Metabolic Characterization of Two Flor Yeasts During Second Fermentation in the Bottle for Sparkling Wine Production
Abstract
1. Introduction
2. Results
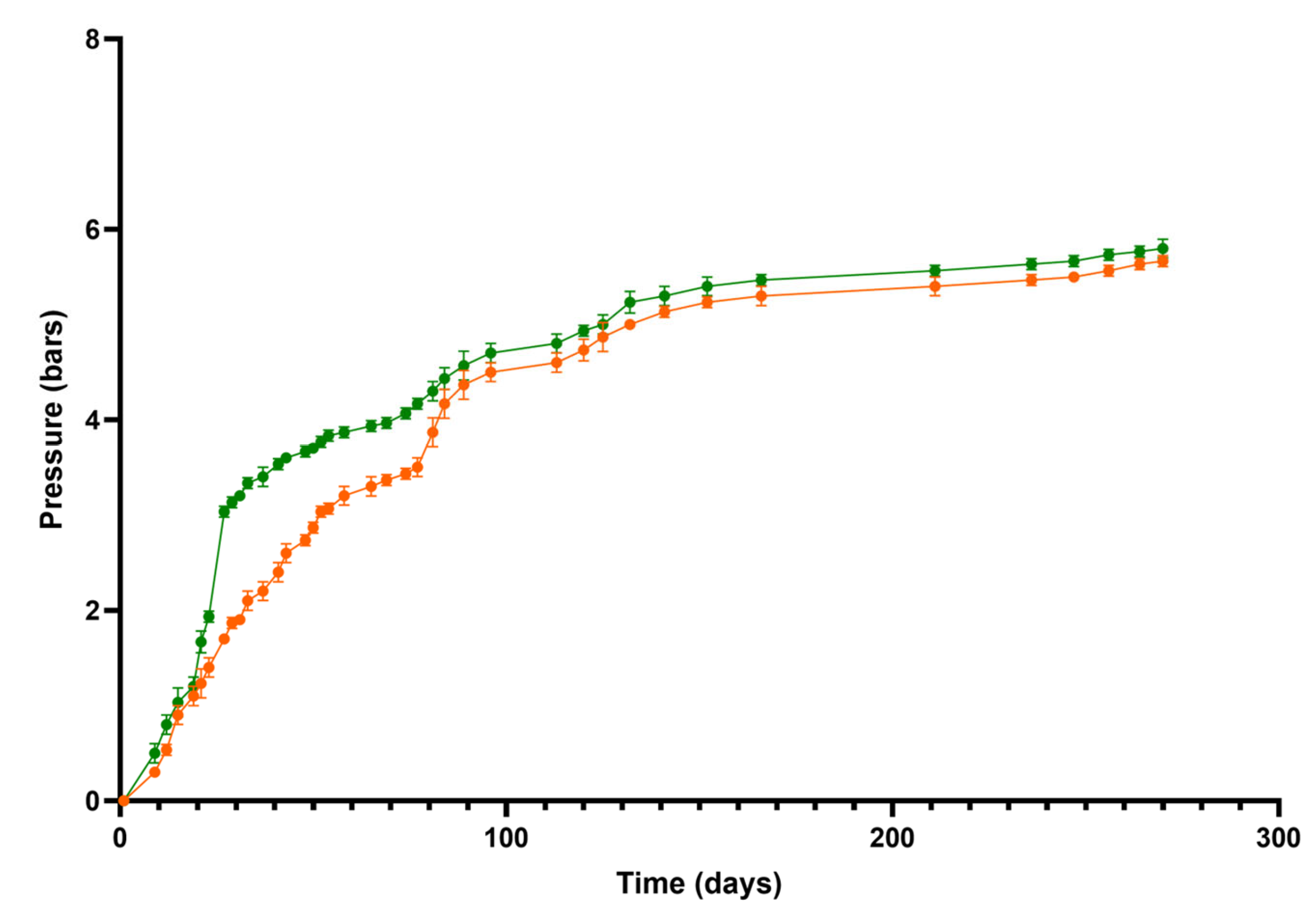
2.1. CO2 Pressure Monitoring of Second Fermentation and General Oenological Parameters
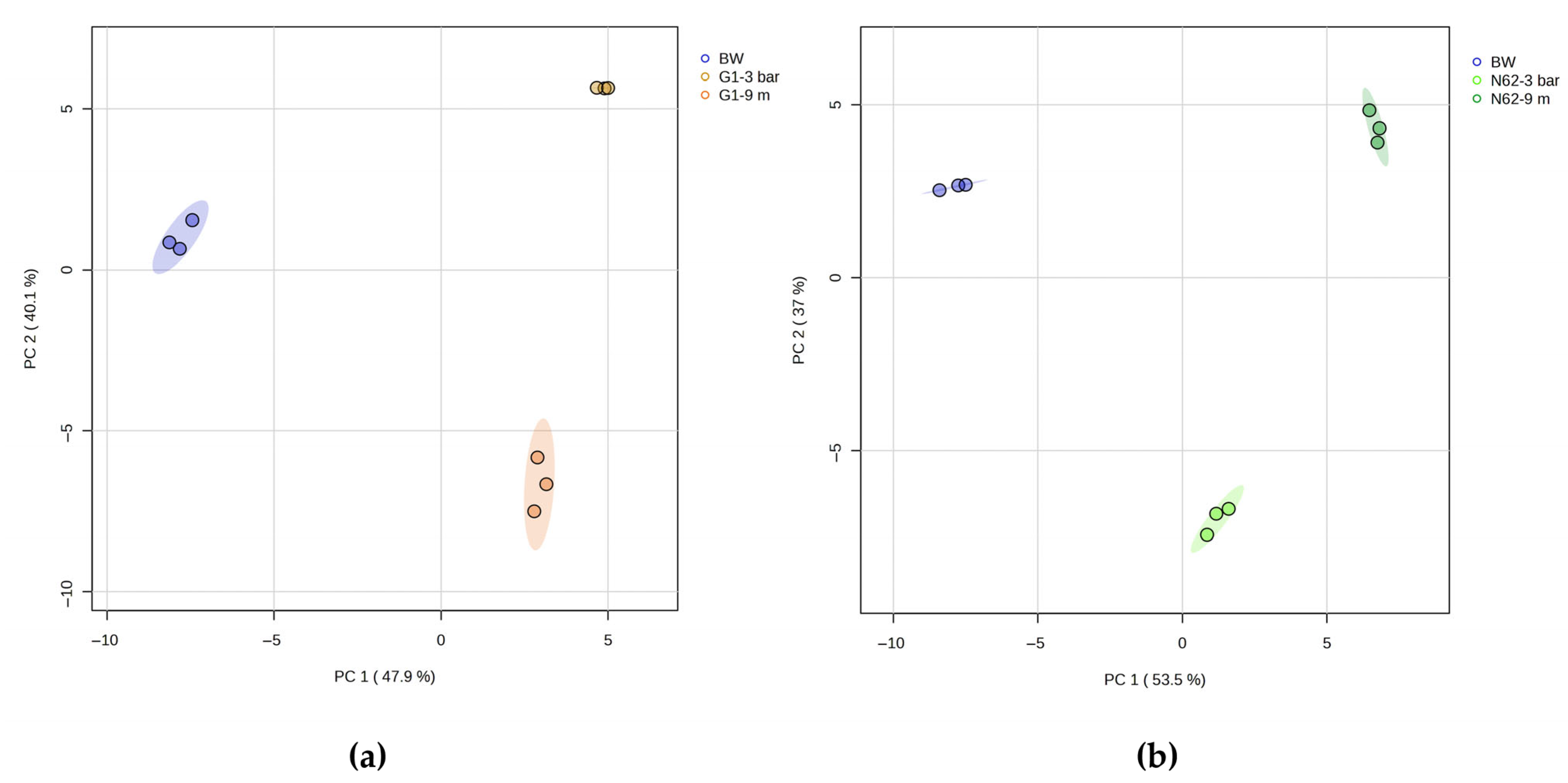
2.2. Changes in the Metabolic Profiles of Different Wines
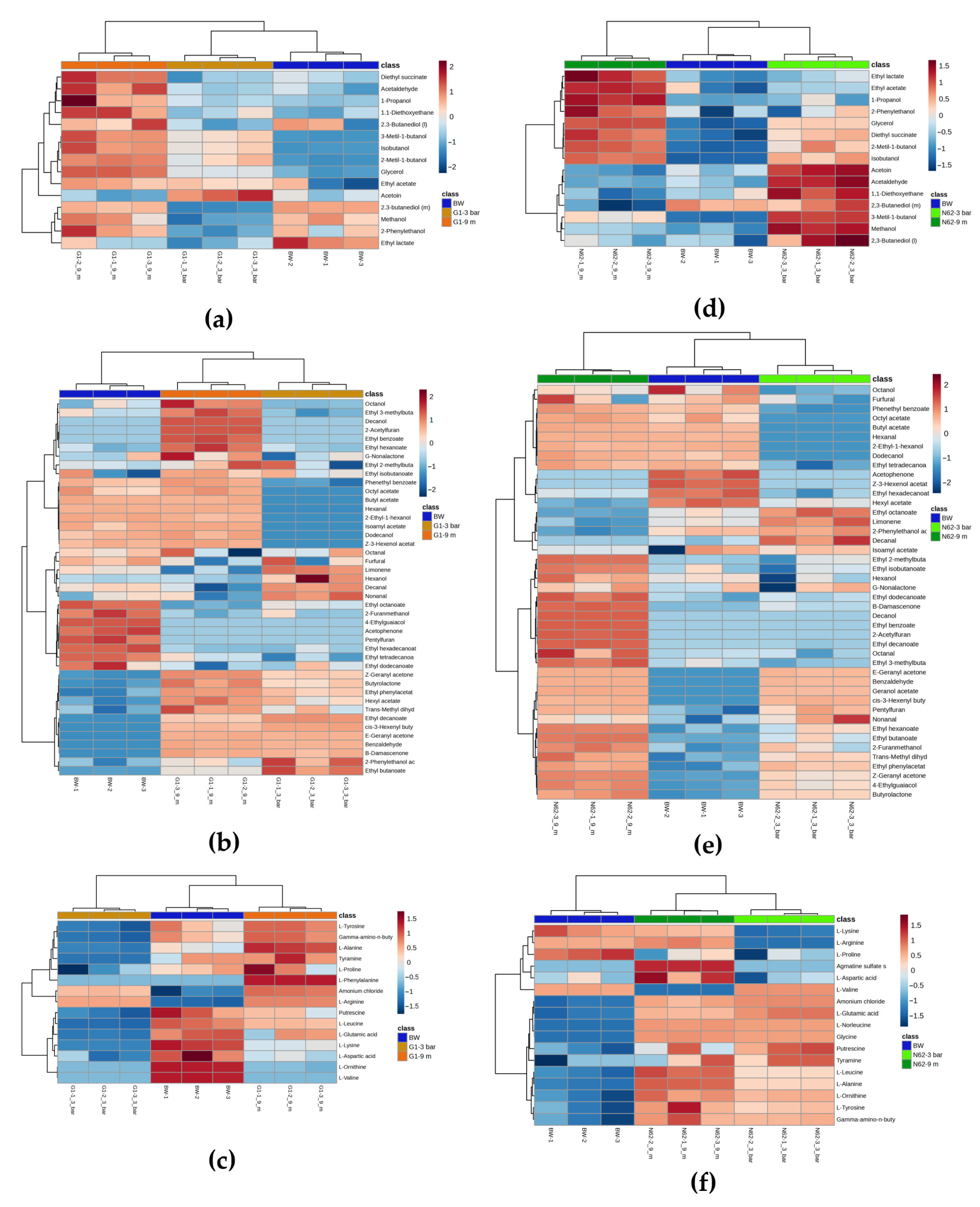
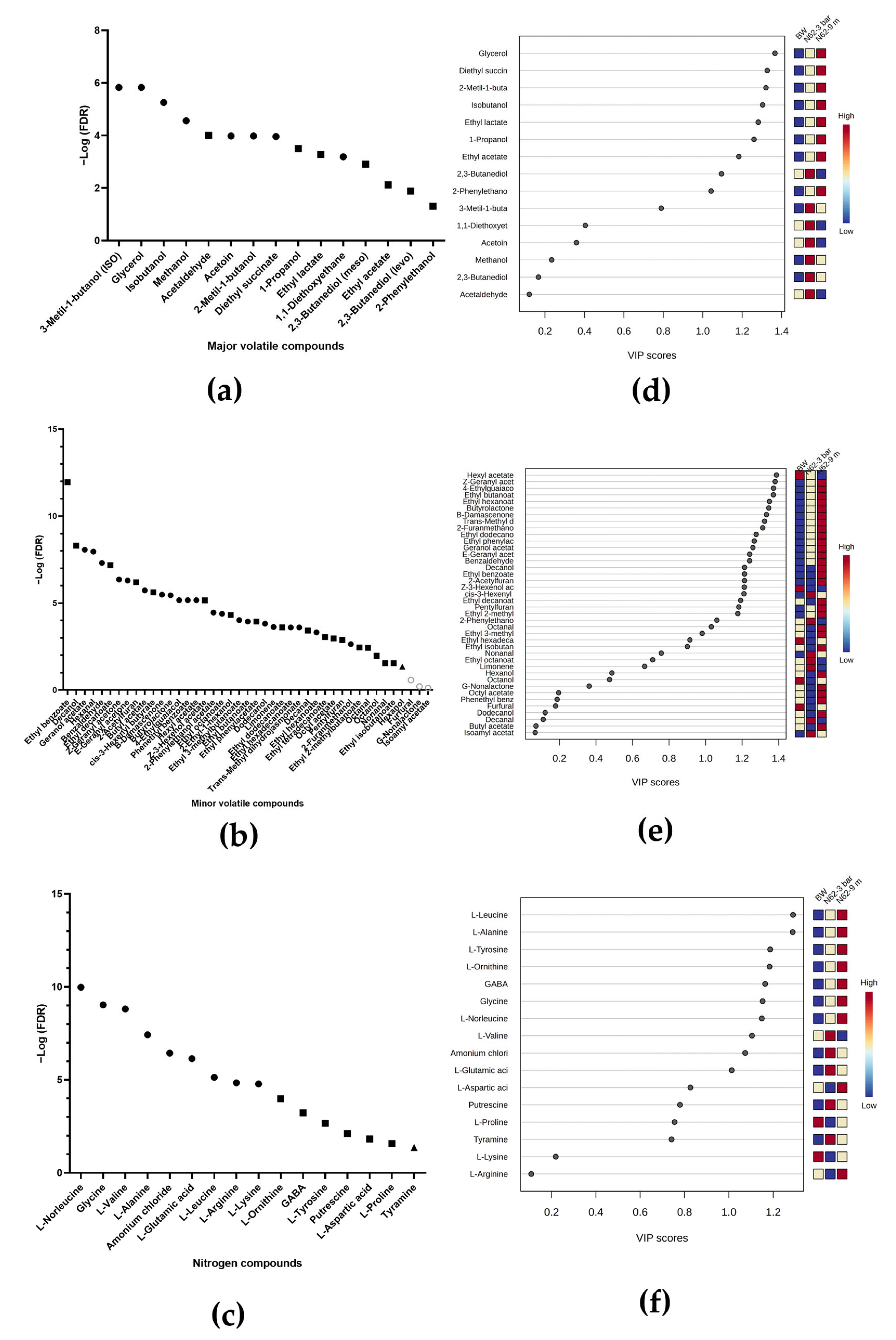
2.3. Metabolic Analysis Throughout the Second Fermentation
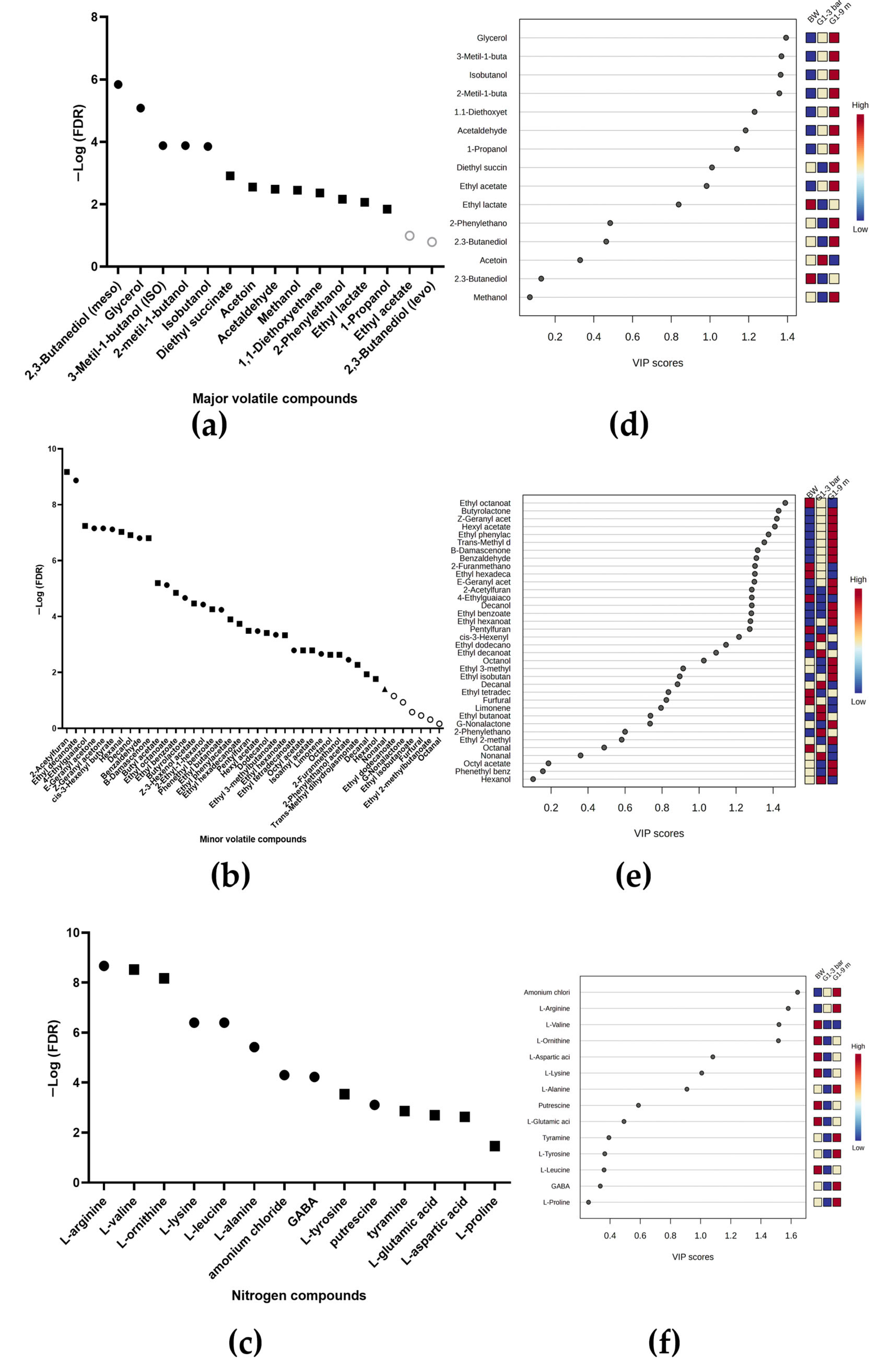
2.3.1. For Wines Inoculated with Strain G1
2.3.2. For Wines Inoculated with Strain N62
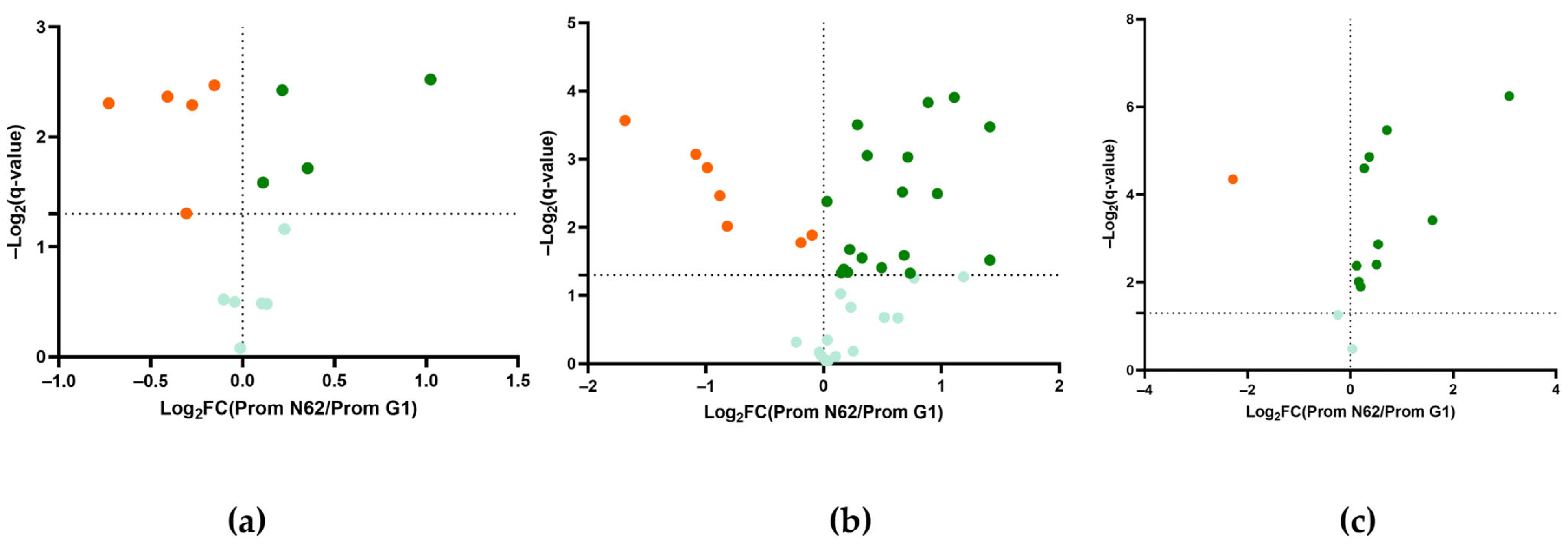
2.4. Comparison of the Metabolomic Profile of Wines at 9 Months
3. Discussion
4. Materials and Methods
4.1. Yeast Strains and Characterization
4.2. Acclimatation Process and Sparkling Wine Production
4.3. Chemical Analysis and Oenological Parameters of Wines
4.4. Cell Counting: Total and Viable
4.5. Analysis of Wine Volatiles
4.6. Quantification of Nitrogen Compounds
4.7. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine (OIV). State of the International Wine Markets in 2024; OIV: Paris, France, 2025. [Google Scholar]
- Amaro, F.; Almeida, J.; Oliveira, A.S.; Furtado, I.; Bastos, M.D.L.; Guedes de Pinho, P.; Pinto, J. Impact of cork closures on the volatile profile of sparkling wines during bottle aging. Foods 2022, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of native yeast strains for in bottle fermentation to face the uniformity in sparkling wine production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef]
- Martínez-García, R.; Roldán-Romero, Y.; Moreno, J.; Puig-Pujol, A.; Mauricio, J.C.; García-Martínez, T. Use of a flor yeast strain for the second fermentation of sparkling wines: Effect of endogenous CO2 over-pressure on the volatilome. Food Chem. 2020, 308, 125555. [Google Scholar] [CrossRef] [PubMed]
- García-García, J.C.; García-Martínez, T.; Román-Camacho, J.J.; Moreno, J.; Mauricio, J.C. Comparative proteomics of two flor yeasts in sparkling wine fermentation: First approach. Foods 2025, 14, 282. [Google Scholar] [CrossRef]
- Buxaderas, S.; López-Tamames, E. Sparkling wines: Features and trends from tradition. Adv. Food Nutr. Res. 2012, 66, 1–45. [Google Scholar]
- Cravero, M. Innovations in sparkling wine production: A review on the sensory aspects and the consumer’s point of view. Beverages 2023, 9, 80. [Google Scholar] [CrossRef]
- Just-Borràs, A.; Moroz, E.; Giménez, P.; Gombau, J.; Ribé, E.; Collado, A.; Cabanillas, P.; Marangon, M.; Fort, F.; Canals, J.M.; et al. Comparison of ancestral and traditional methods for elaborating sparkling wines. Curr. Res. Food Sci. 2024, 8, 100768. [Google Scholar] [CrossRef]
- Charnock, H.M.; Pickering, G.J.; Kemp, B.S. The Maillard reaction in traditional method sparkling wine. Front. Microbiol. 2022, 13, 979866. [Google Scholar] [CrossRef]
- Gianvito, P.; Arfelli, G.; Suzzi, G.; Tofalo, R. New trends in sparkling wine production: Yeast rational selection. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; Volume 7, pp. 347–386. [Google Scholar]
- Pinheiro, S.S.; Campos, F.; Cabrita, M.J.; da Silva, M.G. Exploring the aroma profile of traditional sparkling wines: A review on yeast selection in second fermentation, aging, closures, and analytical strategies. Molecules 2025, 30, 2825. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter cultures for sparkling wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- Tufariello, M.; Palombi, L.; Rizzuti, A.; Musio, B.; Capozzi, V.; Gallo, V.; Mastrorilli, P.; Grieco, F. Volatile and chemical profiles of Bombino sparkling wines produced with autochthonous yeast strains. Food Control 2023, 145, 109462. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, A. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Sommer, S.; Sommer, S.J.; Liu, C.; Burken, O.; Anderson, A.F. The Impact of microbial activity on the chemical composition and aroma profile of traditional sparkling wines. Fermentation 2024, 10, 212. [Google Scholar] [CrossRef]
- Gallardo-Chacón, J.J.; Vichi, S.; Urpí, P.; López-Tamames, E.; Buxaderas, S. Antioxidant activity of lees cell surface during sparkling wine sur lie aging. Int. J. Food Microbiol. 2010, 143, 48–53. [Google Scholar] [CrossRef]
- Ruipérez, V.; Rodríguez-Nogales, J.M.; Fernández-Fernández, E.; Vila-Crespo, J. Impact of β-glucanases and yeast derivatives on chemical and sensory composition of long-aged sparkling wines. J. Food Compos. Anal. 2022, 107, 104385. [Google Scholar] [CrossRef]
- Espinase Nandorfy, D.; Watson, F.; Likos, D.; Siebert, T.; Bindon, K.; Kassara, S.; Shellie, R.; Keast, R.; Francis, I.L. Influence of amino acids, and their interaction with volatiles and polyphenols, on the sensory properties of red wine. Aust. J. Grape Wine Res. 2022, 28, 621–637. [Google Scholar] [CrossRef]
- González-Jiménez, M.C.; Mauricio, J.C.; Moreno-García, J.; Puig-Pujol, A.; Moreno, J.; García-Martínez, T. Differential response of the proteins involved in amino acid metabolism in two Saccharomyces cerevisiae strains during the second fermentation in a sealed bottle. Appl. Sci. 2021, 11, 12165. [Google Scholar] [CrossRef]
- Prokes, K.; Baron, M.; Mlcek, J.; Jurikova, T.; Adamkova, A.; Ercisli, S.; Sochor, J. The influence of traditional and immobilized yeast on the amino-acid content of sparkling wine. Fermentation 2022, 8, 36. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Wine secondary aroma: Understanding yeast production of higher alcohols. Microb. Biotechnol. 2017, 10, 1449. [Google Scholar] [CrossRef]
- Jeandet, P.; Heinzmann, S.S.; Roullier-Gall, C.; Cilindre, C.; Aron, A.; Deville, M.A.; Moritz, F.; Karbowiak, T.; Demarville, D.; Brun, C.; et al. Chemical messages in 170-year-old champagne bottles from the Baltic Sea: Revealing tastes from the past. Proc. Natl. Acad. Sci. USA 2015, 112, 5893–5898. [Google Scholar] [CrossRef]
- Le Menn, N.; Marchand, S.; De Revel, G.; Demarville, D.; Laborde, D.; Marchal, R. N, S, O-Heterocycles in aged Champagne reserve wines and correlation with free amino acid concentrations. J. Agric. Food Chem. 2017, 65, 2345–2356. [Google Scholar] [CrossRef]
- Sartor, S.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Profiling of free amino acids in sparkling wines during over-lees aging and evaluation of sensory properties. LWT 2021, 140, 110847. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of yeast strain on odor-active compounds in Fiano wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Liu, G.; Wu, M.; Li, Y.; Qayyum, N.; Li, X.; Zhang, J.; Wang, C. The effect of different pretreatment methods on jujube juice and lactic acid bacteria-fermented jujube juice. LWT 2023, 181, 114692. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Sottil, C.; Salor-Torregrosa, J.M.; Moreno-Garcia, J.; Peinado, J.; Mauricio, J.C.; Moreno, J.; Garcia-Martinez, T. Using Torulaspora delbrueckii, Saccharomyces cerevisiae and Saccharomyces bayanus wine yeasts as starter cultures for fermentation and quality improvement of mead. Eur. Food Res. Technol. 2019, 245, 2705–2714. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, L.; Liu, A.; Su, L.; Shi, K.; Zhao, H.; Liu, S. Role of amino acids in flavor profiles and foam characteristics of sparkling wines during aging. J. Food Compos. Anal. 2024, 126, 105903. [Google Scholar] [CrossRef]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41 (Suppl. S1), S95–S128. [Google Scholar] [CrossRef]
- Satora, P.; Tuszyński, T. Influence of indigenous yeasts on the fermentation and volatile profile of plum brandies. Food Microbiol. 2010, 27, 418–424. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of Croatian commercial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef]
- Hodson, G.; Wilkes, E.; Azevedo, S.; Battaglene, T. Methanol in wine. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 9, p. 02028. [Google Scholar]
- Xu, X.; Wang, J.; Bao, M.; Niu, C.; Liu, C.; Zheng, F.; Li, Y.; Li, Q. Reverse metabolic engineering in lager yeast: Impact of the NADH/NAD+ ratio on acetaldehyde production during the brewing process. Appl. Microbiol. Biotechnol. 2019, 103, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Guittin, C.; Maçna, F.; Picou, C.; Perez, M.; Barreau, A.; Poitou, X.; Sablayrolles, J.; Mouret, J.; Farines, V. New online monitoring approaches to describe and understand the kinetics of acetaldehyde concentration during wine alcoholic fermentation: Access to production balances. Fermentation 2023, 9, 299. [Google Scholar] [CrossRef]
- Nedović, V.; Gibson, B.; Mantzouridou, T.F.; Bugarski, B.; Djordjević, V.; Kalušević, A.; Paraskevopoulou, A.; Sandell, M.; Smogrovicova, D.; Yilmaztekin, M. Aroma formation by immobilized yeast cells in fermentation processes. Yeast 2015, 32, 173–216. [Google Scholar] [CrossRef]
- Chen, S.; Wang, C.; Qian, M.; Li, Z.; Xu, Y. Characterization of the key aroma compounds in aged Chinese rice wine by comparative aroma extract dilution analysis, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2019, 67, 4876–4884. [Google Scholar] [CrossRef]
- De Lerma, N.L.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of two yeast strains in free, bioimmobilized or immobilized with alginate forms on the aromatic profile of long aged sparkling wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of industrial yeast derivative products on the modification of wine aroma compounds and sensorial profile. A review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef]
- Luchian, C.E.; Scutarașu, E.C.; Colibaba, L.C.; Focea, M.C.; Cotea, V. Trends in reducing the effects of global warming: Applications of reverse osmosis to obtain sparkling wines with moderate alcohol concentrations. In Global Warming and the Wine Industry-Challenges, Innovations and Future Prospects; IntechOpen: London, UK, 2023. [Google Scholar]
- Gao, Y.T.; Zhang, Y.S.; Wen, X.; Song, X.W.; Meng, D.; Li, B.J.; Wang, M.Y.; Tao, Y.Q.; Zhao, H.; Guan, W.Q.; et al. The glycerol and ethanol production kinetics in low-temperature wine fermentation using Saccharomyces cerevisiae yeast strains. Int. J. Food Sci. Technol. 2019, 54, 102–110. [Google Scholar] [CrossRef]
- Ochando, T.; Mouret, J.R.; Humbert-Goffard, A.; Aguera, E.; Sablayrolles, J.M.; Farines, V. Comprehensive study of the dynamic interaction between SO2 and acetaldehyde during alcoholic fermentation. Food Res. Int. 2020, 136, 109607. [Google Scholar] [CrossRef]
- Wang, X.; Fan, G.; Peng, Y.; Xu, N.; Xie, Y.; Zhou, H.; Liang, H.; Zhan, J.; Huang, W.; You, Y. Mechanisms and effects of non-Saccharomyces yeast fermentation on the aromatic profile of wine. J. Food Compos. Anal. 2023, 124, 105660. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the usefulness of aroma networks to explain wine aroma properties: A case study of Portuguese wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef]
- Huang, Y.; Zhong, M.; Mu, S.; Han, Y.; Shi, Y.; Li, X.; Li, D. Assessment of the contributions of Saccharomyces cerevisiae, Hansenula sp. and Pichia kudriavzevii to volatile organic compounds and sensory characteristics of waxy rice wine. Eur. Food Res. Technol. 2023, 249, 685–697. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-Martínez, T.; Mauricio, J.C.; Moreno, J.; Peinado, R.A. Influence of terroir on microbial diversity and wine volatilome. Appl. Sci. 2025, 15, 3237. [Google Scholar] [CrossRef]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Badura, J.; Medić, M.; Wyk, N.V.; Krause, B.; Semmler, H.; Brezina, S.; Pretorius, I.S.; Rauhut, D.; Wallbrunn, C.V. Synthesis of aroma compounds as a function of different nitrogen sources in fermentations using non-Saccharomyces wine yeasts. Microorganisms 2022, 11, 14. [Google Scholar] [CrossRef]
- Voce, S.; Škrab, D.; Vrhovsek, U.; Battistutta, F.; Comuzzo, P.; Sivilotti, P. Compositional characterization of commercial sparkling wines from cv. Ribolla Gialla produced in Friuli Venezia Giulia. Eur. Food Res. Technol. 2020, 245, 2279–2292, Erratum in Eur. Food Res. Technol. 2021, 247, 295. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.J.; Muñoz-Redondo, J.M.; Cuevas, F.J.; Marrufo-Curtido, A.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M. The influence of pre-fermentative maceration and ageing factors on ester profile and marker determination of Pedro Ximenez sparkling wines. Food Chem. 2017, 230, 697–704. [Google Scholar] [CrossRef]
- Lambert-Royo, M.I.; Ubeda, C.; Del Barrio-Galán, R.; Sieczkowski, N.; Canals, J.M.; Peña-Neira, Á.; i Cortiella, M.G. The diversity of effects of yeast derivatives during sparkling wine aging. Food Chem. 2022, 390, 133174. [Google Scholar] [CrossRef]
- Benucci, I.; Cerreti, M.; Maresca, D.; Mauriello, G.; Esti, M. Yeast cells in double layer calcium alginate–chitosan microcapsules for sparkling wine production. Food Chem. 2019, 300, 125174. [Google Scholar] [CrossRef]
- Cotea, V.D.; Zănoagă, C.; Cotea, V.V. Treatise of Oenochemistry; Editura Academiei Române: București, Romania, 2014; Volume I. [Google Scholar]
- Liu, S.; Simonato, B.; Rizzi, C.; Zapparoli, G.; Bianchi, F.; Vincenzi, S. Sustainable Sparkling Cherry Wine Production from Early and Late Varieties: Insights into Technological Properties and Volatile Compounds. Food Bioprocess Technol. 2025, 18, 7083–7094. [Google Scholar] [CrossRef]
- Martín-Garcia, A.; Abarca-Rivas, C.; Riu-Aumatell, M.; López-Tamames, E. Comparison of volatile compounds during biological ageing and commercial storage of Cava (Spanish sparkling wine): The role of lees. Heliyon 2023, 9, e19306. [Google Scholar] [CrossRef] [PubMed]
- Mu, Z.; Yang, Y.; Xia, Y.; Zhang, H.; Ni, B.; Ni, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; et al. Enhancement of the aromatic alcohols and health properties of Chinese rice wine by using a potentially probiotic Saccharomyces cerevisiae BR14. LWT 2023, 181, 114748. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef]
- Demirgül, F.; Şimşek, Ö.; Bozkurt, F.; Dertli, E.; Sağdıç, O. Production and characterization of yeast extracts produced by Saccharomyces cerevisiae, Saccharomyces boulardii and Kluyveromyces marxianus. Prep. Biochem. Biotechnol. 2022, 52, 657–667. [Google Scholar] [CrossRef]
- Minebois, R.; Lairón-Peris, M.; Barrio, E.; Pérez-Torrado, R.; Querol, A. Metabolic differences between a wild and a wine strain of Saccharomyces cerevisiae during fermentation unveiled by multi-omic analysis. Environ. Microbiol. 2021, 23, 3059–3076. [Google Scholar] [CrossRef] [PubMed]
- Greifová, G.; Májeková, H.; Greif, G.; Body, P.; Greifová, M.; Dubničková, M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. 2017, 62, 515–524. [Google Scholar] [CrossRef]
- OIV. 2021. Available online: https://www.oiv.int/en/ (accessed on 25 August 2025).
- Martínez-García, R.; Mauricio, J.C.; García-Martínez, T.; Peinado, R.A.; Moreno, J. Towards a better understanding of the evolution of odour-active compounds and the aroma perception of sparkling wines during ageing. Food Chem. 2021, 357, 129784. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC Analysis of biogenic amines, amino acids, and ammonium ion as aminoenone derivatives in wine and beer samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
| G1 (3 Bars) | G1 (9 Months) | N62 (3 Bars) | N62 (9 Months) | |
|---|---|---|---|---|
| pH | 3.022 a ± 0.071 | 3.276 b ± 0.061 | 3.132 a ± 0.061 | 3.302 b ± 0.082 |
| Titratable acidity (g/L) | 4.343 ± 0.613 | 4.022 ± 0.533 | 4.260 ± 0.111 | 3.949 ± 0.105 |
| Ethanol (% v/v) | 11.929 a ± 0.080 | 12.134 b ± 0.090 | 11.100 c ± 0.080 | 11.987 ab ± 0.090 |
| Volatile acidity (g/L) | 0.224 a ± 0.021 | 0.282 a ± 0.023 | 0.421 b ± 0.111 | 0.497 b ± 0.131 |
| Reducing sugar (g/L) | 0.235 a ± 0.004 | 0.197 b ± 0.004 | 0.221 c ± 0.003 | 0.178 d ± 0.003 |
| Malic acid (g/L) | 0.220 a ± 0.010 | 0.199 b ± 0.010 | 0.010 c ± 0.000 | 0.010 c ± 0.000 |
| Lactic acid (g/L) | 1.213 a ± 0.010 | 1.989 b ± 0.111 | 0.330 c ± 0.010 | 1.334 d ± 0.032 |
| G1 (3 Bars) | G1 (9 Months) | N62 (3 Bars) | N62 (9 Months) | |
|---|---|---|---|---|
| Viable (× 106 cells/mL) | 0.730 a ± 0.035 | 0.060 b ± 0.010 | 3.900 c ± 0.265 | 0.830 a ± 0.040 |
| Total (× 106 cells/mL) | 21.000 a ± 1.730 | 23.000 a ± 1.832 | 46.300 b ± 3.210 | 44.86 c ± 2.340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, J.C.; Alcalá-Jiménez, M.T.; Mauricio, J.C.; Campos-Vázquez, C.; Santos-Dueñas, I.M.; Moreno, J.; García-Martínez, T. Metabolic Characterization of Two Flor Yeasts During Second Fermentation in the Bottle for Sparkling Wine Production. Int. J. Mol. Sci. 2025, 26, 10457. https://doi.org/10.3390/ijms262110457
García-García JC, Alcalá-Jiménez MT, Mauricio JC, Campos-Vázquez C, Santos-Dueñas IM, Moreno J, García-Martínez T. Metabolic Characterization of Two Flor Yeasts During Second Fermentation in the Bottle for Sparkling Wine Production. International Journal of Molecular Sciences. 2025; 26(21):10457. https://doi.org/10.3390/ijms262110457
Chicago/Turabian StyleGarcía-García, Juan Carlos, María Trinidad Alcalá-Jiménez, Juan Carlos Mauricio, Cristina Campos-Vázquez, Inés M. Santos-Dueñas, Juan Moreno, and Teresa García-Martínez. 2025. "Metabolic Characterization of Two Flor Yeasts During Second Fermentation in the Bottle for Sparkling Wine Production" International Journal of Molecular Sciences 26, no. 21: 10457. https://doi.org/10.3390/ijms262110457
APA StyleGarcía-García, J. C., Alcalá-Jiménez, M. T., Mauricio, J. C., Campos-Vázquez, C., Santos-Dueñas, I. M., Moreno, J., & García-Martínez, T. (2025). Metabolic Characterization of Two Flor Yeasts During Second Fermentation in the Bottle for Sparkling Wine Production. International Journal of Molecular Sciences, 26(21), 10457. https://doi.org/10.3390/ijms262110457







