Multiomics Investigation of Exhausted T Cells in Glioblastoma Tumor Microenvironment: CCL5 as a Prognostic and Therapeutic Target
Abstract
1. Introduction
2. Results
2.1. Mendelian Randomization Analysis
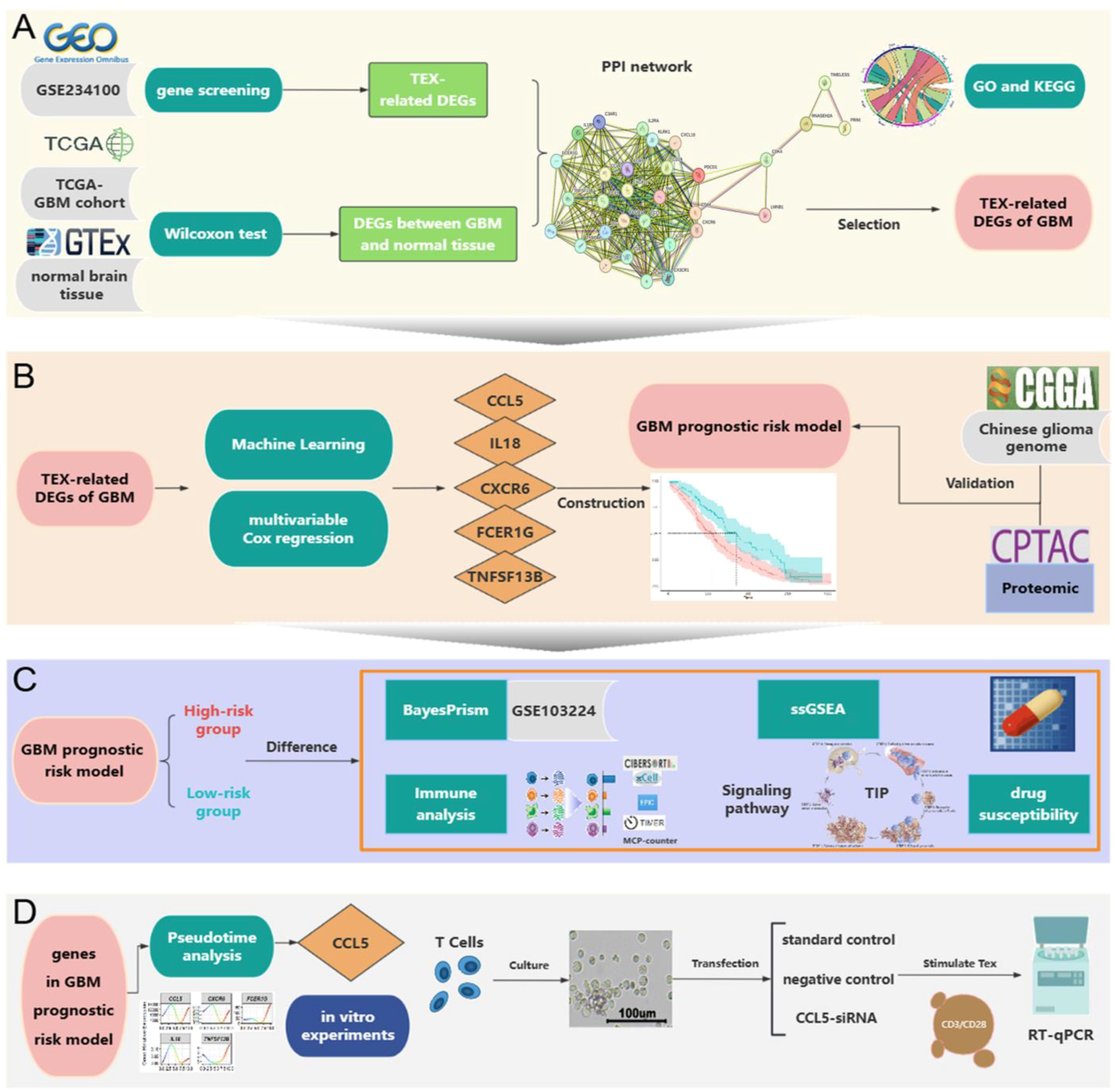
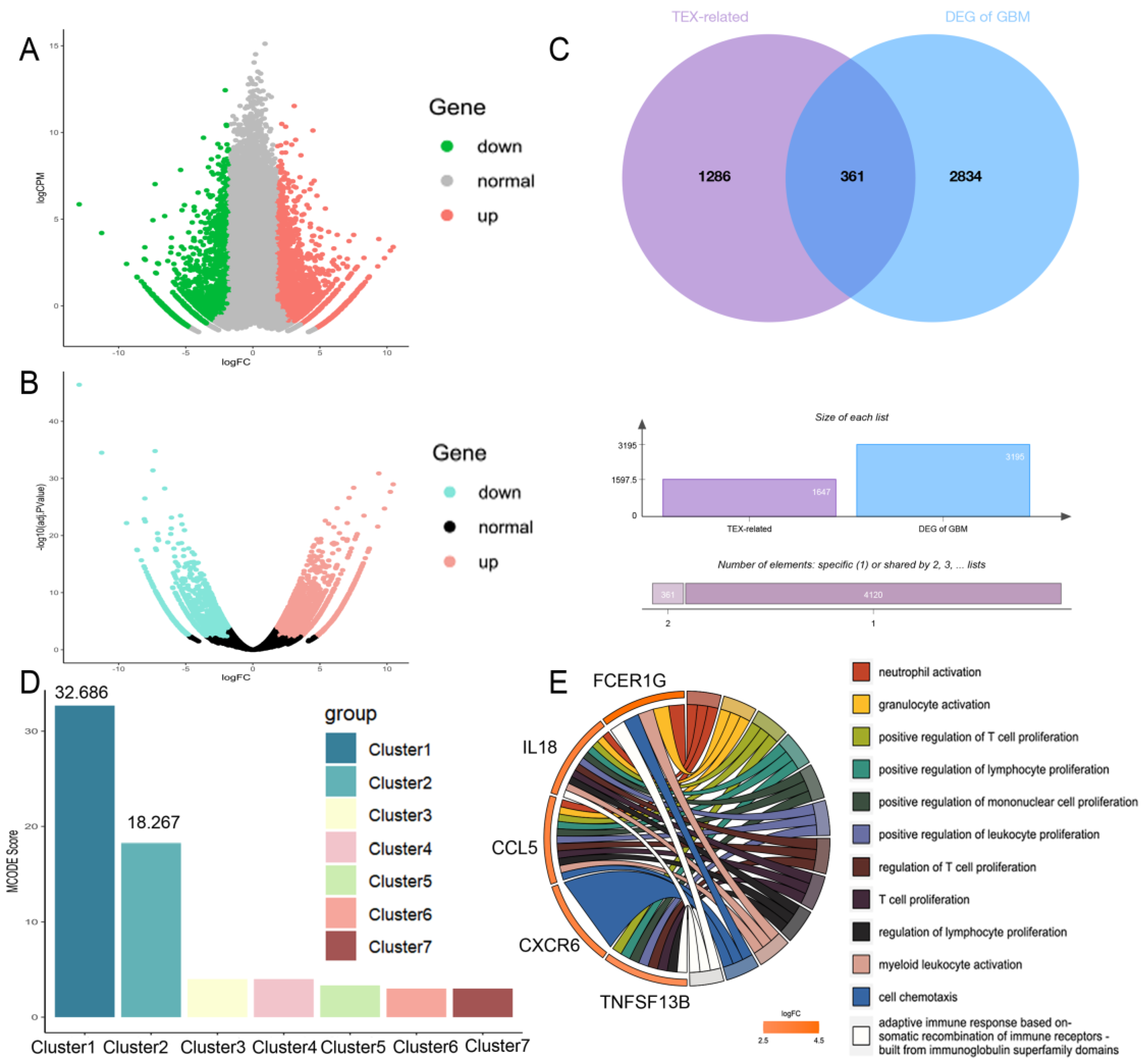
2.2. Construction of TEX-Related Gene Set in GBM
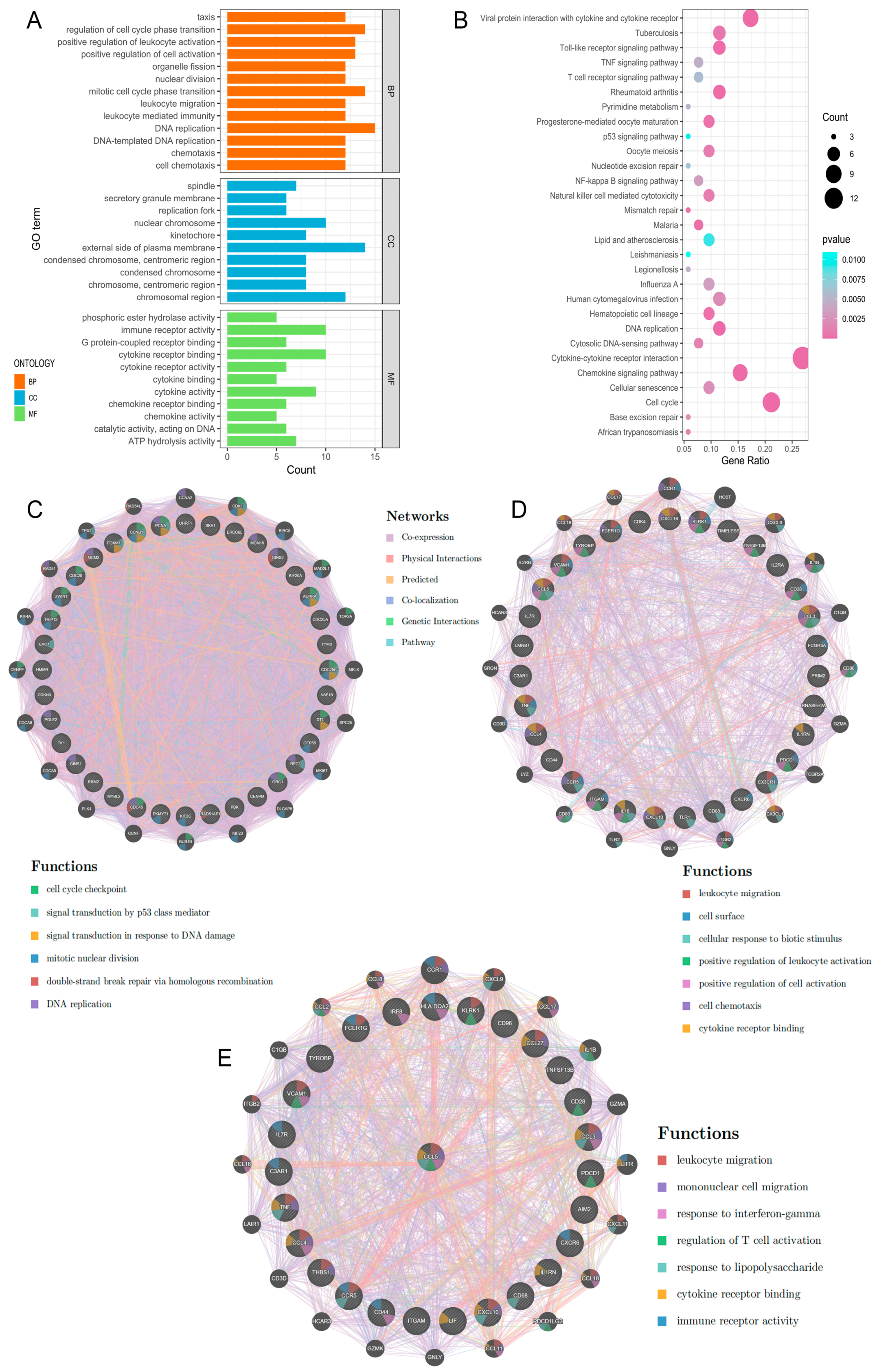
2.3. PPI Network Construction and Hub Gene Selection
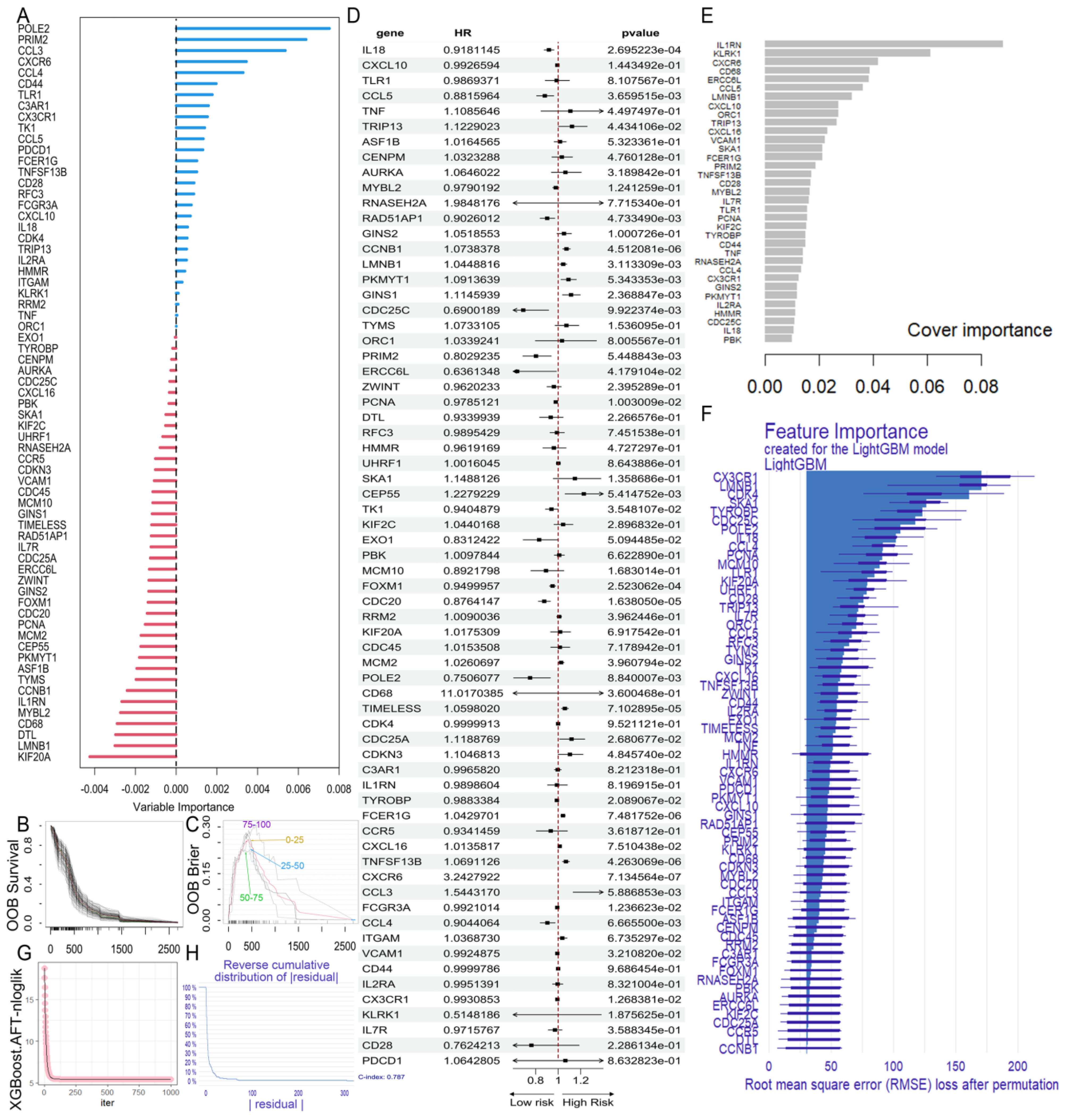
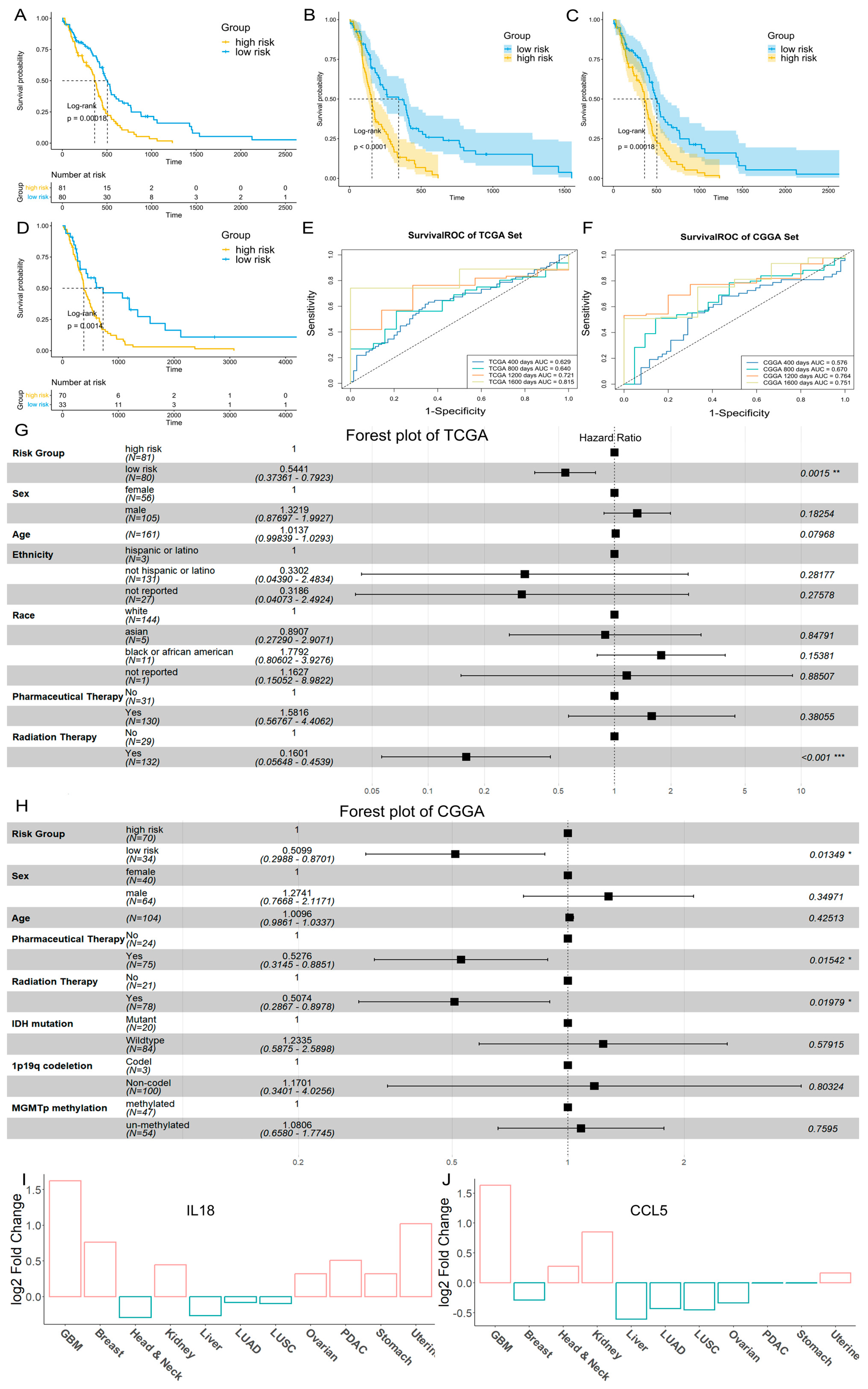
2.4. Construction of the GBM Prognostic Risk Model
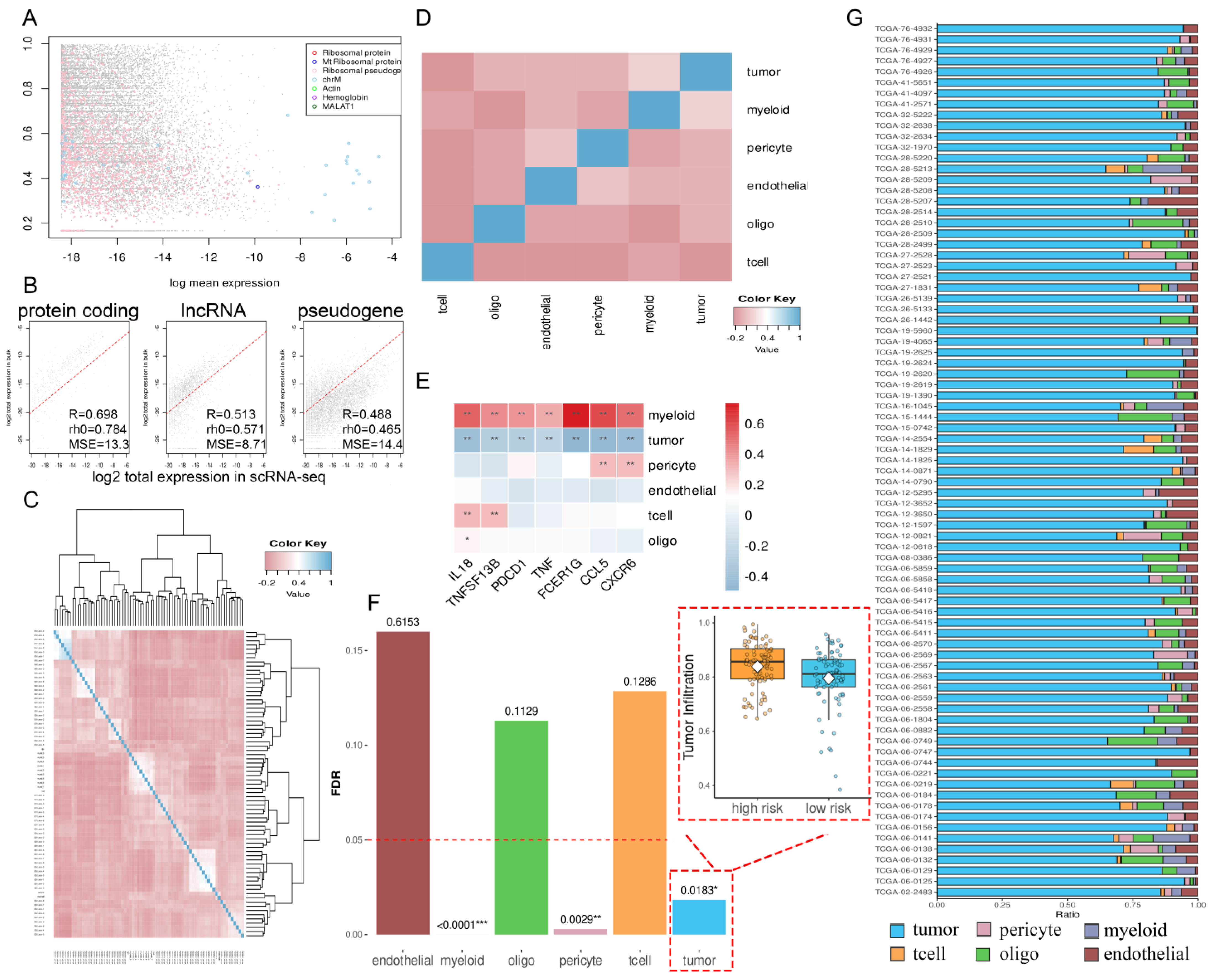
2.5. Proteomic Results
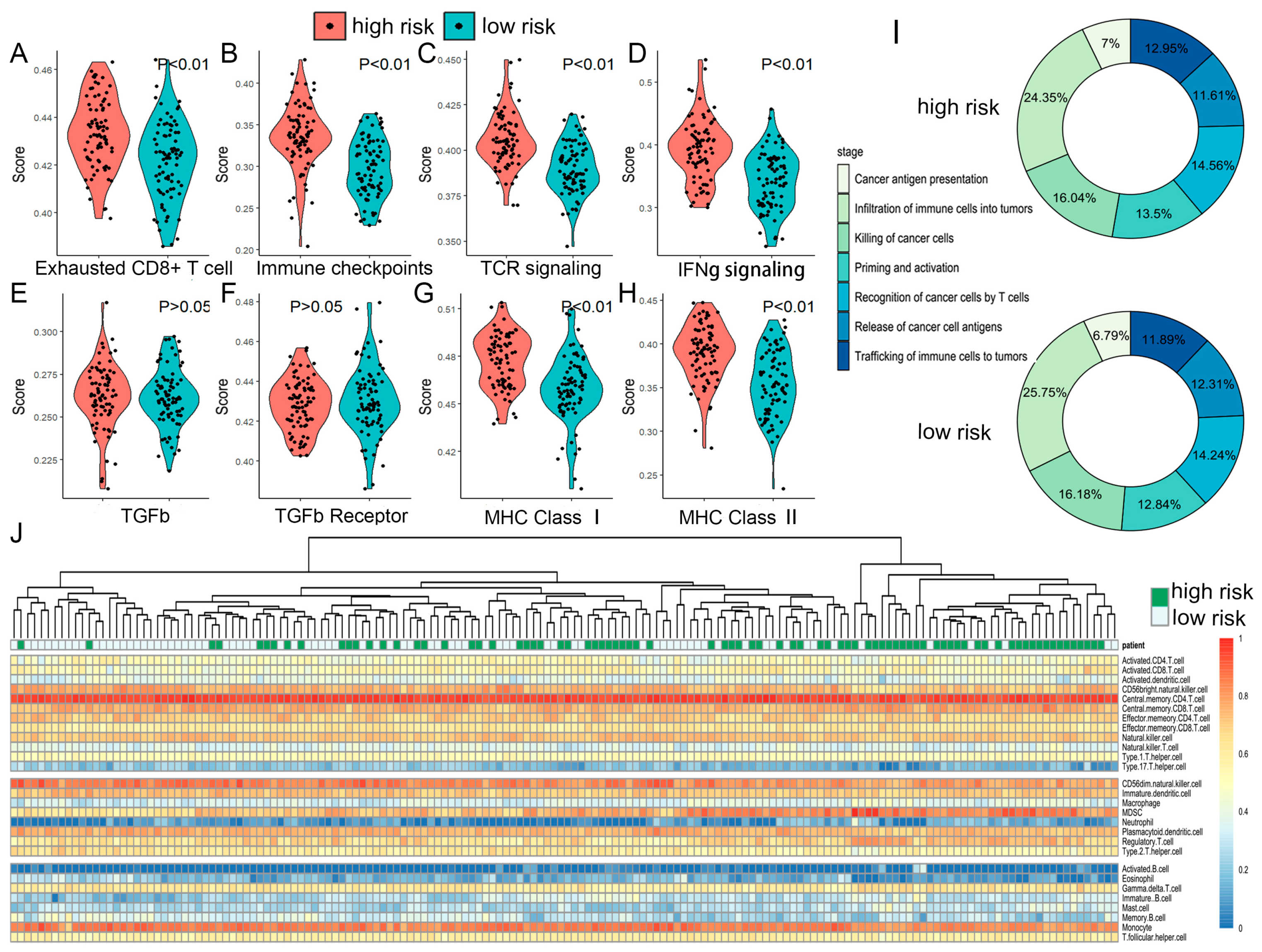
2.6. The TME and Functional Features of Two Risk Groups
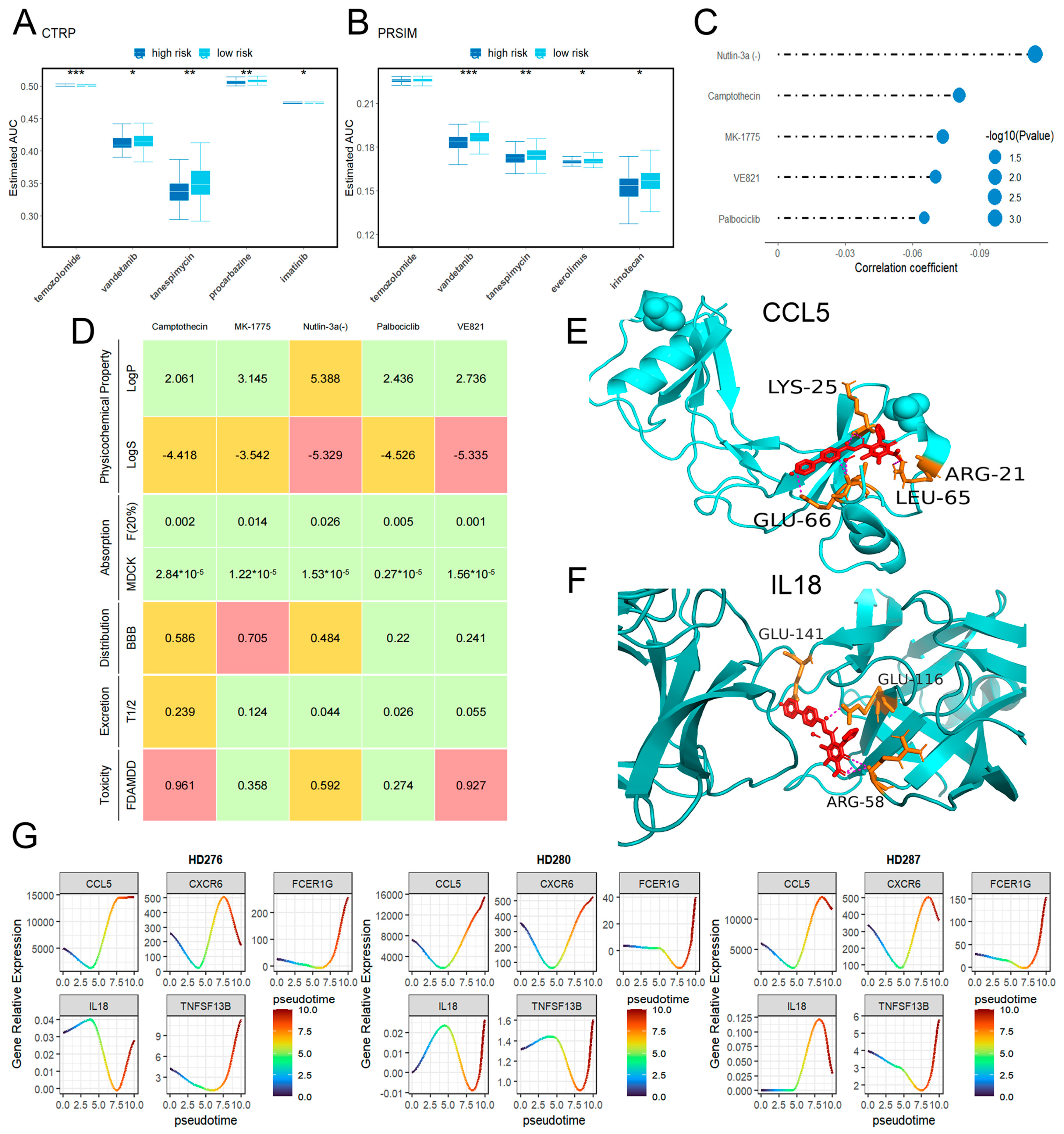
2.7. Drug Sensitivity
2.8. Pseudotime Analysis Results
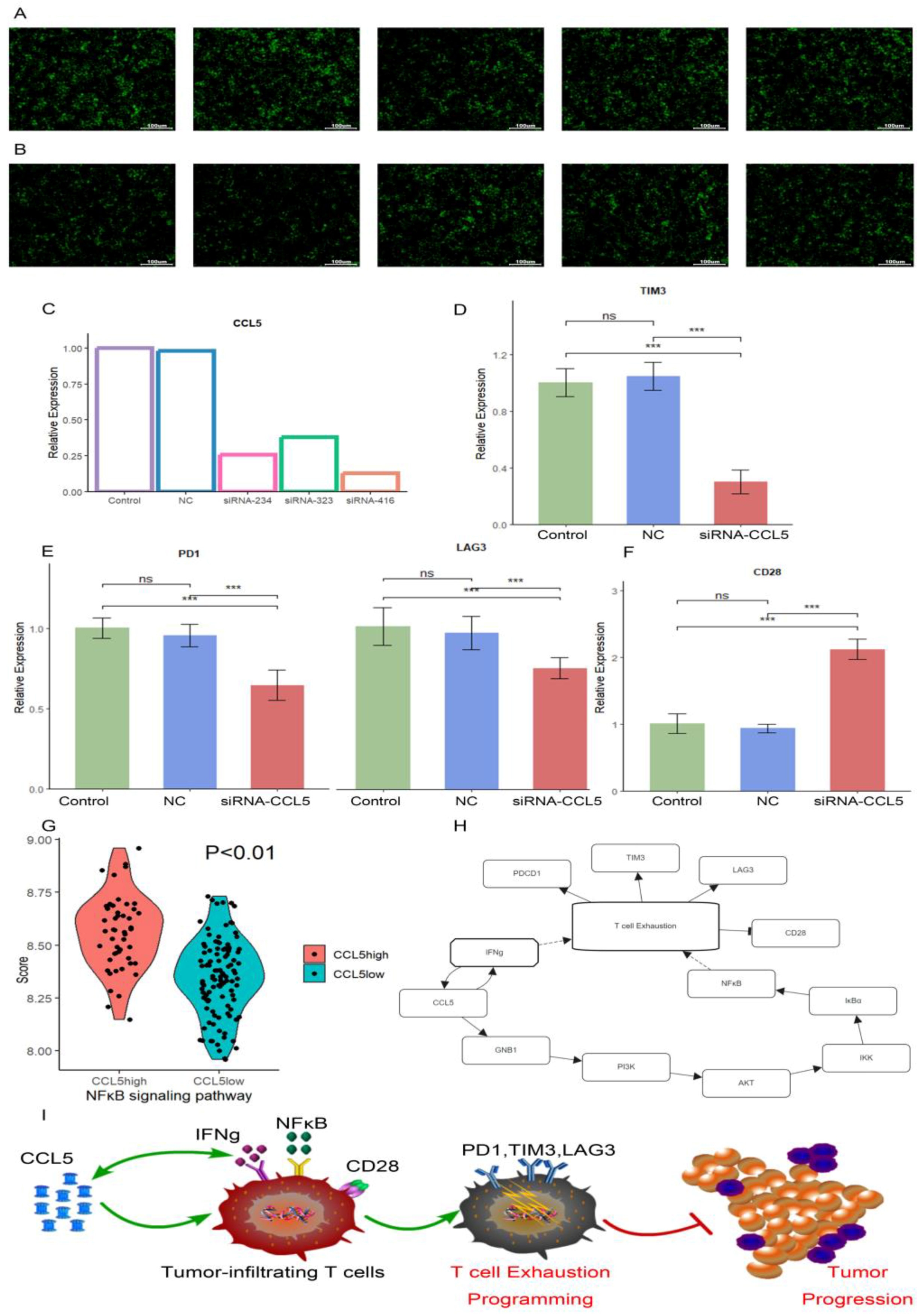
2.9. Verification by RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Dataset Collection
4.2. Mendelian Randomization
4.3. Identification of TEX-Related DEGs
4.4. Identification of Common DEGs in GBM
4.5. PPI Network, GO, and KEGG Analysis Based on Common DEGs
4.6. Survival Analysis
4.7. Proteomics Analysis
4.8. Cell Proportion Reconstruction and Function Analysis
4.9. Candidate Drug Analysis
4.10. Pseudotime Analysis
4.11. Transfection and Quantitative Real-Time Polymerase Chain Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GBM | Glioblastoma Multiforme |
| TEX | Exhausted T |
| PPI | Protein–protein interaction |
| scRNA-seq | Single-cell RNA sequencing |
| TIP | Tumor immune penetration |
| TME | Tumor microenvironment |
| OS | Overall survival times |
| PFS | Progression-free survival times |
| TMZ | Temozolomide |
| TEFF | Activated effector T cells |
| MR | Mendelian randomization |
| IVW | Inverse variance weighted |
| DEGs | Differentially expressed genes |
| FDR | False discovery rate |
| BCV | Biological coefficient of variation |
| ssGSEA | Single sample gene set enrichment analysis |
| k-NN | k-nearest neighbor |
| GWAS | Genome-wide association studies |
| VIMP | Variable Importance |
| AUC | Area under the ROC curve |
| Trest | Resting T cells |
| Ttumor | Tumor-infiltrating T cells |
| Temra | Terminally differentiated effector memory T cell |
| BBB | Blood–brain barrier |
References
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Baxter, A.E.; Huang, H.; Giles, J.R.; Chen, Z.; Wu, J.E.; Drury, S.; Dalton, K.; Park, S.L.; Torres, L.; Simone, B.W.; et al. The SWI/SNF chromatin remodeling complexes BAF and PBAF differentially regulate epigenetic transitions in exhausted CD8(+) T cells. Immunity 2023, 56, 1320–1340.e10. [Google Scholar] [CrossRef]
- Beltra, J.C.; Manne, S.; Abdel-Hakeem, M.S.; Kurachi, M.; Giles, J.R.; Chen, Z.; Casella, V.; Ngiow, S.F.; Khan, O.; Huang, Y.J.; et al. Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 2020, 52, 825–841.e8. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016, 354, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.; Perica, K.; Klebanoff, C.A.; Wolchok, J.D. Clinical implications of T cell exhaustion for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Castellani, G.; Croese, T.; Peralta Ramos, J.M.; Schwartz, M. Transforming the understanding of brain immunity. Science 2023, 380, eabo7649. [Google Scholar] [CrossRef]
- Noor, L.; Upadhyay, A.; Joshi, V. Role of T Lymphocytes in Glioma Immune Microenvironment: Two Sides of a Coin. Biology 2024, 13, 846. [Google Scholar] [CrossRef]
- Korn, T.; Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 2017, 17, 179–194. [Google Scholar] [CrossRef]
- Woroniecka, K.I.; Rhodin, K.E.; Dechant, C.; Cui, X.; Chongsathidkiet, P.; Wilkinson, D.; Waibl-Polania, J.; Sanchez-Perez, L.; Fecci, P.E. 4-1BB Agonism Averts TIL Exhaustion and Licenses PD-1 Blockade in Glioblastoma and Other Intracranial Cancers. Clin. Cancer Res. 2020, 26, 1349–1358. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016–2020. Neuro Oncol 2023, 25 (Suppl. S2), iv1–iv99. [Google Scholar] [CrossRef]
- Huang, W.; Wang, J.; Zhang, D.; Chen, W.; Hou, L.; Wu, X.; Lu, Y. Inhibition of KIF14 Suppresses Tumor Cell Growth and Promotes Apoptosis in Human Glioblastoma. Cell Physiol. Biochem. 2015, 37, 1659–1670. [Google Scholar] [CrossRef]
- Zeng, A.; Yin, J.; Li, Y.; Li, R.; Wang, Z.; Zhou, X.; Jin, X.; Shen, F.; Yan, W.; You, Y. miR-129-5p targets Wnt5a to block PKC/ERK/NF-kappaB and JNK pathways in glioblastoma. Cell Death Dis. 2018, 9, 394. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Boisserand, L.S.B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.-L.; Iwasaki, A. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Q.; Chen, Z.; Wu, M.; Zhang, C.; Su, J.; Li, Y.; Zhang, C. Hsa_circ_0110757 upregulates ITGA1 to facilitate temozolomide resistance in glioma by suppressing hsa-miR-1298-5p. Cell Death Dis. 2021, 12, 252. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Dos Santos Rodrigues, B.; Sun, C.; Singh, J. Biodistribution of TAT or QLPVM coupled to receptor targeted liposomes for delivery of anticancer therapeutics to brain in vitro and in vivo. Nanomedicine 2020, 23, 102112. [Google Scholar] [CrossRef] [PubMed]
- Ciechomska, I.A.; Wojnicki, K.; Wojtas, B.; Szadkowska, P.; Poleszak, K.; Kaza, B.; Jaskula, K.; Dawidczyk, W.; Czepko, R.; Banach, M.; et al. Exploring Novel Therapeutic Opportunities for Glioblastoma Using Patient-Derived Cell Cultures. Cancers 2023, 15, 1562. [Google Scholar] [CrossRef]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef]
- Allahmoradi, E.; Mohammadi, R.; Kheirandish Zarandi, P.; Alavian, S.M.; Heiat, M. The CD8+ T cell exhaustion mechanisms in chronic hepatitis B infection and immunotherapeutic strategies: A systematic review. Expert. Rev. Clin. Immunol. 2023, 19, 671–688. [Google Scholar] [CrossRef]
- Pham, T.N.; Spaulding, C.; Shields, M.A.; Metropulos, A.E.; Shah, D.N.; Khalafalla, M.G.; Principe, D.R.; Bentrem, D.J.; Munshi, H.G. Inhibition of MNKs promotes macrophage immunosuppressive phenotype to limit CD8+ T cell antitumor immunity. JCI Insight 2022, 7, e152731. [Google Scholar] [CrossRef]
- Xia, P.; Zhang, H.; Xu, K.; Jiang, X.; Gao, M.; Wang, G.; Liu, Y.; Yao, Y.; Chen, X.; Ma, W.; et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis. 2021, 12, 691. [Google Scholar] [CrossRef]
- van de Kooij, B.; Schreuder, A.; Pavani, R.; Garzero, V.; Uruci, S.; Wendel, T.J.; van Hoeck, A.; San Martin Alonso, M.; Everts, M.; Koerse, D.; et al. EXO1 protects BRCA1-deficient cells against toxic DNA lesions. Mol. Cell 2024, 84, 659–674.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, K.; Gray, S.; Zhang, Y.; Tang, C.; Morrish, R.B.; Tosti, E.; van Oers, J.; Amin, M.R.; Cohen, P.E.; et al. Role of EXO1 nuclease activity in genome maintenance, the immune response and tumor suppression in Exo1D173A mice. Nucleic Acids Res. 2022, 50, 8093–8106. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, A.O.; Wieland, A.; Nasti, T.; Yang, S.; Zhang, R.; Barber, D.L.; Konieczny, B.T.; Daugherty, C.Z.; Koenig, L.; Yu, K.; et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017, 355, 1423–1427. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef]
- Innocenti, F.; Cooper, G.M.; Stanaway, I.B.; Gamazon, E.R.; Smith, J.D.; Mirkov, S.; Ramirez, J.; Liu, W.; Lin, Y.S.; Moloney, C.; et al. Identification, replication, and functional fine-mapping of expression quantitative trait loci in primary human liver tissue. PLoS Genet. 2011, 7, e1002078. [Google Scholar] [CrossRef]
- Mallon, B.S.; Chenoweth, J.G.; Johnson, K.R.; Hamilton, R.S.; Tesar, P.J.; Yavatkar, A.S.; Tyson, L.J.; Park, K.; Chen, K.G.; Fann, Y.C.; et al. StemCellDB: The human pluripotent stem cell database at the National Institutes of Health. Stem Cell Res. 2013, 10, 57–66. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Liu, H.; Ding, K.; Shen, H.; Zhao, X.; Fu, R. Targeting NKG2D/NKG2DL axis in multiple myeloma therapy. Cytokine Growth Factor. Rev. 2024, 76, 1–11. [Google Scholar] [CrossRef]
- Vivier, E.; Nunes, J.A.; Vely, F. Natural killer cell signaling pathways. Science 2004, 306, 1517–1519. [Google Scholar] [CrossRef]
- Knopf, P.; Stowbur, D.; Hoffmann, S.H.L.; Hermann, N.; Maurer, A.; Bucher, V.; Poxleitner, M.; Tako, B.; Sonanini, D.; Krishnamachary, B.; et al. Acidosis-mediated increase in IFN-gamma-induced PD-L1 expression on cancer cells as an immune escape mechanism in solid tumors. Mol. Cancer 2023, 22, 207. [Google Scholar] [CrossRef]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef]
- Liu, C.; Yao, Z.; Wang, J.; Zhang, W.; Yang, Y.; Zhang, Y.; Qu, X.; Zhu, Y.; Zou, J.; Peng, S.; et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. 2020, 27, 1765–1781. [Google Scholar] [CrossRef] [PubMed]
- Mielcarska, S.; Kula, A.; Dawidowicz, M.; Kiczmer, P.; Chrabanska, M.; Rynkiewicz, M.; Wziatek-Kuczmik, D.; Swietochowska, E.; Waniczek, D. Assessment of the RANTES Level Correlation and Selected Inflammatory and Pro-Angiogenic Molecules Evaluation of Their Influence on CRC Clinical Features: A Preliminary Observational Study. Medicina 2022, 58, 203. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.Y.; Park, S.J.; Jo, E.; Hwang, I.-H.; Lee, K.-B.; Kim, S.-W.; Kim, D.J.; Joo, J.C.; Hong, S.H.; Lee, M.-G.; et al. Cordycepin induces apoptosis of human ovarian cancer cells by inhibiting CCL5-mediated Akt/NF-κB signaling pathway. Cell Death Discov. 2018, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Pichler, A.C.; Carrie, N.; Cuisinier, M.; Ghazali, S.; Voisin, A.; Axisa, P.P.; Tosolini, M.; Mazzotti, C.; Golec, D.P.; Maheo, S.; et al. TCR-independent CD137 (4-1BB) signaling promotes CD8(+)-exhausted T cell proliferation and terminal differentiation. Immunity 2023, 56, 1631–1648.e10. [Google Scholar] [CrossRef]
- Grinberg-Bleyer, Y.; Oh, H.; Desrichard, A.; Bhatt, D.M.; Caron, R.; Chan, T.A.; Schmid, R.M.; Klein, U.; Hayden, M.S.; Ghosh, S. NF-kappaB c-Rel Is Crucial for the Regulatory T Cell Immune Checkpoint in Cancer. Cell 2017, 170, 1096–1108.e13. [Google Scholar] [CrossRef]
- Dangaj, D.; Bruand, M.; Grimm, A.J.; Ronet, C.; Barras, D.; Duttagupta, P.A.; Lanitis, E.; Duraiswamy, J.; Tanyi, J.L.; Benencia, F.; et al. Cooperation between Constitutive and Inducible Chemokines Enables T Cell Engraftment and Immune Attack in Solid Tumors. Cancer Cell 2019, 35, 885–900.e10. [Google Scholar] [CrossRef]
- Pacioni, S.; D’Alessandris, Q.G.; Buccarelli, M.; Boe, A.; Martini, M.; Larocca, L.M.; Bolasco, G.; Ricci-Vitiani, L.; Falchetti, M.L.; Pallini, R. Brain Invasion along Perivascular Spaces by Glioma Cells: Relationship with Blood-Brain Barrier. Cancers 2019, 12, 18. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, C.; Shi, Y.; Wu, Q.; Gimple, R.C.; Fang, X.; Huang, Z.; Zhai, K.; Ke, S.Q.; Ping, Y.F.; et al. Targeting Glioma Stem Cell-Derived Pericytes Disrupts the Blood-Tumor Barrier and Improves Chemotherapeutic Efficacy. Cell Stem Cell 2017, 21, 591–603.e4. [Google Scholar] [CrossRef]
- Hart, M.G.; Garside, R.; Rogers, G.; Stein, K.; Grant, R. Temozolomide for high grade glioma. Cochrane Database Syst. Rev. 2013, 2013, CD007415. [Google Scholar] [CrossRef]
- Zhang, J.; Stevens, M.F.; Bradshaw, T.D. Temozolomide: Mechanisms of action, repair and resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, Y.; Luo, L.; You, Z.; Chen, H.; Wang, J.; Zhang, F.; Liu, Y.; Ke, Y. Glioblastoma stem cells deliver ABCB4 transcribed by ATF3 via exosomes conferring glioblastoma resistance to temozolomide. Cell Death Dis. 2024, 15, 318. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Q.; Gao, G.; Cao, Z.; Guan, Z.; Jia, B.; Wang, W.; Zhang, K.; Zhang, W.; Wang, S.; et al. Exosome-transmitted circCABIN1 promotes temozolomide resistance in glioblastoma via sustaining ErbB downstream signaling. J. Nanobiotechnol. 2023, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Peralta, R.M.; Xie, B.; Lontos, K.; Nieves-Rosado, H.; Spahr, K.; Joshi, S.; Ford, B.R.; Quann, K.; Frisch, A.T.; Dean, V.; et al. Dysfunction of exhausted T cells is enforced by MCT11-mediated lactate metabolism. Nat. Immunol. 2024, 25, 2297–2307. [Google Scholar] [CrossRef]
- Jeon, S.H.; You, G.; Park, J.; Chung, Y.; Park, K.; Kim, H.; Jeon, J.; Kim, Y.; Son, W.-C.; Jeong, D.S.; et al. Anti–4-1BB×PDL1 Bispecific Antibody Reinvigorates Tumor-Specific Exhausted CD8+ T Cells and Enhances the Efficacy of Anti-PD1 Blockade. Clin. Cancer Res. 2024, 30, 4155–4166. [Google Scholar] [CrossRef]
- Zhang, X.F.; Zhang, X.L.; Wang, Y.J.; Fang, Y.; Li, M.L.; Liu, X.Y.; Luo, H.Y.; Tian, Y. The regulatory network of the chemokine CCL5 in colorectal cancer. Ann. Med. 2023, 55, 2205168. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Peng, X.; Chen, C.; Chen, J.; Zhong, L.; Li, Y.; Sui, S. High expression of CCL3/CCL4/CCL5/CCR5 promotes exhausted CD8(+) T cells terminal differentiation and is associated with poor prognosis in pediatric B-ALL patients. Int. J. Immunopathol. Pharmacol. 2025, 39, 3946320251346823. [Google Scholar] [CrossRef]
- Kaplanski, G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol. Rev. 2018, 281, 138–153. [Google Scholar] [CrossRef]
- Lesch, S.; Blumenberg, V.; Stoiber, S.; Gottschlich, A.; Ogonek, J.; Cadilha, B.L.; Dantes, Z.; Rataj, F.; Dorman, K.; Lutz, J.; et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat. Biomed. Eng. 2021, 5, 1246–1260. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Liang, L.; Ao, S.; Zhang, J.; Chang, Z.; Wang, Z.; Zhou, Y.; Duan, X.; Deng, T. Fc Fragment of IgE Receptor Ig (FCER1G) acts as a key gene involved in cancer immune infiltration and tumour microenvironment. Immunology 2023, 168, 302–319. [Google Scholar] [CrossRef]
- Ma, T.; Meng, L.; Wang, X.; Tian, Z.; Wang, J.; Liu, X.; Zhang, W.; Zhang, Y. TNFSF13B and PPARGC1A expression is associated with tumor-infiltrating immune cell abundance and prognosis in clear cell renal cell carcinoma. Am. J. Transl. Res. 2021, 13, 11048–11064. [Google Scholar] [PubMed]
- Abusanad, A.; Al Hashem, H. A Substantial Response from Adding Palbociclib to Endocrine Therapy in Brain Metastasis from Hormone Receptor-Positive, HER2-Negative Breast Cancer: Case Reports. Case Rep. Oncol. 2021, 14, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Steiger, H.J.; Vollmer, K.; Rogers, S.; Schwyzer, L. State of affairs regarding targeted pharmacological therapy of cancers metastasized to the brain. Neurosurg. Rev. 2022, 45, 3119–3138. [Google Scholar] [CrossRef] [PubMed]
- Trefny, M.P.; Kirchhammer, N.; Auf der Maur, P.; Natoli, M.; Schmid, D.; Germann, M.; Fernandez Rodriguez, L.; Herzig, P.; Lotscher, J.; Akrami, M.; et al. Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy. Nat. Commun. 2023, 14, 86. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zeng, Y.; Chen, K.; Zhang, Q.; Wang, L.; Huang, Y.; Jian, S. The significance of CD8(+) tumor-infiltrating lymphocytes exhaustion heterogeneity and its underlying mechanism in diffuse large B-cell lymphoma. Int. Immunopharmacol. 2024, 137, 112447. [Google Scholar] [CrossRef]
- Melese, E.S.; Franks, E.; Cederberg, R.A.; Harbourne, B.T.; Shi, R.; Wadsworth, B.J.; Collier, J.L.; Halvorsen, E.C.; Johnson, F.; Luu, J.; et al. CCL5 production in lung cancer cells leads to an altered immune microenvironment and promotes tumor development. Oncoimmunology 2022, 11, 2010905. [Google Scholar] [CrossRef]
- Araujo, J.M.; Gomez, A.C.; Aguilar, A.; Salgado, R.; Balko, J.M.; Bravo, L.; Doimi, F.; Bretel, D.; Morante, Z.; Flores, C.; et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci. Rep. 2018, 8, 4899. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Matsuura, Y.; Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res. 2024, 53, D672–D677. [Google Scholar] [CrossRef]
- Sollis, E.; Mosaku, A.; Abid, A.; Buniello, A.; Cerezo, M.; Gil, L.; Groza, T.; Gunes, O.; Hall, P.; Hayhurst, J.; et al. The NHGRI-EBI GWAS Catalog: Knowledgebase and deposition resource. Nucleic Acids Res. 2023, 51, D977–D985. [Google Scholar] [CrossRef]
- Melin, B.S.; Barnholtz-Sloan, J.S.; Wrensch, M.R.; Johansen, C.; Il’yasova, D.; Kinnersley, B.; Ostrom, Q.T.; Labreche, K.; Chen, Y.; Armstrong, G.; et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat. Genet. 2017, 49, 789–794. [Google Scholar] [CrossRef]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018, 7, e34408. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Glioma Patients. Genom. Proteom. Bioinform. 2021, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, F.; Wang, W.; Wang, Z.; Zhang, C.; Jiang, T. Comprehensive RNA-seq transcriptomic profiling in the malignant progression of gliomas. Sci. Data 2017, 4, 170024. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.S.; Chen, H.M.; Yang, M.Y.; Zhang, C.B.; Yu, K.; Ye, W.L.; Hu, B.Q.; Yan, W.; Zhang, W.; Akers, J.; et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome Res. 2014, 24, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.T. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science 2015, 348, 648–660. [Google Scholar] [CrossRef]
- Gillotay, P.; Shankar, M.; Haerlingen, B.; Sema Elif, E.; Pozo-Morales, M.; Garteizgogeascoa, I.; Reinhardt, S.; Krankel, A.; Blasche, J.; Petzold, A.; et al. Single-cell transcriptome analysis reveals thyrocyte diversity in the zebrafish thyroid gland. EMBO Rep. 2020, 21, e50612. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, H.; Zhu, Y.; Hong, Y.; Zha, J.; Lin, Z.; Li, Z.; Wang, C.; Fang, Z.; Yu, X.; et al. Loss of CD28 expression associates with severe T-cell exhaustion in acute myeloid leukemia. Front. Immunol. 2023, 14, 1139517. [Google Scholar] [CrossRef]
- Akbar, A.N.; Terry, L.; Timms, A.; Beverley, P.C.; Janossy, G. Loss of CD45RA and gain of UCHL1 reactivity is a feature of primed T cells. J. Immunol. 1988, 140, 2171–2178. [Google Scholar] [CrossRef]
- Gattinoni, L.; Lugli, E.; Ji, Y.; Pos, Z.; Paulos, C.M.; Quigley, M.F.; Almeida, J.R.; Gostick, E.; Yu, Z.; Carpenito, C.; et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011, 17, 1290–1297. [Google Scholar] [CrossRef]
- Palmer, T.M.; Lawlor, D.A.; Harbord, R.M.; Sheehan, N.A.; Tobias, J.H.; Timpson, N.J.; Davey Smith, G.; Sterne, J.A. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat. Methods Med. Res. 2012, 21, 223–242. [Google Scholar] [CrossRef]
- Levin, M.G.; Judy, R.; Gill, D.; Vujkovic, M.; Verma, S.S.; Bradford, Y.; Regeneron Genetics, C.; Ritchie, M.D.; Hyman, M.C.; Nazarian, S.; et al. Genetics of height and risk of atrial fibrillation: A Mendelian randomization study. PLoS Med. 2020, 17, e1003288. [Google Scholar] [CrossRef]
- Zhong, S.; Yang, W.; Zhang, Z.; Xie, Y.; Pan, L.; Ren, J.; Ren, F.; Li, Y.; Xie, H.; Chen, H.; et al. Association between viral infections and glioma risk: A two-sample bidirectional Mendelian randomization analysis. BMC Med. 2023, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lun, A.T.; Smyth, G.K. From reads to genes to pathways: Differential expression analysis of RNA-Seq experiments using Rsubread and the edgeR quasi-likelihood pipeline. F1000Res 2016, 5, 1438. [Google Scholar] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Kersten, K.; Hu, K.H.; Combes, A.J.; Samad, B.; Harwin, T.; Ray, A.; Rao, A.A.; Cai, E.; Marchuk, K.; Artichoker, J.; et al. Spatiotemporal co-dependency between macrophages and exhausted CD8(+) T cells in cancer. Cancer Cell 2022, 40, 624–638.e9. [Google Scholar] [CrossRef]
- Li, Y.; Ge, X.; Peng, F.; Li, W.; Li, J.J. Exaggerated false positives by popular differential expression methods when analyzing human population samples. Genome Biol. 2022, 23, 79. [Google Scholar] [CrossRef]
- Ishwaran, H.; Kogalur, U.B.; Blackstone, E.H.; Lauer, M.S. Random survival forests. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2008, 2, 841–860. [Google Scholar] [CrossRef]
- Chen, T.; Guestrin, C. XGBoost: A Scalable Tree Boosting System. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 785–794. [Google Scholar]
- Zhang, J.; Mucs, D.; Norinder, U.; Svensson, F. LightGBM: An Effective and Scalable Algorithm for Prediction of Chemical Toxicity-Application to the Tox21 and Mutagenicity Data Sets. J. Chem. Inf. Model. 2019, 59, 4150–4158. [Google Scholar] [CrossRef]
- Thangudu, R.R.; Rudnick, P.A.; Holck, M.; Singhal, D.; MacCoss, M.J.; Edwards, N.J.; Ketchum, K.A.; Kinsinger, C.R.; Kim, E.; Basu, A. Abstract LB-242: Proteomic Data Commons: A resource for proteogenomic analysis. Cancer Res. 2020, 80 (Suppl. S16), LB-242. [Google Scholar] [CrossRef]
- Chu, T.; Wang, Z.; Pe’er, D.; Danko, C.G. Cell type and gene expression deconvolution with BayesPrism enables Bayesian integrative analysis across bulk and single-cell RNA sequencing in oncology. Nat. Cancer 2022, 3, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.M.; Liu, C.L.; Green, M.R.; Gentles, A.J.; Feng, W.; Xu, Y.; Hoang, C.D.; Diehn, M.; Alizadeh, A.A. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 2015, 12, 453–457. [Google Scholar] [CrossRef]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef]
- Racle, J.; Gfeller, D. EPIC: A Tool to Estimate the Proportions of Different Cell Types from Bulk Gene Expression Data. Methods Mol. Biol. 2020, 2120, 233–248. [Google Scholar]
- Racle, J.; de Jonge, K.; Baumgaertner, P.; Speiser, D.E.; Gfeller, D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife 2017, 6, e26476. [Google Scholar] [CrossRef]
- Lindskrog, S.V.; Prip, F.; Lamy, P.; Taber, A.; Groeneveld, C.S.; Birkenkamp-Demtroder, K.; Jensen, J.B.; Strandgaard, T.; Nordentoft, I.; Christensen, E.; et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat. Commun. 2021, 12, 2301. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef]
- Shi, R.; Wang, X.; Wu, Y.; Xu, B.; Zhao, T.; Trapp, C.; Wang, X.; Unger, K.; Zhou, C.; Lu, S.; et al. APOBEC-mediated mutagenesis is a favorable predictor of prognosis and immunotherapy for bladder cancer patients: Evidence from pan-cancer analysis and multiple databases. Theranostics 2022, 12, 4181–4199. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Y.; Chen, J.; Lin, X.; Zhang, H.; Wang, H.; Wang, H.; Bie, X.; Jiang, J.; Feng, X.; et al. Dynamic chromatin regulatory programs during embryogenesis of hexaploid wheat. Genome Biol. 2023, 24, 7. [Google Scholar] [CrossRef]
- Liu, X.; Bie, X.M.; Lin, X.; Li, M.; Wang, H.; Zhang, X.; Yang, Y.; Zhang, C.; Zhang, X.S.; Xiao, J. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 2023, 9, 908–925. [Google Scholar] [CrossRef]
- Corselli, M.; Saksena, S.; Nakamoto, M.; Lomas III, W.E.; Taylor, I.; Chattopadhyay, P.K. Single cell multiomic analysis of T cell exhaustion in vitro. Cytom. Part A 2022, 101, 27–44. [Google Scholar] [CrossRef]
- Wu, J.E.; Manne, S.; Ngiow, S.F.; Baxter, A.E.; Huang, H.; Freilich, E.; Clark, M.L.; Lee, J.H.; Chen, Z.; Khan, O.; et al. In Vitro modeling of CD8+ T cell exhaustion enables CRISPR screening to reveal a role for BHLHE40. Sci. Immunol. 2023, 8, eade3369. [Google Scholar] [CrossRef]

| Outcome | β | Se | p-Value | |
|---|---|---|---|---|
| meta-analysis of published GWAS (glioma) | ||||
| CD28− CD8+ T cell Absolute Count | −0.0482 | 0.0299 | 0.1062 | |
| CD28− CD8+ T cell % CD8+ T cell | −0.0471 | 0.0255 | 0.0652 | |
| CD45RA− CD28− CD8+ T cell Absolute Count | −5.3750 | 3.2070 | 0.0937 | |
| CD45RA− CD28− CD8+ T cell % CD8+ T cell | −0.7579 | 0.3340 | 0.0233 * | |
| UCSF/Mayo study (GBM) | ||||
| CD28− CD8+ T cell Absolute Count | −0.0990 | 0.0535 | 0.0642 | |
| CD28− CD8+ T cell % CD8+ T cell | −0.0964 | 0.0481 | 0.0449 * | |
| CD45RA− CD28− CD8+ T cell Absolute Count | −11.9088 | 5.6194 | 0.0341 * | |
| CD45RA− CD28− CD8+ T cell % CD8+ T cell | −1.0279 | 0.6422 | 0.1095 | |
| GICC study (GBM) | ||||
| CD28− CD8+ T cell Absolute Count | −0.0603 | 0.0348 | 0.0833 | |
| CD28− CD8+ T cell % CD8+ T cell | −0.0547 | 0.0256 | 0.0329 * | |
| CD45RA− CD28− CD8+ T cell Absolute Count | −4.2093 | 3.1080 | 0.1756 | |
| CD45RA− CD28− CD8+ T cell % CD8+ T cell | −0.3285 | 0.3367 | 0.3293 |
| Gene | Ensembl IDs | Location | Hazard Ratios | 95% CI | p-Value |
|---|---|---|---|---|---|
| IL18 | ENSG00000150782 | Chromosome 11: 112,143,253–112,164,096 reverse strand | 0.9181 | 0.8769, 0.9613 | 0.0003 |
| CXCR6 | ENSG00000172215 | Chromosome 3: 45,940,933–45,948,351 forward strand | 3.2428 | 2.0367, 5.1630 | p < 0.0001 |
| CCL5 | ENSG00000271503 | Chromosome 17: 35,871,491–35,880,793 reverse strand | 0.8816 | 0.8098, 0.9598 | 0.0037 |
| FCER1G | ENSG00000158869 | Chromosome 1: 161,215,234–161,220,699 forward strand | 1.0430 | 1.0239, 1.0623 | p < 0.0001 |
| TNFSF13B | ENSG00000102524 | Chromosome 13: 108,251,240–108,308,484 forward strand | 1.0691 | 1.0391, 1.1000 | p < 0.0001 |
| Data Name | Database | Type | Detail | Data |
|---|---|---|---|---|
| Meta-analysis of published GWAS about glioma | NHGRI-EBI GWAS catalog | GWAS | A meta-analysis of published GWAS covering phenotypes of non-glioblastoma glioma, glioma, and glioma (high-grade) [61] | 28 March 2024 |
| GICC study (GBM) | Glioma International Case Control Consortium | GWAS | A GWAS of 4572 cases and 3286 controls performed by the Glioma International Case Control Consortium [62] | |
| UCSF/Mayo study (GBM) | University of California, San Francisco (UCSF)-Mayo | GWAS | A GWAS of 1591 cases and 804 controls from the University of California, San Francisco (UCSF)-Mayo [62] | |
| Public GWAS related to TEX | MR Base GWAS catalog | GWAS | A report about 731 immune cell traits in a cohort of 3757 Sardinians [63] (GCST90001686,GCST90001687,GCST90001695,GCST90001696) | 28 March 2024 |
| TCGA-GBM | TCGA | bulk RNA-seq | The project of The Cancer Genome Atlas included 167 GBM patients | 15 November 2023 |
| GSE234100 | GEO | bulk RNA-seq | Primary human T cells from three healthy donors were TCR-transduced and stimulated with cognate antigen (NY-ESO-1) to generate effector cells (TEFF, 1× stimulation) and exhausted cells (TEX, 4× stimulation) [19] | 1 July 2023 |
| GSE103224 | GEO | single-cell RNA-seq | Performed single-cell RNA-seq on tens of thousands of dissociated high-grade glioma tissue cells from 8 human patients [68] | 2 July 2018 |
| GSE210534 | GEO | bulk RNA-seq | Four human healthy donor T cells were isolated, transduced with an NY-ESO-1 TCR lentivirus construct, stimulated in four different conditions (Trested, Ttumor, TEX, Teff) [56] | 7 November 2022 |
| CGGA.mRNAseq_325.ReadCounts-genes | CGGA | bulk RNA-seq | The first batch of sequencing data released by Chinese Glioma Genome Atlas includes 325 samples from Chinese cohort [64,65,66] | 20 June 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Hua, M.; Wang, Y.; Zhang, Q.; Cao, Y.; Dai, Y.; Ma, C.; Zheng, X.; Ge, K.; Zhang, H.; et al. Multiomics Investigation of Exhausted T Cells in Glioblastoma Tumor Microenvironment: CCL5 as a Prognostic and Therapeutic Target. Int. J. Mol. Sci. 2025, 26, 9920. https://doi.org/10.3390/ijms26209920
Qin R, Hua M, Wang Y, Zhang Q, Cao Y, Dai Y, Ma C, Zheng X, Ge K, Zhang H, et al. Multiomics Investigation of Exhausted T Cells in Glioblastoma Tumor Microenvironment: CCL5 as a Prognostic and Therapeutic Target. International Journal of Molecular Sciences. 2025; 26(20):9920. https://doi.org/10.3390/ijms26209920
Chicago/Turabian StyleQin, Ruihao, Menglei Hua, Yaru Wang, Qi Zhang, Yong Cao, Yanyan Dai, Chenjing Ma, Xiaohan Zheng, Kaiyuan Ge, Huimin Zhang, and et al. 2025. "Multiomics Investigation of Exhausted T Cells in Glioblastoma Tumor Microenvironment: CCL5 as a Prognostic and Therapeutic Target" International Journal of Molecular Sciences 26, no. 20: 9920. https://doi.org/10.3390/ijms26209920
APA StyleQin, R., Hua, M., Wang, Y., Zhang, Q., Cao, Y., Dai, Y., Ma, C., Zheng, X., Ge, K., Zhang, H., Li, S., Liu, Y., Cao, L., & Wang, L. (2025). Multiomics Investigation of Exhausted T Cells in Glioblastoma Tumor Microenvironment: CCL5 as a Prognostic and Therapeutic Target. International Journal of Molecular Sciences, 26(20), 9920. https://doi.org/10.3390/ijms26209920





