The Role of Mast Cells in Healing Purulent Wounds Using a Drug from the Polyhexamethylene Guanidine Group with the Antiseptic Polyhexanide: An Ultrastructural Study
Abstract
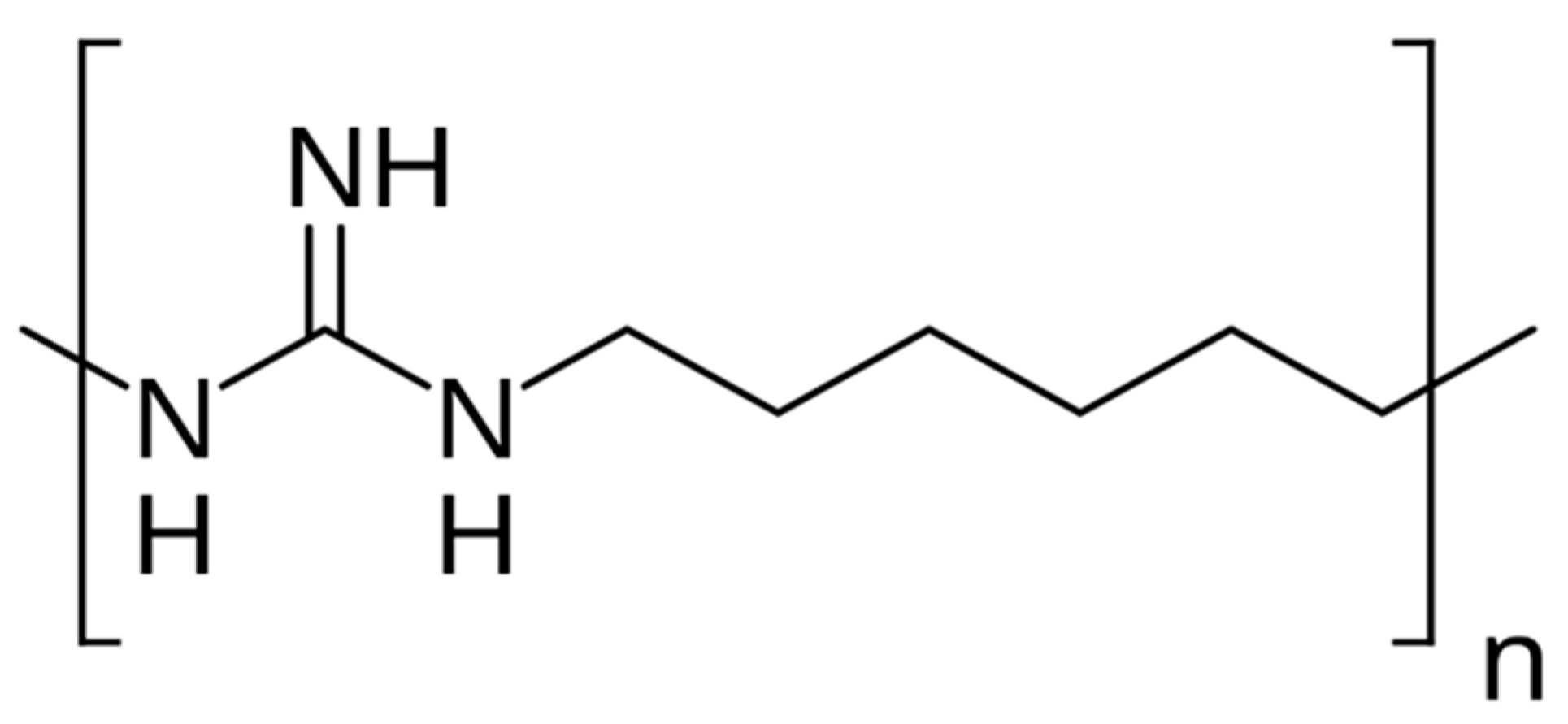
1. Introduction
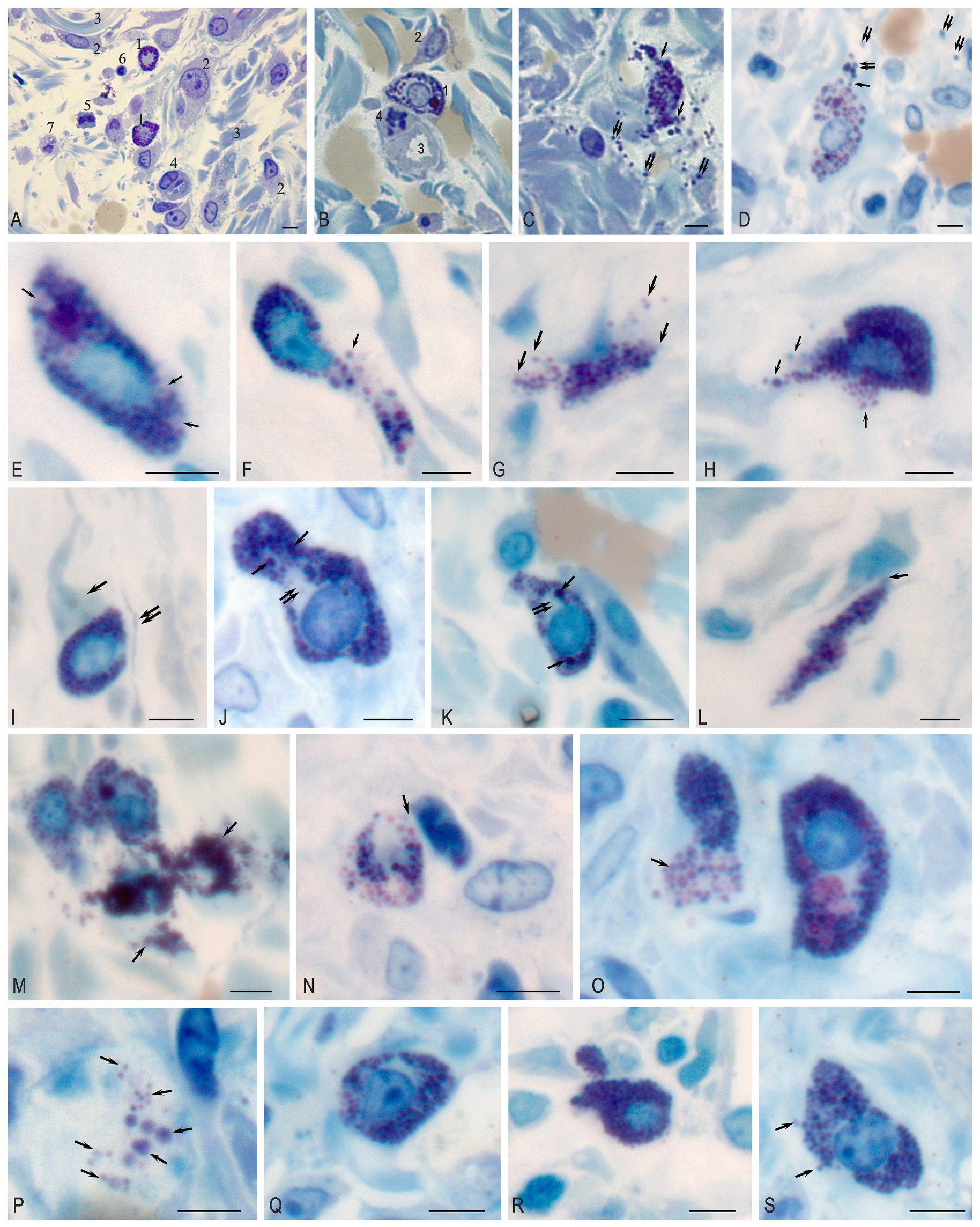
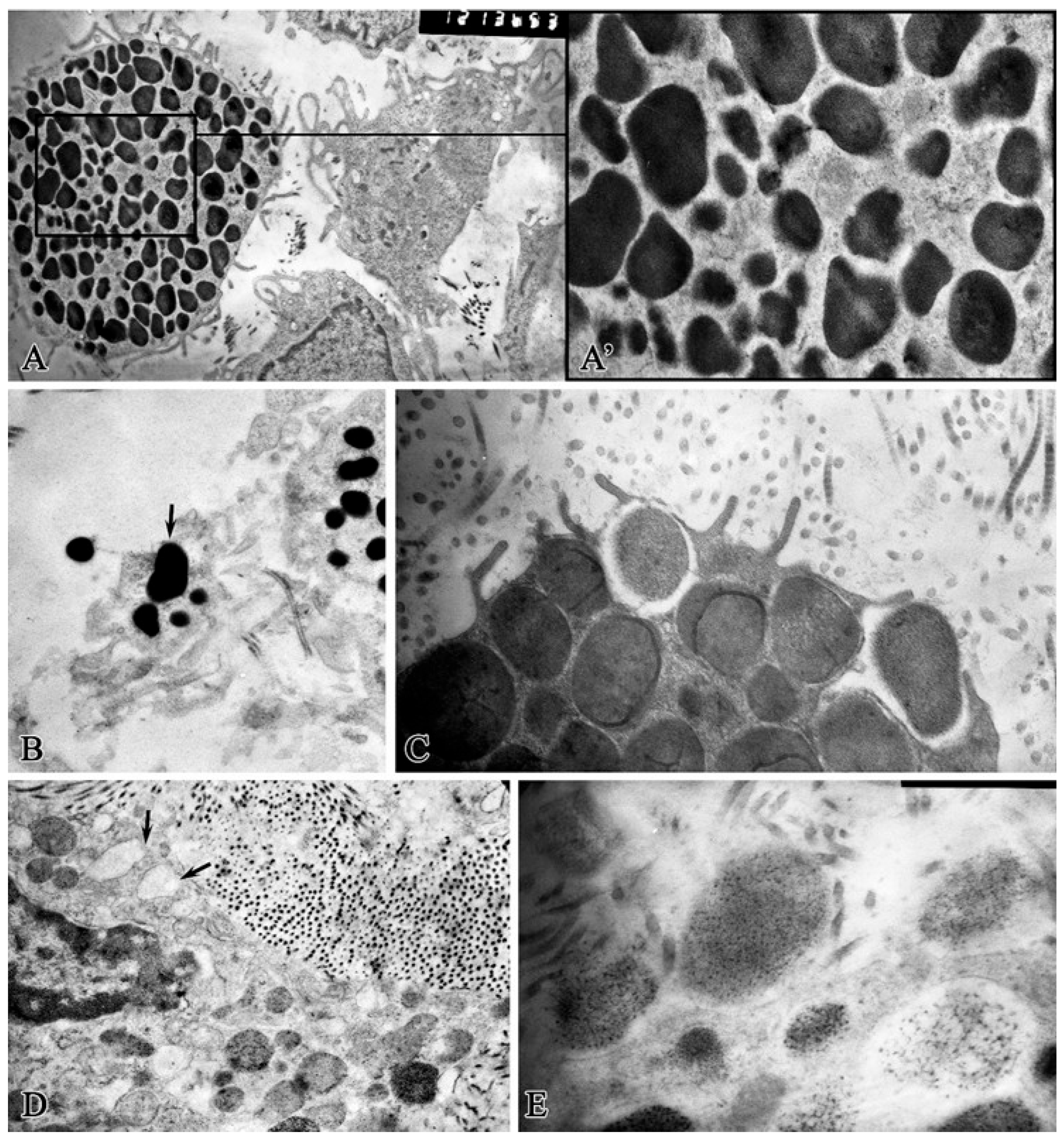
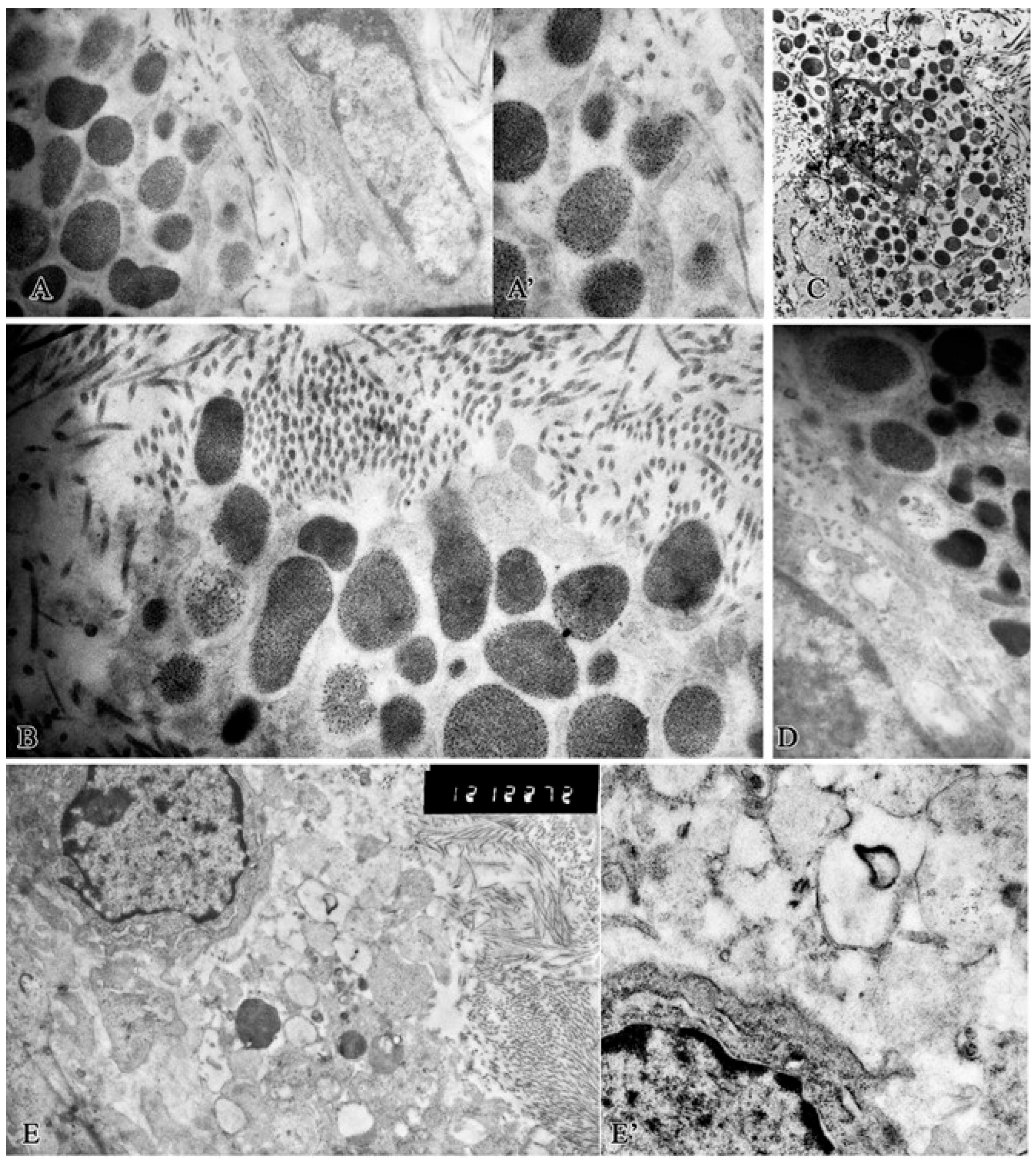
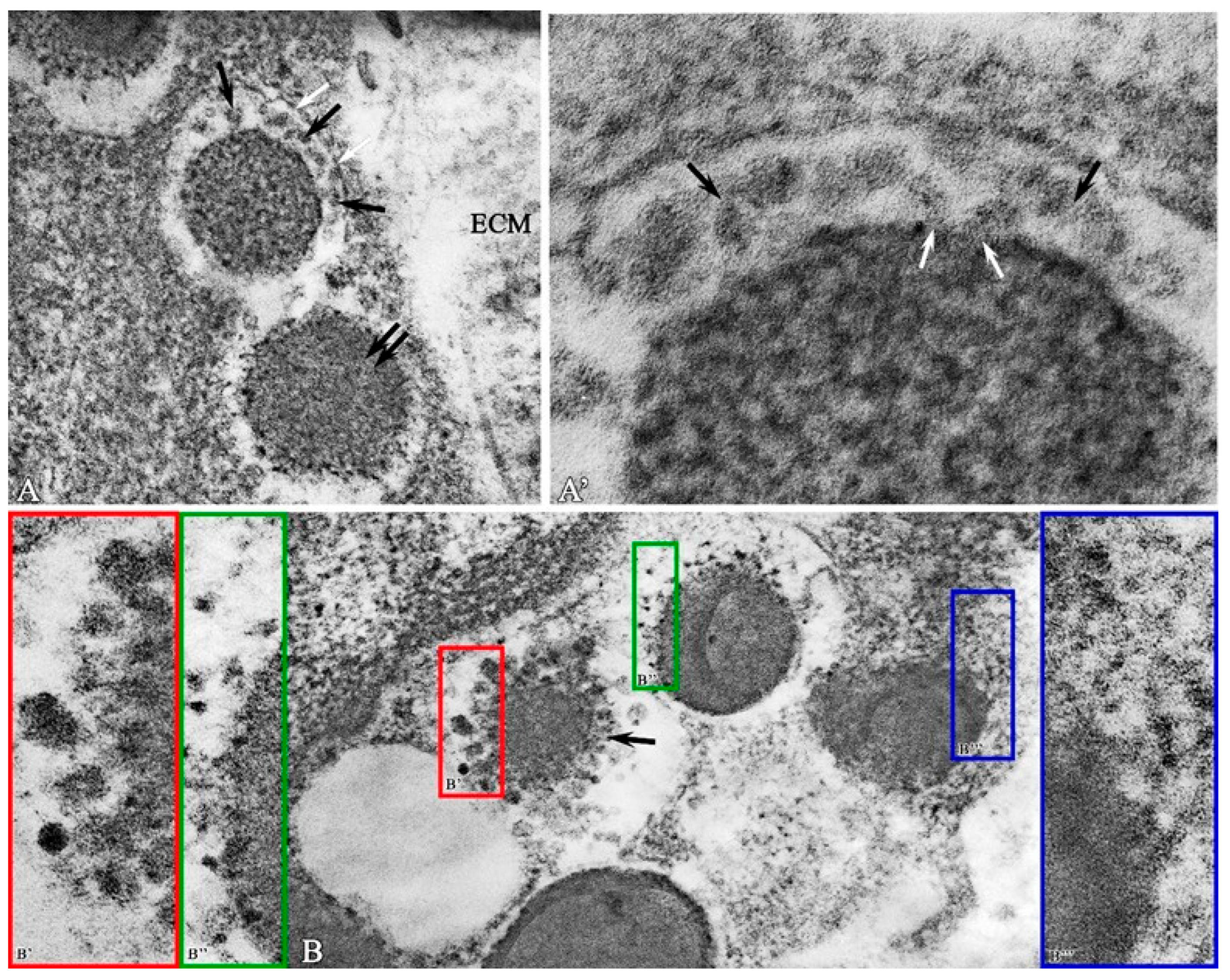
2. Results
Morphological Study
3. Discussion
| Phase | Mediators | Biological Effects |
|---|---|---|
| Hemostasis | Heparin | Inhibits the enzymatic and synthetic action of thrombin [99]. Binds molecules produced by mast cells [100]. |
| Tryptase | Suppresses thrombin-induced fibrinogen activity, which is responsible for blood clotting [25]. | |
| TNF-α | Has a positive effect on the expression of coagulation factor XIIIa (fibrin stabilizing factor), so it promotes coagulation [84,101]. | |
| Inflammation | MCP-1s | Affects the phagocyte morphology [102]. |
| Proliferation | Tryptase | Stimulates angiogenesis Cleaves fibronectin and activates PAR-2 [103,104]. |
| VEGF, TGF-β, Chymase | Stimulate angiogenesis [19,30,31,32,91]. | |
| Heparin | Inhibits angiogenesis [30,32]. | |
| Cytokines | Affects the phenotypic characteristics of activated fibroblasts [4]. | |
| Remodeling of the extracellular matrix | Tryptase | Promotes the synthesis of type I collagen [25,105]. |
| Histamine | Regulates skin remodeling processes [106,107]. Produces proteolytic enzymes [9,108]. | |
| TGF-β1, TNF-α, IL-4 | Regulates fibroblast proliferation [109]. |
4. Materials and Methods
4.1. Research Design
4.2. Electron Microscopy
4.3. Quantitative Analysis
4.4. Statistical Analysis
4.5. Ethical Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MC | Mast Cell |
| CFU | Colony-Forming Unit |
| Ci | Curie |
References
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin tissue engineering: Wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef]
- Martin, R.F. Wound Healing. Surg. Clin. N. Am. 2020, 100, ix–xi. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Bacci, S. Fine Regulation during Wound Healing by Mast Cells, a Physiological Role Not Yet Clarified. Int. J. Mol. Sci. 2022, 23, 1820. [Google Scholar] [CrossRef]
- Fernandez-Guarino, M.; Bacci, S. Mast cells and wound healing: Still an open question. Histol. Histopathol. 2025, 40, 21–30. [Google Scholar] [CrossRef]
- Guth, C.; Limjunyawong, N.; Pundir, P. The evolving role of mast cells in wound healing: Insights from recent research and diverse models. Immunol. Cell Biol. 2024, 102, 878–890. [Google Scholar] [CrossRef]
- Heidarzadeh-Asl, S.; Maurer, M.; Kiani, A.; Atiakshin, D.; Stahl Skov, P.; Elieh-Ali-Komi, D. Novel insights on the biology and immunologic effects of histamine: A road map for allergists and mast cell biologists. J. Allergy Clin. Immunol. 2024, 155, 1095–1114. [Google Scholar] [CrossRef]
- Galvan-Morales, M.A.; Vizuet-de-Rueda, J.C.; Montero-Vargas, J.M.; Teran, L.M. Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies. Int. J. Mol. Sci. 2025, 26, 8895. [Google Scholar] [CrossRef]
- Montero-Hernandez, J.E.; Zhang, K.; Blank, U.; Menasche, G. LRO biogenesis and function: What can we learn from mast cells? Front. Cell Dev. Biol. 2025, 13, 1613677. [Google Scholar] [CrossRef]
- Sagi-Eisenberg, R. Biogenesis and homeostasis of mast cell lysosome related secretory granules. Front. Cell Dev. Biol. 2025, 13, 1603999. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Shafaghat, F.; Alipoor, S.D.; Kazemi, T.; Atiakshin, D.; Pyatilova, P.; Maurer, M. Immunomodulatory Significance of Mast Cell Exosomes (MC-EXOs) in Immune Response Coordination. Clin. Rev. Allergy Immunol. 2025, 68, 20. [Google Scholar] [CrossRef]
- Bacci, S. The evolution of mast cells across all vertebrate classes: The mystery continues. Histol. Histopathol. 2025, 18926. [Google Scholar] [CrossRef]
- Papa, V.; Li Pomi, F.; Di Gioacchino, M.; Mangifesta, R.; Borgia, F.; Gangemi, S. Mast Cells and Microbiome in Health and Disease. Front. Biosci. 2025, 30, 26283. [Google Scholar] [CrossRef]
- Fukuishi, N.; Takahama, K.; Kurosaki, H.; Ono, S.; Asai, H. The Role of Endogenous Specialized Proresolving Mediators in Mast Cells and Their Involvement in Inflammation and Resolution. Int. J. Mol. Sci. 2025, 26, 1491. [Google Scholar] [CrossRef]
- Pahima, H.T.; Dwyer, D.F. Update on mast cell biology. J. Allergy Clin. Immunol. 2025, 155, 1115–1123. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cell proteases and metastasis. Pathol. Res. Pract. 2025, 266, 155801. [Google Scholar] [CrossRef]
- Zhao, Y.; Abzalimov, R.R.; Kaltashov, I.A. Interactions of Intact Unfractionated Heparin with Its Client Proteins Can Be Probed Directly Using Native Electrospray Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 1711–1718. [Google Scholar] [CrossRef]
- Norrby, K. On Connective Tissue Mast Cells as Protectors of Life, Reproduction, and Progeny. Int. J. Mol. Sci. 2024, 25, 4499. [Google Scholar] [CrossRef]
- Hao, C.; Xu, H.; Yu, L.; Zhang, L. Heparin: An essential drug for modern medicine. Prog. Mol. Biol. Transl. Sci. 2019, 163, 1–19. [Google Scholar] [CrossRef]
- Weiss, R.J.; Esko, J.D.; Tor, Y. Targeting heparin and heparan sulfate protein interactions. Org. Biomol. Chem. 2017, 15, 5656–5668. [Google Scholar] [CrossRef]
- Mulloy, B.; Lever, R.; Page, C.P. Mast cell glycosaminoglycans. Glycoconj. J. 2017, 34, 351–361. [Google Scholar] [CrossRef]
- Atiakshin, D.; Patsap, O.; Kostin, A.; Mikhalyova, L.; Buchwalow, I.; Tiemann, M. Mast Cell Tryptase and Carboxypeptidase A3 in the Formation of Ovarian Endometrioid Cysts. Int. J. Mol. Sci. 2023, 24, 6498. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef]
- Hellman, L.; Akula, S.; Fu, Z.; Wernersson, S. Mast Cell and Basophil Granule Proteases—In Vivo Targets and Function. Front. Immunol. 2022, 13, 918305. [Google Scholar] [CrossRef]
- Agier, J.; Pastwinska, J.; Brzezinska-Blaszczyk, E. An overview of mast cell pattern recognition receptors. Inflamm. Res. 2018, 67, 737–746. [Google Scholar] [CrossRef]
- Gebremeskel, S.; Schanin, J.; Coyle, K.M.; Butuci, M.; Luu, T.; Brock, E.C.; Xu, A.; Wong, A.; Leung, J.; Korver, W.; et al. Mast Cell and Eosinophil Activation Are Associated With COVID-19 and TLR-Mediated Viral Inflammation: Implications for an Anti-Siglec-8 Antibody. Front. Immunol. 2021, 12, 650331. [Google Scholar] [CrossRef]
- Ribatti, D. Mast cells are at the interface between the external environment and the inner organism. Front. Med. 2023, 10, 1332047. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.; Wang, W.; Zhang, D.; Ma, Y.; Zhang, Y.; Wang, X. The Auxiliary Role of Heparin in Bone Regeneration and its Application in Bone Substitute Materials. Front. Bioeng. Biotechnol. 2022, 10, 837172. [Google Scholar] [CrossRef]
- Huber, R.; Attili/Abedalkhader, R.; Kuper, D.; Hauke, L.; Luns, B.; Brand, K.; Weissenborn, K.; Lichtinghagen, R. Cellular and Molecular Effects of High-Molecular-Weight Heparin on Matrix Metalloproteinase 9 Expression. Int. J. Mol. Sci. 2019, 20, 1595. [Google Scholar] [CrossRef]
- Shastri, M.D.; Stewart, N.; Eapen, M.; Peterson, G.M.; Zaidi, S.T.; Gueven, N.; Sohal, S.S.; Patel, R.P. Opposing effects of low molecular weight heparins on the release of inflammatory cytokines from peripheral blood mononuclear cells of asthmatics. PLoS ONE 2015, 10, e0118798. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cells and collagen fibrillogenesis. Histochem. Cell Biol. 2020, 154, 21–40. [Google Scholar] [CrossRef]
- Poto, R.; Patella, V.; Criscuolo, G.; Marone, G.; Coscioni, E.; Varricchi, G. Autoantibodies to IgE can induce the release of proinflammatory and vasoactive mediators from human cardiac mast cells. Clin. Exp. Med. 2023, 23, 1265–1276. [Google Scholar] [CrossRef]
- Atiakshin, D.; Soboleva, M.; Nikityuk, D.; Alexeeva, N.; Klochkova, S.; Kostin, A.; Shishkina, V.; Buchwalow, I.; Tiemann, M. Mast Cells in Regeneration of the Skin in Burn Wound with Special Emphasis on Molecular Hydrogen Effect. Pharmaceuticals 2023, 16, 348. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Ud-Din, S.; Bayat, A. A Review of the Evidence for and against a Role for Mast Cells in Cutaneous Scarring and Fibrosis. Int. J. Mol. Sci. 2020, 21, 9673. [Google Scholar] [CrossRef]
- Andriessen, A.E.; Eberlein, T. Assessment of a wound cleansing solution in the treatment of problem wounds. Wounds 2008, 20, 171–175. [Google Scholar]
- Kaehn, K.; Eberlein, T. In-vitro test for comparing the efficacy of wound rinsing solutions. Br. J. Nurs. 2009, 18, S4, S6–S8, S10. [Google Scholar] [CrossRef]
- Rafael, A.; Mendoza, J.-C.H.; Galiano, R.D. The Impact of Biofilm Formation on Wound Healing [Internet]. In Wound Healing—Current Perspectives; Dogan, K.H., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Zaitsev, A.; Asanov, O.; Chekmareva, I. Analysis of the effectiveness of the erbium laser in the treatment of trophic purulent wounds in an experiment. Med. News North Cauc. 2023, 18, 394–397. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and wound healing: From bench to bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Tamrazova, O.B.; Stadnikova, A.S.; Gureeva, M.A.; Nikitin, I.S. Modern aspects of treatment of purulent wounds with combined drugs. Klin. Dermatol. I Venerol. 2020, 19, 905. [Google Scholar] [CrossRef]
- Grigoryan, A.Y.; Bezhin, A.I.; Pankrusheva, T.A.; Sukovatykh, B.S. Local management of purulent wounds with wound dressings. Khirurgiia 2022, 42–48. [Google Scholar] [CrossRef]
- Bystrov, S.A.; Bezborodov, A.I.; Katorkin, S.E. Treatment of purulent wounds with wound dressing on a foamy basis with Hydrofiber technology. Khirurgiia 2017, 49–53. [Google Scholar] [CrossRef]
- Dowsett, C. Managing wound exudate: Role of Versiva XC gelling foam dressing. Br. J. Nurs. 2008, 17, S38–S40. [Google Scholar] [CrossRef]
- Kouketsu, A.; Shimizu, Y.; Nogami, S.; Yamada-Fujiwara, M.; Nagai, H.; Yamauchi, K.; Miyashita, H.; Saito, H.; Odashima, K.; Yanagisawa, Y.; et al. Wound healing effect of autologous fibrin glue and polyglycolic acid sheets in a rat back skin defect model. Transfus. Apher. Sci. 2021, 60, 103144. [Google Scholar] [CrossRef]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Dermatol. Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef]
- Lawson, M.C.; Hoth, K.C.; Deforest, C.A.; Bowman, C.N.; Anseth, K.S. Inhibition of Staphylococcus epidermidis biofilms using polymerizable vancomycin derivatives. Clin. Orthop. Relat. Res. 2010, 468, 2081–2091. [Google Scholar] [CrossRef]
- Percival, S.L.; Thomas, J.; Linton, S.; Okel, T.; Corum, L.; Slone, W. The antimicrobial efficacy of silver on antibiotic-resistant bacteria isolated from burn wounds. Int. Wound J. 2012, 9, 488–493. [Google Scholar] [CrossRef]
- Lindsay, S.; Oates, A.; Bourdillon, K. The detrimental impact of extracellular bacterial proteases on wound healing. Int. Wound J. 2017, 14, 1237–1247. [Google Scholar] [CrossRef]
- Liu, M.; Huang, L.; Xu, X.; Wei, X.; Yang, X.; Li, X.; Wang, B.; Xu, Y.; Li, L.; Yang, Z. Copper Doped Carbon Dots for Addressing Bacterial Biofilm Formation, Wound Infection, and Tooth Staining. ACS Nano 2022, 16, 9479–9497. [Google Scholar] [CrossRef]
- Grigorian, A.; Bezhin, A.; Pankrusheva, T.; Zhilyaeva, L. New methods of local medical treatment of purulent wounds. Res. Pract. Med. J. 2020, 7, 56–63. [Google Scholar] [CrossRef]
- Kenawy, M.; Abdel-Hamid, Y. Maggot Therapy “Use of Fly Larvae for Treatment of Wounds”—A Review. Egypt. Acad. J. Biol. Sci. E Med. Entomol. Parasitol. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Urakov, A.L.; Urakova, N.A.; Reshetnikov, A.P.; Shabanov, P.D.; Wang, Y.; Bodduluri, P.V.; Samorodov, A.V.; Rozov, R.A.; Shchemeleva, A.A.; Novikov, V.E.; et al. Pyolytics as a product of the physical–chemical repurposing of antiseptics and an alternative to larval therapy for chronic wounds. Rev. Clin. Pharmacol. Drug Ther. 2023, 21, 287–297. [Google Scholar] [CrossRef]
- Urakov, A.; Urakova, N.; Fisher, E.; Shchemeleva, A.; Stolyarenko, A.; Martiusheva, V.; Zavarzina, M. Antiseptic pyolytics and warming wet compresses improve the prospect of healing chronic wounds. Explor. Med. 2023, 4, 747–754. [Google Scholar] [CrossRef]
- Lebed-Sharlevich, Y.I.; Mamonov, R.A. Safety issues in the use of disinfectants based on polyhexamethyleneguanidine (literature review). Hyg. Sanit. 2023, 102, 981–986. [Google Scholar] [CrossRef]
- Dias, F.G.G.; Pereira, L.F.; Parreira, R.L.T.; Veneziani, R.C.S.; Bianchi, T.C.; Fontes, V.; Galvani, M.C.; Cerce, D.D.P.; Martins, C.H.G.; Rinaldi-Neto, F.; et al. Evaluation of the antiseptic and wound healing potential of polyhexamethylene guanidine hydrochloride as well as its toxic effects. Eur. J. Pharm. Sci. 2021, 160, 105739. [Google Scholar] [CrossRef]
- Wikipedia. Polyhexamethylene Guanidine. Available online: https://en.wikipedia.org/w/index.php?title=Polyhexamethylene_guanidine&oldid=1308563462 (accessed on 30 August 2025).
- Stoliarov, E.A.; Ivanova, V.D.; Kolsanov, A.V. Healing of purulent wounds of soft tissues in local treatment. Khirurgiia 2003, 28–32. [Google Scholar]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef]
- Carlier, A.; Pessi, G.; Eberl, L. Microbial Biofilms and Quorum Sensing. In Principles of Plant-Microbe Interactions; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Vani, S.; Vadakkan, K.; Mani, B. A narrative review on bacterial biofilm: Its formation, clinical aspects and inhibition strategies. Future J. Pharm. Sci. 2023, 9, 50. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Pundir, P.; Liu, R.; Vasavda, C.; Serhan, N.; Limjunyawong, N.; Yee, R.; Zhan, Y.; Dong, X.; Wu, X.; Zhang, Y.; et al. A Connective Tissue Mast-Cell-Specific Receptor Detects Bacterial Quorum-Sensing Molecules and Mediates Antibacterial Immunity. Cell Host Microbe 2019, 26, 114–122 e118. [Google Scholar] [CrossRef]
- Kunder, C.A.; St John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Anderson, C.C.; Fudge, D.H.; Roede, J.R.; Brown, J.M. Metabolic Consequences of IgE- and Non-IgE-Mediated Mast Cell Degranulation. J. Immunol. 2021, 207, 2637–2648. [Google Scholar] [CrossRef]
- Nassar, A.; Wagura, E.; Loukas, M. Mast cells and arteriogenesis: A systematic review. Cardiovasc. Pathol. 2025, 75, 107716. [Google Scholar] [CrossRef]
- Sengul, T.; Kirkland-Kyhn, H.; Karadag, A. Chronic Wounds and Dressings: An Overview of Management and Effectiveness. Nurs. Clin. N. Am. 2025, 60, 1–13. [Google Scholar] [CrossRef]
- Iba, Y.; Shibata, A.; Kato, M.; Masukawa, T. Possible involvement of mast cells in collagen remodeling in the late phase of cutaneous wound healing in mice. Int. Immunopharmacol. 2004, 4, 1873–1880. [Google Scholar] [CrossRef]
- Galli, S.J.; Grimbaldeston, M.; Tsai, M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 2008, 8, 478–486. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast cell-mediated immune regulation in health and disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef]
- Tsai, M.; Grimbaldeston, M.; Galli, S.J. Mast cells and immunoregulation/immunomodulation. Adv. Exp. Med. Biol. 2011, 716, 186–211. [Google Scholar] [CrossRef]
- Kostin, A.; Lyundup, A.; Alekhnovich, A.; Prikhodko, A.; Patsap, O.; Gronskaia, S.; Belaya, Z.; Lesnyak, O.; Melnichenko, G.; Mokrysheva, N.; et al. Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23. Med. Sci. 2025, 13, 195. [Google Scholar] [CrossRef]
- Pal, S.; Nath, S.; Meininger, C.J.; Gashev, A.A. Emerging Roles of Mast Cells in the Regulation of Lymphatic Immuno-Physiology. Front. Immunol. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Jimenez, M.; Cervantes-Garcia, D.; Cordova-Davalos, L.E.; Perez-Rodriguez, M.J.; Gonzalez-Espinosa, C.; Salinas, E. Responses of Mast Cells to Pathogens: Beneficial and Detrimental Roles. Front. Immunol. 2021, 12, 685865. [Google Scholar] [CrossRef]
- Kasuya, A.; Tokura, Y. Attempts to accelerate wound healing. J. Dermatol. Sci. 2014, 76, 169–172. [Google Scholar] [CrossRef]
- Soliman, M.; Kim, D.S.; Park, J.G.; Kim, J.Y.; Alfajaro, M.M.; Baek, Y.B.; Cho, E.H.; Park, C.H.; Kang, M.I.; Park, S.I.; et al. Phosphatidylinositol 3-Kinase/Akt and MEK/ERK Signaling Pathways Facilitate Sapovirus Trafficking and Late Endosomal Acidification for Viral Uncoating in LLC-PK Cells. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef]
- Zhu, C.; Bao, N.R.; Chen, S.; Zhao, J.N. HBD-3 regulation of the immune response and the LPS/TLR4-mediated signaling pathway. Exp. Ther. Med. 2016, 12, 2150–2154. [Google Scholar] [CrossRef][Green Version]
- Heukels, P.; Moor, C.C.; von der Thusen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef]
- Veerappan, A.; O’Connor, N.J.; Brazin, J.; Reid, A.C.; Jung, A.; McGee, D.; Summers, B.; Branch-Elliman, D.; Stiles, B.; Worgall, S.; et al. Mast cells: A pivotal role in pulmonary fibrosis. DNA Cell Biol. 2013, 32, 206–218. [Google Scholar] [CrossRef]
- Dudeck, J.; Froebel, J.; Kotrba, J.; Lehmann, C.H.K.; Dudziak, D.; Speier, S.; Nedospasov, S.A.; Schraven, B.; Dudeck, A. Engulfment of mast cell secretory granules on skin inflammation boosts dendritic cell migration and priming efficiency. J. Allergy Clin. Immunol. 2019, 143, 1849–1864. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Savage, A.; Risquez, C.; Gomi, K.; Schreiner, R.; Borczuk, A.C.; Worgall, S.; Silver, R.B. The mast cell exosome-fibroblast connection: A novel pro-fibrotic pathway. Front. Med. 2023, 10, 1139397. [Google Scholar] [CrossRef]
- Shimbori, C.; Upagupta, C.; Bellaye, P.S.; Ayaub, E.A.; Sato, S.; Yanagihara, T.; Zhou, Q.; Ognjanovic, A.; Ask, K.; Gauldie, J.; et al. Mechanical stress-induced mast cell degranulation activates TGF-beta1 signalling pathway in pulmonary fibrosis. Thorax 2019, 74, 455–465. [Google Scholar] [CrossRef]
- Spoerl, D.; Nigolian, H.; Czarnetzki, C.; Harr, T. Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”? Int. J. Mol. Sci. 2017, 18, 1223. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
- Rousselle, P.; Braye, F.; Dayan, G. Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Adv. Drug Deliv. Rev. 2019, 146, 344–365. [Google Scholar] [CrossRef]
- Berlin, F.; Mogren, S.; Tutzauer, J.; Andersson, C.K. Mast Cell Proteases Tryptase and Chymase Induce Migratory and Morphological Alterations in Bronchial Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5250. [Google Scholar] [CrossRef]
- Mogren, S.; Berlin, F.; Ramu, S.; Sverrild, A.; Porsbjerg, C.; Uller, L.; Andersson, C.K. Mast cell tryptase enhances wound healing by promoting migration in human bronchial epithelial cells. Cell Adh. Migr. 2021, 15, 202–214. [Google Scholar] [CrossRef]
- Chekmaryova, I.; Kalinin, D.; Kostin, A.; Buchwalow, I.; Tiemann, M.; Elieh-Ali-Komi, D.; Atiakshin, D. Ultrastructural features of tumor-associated mast cells in parasympathetic paragangliomas (chemodectomas) of the neck. Microsc. Res. Tech. 2024, 87, 1373–1383. [Google Scholar] [CrossRef]
- Crivellato, E.; Nico, B.; Mallardi, F.; Beltrami, C.A.; Ribatti, D. Piecemeal degranulation as a general secretory mechanism? Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 274, 778–784. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Cochrane, D.E. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol. 2004, 146, 1–12. [Google Scholar] [CrossRef]
- Puxeddu, I.; Piliponsky, A.M.; Bachelet, I.; Levi-Schaffer, F. Mast cells in allergy and beyond. Int. J. Biochem. Cell Biol. 2003, 35, 1601–1607. [Google Scholar] [CrossRef]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- D’Inca, F.; Pucillo, C.E. Exosomes: Tiny clues for mast cell communication. Front. Immunol. 2015, 6, 73. [Google Scholar] [CrossRef]
- Hemker, H.C.; Al Dieri, R.; Beguin, S. Heparins: A Shift of Paradigm. Front. Med. 2019, 6, 254. [Google Scholar] [CrossRef]
- Kondashevskaya, M. Mast Cells Heparin—New Information on the Old Component (Review). Ann. Russ. Acad. Med. Sci. 2021, 76, 149–158. [Google Scholar] [CrossRef]
- Tereshchenko, I.V.; Kayushev, P.E. Tumor necrosis factor α and its role in pathologies. Russ. Med. Inq. 2022, 6, 523–527. (In Russian) [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Kaieda, S.; Shin, K.; Nigrovic, P.A.; Seki, K.; Lee, R.T.; Stevens, R.L.; Lee, D.M. Synovial fibroblasts promote the expression and granule accumulation of tryptase via interleukin-33 and its receptor ST-2 (IL1RL1). J. Biol. Chem. 2010, 285, 21478–21486. [Google Scholar] [CrossRef]
- Ma, C.; Li, H.; Lu, S.; Li, X.; Wang, S.; Wang, W. Tryptase and Exogenous Trypsin: Mechanisms and Ophthalmic Applications. J. Inflamm. Res. 2023, 16, 927–939. [Google Scholar] [CrossRef]
- Villar, J.; Cabrera-Benitez, N.E.; Valladares, F.; Garcia-Hernandez, S.; Ramos-Nuez, A.; Martin-Barrasa, J.L.; Muros, M.; Kacmarek, R.M.; Slutsky, A.S. Tryptase is involved in the development of early ventilator-induced pulmonary fibrosis in sepsis-induced lung injury. Crit. Care 2015, 19, 138. [Google Scholar] [CrossRef]
- Yang, L.; Murota, H.; Serada, S.; Fujimoto, M.; Kudo, A.; Naka, T.; Katayama, I. Histamine contributes to tissue remodeling via periostin expression. J. Investig. Dermatol. 2014, 134, 2105–2113. [Google Scholar] [CrossRef]
- Patel, A.; Vasanthan, V.; Fu, W.; Fahlman, R.P.; MacTavish, D.; Jhamandas, J.H. Histamine induces the production of matrix metalloproteinase-9 in human astrocytic cultures via H1-receptor subtype. Brain Struct. Funct. 2016, 221, 1845–1860. [Google Scholar] [CrossRef]
- Jurado-León, R.; Rodríguez-Fragoso, L.; Reyes Esparza, J. Possible contribution of histamine in the pathophysiology of hepatic fibrosis: Modulation of proteolytic activity. Biotecnol. Apl. 2005, 22, 221–226. [Google Scholar]
- Wang, Z.C.; Zhao, W.Y.; Cao, Y.; Liu, Y.Q.; Sun, Q.; Shi, P.; Cai, J.Q.; Shen, X.Z.; Tan, W.Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef]

| Observation Day | Control | Experimental |
|---|---|---|
| 1 | 100 | 100 |
| 3 | 80 | 40 |
| 7 | 80 | 20 * |
| 12 | 60 | 0 * |
| Phase of Wound Healing | Control | Experimental |
|---|---|---|
| Cleaning | 9.6 ± 0.2 | 5.3 ± 0.3 * |
| Onset of granulation | 11.7 ± 0.2 | 7.0 ± 0.3 * |
| Observation Day | Control | Experimental |
|---|---|---|
| 1 | 421.5 ± 0.9 | 421.2 ± 0.9 |
| 3 | 390.6 ± 0.9 | 370.5 ± 2.3 |
| 7 | 300.2 ± 2.3 | 241.8 ± 2.2 * |
| 12 | 250.8 ± 4.2 | 150.8 ± 3.6 * |
| Observation Day | Control | Experimental | ||
|---|---|---|---|---|
| Number of MCs, pc | MC Degranulation Index, % | Number of MCs, pc | MC Degranulation Index, % | |
| 1 | 35.48 ± 5.66 | 44.0 | 35.48 ± 5.66 | 44.0 |
| 3 | 48.39 ± 8.71 | 63.2 | 64.52 ± 9.35 | 70.6 |
| 7 | 54.84 ± 7.74 | 66.7 | 80.65 ± 122.25 * ” | 80.1 |
| 12 | 77.40 ± 7.74 | 71.8 | 48.39 ± 9.35 * ” | 57.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chekmareva, I.; Emaimo John, A.; Kostin, A.; Alekhnovich, A.; Volodkin, A.; Klabukov, I.; Baranovskii, D.; Shishkina, V.; Buchwalow, I.; Tiemann, M.; et al. The Role of Mast Cells in Healing Purulent Wounds Using a Drug from the Polyhexamethylene Guanidine Group with the Antiseptic Polyhexanide: An Ultrastructural Study. Int. J. Mol. Sci. 2025, 26, 10405. https://doi.org/10.3390/ijms262110405
Chekmareva I, Emaimo John A, Kostin A, Alekhnovich A, Volodkin A, Klabukov I, Baranovskii D, Shishkina V, Buchwalow I, Tiemann M, et al. The Role of Mast Cells in Healing Purulent Wounds Using a Drug from the Polyhexamethylene Guanidine Group with the Antiseptic Polyhexanide: An Ultrastructural Study. International Journal of Molecular Sciences. 2025; 26(21):10405. https://doi.org/10.3390/ijms262110405
Chicago/Turabian StyleChekmareva, Irina, Atim Emaimo John, Andrey Kostin, Alexander Alekhnovich, Artem Volodkin, Ilya Klabukov, Denis Baranovskii, Viktoria Shishkina, Igor Buchwalow, Markus Tiemann, and et al. 2025. "The Role of Mast Cells in Healing Purulent Wounds Using a Drug from the Polyhexamethylene Guanidine Group with the Antiseptic Polyhexanide: An Ultrastructural Study" International Journal of Molecular Sciences 26, no. 21: 10405. https://doi.org/10.3390/ijms262110405
APA StyleChekmareva, I., Emaimo John, A., Kostin, A., Alekhnovich, A., Volodkin, A., Klabukov, I., Baranovskii, D., Shishkina, V., Buchwalow, I., Tiemann, M., & Atiakshin, D. (2025). The Role of Mast Cells in Healing Purulent Wounds Using a Drug from the Polyhexamethylene Guanidine Group with the Antiseptic Polyhexanide: An Ultrastructural Study. International Journal of Molecular Sciences, 26(21), 10405. https://doi.org/10.3390/ijms262110405









