Microbial Biotransformation of Chicory by Bacteroides fragilis: In Vitro Implications for Obesity-Related Psoriasis
Abstract
1. Introduction
2. Results
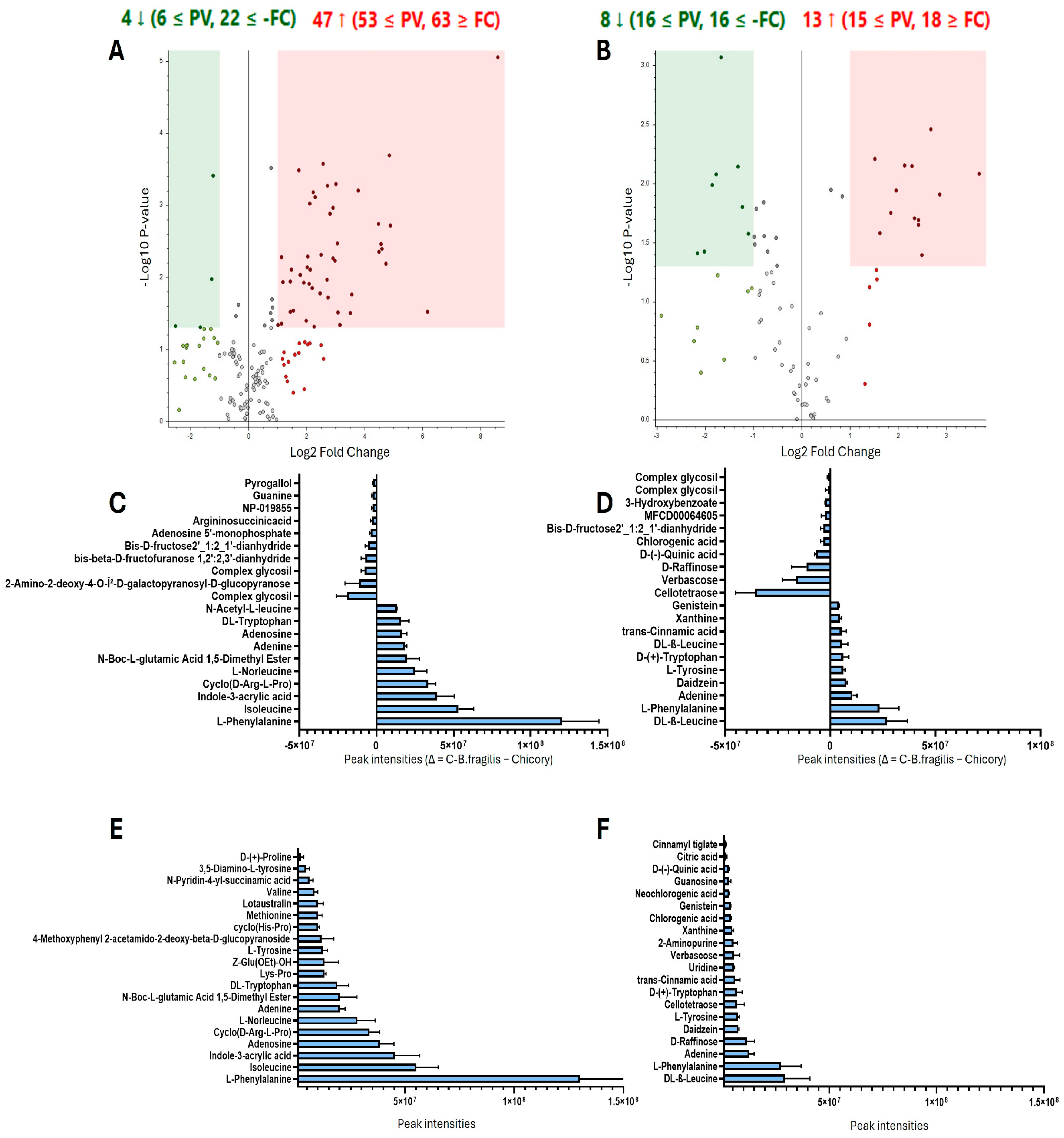
2.1. Metabolite Identification in C-B. fragilis Extract
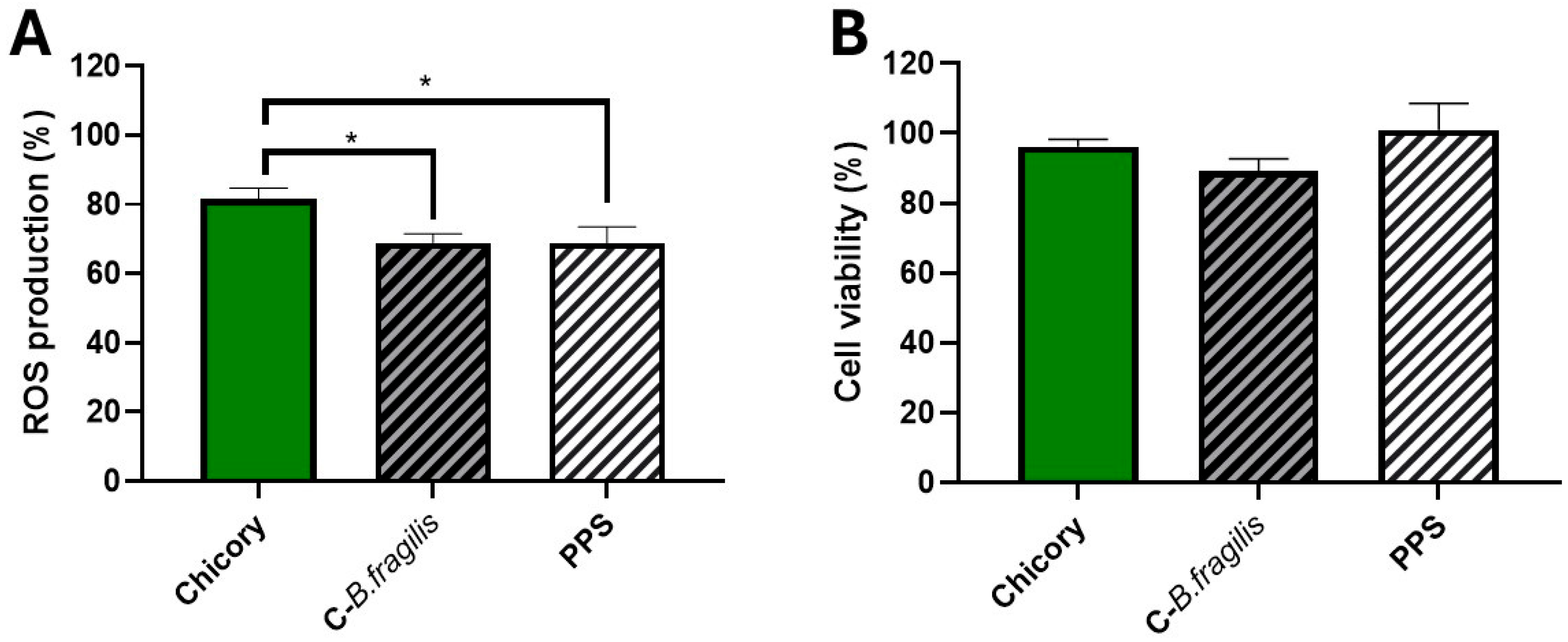
2.2. C-B. fragilis Extract and Its Supernatant (PPS) Inhibited ROS Production by Blood Leukocytes
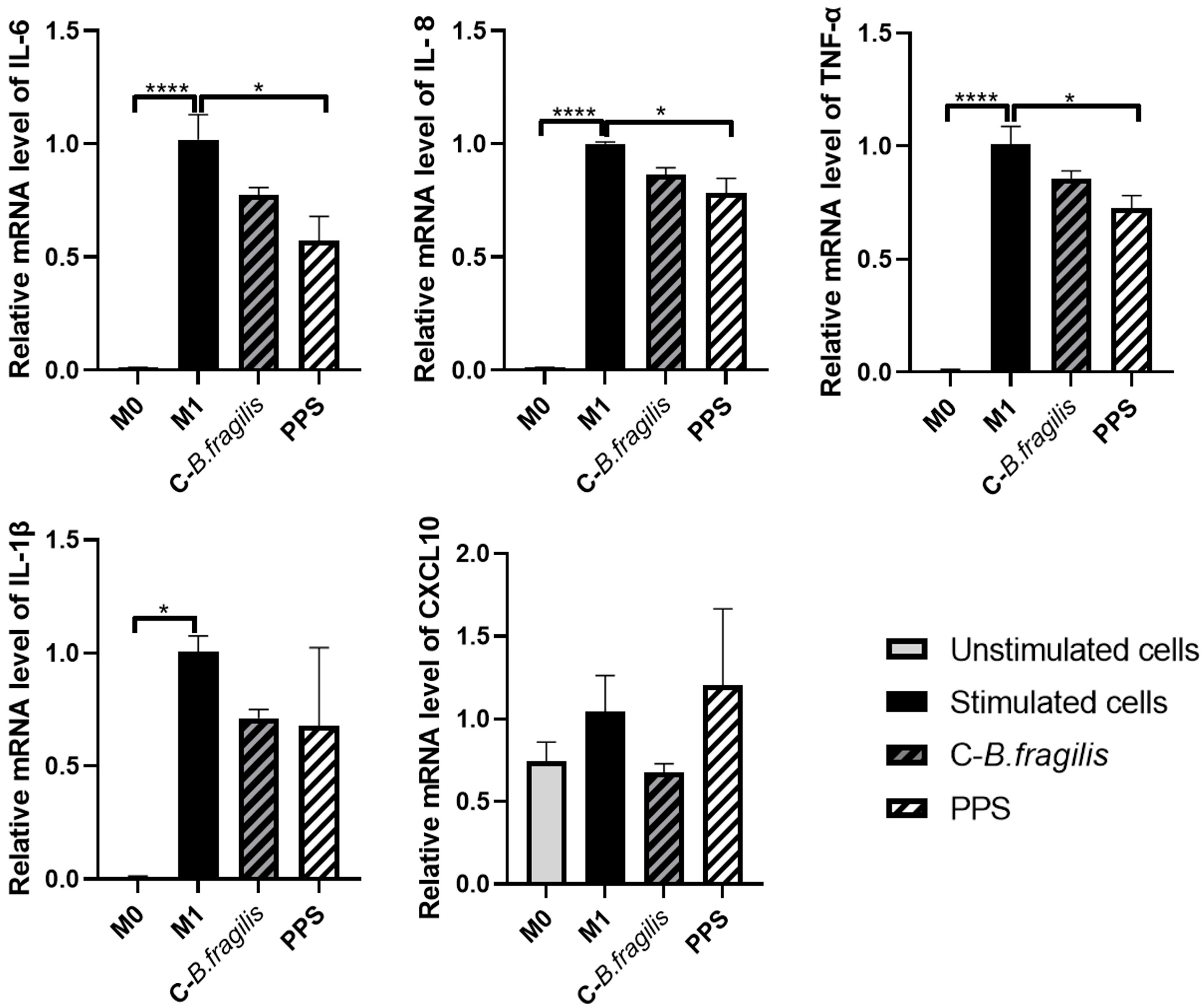
2.3. PPS Decreased the Expression of Genes Involved in M1-like Macrophage Polarization
2.4. C-B. fragilis and PPS Extract Modestly Decreased IFNγ, IL-17A and IL-1β Secretion in LPS-Stimulated PBMCs
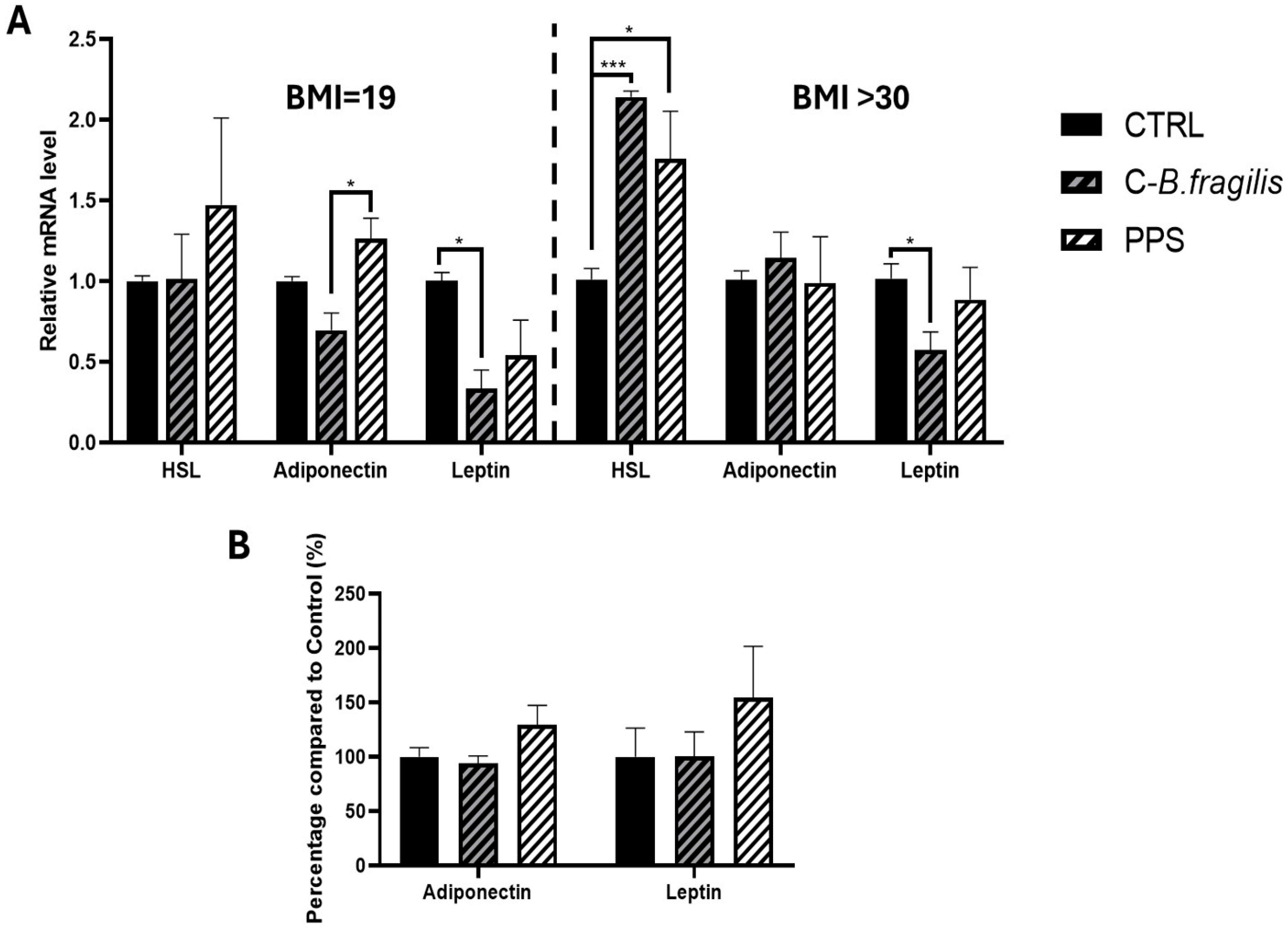
2.5. C-B. fragilis Affected the Expression of Leptin and Hormone-Sensitive Lipase (HSL) by Human Adipospheroids, and PPS Decreased Adipogenesis
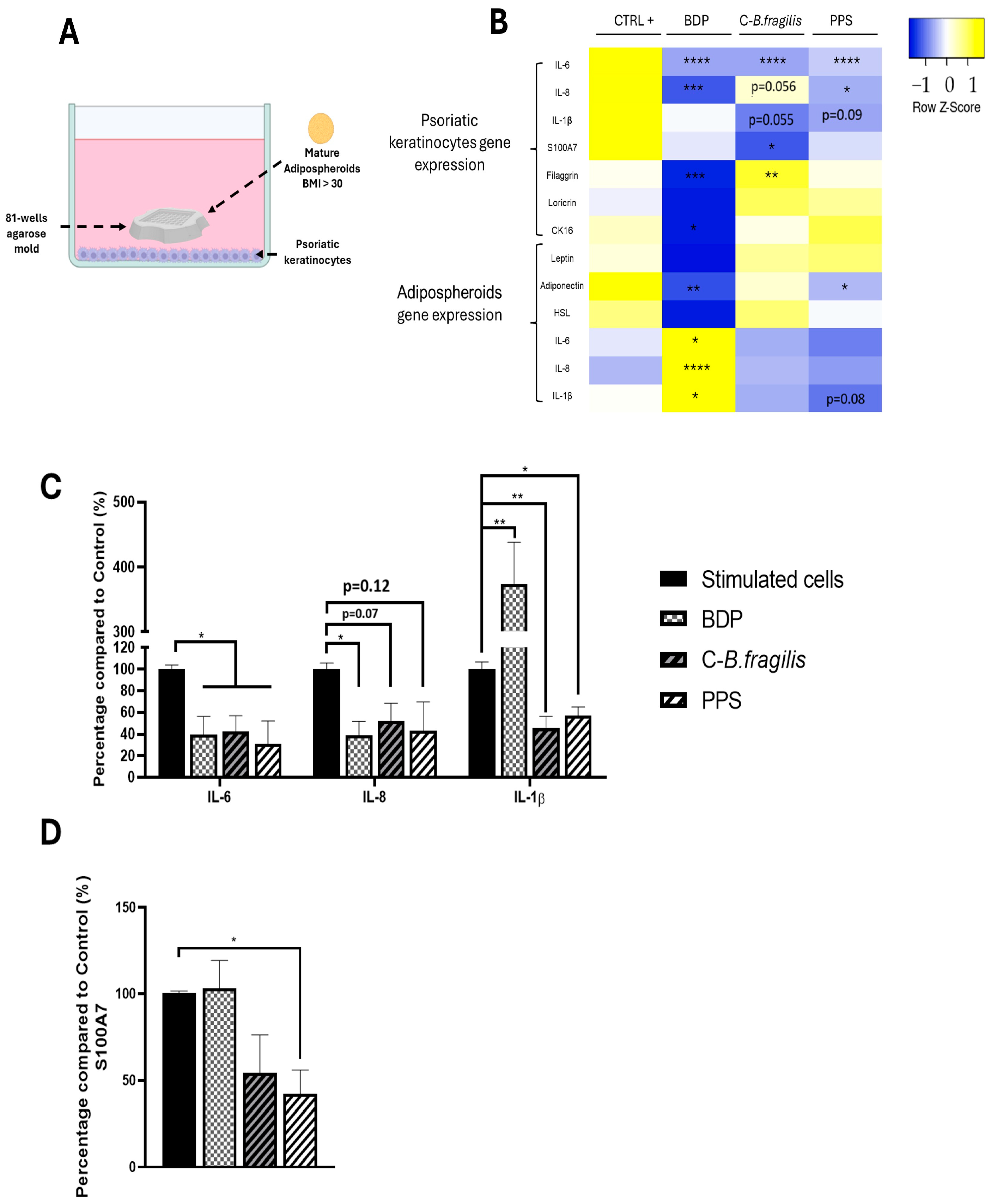
2.6. C-B. fragilis and PPS Extract Affected the Expression and Secretion of Pro-Inflammatory Cytokines in Human Psoriatic Keratinocytes
2.7. Effect of C-B. fragilis and PPS Extract on the Interaction Between Adipocyte/Psoriatic Keratinocytes in a Co-Culture Model
3. Discussion
4. Materials and Methods
4.1. Generation of a Chicory Extract Fermented with Bacteroides fragilis (C-B. fragilis)
4.2. Untargeted Metabolomic
4.3. Blood Leukocyte Preparation
4.4. Peripheral Blood Mononuclear Cells (PBMCs) Preparation from Human Blood
4.5. Reactive Oxygen Species (ROS) Generation by Leukocytes
4.6. Leucocyte Viability
4.7. Determination of Cytokine Concentrations
4.8. Human Monocytic Leukemia Cells
4.9. Adipose Cell Culture
4.10. Quantification of Lipid Accumulation
4.11. Generation of Adipospheroids
4.12. Human Psoriatic Keratinocyte Culture
4.13. Co-Culture Between Adipocytes and Psoriatic Keratinocytes
4.14. Real-Time Quantitative PCR (RT-qPCR)
4.15. ELISA Assays
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pi-Sunyer, X. The Medical Risks of Obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Lingvay, I.; Cohen, R.V.; le Roux, C.W.; Sumithran, P. Obesity in adults. Lancet 2024, 404, 972–987. [Google Scholar] [CrossRef]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 April 2024).
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Castoldi, A.; Naffah de Souza, C.; Câmara, N.O.S.; Moraes-Vieira, P.M. The Macrophage Switch in Obesity Development. Front. Immunol. 2015, 6, 637. [Google Scholar] [CrossRef]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between Adipocyte Size and Adipokine Expression and Secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Must, A.; Spadano, J.; Coakley, E.H.; Field, A.E.; Colditz, G.; Dietz, W.H. The Disease Burden Associated With Overweight and Obesity. JAMA 1999, 282, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.; Baker, B.S. Triggering psoriasis: The role of infections and medications. Clin. Dermatol. 2007, 25, 606–615. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Pistoia, E.S.; Artosi, F.; Shumack, R.G.; Borselli, C.; Rivieccio, A.; Caputo, V.; Favaro, M.; Sorge, R.; et al. Prevalence of fungal colonization among patients with psoriasis in difficult-to-treat areas: Impact of apremilast on mycotic burden and clinical outcomes. Front. Immunol. 2024, 15, 1508489. [Google Scholar] [CrossRef]
- Lindegård, B. Diseases Associated with Psoriasis in a General Population of 159,200 Middle-Aged, Urban, Native Swedes. Dermatologica 2009, 172, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yoo, J.A.; Yoon, H.; Han, T.; Yoon, J.; An, S.; Cho, J.Y.; Lee, J. The Role of Leptin in the Association between Obesity and Psoriasis. Biomol. Ther. 2021, 29, 11–21. [Google Scholar] [CrossRef]
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The role of leptin in selected skin diseases. Lipids Health Dis. 2020, 19, 215. [Google Scholar] [CrossRef]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef]
- Qu, D.; Sun, F.; Feng, S.; Yu, L.; Tian, F.; Zhang, H.; Chen, W.; Zhai, Q. Protective effects of Bacteroides fragilis against lipopolysaccharide-induced systemic inflammation and their potential functional genes. Food Funct. 2022, 13, 1015–1025. [Google Scholar] [CrossRef]
- Troy, E.B.; Kasper, D.L. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front. Biosci. J. Virtual Libr. 2010, 15, 25–34. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef]
- Chervet, A.; Nehme, R.; Defois-Fraysse, C.; Decombat, C.; Blavignac, C.; Auxenfans, C.; Evrard, B.; Michel, S.; Filaire, E.; Berthon, J.-Y.; et al. Development and characterization of a chicory extract fermented by Akkermansia muciniphila: An in vitro study on its potential to modulate obesity-related inflammation. Curr. Res. Food Sci. 2025, 10, 100974. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Skov, L. Psoriasis and Obesity. Dermatology 2017, 232, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Liu, L.; Wang, C.; Sun, X.; Zhou, Y.; Hong, S.; Cai, X.; Xu, W.; Li, X. Global prevalence of obesity in patients with psoriasis: An analysis in the past two decades. Autoimmun. Rev. 2024, 23, 103577. [Google Scholar] [CrossRef]
- Cohen, A.D.; Sherf, M.; Vidavsky, L.; Vardy, D.A.; Shapiro, J.; Meyerovitch, J. Association between Psoriasis and the Metabolic Syndrome: A Cross-Sectional Study. Dermatology 2008, 216, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Strbo, N. Effects of Obesity on Infections with Emphasis on Skin Infections and Wound Healing. J. Dermatol. Skin Sci. 2022, 4, 5–10. [Google Scholar] [CrossRef]
- Darlenski, R.; Mihaylova, V.; Handjieva-Darlenska, T. The Link Between Obesity and the Skin. Front. Nutr. 2022, 9, 855573. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Liang, Y.; Jiao, X.; Zhao, C. Beneficial Effect of Intestinal Fermentation of Natural Polysaccharides. Nutrients 2018, 10, 1055. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, F.; Chen, H.; Zheng, Q. The efficacy and safety of probiotics in the adjuvant treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2024, 11, 1448626. [Google Scholar] [CrossRef] [PubMed]
- Rather, I.A.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K.; Bhat, E.A.; Lim, J.; Paek, W.K.; Park, Y.-H. Probiotic Lactobacillus sakei proBio-65 Extract Ameliorates the Severity of Imiquimod Induced Psoriasis-Like Skin Inflammation in a Mouse Model. Front. Microbiol. 2018, 9, 1021. [Google Scholar] [CrossRef]
- Cang, W.; Li, X.; Tang, J.; Wang, Y.; Mu, D.; Wu, C.; Shi, H.; Shi, L.; Wu, J.; Wu, R. Therapeutic Potential of Bacteroides fragilis SNBF-1 as a Next-Generation Probiotic: In Vitro Efficacy in Lipid and Carbohydrate Metabolism and Antioxidant Activity. Foods 2024, 13, 735. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Arboleya, S.; Hernandez-Barranco, A.M.; Alvarez-Buylla, J.R.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G. Interactions between Bifidobacterium and Bacteroides Species in Cofermentations Are Affected by Carbon Sources, Including Exopolysaccharides Produced by Bifidobacteria. Appl. Environ. Microbiol. 2013, 79, 7518–7524. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Sánchez, B.; Salazar, N.; Martínez, N.; Redruello, B.; Gueimonde, M.; de los Reyes-Gavilán, C.G. Different metabolic features of Bacteroides fragilis growing in the presence of glucose and exopolysaccharides of bifidobacteria. Front. Microbiol. 2015, 6, 825. [Google Scholar] [CrossRef]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Jeong, Y.; Kang, C.-H. Antioxidant Activity and Probiotic Properties of Lactic Acid Bacteria. Fermentation 2022, 8, 29. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.-S.; Kim, Y.; Jeong, Y.; Kim, J.-E.; Paek, N.-S.; Kang, C.-H. Antioxidant and Probiotic Properties of Lactobacilli and Bifidobacteria of Human Origins. Biotechnol. Bioprocess Eng. 2020, 25, 421–430. [Google Scholar] [CrossRef]
- Appari, M.; Channon, K.M.; McNeill, E. Metabolic Regulation of Adipose Tissue Macrophage Function in Obesity and Diabetes. Antioxid. Redox Signal. 2018, 29, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.-H.; Chung, H.-T.; Kang, D.G.; Pae, H.-O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.-Q.; Gao, Y.; Pan, Q.-W.; Cao, J.-S. Extracellular vesicles of Bacteroides fragilis regulated macrophage polarization through promoted Sema7a expression. Microb. Pathog. 2024, 187, 106527. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, Z.; Tan, Y.; Guo, Z.; Liu, Y.; Wang, Y.; Yuan, Y.; Yang, R.; Bi, Y.; Bai, Y.; et al. A novel strain of Bacteroides fragilis enhances phagocytosis and polarises M1 macrophages. Sci. Rep. 2016, 6, 29401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, C.; Xun, T.; Mo, L.; Tong, Y.; Ni, W.; Huang, S.; Liu, B.; Zhan, X.; Yang, X. Bacteroides fragilis Polysaccharide A Ameliorates Abnormal Voriconazole Metabolism Accompanied with the Inhibition of TLR4/NF-κB Pathway. Front. Pharmacol. 2021, 12, 663325. [Google Scholar] [CrossRef]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Olaniyan, O.T.; Emeruwa, C.N.; Saha, S.; Saso, L.; Tucci, P. An insight into role of amino acids as antioxidants via NRF2 activation. Amino Acids 2024, 56, 23. [Google Scholar] [CrossRef]
- Peter, K.; Rehli, M.; Singer, K.; Renner-Sattler, K.; Kreutz, M. Lactic acid delays the inflammatory response of human monocytes. Biochem. Biophys. Res. Commun. 2015, 457, 412–418. [Google Scholar] [CrossRef]
- Yang, H.; Meng, L.; Ai, D.; Hou, N.; Li, H.; Shuai, X.; Peng, X. Acetic acid alleviates the inflammatory response and liver injury in septic mice by increasing the expression of TRIM40. Exp. Ther. Med. 2019, 17, 2789–2798. [Google Scholar] [CrossRef]
- Singh, S.K.; Kaldate, R.; Bisht, A. Chapter4.5—Citric acid, antioxidant effects in health. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. ISBN 978-0-12-819096-8. [Google Scholar]
- Harber, K.J.; de Goede, K.E.; Verberk, S.G.S.; Meinster, E.; de Vries, H.E.; van Weeghel, M.; de Winther, M.P.J.; Van den Bossche, J. Succinate Is an Inflammation-Induced Immunoregulatory Metabolite in Macrophages. Metabolites 2020, 10, 372. [Google Scholar] [CrossRef]
- Ji, Z.; Feng, X.; Han, C.; Li, S.; Wu, B.; Zhang, X.; Zhu, S.; Tong, W.; Xu, W. The malic acid inhibiting inflammation in ankylosing spondylitis by interfering M1 macrophage polarization. Int. Immunopharmacol. 2025, 144, 113653. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-κB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit only NF-κB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediators Inflamm. 2007, 2007, 45673. [Google Scholar] [CrossRef]
- Nakajima, H.; Nakajima, K.; Tarutani, M.; Morishige, R.; Sano, S. Kinetics of circulating Th17 cytokines and adipokines in psoriasis patients. Arch. Dermatol. Res. 2011, 303, 451–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnston, A.; Arnadottir, S.; Gudjonsson, J.E.; Aphale, A.; Sigmarsdottir, A.A.; Gunnarsson, S.I.; Steinsson, J.T.; Elder, J.T.; Valdimarsson, H. Obesity in psoriasis: Leptin and resistin as mediators of cutaneous inflammation. Br. J. Dermatol. 2008, 159, 342–350. [Google Scholar] [CrossRef]
- Kobyliak, N.; Conte, C.; Cammarota, G.; Haley, A.P.; Styriak, I.; Gaspar, L.; Fusek, J.; Rodrigo, L.; Kruzliak, P. Probiotics in prevention and treatment of obesity: A critical view. Nutr. Metab. 2016, 13, 14. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Cho, M.; Kang, D.-J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.-J.; Lee, H.; Kim, M.; Lee, J.W.; Saeed, M.; Lee, H.; Jung, S.-H.; Shim, J.-J.; Lee, J.-L.; Heo, K.; et al. Development and metabolic profiling of a postbiotic complex exhibiting antibacterial activity against skin microorganisms and anti-inflammatory effect on human keratinocytes. Food Sci. Biotechnol. 2022, 31, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, H.; Lin, W.; Lu, L.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target. Ther. 2023, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, L.; Srivastava, A.; Meisgen, F.; Mahapatra, K.D.; Xia, P.; Landén, N.X.; Pivarcsi, A.; Sonkoly, E. The Keratinocyte Transcriptome in Psoriasis: Pathways Related to Immune Responses, Cell Cycle and Keratinization. Acta Derm. Venereol. 2019, 99, 196–205. [Google Scholar] [CrossRef]
- Chervet, A.; Nehme, R.; Decombat, C.; Longechamp, L.; Habanjar, O.; Rousset, A.; Fraisse, D.; Blavignac, C.; Filaire, E.; Berthon, J.-Y.; et al. Exploring the Therapeutic Potential of Ampelopsis grossedentata Leaf Extract as an Anti-Inflammatory and Antioxidant Agent in Human Immune Cells. Int. J. Mol. Sci. 2023, 25, 416. [Google Scholar] [CrossRef]
- Habanjar, O.; Maurin, A.-C.; Vituret, C.; Vachias, C.; Longechamp, L.; Garnier, C.; Decombat, C.; Bourgne, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; et al. A bicellular fluorescent ductal carcinoma in situ (DCIS)-like tumoroid to study the progression of carcinoma: Practical approaches and optimization. Biomater. Sci. 2023, 11, 3308–3320. [Google Scholar] [CrossRef]



| Gene | Species | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|---|
| GAPDH | Human | CACATGGCCTCCAAGGAGTAA | TGAGGGTCTCTCTCTTCCTCTTGT |
| IL-8 | Human | CTGGCCGTGGCTCTCTTG | CCTTGGCAAAACTGCACCTT |
| IL-1β | Human | CCTGTCCTGCGTGTTGAAAGA | GGGAACTGGGCAGACTCAAA |
| IL-6 | Human | GCTGCAGGCACAGAACCA | ACTCCTTAAAGCTGCGCAGAA |
| TNFα | Human | TCTTCTCGAACCCCGAGTGA | GGAGCTGCCCCTCAGCTT |
| CXCL10 | Human | GGAAATCGTGCGTGACATTA | AGGAAGGAAGGCTGGAAGAG |
| Leptin | Human | CGGAGAGTACAGTGAGCCA | CGGAATCTCGCTCTGTCAT |
| Adiponectin | Human | CCCAAAGAGGAGAGGAA | TCAGAAACAGGACACAAC |
| HSL | Human | GCCTGGGCTTCCAGTTCAC | CCTGTCTCGTTGCGTTTGTAGT |
| Loricrin | Human | GTCTGCGGAGGTGGTTCCTCT | TGCTGGGTCTGGTGGCAGATC |
| Filaggrin | Human | CATGGCAGCTATGGTAGTGCAGA | ACCAAACGCACTTGCTTTACAGA |
| Cytokeratin16 | Human | CTACCTGAGGAAGAACCACGAG | CTCGTACTGGTGACGCATCTGA |
| S100A7 | Human | GCACAAATTACCTCGCCGAT | GACATTTTATTGTTCCTGGGGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chervet, A.; Nehme, R.; Defois-Fraysse, C.; Decombat, C.; Auxenfans, C.; Evrard, B.; Michel, S.; Filaire, E.; Berthon, J.-Y.; Dreux-Zigha, A.; et al. Microbial Biotransformation of Chicory by Bacteroides fragilis: In Vitro Implications for Obesity-Related Psoriasis. Int. J. Mol. Sci. 2025, 26, 10428. https://doi.org/10.3390/ijms262110428
Chervet A, Nehme R, Defois-Fraysse C, Decombat C, Auxenfans C, Evrard B, Michel S, Filaire E, Berthon J-Y, Dreux-Zigha A, et al. Microbial Biotransformation of Chicory by Bacteroides fragilis: In Vitro Implications for Obesity-Related Psoriasis. International Journal of Molecular Sciences. 2025; 26(21):10428. https://doi.org/10.3390/ijms262110428
Chicago/Turabian StyleChervet, Arthur, Rawan Nehme, Clemence Defois-Fraysse, Caroline Decombat, Celine Auxenfans, Bertrand Evrard, Solene Michel, Edith Filaire, Jean-Yves Berthon, Assia Dreux-Zigha, and et al. 2025. "Microbial Biotransformation of Chicory by Bacteroides fragilis: In Vitro Implications for Obesity-Related Psoriasis" International Journal of Molecular Sciences 26, no. 21: 10428. https://doi.org/10.3390/ijms262110428
APA StyleChervet, A., Nehme, R., Defois-Fraysse, C., Decombat, C., Auxenfans, C., Evrard, B., Michel, S., Filaire, E., Berthon, J.-Y., Dreux-Zigha, A., Delort, L., & Caldefie-Chezet, F. (2025). Microbial Biotransformation of Chicory by Bacteroides fragilis: In Vitro Implications for Obesity-Related Psoriasis. International Journal of Molecular Sciences, 26(21), 10428. https://doi.org/10.3390/ijms262110428







