Long-Term Effects of Semaglutide and Sitagliptin on Circulating IGFBP-1, IGFBP-3 and IGFBP-rp1: Results from a One-Year Study in Type 2 Diabetes
Abstract
1. Introduction
2. Results
2.1. Baseline Anthropometric and Routine Laboratory Parameters of the Enrolled T2DM Patients and Non-Diabetic Controls
2.2. Changes in Anthropometric and Routine Laboratory Parameters of T2DM Patients After a 52-Week Semaglutide Treatment
2.3. Changes in Anthropometric and Routine Laboratory Parameters of T2DM Patients After 52-Week Sitagliptin Treatment
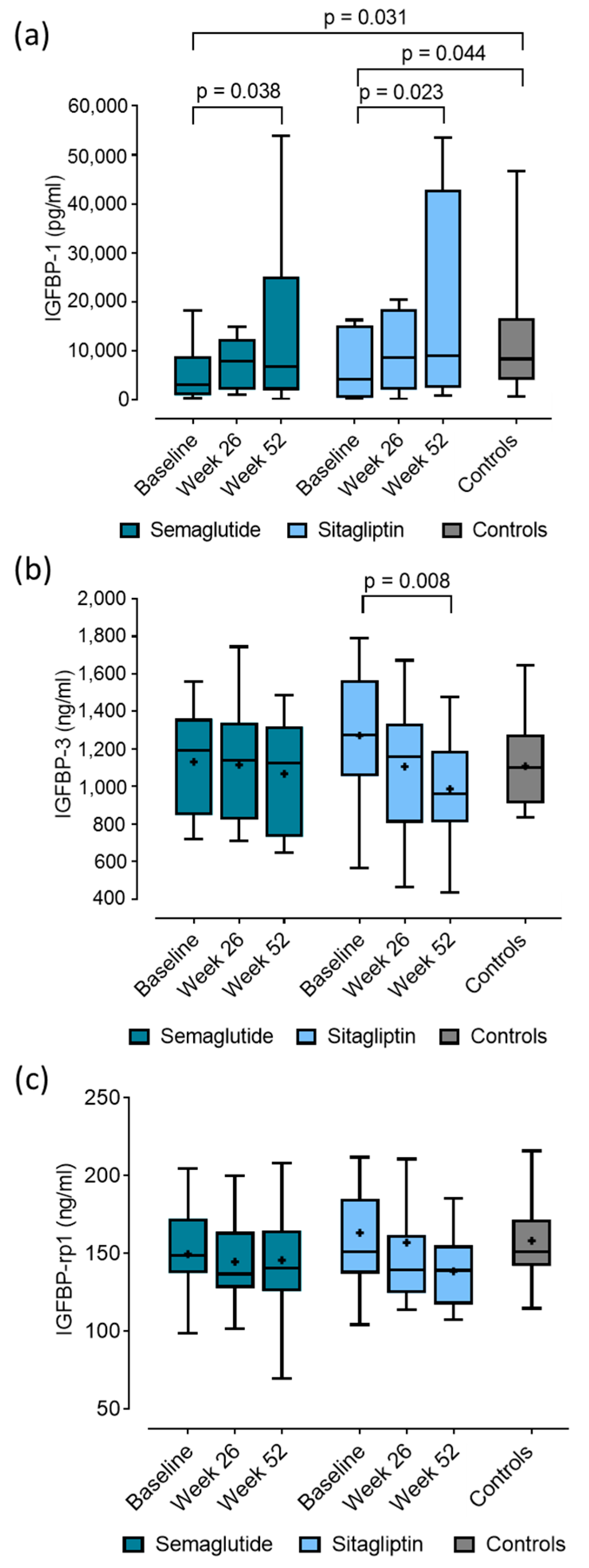
2.4. Baseline Concentrations and Changes in IGFBP-1, IGFBP-3 and IGFBP-rp1 in Patients with T2DM After 52-Week Semaglutide and Sitagliptin Treatment
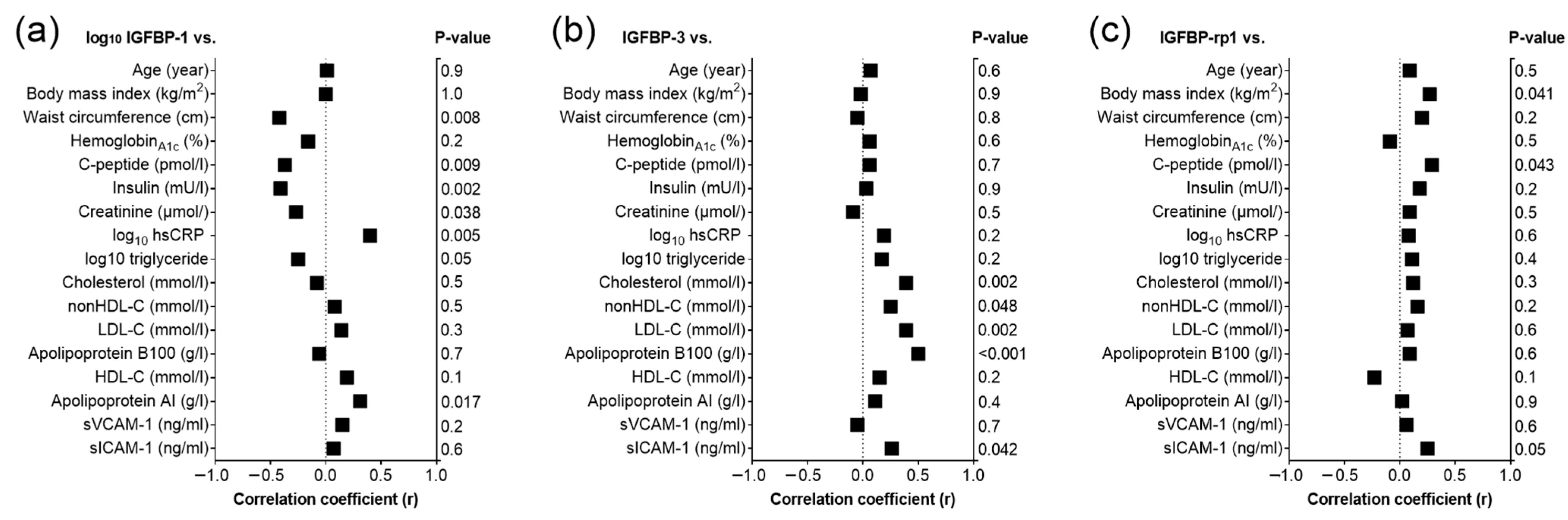
2.5. Correlations of Baseline IGFBP-1, IGFBP-3 and IGFBP-rp1 in T2DM Patients and Controls
2.6. Multiple Regression Analyses
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment
4.2. Inclusion and Exclusion Criteria
4.3. Laboratory Analyses
4.4. Determination of IGFBP-1, IGFBP-3, and IGFBP-rp1 Levels
4.5. Measurement of sICAM-1 and sVCAM-1 Levels
4.6. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, M.; Zulfiqar, E.; Shafiq, A.; Shahzad, M.; Hashmi, T.M.; Ahmed, R.; Rana, J.S.; Sidney, S.; Greene, S.J.; Mentz, R.J.; et al. Type 2 Diabetes Mellitus-Related Mortality in the United States, 1999 to 2023. JACC Adv. 2025, 4, 101882. [Google Scholar] [CrossRef]
- Rankovic, M.; Jeremic, N.; Srejovic, I.; Radonjic, K.; Stojanovic, A.; Glisic, M.; Bolevich, S.; Jakovljevic, V. Dipeptidyl peptidase-4 inhibitors as new tools for cardioprotection. Heart Fail. Rev. 2021, 26, 437–450. [Google Scholar] [CrossRef]
- Sztanek, F.; Tóth, L.I.; Pető, A.; Hernyák, M.; Diószegi, Á.; Harangi, M. New Developments in Pharmacological Treatment of Obesity and Type 2 Diabetes—Beyond and within GLP-1 Receptor Agonists. Biomedicines 2024, 12, 1320. [Google Scholar] [CrossRef]
- Tóth, L.I.; Harsányi, A.; Csiha, S.; Molnár, Á.; Lőrincz, H.; Nagy, A.C.; Paragh, G.; Harangi, M.; Sztanek, F. Semaglutide Improves Lipid Subfraction Profiles in Type 2 Diabetes: Insights from a One-Year Follow-Up Study. Int. J. Mol. Sci. 2025, 26, 5951. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef]
- Haywood, N.J.; Slater, T.A.; Matthews, C.J.; Wheatcroft, S.B. The insulin like growth factor and binding protein family: Novel therapeutic targets in obesity & diabetes. Mol. Metab. 2019, 19, 86–96. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Heald, A.H.; Cruickshank, J.K.; Riste, L.K.; Cade, J.E.; Anderson, S.; Greenhalgh, A.; Sampayo, J.; Taylor, W.; Fraser, W.; White, A.; et al. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia 2001, 44, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Travers, S.H.; Labarta, J.I.; Gargosky, S.E.; Rosenfeld, R.G.; Jeffers, B.W.; Eckel, R.H. Insulin-like growth factor binding protein-I levels are strongly associated with insulin sensitivity and obesity in early pubertal children. J. Clin. Endocrinol. Metab. 1998, 83, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Lewitt, M.S.; Hilding, A.; Brismar, K.; Efendic, S.; Ostenson, C.G.; Hall, K. IGF-binding protein 1 and abdominal obesity in the development of type 2 diabetes in women. Eur. J. Endocrinol. 2010, 163, 233–242. [Google Scholar] [CrossRef]
- Yeap, B.B.; Chubb, S.A.; Ho, K.K.; Setoh, J.W.; McCaul, K.A.; Norman, P.E.; Jamrozik, K.; Flicker, L. IGF1 and its binding proteins 3 and 1 are differentially associated with metabolic syndrome in older men. Eur. J. Endocrinol. 2010, 162, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Ali, O.; Shim, M.; Lee, K.W.; Vuguin, P.; Muzumdar, R.; Barzilai, N.; Cohen, P. Insulin-like growth factor binding protein-3 induces insulin resistance in adipocytes in vitro and in rats in vivo. Pediatr. Res. 2007, 61, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Silha, J.V.; Gui, Y.; Murphy, L.J. Impaired glucose homeostasis in insulin-like growth factor-binding protein-3-transgenic mice. Am. J. Physiol.-Endocrinol. Metab. 2002, 283, E937–E945. [Google Scholar] [CrossRef]
- Gu, H.F.; Gu, T.; Hilding, A.; Zhu, Y.; Kärvestedt, L.; Ostenson, C.G.; Lai, M.; Kutsukake, M.; Frystyk, J.; Tamura, K.; et al. Evaluation of IGFBP-7 DNA methylation changes and serum protein variation in Swedish subjects with and without type 2 diabetes. Clin. Epigenetics 2013, 5, 20. [Google Scholar] [CrossRef]
- López-Bermejo, A.; Khosravi, J.; Fernández-Real, J.M.; Hwa, V.; Pratt, K.L.; Casamitjana, R.; Garcia-Gil, M.M.; Rosenfeld, R.G.; Ricart, W. Insulin resistance is associated with increased serum concentration of IGF-binding protein-related protein 1 (IGFBP-rP1/MAC25). Diabetes 2006, 55, 2333–2339. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Dent, M.S.; Hall, K. The Insulin-Like Growth Factor System in Obesity, Insulin Resistance and Type 2 Diabetes Mellitus. J. Clin. Med. 2014, 3, 1561–1574. [Google Scholar] [CrossRef]
- Gibson, J.M.; Westwood, M.; Crosby, S.R.; Gordon, C.; Holly, J.M.; Fraser, W.; Anderson, C.; White, A.; Young, R.J. Choice of treatment affects plasma levels of insulin-like growth factor-binding protein-1 in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 1995, 80, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Cho, H.C.; Lee, J.H.; Oh, G.T.; Koo, S.H.; Park, B.H.; Lee, I.K.; Choi, H.S.; Song, D.K.; Im, S.S. Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states. Sci. Rep. 2016, 6, 23665. [Google Scholar] [CrossRef]
- Chikata, Y.; Iwata, H.; Miyosawa, K.; Koike, T.; Yasuda, H.; Funamizu, T.; Doi, S.; Endo, H.; Wada, H.; Naito, R.; et al. Dipeptidyl peptidase-4 inhibitors reduced long-term cardiovascular risk in diabetic patients after percutaneous coronary intervention via insulin-like growth factor-1 axis. Sci. Rep. 2022, 12, 5129. [Google Scholar] [CrossRef]
- Ferrannini, E.; Murthy, A.C.; Lee, Y.H.; Muscelli, E.; Weiss, S.; Ostroff, R.M.; Sattar, N.; Williams, S.A.; Ganz, P. Mechanisms of Sodium-Glucose Cotransporter 2 Inhibition: Insights from Large-Scale Proteomics. Diabetes Care 2020, 43, 2183–2189. [Google Scholar] [CrossRef]
- Mohebi, R.; Liu, Y.; Hansen, M.K.; Yavin, Y.; Sattar, N.; Pollock, C.A.; Butler, J.; Jardine, M.; Masson, S.; Heerspink, H.J.L.; et al. Insulin growth factor axis and cardio-renal risk in diabetic kidney disease: An analysis from the CREDENCE trial. Cardiovasc. Diabetol. 2023, 22, 176. [Google Scholar] [CrossRef]
- Thomas, M.K.; Nikooienejad, A.; Bray, R.; Cui, X.; Wilson, J.; Duffin, K.; Milicevic, Z.; Haupt, A.; Robins, D.A. Dual GIP and GLP-1 Receptor Agonist Tirzepatide Improves Beta-cell Function and Insulin Sensitivity in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, 388–396. [Google Scholar] [CrossRef]
- Wheatcroft, S.B.; Kearney, M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: Implications for metabolic homeostasis. Trends Endocrinol. Metab. 2009, 20, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Brismar, K.; Hilding, A.; Ansurudeen, I.; Flyvbjerg, A.; Frystyk, J.; Östenson, C.G. Adiponectin, IGFBP-1 and -2 are independent predictors in forecasting prediabetes and type 2 diabetes. Front. Endocrinol. 2022, 13, 1092307. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; He, M.; Sun, Q.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Gunter, M.J.; Pollak, M.; Kim, M.; Pessin, J.E.; et al. Insulin-like growth factor axis and risk of type 2 diabetes in women. Diabetes 2012, 61, 2248–2254. [Google Scholar] [CrossRef]
- Meyer, N.M.T.; Kabisch, S.; Dambeck, U.; Honsek, C.; Kemper, M.; Gerbracht, C.; Arafat, A.M.; Birkenfeld, A.L.; Schwarz, P.E.H.; Machann, J.; et al. Low IGF1 and high IGFBP1 predict diabetes onset in prediabetic patients. Eur. J. Endocrinol. 2022, 187, 555–565. [Google Scholar] [CrossRef]
- Lu, J.; Liu, K.C.; Schulz, N.; Karampelias, C.; Charbord, J.; Hilding, A.; Rautio, L.; Bertolino, P.; Östenson, C.G.; Brismar, K.; et al. IGFBP1 increases β-cell regeneration by promoting α- to β-cell transdifferentiation. EMBO J. 2016, 35, 2026–2044. [Google Scholar] [CrossRef]
- Leinonen, E.S.; Salonen, J.T.; Salonen, R.M.; Koistinen, R.A.; Leinonen, P.J.; Sarna, S.S.; Taskinen, M.R. Reduced IGFBP-1 is associated with thickening of the carotid wall in type 2 diabetes. Diabetes Care 2002, 25, 1807–1812. [Google Scholar] [CrossRef]
- Varma Shrivastav, S.; Bhardwaj, A.; Pathak, K.A.; Shrivastav, A. Insulin-Like Growth Factor Binding Protein-3 (IGFBP-3): Unraveling the Role in Mediating IGF-Independent Effects Within the Cell. Front. Cell Dev. Biol. 2020, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Twigg, S.M.; Firth, S.M.; Baxter, R.C. Insulin-like growth factor binding protein-3 leads to insulin resistance in adipocytes. J. Clin. Endocrinol. Metab. 2005, 90, 6588–6595. [Google Scholar] [CrossRef]
- Aneke-Nash, C.S.; Xue, X.; Qi, Q.; Biggs, M.L.; Cappola, A.; Kuller, L.; Pollak, M.; Psaty, B.M.; Siscovick, D.; Mukamal, K.; et al. The Association Between IGF-I and IGFBP-3 and Incident Diabetes in an Older Population of Men and Women in the Cardiovascular Health Study. J. Clin. Endocrinol. Metab. 2017, 102, 4541–4547. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Al-Sabah, R.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Profiling of Insulin-Like Growth Factor Binding Proteins (IGFBPs) in Obesity and Their Association with Ox-LDL and Hs-CRP in Adolescents. Front. Endocrinol. 2021, 12, 727004. [Google Scholar] [CrossRef]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef]
- Chan, S.S.; Schedlich, L.J.; Twigg, S.M.; Baxter, R.C. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am. J. Physiol.-Endocrinol. Metab. 2009, 296, E654–E663. [Google Scholar] [CrossRef]
- de Silva, H.C.; Firth, S.M.; Twigg, S.M.; Baxter, R.C. Interaction between IGF binding protein-3 and TGFβ in the regulation of adipocyte differentiation. Endocrinology 2012, 153, 4799–4807. [Google Scholar] [CrossRef] [PubMed]
- Eggert, M.L.; Wallaschofski, H.; Grotevendt, A.; Nauck, M.; Völzke, H.; Samietz, S.; Friedrich, N. Cross-sectional and longitudinal relation of IGF1 and IGF-binding protein 3 with lipid metabolism. Eur. J. Endocrinol. 2014, 171, 9–19. [Google Scholar] [CrossRef]
- Kawachi, S.; Takeda, N.; Sasaki, A.; Kokubo, Y.; Takami, K.; Sarui, H.; Hayashi, M.; Yamakita, N.; Yasuda, K. Circulating insulin-like growth factor-1 and insulin-like growth factor binding protein-3 are associated with early carotid atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 617–621. [Google Scholar] [CrossRef]
- Lit, K.K.; Zhirenova, Z.; Blocki, A. Insulin-like growth factor-binding protein 7 (IGFBP7): A microenvironment-dependent regulator of angiogenesis and vascular remodeling. Front. Cell Dev. Biol. 2024, 12, 1421438. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, M.; Ling, J.; Cai, L.; Zhang, D.; Gu, H.F.; Wang, H.; Zhu, Y.; Lai, M. Serum IGFBP7 levels associate with insulin resistance and the risk of metabolic syndrome in a Chinese population. Sci. Rep. 2015, 5, 10227. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ragan, B.G.; Park, J.H. Issues in outcomes research: An overview of randomization techniques for clinical trials. J. Athl. Train. 2008, 43, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. How to randomise. BMJ 1999, 319, 703–704. [Google Scholar] [CrossRef] [PubMed]
| Patients with T2DM | Controls | ||
|---|---|---|---|
| Semaglutide Group (Baseline) | Sitagliptin Group (Baseline) | ||
| Number of patients | 18 (12 m/6 f) | 16 (7 m/9 f) | 31 (10 m/21 f) |
| Age (year) | 57.9 ± 10.6 | 59.4 ± 11.9 | 55.2 ± 5.0 |
| Body mass index (kg/m2) | 37.96 ± 10.64 | 31.26 ± 2.75 | 37.89 ± 9.64 |
| Waist circumference (cm) | 126.4 ± 21.8 | 126.6 ± 25.1 | 114.8 ± 21.0 |
| Fasting glucose (mmol/L) | 8.1 (7.10–11.80) # | 8.90 (7.60–10.80) § | 5.35 (4.95–6.05) |
| Fructosamine (mmol/L) | 322.39 ± 87.95 # | 299.00 ± 68.80 § | 239.35 ± 55.37 |
| HemoglobinA1c (%) | 8.1 ± 1.7 # | 8.1 ± 1.3 § | 6.0 ± 1.3 |
| Insulin (mU/L) | 19.4 (15.2–27.5) | 26.2 (20.5–41.6) § | 13.7 (8.9–19.8) |
| C-peptide (pmol/L) | 1370 (1270–1800) # | 1430 (1140–2730) § | 826.5 (502–976) |
| Creatinine (µmol/L) | 78.50 ± 15.07 | 71.47 ± 10.18 | 68.41 ± 15.21 |
| eGFR (mL/min/1.73 m2) | 87 (74–90) | 90 (73–90) | 90 (89–90) |
| hsCRP (mg/L) | 2.3 (1.7–5.8) # | 5.5 (2.9–13.8) | 11.6 (3.0–18.0) |
| AST (U/L) | 21 (16–25) | 27 (21–35) | 20.5 (17–24) |
| ALT (U/L) | 24 (17–37) | 34 (23–46) | 25 (16–37) |
| GGT (U/L) | 33 (24–49) | 38 (22–87) | 27 (18–39) |
| Triglyceride (mmol/L) | 1.72 (1.50–3.11) | 2.00 (1.30–3.40) | 1.70 (1.0–2.28) |
| Total cholesterol (mmol/L) | 5.49 ± 1.36 | 5.85 ± 2.24 | 5.14 ± 0.99 |
| HDL-C (mmol/L) | 1.27 ± 0.30 | 1.34 ± 0.52 | 1.30 ± 0.32 |
| Apolipoprotein AI (g/L) | 1.55 ± 0.24 | 1.62 ± 0.33 | 1.59 ± 0.27 |
| non-HDL-C (mmol/L) | 3.98 ± 0.95 | 4.09 ± 1.95 | 3.58 ± 1.29 |
| LDL-C (mmol/L) | 3.13 ± 0.88 | 3.28 ± 1.51 | 3.28 ± 0.84 |
| Apolipoprotein B100 (g/L) | 1.03 ± 0.28 | 1.26 ± 0.50 | 1.04 ± 0.25 |
| sICAM-1 (ng/mL) | 262.1 ± 65.4 | 277.6 ± 63.8 | 280.4 ± 51.7 |
| sVCAM-1 (ng/mL) | 783.9 ± 212.2 | 771.1 ± 181.9 | 640.4 ± 169.1 |
| Baseline | Week 26 | Week 52 | |
|---|---|---|---|
| Number of patients | 18 (12 m/6 f) | ||
| Age (year) | 57.9 ± 10.6 | ||
| Body mass index (kg/m2) | 37.96 ± 10.64 | 35.28 ± 9.43 † | 34.88 ± 10.22 § |
| Waist circumference (cm) | 126.4 ± 21.8 | 119.7 ± 21.6 † | 115.5 ± 20.9 § |
| Fasting glucose (mmol/L) | 8.1 (7.1–11.8) | 7.5 (5.0–8.6) † | 7.7 (5.3–10.4) § |
| Fructosamine (mmol/L) | 322.4 ± 87.9 | 260.4 ± 39.2 † | 251.5 ± 37.7 § |
| HemoglobinA1c (%) | 8.08 ± 1.65 | 6.86 ± 1.12 † | 6.57 ± 0.95 § |
| Insulin (mU/L) | 19.4 (15.2–27.5) | 21.6 (12.3–28.1) | 19.3 (9.1–30.2) |
| C-peptide (pmol/L) | 1370 (1270–1800) | 1340 (733–2110) | 1240 (1040–1610) |
| Creatinine (µmol/L) | 78.50 ± 15.07 | 77.94 ± 30.64 | 71.56 ± 16.67 |
| eGFR (mL/min/1.73 m2) | 86.5 (74–90) | 90 (78–90) | 90 (84–90) |
| hsCRP (mg/L) | 2.3 (1.7–5.8) | 2.30 (1.30–6.26) | 2.10 (1.10–2.40) |
| AST (U/L) | 21 (17–25) | 20.5 (14–28) | 17 (15–26) |
| ALT (U/L) | 27 (21–37) | 26 (18–32) | 21 (16–31) |
| GGT (U/L) | 34 (27–56) | 33 (23–45) | 35 (22–50) |
| Triglyceride (mmol/L) | 1.72 (1.50–3.11) | 1.575 (1.00–2.17) | 1.585 (1.0–2.50) |
| Total cholesterol (mmol/L) | 5.49 ± 1.36 | 4.83 ± 1.31 | 4.79 ± 1.00 |
| HDL-C (mmol/L) | 1.27 ± 0.30 | 1.40 ± 0.37 † | 1.43 ± 0.38 § |
| Apolipoprotein AI (g/L) | 1.55 ± 0.24 | 1.49 ± 0.23 | 1.54 ± 0.27 |
| non-HDL-C (mmol/L) | 3.98 ± 0.95 | 3.34 ± 1.08 | 3.35 ± 0.82 § |
| LDL-C (mmol/L) | 3.13 ± 0.88 | 2.85 ± 1.06 | 2.72 ± 0.84 § |
| Apolipoprotein B100 (g/L) | 1.03 ± 0.28 | 0.96 ± 0.31 | 0.99 ± 0.25 |
| sICAM-1 (ng/mL) | 262.1 ± 65.4 | 238.9 ± 59.7 | 250.8 ± 49.7 |
| sVCAM-1 (ng/mL) | 783.9 ± 212.2 | 763.9 ± 162.1 | 748.3 ± 150.1 |
| Baseline | Week 26 | Week 52 | |
|---|---|---|---|
| Number of patients | 16 (7 m/9 f) | ||
| Age (year) | 59.4 ± 11.9 | ||
| Body mass index (kg/m2) | 31.26 ± 2.75 | 31.10 ± 2.98 | 30.911 ± 2.9 § |
| Waist circumference (cm) | 126.60 ± 25.08 | 125.25 ± 29.18 | 124.25 ± 29.58 |
| Fasting glucose (mmol/L) | 8.90 (7.60–10.80) | 7.50 (5–7.9) † | 8.00 (5.7–9.3) |
| Fructosamine (mmol/L) | 299.0 ± 68.8 | 262.6 ± 51.2 | 253.4 ± 28.7 § |
| HemoglobinA1c (%) | 8.13 ± 1.29 | 6.91 ± 0.95 † | 7.11 ± 1.07 § |
| Insulin (mU/L) | 26.2 (20.5–41.6) | 26.0 (18.7–40.2) | 21.95 (12.5–26.5) |
| C-peptide (pmol/L) | 1430 (1140–2730) | 1870 (966–2020) | 1110 (904.5–1675) |
| Creatinine (µmol/L) | 71.47 ± 10.18 | 70.571 ± 15.301 | 71.20 ± 14.26 |
| eGFR (mL/min/1.73 m2) | 90 (73–90) | 90 (85–90) | 90 (85–90) |
| hsCRP (mg/L) | 5.5 (2.9–13.8) | 4.7 (2.3–11.7) | 8.7 (2.4–14.7) |
| AST (U/L) | 27 (21–35) | 23 (18–32) | 28.5 (16–36) |
| ALT (U/L) | 34 (23–46) | 29.5 (21–43) | 36 (25–46) |
| GGT (U/L) | 38 (22–87) | 39 (20–52) | 39 (25–44) |
| Triglyceride (mmol/L) | 2.00 (1.30–3.40) | 2.08 (1.55–2.9) | 1.85 (1.3–3.1) |
| Total cholesterol (mmol/L) | 5.85 ± 2.24 | 5.264 ± 1.32 | 5.31 ± 1.57 |
| HDL-C (mmol/L) | 1.34 ± 0.52 | 1.303 ± 0.44 | 1.338 ± 0.34 |
| Apolipoprotein AI (g/L) | 1.62 ± 0.33 | 1.53 ± 0.39 † | 1.48 ± 0.34 § |
| non-HDL-C (mmol/L) | 4.09 ± 1.95 | 3.962 ± 1.34 | 4.00 ± 1.52 |
| LDL-C (mmol/L) | 3.28 ± 1.51 | 3.09 ± 1.08 | 3.21 ± 1.2 |
| Apolipoprotein B100 (g/L) | 1.26 ± 0.50 | 1.10 ± 0.4 | 1.18 ± 0.41 |
| sICAM-1 (ng/mL) | 277.6 ± 63.8 | 291.9 ± 64.5 | 284.0 ± 43.4 |
| sVCAM-1 (ng/mL) | 771.1 ± 181.9 | 860.2 ± 547.1 | 691.9 ± 75.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dániel, E.; Sztanek, F.; Csiha, S.; Ratku, B.; Somodi, S.; Paragh, G.; Harangi, M.; Lőrincz, H. Long-Term Effects of Semaglutide and Sitagliptin on Circulating IGFBP-1, IGFBP-3 and IGFBP-rp1: Results from a One-Year Study in Type 2 Diabetes. Int. J. Mol. Sci. 2025, 26, 10404. https://doi.org/10.3390/ijms262110404
Dániel E, Sztanek F, Csiha S, Ratku B, Somodi S, Paragh G, Harangi M, Lőrincz H. Long-Term Effects of Semaglutide and Sitagliptin on Circulating IGFBP-1, IGFBP-3 and IGFBP-rp1: Results from a One-Year Study in Type 2 Diabetes. International Journal of Molecular Sciences. 2025; 26(21):10404. https://doi.org/10.3390/ijms262110404
Chicago/Turabian StyleDániel, Eszter, Ferenc Sztanek, Sára Csiha, Balázs Ratku, Sándor Somodi, György Paragh, Mariann Harangi, and Hajnalka Lőrincz. 2025. "Long-Term Effects of Semaglutide and Sitagliptin on Circulating IGFBP-1, IGFBP-3 and IGFBP-rp1: Results from a One-Year Study in Type 2 Diabetes" International Journal of Molecular Sciences 26, no. 21: 10404. https://doi.org/10.3390/ijms262110404
APA StyleDániel, E., Sztanek, F., Csiha, S., Ratku, B., Somodi, S., Paragh, G., Harangi, M., & Lőrincz, H. (2025). Long-Term Effects of Semaglutide and Sitagliptin on Circulating IGFBP-1, IGFBP-3 and IGFBP-rp1: Results from a One-Year Study in Type 2 Diabetes. International Journal of Molecular Sciences, 26(21), 10404. https://doi.org/10.3390/ijms262110404










