Rat Glioma 101.8 Tissue Strain: Molecular and Morphological Features
Abstract
1. Introduction
2. Results
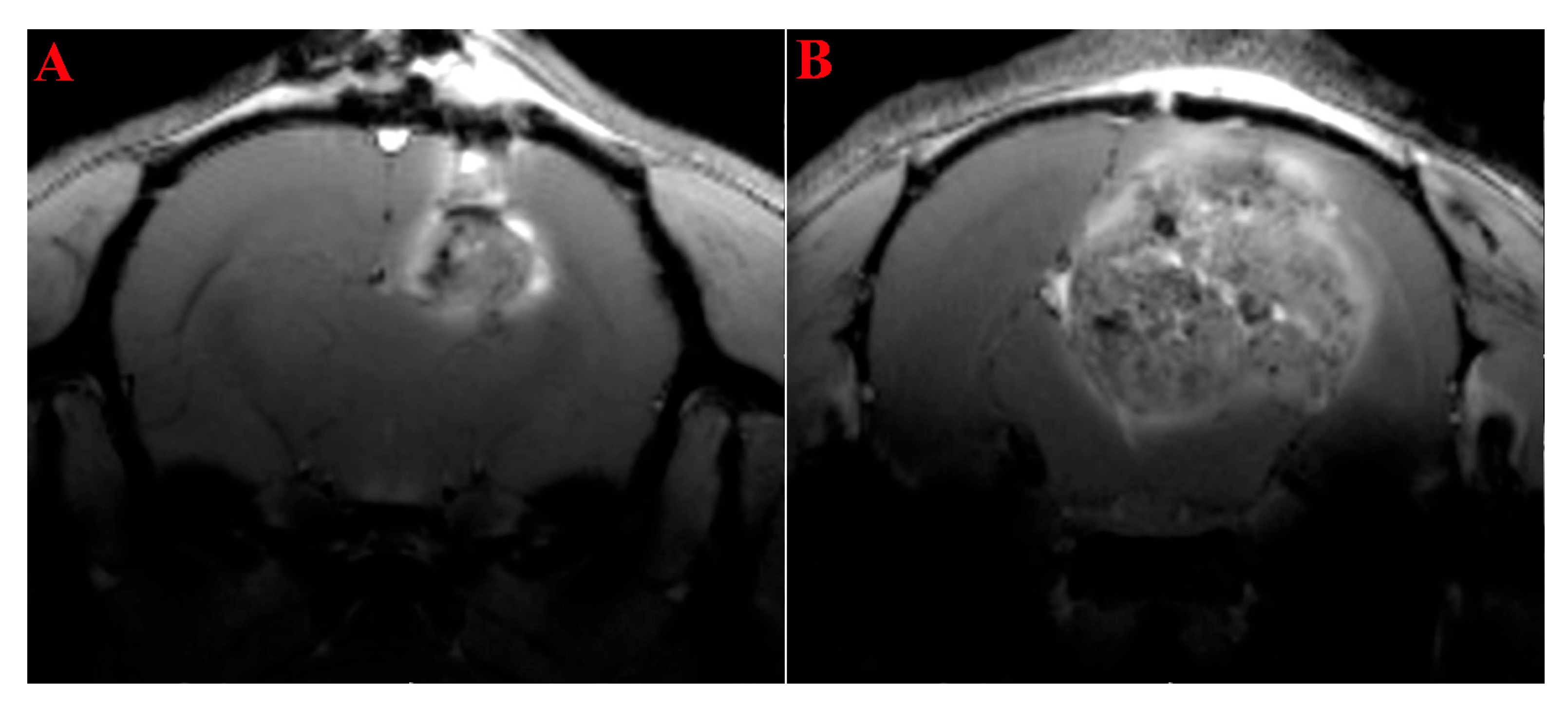
2.1. MRI Study of the Rats’ Brain with Transplanted Glioma 101.8
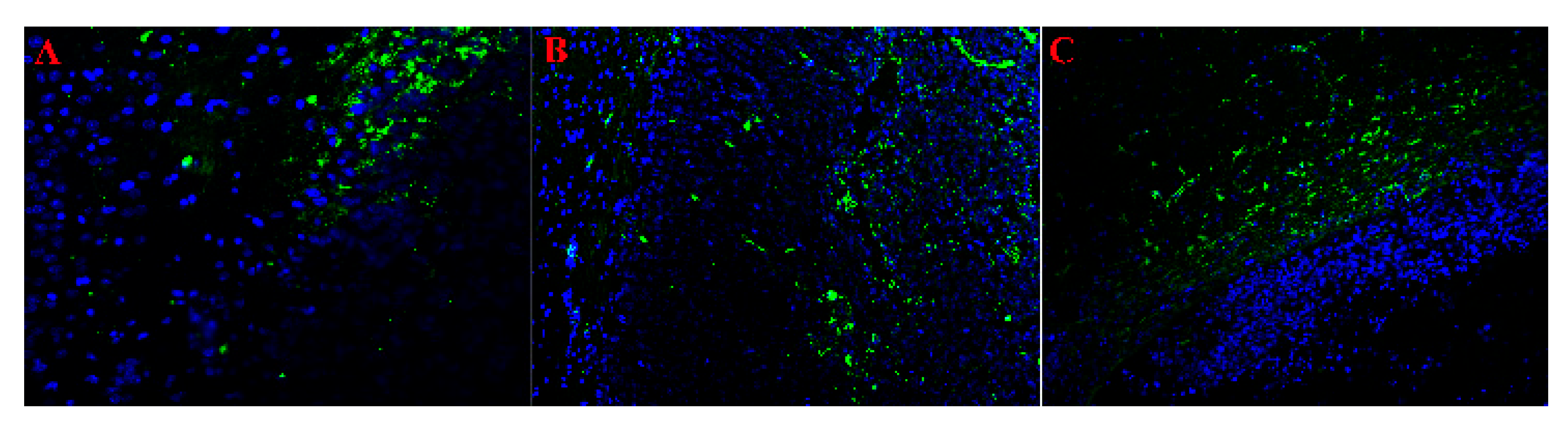
2.2. Histologic Characterization of GB 101.8
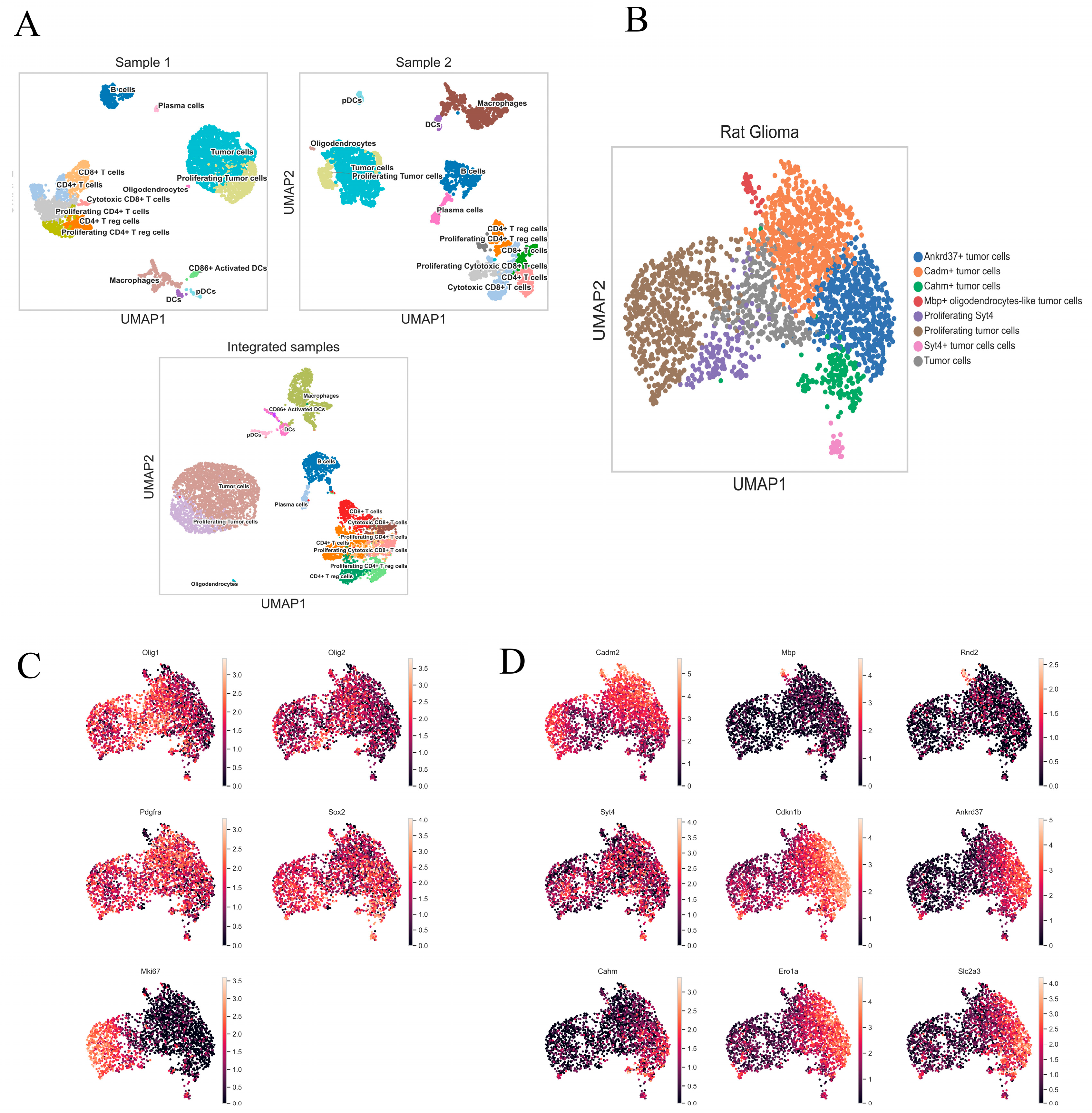
2.3. Characterization of Rat Glioma 101.8 Cell Subpopulations and Their Microenvironment
2.4. Integrated Heterogeneity Analysis of the Tumor Cluster
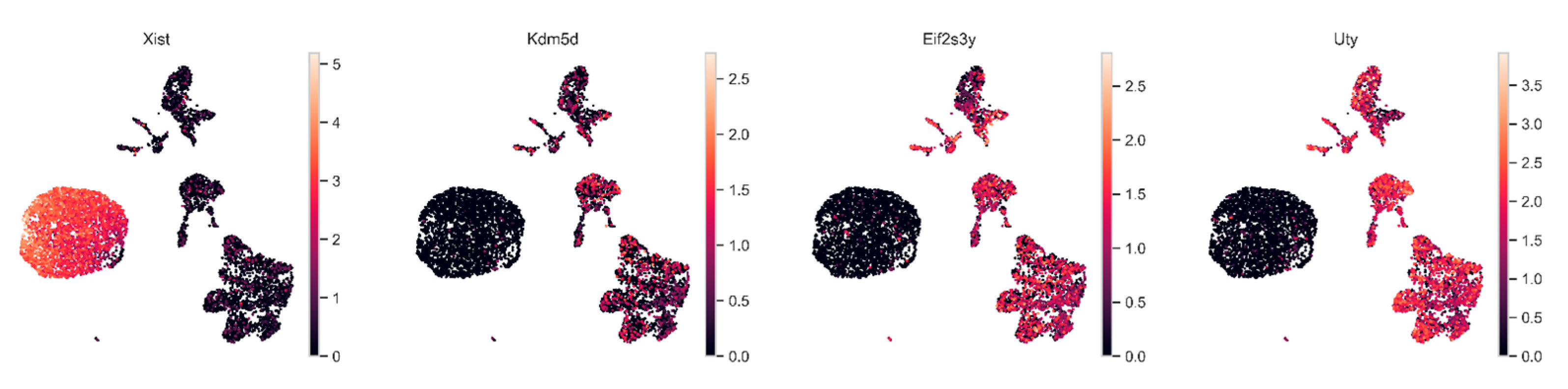
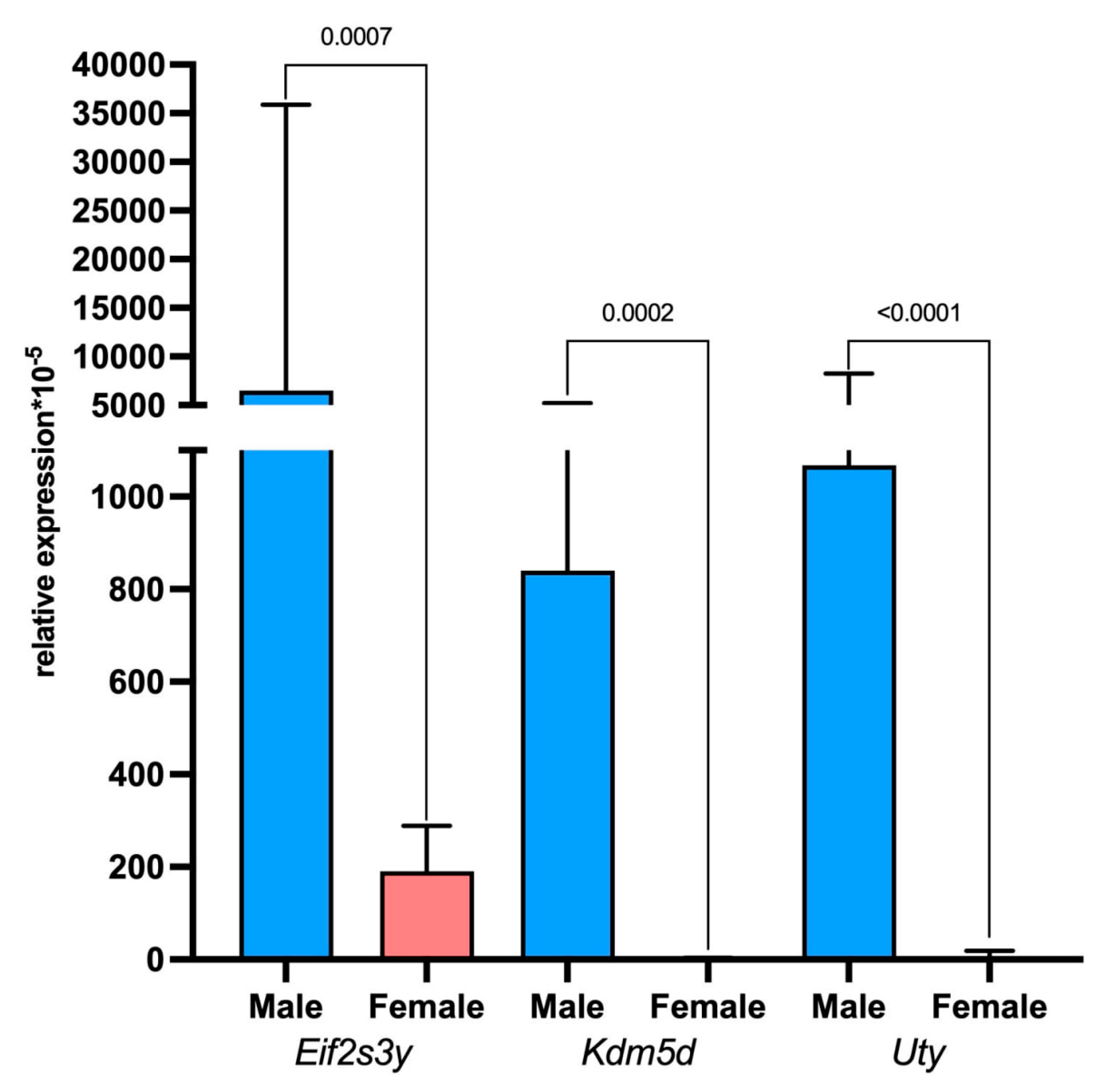
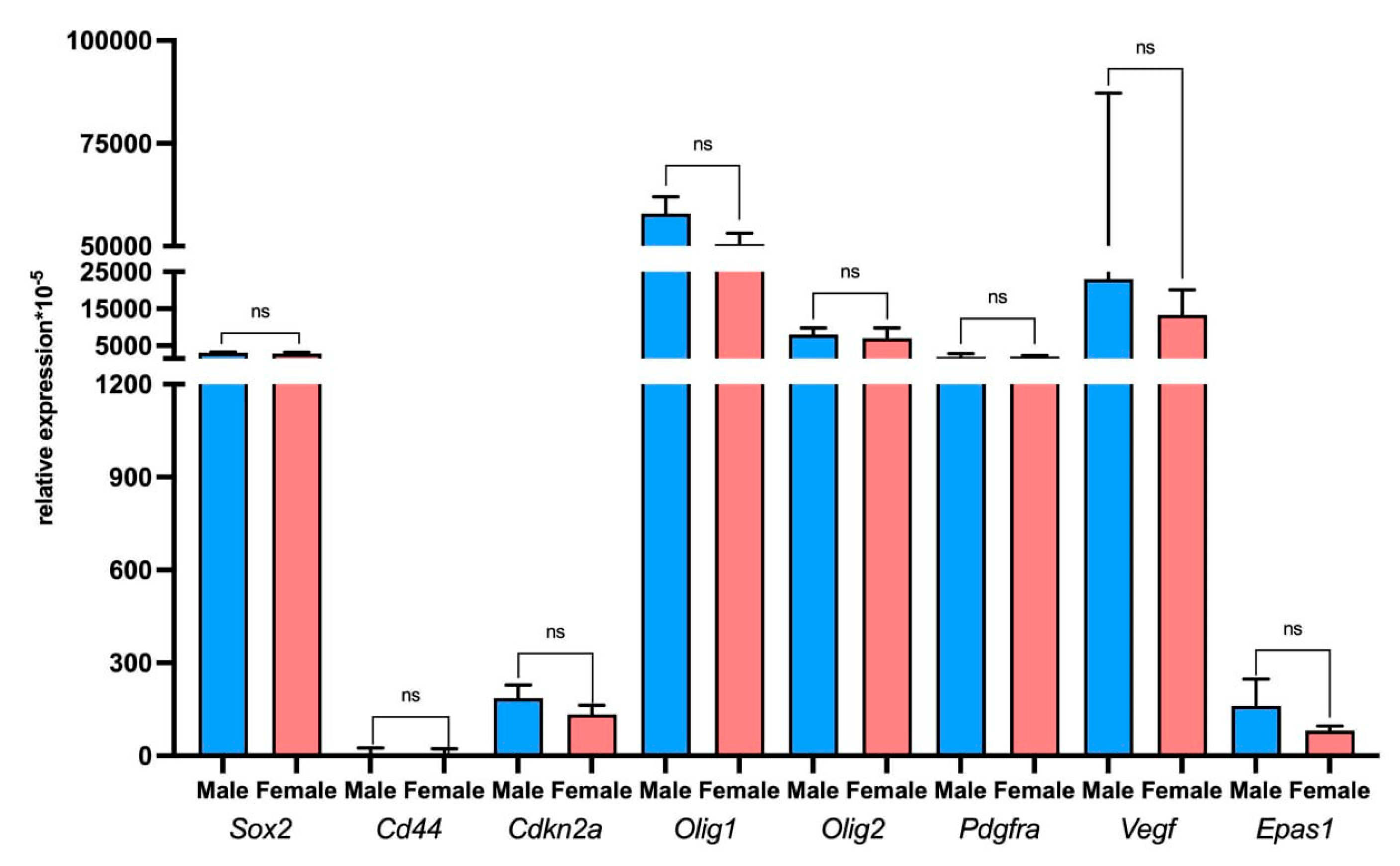
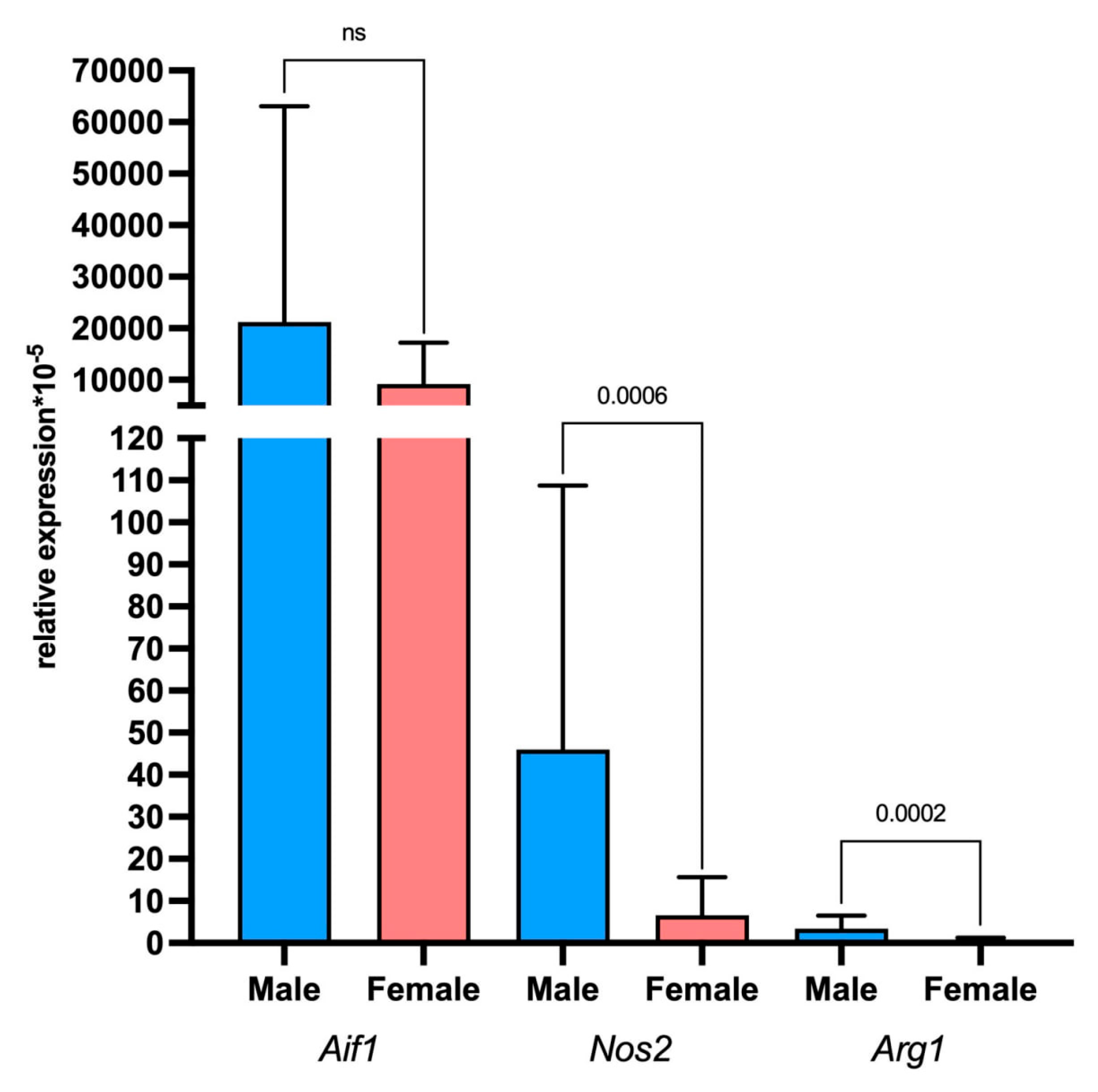
2.5. Assessment of Tumor and Microenvironment Cells Identitied Using Sex-Linked Gene Expression Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Revitalization and Transplantation of Tissue Derived from the GB 101.8 Tumor Strain
4.3. MRI Imaging
4.4. Preparation of Rat Glioma 101.8 Tissue Samples
4.5. Single-Cell RNA Sequencing of Rat Glioma 101.8 Tumor Samples
4.6. PCR-RT
4.7. Histological Examination
4.8. Immunohistochemical Analysis
4.9. Statistical Analysis
5. Conclusions
Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nafe, R.; Porto, L.; Samp, P.-F.; You, S.-J.; Hattingen, E. Adult-type and Pediatric-type Diffuse Gliomas. Clin. Neuroradiol. 2023, 33, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Fatahian, R.; Kazeminia, M.; Hosseinian-Far, A.; Shohaimi, S.; Mohammadi, M. Patients’ Survival with Astrocytoma After Treatment: A Systematic Review and Meta-analysis of Clinical Trial Studies. Indian J. Surg. Oncol. 2022, 13, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.; Dinesan, M.; Ajayakumar, T. Survival and quality of life analysis in glioblastoma multiforme with adjuvant chemoradiotherapy: A retrospective study. Rep. Pract. Oncol. Radiother. 2022, 27, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Liang, H.; Cheng, P.; Yang, H.; Zhao, P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients With High-Grade Glioma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 151. [Google Scholar] [CrossRef]
- Taal, W.; Bromberg, J.E.; Bent, M.J.v.D. Chemotherapy in glioma. CNS Oncol. 2015, 4, 179–192. [Google Scholar] [CrossRef]
- Piccioni, D.E.; Selfridge, J.; Mody, R.R.; Chowdhury, R.; Li, S.; Lalezari, S.; Wawrzynski, J.; Quan, J.; Zurayk, M.; Chou, A.P.; et al. Deferred use of bevacizumab for recurrent glioblastoma is not associated with diminished efficacy. Neuro-Oncology 2014, 16, 815–822. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 1–32. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ahmad, Z.; Rahim, S.; Abdul-Ghafar, J.; Chundriger, Q.; Din, N.U. Events in CNS Tumor Pathology Post-2016 WHO CNS: cIMPACT-NOW Updates and Other Advancements: A Comprehensive Review Plus a Summary of the Salient Features of 2021 WHO CNS 5. Int. J. Gen. Med. 2023, ume 16, 107–127. [Google Scholar] [CrossRef]
- Liffers, K.; Lamszus, K.; Schulte, A. EGFR Amplification and Glioblastoma Stem-Like Cells. Stem Cells Int. 2015, 2015, 427518. [Google Scholar] [CrossRef]
- Calabrese, E.; Rudie, J.D.; Rauschecker, A.M.; Villanueva-Meyer, J.E.; Clarke, J.L.; Solomon, D.A.; Cha, S. Combining radiomics and deep convolutional neural network features from preoperative MRI for predicting clinically relevant genetic biomarkers in glioblastoma. Neuro-Oncol. Adv. 2022, 4, vdac060. [Google Scholar] [CrossRef]
- Cole, A.P.; Hoffmeyer, E.; Chetty, S.L.; Cruz-Cruz, J.; Hamrick, F.; Youssef, O.; Cheshier, S.; Mitra, S.S. Microglia in the Brain Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Begley, S.L.; O’rOurke, D.M.; Binder, Z.A. CAR T cell therapy for glioblastoma: A review of the first decade of clinical trials. Mol. Ther. 2025, 33, 2454–2461. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, L.; Luo, L.; Shu, L.; Si, X.; Chen, Z.; Xia, W.; Huang, J.; Liu, Y.; Shao, A.; et al. Opportunities and challenges of glioma organoids. Cell Commun. Signal. 2021, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef]
- Alekseeva, A.I.; Khalansky, A.S.; Miroshnichenko, E.A.; Gerasimov, A.D.; Sentyabreva, A.V.; Kudelkina, V.V.; Osipova, N.S.; Gulyaev, M.V.; Gelperina, S.E.; Kosyreva, A.M. The Effect of Therapy Regimen on Antitumor Efficacy of the Nanosomal Doxorubicin against Rat Glioblastoma 101.8. Bull. Exp. Biol. Med. 2024, 176, 697–702. [Google Scholar] [CrossRef]
- Kucheryavenko, A.S.; Chernomyrdin, N.V.; Gavdush, A.A.; Alekseeva, A.I.; Nikitin, P.V.; Dolganova, I.N.; Karalkin, P.A.; Khalansky, A.S.; Spektor, I.E.; Skorobogatiy, M.; et al. Terahertz dielectric spectroscopy and solid immersion microscopy of ex vivo glioma model 101.8: Brain tissue heterogeneity. Biomed. Opt. Express 2021, 12, 5272–5289. [Google Scholar] [CrossRef]
- Kovshova, T.; Osipova, N.; Alekseeva, A.; Malinovskaya, J.; Belov, A.; Budko, A.; Pavlova, G.; Maksimenko, O.; Nagpal, S.; Braner, S.; et al. Exploring the Interplay between Drug Release and Targeting of Lipid-Like Polymer Nanoparticles Loaded with Doxorubicin. Molecules 2021, 26, 831. [Google Scholar] [CrossRef]
- Sentyabreva, A.; Miroshnichenko, E.; Artemova, D.; Alekseeva, A.; Kosyreva, A. Morphological and Molecular Biological Characteristics of Experimental Rat Glioblastoma Tissue Strains Induced by Different Carcinogenic Chemicals. Biomedicines 2024, 12, 713. [Google Scholar] [CrossRef]
- Szu, J.; Wojcinski, A.; Jiang, P.; Kesari, S. Impact of the Olig Family on Neurodevelopmental Disorders. Front. Neurosci. 2021, 15, 659601. [Google Scholar] [CrossRef]
- Meijer, D.H.; Kane, M.F.; Mehta, S.; Liu, H.; Harrington, E.; Taylor, C.M.; Stiles, C.D.; Rowitch, D.H. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nat. Rev. Neurosci. 2012, 13, 819–831. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Aguirre-Cruz, L.; Mokhtari, K.; Marie, Y.; Sanson, M. OLIG-1 and 2 gene expression and oligodendroglial tumours. Neuropathol. Appl. Neurobiol. 2002, 28, 89–94. [Google Scholar] [CrossRef]
- Rissi, D.R.; Donovan, T.A.; Porter, B.F.; Frank, C.; Miller, A.D. Canine Gliomatosis Cerebri: Morphologic and Immunohistochemical Characterization Is Supportive of Glial Histogenesis. Veter- Pathol. 2020, 58, 293–304. [Google Scholar] [CrossRef]
- Aguirre-Cruz, L.; Mokhtari, K.; Hoang-Xuan, K.; Marie, Y.; Criniere, E.; Taillibert, S.; Lopes, M.; Delattre, J.-Y.; Sanson, M. Analysis of the bHLH Transcription Factors Olig1 and Olig2 in Brain Tumors. J. Neuro-Oncol. 2004, 67, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.L.; Brayer, K.J.; Paez-Beltran, L.E.; Villicana, E.; Keith, M.S.; Suzuki, H.; Newville, J.; Anderson, R.H.; Lo, Y.; Mertz, C.M.; et al. Transcription factors ASCL1 and OLIG2 drive glioblastoma initiation and co-regulate tumor cell types and migration. Nat. Commun. 2024, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Hede, S.-M.; He, X.; Hedrén, A.; Thompson, J.; Lindström, M.S.; Nistér, M. PDGF and PDGF receptors in glioma. Upsala J. Med. Sci. 2012, 117, 99–112. [Google Scholar] [CrossRef]
- Dufour, C.; Perbet, R.; Leblond, P.; Vasseur, R.; Stechly, L.; Pierache, A.; Reyns, N.; Touzet, G.; Le Rhun, E.; Vinchon, M.; et al. Identification of prognostic markers in diffuse midline gliomas H3K27M-mutant. Brain Pathol. 2019, 30, 179–190. [Google Scholar] [CrossRef]
- Smith, J.S.; Wang, X.-Y.; Qian, J.; Hosek, S.M.; Scheithauer, B.W.; Jenkins, R.B.; James, C.D. Amplification of the Platelet-Derived Growth Factor Receptor-A (PDGFRA) Gene Occurs in Oligodendrogliomas with Grade IV Anaplastic Features. J. Neuropathol. Exp. Neurol. 2000, 59, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Garros-Regulez, L.; Garcia, I.; Carrasco-Garcia, E.; Lantero, A.; Aldaz, P.; Moreno-Cugnon, L.; Arrizabalaga, O.; Undabeitia, J.; Torres-Bayona, S.; Villanua, J.; et al. Targeting SOX2 as a Therapeutic Strategy in Glioblastoma. Front. Oncol. 2016, 6, 222. [Google Scholar] [CrossRef]
- Yu, W.; Ren, X.; Hu, C.; Tan, Y.; Shui, Y.; Chen, Z.; Zhang, L.; Peng, J.; Wei, Q. Glioma SOX2 expression decreased after adjuvant therapy. BMC Cancer 2019, 19, 1087. [Google Scholar] [CrossRef]
- Berezovsky, A.D.; Poisson, L.M.; Cherba, D.; Webb, C.P.; Transou, A.D.; Lemke, N.W.; Hong, X.; Hasselbach, L.A.; Irtenkauf, S.M.; Mikkelsen, T.; et al. Sox2 Promotes Malignancy in Glioblastoma by Regulating Plasticity and Astrocytic Differentiation. Neoplasia 2014, 16, 193–206.e25, Erratum in Neoplasia 2014, 16, 461. [Google Scholar] [CrossRef]
- Riddick, G.; Kotliarova, S.; Rodriguez, V.; Kim, H.S.; Linkous, A.; Storaska, A.J.; Ahn, S.; Walling, J.; Belova, G.; Fine, H.A. A Core Regulatory Circuit in Glioblastoma Stem Cells Links MAPK Activation to a Transcriptional Program of Neural Stem Cell Identity. Sci. Rep. 2017, 7, srep43605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Xu, C.; Wang, D.; Ma, C.; Wang, Z.; Li, Y.; Li, Z. Unveiling novel cell clusters and biomarkers in glioblastoma and its peritumoral microenvironment at the single-cell perspective. J. Transl. Med. 2024, 22, 551. [Google Scholar] [CrossRef] [PubMed]
- Na Lee, H.; Jeong, M.S.; Jang, S.B. Molecular Characteristics of Amyloid Precursor Protein (APP) and Its Effects in Cancer. Int. J. Mol. Sci. 2021, 22, 4999. [Google Scholar] [CrossRef]
- Drucker, K.L.; Gianinni, C.; Decker, P.A.; Diamandis, E.P.; Scarisbrick, I.A. Prognostic significance of multiple kallikreins in high-grade astrocytoma. BMC Cancer 2015, 15, 565. [Google Scholar] [CrossRef]
- Yang, C.; Yuan, H.; Gu, J.; Xu, D.; Wang, M.; Qiao, J.; Yang, X.; Zhang, J.; Yao, M.; Gu, J.; et al. ABCA8-mediated efflux of taurocholic acid contributes to gemcitabine insensitivity in human pancreatic cancer via the S1PR2-ERK pathway. Cell Death Discov. 2021, 7, 6. [Google Scholar] [CrossRef]
- Zhu, C.; Soto-Feliciano, Y.M.; Morris, J.P.; Huang, C.-H.; Koche, R.P.; Ho, Y.-J.; Banito, A.; Chen, C.-W.D.; Shroff, A.; Tian, S.; et al. MLL3 regulates the CDKN2A tumor suppressor locus in liver cancer. eLife 2023, 12, e80854. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lv, G.; Li, Y.; Li, L.; Wang, B. Downregulation of CDKN2A and suppression of cyclin D1 gene expressions in malignant gliomas. J. Exp. Clin. Cancer Res. 2011, 30, 76. [Google Scholar] [CrossRef]
- Zhang, A.; Huang, Z.; Tao, W.; Zhai, K.; Wu, Q.; Rich, J.N.; Zhou, W.; Bao, S. USP33 deubiquitinates and stabilizes HIF-2alpha to promote hypoxia response in glioma stem cells. EMBO J. 2022, 41, e109187. [Google Scholar] [CrossRef]
- Johansson, E.; Grassi, E.S.; Pantazopoulou, V.; Tong, B.; Lindgren, D.; Berg, T.J.; Pietras, E.J.; Axelson, H.; Pietras, A. CD44 Interacts with HIF-2α to Modulate the Hypoxic Phenotype of Perinecrotic and Perivascular Glioma Cells. Cell Rep. 2017, 20, 1641–1653. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Wang, Y.; Gao, J.; Yang, H.; Li, H. MiR-17-5p Targets and Downregulates CADM2, Activating the Malignant Phenotypes of Colon Cancer Cells. Mol. Biotechnol. 2022, 64, 1388–1400. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jo, S.; Paek, S.H.; Kang, S.S.; Chung, H. SUZ12 inhibition attenuates cell proliferation of glioblastoma via post-translational regulation of CDKN1B. Genes Genom. 2023, 45, 1623–1632. [Google Scholar] [CrossRef]
- Voronkova, M.A.; Johnson, B.; Gandhi, N.; Koomen, J.M.; Patrick, M.; Bhupathi, S.S.; Wu, V.M.; Elliott, A.; Vanderwalde, A.; Halmos, B.; et al. ERO1A levels are a prognostic indicator in EGFR mutated non small cell lung cancer. npj Precis. Oncol. 2024, 8, 250. [Google Scholar] [CrossRef]
- Jiang, S.; Zhu, L.; Jiang, C.; Yu, S.; Wang, B.; Ren, Y. Prognostic and immune roles of synaptotagmin-4 in gastric cancer and brain lower-grade gliom. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Deng, M.; Zhang, W.; Yuan, L.; Tan, J.; Chen, Z. HIF-1a regulates hypoxia-induced autophagy via translocation of ANKRD37 in colon cancer. Exp. Cell Res. 2020, 395, 112175. [Google Scholar] [CrossRef]
- Libby, C.J.; Gc, S.; Benavides, G.A.; Fisher, J.L.; Williford, S.E.; Zhang, S.; Tran, A.N.; Gordon, E.R.; Jones, A.B.; Tuy, K.; et al. A role for GLUT3 in glioblastoma cell invasion that is not recapitulated by GLUT1. Cell Adhes. Migr. 2021, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Steele, R.; Ryerse, J.; Ray, R.B. Tumor-Suppressive Effects of MBP-1 in Non–Small Cell Lung Cancer Cells. Cancer Res. 2006, 66, 11907–11912. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Singh, J.; Pillai, P.P. MeCP2 Differentially Regulate the Myelin MBP and PLP Protein Expression in Oligodendrocytes and C6 Glioma. J. Mol. Neurosci. 2018, 65, 343–350. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Q.; Yuan, F.; Dong, H.; Zhang, H.; Geng, R.; Qi, Y.; Xiong, X.; Chen, Q.; Liu, B. RND2 attenuates apoptosis and autophagy in glioblastoma cells by targeting the p38 MAPK signalling pathway. J. Exp. Clin. Cancer Res. 2020, 39, 74. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Huai, T.; Huo, X.; Wang, H.; Bian, E.; Zhao, B. DNMT1 Mediated CAHM Repression Promotes Glioma Invasion via SPAK/JNK Pathway. Cell. Mol. Neurobiol. 2021, 42, 2643–2653. [Google Scholar] [CrossRef]
- Gayen, S.; Maclary, E.; Hinten, M.; Kalantry, S. Sex-specific silencing of X-linked genes by Xist RNA. Proc. Natl. Acad. Sci. USA 2016, 113, E309–E318. [Google Scholar] [CrossRef]
- Li, Y.; Wu, W.; Xu, W.; Wang, Y.; Wan, S.; Chen, W.; Yang, D.; Zhang, M.; Wu, X.; Yang, X.; et al. Eif2s3y alleviated LPS-induced damage to mouse testis and maintained spermatogenesis by negatively regulating Adamts5. Theriogenology 2023, 211, 65–75. [Google Scholar] [CrossRef]
- Li, J.; Lan, Z.; Liao, W.; Horner, J.W.; Xu, X.; Liu, J.; Yoshihama, Y.; Jiang, S.; Shim, H.S.; Slotnik, M.; et al. Histone demethylase KDM5D upregulation drives sex differences in colon cancer. Nature 2023, 619, 632–639. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yoo, H.-B.; Roe, J.-S. Current advances and future directions in targeting histone demethylases for cancer therapy. Mol. Cells 2025, 48, 100192. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef]
- Lisi, L.; Ciotti, G.M.P.; Braun, D.; Kalinin, S.; Currò, D.; Dello Russo, C.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.L.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef]
- Nelson, L.H.; Lenz, K.M. The immune system as a novel regulator of sex differences in brain and behavioral development. J. Neurosci. Res. 2016, 95, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Nissen, J.C. Microglial Function across the Spectrum of Age and Gender. Int. J. Mol. Sci. 2017, 18, 561. [Google Scholar] [CrossRef] [PubMed]
- Ochocka, N.; Segit, P.; Walentynowicz, K.A.; Wojnicki, K.; Cyranowski, S.; Swatler, J.; Mieczkowski, J.; Kaminska, B. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun. 2021, 12, 1151. [Google Scholar] [CrossRef]
- Tharp, M.E.; Han, C.Z.; Talukdar, M.; Balak, C.D.; Fitzpatrick, C.; Connor, C.O.; Preissl, S.; Buchanan, J.; Nott, A.; Escoubet, L.; et al. The inactive X chromosome drives sex differences in microglial inflammatory activity in human glioblastoma. bioRxiv 2025. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Sun, M.; Li, J.; Zheng, W.; Yang, F.; Zhang, Z. Olig1/2 Drive Astrocytic Glioblastoma Proliferation Through Transcriptional Co-Regulation of Various Cyclins. Genes 2025, 16, 573. [Google Scholar] [CrossRef] [PubMed]
- Szu, J.I.; Tsigelny, I.F.; Wojcinski, A.; Kesari, S. Biological functions of the Olig gene family in brain cancer and therapeutic targeting. Front. Neurosci. 2023, 17, 1129434. [Google Scholar] [CrossRef]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Anthony, C.; Mladkova-Suchy, N.; Adamson, D.C. The evolving role of antiangiogenic therapies in glioblastoma multiforme: Current clinical significance and future potential. Expert Opin. Investig. Drugs 2019, 28, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Alekseeva, A.I.; Gerasimov, A.D.; Kudelkina, V.V.; Osipova, N.S.; Drozd, S.F.; Pavlova, G.V.; Kosyreva, A.M.; Fatkhudinov, T.K. Changes in Oncogene Expression in Experimental Glioblastoma 101.8 Rats during Therapy with PLGA Nanoparticles Loaded with Doxorubicin. Bull. Exp. Biol. Med. 2023, 174, 518–522. [Google Scholar] [CrossRef]
- Lin, F.; de Gooijer, M.C.; Roig, E.M.; Buil, L.C.; Christner, S.M.; Beumer, J.H.; Würdinger, T.; Beijnen, J.H.; van Tellingen, O. ABCB1, ABCG2, and PTEN Determine the Response of Glioblastoma to Temozolomide and ABT-888 Therapy. Clin. Cancer Res. 2014, 20, 2703–2713. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, S.; Cui, X.; Wang, Q.; Yang, E.; Tong, F.; Hong, B.; Xiao, M.; Xin, L.; Xu, C.; et al. A novel compound EPIC-0412 reverses temozolomide resistance via inhibiting DNA repair/MGMT in glioblastoma. Neuro-Oncology 2022, 25, 857–870. [Google Scholar] [CrossRef]
- Syed, W.; Ibatullin, M. Glioblastoma: Overview and Magnetic Resonance Spectroscopy Analysis for Treatment. Cureus 2024, 16, e66390. [Google Scholar] [CrossRef]


| Cell Type | Marker Genes |
|---|---|
| Glioma cells | Sox2, Olig2, Pdgfra, Cspg4, and Nes |
| B lymphocytes | Cd79a, Ms4a1, Pax5, Cd24, Cd27, and Cd19 |
| Plasmacytoid dendritic cells | Bst2, Tcf4, Irf7, and Ly6c |
| Macrophages | Cd68, Adgre1, Mrc1, Ccl2, Cd86, and Itgam |
| Dendritic cells | Clec9A, Cadm1, Xcr1, Itgax, Ccr7, Cd80, and Cd86 |
| Plasma cells | Mzb1 and Prdm1 |
| Microglia | Itgam, Ptprc, Cx3cr1, P2ry12, and Hexb |
| T lymphocytes | Cd3e, Cd4, Cd8a, Foxp3, Il2, Icos, Gzmb, and Prf1 |
| T-regulatory lymphocytes | Foxp3, Il2rA, Tgfb1, Ctla4, Ikzf2, Tigit, Lag3, and Pdcd1 |
| Proliferating cells | Mki67, Top2a, and Pcna |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, A.I.; Sentyabreva, A.V.; Kudelkina, V.V.; Miroshnichenko, E.A.; Ikonnikov, A.V.; Kopantseva, E.E.; Kosyreva, A.M.; Fatkhudinov, T.K. Rat Glioma 101.8 Tissue Strain: Molecular and Morphological Features. Int. J. Mol. Sci. 2025, 26, 8992. https://doi.org/10.3390/ijms26188992
Alekseeva AI, Sentyabreva AV, Kudelkina VV, Miroshnichenko EA, Ikonnikov AV, Kopantseva EE, Kosyreva AM, Fatkhudinov TK. Rat Glioma 101.8 Tissue Strain: Molecular and Morphological Features. International Journal of Molecular Sciences. 2025; 26(18):8992. https://doi.org/10.3390/ijms26188992
Chicago/Turabian StyleAlekseeva, Anna Igorevna, Alexandra Vladislavovna Sentyabreva, Vera Vladimirovna Kudelkina, Ekaterina Alexandrovna Miroshnichenko, Alexandr Vladimirovich Ikonnikov, Elena Evgenievna Kopantseva, Anna Mikhailovna Kosyreva, and Timur Khaysamudinovich Fatkhudinov. 2025. "Rat Glioma 101.8 Tissue Strain: Molecular and Morphological Features" International Journal of Molecular Sciences 26, no. 18: 8992. https://doi.org/10.3390/ijms26188992
APA StyleAlekseeva, A. I., Sentyabreva, A. V., Kudelkina, V. V., Miroshnichenko, E. A., Ikonnikov, A. V., Kopantseva, E. E., Kosyreva, A. M., & Fatkhudinov, T. K. (2025). Rat Glioma 101.8 Tissue Strain: Molecular and Morphological Features. International Journal of Molecular Sciences, 26(18), 8992. https://doi.org/10.3390/ijms26188992






