Pleiotropic Effects of Polymorphisms in the BCL11A Gene on Laboratory Parameters in Sickle Cell Anemia
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
2.2. Allelic and Genotypic Frequencies of Polymorphisms
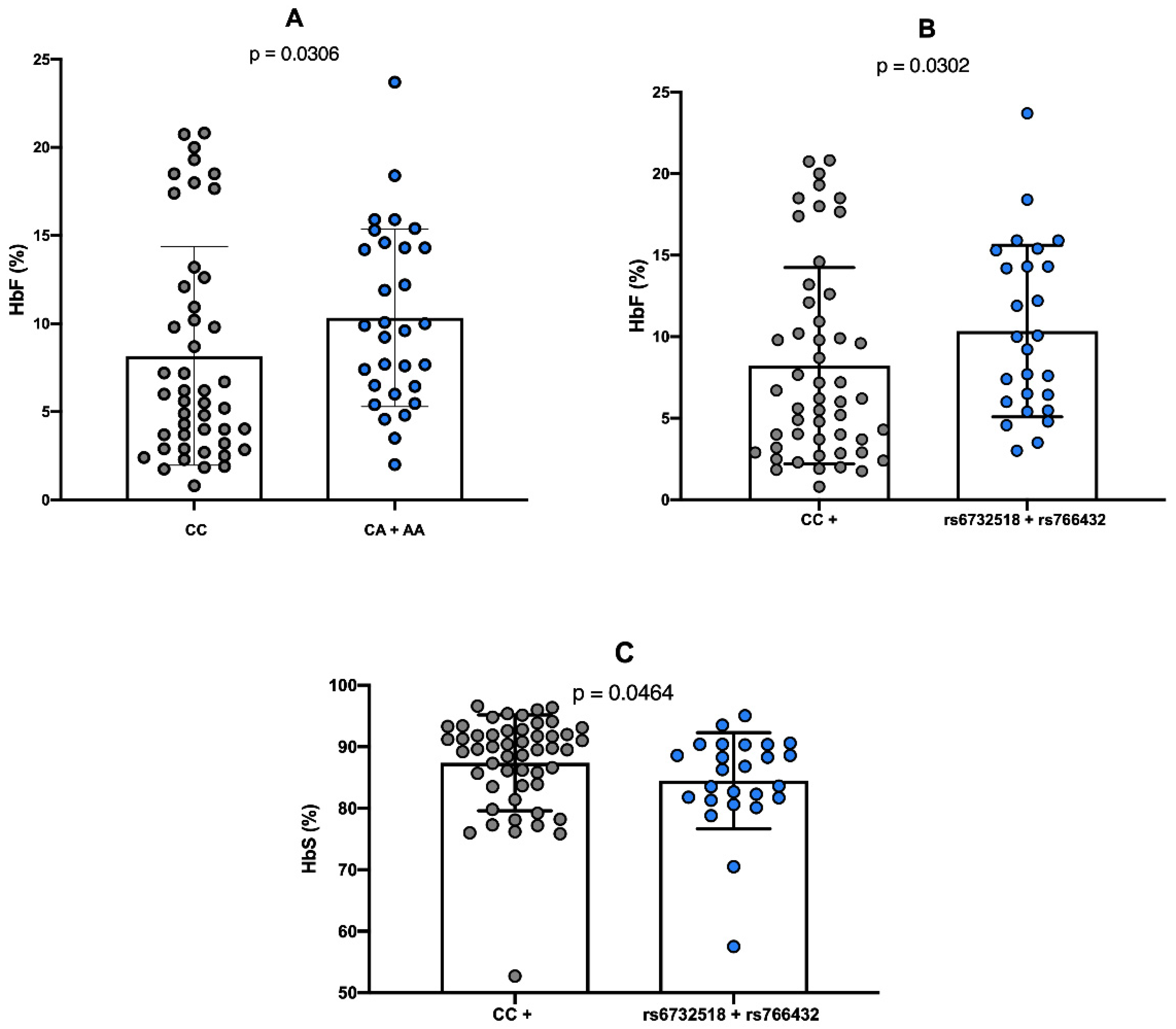
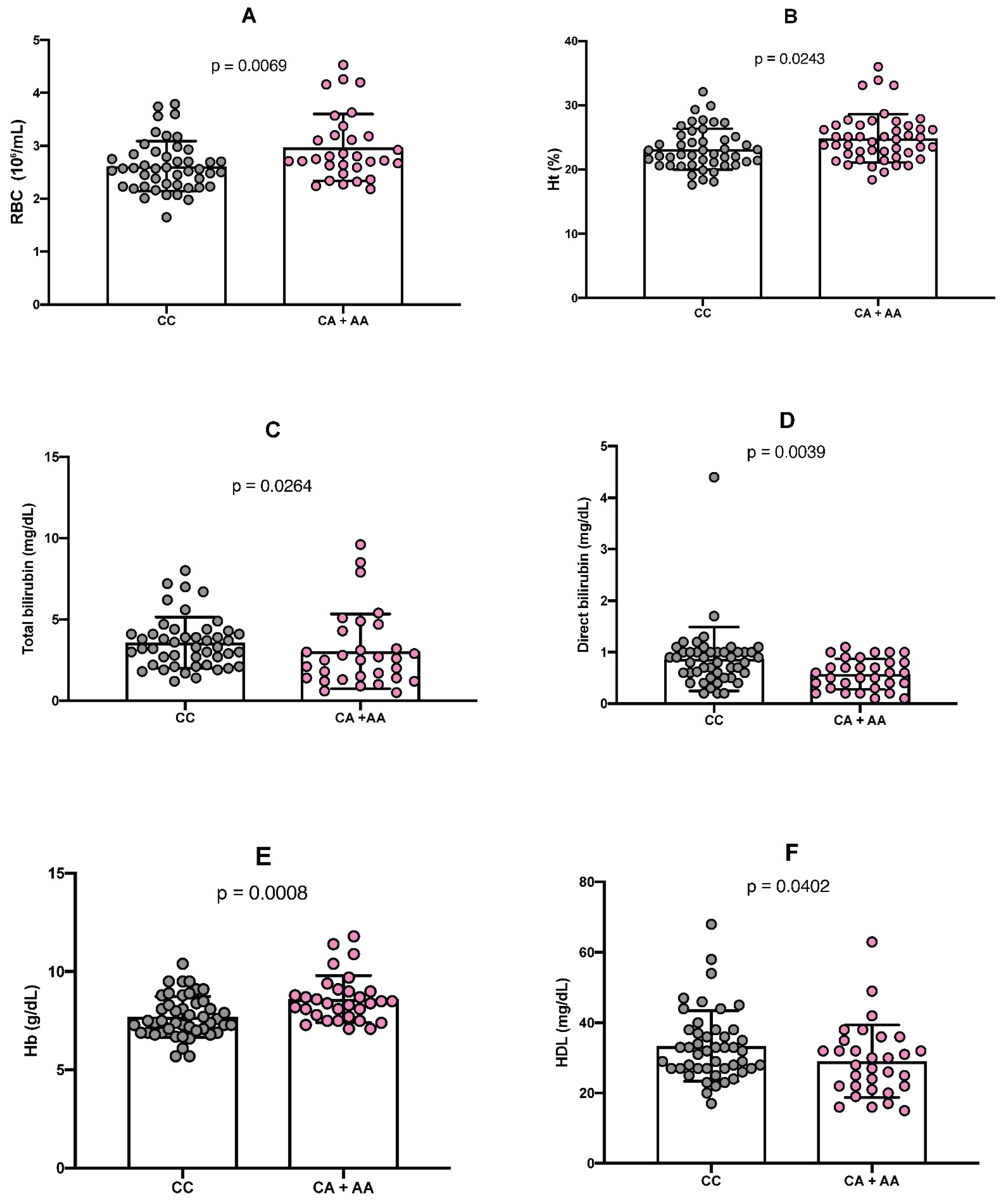
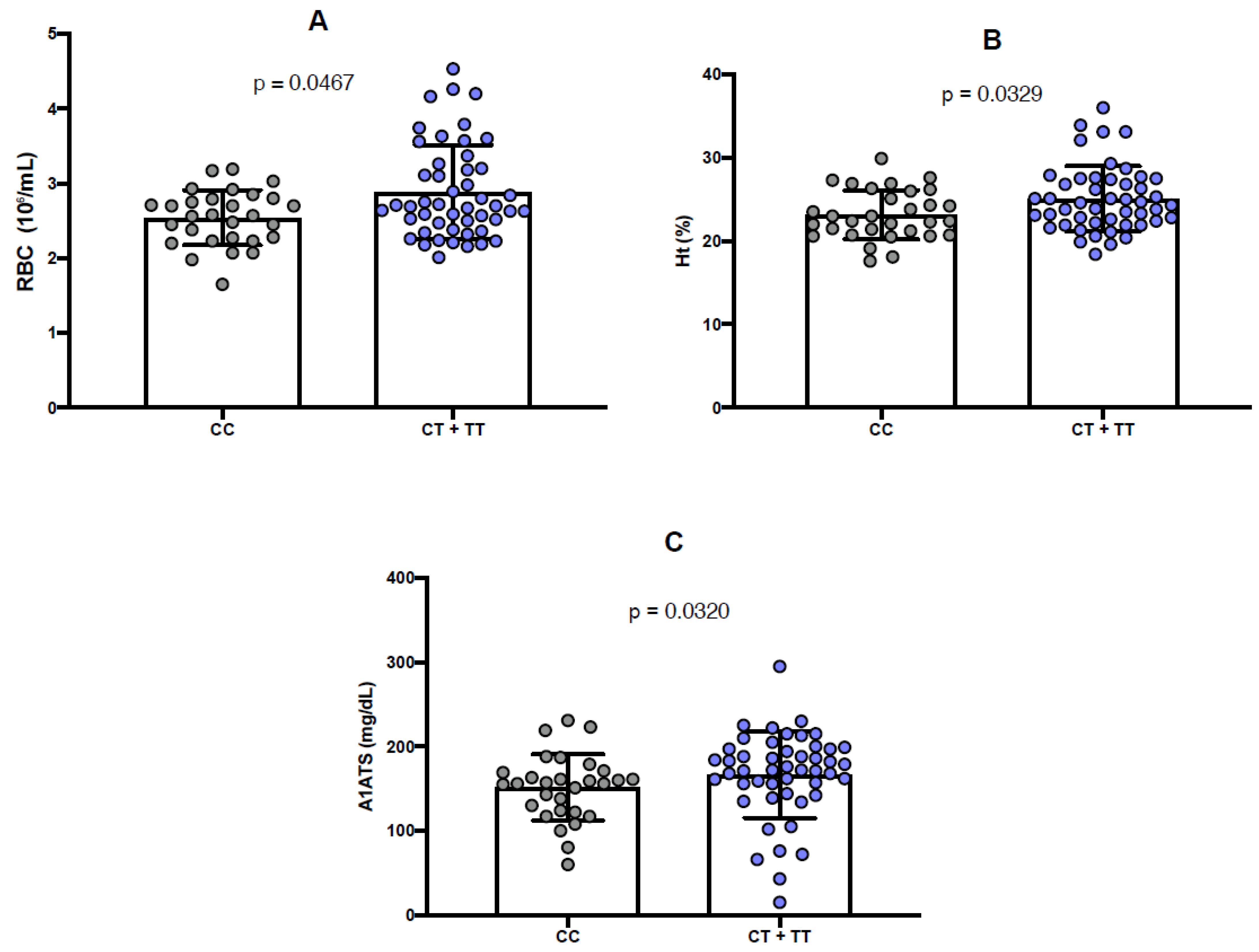
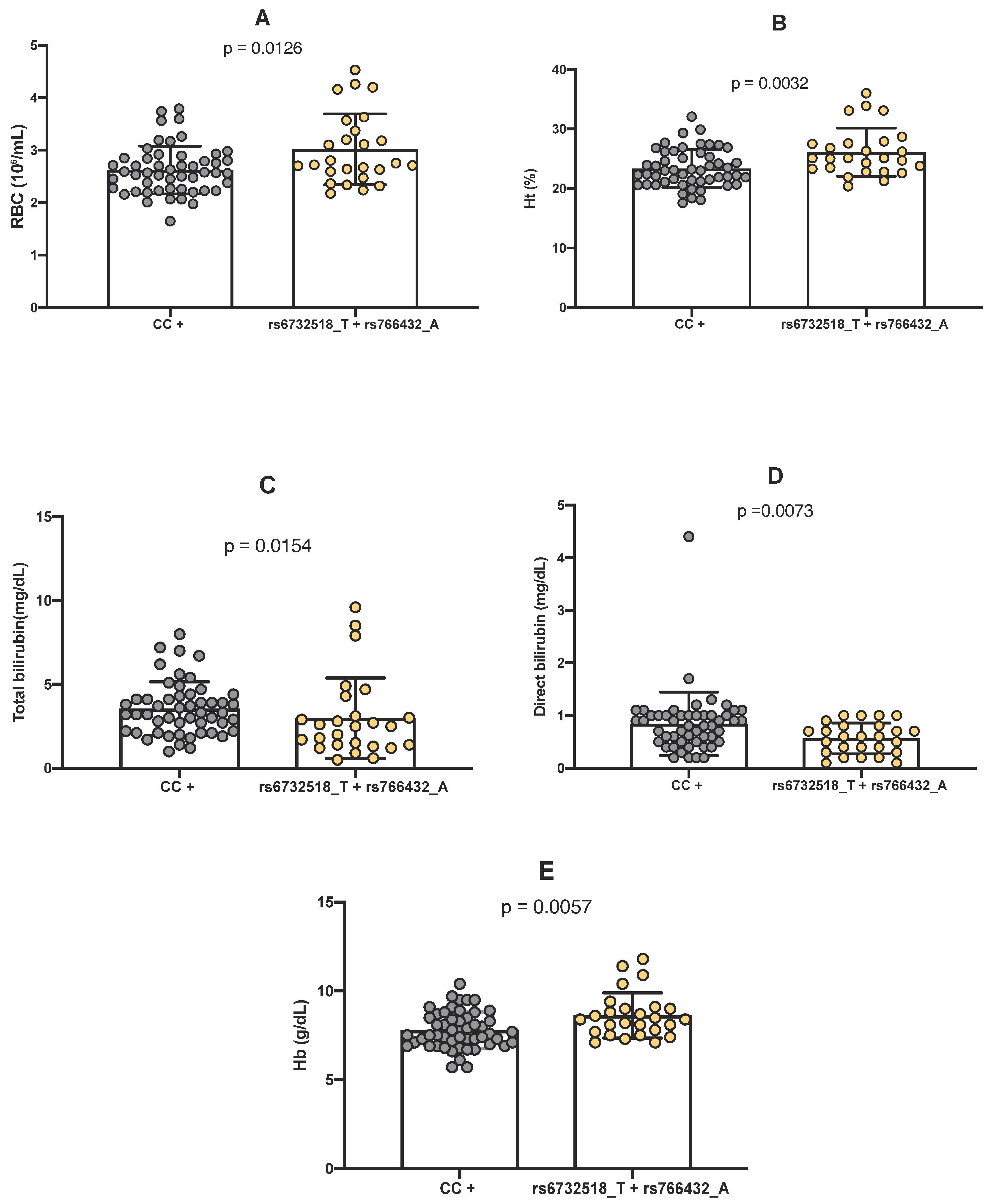
2.3. Comparison Between Genotypes of Polymorphisms and Hemoglobin Levels
2.4. Comparison Between Genotypes of Polymorphisms and Laboratory Parameters
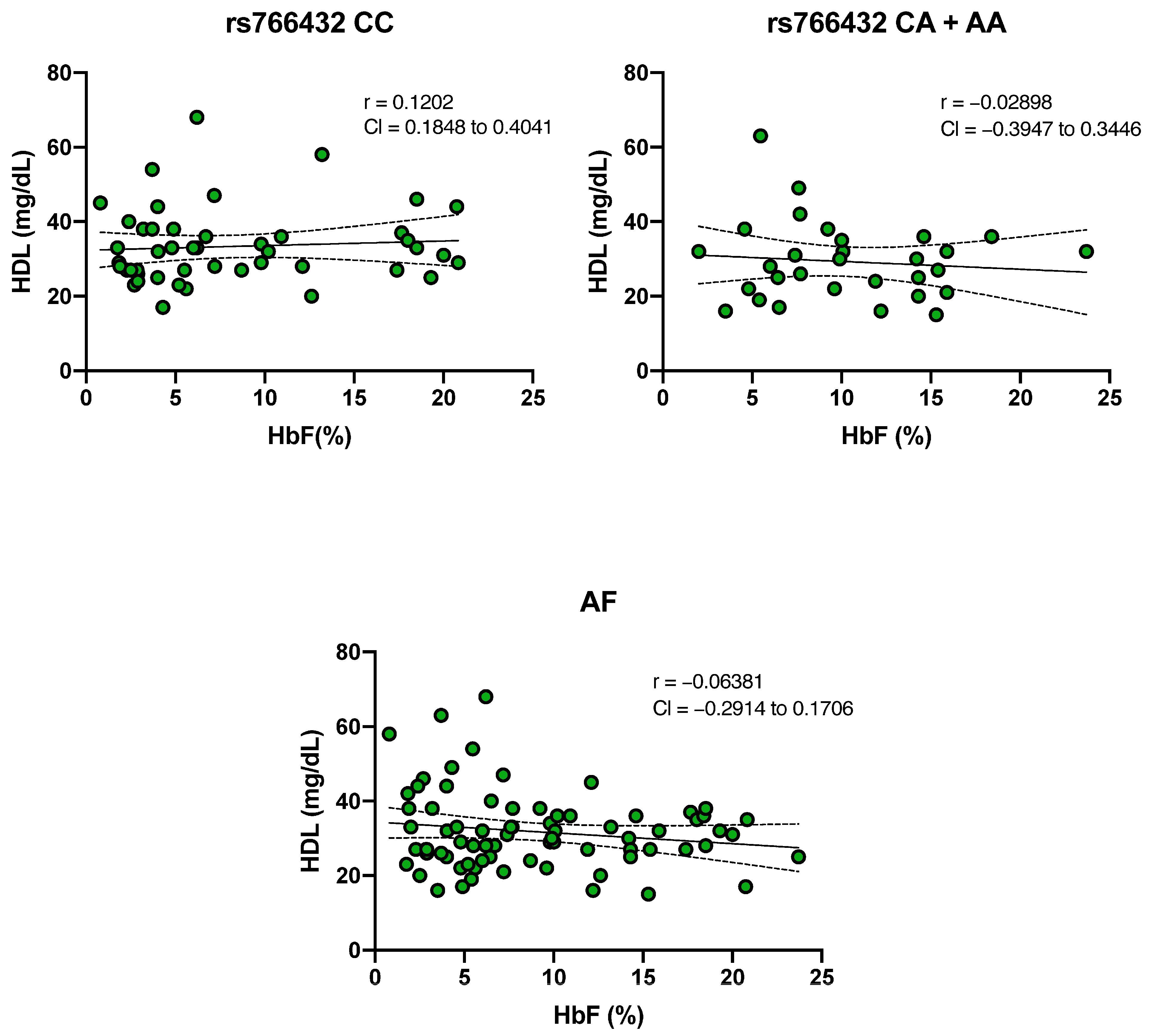
2.5. Evaluation of the Relationship Between HbF and HDL Cholesterol Levels
3. Discussion
4. Materials and Methods
4.1. Patient Recruitment
4.2. Sample Collection
4.3. Hematological and Biochemical Analyses
4.4. Genetic Analyses
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esoh, K.; Wonkam, A. Evolutionary history of sickle-cell mutation: Implications for global genetic medicine. Hum. Mol. Genet. 2021, 30, R119–R128. [Google Scholar] [CrossRef]
- Sikora, J.; Orlov, S.N.; Furuya, K.; Grygorczyk, R. Hemolysis is a primary ATP-release mechanism in human erythrocytes. Blood 2014, 124, 2150–2157. [Google Scholar] [CrossRef]
- Kato, G.J.; Steinberg, M.H.; Gladwin, M.T. Intravascular hemolysis and the pathophysiology of sickle cell disease. J. Clin. Investig. 2017, 127, 750–760. [Google Scholar] [CrossRef]
- Uwaezuoke, S.N.; Ayuk, A.C.; Ndu, I.K.; Eneh, C.; Mbanefo, N.R.; Ezenwosu, O.U. Vaso-occlusive crisis in sickle cell disease: Current paradigm on pain management. J. Pain Res. 2018, 11, 3141–3150. [Google Scholar] [CrossRef]
- Pecker, L.H.; Little, J. Clinical manifestations of sickle cell disease across the lifespan. In Sickle Cell Disease and Hematopoietic Stem Cell Transplantation; Springer: Cham, Switzerland, 2018; pp. 3–39. [Google Scholar]
- Bauer, D.E.; Orkin, S.H. Hemoglobin switching’s surprise: The versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr. Opin. Genet. Dev. 2015, 33, 62–70. [Google Scholar] [CrossRef]
- López Rubio, M.; Argüello Marina, M. The current role of hydroxyurea in the treatment of sickle cell anemia. J. Clin. Med. 2024, 13, 6404. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wyszynski, D.F.; Farrell, J.J.; Kutlar, A.; A Farrer, L.; Baldwin, C.T.; Steinberg, M.H. Fetal hemoglobin in sickle cell anemia: Genetic determinants of response to hydroxyurea. Pharmacogenomics J. 2007, 7, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Pule, G.D.; Mowla, S.; Novitzky, N.; Wiysonge, C.S.; Wonkam, A. A systematic review of known mechanisms of hydroxyurea-induced fetal hemoglobin for treatment of sickle cell disease. Expert Rev. Hematol. 2015, 8, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, D.; Engel, J.D.; Saunthararajah, Y. Fetal hemoglobin induction by epigenetic drugs. In Seminars in Hematology; W.B. Saunders: Philadelphia, PA, USA, 2018; pp. 60–67. [Google Scholar]
- Esrick, E.B.; Lehmann, L.E.; Biffi, A.; Achebe, M.; Brendel, C.; Ciuculescu, M.F.; Daley, H.; MacKinnon, B.; Morris, E.; Federico, A.; et al. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. N. Engl. J. Med. 2021, 384, 205–215. [Google Scholar] [CrossRef]
- Frangoul, H.; Altshuler, D.; Cappellini, M.D.; Chen, Y.-S.; Domm, J.; Eustace, B.K.; Foell, J.; De La Fuente, J.; Grupp, S.; Handgretinger, R.; et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med. 2021, 384, 252–260. [Google Scholar]
- Iarovaia, O.V.; Kovina, A.P.; Petrova, N.V.; Razin, S.V.; Ioudinkova, E.S.; Vassetzky, Y.S.; Ulianov, S.V. Genetic and epigenetic mechanisms of β-globin gene switching. Biochemistry 2018, 83, 381–392. [Google Scholar] [CrossRef]
- Cao, A.; Moi, P. Regulation of the globin genes. Pediatr. Res. 2002, 51, 415–421. [Google Scholar] [CrossRef][Green Version]
- Ginder, G.D. Epigenetic regulation of fetal globin gene expression in adult erythroid cells. Transl. Res. 2015, 165, 115–125. [Google Scholar] [CrossRef]
- Liu, N.; Hargreaves, V.V.; Zhu, Q.; Kurland, J.V.; Hong, J.; Kim, W.; Sher, F.; Macias-Trevino, C.; Rogers, J.M.; Kurita, R.; et al. Direct promoter repression by BCL11A controls the fetal to adult hemoglobin switch. Cell 2018, 173, 430–442.e17. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.C.; Luc, S.; Croney, D.M.; Woodworth, M.B.; Greig, L.C.; Fujiwara, Y.; Nguyen, M.; Sher, F.; Macklis, J.D.; Bauer, D.E.; et al. Strict in vivo specificity of the Bcl11a erythroid enhancer. Blood 2016, 128, 2338–2342. [Google Scholar] [CrossRef]
- Yin, J.; Xie, X.; Ye, Y.; Wang, L.; Che, F. BCL11A: A potential diagnostic biomarker and therapeutic target in human diseases. Biosci. Rep. 2019, 39, BSR20190604. [Google Scholar] [CrossRef]
- Thein, S.L.; Menzel, S.; Lathrop, M.; Garner, C. Control of fetal hemoglobin: New insights emerging from genomics and clinical implications. Hum. Mol. Genet. 2009, 18, R216–R223. [Google Scholar] [CrossRef] [PubMed]
- Métais, J.-Y.; Doerfler, P.A.; Mayuranathan, T.; Bauer, D.E.; Fowler, S.C.; Hsieh, M.M.; Katta, V.; Keriwala, S.; Lazzarotto, C.R.; Luk, K.; et al. Genome editing of HBG1 and HBG2 to induce fetal hemoglobin. Blood Adv. 2019, 3, 3379–3392. [Google Scholar] [CrossRef]
- Allard, P.; Alhaj, N.; Lobitz, S.; Cario, H.; Jarisch, A.; Grosse, R.; Oevermann, L.; Hakimeh, D.; Tagliaferri, L.; Kohne, E.; et al. Genetic modifiers of fetal hemoglobin affect the course of sickle cell disease in patients treated with hydroxyurea. Haematologica 2021, 107, 1577. [Google Scholar] [CrossRef]
- Abdulazeez, S.; Sultana, S.; Almandil, N.B.; Almohazey, D.; Bency, B.J.; Borgio, J.F. The rs61742690 (S783N) single nucleotide polymorphism is a suitable target for disrupting BCL11A-mediated foetal-to-adult globin switching. PLoS ONE 2019, 14, e0212492. [Google Scholar] [CrossRef] [PubMed]
- Abdulazeez, S. Molecular simulation studies on B-cell lymphoma/leukaemia 11A (BCL11A). Am. J. Transl. Res. 2019, 11, 3689. [Google Scholar]
- Solovieff, N.; Milton, J.N.; Hartley, S.W.; Sherva, R.; Sebastiani, P.; Dworkis, D.A.; Klings, E.S.; Farrer, L.A.; Garrett, M.E.; Ashley-Koch, A.; et al. Fetal hemoglobin in sickle cell anemia: Genome-wide association studies suggest a regulatory region in the 5′ olfactory receptor gene cluster. Blood 2010, 115, 1815–1822. [Google Scholar] [CrossRef]
- Banan, M.; Bayat, H.; Namdar-Aligoodarzi, P.; Azarkeivan, A.; Kamali, K.; Daneshmand, P.; Zaker-Kandjani, B.; Najmabadi, H. Utility of the multivariate approach in predicting β-thalassemia intermedia or β-thalassemia major types in Iranian patients. Hemoglobin 2013, 37, 413–422. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Purvis, S.; Barron-Casella, E.; DeBaun, M.R.; Casella, J.F.; E Arking, D.; Keefer, J.R. Genome-wide association study identifies genetic variants influencing F-cell levels in sickle-cell patients. J. Hum. Genet. 2011, 56, 316–323. [Google Scholar] [CrossRef]
- Aleluia, M.M.; Santiago, R.P.; da Guarda, C.C.; Fonseca, T.C.C.; Neves, F.I.; Quinto, R.S.; Figueiredo, C.V.B.; Yahouédéhou, S.C.M.A.; Oliveira, R.M.; Ferreira, J.R.D.; et al. Genetic modulation of fetal hemoglobin in hydroxyurea-treated sickle cell anemia. Am. J. Hematol. 2017, 92, E70. [Google Scholar] [CrossRef]
- Namipashaki, A.; Razaghi-Moghaddam, Z.; Ansari-Pour, N. The essentiality of reporting Hardy–Weinberg equilibrium calculations in population-based genetic association studies. Cell J. 2015, 17, 187. [Google Scholar] [PubMed]
- Bosco, F.; Castro, D.; Briones, M.R.S. Neutral and stable equilibria of genetic systems and the Hardy–Weinberg principle: Limitations of the chi-square test and advantages of auto-correlation functions of allele frequencies. Front. Genet. 2012, 3, 276. [Google Scholar] [CrossRef] [PubMed]
- Chaouch, L.; Sellami, H.; Kalai, M.; Darragi, I.; Boudrigua, I.; Chaouachi, D.; Abbes, S.; Mnif, S. New deletion at promoter of HBG1 gene in sickle cell disease patients with high HbF level. J. Pediatr. Hematol. Oncol. 2020, 42, 20–22. [Google Scholar] [CrossRef]
- Chaouch, L.; Moumni, I.; Ouragini, H.; Darragi, I.; Kalai, M.; Chaouachi, D.; Boudrigua, I.; Hafsia, R.; Abbes, S. rs11886868 and rs4671393 of BCL11A associated with HbF level variation and modulate clinical events among sickle cell anemia patients. Hematology 2016, 21, 425–429. [Google Scholar]
- Quinn, C.T.; Ware, R.E. The modern use of hydroxyurea for children with sickle cell anemia. Haematologica 2025, 110, 1061. [Google Scholar] [CrossRef] [PubMed]
- Nagel, R.L.; Steinberg, M.H. Role of epistatic (modifier) genes in the modulation of the phenotypic diversity of sickle cell anemia. Pediatr. Pathol. Mol. Med. 2001, 20, 123–136. [Google Scholar]
- Da Guarda, C.C.; Yahouédéhou, S.C.M.A.; Santiago, R.P.; Dos Santos Neres, J.S.; de Lima Fernandes, C.F.; Aleluia, M.M.; Figueiredo, C.V.B.; Fiuza, L.M.; Carvalho, S.P.; de Oliveira, R.M.; et al. Sickle cell disease: A distinction of two most frequent genotypes (HbSS and HbSC). PLoS ONE 2020, 15, e0228399. [Google Scholar] [CrossRef]
- Olatunya, O.S.; Lanaro, C.; Longhini, A.L.; Penteado, C.F.F.; Fertrin, K.Y.; Adekile, A.; Saad, S.T.O.; Costa, F.F. Red blood cell microparticles are associated with hemolysis markers and may contribute to clinical events among sickle cell disease patients. Ann. Hematol. 2019, 98, 2507–2521. [Google Scholar] [CrossRef] [PubMed]
- Romero-Tlalolini, M.L.Á.; Aguilar-Ruiz, S.R.; Baltiérrez-Hoyos, R.; Vargas-Arzola, J.; Hernández-Osorio, L.A.; Vásquez-Garzón, V.R.; Bernardino-Hernández, H.U.; Torres-Aguilar, H. Detection of Asymptomatic Sickle Cell Hemoglobin Carriers and Fetal Hemoglobin Regulating Genetic Variants in African Descendants from Oaxaca, Mexico. Anemia 2024, 2024, 4940760. [Google Scholar] [CrossRef]
- Sales, R.R.; Nogueira, B.L.; Luizon, M.R. Pharmacogenomics of hydroxyurea therapy and fetal hemoglobin (HbF) levels in sickle cell anemia. Pharmacogenomics 2022, 23, 393–396. [Google Scholar] [PubMed]
- Groenen, A.G.; Halmos, B.; Tall, A.R.; Westerterp, M. Cholesterol efflux pathways, inflammation, and atherosclerosis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Ogunsile, F.J.; Fowotade, A.; Bolarinwa, R.A. Metabolic syndrome among adults living with sickle cell disease. J. Clin. Pathol. 2019, 72, 91047. [Google Scholar]
- Khadayat, S.; Shrestha, R.; Gupta, S. Prevalence of metabolic syndrome in sickle cell disease. BMC Pediatr. 2024, 24, 4388. [Google Scholar]
- Barker, G.; Winer, J.R.; Guirgis, F.W.; Reddy, S. HDL and persistent inflammation immunosuppression and catabolism syndrome. Curr. Opin. Lipidol. 2021, 32, 315–322. [Google Scholar]
- Seixas, M.O.; Rocha, L.C.; Carvalho, M.B.; Menezes, J.F.; Lyra, I.M.; Nascimento, V.M.; Couto, R.D.; Atta, Á.M.; Reis, M.G.; Goncalves, M.S. Levels of high-density lipoprotein cholesterol (HDL-C) among children with steady-state sickle cell disease. Lipids Health Dis. 2010, 9, 91. [Google Scholar] [CrossRef]
- Ataga, K.I.; Hinderliter, A.; Brittain, J.E.; Jones, S.; Xu, H.; Cai, J.; Kim, S.; Pritchard, K.A.; Hillery, C.A. Association of pro-inflammatory high-density lipoprotein cholesterol with clinical and laboratory variables in sickle cell disease. Hematology 2015, 20, 289–296. [Google Scholar] [CrossRef]
- Rahimi, Z.; Merat, A.; Haghshenass, M.; Madani, H.; Rezaei, M.; Nagel, R.L. Plasma lipids in Iranians with sickle cell disease: Hypocholesterolemia in sickle cell anemia and increase of HDL-cholesterol in sickle cell trait. Clin. Chim. Acta 2006, 365, 217–220. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Unal, S.; Oztas, Y. Altered HDL particle in sickle cell disease: Decreased cholesterol content is associated with hemolysis, whereas decreased apolipoprotein A1 is linked to inflammation. Lipids Health Dis. 2019, 18, 225. [Google Scholar] [CrossRef]
- Pincez, T.; Bertrand, Y.; Labopin, M.; Garrett, M.E.; Brugnara, C.; Ashley-Koch, A.E.; Telen, M.J.; Galacteros, F.; Joly, P.; Bartolucci, P.; et al. Variation and impact of polygenic hematologic traits in monogenic sickle cell disease. Haematologica 2022, 108, 870. [Google Scholar] [CrossRef]
- Bergin, D.A.; Hurley, K.; McElvaney, N.G.; Reeves, E.P. Alpha-1 antitrypsin: A potent anti-inflammatory and potential novel therapeutic agent. Arch. Immunol. Ther. Exp. 2012, 60, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Brantly, M. α1-Antitrypsin: Not just an antiprotease: Extending the half-life of a natural anti-inflammatory molecule by conjugation with polyethylene glycol. Am. J. Respir. Cell Mol. Biol. 2002, 27, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Churg, A.; Dai, J.; Zay, K.; Karsan, A.; Hendricks, R.; Yee, C.; Martin, R.; MacKenzie, R.; Xie, C.; Zhang, L.; et al. Alpha-1-antitrypsin and a broad-spectrum metalloprotease inhibitor, RS113456, have similar acute anti-inflammatory effects. Lab. Investig. 2001, 81, 1119–1131. [Google Scholar] [CrossRef]
- Gordon, S.M.; McKenzie, B.; Kemeh, G.; Sampson, M.; Perl, S.; Young, N.S.; Fessler, M.B.; Remaley, A.T. Rosuvastatin alters the proteome of high-density lipoproteins: Generation of alpha-1-antitrypsin enriched particles with anti-inflammatory properties. Mol. Cell. Proteom. 2015, 14, 3247–3257. [Google Scholar] [CrossRef]
- Carvalho, M.O.S.; Souza, A.L.C.S.; Carvalho, M.B.; Pacheco, A.P.A.S.; Rocha, L.C.; do Nascimento, V.M.L.; Figueiredo, C.V.B.; Guarda, C.C.; Santiago, R.P.; Adekile, A.; et al. Evaluation of alpha-1 antitrypsin levels and SERPINA1 gene polymorphisms in sickle cell disease. Front. Immunol. 2017, 8, 1491. [Google Scholar] [CrossRef] [PubMed]
| N | Mean | SD | |
|---|---|---|---|
| Gender | 77 | ||
| Female | 33 | ||
| Male | 44 | ||
| Age | 77 | 13.98 | 9.932 |
| Hematological parameters | |||
| Hemoglobin S, % | 77 | 86.43 | 7.870 |
| Fetal hemoglobin, % | 76 | 9.019 | 5.828 |
| Hemoglobin, g/dL | 77 | 8.071 | 1.186 |
| Hematocrit, % | 77 | 24.29 | 3.697 |
| RBC, 106/mL | 77 | 5.418 | 23.28 |
| Biochemical parameters | |||
| Total bilirubin, mg/dL | 77 | 3.360 | 1.906 |
| Direct bilirubin, mg/dL | 77 | 0.7496 | 0.5337 |
| Lipid metabolism | |||
| HDL, mg/dL | 77 | 31.65 | 10.32 |
| Polymorphism | Genotype | Freq. (%) | Allele | Freq. | EHW | X2 |
|---|---|---|---|---|---|---|
| rs766432 C>A | CC | 59.74 | C | 0.77 | no | 0.0002 |
| CA | 35.06 | A | 0.23 | |||
| AA | 5.19 | |||||
| rs6732518 C>T | CC | 37.66 | C | 0.38 | yes | 0.20032 |
| CT | 49.35 | T | 0.62 | |||
| TT | 12.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, A.M.; Fiuza, L.; Figueiredo, C.; Guarda, C.; Santiago, R.; Yahouédéhou, S.; Carvalho, S.; Pacheco, A.P.; Lyra, I.; Adorno, E.V.; et al. Pleiotropic Effects of Polymorphisms in the BCL11A Gene on Laboratory Parameters in Sickle Cell Anemia. Int. J. Mol. Sci. 2025, 26, 10458. https://doi.org/10.3390/ijms262110458
Oliveira AM, Fiuza L, Figueiredo C, Guarda C, Santiago R, Yahouédéhou S, Carvalho S, Pacheco AP, Lyra I, Adorno EV, et al. Pleiotropic Effects of Polymorphisms in the BCL11A Gene on Laboratory Parameters in Sickle Cell Anemia. International Journal of Molecular Sciences. 2025; 26(21):10458. https://doi.org/10.3390/ijms262110458
Chicago/Turabian StyleOliveira, Antonio Mateus, Luciana Fiuza, Camylla Figueiredo, Caroline Guarda, Rayra Santiago, Sètondji Yahouédéhou, Suéllen Carvalho, Ana Paula Pacheco, Isa Lyra, Elisângela Vitória Adorno, and et al. 2025. "Pleiotropic Effects of Polymorphisms in the BCL11A Gene on Laboratory Parameters in Sickle Cell Anemia" International Journal of Molecular Sciences 26, no. 21: 10458. https://doi.org/10.3390/ijms262110458
APA StyleOliveira, A. M., Fiuza, L., Figueiredo, C., Guarda, C., Santiago, R., Yahouédéhou, S., Carvalho, S., Pacheco, A. P., Lyra, I., Adorno, E. V., Barbosa, C., & Gonçalves, M. (2025). Pleiotropic Effects of Polymorphisms in the BCL11A Gene on Laboratory Parameters in Sickle Cell Anemia. International Journal of Molecular Sciences, 26(21), 10458. https://doi.org/10.3390/ijms262110458





