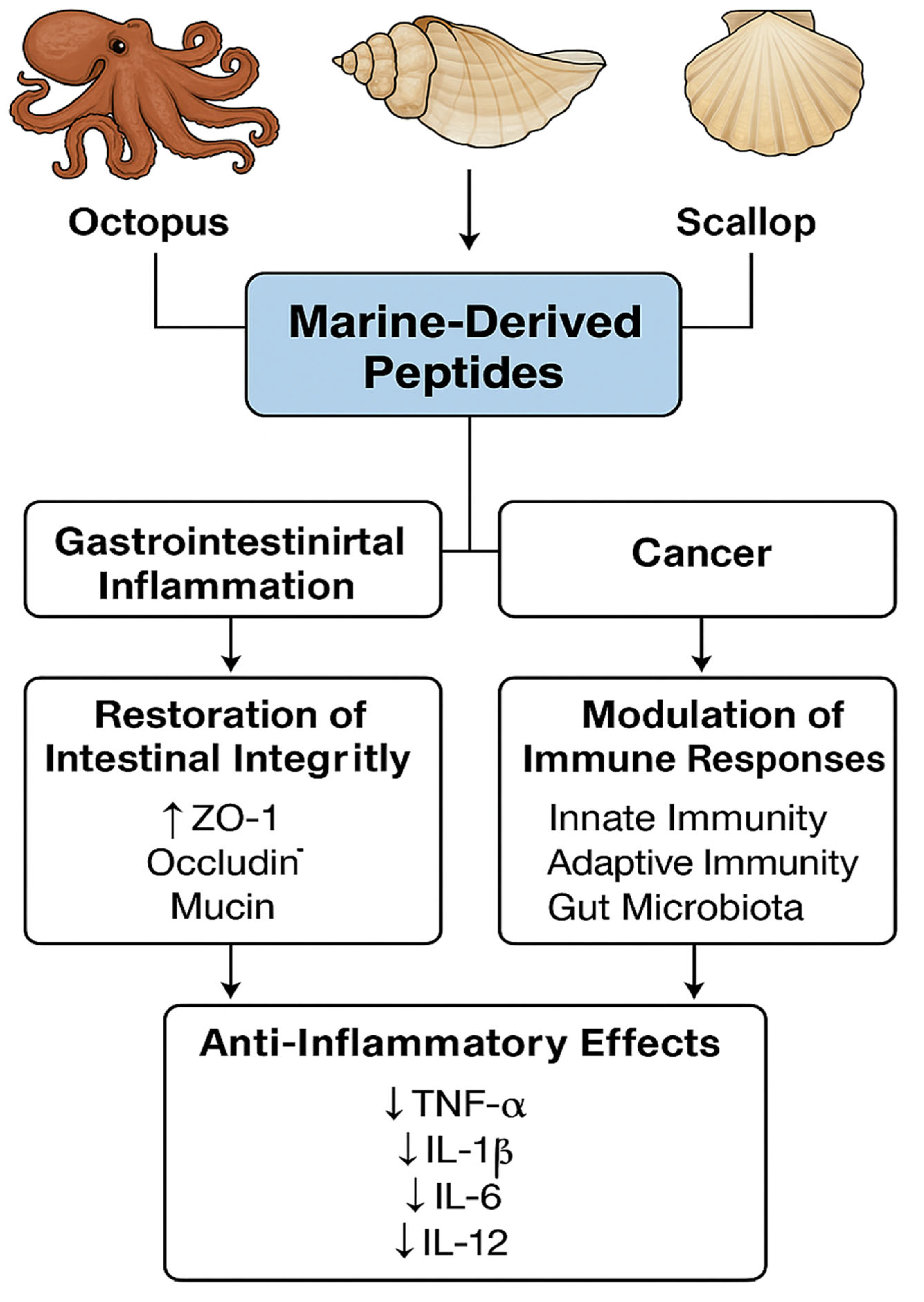
3. Bioactivities of Marine-Derived Peptides
3.1. Antioxidant Activity
3.2. Immunomodulatory Effects
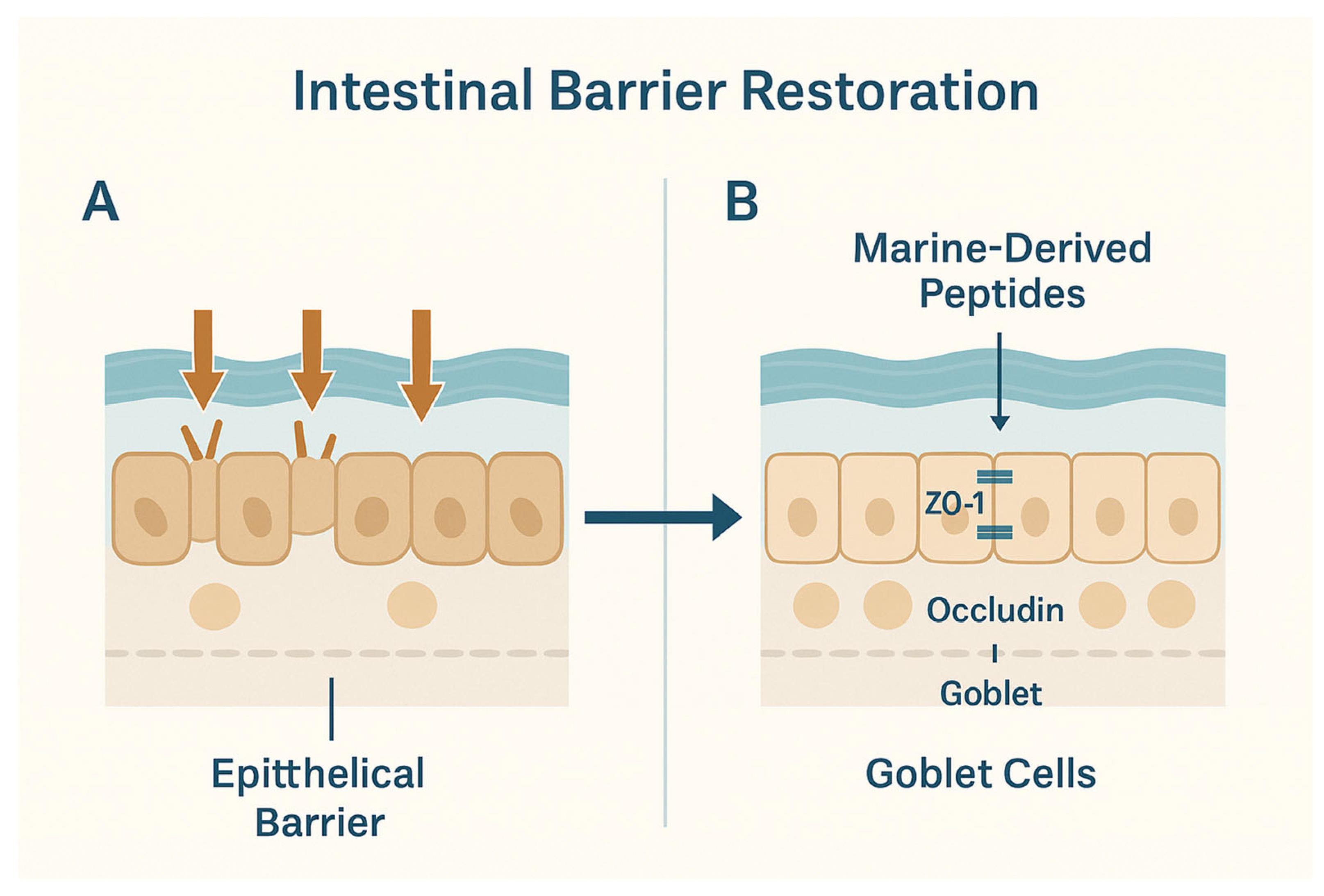
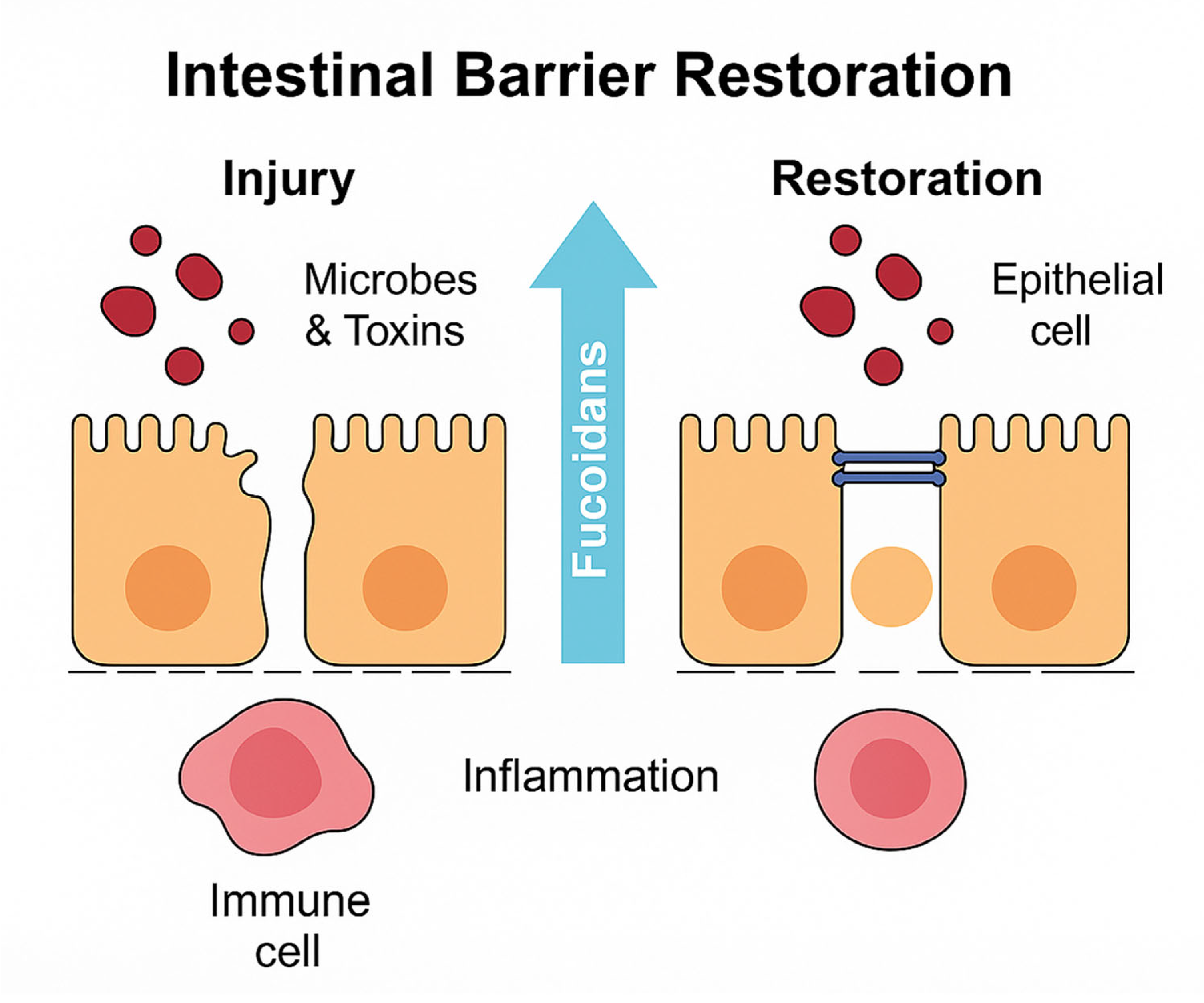
4. Restoration of Intestinal Barrier Integrity
5. Gut Microbiota Modulation: Development of New Therapeutics Using Bioactive Peptides
6. Potential Application in Disease Models
6.1. Chemotherapy-Induced Immunosuppression
6.2. Implications for Cancer Therapy Support
6.3. Translational and Therapeutic Potential Perspectives of Peptides Derived from Marine Organisms with Anticancer Potential
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | Inflammatory Bowel Diseases |
| CRC | Colorectal Cancer |
| DSS | Dextran Sulfate Sodium |
| CTX | Cyclophosphamide |
| ZO-1 | Zonula Occludens-1 |
| NF-Κb | Nuclear Factor Kappa B |
| TLR-4 | Toll-Like Receptor 4 |
| IL | Interleukin |
| TNF-α | Tumor Necrosis Factor Alpha |
| H2O2 | Hydrogen |
| MUC2 | Mucin 2 |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NK | Nature Killer |
| NO | Nitric Oxide |
| SCFA | Short-Chain Fatty Acid |
| ROS | Reactive Oxygen Species |
References
- Okeke, E.S.; Okagu, I.U.; Chukwudozie, K.; Ezike, T.C.; Ezeorba, T.P.C. Marine-derived bioactive proteins and peptides: A review of current knowledge on anticancer potentials, clinical trials, and future prospects. Nat. Prod. Commun. 2024, 19, 1934578X241239825. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Rafieezadeh, D.; Esfandyari, G. Marine bioactive peptides with anticancer potential, a narrative review. Int. J. Biochem. Mol. Biol. 2024, 15, 118–126. [Google Scholar] [CrossRef]
- Cunha, S.A.; Pintado, M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022, 119, 348–370. [Google Scholar] [CrossRef]
- Du, J.; Xiao, M.; Sudo, N.; Liu, Q. Bioactive peptides of marine organisms: Roles in the reduction and control of cardiovascular diseases. Food Sci. Nutr. 2024, 12, 5271–5284. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Proteins and bioactive peptides from algae: Insights into antioxidant, anti-hypertensive, anti-diabetic and anti-cancer activities. Trends Food Sci. Technol. 2024, 145, 104352. [Google Scholar] [CrossRef]
- Ullah, H.; Alioui, Y.; Ali, M.; Ali, S.; Farooqui, N.A.; Siddiqui, N.Z.; Alsholi, D.M.; Ilyas, M.; Rahman, M.U.; Xin, Y.; et al. Sea conch (Rapana venosa) peptide hydrolysate regulates NF-κB pathway and restores intestinal immune homeostasis in DSS-induced colitis mice. Food Sci. Nutr. 2024, 12, 10070–10086. [Google Scholar] [CrossRef]
- Ullah, H.; Deng, T.; Ali, M.; Farooqui, N.A.; Alsholi, D.M.; Siddiqui, N.Z.; Rehman, A.U.; Ali, S.; Ilyas, M.; Wang, L.; et al. Sea conch peptides hydrolysate alleviates DSS-induced colitis in mice through immune modulation and gut microbiota restoration. Molecules 2023, 28, 6849. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Ullah, H.; Farooqui, N.A.; Deng, T.; Siddiqui, N.Z.; Ilyas, M.; Ali, S.; Rahman, M.U.; Rehman, A.U.; Alioui, Y.; et al. NF-κB pathway activation by Octopus peptide hydrolysate ameliorates gut dysbiosis and enhances immune response in cyclophosphamide-induced mice. Heliyon 2024, 10, e38370. [Google Scholar] [CrossRef]
- Ilyas, M.; Rahman, M.U.; Ali, M.; Deng, T.; Farooqui, N.A.; Ali, S.; Ullah, H.; Alioui, Y.; Ma, R.; Lu, S.; et al. Effect of Marine-Derived Scallop Peptide Hydrolysate on Immune Modulation and Gut Microbiota Restoration in Cyclophosphamide-Induced Immunosuppressed Mice. Food Sci Nutr. 2025, 13, e70421. [Google Scholar] [CrossRef]
- Macedo, M.W.F.S.; Cunha, N.B.d.; Carneiro, J.A.; Costa, R.A.d.; Alencar, S.A.d.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine organisms as a rich source of biologically active peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Cermeño, M.; Kleekayai, T.; Amigo-Benavent, M.; Harnedy-Rothwell, P.; FitzGerald, R.J. Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 2020, 41, 1694–1717. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Long, Y.; Tang, L.; Zhou, Y.; Zhao, S.; Zhu, H. Causal relationship between gut microbiota and cancers: A two-sample Mendelian randomisation study. BMC Med. 2023, 21, 66. [Google Scholar] [CrossRef]
- Ning, S.; Zhang, Z.; Zhou, C.; Wang, B.; Liu, Z.; Feng, B. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: A dynamic interplay influencing pathogenesis and therapy. Front. Med. 2024, 11, 1457218. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, A.I.; Xin, Y.; Liang, W. Morchella esculenta polysaccharide attenuate obesity, inflammation and modulate gut microbiota. AMB Express. 2022, 12, 114. [Google Scholar] [CrossRef]
- Xiang, X.-W.; Zhou, X.-L.; Wang, R.; Shu, C.-H.; Zhou, Y.-F.; Ying, X.-G.; Zheng, B. Protective effect of tuna bioactive peptide on dextran sulfate sodium-induced colitis in mice. Mar. Drugs 2021, 19, 127. [Google Scholar] [CrossRef]
- Alioui, Y.; Ullah, H.; Ali, S.; Rahman, M.U.; Elkharti, M.; Farooqui, N.A.; Rehman, A.U.; Ilyas, M.; Alsholi, D.M.; Siddiqi, N.Z.; et al. Polysaccharides derived from golden mushroom (Cantharellus cibarius Fr.) modulate gut microbiota and enhance intestinal barrier function to ameliorate dextran sulfate sodium-induced colitis in mice. Front. Pharmacol. 2024, 15, 1498625. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Santhiravel, S. Novel marine bioactives: Application in functional foods, nutraceuticals, and pharmaceuticals. J. Food Bioact. 2022, 19, 4–96. [Google Scholar] [CrossRef]
- Farooq, S.; Ahmad, M.I.; Zheng, S.; Ali, U.; Li, Y.; Shixiu, C.; Zhang, H. A review on marine collagen: Sources, extraction methods, colloids properties, and food applications. Collagen Leather 2024, 6, 11. [Google Scholar] [CrossRef]
- Zheng, S.-L.; Wang, Y.-Z.; Zhao, Y.-Q.; Chi, C.-F.; Zhu, W.-Y.; Wang, B. High Fischer ratio oligopeptides from hard-shelled mussel: Preparation and hepatoprotective effect against acetaminophen-induced liver injury in mice. Food Biosci. 2023, 53, 102638. [Google Scholar] [CrossRef]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; Alashi, M.A.; Aluko, R.E.; FitzGerald, R.J. Peptide identification in a salmon gelatin hydrolysate with antihypertensive, dipeptidyl peptidase IV inhibitory and antioxidant activities. Food Res Int. 2017, 100, 112–120. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stefan, L.M.; Anton, E.D.; Berger, D.; Matei, C.; Negreanu-Pirjol, T.; Moldovan, L. Physicochemical and biological properties of gelatin extracted from marine snail Rapana venosa. Mar. Drugs 2019, 17, 589. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Januka, T.; Kumari, D.; de Camargo, A.C.; Shahidi, F. Phenolic antioxidants of bael fruit herbal tea and effects on postprandial glycemia and plasma antioxidant status in healthy adults. J. Food Bioact. 2020, 11, 75–83. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, J.; Zhong, H.; Abdullah; Zhuang, J.; Zhang, J.; Wang, J.; Zhang, X.; Feng, F. High-degree hydrolysis sea cucumber peptides improve exercise performance and exert antifatigue effect via activating the NRF2 and AMPK signaling pathways in mice. J. Funct. Foods 2021, 86, 104677. [Google Scholar] [CrossRef]
- Jegani, K.T.; Balde, A.; Nazeer, R.A. A review on anti-inflammatory and antioxidant peptides derived from marine organisms: Mechanism of action and therapeutic applications. Food Biosci. 2024, 63, 105745. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Oh, J.-Y.; Lee, D.-S.; Yang, H.-W.; Kang, M.-C.; Kim, E.-A.; Kang, N.; Kim, J.; Heo, S.-J.; et al. Potential antioxidant properties of enzymatic hydrolysates from Stichopus japonicus against hydrogen peroxide-induced oxidative stress. Antioxidants 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, M.; Li, M.; Hou, J.; Zhang, X.; Gao, Y.; Chachar, B.; Li, X. Dietary bioactive peptide alanyl-glutamine attenuates dextran sodium sulfate-induced colitis by modulating gut microbiota. Oxid. Med. Cell Longev. 2021, 2021, 5543003. [Google Scholar] [CrossRef]
- Xia, G.; Zhang, X.; Dong, Z.; Shen, X. Comparative study on the antioxidant activity of peptides from pearl oyster (Pinctada martensii) mantle type V collagen and tilapia (Oreochromis niloticus) scale type I collagen. J. Ocean Univ. China 2017, 16, 1175–1182. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Isolation, purification and identification of three novel antioxidative peptides from patin (Pangasius sutchi) myofibrillar protein hydrolysates. LWT Food Sci. Technol. 2015, 60, 452–461. [Google Scholar] [CrossRef]
- Bashir, K.M.I.; Sohn, J.H.; Kim, J.-S.; Choi, J.-S. Identification and characterization of novel antioxidant peptides from mackerel (Scomber japonicus) muscle protein hydrolysates. Food Chem. 2020, 323, 126809. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber Acaudina molpadioides obtained from Zhejiang province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.; Silva, M.; Silva, C.; Prata, M.; Rodrigues, F.; Siqueira, R.; Lima, A.; Santos, A.; Havt, A. Alanyl-glutamine protects the intestinal barrier function in trained rats against the impact of acute exhaustive exercise. Braz. J. Med. Biol. Res. 2020, 53, e9211. [Google Scholar] [CrossRef]
- Lima, K.; Quadros, C.; Rocha, M.; Lacerda, J.; Juliano, M.; Dias, M.; Mendes, M.; Prentice, C. Bioactivity and bioaccessibility of protein hydrolysates from industrial by-products of stripped weakfish (Cynoscion guatucupa). LWT-Food Sci. Technol. 2019, 408–413. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Li, J.; Zhou, B. Peptides derived from Rhopilema esculentum hydrolysate exhibit angiotensin converting enzyme (ACE) inhibitory and antioxidant abilities. Molecules 2014, 19, 13587–13602. [Google Scholar] [CrossRef]
- Li, X.; Tan, C.-P.; Liu, Y.-F.; Xu, Y.-J. Interactions between food hazards and intestinal barrier: Impact on foodborne diseases. J. Agric. Food Chem. 2020, 68, 14728–14738. [Google Scholar] [CrossRef]
- Galdiero, E.; Lombardi, L.; Falanga, A.; Libralato, G.; Guida, M.; Carotenuto, R. Biofilms: Novel strategies based on antimicrobial peptides. Pharmaceutics 2019, 11, 322. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- He, L.; Zhang, Z.; Sun, B.; Chen, Q.; Liu, R.; Ren, J.; Wang, J.; Li, Y. Sea cucumber (Codonopsis pilosula) oligopeptides: Immunomodulatory effects based on stimulating Th cells, cytokine secretion and antibody production. Food Funct. 2016, 7, 1208–1216. [Google Scholar] [CrossRef]
- Du, X.; Lian, F.; Li, Y.; Li, D.; Wu, D.; Feng, Q.; Feng, Z.; Li, Y.; Bu, G.; Meng, F.; et al. Peptides from Colochirus robustus Enhance Immune Function via Activating CD3ζ- and ZAP-70-Mediated Signaling in C57BL/6 Mice. Int. J. Mol. Sci. 2017, 18, 2110. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.; Wang, Y.; Fan, R.; Hu, X.; Zhang, F.; Yang, J.; Chen, J. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef]
- Xi, P.; Jiang, Z.; Zheng, C.; Lin, Y.; Wu, G. Regulation of protein metabolism by glutamine: Implications for nutrition and health. Front. Biosci. (Landmark Ed.) 2011, 16, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Haertel, F.; Nuding, S.; Reisberg, D.; Peters, M.; Werdan, K.; Schulze, P.C.; Ebelt, H. The prognostic value of a liver function test using indocyanine green (ICG) clearance in patients with multiple organ dysfunction syndrome (MODS). J. Clin. Med. 2024, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, B.; Lin, M.; Zhou, P.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets. Asian-Australas J. Anim. Sci. 2016, 30, 236. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Y.; Li, S.; Huang, X.; Bao, X.; Wang, J.; Zheng, N. Modulation of intestinal epithelial permeability and mucin mRNA (MUC2, MUC5AC, and MUC5B) expression and protein secretion in Caco-2/HT29-MTX co-cultures exposed to aflatoxin M1, ochratoxin A, and zearalenone individually or collectively. Toxicol. Lett. 2019, 309, 1–9. [Google Scholar] [CrossRef]
- Gu, A.; Yang, L.; Wang, J.; Li, J.; Shan, A. Protective effect of glutamine and alanyl-glutamine against zearalenone-induced intestinal epithelial barrier dysfunction in IPEC-J2 cells. Res. Vet. Sci. 2021, 137, 48–55. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Hu, H.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. The protective role of glutamine on enteropathy induced by high dose of soybean meal in turbot, Scophthalmus maximus L. Aquaculture 2018, 497, 510–519. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Chu, C.-C.; Ko, T.-L.; Yeh, C.-L.; Yeh, S.-L. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur. J. Nutr. 2013, 52, 1089–1098. [Google Scholar] [CrossRef]
- Hao, W.; Hao, C.; Wu, C.; Xu, Y.; Jin, C. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere 2022, 288, 132556. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M.C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Natividad, J.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Kang, W.; Li, W.; Chen, S.; Gao, Y. Oral delivery of protein and peptide drugs: From non-specific formulation approaches to intestinal cell targeting strategies. Theranostics 2022, 12, 1419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.; Li, W.; Ma, L.; Liu, X.; Ding, Q.; Yu, W.; Yu, T.; Ding, C.; Liu, W. Research progress of natural plant polysaccharides inhibiting inflammatory signaling pathways and regulating intestinal flora and metabolism to protect inflammatory bowel disease. Int. J. Biol. Macromol. 2023, 253, 126799. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Xu, B.; Yin, B.; Xu, X.; Niu, Y.; Tang, Y.; Wang, X.; Xie, C.; Yang, T.; Zhou, S.; et al. Modulation of gut microbial community and metabolism by dietary glycyl-glutamine supplementation may favor weaning transition in piglets. Front. Microbiol. 2020, 10, 3125. [Google Scholar] [CrossRef]
- Fan, L.; Liu, X.; Deng, Y.; Zheng, X. Preparation of glutamine-enriched fermented feed from corn gluten meal and its functionality evaluation. Foods 2023, 12, 4336. [Google Scholar] [CrossRef]
- Huo, J.; Wu, Z.; Sun, W.; Wang, Z.; Wu, J.; Huang, M.; Wang, B.; Sun, B. Protective effects of natural polysaccharides on intestinal barrier injury: A review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Toalá, J.; Santiago-López, L.; Peres, C.; Peres, C.; Garcia, H.; Vallejo-Cordoba, B.; González-Córdova, A.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Gastrointestinal digestion of food proteins under the effects of released bioactive peptides on digestive health. Mol. Nutr. Food. Res. 2020, 64, 2000401. [Google Scholar] [CrossRef]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef]
- Gilmore, K.; Chen, P.; Leung, K.P. Anti-microbial peptides for plaque control and beyond. J. Calif. Dent. Assoc. 2009, 37, 779–788. [Google Scholar] [CrossRef]
- Kharidia, R.; Tu, Z.; Chen, L.; Liang, J. Activity and selectivity of histidine-containing lytic peptides to antibiotic-resistant bacteria. Arch. Microbiol. 2012, 194, 769–778. [Google Scholar] [CrossRef]
- Liu, W.; Lin, J.; Zhang, C.; Yang, Z.; Shan, H.; Jiang, J.; Wan, X.; Wang, Z. Effect of Dietary Casein Phosphopeptide Addition on the Egg Production Performance, Egg Quality, and Eggshell Ultrastructure of Late Laying Hens. Foods 2023, 12, 1712. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Zhang, W.; Li, X. Bioactive peptides for anticancer therapies. Biomater Transl. 2023, 4, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Lee, J.-E.; An, B.J.; Jo, C.; Min, B.; Paik, H.-D.; Ahn, D.U. The elastase and melanogenesis inhibitory and anti-inflammatory activities of phosvitin phosphopeptides produced using high-temperature and mild-pressure (HTMP) pretreatment and enzyme hydrolysis combinations. Poult. Sci. 2023, 102, 102680. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; He, K.; Dong, X.; Zhang, Z.; Wang, F.; Tang, Y.; Chen, Y.; Ding, G. Immunomodulatory activity of low molecular-weight peptides from Nibea japonica skin in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2020, 68, 103888. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.F.; Hrelia, S. Bioactive peptides in cereals and legumes: Agronomical, biochemical and clinical aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, B.; Berrada, H.; Barba, F.J. Marine resources and cancer therapy: From current evidence to challenges for functional foods development. Curr. Opin. Food Sci. 2022, 44, 100805. [Google Scholar] [CrossRef]
- Kandyliari, A.; Golla, J.P.; Chen, Y.; Papandroulakis, N.; Kapsokefalou, M.; Vasiliou, V. Antiproliferative activity of protein hydrolysates derived from fish by-products on human colon and breast cancer cells. Proc. Nutr. Soc. 2020, 79, E282. [Google Scholar] [CrossRef]
- Eghtedari, M.; Porzani, S.J.; Nowruzi, B. Anticancer potential of natural peptides from terrestrial and marine environments: A review. Phytochem. Lett. 2021, 42, 87–103. [Google Scholar] [CrossRef]
- Ting, C.-H.; Chen, J.-Y. Nile tilapia derived TP4 shows broad cytotoxicity toward to non-small-cell lung cancer cells. Mar. Drugs. 2018, 16, 506. [Google Scholar] [CrossRef]
- Su, B.-C.; Wu, T.-H.; Hsu, C.-H.; Chen, J.-Y. Distribution of positively charged amino acid residues in antimicrobial peptide epinecidin-1 is crucial for in vitro glioblastoma cytotoxicity and its underlying mechanisms. Chem. Biol. Interact. 2020, 315, 108904. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zhang, Y.; Ye, L.; Tang, Y.; Ding, G.; Zhang, X.; Yang, Z. A novel anti-proliferative pentapeptide (ILYMP) isolated from Cyclina sinensis protein hydrolysate induces apoptosis of DU-145 prostate cancer cells. Mol. Med. Rep. 2018, 18, 771–778. [Google Scholar] [CrossRef]
- Qu, B.; Yuan, J.; Liu, X.; Zhang, S.; Ma, X.; Lu, L. Anticancer activities of natural antimicrobial peptides from animals. Front. Microbiol. 2024, 14, 1321386. [Google Scholar] [CrossRef]
- Kang, H.K.; Choi, M.-C.; Seo, C.H.; Park, Y. Therapeutic properties and biological benefits of marine-derived anticancer peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef]
- Hansen, I.K.; Isaksson, J.; Poth, A.G.; Hansen, K.Ø.; Andersen, A.J.; Richard, C.S.; Blencke, H.-M.; Stensvåg, K.; Craik, D.J.; Haug, T. Isolation and characterization of antimicrobial peptides with unusual disulfide connectivity from the colonial ascidian Synoicum turgens. Mar. Drugs. 2020, 18, 51. [Google Scholar] [CrossRef]
- Mao, J.; Zhang, Z.; Chen, Y.; Wu, T.; Fersht, V.; Jin, Y.; Meng, J.; Zhang, M. Sea cucumber peptides inhibit the malignancy of NSCLC by regulating miR-378a-5p targeted TUSC2. Food Funct. 2021, 12, 12362–12371. [Google Scholar] [CrossRef] [PubMed]
- Wargasetia, T.L.; Ratnawati, H.; Widodo, N.; Widyananda, M.H. Bioinformatics study of sea cucumber peptides as antibreast cancer through inhibiting the activity of overexpressed protein (EGFR, PI3K, AKT1, and CDK4). Cancer Inform. 2021, 20, 11769351211031864. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar Drugs. 2014, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From ocean to medicine: Pharmaceutical applications of metabolites from marine bacteria. Antibiotics 2020, 9, 455. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, F.; Li, D.; Su, Y.; Chen, L.; Cheng, X.; Zheng, X.; Mao, J. Bio-Active Peptides from Marine Sources: Mechanistic Insights into Immune Regulation, Microbiota Modulation, and Intestinal Barrier Protection. Int. J. Mol. Sci. 2025, 26, 10508. https://doi.org/10.3390/ijms262110508
Ali F, Li D, Su Y, Chen L, Cheng X, Zheng X, Mao J. Bio-Active Peptides from Marine Sources: Mechanistic Insights into Immune Regulation, Microbiota Modulation, and Intestinal Barrier Protection. International Journal of Molecular Sciences. 2025; 26(21):10508. https://doi.org/10.3390/ijms262110508
Chicago/Turabian StyleAli, Farman, Dailin Li, Yunpeng Su, Lixue Chen, Xiaoxin Cheng, Xu Zheng, and Jun Mao. 2025. "Bio-Active Peptides from Marine Sources: Mechanistic Insights into Immune Regulation, Microbiota Modulation, and Intestinal Barrier Protection" International Journal of Molecular Sciences 26, no. 21: 10508. https://doi.org/10.3390/ijms262110508
APA StyleAli, F., Li, D., Su, Y., Chen, L., Cheng, X., Zheng, X., & Mao, J. (2025). Bio-Active Peptides from Marine Sources: Mechanistic Insights into Immune Regulation, Microbiota Modulation, and Intestinal Barrier Protection. International Journal of Molecular Sciences, 26(21), 10508. https://doi.org/10.3390/ijms262110508





