Genomic Confluence: When Cerebrotendinous Xanthomatosis, Klinefelter Syndrome, and a BRCA2 Variant Intersect
Abstract
1. Introduction
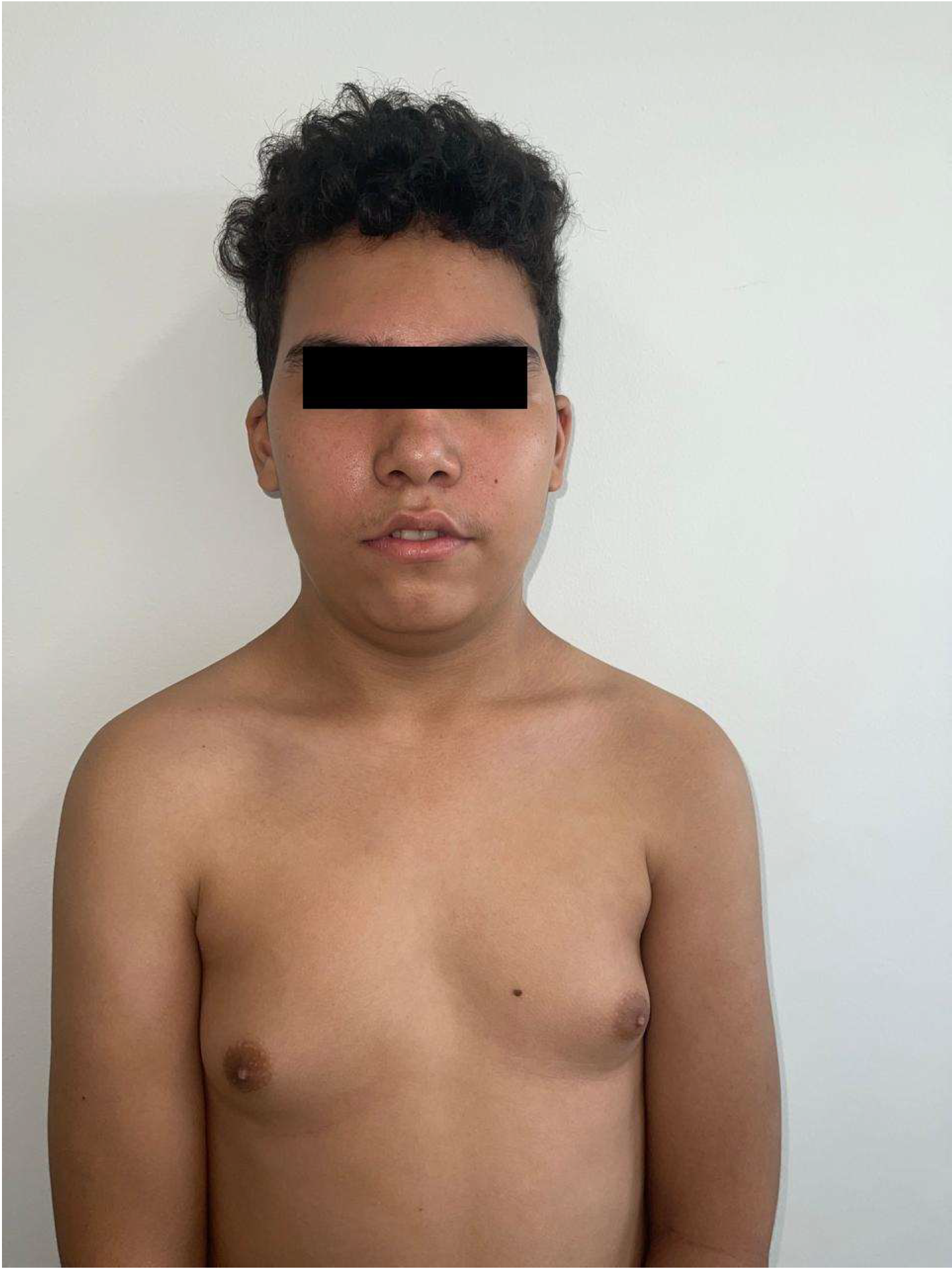
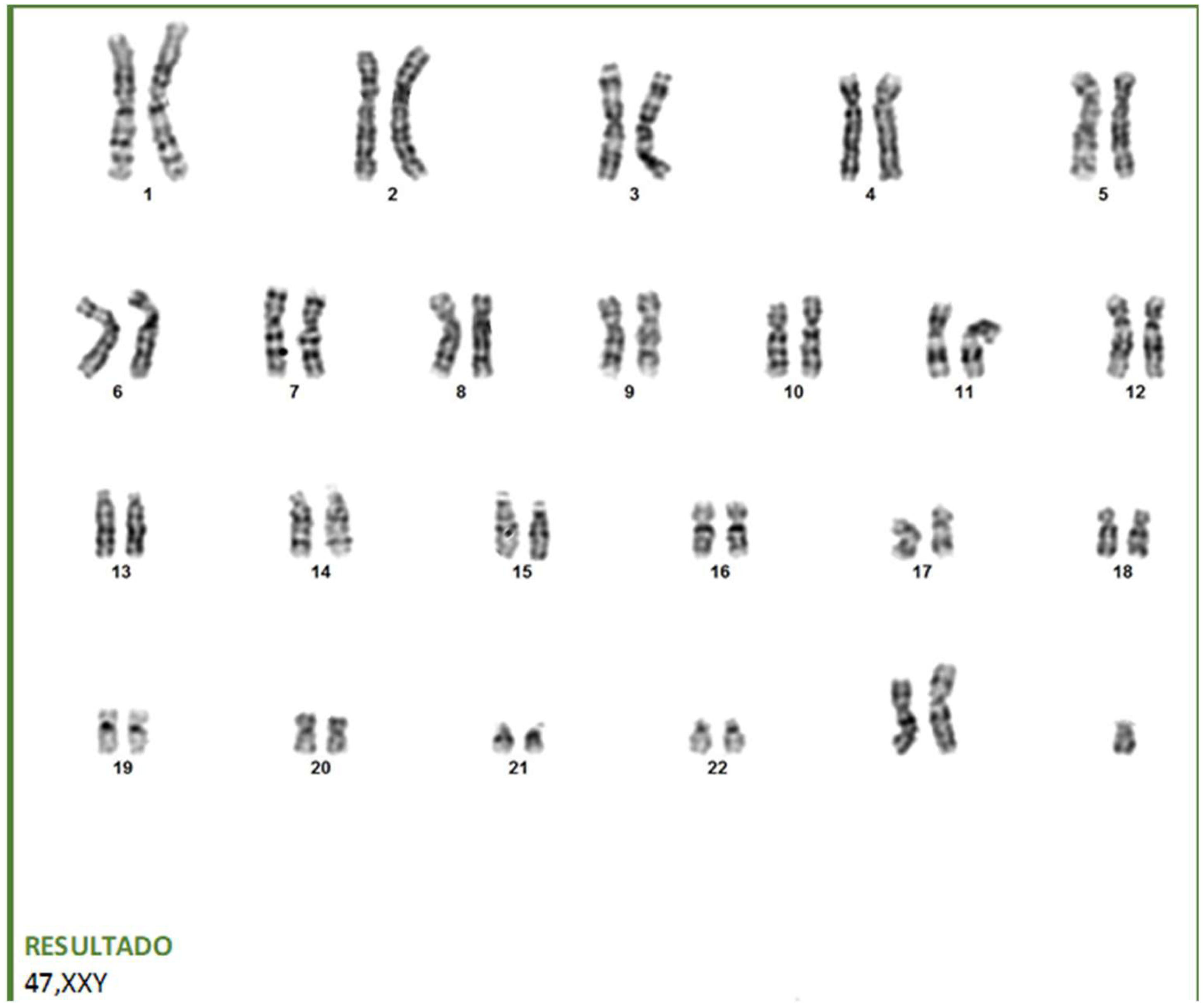
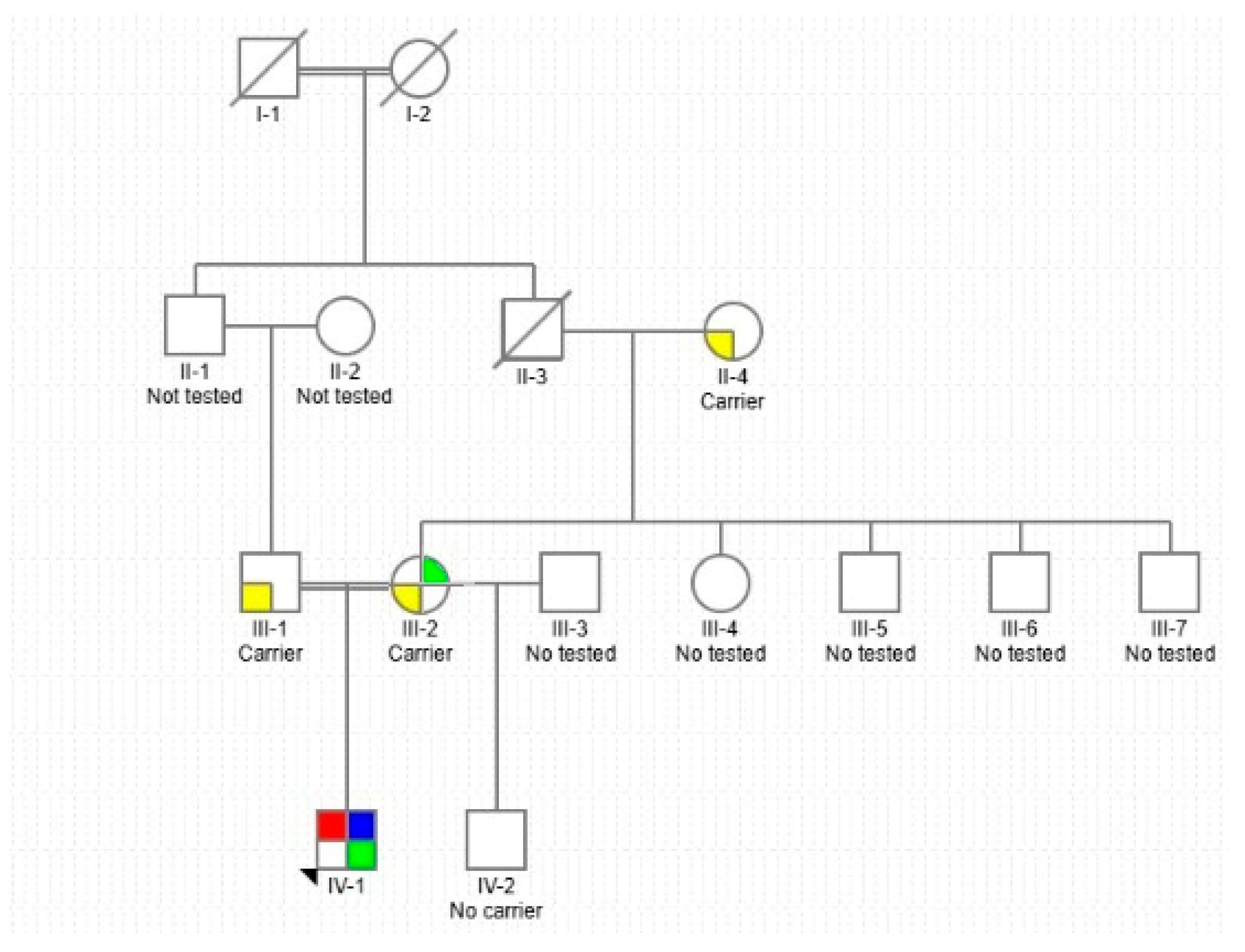
2. Case Description
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitani, T.; Isikay, S.; Gezdirici, A.; Gulec, E.Y.; Punetha, J.; Fatih, J.M.; Herman, I.; Akay, G.; Du, H.; Calame, D.G.; et al. High prevalence of multilocus pathogenic variation in neurodevelopmental disorders in the Turkish population. Am. J. Hum. Genet. 2021, 108, 1981–2005. [Google Scholar] [CrossRef] [PubMed]
- Posey, J.E.; Harel, T.; Liu, P.; Rosenfeld, J.A.; James, R.A.; Akdemir, Z.H.C.; Walkiewicz, M.; Bi, W.; Xiao, R.; Ding, Y.; et al. Resolution of Disease Phenotypes Resulting from Multilocus Genomic Variation. N. Engl. J. Med. 2017, 376, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Herman, I.; Jolly, A.; Du, H.; Dawood, M.; Abdel-Salam, G.M.H.; Marafi, D.; Mitani, T.; Calame, D.G.; Coban-Akdemir, Z.; Fatih, J.M.; et al. Quantitative dissection of multilocus pathogenic variation in an Egyptian infant with severe neurodevelopmental disorder resulting from multiple molecular diagnoses. Am. J. Med. Genet. A 2022, 188, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Espada-Musitu, D.; Manero-Azua, Á.; Vado, Y.; de Nanclares, G.P. Genetic counselling in the era of next generation sequencing. An. Pediatr. Engl. Ed. 2025, 102, 503712. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.L.; Udyawar, D.; Kaur, P.; Sharma, S.; Suresh, N.; Nampoothiri, S.; Rosario, M.C.D.; Somashekar, P.H.; Rao, L.P.; Kausthubham, N.; et al. Multilocus disease-causing genomic variations for Mendelian disorders: Role of systematic phenotyping and implications on genetic counselling. Eur. J. Hum. Genet. 2021, 29, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, P.R.; Bernardes, A.M.; Ribeiro, R.M.; Vasconcelos, S.C.; Araújo, D.A.B.S.; Gama, V.C.d.V.; Fussiger, H.; Santos, C.d.F.; Dias, D.A.; Pessoa, A.L.S.; et al. Cerebrotendinous Xanthomatosis: A practice review of pathophysiology, diagnosis, and treatment. Front. Neurol. 2022, 13, 1049850. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.; Hoggard, N.; Hadjivassiliou, M. Cerebrotendinous Xanthomatosis: Diversity of presentation and refining treatment with chenodeoxycholic acid. Cerebellum Ataxias 2021, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.; Ramakrishnan, A.; Graham, C.; Bambang, K.; Sriranglingam, U.; Senniappan, S. Klinefelter Syndrome: A Review. Clin. Endocrinol. 2025, 102, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Butler, G.; Srirangalingam, U.; Faithfull, J.; Sangster, P.; Senniappan, S.; Mitchell, R. Klinefelter syndrome: Going beyond the diagnosis. Arch. Dis. Child. 2023, 108, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Silvestri, V.; Leslie, G.; Rebbeck, T.R.; Neuhausen, S.L.; Hopper, J.L.; Nielsen, H.R.; Lee, A.; Yang, X.; McGuffog, L.; et al. Cancer Risks Associated with BRCA1 and BRCA2 Pathogenic Variants. J. Clin. Oncol. 2022, 40, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Baldridge, D.; Heeley, J.; Vineyard, M.; Manwaring, L.; Toler, T.L.; Fassi, E.; Fiala, E.; Brown, S.; Goss, C.W.; Willing, M.; et al. The Exome Clinic and the role of medical genetics expertise in the interpretation of exome sequencing results. Genet. Med. 2017, 19, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Karaca, E.; Posey, J.E.; Coban Akdemir, Z.; Pehlivan, D.; Harel, T.; Jhangiani, S.N.; Bayram, Y.; Song, X.; Bahrambeigi, V.; Yuregir, O.O.; et al. Phenotypic expansion illuminates multilocus pathogenic variation. Genet. Med. 2018, 20, 1528–1537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.; Chen, Z.; He, R.; Zhang, Y.; Dong, H.; Ma, Y.; Wu, T.; Wang, Q.; Ding, Y.; et al. Dual rare genetic diseases in five pediatric patients: Insights from next-generation diagnostic methods. Orphanet J. Rare Dis. 2024, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Berginer, V.M.; Salen, G.; Shefer, S. Long-Term Treatment of Cerebrotendinous Xanthomatosis with Chenodeoxycholic Acid. N. Engl. J. Med. 1984, 311, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- Groth, K.A.; Skakkebæk, A.; Høst, C.; Gravholt, C.H.; Bojesen, A. Klinefelter Syndrome—A Clinical Update. J. Clin. Endocrinol. Metab. 2013, 98, 20–30. [Google Scholar] [CrossRef]
- Gupta, S.; Weiss, J.M.; Burke, C.A.; Hodan, R.; Axell, L.; Chen, L.-M.; Chung, D.C.; Clayback, K.M.; Felder, S.; Foda, Z.; et al. NCCN Guidelines Version 4.2024 Genetic/Familial High-Risk Assessment: Colorectal, Endometrial, and Gastric Continue. 2025. Available online: https://www.nccn.org/home/ (accessed on 15 September 2025).
- Dumbuya, J.S.; Zeng, C.; Deng, L.; Li, Y.; Chen, X.; Ahmad, B.; Lu, J. The impact of rare diseases on the quality of life in paediatric patients: Current status. Front. Public Health 2025, 13, 1531583. [Google Scholar] [CrossRef]
- Pramparo, T.; Steiner, R.D.; Rodems, S.; Jenkinson, C. Allelic prevalence and geographic distribution of cerebrotendinous xanthomatosis. Orphanet J. Rare Dis. 2023, 18, 13. [Google Scholar] [CrossRef]
- Ridder, L.O.; Berglund, A.; Stochholm, K.; Chang, S.; Gravholt, C.H. Morbidity, mortality, and socioeconomics in Klinefelter syndrome and 47,XYY syndrome: A comparative review. Endocr. Connect. 2023, 12, e230024. [Google Scholar] [CrossRef] [PubMed]
- Matta, B.P.; Gomes, R.; Mattos, D.; Olicio, R.; Nascimento, C.M.; Ferreira, G.M.; Brant, A.C.; Boroni, M.; Furtado, C.; Lima, V.; et al. Familial history and prevalence of BRCA1, BRCA2 and TP53 pathogenic variants in HBOC Brazilian patients from a public healthcare service. Sci. Rep. 2022, 12, 18629. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Lee, K.; Abul-Husn, N.S.; Amendola, L.M.; Brothers, K.; Chung, W.K.; Gollob, M.H.; Gordon, A.S.; Harrison, S.M.; Hershberger, R.E.; et al. ACMG SF v3.2 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2023, 25, 100866. [Google Scholar] [CrossRef] [PubMed]
| CTX | Klinefelter Syndrome | BRCA2 Variant | Observed in Patient | Interpretation/Likely Contribution | |
|---|---|---|---|---|---|
| Neurological | |||||
| Seizures | + | Rare | − | + | Explained by CTX (neurological involvement) |
| Cognitive impairment | + | + | − | + | Blended effect of CTX and Klinefelter |
| Behavioral difficulties/inattention | + | + | − | + | Likely additive contribution |
| MRI brain lesion | + | − | − | + | Consistent with CTX |
| Endocrine/Metabolic | |||||
| Gynecomastia | − | + | − | + | Typical for Klinefelter syndrome |
| Hypogonadism | − | + | − | + | Klinefelter-related endocrine dysfunction |
| Elevated cholesterol/LDL | + | − | − | + | Typical of CTX lipid metabolism |
| Growth parameters (normal) | Variable | Often tall stature | − | + | Compatible with Klinefelter |
| Gastrointestinal | |||||
| Chronic diarrhea | + | − | − | + | Characteristics of CTX |
| Dysmorphic/Physical | |||||
| Micrognathia | Rare | + | − | + | Likely Klinefelter-associated |
| Synophrys/ptosis/epicanthal folds | Variable | Mild | − | + | Possibly overlapping or nonspecific |
| Webbed neck/pectus excavatum | Rare | Occasional | − | + | Possibly related to Klinefelter |
| Café-au-lait spot/telangiectasias | − | − | − | + | Incidental, unrelated |
| Genetic Findings | |||||
| CYP27A1 homozygous variant (c.1183C>T) | Diagnostic | − | − | + | Confirms CTX |
| BRCA2 heterozygous variant (c.4936_4939del) | − | − | Diagnostic | + | Incidental, cancer predisposition |
| 47,XXY karyotype | − | Diagnostic | − | + | Confirms Klinefelter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pachajoa, H.; Bonilla, S.; Nieva-Posso, D.A. Genomic Confluence: When Cerebrotendinous Xanthomatosis, Klinefelter Syndrome, and a BRCA2 Variant Intersect. Int. J. Mol. Sci. 2025, 26, 10510. https://doi.org/10.3390/ijms262110510
Pachajoa H, Bonilla S, Nieva-Posso DA. Genomic Confluence: When Cerebrotendinous Xanthomatosis, Klinefelter Syndrome, and a BRCA2 Variant Intersect. International Journal of Molecular Sciences. 2025; 26(21):10510. https://doi.org/10.3390/ijms262110510
Chicago/Turabian StylePachajoa, Harry, Sebastián Bonilla, and Daniel Andrés Nieva-Posso. 2025. "Genomic Confluence: When Cerebrotendinous Xanthomatosis, Klinefelter Syndrome, and a BRCA2 Variant Intersect" International Journal of Molecular Sciences 26, no. 21: 10510. https://doi.org/10.3390/ijms262110510
APA StylePachajoa, H., Bonilla, S., & Nieva-Posso, D. A. (2025). Genomic Confluence: When Cerebrotendinous Xanthomatosis, Klinefelter Syndrome, and a BRCA2 Variant Intersect. International Journal of Molecular Sciences, 26(21), 10510. https://doi.org/10.3390/ijms262110510






