Immunology of Hypertension: Pathophysiological and Therapeutic Aspects
Abstract
1. Introduction
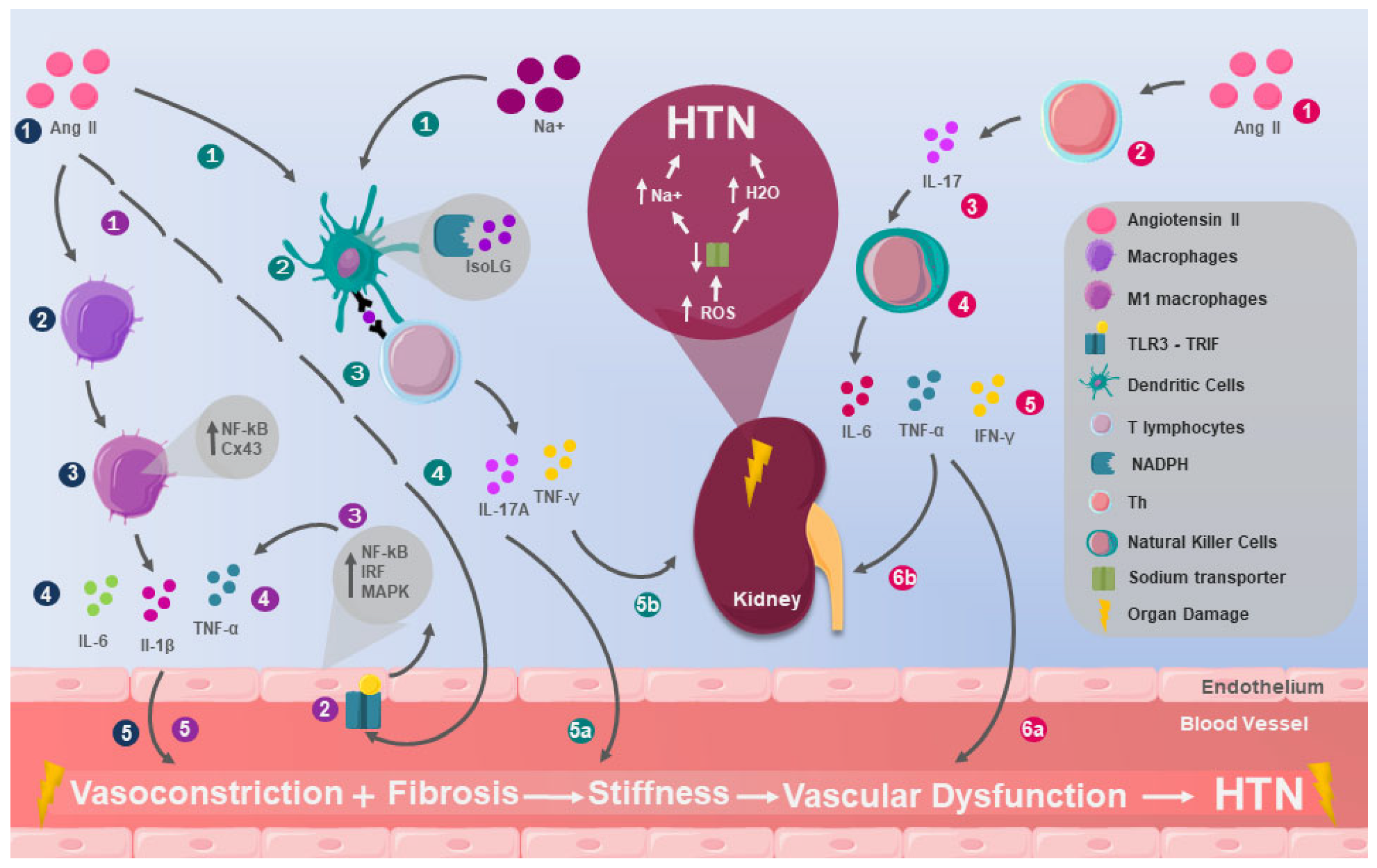
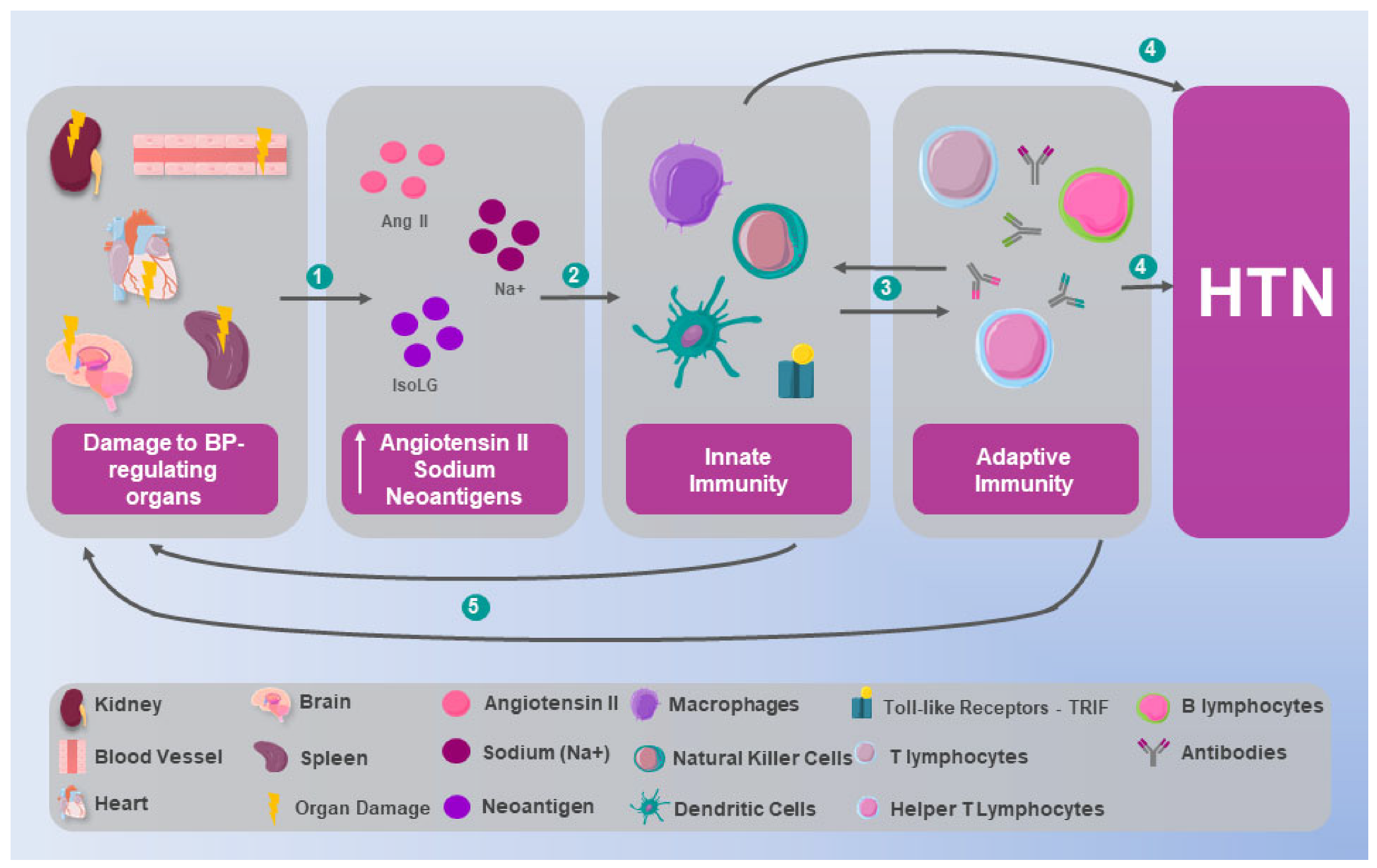
2. Immunological Mechanisms in Hypertension: Preclinical Evidence
2.1. Innate Immunity
| Authors | Type of immunity | Description | Results |
|---|---|---|---|
| Elfarra et al. [27] | Innate NK Cells | Three groups of pregnant rats were studied: a control group, a group induced with RUPP, and another group induced with RUPP and NK cell depletion. NK cell levels, mean arterial pressure, fetal weight, and cytokines were monitored. | Mean arterial pressure (MAP) was measured and compared among groups: in normal rats, MAP was 108 ± 2 mmHg; in RUPP rats, 125 ± 2 mmHg; and in RUPP + NK depletion rats, 122 ± 2 mmHg. |
| Wu et al. [31] | Innate Macrophages | RAW264.7 macrophages were stimulated with Ang II to simulate the inflammatory process. | Macrophage polarization to the M1 type was observed, promoting the release of iNOS, TNF-α, IL-1β, and IL-6, as well as increasing the levels of connexin 43 (Cx43) and NF-κB (p65) in macrophages. |
| Ao et al. [34] | Innate Receptors | The interaction between innate immune receptor signaling adapters was studied in human, porcine, bovine, caprine, equine, murine, and avian renal cell cultures. | In all these species, TLRs activate a signaling pathway that leads to the production of NF-κB, IRF, and MAPK, culminating in the release of pro-inflammatory cytokines. |
| Lu et al. [35] | Innate | Two groups of mice were studied: a wild-type group and a group with FLT3L deficiency. Both received continuous Ang II infusions. | There was a significant increase in DC and T cells, along with pro-inflammatory cytokines in the kidneys of wild-type mice, accompanied by elevated MAP, compared to FLT3L-deficient mice (FLT3L−/−). |
| Rodriguez et al. [40] | Adaptive T Lymphocytes | Three groups of genetically hypertensive mice were studied: one receiving a vehicle, one infused with MMF, and a control group. | The MMF-infused group showed normalization of blood pressure; a reduction in lymphocytes, macrophages, and Ang II-positive cells infiltrating the kidney; and reduced oxidative stress. |
| Mikolajczyk et al. [41] | Adaptive T Lymphocytes | Ang II was chronically infused into the perivascular adipose tissue of mice. | Increased presence of T cells, particularly those with CC chemokine receptors (CCR1, CCR3, and CCR5 for RANTES), as well as increased macrophage and dendritic cell infiltration. RANTES (−/−) knockout protected against T cell infiltration. |
| Guzik et al. [42] | Adaptive T Lymphocytes | Ang II or DOCA was infused into RAG-1 mice, which were then transferred with T cells but not B cells. | RAG-1 mice did not develop vascular alterations upon infusion of Ang II or DOCA. However, upon T cell transfer, vascular alterations became apparent. |
| Sun et al. [43] | Adaptive T Lymphocytes | Hypertension was induced by angiotensin II (Ang II) infusion in mice with T cell-mediated reactivity (T cell MR) knockout. | MR deficiency in T cells reduced systolic and diastolic blood pressure, pre-existing vascular damage, and IFN-γ levels from T cells in the kidneys and aorta. |
| Chan et al. [44] | Adaptive B Lymphocytes | Ang II was infused into BAFFR −/− mice. | The hypertensive response to Ang II administration was attenuated. |
| Parra et al. [45] | Autoimmune | NOS inhibition was performed in a salt-sensitive hypertensive mouse model, followed by the infusion of HSP70 into the peritoneum. | A regulatory T cell response was observed, correcting HSP70 immune activity, reducing immune cell infiltration in renal tissue, and preventing salt-induced hypertension. |
2.2. Adaptive Immunity
2.3. Autoimmunity in Hypertension
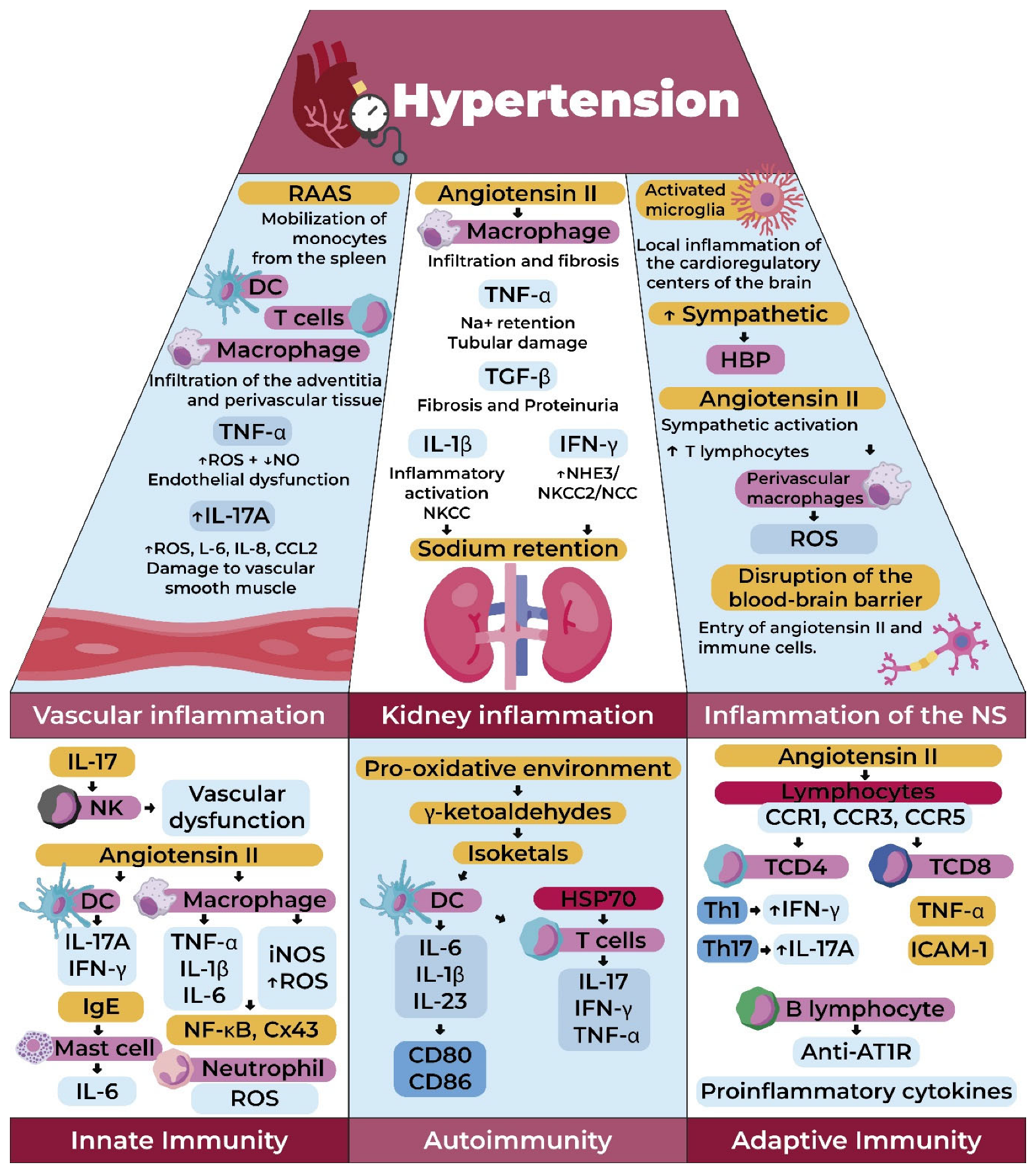
3. Inflammation and Hypertension: From Immunological Alteration to Multisystemic Involvement
3.1. Renal Inflammation in Hypertension
3.2. Vascular Inflammation in Hypertension
3.3. CNS and SNS Inflammation in Hypertension
3.4. Omics and Genetic Findings in the Immunology of Hypertension
4. The Management of Hypertension as an Immunological Disease: Are There Possibilities for New Therapeutic Targets?
4.1. Immunosuppressant Agents
4.1.1. Mycophenolate Mofetil
4.1.2. Methotrexate
4.2. Selected Anti-Cytokine Therapies
4.2.1. Anti-TNF-α
4.2.2. IL-1β
4.3. The Role of the Gut Microbiome in Immune Regulation and Hypertension Therapy
4.3.1. Fiber
4.3.2. Probiotics
4.4. Vaccination as an Innovative Therapeutic Strategy for Hypertension
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hypertension. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 3 March 2025).
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies from 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef] [PubMed]
- 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8)|Hypertension|JAMA|JAMA Network. Available online: https://jamanetwork.com/journals/jama/fullarticle/1791497 (accessed on 3 March 2025).
- 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines|Hypertension. Available online: https://www.ahajournals.org/doi/10.1161/HYP.0000000000000065 (accessed on 3 March 2025).
- Vallée, A.; Safar, M.E.; Blacher, J. Hypertension Artérielle Permanente Essentielle: Définitions et Revue Hémodynamique, Clinique et Thérapeutique. Presse Médicale 2019, 48 Pt 1, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Interactions Between the Sympathetic Nervous System and the RAAS in Heart Failure|Current Heart Failure Reports. Available online: https://link.springer.com/article/10.1007/s11897-004-0024-5 (accessed on 3 March 2025).
- Salt, Hypertension, and Immunity|Annual Reviews. Available online: https://www.annualreviews.org/content/journals/10.1146/annurev-physiol-021317-121134 (accessed on 3 March 2025).
- Su, Z.; Tian, S.; Liang, W. Circulating CTRP1 Levels Are Increased and Associated with the STOD in Essential Hypertension in Chinese Patients. Cardiovasc. Ther. 2019, 2019, 4183781. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rahimi, K.; Bautista, L.E.; Nazarzadeh, M.; Zargar, M.S.; Shab-Bidar, S. Inflammation Markers and Risk of Developing Hypertension: A Meta-Analysis of Cohort Studies. Heart 2019, 105, 686–692. [Google Scholar] [CrossRef]
- Wolf, V.L.; Ryan, M.J. Autoimmune Disease-Associated Hypertension. Curr. Hypertens. Rep. 2019, 21, 10. [Google Scholar] [CrossRef]
- Guzik, T.J.; Nosalski, R.; Maffia, P.; Drummond, G.R. Immune and Inflammatory Mechanisms in Hypertension. Nat. Rev. Cardiol. 2024, 21, 396–416. [Google Scholar] [CrossRef]
- Xiao, L.; Kirabo, A.; Wu, J.; Saleh, M.A.; Zhu, L.; Wang, F.; Takahashi, T.; Loperena, R.; Foss, J.D.; Mernaugh, R.L.; et al. Renal Denervation Prevents Immune Cell Activation and Renal Inflammation in Angiotensin II–Induced Hypertension. Circ. Res. 2015, 117, 547–557. [Google Scholar] [CrossRef]
- Ebringer, A.; Doyle, A.E. Raised Serum IgG Levels in Hypertension. Br. Med. J. 1970, 2, 146–148. [Google Scholar] [CrossRef]
- Adlin, E.V.; Moctezuma, J.; Marks, A.D.; Moctezuma, J.; Channick, B.J. Serum Immunoglobulins in Hypertension. Hypertension 1979, 1, 650–653. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Mohebati, M.; Feiz Disfani, M.; Saberi Karimian, M.; Ebrahimi, M.; Avan, A.; Eslami, S.; Pasdar, A.; Rooki, H.; Esmaeili, H.; et al. An Imbalance in Serum Concentrations of Inflammatory and Anti-Inflammatory Cytokines in Hypertension. J. Am. Soc. Hypertens. JASH 2014, 8, 614–623. [Google Scholar] [CrossRef]
- Sesso, H.D.; Buring, J.E.; Rifai, N.; Blake, G.J.; Gaziano, J.M.; Ridker, P.M. C-Reactive Protein and the Risk of Developing Hypertension. JAMA 2003, 290, 2945–2951. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.E.; López-Jaramillo, P.; Vera, L.M.; Casas, J.P.; Otero, A.P.; Guaracao, A.I. Is C-Reactive Protein an Independent Risk Factor for Essential Hypertension? J. Hypertens. 2001, 19, 857–861. [Google Scholar] [CrossRef]
- Youn, J.-C.; Yu, H.T.; Lim, B.J.; Koh, M.J.; Lee, J.; Chang, D.-Y.; Choi, Y.S.; Lee, S.-H.; Kang, S.-M.; Jang, Y.; et al. Immunosenescent CD8+ T Cells and C-X-C Chemokine Receptor Type 3 Chemokines Are Increased in Human Hypertension. Hypertension 2013, 62, 126–133. [Google Scholar] [CrossRef]
- Navarro-González, J.F.; Mora, C.; Muros, M.; Jarque, A.; Herrera, H.; García, J. Association of Tumor Necrosis Factor-Alpha with Early Target Organ Damage in Newly Diagnosed Patients with Essential Hypertension. J. Hypertens. 2008, 26, 2168–2175. [Google Scholar] [CrossRef]
- Carpenter, S.; O’Neill, L.A.J. From Periphery to Center Stage: 50 Years of Advancements in Innate Immunity. Cell 2024, 187, 2030–2051. [Google Scholar] [CrossRef]
- Monserrat Sanz, J.; Gómez Lahoz, A.M.; Sosa Reina, M.D.; Prieto Martín, A. Introducción al Sistema Inmune. Componentes Celulares Del Sistema Inmune Innato. Med.-Programa Form. Méd. Contin. Acreditado 2017, 12, 1369–1378. [Google Scholar] [CrossRef]
- Lopez Gelston, C.A.; Mitchell, B.M. Recent Advances in Immunity and Hypertension. Am. J. Hypertens. 2017, 30, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N.; et al. Critical Role of Interleukin (IL)-17 in Inflammatory and Immune Disorders: An Updated Review of the Evidence Focusing in Controversies. Autoimmun. Rev. 2020, 19, 102429. [Google Scholar] [CrossRef]
- Travis, O.K.; White, D.; Pierce, W.A.; Ge, Y.; Stubbs, C.Y.; Spradley, F.T.; Williams, J.M.; Cornelius, D.C. Chronic Infusion of Interleukin-17 Promotes Hypertension, Activation of Cytolytic Natural Killer Cells, and Vascular Dysfunction in Pregnant Rats. Physiol. Rep. 2019, 7, e14038. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.A.; McCalmon, M.; Ibrahim, T.; White, D.L.; Williams, J.M.; LaMarca, B.; Cornelius, D.C. Placental Ischemia-Stimulated T-Helper 17 Cells Induce Preeclampsia-Associated Cytolytic Natural Killer Cells During Pregnancy. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R336–R343. Available online: https://pubmed.ncbi.nlm.nih.gov/29718698/ (accessed on 3 March 2025). [CrossRef]
- Elfarra, J.; Amaral, L.M.; McCalmon, M.; Scott, J.D.; Cunningham, M.W.; Gnam, A.; Ibrahim, T.; LaMarca, B.; Cornelius, D.C. Natural Killer Cells Mediate Pathophysiology in Response to Reduced Uterine Perfusion Pressure. Clin. Sci. 2017, 131, 2753–2762. [Google Scholar] [CrossRef]
- Fujiu, K.; Shibata, M.; Nakayama, Y.; Ogata, F.; Matsumoto, S.; Noshita, K.; Iwami, S.; Nakae, S.; Komuro, I.; Nagai, R.; et al. A Heart-Brain-Kidney Network Controls Adaptation to Cardiac Stress through Tissue Macrophage Activation. Nat. Med. 2017, 23, 611–622. [Google Scholar] [CrossRef]
- Nikitina, E.; Larionova, I.; Choinzonov, E.; Kzhyshkowska, J. Monocytes and Macrophages as Viral Targets and Reservoirs. Int. J. Mol. Sci. 2018, 19, 2821. [Google Scholar] [CrossRef]
- Sivalingam, A.M.; Pandian, A. IL-1β and Vascular Inflammation in Hypertension and Metabolic Diseases? Hypertens. Res. 2024, 47, 3284–3285. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, K.; Xiao, J.; Xin, J.; Zhang, L.; Li, X.; Li, L.; Si, J.; Wang, L.; Ma, K. Angiotensin II Induces RAW264.7 Macrophage Polarization to the M1-type through the Connexin 43/NF-κB Pathway. Mol. Med. Rep. 2020, 21, 2103–2112. [Google Scholar] [CrossRef]
- Hernanz, R.; Martínez-Revelles, S.; Palacios, R.; Martín, A.; Cachofeiro, V.; Aguado, A.; García-Redondo, L.; Barrús, M.T.; de Batista, P.R.; Briones, A.M.; et al. Toll-like Receptor 4 Contributes to Vascular Remodelling and Endothelial Dysfunction in Angiotensin II-Induced Hypertension. Br. J. Pharmacol. 2015, 172, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.V.; Cicha, M.Z.; Nunez, S.; Meyerholz, D.K.; Chapleau, M.W.; Abboud, F.M. Angiotensin II-Induced Hypertension and Cardiac Hypertrophy Are Differentially Mediated by TLR3- and TLR4-Dependent Pathways. Am. J. Physiol.-Heart Circ. Physiol. 2019, 316, H1027–H1038. [Google Scholar] [CrossRef]
- Ao, D.; Li, S.; Jiang, S.; Luo, J.; Chen, N.; Meurens, F.; Zhu, J. Inter-Relation Analysis of Signaling Adaptors of Porcine Innate Immune Pathways. Mol. Immunol. 2020, 121, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Rudemiller, N.P.; Privratsky, J.R.; Ren, J.; Wen, Y.; Griffiths, R.; Crowley, S.D. Classical Dendritic Cells Mediate Hypertension by Promoting Renal Oxidative Stress and Fluid Retention. Hypertension 2020, 75, 131–138. [Google Scholar] [CrossRef]
- Barbaro, N.R.; Foss, J.D.; Kryshtal, D.O.; Tsyba, N.; Kumaresan, S.; Xiao, L.; Mernaugh, R.L.; Itani, H.A.; Loperena, R.; Chen, W.; et al. Dendritic Cell Amiloride-Sensitive Channels Mediate Sodium-Induced Inflammation and Hypertension. Cell Rep. 2017, 21, 1009–1020. [Google Scholar] [CrossRef]
- Foss, J.D.; Fiege, J.; Shimizu, Y.; Collister, J.P.; Mayerhofer, T.; Wood, L.; Osborn, J.W. Role of Afferent and Efferent Renal Nerves in the Development of AngII-salt Hypertension in Rats. Physiol. Rep. 2018, 6, e13602. [Google Scholar] [CrossRef] [PubMed]
- Van Beusecum, J.P.; Barbaro, N.R.; McDowell, Z.; Aden, L.A.; Xiao, L.; Pandey, A.K.; Itani, H.A.; Himmel, L.E.; Harrison, D.G.; Kirabo, A. High Salt Activates CD11c+ Antigen Presenting Cells Via Serum Glucocorticoid Kinase 1 to Promote Renal Inflammation and Salt-Sensitive Hypertension. Hypertension 2019, 74, 555–563. [Google Scholar] [CrossRef]
- Nguyen, B.A.; Alexander, M.R.; Harrison, D.G. Immune Mechanisms in the Pathophysiology of Hypertension. Nat. Rev. Nephrol. 2024, 20, 530–540. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Lanaspa, M.A.; Johnson, R.J. The Role of Autoimmune Reactivity Induced by Heat Shock Protein 70 in the Pathogenesis of Essential Hypertension. Br. J. Pharmacol. 2019, 176, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Guzik, T.J. Adaptive Immunity in Hypertension. Curr. Hypertens. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T Cell in the Genesis of Angiotensin II Induced Hypertension and Vascular Dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-N.; Li, C.; Liu, Y.; Du, L.-J.; Zeng, M.-R.; Zheng, X.-J.; Zhang, W.-C.; Liu, Y.; Zhu, M.; Kong, D.; et al. T-Cell Mineralocorticoid Receptor Controls Blood Pressure by Regulating Interferon-Gamma. Circ. Res. 2017, 120, 1584–1597. [Google Scholar] [CrossRef]
- Chan, C.T.; Sobey, C.G.; Lieu, M.; Ferens, D.; Kett, M.M.; Diep, H.; Kim, H.A.; Krishnan, S.M.; Lewis, C.V.; Salimova, E.; et al. Obligatory Role for B Cells in the Development of Angiotensin II–Dependent Hypertension. Hypertension 2015, 66, 1023–1033. [Google Scholar] [CrossRef]
- Parra, G.; Quiroz, Y.; Salazar, J.; Bravo, Y.; Pons, H.; Chavez, M.; Johnson, R.J.; Rodriguez-Iturbe, B. Experimental Induction of Salt-Sensitive Hypertension Is Associated with Lymphocyte Proliferative Response to HSP70. Kidney Int. Suppl. 2008, 111, S55–S59. [Google Scholar] [CrossRef]
- Shokoples, B.G.; Paradis, P.; Schiffrin, E.L. Immunological Insights into Hypertension: Unraveling Triggers and Potential Therapeutic Avenues. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2024, 47, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Crowley, S.D. Role of T-Cell Activation in Salt-Sensitive Hypertension. Am. J. Physiol.—Heart Circ. Physiol. 2019, 316, H1345–H1353. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Iturbe, B.; Quiroz, Y.; Nava, M.; Bonet, L.; Chávez, M.; Herrera-Acosta, J.; Johnson, R.J.; Pons, H.A. Reduction of Renal Immune Cell Infiltration Results in Blood Pressure Control in Genetically Hypertensive Rats. Am. J. Physiol. Renal Physiol. 2002, 282, F191–F201. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Nangaku, M.; Miyata, T.; Inagi, R.; Yamada, K.; Kurokawa, K.; Fujita, T. Imbalance of T-Cell Subsets in Angiotensin II–Infused Hypertensive Rats With Kidney Injury. Hypertension 2003, 42, 31–38. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Mian, M.O.R.; Paradis, P.; Schiffrin, E.L. Dual Opposing Roles of Adaptive Immunity in Hypertension. Eur. Heart J. 2014, 35, 1238–1244. [Google Scholar] [CrossRef]
- Mikolajczyk, T.P.; Nosalski, R.; Szczepaniak, P.; Budzyn, K.; Osmenda, G.; Skiba, D.; Sagan, A.; Wu, J.; Vinh, A.; Marvar, P.J.; et al. Role of Chemokine RANTES in the Regulation of Perivascular Inflammation, T-Cell Accumulation, and Vascular Dysfunction in Hypertension. FASEB J. 2016, 30, 1987–1999. [Google Scholar] [CrossRef]
- Schüler, R.; Efentakis, P.; Wild, J.; Lagrange, J.; Garlapati, V.; Molitor, M.; Kossmann, S.; Oelze, M.; Stamm, P.; Li, H.; et al. T Cell-Derived IL-17A Induces Vascular Dysfunction via Perivascular Fibrosis Formation and Dysregulation of ·NO/cGMP Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 6721531. [Google Scholar] [CrossRef]
- Small, H.Y.; Migliarino, S.; Czesnikiewicz-Guzik, M.; Guzik, T.J. Hypertension: Focus on Autoimmunity and Oxidative Stress. Free Radic. Biol. Med. 2018, 125, 104–115. [Google Scholar] [CrossRef]
- Kirabo, A.; Fontana, V.; de Faria, A.P.C.; Loperena, R.; Galindo, C.L.; Wu, J.; Bikineyeva, A.T.; Dikalov, S.; Xiao, L.; Chen, W.; et al. DC Isoketal-Modified Proteins Activate T Cells and Promote Hypertension. J. Clin. Investig. 2014, 124, 4642–4656. [Google Scholar] [CrossRef] [PubMed]
- Oyagbemi, A.A.; Omobowale, T.O.; Ola-Davies, O.E.; Asenuga, E.R.; Ajibade, T.O.; Adejumobi, O.A.; Arojojoye, O.A.; Afolabi, J.M.; Ogunpolu, B.S.; Falayi, O.O.; et al. Quercetin Attenuates Hypertension Induced by Sodium Fluoride via Reduction in Oxidative Stress and Modulation of HSP 70/ERK/PPARγ Signaling Pathways. BioFactors 2018, 44, 465–479. [Google Scholar] [CrossRef]
- Pons, H.; Ferrebuz, A.; Quiroz, Y.; Romero-Vasquez, F.; Parra, G.; Johnson, R.J.; Rodriguez-Iturbe, B. Immune Reactivity to Heat Shock Protein 70 Expressed in the Kidney Is Cause of Salt-Sensitive Hypertension. Am. J. Physiol. Renal Physiol. 2013, 304, F289–F299. [Google Scholar] [CrossRef]
- Srivastava, K.; Narang, R.; Bhatia, J.; Saluja, D. Expression of Heat Shock Protein 70 Gene and Its Correlation with Inflammatory Markers in Essential Hypertension. PLoS ONE 2016, 11, e0151060. [Google Scholar] [CrossRef]
- Zheng, J.-P.; Lyu, Y.; Li, R.; Tian, F.; Mu, J. Interaction of Heat Shock Protein 70 (HSP70) Polymorphisms and Occupational Hazards Increases the Risk of Hypertension in Coke Oven Workers. Occup. Environ. Med. 2018, 75, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Granger, J.P.; do Carmo, J.M.; da Silva, A.A.; Dubinion, J.; George, E.; Hamza, S.; Speed, J.; Hall, M.E. Hypertension: Physiology and Pathophysiology. Compr. Physiol. 2012, 2, 2393–2442. [Google Scholar] [CrossRef]
- Justin Rucker, A.; Crowley, S.D. The Role of Macrophages in Hypertension and Its Complications. Pflugers Arch. 2017, 469, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, E.R.; Mackey-Bojack, S.M. Histologic Examination of the Heart in the Forensic Autopsy. Acad. Forensic Pathol. 2018, 8, 565–615. [Google Scholar] [CrossRef]
- Rodríguez-Iturbe, B.; Pons, H.; Quiroz, Y.; Gordon, K.; Rincón, J.; Chávez, M.; Parra, G.; Herrera-Acosta, J.; Gómez-Garre, D.; Largo, R.; et al. Mycophenolate Mofetil Prevents Salt-Sensitive Hypertension Resulting from Angiotensin II Exposure. Kidney Int. 2001, 59, 2222–2232. [Google Scholar] [CrossRef]
- Wenzel, P.; Knorr, M.; Kossmann, S.; Stratmann, J.; Hausding, M.; Schuhmacher, S.; Karbach, S.H.; Schwenk, M.; Yogev, N.; Schulz, E.; et al. Lysozyme M–Positive Monocytes Mediate Angiotensin II–Induced Arterial Hypertension and Vascular Dysfunction. Circulation 2011, 124, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Crowley, S.D. Renal Effects of Cytokines in Hypertension. Curr. Opin. Nephrol. Hypertens. 2018, 27, 70–76. [Google Scholar] [CrossRef]
- Lu, X.; Crowley, S.D. Inflammation in Salt-Sensitive Hypertension and Renal Damage. Curr. Hypertens. Rep. 2018, 20, 103. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, M.B.; Griffiths, R.; Mao, A.; Song, Y.; Karlovich, N.S.; Sparks, M.A.; Jin, H.; Wu, M.; Lin, E.E.; et al. Tumor Necrosis Factor-α Produced in the Kidney Contributes to Angiotensin II-Dependent Hypertension. Hypertension 2014, 64, 1275–1281. [Google Scholar] [CrossRef]
- Pukajło-Marczyk, A.; Zwolińska, D. The Role of TNF-α in the Pathogenesis of Idiopathic Nephrotic Syndrome and Its Usefulness as a Marker of the Disease Course. J. Clin. Med. 2024, 13, 1888. [Google Scholar] [CrossRef]
- Venegas-Pont, M.; Manigrasso, M.B.; Grifoni, S.C.; LaMarca, B.B.; Maric, C.; Racusen, L.C.; Glover, P.H.; Jones, A.V.; Drummond, H.A.; Ryan, M.J. Tumor Necrosis Factor-α Antagonist Etanercept Decreases Blood Pressure and Protects the Kidney in a Mouse Model of Systemic Lupus Erythematosus. Hypertension 2010, 56, 643–649. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, Y.; Usa, K.; Liu, Y.; Baker, M.A.; Mattson, D.L.; He, Y.; Wang, N.; Liang, M. Renal Tumor Necrosis Factor α Contributes to Hypertension in Dahl Salt-Sensitive Rats. Sci. Rep. 2016, 6, 21960. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bahrami, L.; Castillo, A.; Majid, D.S.A. TNF-α Type 2 Receptor Mediates Renal Inflammatory Response to Chronic Angiotensin II Administration with High Salt Intake in Mice. Am. J. Physiol. Renal Physiol. 2013, 304, F991–F999. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Smith, D.E. The IL-1 Family: Regulators of Immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef]
- Krishnan, S.M.; Dowling, J.K.; Ling, Y.H.; Diep, H.; Chan, C.T.; Ferens, D.; Kett, M.M.; Pinar, A.; Samuel, C.S.; Vinh, A.; et al. Inflammasome Activity Is Essential for One Kidney/Deoxycorticosterone Acetate/Salt-Induced Hypertension in Mice. Br. J. Pharmacol. 2016, 173, 752–765. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, Y.; Tang, T.; Lv, L.; Liu, H.; Ma, K.; Liu, B. NLRP3 Inflammasome Activation Is Involved in Ang II-Induced Kidney Damage via Mitochondrial Dysfunction. Oncotarget 2016, 7, 54290–54302. [Google Scholar] [CrossRef]
- Takahashi, H.; Nishimura, M.; Sakamoto, M.; Ikegaki, I.; Nakanishi, T.; Yoshimura, M. Effects of Interleukin-1 Beta on Blood Pressure, Sympathetic Nerve Activity, and Pituitary Endocrine Functions in Anesthetized Rats. Am. J. Hypertens. 1992, 5 Pt 1, 224–229. [Google Scholar] [CrossRef]
- Moes, A.D.; Severs, D.; Verdonk, K.; van der Lubbe, N.; Zietse, R.; Danser, A.H.J.; Hoorn, E.J. Mycophenolate Mofetil Attenuates DOCA-Salt Hypertension: Effects on Vascular Tone. Front. Physiol. 2018, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Kamat, N.V.; Thabet, S.R.; Xiao, L.; Saleh, M.A.; Kirabo, A.; Madhur, M.S.; Delpire, E.; Harrison, D.G.; McDonough, A.A. Renal Transporter Activation During Angiotensin-II Hypertension Is Blunted in Interferon-Γ−/− and Interleukin-17A−/− Mice. Hypertension 2015, 65, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; McMaster, W.G.; Wu, J.; Norlander, A.E.; Funt, S.A.; Thabet, S.R.; Kirabo, A.; Xiao, L.; Chen, W.; Itani, H.A.; et al. Lymphocyte Adaptor Protein LNK Deficiency Exacerbates Hypertension and End-Organ Inflammation. J. Clin. Investig. 2015, 125, 1189–1202. [Google Scholar] [CrossRef]
- Markó, L.; Kvakan, H.; Park, J.-K.; Qadri, F.; Spallek, B.; Binger, K.J.; Bowman, E.P.; Kleinewietfeld, M.; Fokuhl, V.; Dechend, R.; et al. Interferon-γ Signaling Inhibition Ameliorates Angiotensin II-Induced Cardiac Damage. Hypertension 2012, 60, 1430–1436. [Google Scholar] [CrossRef]
- Kagami, S.; Border, W.A.; Miller, D.E.; Noble, N.A. Angiotensin II Stimulates Extracellular Matrix Protein Synthesis through Induction of Transforming Growth Factor-Beta Expression in Rat Glomerular Mesangial Cells. J. Clin. Investig. 1994, 93, 2431–2437. [Google Scholar] [CrossRef]
- Mozes, M.M.; Böttinger, E.P.; Jacot, T.A.; Kopp, J.B. Renal Expression of Fibrotic Matrix Proteins and of Transforming Growth Factor-β (TGF-β) Isoforms in TGF-β Transgenic Mice. J. Am. Soc. Nephrol. 1999, 10, 271–280. [Google Scholar] [CrossRef]
- Ledbetter, S.; Kurtzberg, L.; Doyle, S.; Pratt, B.M. Renal Fibrosis in Mice Treated with Human Recombinant Transforming Growth Factor-Beta2. Kidney Int. 2000, 58, 2367–2376. [Google Scholar] [CrossRef]
- Noble, N.A.; Border, W.A. Angiotensin II in Renal Fibrosis: Should TGF-Beta Rather than Blood Pressure Be the Therapeutic Target? Semin. Nephrol. 1997, 17, 455–466. [Google Scholar]
- Sanders, P.W. Vascular Consequences of Dietary Salt Intake. Am. J. Physiol. Renal Physiol. 2009, 297, F237–F243. [Google Scholar] [CrossRef]
- Ying, W.-Z.; Aaron, K.; Sanders, P.W. Mechanism of Dietary Salt-Mediated Increase in Intravascular Production of TGF-Β1. Am. J. Physiol.—Ren. Physiol. 2008, 295, F406–F414. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; Dahly-Vernon, A.J.; Dunn, K.M.J.; Chen, C.C.A.; Ledbetter, S.R.; Williams, J.M.; Roman, R.J. Renoprotective Effects of Anti-TGF-β Antibody and Antihypertensive Therapies in Dahl S Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R57–R69. [Google Scholar] [CrossRef] [PubMed]
- Barhoumi, T.; Kasal, D.A.; Li, M.W.; Shbat, L.; Laurant, P.; Neves, M.F.; Paradis, P.; Schiffrin, E.L. T Regulatory Lymphocytes Prevent Angiotensin II–Induced Hypertension and Vascular Injury. Hypertension 2011, 57, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Kasal, D.A.; Barhoumi, T.; Li, M.W.; Yamamoto, N.; Zdanovich, E.; Rehman, A.; Neves, M.F.; Laurant, P.; Paradis, P.; Schiffrin, E.L. T Regulatory Lymphocytes Prevent Aldosterone-Induced Vascular Injury. Hypertension 2012, 59, 324–330. [Google Scholar] [CrossRef]
- Kvakan, H.; Kleinewietfeld, M.; Qadri, F.; Park, J.-K.; Fischer, R.; Schwarz, I.; Rahn, H.-P.; Plehm, R.; Wellner, M.; Elitok, S.; et al. Regulatory T Cells Ameliorate Angiotensin II–Induced Cardiac Damage. Circulation 2009, 119, 2904–2912. [Google Scholar] [CrossRef]
- Lob, H.E.; Marvar, P.J.; Guzik, T.J.; Sharma, S.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 2010, 55, 277. [Google Scholar] [CrossRef]
- De Ciuceis, C.; Amiri, F.; Brassard, P.; Endemann, D.H.; Touyz, R.M.; Schiffrin, E.L. Reduced Vascular Remodeling, Endothelial Dysfunction, and Oxidative Stress in Resistance Arteries of Angiotensin II–Infused Macrophage Colony-Stimulating Factor–Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2106–2113. [Google Scholar] [CrossRef]
- Chan, C.T.; Moore, J.P.; Budzyn, K.; Guida, E.; Diep, H.; Vinh, A.; Jones, E.S.; Widdop, R.E.; Armitage, J.A.; Sakkal, S.; et al. Reversal of Vascular Macrophage Accumulation and Hypertension by a CCR2 Antagonist in Deoxycorticosterone/Salt-Treated Mice. Hypertension 2012, 60, 1207–1212. [Google Scholar] [CrossRef]
- Elmarakby, A.A.; Quigley, J.E.; Olearczyk, J.J.; Sridhar, A.; Cook, A.K.; Inscho, E.W.; Pollock, D.M.; Imig, J.D. Chemokine Receptor 2b Inhibition Provides Renal Protection in Angiotensin II–Salt Hypertension. Hypertension 2007, 50, 1069–1076. [Google Scholar] [CrossRef]
- Ko, E.A.; Amiri, F.; Pandey, N.R.; Javeshghani, D.; Leibovitz, E.; Touyz, R.M.; Schiffrin, E.L. Resistance Artery Remodeling in Deoxycorticosterone Acetate-Salt Hypertension Is Dependent on Vascular Inflammation: Evidence from m-CSF-Deficient Mice. Am. J. Physiol.-Heart Circ. Physiol. 2007, 292, H1789–H1795. [Google Scholar] [CrossRef]
- Kossmann, S.; Hu, H.; Steven, S.; Schönfelder, T.; Fraccarollo, D.; Mikhed, Y.; Brähler, M.; Knorr, M.; Brandt, M.; Karbach, S.H.; et al. Inflammatory Monocytes Determine Endothelial Nitric-Oxide Synthase Uncoupling and Nitro-Oxidative Stress Induced by Angiotensin II. J. Biol. Chem. 2014, 289, 27540–27550. [Google Scholar] [CrossRef] [PubMed]
- Höfer, T.; Busch, K.; Klapproth, K.; Rodewald, H.-R. Fate Mapping and Quantitation of Hematopoiesis In Vivo. Annu. Rev. Immunol. 2016, 34, 449–478. [Google Scholar] [CrossRef] [PubMed]
- Kintscher, U.; Wakino, S.; Kim, S.; Fleck, E.; Hsueh, W.A.; Law, R.E. Angiotensin II Induces Migration and Pyk2/Paxillin Phosphorylation of Human Monocytes. Hypertension 2001, 37 Pt 2, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.-L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of Splenic Reservoir Monocytes and Their Deployment to Inflammatory Sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Alonso, J.; Sánchez de Miguel, L.; Montón, M.; Casado, S.; López-Farré, A. Endothelial Cytosolic Proteins Bind to the 3’ Untranslated Region of Endothelial Nitric Oxide Synthase mRNA: Regulation by Tumor Necrosis Factor Alpha. Mol. Cell. Biol. 1997, 17, 5719–5726. [Google Scholar] [CrossRef]
- Landry, D.B.; Couper, L.L.; Bryant, S.R.; Lindner, V. Activation of the NF-Kappa B and I Kappa B System in Smooth Muscle Cells after Rat Arterial Injury. Induction of Vascular Cell Adhesion Molecule-1 and Monocyte Chemoattractant Protein-1. Am. J. Pathol. 1997, 151, 1085–1095. [Google Scholar]
- Neumann, P.; Gertzberg, N.; Johnson, A. TNF-Alpha Induces a Decrease in eNOS Promoter Activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L452–L459. [Google Scholar] [CrossRef]
- Sun, H.-X.; Zeng, D.-Y.; Li, R.-T.; Pang, R.-P.; Yang, H.; Hu, Y.-L.; Zhang, Q.; Jiang, Y.; Huang, L.-Y.; Tang, Y.-B.; et al. Essential Role of microRNA-155 in Regulating Endothelium-Dependent Vasorelaxation by Targeting Endothelial Nitric Oxide Synthase. Hypertension 2012, 60, 1407–1414. [Google Scholar] [CrossRef]
- Sag, C.M.; Schnelle, M.; Zhang, J.; Murdoch, C.E.; Kossmann, S.; Protti, A.; Santos, C.X.C.; Sawyer, G.; Zhang, X.; Mongue-Din, H.; et al. Distinct Regulatory Effects of Myeloid Cell and Endothelial Cell NAPDH Oxidase 2 on Blood Pressure. Circulation 2017, 135, 2163–2177. [Google Scholar] [CrossRef]
- Matsuno, K.; Yamada, H.; Iwata, K.; Jin, D.; Katsuyama, M.; Matsuki, M.; Takai, S.; Yamanishi, K.; Miyazaki, M.; Matsubara, H.; et al. Nox1 Is Involved in Angiotensin II-Mediated Hypertension: A Study in Nox1-Deficient Mice. Circulation 2005, 112, 2677–2685. [Google Scholar] [CrossRef]
- Dikalova, A.; Clempus, R.; Lassègue, B.; Cheng, G.; McCoy, J.; Dikalov, S.; San Martin, A.; Lyle, A.; Weber, D.S.; Weiss, D.; et al. Nox1 Overexpression Potentiates Angiotensin II-Induced Hypertension and Vascular Smooth Muscle Hypertrophy in Transgenic Mice. Circulation 2005, 112, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Park, Y.-J.; Chung, Y. Targeting IL-17 in Autoimmunity and Inflammation. Arch. Pharm. Res. 2016, 39, 1537–1547. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.E.; Rao, D.A.; Zhou, J.; Lo, S.L.; Ranjbaran, H.; Gallo, A.; Sokol, S.I.; Pfau, S.; Pober, J.S.; Tellides, G. Interleukin-17 and Interferon-γ Are Produced Concomitantly by Human Coronary Artery-Infiltrating T Cells and Act Synergistically on Vascular Smooth Muscle Cells. Circulation 2009, 119, 1424–1432. [Google Scholar] [CrossRef]
- Pietrowski, E.; Bender, B.; Huppert, J.; White, R.; Luhmann, H.J.; Kuhlmann, C.R.W. Pro-Inflammatory Effects of Interleukin-17A on Vascular Smooth Muscle Cells Involve NAD(P)H- Oxidase Derived Reactive Oxygen Species. J. Vasc. Res. 2011, 48, 52–58. [Google Scholar] [CrossRef]
- Madhur, M.S.; Lob, H.E.; McCann, L.A.; Iwakura, Y.; Blinder, Y.; Guzik, T.J.; Harrison, D.G. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 2010, 55, 500. [Google Scholar] [CrossRef]
- Nguyen, H.; Chiasson, V.L.; Chatterjee, P.; Kopriva, S.E.; Young, K.J.; Mitchell, B.M. Interleukin-17 Causes Rho-Kinase-Mediated Endothelial Dysfunction and Hypertension. Cardiovasc. Res. 2013, 97, 696–704. [Google Scholar] [CrossRef]
- Saleh, M.A.; Norlander, A.E.; Madhur, M.S. Inhibition of Interleukin-17A, But Not Interleukin-17F, Signaling Lowers Blood Pressure, and Reduces End-Organ Inflammation in Angiotensin II–Induced Hypertension. JACC Basic Transl. Sci. 2016, 1, 606–616. [Google Scholar] [CrossRef]
- Caillon, A.; Mian, M.O.R.; Fraulob-Aquino, J.C.; Huo, K.-G.; Barhoumi, T.; Ouerd, S.; Sinnaeve, P.R.; Paradis, P.; Schiffrin, E.L. Γδ T Cells Mediate Angiotensin II-Induced Hypertension and Vascular Injury. Circulation 2017, 135, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Norlander, A.E.; Saleh, M.A.; Kamat, N.V.; Ko, B.; Gnecco, J.; Zhu, L.; Dale, B.L.; Iwakura, Y.; Hoover, R.S.; McDonough, A.A.; et al. Interleukin-17A Regulates Renal Sodium Transporters and Renal Injury in Angiotensin II-Induced Hypertension. Hypertension 2016, 68, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Krebs, C.F.; Lange, S.; Niemann, G.; Rosendahl, A.; Lehners, A.; Meyer-Schwesinger, C.; Stahl, R.A.K.; Benndorf, R.A.; Velden, J.; Paust, H.-J.; et al. Deficiency of the Interleukin 17/23 Axis Accelerates Renal Injury in Mice With Deoxycorticosterone Acetate+Angiotensin II–Induced Hypertension. Hypertension 2014, 63, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Thunhorst, R.L.; Yu, Y.; Guo, F.; Beltz, T.G.; Felder, R.B.; Johnson, A.K. Central Renin-Angiotensin System Activation and Inflammation Induced by High-Fat Diet Sensitize Angiotensin II-Elicited Hypertension. Hypertension 2016, 67, 163–170. [Google Scholar] [CrossRef]
- Hanoun, M.; Maryanovich, M.; Arnal-Estapé, A.; Frenette, P.S. Neural Regulation of Hematopoiesis, Inflammation, and Cancer. Neuron 2015, 86, 360–373. [Google Scholar] [CrossRef]
- Afan, A.M.; Broome, C.S.; Nicholls, S.E.; Whetton, A.D.; Miyan, J.A. Bone Marrow Innervation Regulates Cellular Retention in the Murine Haemopoietic System. Br. J. Haematol. 1997, 98, 569–577. [Google Scholar] [CrossRef]
- Santisteban, M.M.; Kim, S.; Pepine, C.J.; Raizada, M.K. Brain–Gut–Bone Marrow Axis. Circ. Res. 2016, 118, 1327–1336. [Google Scholar] [CrossRef]
- Ganta, C.K.; Lu, N.; Helwig, B.G.; Blecha, F.; Ganta, R.R.; Zheng, L.; Ross, C.R.; Musch, T.I.; Fels, R.J.; Kenney, M.J. Central Angiotensin II-Enhanced Splenic Cytokine Gene Expression Is Mediated by the Sympathetic Nervous System. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H1683–H1691. [Google Scholar] [CrossRef]
- Shen, X.Z.; Li, Y.; Li, L.; Shah, K.H.; Bernstein, K.E.; Lyden, P.; Shi, P. Microglia Participate in Neurogenic Regulation of Hypertension. Hypertension 2015, 66, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Marvar, P.J.; Thabet, S.R.; Guzik, T.J.; Lob, H.E.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Central and Peripheral Mechanisms of T-Lymphocyte Activation and Vascular Inflammation Produced by Angiotensin II–Induced Hypertension. Circ. Res. 2010, 107, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of Inflammation, Immunity, and Oxidative Stress in Hypertension: New Insights and Potential Therapeutic Targets. Front. Immunol. 2023, 13, 1098725. [Google Scholar] [CrossRef]
- Pollow, D.P.; Uhrlaub, J.; Romero-Aleshire, M.J.; Sandberg, K.; Nikolich-Zugich, J.; Brooks, H.L.; Hay, M. Sex Differences in T-Lymphocyte Tissue Infiltration and Development of Angiotensin II Hypertension. Hypertension 2014, 64, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Diez-Freire, C.; Jun, J.Y.; Qi, Y.; Katovich, M.J.; Li, Q.; Sriramula, S.; Francis, J.; Sumners, C.; Raizada, M.K. Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension 2010, 56, 297–303. [Google Scholar] [CrossRef]
- Sriramula, S.; Cardinale, J.P.; Francis, J. Inhibition of TNF in the Brain Reverses Alterations in RAS Components and Attenuates Angiotensin II-Induced Hypertension. PLoS ONE 2013, 8, e63847. [Google Scholar] [CrossRef]
- Coppolino, G.; Pisano, A.; Rivoli, L.; Bolignano, D. Renal Denervation for Resistant Hypertension. Cochrane Database Syst. Rev. 2017, 2, CD011499. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. Precision Hypertension. Hypertension 2024, 81, 702–708. [Google Scholar] [CrossRef]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic Analysis of over One Million People Identifies 535 New Loci Associated with Blood Pressure Traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Dominiczak, A.F. Genomics of Hypertension: The Road to Precision Medicine. Nat. Rev. Cardiol. 2021, 18, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Olczak, K.J.; Taylor-Bateman, V.; Nicholls, H.L.; Traylor, M.; Cabrera, C.P.; Munroe, P.B. Hypertension Genetics Past, Present and Future Applications. J. Intern. Med. 2021, 290, 1130–1152. [Google Scholar] [CrossRef]
- Alexander, M.R.; Edwards, T.L.; Harrison, D.G. GWAS for Defining the Pathogenesis of Hypertension: Have They Delivered? Hypertension 2025, 82, 573–582. [Google Scholar] [CrossRef]
- Mani, A. Update in Genetic and Epigenetic Causes of Hypertension. Cell. Mol. Life Sci. CMLS 2024, 81, 201. [Google Scholar] [CrossRef]
- R Muralitharan, R.; Marques, F.Z.; O’Donnell, J.A. Recent Advancements in Targeting the Immune System to Treat Hypertension. Eur. J. Pharmacol. 2024, 983, 177008. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, H.; Hayashi, T.; Iuchi, H.; Isaka, T.; Sakamoto, N.; Kayama, Y.; Tojo, K.; Yoshimura, M.; Utsunomiya, K. Effects of Candesartan in Hypertensive Patients with Type 2 Diabetes Mellitus on Inflammatory Parameters and Their Relationship to Pulse Pressure. Cardiovasc. Diabetol. 2012, 11, 118. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P.; Salvadeo, S.A.T.; Ferrari, I.; Gravina, A.; Mereu, R.; Palumbo, I.; D’Angelo, A.; Cicero, A.F.G. Candesartan Effect on Inflammation in Hypertension. Hypertens. Res. 2010, 33, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sakamoto, M.; Hayashi, T.; Iuchi, H.; Ohashi, K.; Isaka, T.; Sakamoto, N.; Kayama, Y.; Tojo, K.; Yoshimura, M.; et al. Effects of Co-Administration of Candesartan with Pioglitazone on Inflammatory Parameters in Hypertensive Patients with Type 2 Diabetes Mellitus: A Preliminary Report. Cardiovasc. Diabetol. 2013, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Vollenweider, P.; Waeber, G. Angiotensin Receptor Blockers Are Not Associated with Reduced Inflammatory Markers in the General Population. J. Hypertens. 2015, 33, 2173. [Google Scholar] [CrossRef]
- Herrera, J.; Ferrebuz, A.; MacGregor, E.G.; Rodriguez-Iturbe, B. Mycophenolate Mofetil Treatment Improves Hypertension in Patients with Psoriasis and Rheumatoid Arthritis. J. Am. Soc. Nephrol. 2006, 17 (Suppl. 3), S218. [Google Scholar] [CrossRef]
- Lembo, G. From Clinical Observations to Molecular Mechanisms and Back to Patients: The Successful Circuit of the CANTOS Study. Cardiovasc. Res. 2018, 114, e3–e5. [Google Scholar] [CrossRef]
- Leslie, M. Restraining Immunity Could Lower High Blood Pressure. Science 2018, 359, 966–967. [Google Scholar] [CrossRef]
- Varatharajan, N.; Lim, I.G.S.; Anandacoomarasamy, A.; Russo, R.; Byth, K.; Spencer, D.G.; Manolios, N.; Howe, G.B. Methotrexate: Long-Term Safety and Efficacy in an Australian Consultant Rheumatology Practice. Intern. Med. J. 2009, 39, 228–236. [Google Scholar] [CrossRef]
- Kinder, A.J.; Hassell, A.B.; Brand, J.; Brownfield, A.; Grove, M.; Shadforth, M.F. The Treatment of Inflammatory Arthritis with Methotrexate in Clinical Practice: Treatment Duration and Incidence of Adverse Drug Reactions. Rheumatology 2005, 44, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, A.A.; Baghdadi, L.R.; Shanahan, E.M.; Wiese, M.D.; Tommasi, S.; Elliot, D.; Woodman, R.J. Methotrexate, Blood Pressure and Markers of Arterial Function in Patients with Rheumatoid Arthritis: A Repeated Cross-Sectional Study. Ther. Adv. Musculoskelet. Dis. 2017, 9, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Klarenbeek, N.B.; van der Kooij, S.M.; Huizinga, T.J.W.; Goekoop-Ruiterman, Y.P.M.; Hulsmans, H.M.J.; van Krugten, M.V.; Speyer, I.; de Vries-Bouwstra, J.K.; Kerstens, P.J.S.M.; Huizinga, T.W.J.; et al. Blood Pressure Changes in Patients with Recent-Onset Rheumatoid Arthritis Treated with Four Different Treatment Strategies: A Post Hoc Analysis from the BeSt Trial. Ann. Rheum. Dis. 2010, 69, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Takeuchi, T.; Kotani, T.; Yamamoto, N.; Hata, K.; Nagai, K.; Shoda, T.; Takai, S.; Makino, S.; Hanafusa, T. Infliximab, a TNF-α Inhibitor, Reduces 24-h Ambulatory Blood Pressure in Rheumatoid Arthritis Patients. J. Hum. Hypertens. 2014, 28, 165–169. [Google Scholar] [CrossRef]
- Tam, L.-S.; Shang, Q.; Li, E.K.; Wang, S.; Li, R.-J.; Lee, K.-L.; Leung, Y.-Y.; Ying, K.-Y.; Yim, C.-W.; Kun, E.W.; et al. Infliximab Is Associated with Improvement in Arterial Stiffness in Patients with Early Rheumatoid Arthritis—A Randomized Trial. J. Rheumatol. 2012, 39, 2267–2275. [Google Scholar] [CrossRef]
- Van Doornum, S.; McColl, G.; Wicks, I.P. Tumour Necrosis Factor Antagonists Improve Disease Activity but Not Arterial Stiffness in Rheumatoid Arthritis. Rheumatology 2005, 44, 1428–1432. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Aday, A.W.; Ridker, P.M. Antiinflammatory Therapy in Clinical Care: The CANTOS Trial and Beyond. Front. Cardiovasc. Med. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Maki, K.A.; Marques, F.Z.; Cai, J.; Joe, B.; Pepine, C.J.; Pluznick, J.L.; Meyer, K.A.; Kirabo, A.; Bennett, B.J.; et al. Hypertension and the Gut Microbiome: A Science Advisory From the American Heart Association. Hypertension 2025. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M. Dietary Fiber Future Directions: Integrating New Definitions and Findings to Inform Nutrition Research and Communication12. Adv. Nutr. 2013, 4, 8–15. [Google Scholar] [CrossRef]
- Xie, L.; Alam, M.J.; Marques, F.Z.; Mackay, C.R. A Major Mechanism for Immunomodulation: Dietary Fibres and Acid Metabolites. Semin. Immunol. 2023, 66, 101737. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The Gut Microbiome and Hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef]
- Jama, H.A.; Rhys-Jones, D.; Nakai, M.; Yao, C.K.; Climie, R.E.; Sata, Y.; Anderson, D.; Creek, D.J.; Head, G.A.; Kaye, D.M.; et al. Prebiotic Intervention with HAMSAB in Untreated Essential Hypertensive Patients Assessed in a Phase II Randomized Trial. Nat. Cardiovasc. Res. 2023, 2, 35–43. [Google Scholar] [CrossRef]
- Gill, P.A.; Muir, J.G.; Gibson, P.R.; van Zelm, M.C. A Randomized Dietary Intervention to Increase Colonic and Peripheral Blood SCFAs Modulates the Blood B- and T-Cell Compartments in Healthy Humans. Am. J. Clin. Nutr. 2022, 116, 1354–1367. [Google Scholar] [CrossRef]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Morenga, L.T. Carbohydrate Quality and Human Health: A Series of Systematic Reviews and Meta-Analyses. Lancet 2019, 393, 434–445, Erratum in Lancet 2019, 393, 406. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Romero, M.; Jiménez, R.; Sánchez, M.; Pérez-Vizcaíno, F.; Duarte, J. Antihypertensive Effects of Probiotics. Curr. Hypertens. Rep. 2017, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Musazadeh, V.; Ghalichi, F.; Kavyani, Z.; Nasernia, R.; Parang, M.; Jamilian, P.; Jamilian, P.; Fakhr, L.; Ostadrahimi, A.; et al. Effects of Probiotics Supplementation on Blood Pressure: An Umbrella Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, S.; Sun, J.; Buys, N.; Jayasinghe, R. Effect of Probiotics on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Hypertension 2014, 64, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Li, C.; Wu, D.; Buys, N.; Wang, W.; Fan, H.; Sun, J. Effects of Probiotics on Patients with Hypertension: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 34. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, H.; Zhuang, R.; Fan, Q. Immunotherapy for Hypertensive End-Organ Damage: A New Therapeutic Strategy. Essays Biochem. 2025, EBC20243000. [Google Scholar] [CrossRef]

| Authors | Methodology | Results |
|---|---|---|
| Ebringer et al. [14] | A case–control study measuring IgG levels in 118 patients with severe hypertension and 163 normotensive individuals. | Serum IgG levels were significantly higher in 118 patients with severe hypertension compared to a group of 163 normotensive blood donors. |
| Adlin et al. [15] | Case–control study measuring serum immunoglobulin levels in 52 hypertensive patients and 52 normotensive controls. | Contrary to previous reports, hypertensive subjects did not have higher levels of IgG or IgA than controls. The authors attributed this to the mild elevation of blood pressure. |
| Mirhafez et al. [16] | A case–control study measuring blood levels of 12 cytokines and growth factors in 155 individuals with hypertension and 148 normotensive individuals. | Hypertensive subjects had higher concentrations of IL-1α, -2, -8, TNF-α, IFN-γ, MCP-1, EGF, and VEGF. They also had lower levels of the anti-inflammatory cytokine IL-10 (p < 0.05) compared to healthy individuals. |
| Sesso et al. [17] | Prospective cohort study starting in 1992 with 20,525 U.S. healthcare professionals aged 45 or older, aiming to examine CRP levels. | During follow-up, 5365 women developed hypertension. C-reactive protein was significantly associated with an increased risk of developing hypertension in all prespecified subgroups evaluated, including those with very low baseline blood pressure and those without traditional coronary risk factors. |
| Bautista et al. [18] | A cross-sectional study in 300 individuals evaluating whether circulating CRP levels are independently related to essential hypertension. | Plasma CRP level is an independent risk factor for hypertension. The unadjusted prevalence of hypertension was 58.7% in the highest quartile of CRP, compared to only 34.7% in the lowest quartile. |
| Youn et al. [19] | Case–control study evaluating renal cell infiltration through immunohistochemical staining in kidney biopsy samples from 71 patients with hypertensive nephrosclerosis and 71 control subjects. | Higher numbers of CD4+ and CD8+ T cells were found infiltrating the tubulointerstitial system of hypertensive nephrosclerosis patients compared to normotensive control subjects. |
| Navarro et al. [20] | Case–control study evaluating the relationship between inflammatory parameters (CRP, serum, and urinary TNF-α) and markers of preclinical TOD (LVH and microalbuminuria) in 40 newly diagnosed, never-treated essential hypertension patients, compared to 21 healthy controls. | Urinary TNF-alpha is independently correlated with urinary albumin excretion, suggesting inflammation may contribute to TOD development. Additionally, urinary TNF-α excretion could be an early marker of preclinical TOD in hypertensive patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano, A.; Parra, H.; Ariza, D.; Marquina, M.; Duran, P.; Calvo, M.J.; Nava, M.; Ross, O.; Contreras-Velásquez, J.C.; Rivera-Porras, D.; et al. Immunology of Hypertension: Pathophysiological and Therapeutic Aspects. Int. J. Mol. Sci. 2025, 26, 9921. https://doi.org/10.3390/ijms26209921
Manzano A, Parra H, Ariza D, Marquina M, Duran P, Calvo MJ, Nava M, Ross O, Contreras-Velásquez JC, Rivera-Porras D, et al. Immunology of Hypertension: Pathophysiological and Therapeutic Aspects. International Journal of Molecular Sciences. 2025; 26(20):9921. https://doi.org/10.3390/ijms26209921
Chicago/Turabian StyleManzano, Alexander, Heliana Parra, Daniela Ariza, Maria Marquina, Pablo Duran, María J. Calvo, Manuel Nava, Omar Ross, Julio César Contreras-Velásquez, Diego Rivera-Porras, and et al. 2025. "Immunology of Hypertension: Pathophysiological and Therapeutic Aspects" International Journal of Molecular Sciences 26, no. 20: 9921. https://doi.org/10.3390/ijms26209921
APA StyleManzano, A., Parra, H., Ariza, D., Marquina, M., Duran, P., Calvo, M. J., Nava, M., Ross, O., Contreras-Velásquez, J. C., Rivera-Porras, D., & Bermúdez, V. (2025). Immunology of Hypertension: Pathophysiological and Therapeutic Aspects. International Journal of Molecular Sciences, 26(20), 9921. https://doi.org/10.3390/ijms26209921






