Scorpion Venom as a Source of Cancer Drugs: A Comprehensive Proteomic Analysis and Therapeutic Potential
Abstract
1. Introduction
1.1. The Global Cancer Burden
1.2. Natural Products and Venom-Drived Compounds
1.3. Historical Use of Venom in Medicine
2. Literature Search Strategy and Scope of the Review
3. Composition and Biochemistry of Scorpion Venom
3.1. Proteomic Characterization of Scorpion Venom
3.2. Major Protein and Peptide Components of Scorpion Venom
3.2.1. Neurotoxins
3.2.2. Antimicrobial and Cytolytic Peptides
3.2.3. Enzyme Inhibitors
3.2.4. Disulfide-Rich Peptides
3.2.5. Enzymatic Components
4. Molecular Mechanisms of Anticancer Activity
4.1. Induction of Apoptosis
4.2. Cellular Signaling and Cycle Disruption
5. Scorpion Venom Peptides: Anticancer Activities and Mechanisms of Tumor Targeting
5.1. Apoptosis Induction
5.1.1. TsAP-1 and TsAP-2 from Tityus serrulatus
5.1.2. Bengalin from Heterometrus bengalensis and Other Novel Peptides
5.1.3. Neopladine 1 and Neopladine 2 from Tityus discrepans
5.2. Ion Channel Modulation
5.2.1. AGAP-SYPU2 from Buthus martensii Karsch
5.2.2. BotCl from Buthus occitanus tunetanus
5.2.3. Iberiotoxin (IbTX) from Hottentotta tamulus
5.3. Cell Cycle Arrest
5.3.1. Gonearrestide from Androctonus mauritanicus
5.3.2. PESV from Buthus martensii Karsch
5.4. Membrane Disruption and Tumor Microenvironment
5.4.1. Hyaluronidase BmHYA1 from Buthus martensii Karsch
5.4.2. RK1 from Buthus occitanus tunetanus
5.4.3. Vmct1 from Vaejovis mexicanus
5.4.4. AcrAP1/AcrAP2 from Androctonus crassicauda
5.4.5. Pantinins 1-3 from Pandinus imperator
5.5. Multifunctional Peptides
5.5.1. Chlorotoxin from Leiurus hebraeus and Derivatives
5.5.2. Maurocalcine from Scorpio maurus palmatus and Related Peptides
5.5.3. AsTs-1 from Androctonus australis
6. Immunomodulatory Effects
6.1. Innate Immune Response Modulation
6.2. Adaptive Immunity Enhancement
6.3. Cytokine Profile Alterations
6.4. Immune Cell Activation and Regulation
6.5. Potential for Immunotherapy Enhancement
7. Diagnostic Applications
7.1. Early Detection Methods
7.2. Biomarker Development
7.3. Therapeutic Monitoring
7.4. Integration with Current Diagnostic Tools
8. Drug Development and Delivery Systems
8.1. Peptide Modification Strategies
8.2. Nanoparticle-Based Delivery
8.3. Targeted Delivery Systems
9. Clinical Studies and Trials
9.1. Preclinical Studies
9.2. Clinical Trials
9.2.1. TM-601 (131I-Chlorotoxin Conjugate)
9.2.2. BLZ-100 (Tozuleristide, “Tumor Paint”)
10. Current Challenges and Future Directions
10.1. Production and Scale-Up Issues
10.2. Regulatory Considerations
10.3. Cost and Accessibility
10.4. Research Gaps
10.5. Future Research Directions
11. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ΔΨm | Mitochondrial membrane potential. |
| A2780 | Human Ovarian Cancer Cell Line A2780. |
| Aah II | Aah II neurotoxin. |
| AaTs-1 | Androctonus australis Toxin-1. |
| ABC | ATP-binding cassette. |
| AcrAP1 | Androctonus crassicauda Antimicrobial Peptides 1. |
| AcrAP2 | Androctonus crassicauda Antimicrobial Peptides 2. |
| AGAP | Analgesic–Antitumor Peptide. |
| AGAP-SYPU2 | Analgesic–Antitumor Peptide Variant SYPU2. |
| AI | Artificial intelligence. |
| AK | Adenylate kinase. |
| Akt | Protein Kinase B. |
| AMPK | AMP-Activated Protein Kinase. |
| AMPs | Antimicrobial Peptides. |
| AP-1 | Activator Protein 1. |
| Arg1 | Arginase-1. |
| ATMs | Tumor-Associated Macrophages. |
| ATP | Adenosine Triphosphate. |
| AuNP-antibody | Gold nanoparticle conjugated to antibody. |
| B16-F10 | Murine Melanoma Cell Line B16-F10. |
| Bax | Bcl-2-Associated X Protein. |
| BBB | Blood–Brain Barrier. |
| Bcl-2 | B-cell Lymphoma 2. |
| BKCa | Large-Conductance Calcium-Activated Potassium Channel. |
| BLZ-100 | Chlorotoxin-based Tumor Paint Bioconjugate. |
| BmHYA1 | Buthus martensii Karsch Hyaluronidase 1. |
| BmK | Buthus martensii Karsch. |
| BmKCT | Buthus martensii Karsch chlorotoxin. |
| BmKDfsin4 | Derived Defensin-4 peptide. |
| BmKn1 | Antimicrobial peptides derived from Buthus martensii Karsch venom. |
| BmKn2–7 | Anticancer peptides derived from Buthus martensii Karsch venom. |
| BotCl | Buthus occitanus tunetanus Chloride Channel Blocker. |
| BTB | Blood–tumor barrier. |
| Buthicyclin | Cyclic peptide derived from Defensin-4. |
| C3 | Complement component 3. |
| C6 | Glioma cell line. |
| Ca2+ | Calcium ion. |
| CAM | Chorioallantoic Membrane. |
| Caspase-3 | Effector caspase enzyme. |
| CD | Circular dichroism. |
| CD40L | CD40 ligand. |
| CD44 | Cluster of Differentiation 44. |
| CD44v6 | CD44 Variant Isoform 6. |
| CDK | Cyclin-Dependent Kinase. |
| CDK4 | Cyclin-Dependent Kinase 4. |
| CDKI | Cyclin-Dependent Kinase Inhibitor. |
| CDKIs | Cyclin-Dependent Kinase Inhibitors. |
| CDKs | Cyclin-dependent kinases. |
| cDNA | Complementary DNA. |
| ChTX | Charybdotoxin. |
| CIF8 | Cancer Inhibitory Fractions 8. |
| CIF9 | Cancer Inhibitory Fractions 9. |
| ClC-3 | Chloride Channel Protein 3. |
| Cm28 | Centruroides margaritatus peptide/toxin. |
| CN | Chitosan nanoparticles. |
| CNS | Central nervous system. |
| CORT | Cortistatin. |
| Css54 | Cationic antimicrobial peptide 54. |
| CTX | Chlorotoxin. |
| D3 | Cyclin D3. |
| DISC | Death-inducing signaling complex. |
| DNA | Deoxyribonucleic acid. |
| DU145 | Human Prostate Carcinoma Cell Line DU145. |
| DSB | Disulfide Bonds |
| ECM | Extracellular Matrix. |
| EGFR | Epidermal Growth Factor Receptor. |
| ELISA | Enzyme-linked immunosorbent assay. |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1/2. |
| F3II | Murine mammary adenocarcinoma cell line F3II. |
| FasL | Apoptosis-inducing ligand binding to Fas receptor. |
| FDA | Food and Drug Administration. |
| FoxO | Forkhead Box O. |
| FPRL-1 | Formyl Peptide Receptor-Like 1. |
| FTox-G50 | Scorpion venom fraction Toxin G50. |
| G0 | Cell cycle phases of resting. |
| G1 | Gap 1 phase of the Cell Cycle. |
| GK | Glucokinase. |
| H22 | Murine Hepatocellular Carcinoma Cell Line H22. |
| H460/NCI-H460 | Human Non-Small Cell Lung Carcinoma Cell Line. |
| HA | Hyaluronic Acid. |
| HBsAg-specific | Hepatitis B surface antigen-specific. |
| HCC | Hepatocellular carcinoma. |
| HCT116 | Human Colorectal Carcinoma Cell Line HCT116. |
| HCT-8 | Colorectal cancer cell line. |
| HeLa | Human Cervical Cancer Cell Line HeLa. |
| HER2 | Human epidermal growth receptor 2. |
| HPLC | High-Performance Liquid Chromatography. |
| HSP70 | Heat shock protein 70. |
| HSP90 | Heat shock protein 90. |
| HsTX1 | Heterometrus scaber toxin 1. |
| hTERTC27 | C-terminal 27 kDa polypeptide fragment of human telomerase reverse transcriptase. |
| HUVEC | Human Umbilical Vein Endothelial Cells. |
| IbTX | Iberiotoxin. |
| IC50 | Half Maximal Inhibitory Concentration. |
| IFN-γ | Interferon-gamma. |
| IGR39 | Human Melanoma Cell Line IGR39. |
| IL-10 | Interleukin-10. |
| IL-12p40 | Interleukin-12 subunit p40. |
| IL-12p70 | Biologically active heterodimeric form of interleukin-12. |
| IL-1β | Interleukin-1 beta. |
| IL-23 | Interleukin-23. |
| IL-2R | Interleukin-2 receptor. |
| IL-4 | Interleukin-4. |
| IL-6 | Interleukin-6. |
| IL-8 | Interleukin-8. |
| iTRAQ | Isobaric tags for relative and absolute quantification. |
| JAK | Janus kinase. |
| Kd | Equilibrium Dissociation Constant. |
| Kv1.1 | Voltage-gated Potassium Channel Subfamily A Member 1. |
| Kv1.3 | Voltage-gated Potassium Channel Subfamily A Member 3. |
| Kv10.1 | Voltage-gated potassium channel 10.1. |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry. |
| LD50 | Median lethal dose. |
| LS174 | Human Colon Adenocarcinoma Cell Line LS174. |
| LSPR | Localized plasmon resonance. |
| M1 macrophages | Classically activated macrophages. |
| M2 macrophages | Alternatively activated macrophages. |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. |
| MAP kinases | Mitogen-activated protein kinases. |
| MAPK p38 | p38 Mitogen-Activated Protein Kinase. |
| MCa | Micaelase. |
| MCF-7 | Human Breast Cancer Cell Line MCF-7. |
| MDA-MB-231 | Human Triple-Negative Breast Cancer Cell Line. |
| MDA-MB-435s | Human Breast Carcinoma Cell Line. |
| Meuk7–3 | Peptide fraction Meuk7–3. |
| MgTX | Margatoxin. |
| MiniCTX3 | Charybdotoxin analog peptide. |
| MMP-2 | Matrix metalloproteinase-2. |
| MRI | Magnetic resonance imaging. |
| MS | Mass spectrometry. |
| MS/MS | Tandem mass spectrometry. |
| MT1-MMP | Membrane-Type 1 Matrix Metalloproteinase. |
| mTOR | Mechanistic Target of Rapamycin. |
| MyD88 | Myeloid Differentiation Primary Response 88. |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate. |
| Nav1.4 | Voltage-gated Sodium Channel, Alpha Subunit 1.4. |
| Nav1.5 | Voltage-gated Sodium Channel, Alpha Subunit 1.5. |
| Nav1.7 | Voltage-gated Sodium Channel, Alpha Subunit 1.7. |
| Nav1.8 | Voltage-gated Sodium Channel, Alpha Subunit 1.8. |
| NCI-H460 | Human Lung Cancer Cell Line NCI-H460. |
| NDBP | Non-Disulfide Bridged Peptide. |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer. |
| NGF | Nerve growth factor. |
| NGS | Next-generation sequencing. |
| NOS2 | Inducible Nitric Oxide Synthase. |
| NR | Not reported |
| NRP1 | Neuropilin-1. |
| OGFR | Opioid growth factor receptor. |
| FOXO3a | Forkhead box O3a. |
| p21 | Cyclin-dependent kinase inhibitor 1A. |
| p27 | Cyclin-dependent kinase inhibitor 1B. |
| p53 | Tumor suppressor protein. |
| PAMAM | Polyamidoamine. |
| PARP | Poly polymerase. |
| PC-3 | Human Prostate Carcinoma Cell Line PC-3. |
| PEG | polyethylene glycol. |
| PEG-CTX | Polyethylene glycol–chlorotoxin conjugate. |
| PEGylation | Polyethylene glycol conjugation. |
| PESV | Polypeptide Extract from Scorpion Venom. |
| PI3K | Phosphatidylinositol 3-Kinase. |
| PLA-PEG | Polylactic acid–polyethylene glycol copolymer. |
| PLA2 | Phospholipase A2. |
| PPAR | Peroxisome Proliferator-Activated Receptor. |
| PTEN | Phosphatase and tensin homolog |
| PTMs | Post-translational modifications. |
| RAW264.7 | Macrophage-like cell line. |
| RK1 | Scorpion-derived Peptide from Buthus occitanus tunetanus. |
| ROS | Reactive oxygen species. |
| S1/S2 | Designations of Stigmurin analogs. |
| S180 | Murine Sarcoma Cell Line S180. |
| SDS-PAGE | Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis. |
| SEC | Size exclusion chromatography. |
| SEM | Scanning Electron Microscopy. |
| SHG-44 | Human Glioma Cell Line SHG-44. |
| SPECT | Single-Photon Emission Computed Tomography. |
| SPR | Surface plasmon resonance. |
| STAT3 | Transcription factor. |
| STAT | Signal transducer and activator of transcription. |
| SVS-1 | Anticancer Beta-Hairpin Peptide SVS-1. |
| TAMs | Tumor-associated macrophages. |
| tBid | Truncated Bid. |
| TfR | Transferrin receptor. |
| TGF-β | Transforming Growth Factor Beta. |
| Th1/Th2/Th17 cells | T helper cell subsets. |
| TIMPs | Tissue Inhibitors of Metalloproteinases. |
| TLR2 | Toll-Like Receptors 2. |
| TLR4 | Toll-Like Receptors 4. |
| TM-601 | Synthetic Chlorotoxin Derivative Radiolabeled with Iodine-131. |
| TME | Tumor Microenvironment. |
| TMT | Tandem mass tags. |
| TNF-α | Tumor Necrosis Factor-alpha. |
| TRAIL | Tumor necrosis factor-related apoptosis-inducing ligand. |
| TRPV1+ | Transient Receptor Potential Vanilloid 1–positive. |
| Ts1 | Major neurotoxin from Tityus serrulatus venom. |
| Tsv | Tityus stigmurus venom. |
| TzII | Tityus zulianus toxin isoforms II. |
| TzIII | Tityus zulianus toxin isoforms III. |
| U87 | Human Glioblastoma Cell Line U87. |
| UHPLC-QTOF-MS | Liquid chromatography coupled to mass spectrometry. |
| VERO | African Green Monkey Kidney Epithelial Cell Line VERO. |
| Vm24 | Peptide from Vaejovis mexicanus. |
| Vmct1 | Vaejovis mexicanus cationic peptide 1. |
| Vmct1-K | Lysine-substituted variant of Vmct1. |
| VRE | Vancomycin-Resistant Enterococcus. |
| α1β1 | Alpha-1 Beta-1 Integrin. |
| αvβ3 | Alpha-V Beta-3 Integrin. |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Amjad, M.T.; Chidharla, A.; Kasi, A. Cancer Chemotherapy; StatPearls [Internet]; Stat Pearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The Challenge of Drug Resistance in Cancer Treatment: A Current Overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef]
- Haider, T.; Pandey, V.; Banjare, N.; Gupta, P.N.; Soni, V. Drug Resistance in Cancer: Mechanisms and Tackling Strategies. Pharmacol. Rep. 2020, 72, 1125–1151. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Shah, B.; Dong, X. Current Status of In Vitro Models of the Blood-Brain Barrier. Curr. Drug Deliv. 2022, 19, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Ferreira, S.H. A Bradykinin-Potentiating Factor (Bpf) Present in Venom of Bothrops jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163. [Google Scholar] [CrossRef]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-Potentiating Peptides: Beyond Captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.R.; Scarborough, R.M. Clinical Pharmacology of Eptifibatide. Am. J. Cardiol. 1997, 80, B11–B20. [Google Scholar] [CrossRef]
- McIntosh, M.; Cruz, L.J.; Hunkapiller, M.W.; Gray, W.R.; Olivera, B.M. Isolation and Structure of a Peptide Toxin from the Marine Snail Conus magus. Arch. Biochem. Biophys. 1982, 218, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.; Kleinman, W.A.; Singh, L.; Singh, G.; Raufman, J.P. Isolation and Characterization of Exendin-4, an Exendin-3 Analogue, from Heloderma suspectum Venom. Further Evidence for an Exendin Receptor on Dispersed Acini from Guinea Pig Pancreas. J. Biol. Chem. 1992, 267, 7402–7405. [Google Scholar] [CrossRef]
- Ahmadi, S.; Knerr, J.M.; Argemi, L.; Bordon, K.C.F.; Pucca, M.B.; Cerni, F.A.; Arantes, E.C.; Çalışkan, F.; Laustsen, A.H. Scorpion Venom: Detriments and Benefits. Biomedicines 2020, 8, 118. [Google Scholar] [CrossRef]
- Ortiz, E.; Gurrola, G.B.; Schwartz, E.F.; Possani, L.D. Scorpion Venom Components as Potential Candidates for Drug Development. Toxicon 2015, 93, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.; Ullah, Z.; Al Balowi, A.; Islam, M. In Vitro Determination of the Efficacy of Scorpion Venoms as Anti-Cancer Agents Against Colorectal Cancer Cells: A Nano-Liposomal Delivery Approach. Int. J. Nanomed. 2017, 12, 559–574. [Google Scholar] [CrossRef]
- Montero-Dominguez, P.A.; Corzo, G. Characterization of the Coupling Mechanism of Scorpion β-Neurotoxins on the Voltage-Gated Sodium Channel HNav1.6. J. Biomol. Struct. Dyn. 2023, 41, 14419–14427. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Quintero-Hernández, V.; Possani, L.D. Scorpion Venom Gland Transcriptomics and Proteomics: An Overview. In Venom Genomics and Proteomics; Springer: Dordrecht, The Netherlands, 2014; pp. 1–17. [Google Scholar]
- Marchi, F.C.; Mendes-Silva, E.; Rodrigues-Ribeiro, L.; Bolais-Ramos, L.G.; Verano-Braga, T. Toxinology in the Proteomics Era: A Review on Arachnid Venom Proteomics. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210034. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Riyasdeen, A.; Islam, M. Scorpion Venom Causes Upregulation of P53 and Downregulation of Bcl-xL and BID Protein Expression by Modulating Signaling Proteins Erk1/2 and STAT3, and DNA Damage in Breast and Colorectal Cancer Cell Lines. Integr. Cancer Ther. 2018, 17, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Hou, X.; Ge, L.; Li, R.; Zhou, M.; Wang, H.; Wang, L.; Wei, M.; Chen, T.; Shaw, C. Cationicity-Enhanced Analogues of the Antimicrobial Peptides, AcrAP1 and AcrAP2, from the Venom of the Scorpion, Androctonus crassicauda, Display Potent Growth Modulation Effects on Human Cancer Cell Lines. Int. J. Biol. Sci. 2014, 10, 1097–1107. [Google Scholar] [CrossRef]
- Pedron, C.N.; de Oliveira, C.S.; da Silva, A.F.; Andrade, G.P.; da Silva Pinhal, M.A.; Cerchiaro, G.; da Silva Junior, P.I.; da Silva, F.D.; Torres, M.D.T.; Oliveira, V.X. The Effect of Lysine Substitutions in the Biological Activities of the Scorpion Venom Peptide VmCT1. Eur. J. Pharm. Sci. 2019, 136, 104952. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A.; Kampo, S.; Sackey, M.; Hechavarria, M.E.; Buunaaim, A.D.B. The Pivotal Potentials of Scorpion Buthus martensii Karsch-Analgesic-Antitumor Peptide in Pain Management and Cancer. Evid.-Based Complement. Altern. Med. 2020, 2020, 4234273. [Google Scholar] [CrossRef]
- Shao, J.-H.; Cui, Y.; Zhao, M.-Y.; Wu, C.-F.; Liu, Y.-F.; Zhang, J.-H. Purification, Characterization, and Bioactivity of a New Analgesic-Antitumor Peptide from Chinese Scorpion Buthus martensii Karsch. Peptides 2014, 53, 89–96. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, X.; Ye, T.; Huo, J.; Liu, C.; Zhang, S.; Cao, P. Analgesic-Antitumor Peptide Inhibits Proliferation and Migration of SHG-44 Human Malignant Glioma Cells. J. Cell Biochem. 2011, 112, 2424–2434. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, S.; Tang, J.; Hou, H.; Wang, L.; Lin, H.; Zhang, X.; Jin, M. Two Small Peptides from Buthus martensii Hydrolysates Exhibit Antitumor Activity Through Inhibition of TNF-α-Mediated Signal Transduction Pathways. Life 2025, 15, 105. [Google Scholar] [CrossRef]
- Baradaran, M.; Pashmforoosh, N. Peptides with Diverse Functions from Scorpion Venom: A Great Opportunity for the Treatment of a Wide Variety of Diseases. Payam-E-Marefat-Kabul Educ. Univ. 2023, 27, 84–99. [Google Scholar] [CrossRef]
- Das Gupta, S.; Halder, B.; Gomes, A.; Gomes, A. Bengalin Initiates Autophagic Cell Death Through ERK–MAPK Pathway Following Suppression of Apoptosis in Human Leukemic U937 Cells. Life Sci. 2013, 93, 271–276. [Google Scholar] [CrossRef]
- Das Gupta, S.; Gomes, A.; Debnath, A.; Saha, A.; Gomes, A. Apoptosis Induction in Human Leukemic Cells by a Novel Protein Bengalin, Isolated from Indian Black Scorpion Venom: Through Mitochondrial Pathway and Inhibition of Heat Shock Proteins. Chem. Biol. Interact. 2010, 183, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-Based Peptide Therapy: Insights into Anti-Cancer Mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef] [PubMed]
- Sarfo-Poku, C.; Eshun, O.; Lee, K.H. Medical Application of Scorpion Venom to Breast Cancer: A Mini-Review. Toxicon 2016, 122, 109–112. [Google Scholar] [CrossRef]
- Feng, L.; Gao, R.; Gopalakrishnakone, P. Isolation and Characterization of a Hyaluronidase from the Venom of Chinese Red Scorpion Buthus martensi. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2008, 148, 250–257. [Google Scholar] [CrossRef]
- Feng, L.; Gao, R.; Meng, J.; Gopalakrishnakone, P. Cloning and Molecular Characterization of BmHYA1, a Novel Hyaluronidase from the Venom of Chinese Red Scorpion Buthus martensi Karsch. Toxicon 2010, 56, 474–479. [Google Scholar] [CrossRef] [PubMed]
- El-Qassas, J.; Abd El-Atti, M.; El-Badri, N. Harnessing the Potency of Scorpion Venom-Derived Proteins: Applications in Cancer Therapy. Bioresour. Bioprocess. 2024, 11, 93. [Google Scholar] [CrossRef]
- Xia, X.; Liu, R.; Li, Y.; Xue, S.; Liu, Q.; Jiang, X.; Zhang, W.; Ding, K. Cloning and Molecular Characterization of Scorpion Buthus martensi Venom Hyaluronidases: A Novel Full-Length and Diversiform Noncoding Isoforms. Gene 2014, 547, 338–345. [Google Scholar] [CrossRef]
- Gao, F.; Li, H.; Chen, Y.-D.; Yu, X.-N.; Wang, R.; Chen, X.-L. Upregulation of PTEN Involved in Scorpion Venom-Induced Apoptosis in a Lymphoma Cell Line. Leuk. Lymphoma 2009, 50, 633–641. [Google Scholar] [CrossRef]
- Rapôso, C. Scorpion and Spider Venoms in Cancer Treatment: State of the Art, Challenges, and Perspectives. J. Clin. Transl. Res. 2017, 3, 233–249. [Google Scholar] [CrossRef]
- Panja, K.; Buranapraditkun, S.; Roytrakul, S.; Kovitvadhi, A.; Lertwatcharasarakul, P.; Nakagawa, T.; Limmanont, C.; Jaroensong, T. Scorpion Venom Peptide Effects on Inhibiting Proliferation and Inducing Apoptosis in Canine Mammary Gland Tumor Cell Lines. Animals 2021, 11, 2119. [Google Scholar] [CrossRef]
- Satitmanwiwat, S.; Changsangfa, C.; Khanuengthong, A.; Promthep, K.; Roytrakul, S.; Arpornsuwan, T.; Saikhun, K.; Sritanaudomchai, H. The Scorpion Venom Peptide BmKn2 Induces Apoptosis in Cancerous but Not in Normal Human Oral Cells. Biomed. Pharmacother. 2016, 84, 1042–1050. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, J.; Qiao, W.; Chen, K. Recent Advances in Diagnosis and Treatment of Gliomas Using Chlorotoxin-Based Bioconjugates. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 385–405. [Google Scholar] [PubMed]
- Dardevet, L.; Rani, D.; El Aziz, T.A.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; De Waard, M. Chlorotoxin: A Helpful Natural Scorpion Peptide to Diagnose Glioma and Fight Tumor Invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef]
- Jlassi, A.; Mekni-Toujani, M.; Ferchichi, A.; Gharsallah, C.; Malosse, C.; Chamot-Rooke, J.; ElAyeb, M.; Ghram, A.; Srairi-Abid, N.; Daoud, S. BotCl, the First Chlorotoxin-Like Peptide Inhibiting Newcastle Disease Virus: The Emergence of a New Scorpion Venom AMPs Family. Molecules 2023, 28, 4355. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Inbar, O.; Zaaroor, M. Glioblastoma Multiforme Targeted Therapy: The Chlorotoxin Story. J. Clin. Neurosci. 2016, 33, 52–58. [Google Scholar] [CrossRef]
- Naseem, M.U.; Carcamo-Noriega, E.; Beltrán-Vidal, J.; Borrego, J.; Szanto, T.G.; Zamudio, F.Z.; Delgado-Prudencio, G.; Possani, L.D.; Panyi, G. Cm28, a Scorpion Toxin Having a Unique Primary Structure, Inhibits KV1.2 and KV1.3 with High Affinity. J. Gen. Physiol. 2022, 154, e202213146. [Google Scholar] [CrossRef]
- Nosouhian, M.; Rastegari, A.A.; Shahanipour, K.; Ahadi, A.M.; Sajjadieh, M.S. Anticancer Potentiality of Hottentotta saulcyi Scorpion Curd Venom Against Breast Cancer: An In Vitro and In Vivo Study. Sci. Rep. 2024, 14, 24607. [Google Scholar] [CrossRef] [PubMed]
- Ghezellou, P.; Jakob, K.; Atashi, J.; Ghassempour, A.; Spengler, B. Mass-Spectrometry-Based Lipidome and Proteome Profiling of Hottentotta saulcyi (Scorpiones: Buthidae) Venom. Toxins 2022, 14, 370. [Google Scholar] [CrossRef]
- Li, B.; Lyu, P.; Xi, X.; Ge, L.; Mahadevappa, R.; Shaw, C.; Kwok, H.F. Triggering of Cancer Cell Cycle Arrest by a Novel Scorpion Venom-Derived Peptide-Gonearrestide. J. Cell. Mol. Med. 2018, 22, 4460–4473. [Google Scholar] [CrossRef]
- Moradi, M.; Najafi, R.; Amini, R.; Solgi, R.; Tanzadehpanah, H.; Esfahani, A.M.; Saidijam, M. Remarkable Apoptotic Pathway of Hemiscorpius lepturus Scorpion Venom on CT26 Cell Line. Cell Biol. Toxicol. 2019, 35, 373–385. [Google Scholar] [CrossRef]
- Lebrun, B.; Romi-Lebrun, R.; Martin-Eauclaire, M.-F.; Yasuda, A.; Ishiguro, M.; Oyama, Y.; Pongs, O.; Nakajima, T. A Four-Disulphide-Bridged Toxin, with High Affinity towards Voltage-Gated K+ Channels, Isolated from Heterometrus spinnifer (Scorpionidae) Venom. Biochem. J. 1997, 328, 321–327. [Google Scholar] [CrossRef]
- Candia, S.; Garcia, M.L.; Latorre, R. Mode of Action of Iberiotoxin, a Potent Blocker of the Large Conductance Ca2+-Activated K+ Channel. Biophys. J. 1992, 63, 583–590. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Le Guennec, J.-Y.; Besson, P. Description and Role in Proliferation of Iberiotoxin-Sensitive Currents in Different Human Mammary Epithelial Normal and Cancerous Cells. Biochim. Biophys. Acta 2004, 1667, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, D.; Mlayah-Bellalouna, S.; Jebali, J.; Abdelkafi-Koubaa, Z.; Souid, S.; Moslah, W.; Othman, H.; Luis, J.; ElAyeb, M.; Marrakchi, N.; et al. Functional Role of Kv1.1 and Kv1.3 Channels in the Neoplastic Progression Steps of Three Cancer Cell Lines, Elucidated by Scorpion Peptides. Int. J. Biol. Macromol. 2018, 111, 1146–1155. [Google Scholar] [CrossRef]
- Koschak, A.; Koch, R.O.; Liu, J.; Kaczorowski, G.J.; Reinhart, P.H.; Garcia, M.L.; Knaus, H.-G. [125I]Iberiotoxin-D19Y/Y36F, the First Selective, High Specific Activity Radioligand for High-Conductance Calcium-Activated Potassium Channels. Biochemistry 1997, 36, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.; Calderone, V.; Chericoni, S.; Morelli, I. Natural Modulators of Large-Conductance Calcium-Activated Potassium Channels. Planta Med. 2003, 69, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.H.; Choi, S.Y.; Ryu, P.D.; Lee, S.Y. Anti-Proliferative Effect of Kv1.3 Blockers in A549 Human Lung Adenocarcinoma In Vitro and In Vivo. Eur. J. Pharmacol. 2011, 651, 26–32. [Google Scholar] [CrossRef]
- Boisseau, S.; Mabrouk, K.; Ram, N.; Garmy, N.; Collin, V.; Tadmouri, A.; Mikati, M.; Sabatier, J.-M.; Ronjat, M.; Fantini, J.; et al. Cell Penetration Properties of Maurocalcine, a Natural Venom Peptide Active on the Intracellular Ryanodine Receptor. Biochim. Biophys. Acta (BBA)—Biomembr. 2006, 1758, 308–319. [Google Scholar] [CrossRef]
- Ram, N.; Aroui, S.; Jaumain, E.; Bichraoui, H.; Mabrouk, K.; Ronjat, M.; Lortat-Jacob, H.; De Waard, M. Direct Peptide Interaction with Surface Glycosaminoglycans Contributes to the Cell Penetration of Maurocalcine. J. Biol. Chem. 2008, 283, 24274–24284. [Google Scholar] [CrossRef]
- Ram, N.; Weiss, N.; Texier-Nogues, I.; Aroui, S.; Andreotti, N.; Pirollet, F.; Ronjat, M.; Sabatier, J.-M.; Darbon, H.; Jacquemond, V.; et al. Design of a Disulfide-Less, Pharmacologically Inert, and Chemically Competent Analog of Maurocalcine for the Efficient Transport of Impermeant Compounds into Cells. J. Biol. Chem. 2008, 283, 27048–27056. [Google Scholar] [CrossRef]
- Aroui, S.; Ram, N.; Appaix, F.; Ronjat, M.; Kenani, A.; Pirollet, F.; De Waard, M. Maurocalcine as a Non Toxic Drug Carrier Overcomes Doxorubicin Resistance in the Cancer Cell Line MDA-MB 231. Pharm. Res. 2009, 26, 836–845. [Google Scholar] [CrossRef] [PubMed]
- D’Suze, G.; Rosales, A.; Salazar, V.; Sevcik, C. Apoptogenic Peptides from Tityus discrepans Scorpion Venom Acting Against the SKBR3 Breast Cancer Cell Line. Toxicon 2010, 56, 1497–1505. [Google Scholar] [CrossRef]
- Zeng, X.-C.; Zhou, L.; Shi, W.; Luo, X.; Zhang, L.; Nie, Y.; Wang, J.; Wu, S.; Cao, B.; Cao, H. Three New Antimicrobial Peptides from the Scorpion Pandinus imperator. Peptides 2013, 45, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Crusca, E.; Basso, L.G.M.; Altei, W.F.; Marchetto, R. Biophysical Characterization and Antitumor Activity of Synthetic Pantinin Peptides from Scorpion’s Venom. Biochim. Biophys. Acta (BBA)—Biomembr. 2018, 1860, 2155–2165. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wu, L.C.; Wang, Z.P.; Wang, Z.X.; Jia, Q.; Jiang, G.S.; Zhang, W.D. Anti-Proliferation Effect of Polypeptide Extracted from Scorpion Venom on Human Prostate Cancer Cells In Vitro. J. Clin. Med. Res. 2009, 1, 24–31. [Google Scholar] [CrossRef]
- Sui, W.-W.; Zhang, W.-D.; Wu, L.-C.; Zhang, Y.-Y.; Wang, Z.-P.; Wang, Z.-X.; Jia, Q. Study on the Mechanism of Polypeptide Extract from Scorpion Venom on Inhibition of Angiogenesis of H 22 Hepatoma. Zhongguo Zhong Xi Yi Jie He Za Zhi 2014, 34, 581–586. [Google Scholar]
- Chai, J.; Yang, W.; Gao, Y.; Guo, R.; Peng, Q.; Abdel-Rahman, M.A.; Xu, X. Antitumor Effects of Scorpion Peptide Smp43 Through Mitochondrial Dysfunction and Membrane Disruption on Hepatocellular Carcinoma. J. Nat. Prod. 2021, 84, 3147–3160. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gao, Y.; Nguyen, T.; Chai, J.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Xu, X.; Chen, X. The Potent Antitumor Activity of Smp43 Against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction. Toxins 2023, 15, 347. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, R.A.; Mohallal, M.E.; Mobarak, Y.M.; Ebaid, H.M.; Haywood-Small, S.; Miller, K.; Strong, P.N.; Abdel-Rahman, M.A. Scorpion Venom Antimicrobial Peptides Induce Caspase-1 Dependant Pyroptotic Cell Death. Front. Pharmacol. 2022, 12, 788874. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, D.; Zhang, K.; Gai, C.; Chai, X.; Zhao, Q.; Zou, Y.; Yin, L. Optimization of Antimicrobial Peptide Smp43 as Novel Inhibitors of Cancer. Bioorg. Chem. 2025, 161, 108561. [Google Scholar] [CrossRef]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a Scorpion-Venom Peptide, Exhibits Potent Antitumor Effects Against Hepatoma HepG2 Cells via Multi-Mechanisms In Vivo and In Vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion Peptide Smp24 Exhibits a Potent Antitumor Effect on Human Lung Cancer Cells by Damaging the Membrane and Cytoskeleton In Vivo and In Vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef]
- Daniele-Silva, A.; Machado, R.J.A.; Monteiro, N.K.V.; Estrela, A.B.; Santos, E.C.G.; Carvalho, E.; Araújo Júnior, R.F.; Melo-Silveira, R.F.; Rocha, H.A.O.; Silva-Júnior, A.A.; et al. Stigmurin and TsAP-2 from Tityus stigmurus Scorpion Venom: Assessment of Structure and Therapeutic Potential in Experimental Sepsis. Toxicon 2016, 121, 10–21. [Google Scholar] [CrossRef]
- Guo, X.; Ma, C.; Du, Q.; Wei, R.; Wang, L.; Zhou, M.; Chen, T.; Shaw, C. Two Peptides, TsAP-1 and TsAP-2, from the Venom of the Brazilian Yellow Scorpion, Tityus serrulatus: Evaluation of Their Antimicrobial and Anticancer Activities. Biochimie 2013, 95, 1784–1794. [Google Scholar] [CrossRef]
- Rates, B.; Ferraz, K.K.F.; Borges, M.H.; Richardson, M.; De Lima, M.E.; Pimenta, A.M.C. Tityus serrulatus Venom Peptidomics: Assessing Venom Peptide Diversity. Toxicon 2008, 52, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Gurrola-Briones, G.; Papp, F.; Rodríguez de la Vega, R.C.; Pedraza-Alva, G.; Tajhya, R.B.; Gaspar, R.; Cardenas, L.; Rosenstein, Y.; Beeton, C.; et al. Vm24, a Natural Immunosuppressive Peptide, Potently and Selectively Blocks Kv1.3 Potassium Channels of Human T Cells. Mol. Pharmacol. 2012, 82, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Cid-Uribe, J.I.; Veytia-Bucheli, J.I.; Romero-Gutierrez, T.; Ortiz, E.; Possani, L.D. Scorpion Venomics: A 2019 Overview. Expert Rev. Proteom. 2020, 17, 67–83. [Google Scholar] [CrossRef]
- Santibáñez-López, C.; Cid-Uribe, J.; Batista, C.; Ortiz, E.; Possani, L. Venom Gland Transcriptomic and Proteomic Analyses of the Enigmatic Scorpion Superstitionia donensis (Scorpiones: Superstitioniidae), with Insights on the Evolution of Its Venom Components. Toxins 2016, 8, 367. [Google Scholar] [CrossRef]
- So, W.L.; Leung, T.C.N.; Nong, W.; Bendena, W.G.; Ngai, S.M.; Hui, J.H.L. Transcriptomic and Proteomic Analyses of Venom Glands from Scorpions Liocheles australasiae, Mesobuthus martensii, and Scorpio maurus palmatus. Peptides 2021, 146, 170643. [Google Scholar] [CrossRef]
- Delgado-Prudencio, G.; Cid-Uribe, J.I.; Morales, J.A.; Possani, L.D.; Ortiz, E.; Romero-Gutiérrez, T. The Enzymatic Core of Scorpion Venoms. Toxins 2022, 14, 248. [Google Scholar] [CrossRef]
- Zeng, X.-C.; Luo, F.; Li, W.-X. Molecular Dissection of Venom from Chinese Scorpion Mesobuthus martensii: Identification and Characterization of Four Novel Disulfide-Bridged Venom Peptides. Peptides 2006, 27, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Verano-Braga, T.; Dutra, A.A.A.; León, I.R.; Melo-Braga, M.N.; Roepstorff, P.; Pimenta, A.M.C.; Kjeldsen, F. Moving Pieces in a Venomic Puzzle: Unveiling Post-Translationally Modified Toxins from Tityus serrulatus. J. Proteome Res. 2013, 12, 3460–3470. [Google Scholar] [CrossRef]
- de Melo-Braga, M.N.; Moreira, R.d.S.; Gervásio, J.H.D.B.; Felicori, L.F. Overview of Protein Posttranslational Modifications in Arthropoda Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2022, 28, e20210047. [Google Scholar] [CrossRef]
- Romey, G.; Chicheportiche, R.; Lazdunski, M.; Rochat, H.; Miranda, F.; Lissitzky, S. Scorpion Neurotoxin—A Presynaptic Toxin Which Affects Both Na+ and K+ Channels in Axons. Biochem. Biophys. Res. Commun. 1975, 64, 115–121. [Google Scholar] [CrossRef]
- Romey, G.; Abita, J.P.; Chicheportiche, R.; Rochat, H.; Lazdunski, M. Scorpion Neurotoxin. Biochim. Biophys. Acta (BBA)—Biomembr. 1976, 448, 607–619. [Google Scholar] [CrossRef]
- Catterall, W.A. Neurotoxins That Act on Voltage-Sensitive Sodium Channels in Excitable Membranes. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 15–43. [Google Scholar] [CrossRef] [PubMed]
- Gurevitz, M.; Froy, O.; Zilberberg, N.; Turkov, M.; Strugatsky, D.; Gershburg, E.; Lee, D.; Adams, M.E.; Tugarinov, V.; Anglister, J.; et al. Sodium Channel Modifiers from Scorpion Venom: Structure–Activity Relationship, Mode of Action and Application. Toxicon 1998, 36, 1671–1682. [Google Scholar] [CrossRef]
- Cestèle, S.; Yarov-Yarovoy, V.; Qu, Y.; Sampieri, F.; Scheuer, T.; Catterall, W.A. Structure and Function of the Voltage Sensor of Sodium Channels Probed by a β-Scorpion Toxin. J. Biol. Chem. 2006, 281, 21332–21344. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Cestèle, S.; Yarov-Yarovoy, V.; Yu, F.H.; Konoki, K.; Scheuer, T. Voltage-Gated Ion Channels and Gating Modifier Toxins. Toxicon 2007, 49, 124–141. [Google Scholar] [CrossRef]
- Kozminsky-Atias, A.; Somech, E.; Zilberberg, N. Isolation of the First Toxin from the Scorpion Buthus occitanus israelis Showing Preference for Shaker Potassium Channels. FEBS Lett. 2007, 581, 2478–2484. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hernández, V.; Jiménez-Vargas, J.M.; Gurrola, G.B.; Valdivia, H.H.; Possani, L.D. Scorpion Venom Components That Affect Ion-Channels Function. Toxicon 2013, 76, 328–342. [Google Scholar] [CrossRef]
- Housley, D.M.; Housley, G.D.; Liddell, M.J.; Jennings, E.A. Scorpion Toxin Peptide Action at the Ion Channel Subunit Level. Neuropharmacology 2017, 127, 46–78. [Google Scholar] [CrossRef]
- Zou, X.; Wu, Y.; Chen, J.; Zhao, F.; Zhang, F.; Yu, B.; Cao, Z. Activation of Sodium Channel by a Novel α-Scorpion Toxin, BmK NT2, Stimulates ERK1/2 and CERB Phosphorylation through a Ca2+ Dependent Pathway in Neocortical Neurons. Int. J. Biol. Macromol. 2017, 104, 70–77. [Google Scholar] [CrossRef]
- Beraldo Neto, E.; de Freitas, L.A.; Pimenta, D.C.; Lebrun, I.; Nencioni, A.L.A. Tb1, a Neurotoxin from Tityus bahiensis Scorpion Venom, Induces Epileptic Seizures by Increasing Glutamate Release. Toxins 2020, 12, 65. [Google Scholar] [CrossRef]
- Díaz-García, A.; Varela, D. Voltage-Gated K+/Na+ Channels and Scorpion Venom Toxins in Cancer. Front. Pharmacol. 2020, 11, 913. [Google Scholar] [CrossRef]
- Wiezel, G.A.; Oliveira, I.S.; Reis, M.B.; Ferreira, I.G.; Cordeiro, K.R.; Bordon, K.C.F.; Arantes, E.C. The Complex Repertoire of Tityus Spp. Venoms: Advances on Their Composition and Pharmacological Potential of Their Toxins. Biochimie 2024, 220, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Alam, M.; Abbasi, A.; Undheim, E.; Fry, B.; Kalbacher, H.; Voelter, W. Structure-Activity Relationship of Chlorotoxin-like Peptides. Toxins 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fan, Z.; Li, W.; Dietel, B.; Wu, Y.; Beckmann, M.W.; Wrosch, J.K.; Buchfelder, M.; Eyupoglu, I.Y.; Cao, Z.; et al. Identification of Two Novel Chlorotoxin Derivatives CA4 and CTX-23 with Chemotherapeutic and Anti-Angiogenic Potential. Sci. Rep. 2016, 6, 19799. [Google Scholar] [CrossRef]
- Majc, B.; Novak, M.; Lah, T.T.; Križaj, I. Bioactive Peptides from Venoms Against Glioma Progression. Front. Oncol. 2022, 12, 965882. [Google Scholar] [CrossRef]
- Samat, R.; Sen, S.; Jash, M.; Ghosh, S.; Garg, S.; Sarkar, J.; Ghosh, S. Venom: A Promising Avenue for Antimicrobial Therapeutics. ACS Infect. Dis. 2024, 10, 3098–3125. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.L.; Abdel-Rahman, M.A.; Strong, P.N.; Tawfik, M.M.; Miller, K. Characterization of Three Alpha-Helical Antimicrobial Peptides from the Venom of Scorpio maurus palmatus. Toxicon 2016, 117, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.L.; Heath, G.R.; Johnson, B.R.G.; Abdel-Rahman, M.A.; Strong, P.N.; Evans, S.D.; Miller, K. Phospholipid Dependent Mechanism of Smp24, an α-Helical Antimicrobial Peptide from Scorpion Venom. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 2737–2744. [Google Scholar] [CrossRef]
- Xia, Z.; He, D.; Wu, Y.; Kwok, H.F.; Cao, Z. Scorpion Venom Peptides: Molecular Diversity, Structural Characteristics, and Therapeutic Use from Channelopathies to Viral Infections and Cancers. Pharmacol. Res. 2023, 197, 106978. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Hu, J.; Yang, W.; Cao, Z.; Zhuo, R.; Li, W.; Wu, Y. SjAPI, the First Functionally Characterized Ascaris-Type Protease Inhibitor from Animal Venoms. PLoS ONE 2013, 8, e57529. [Google Scholar] [CrossRef]
- Zhao, R.; Dai, H.; Qiu, S.; Li, T.; He, Y.; Ma, Y.; Chen, Z.; Wu, Y.; Li, W.; Cao, Z. SdPI, The First Functionally Characterized Kunitz-Type Trypsin Inhibitor from Scorpion Venom. PLoS ONE 2011, 6, e27548. [Google Scholar] [CrossRef]
- Ding, L.; Wang, X.; Liu, H.; San, M.; Xu, Y.; Li, J.; Li, S.; Cao, Z.; Li, W.; Wu, Y.; et al. A New Kunitz-Type Plasmin Inhibitor from Scorpion Venom. Toxicon 2015, 106, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, J.; Wang, X.; Yan, S.; Xu, Y.; San, M.; Tang, W.; Yang, F.; Cao, Z.; Li, W.; et al. Functional Characterization of a New Non-Kunitz Serine Protease Inhibitor from the Scorpion Lychas mucronatus. Int. J. Biol. Macromol. 2015, 72, 158–162. [Google Scholar] [CrossRef]
- Hakim, M.A.; Yang, S. Discoveries of Serine Protease Inhibitors from Scorpions. J. Proteom. Bioinform. 2016, 4, 101–106. [Google Scholar] [CrossRef]
- Song, Y.; Gong, K.; Yan, H.; Hong, W.; Wang, L.; Wu, Y.; Li, W.; Li, W.; Cao, Z. Sj7170, a Unique Dual-Function Peptide with a Specific α-Chymotrypsin Inhibitory Activity and a Potent Tumor-Activating Effect from Scorpion Venom. J. Biol. Chem. 2014, 289, 11667–11680, Erratum in J. Biol. Chem. 2022, 298, 102543. [Google Scholar] [CrossRef]
- Possani, L. Peptides and Genes Coding for Scorpion Toxins That Affect Ion-Channels. Biochimie 2000, 82, 861–868. [Google Scholar] [CrossRef]
- Li, Z.; Hu, P.; Wu, W.; Wang, Y. Peptides with Therapeutic Potential in the Venom of the Scorpion Buthus martensii Karsch. Peptides 2019, 115, 43–50. [Google Scholar] [CrossRef]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic Anticancer Properties of Scorpion Venom Peptides: Rhopalurus princeps Venom as an Anticancer Agent. Drug Des. Devel. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef]
- Aissaoui-Zid, D.; Saada, M.-C.; Moslah, W.; Potier-Cartereau, M.; Lemettre, A.; Othman, H.; Gaysinski, M.; Abdelkafi-Koubaa, Z.; Souid, S.; Marrakchi, N.; et al. AaTs-1: A Tetrapeptide from Androctonus australis Scorpion Venom, Inhibiting U87 Glioblastoma Cells Proliferation by P53 and FPRL-1 Up-Regulations. Molecules 2021, 26, 7610. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Ren, F.; Huang, L.; Xu, J.; Li, F. Protective Effect of Scorpion Venom Heat-Resistant Synthetic Peptide Against PM2.5-Induced Microglial Polarization via TLR4-Mediated Autophagy Activating PI3K/AKT/NF-ΚB Signaling Pathway. J. Neuroimmunol. 2021, 355, 577567. [Google Scholar] [CrossRef] [PubMed]
- You, X.; Wu, Y.; Li, Q.; Sheng, W.; Zhou, Q.; Fu, W. Astragalus-Scorpion Drug Pair Inhibits the Development of Prostate Cancer by Regulating GDPD4-2/PI3K/AKT/MTOR Pathway and Autophagy. Front. Pharmacol. 2022, 13, 895696, Erratum in Front. Pharmacol. 2025, 16, 1417603. [Google Scholar] [CrossRef]
- Ismail, M.; El-Asmar, M.F.; Osman, O.H. Pharmacological Studies with Scorpion (Palamneus gravimanus) Venom: Evidence for the Presence of Histamine. Toxicon 1975, 13, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Gomes, A.; Gomes, A.; Dasgupta, S.C.; Lahiri, S.C. Histamine, 5-HT & Hyaluronidase in the Venom of the Scorpion Lychas laevifrons (Pock). Indian J. Med. Res. 1990, 92, 371–373. [Google Scholar] [PubMed]
- Kwon, N.-Y.; Sung, H.-K.; Park, J.-K. Systematic Review of the Antitumor Activities and Mechanisms of Scorpion Venom on Human Breast Cancer Cells Lines (In Vitro Study). J. Clin. Med. 2025, 14, 3181. [Google Scholar] [CrossRef]
- Evans, E.R.J.; McIntyre, L.; Northfield, T.D.; Daly, N.L.; Wilson, D.T. Small Molecules in the Venom of the Scorpion Hormurus waigiensis. Biomedicines 2020, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, L.; Torres, M.D.T.; de la Fuente-Nunez, C. Biologically Active Peptides from Venoms: Applications in Antibiotic Resistance, Cancer, and Beyond. Int. J. Mol. Sci. 2022, 23, 15437. [Google Scholar] [CrossRef]
- Petricevich, V.L. Scorpion Venom and the Inflammatory Response. Mediat. Inflamm. 2010, 2010, 903295. [Google Scholar] [CrossRef]
- El-Benna, J.; Hurtado-Nedelec, M.; Gougerot-Pocidalo, M.-A.; Dang, P.M.-C. Effects of Venoms on Neutrophil Respiratory Burst: A Major Inflammatory Function. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200179. [Google Scholar] [CrossRef]
- Soltan-Alinejad, P.; Alipour, H.; Soltani, A.; Asgari, Q.; Ramezani, A.; Mehrabani, D.; Azizi, K. Molecular Characterization and In Silico Analyses of Maurolipin Structure as a Secretory Phospholipase A 2 (SPLA2) from Venom Glands of Iranian Scorpio maurus (Arachnida: Scorpionida). J. Trop. Med. 2022, 2022, 1839946. [Google Scholar] [CrossRef]
- Baradaran, M.; Salabi, F. Genome-Wide Identification, Structural Homology Analysis, and Evolutionary Diversification of the Phospholipase D Gene Family in the Venom Gland of Three Scorpion Species. BMC Genom. 2023, 24, 730. [Google Scholar] [CrossRef]
- Salabi, F.; Jafari, H. Whole Transcriptome Sequencing Reveals the Activity of the PLA2 Family Members in Androctonus crassicauda (Scorpionida: Buthidae) Venom Gland. FASEB J. 2024, 38, e23658. [Google Scholar] [CrossRef]
- Díaz, C.; Lomonte, B.; Chang-Castillo, A.; Bonilla, F.; Alfaro-Chinchilla, A.; Triana, F.; Angulo, D.; Fernández, J.; Sasa, M. Venomics of Scorpion Ananteris platnicki (Lourenço, 1993), a New World Buthid That Inhabits Costa Rica and Panama. Toxins 2024, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Tobar, L.L.; Clement, H.; Arenas, I.; Sepulveda-Arias, J.C.; Vargas, J.A.G.; Corzo, G. An Overview of Some Enzymes from Buthid Scorpion Venoms from Colombia: Centruroides margaritatus, Tityus pachyurus, and Tityus n. sp. aff. metuendus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2024, 30, e20230063. [Google Scholar] [CrossRef]
- Venancio, E.J.; Portaro, F.C.V.; Kuniyoshi, A.K.; Carvalho, D.C.; Pidde-Queiroz, G.; Tambourgi, D.V. Enzymatic Properties of Venoms from Brazilian Scorpions of Tityus Genus and the Neutralisation Potential of Therapeutical Antivenoms. Toxicon 2013, 69, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Cajado Carvalho, D.; Kuniyoshi, A.K.; Kodama, R.T.; Oliveira, A.K.; Serrano, S.M.T.; Tambourgi, D.V.; Portaro, F.V. Neuropeptide Y Family-Degrading Metallopeptidases in the Tityus serrulatus Venom Partially Blocked by Commercial Antivenoms. Toxicol. Sci. 2014, 142, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Cajado-Carvalho, D.; da Silva, C.C.F.; Kodama, R.T.; Mariano, D.O.C.; Pimenta, D.C.; Duzzi, B.; Kuniyoshi, A.K.; Portaro, F.V. Purification and Biochemical Characterization of TsMS 3 and TsMS 4: Neuropeptide-Degrading Metallopeptidases in the Tityus serrulatus Venom. Toxins 2019, 11, 194. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, Y.; Zhao, R.; Zhang, W.; He, Y.; Wu, Y.; Cao, Z.; Guo, L.; Li, W. Molecular Diversity of Toxic Components from the Scorpion Heterometrus petersii Venom Revealed by Proteomic and Transcriptome Analysis. Proteomics 2010, 10, 2471–2485. [Google Scholar] [CrossRef]
- Ding, J.; Chua, P.-J.; Bay, B.-H.; Gopalakrishnakone, P. Scorpion Venoms as a Potential Source of Novel Cancer Therapeutic Compounds. Exp. Biol. Med. 2014, 239, 387–393. [Google Scholar] [CrossRef]
- Ricci, J.-E.; Gottlieb, R.A.; Green, D.R. Caspase-Mediated Loss of Mitochondrial Function and Generation of Reactive Oxygen Species during Apoptosis. J. Cell Biol. 2003, 160, 65–75. [Google Scholar] [CrossRef]
- Roy, S.; Nicholson, D.W. Cross-Talk in Cell Death Signaling. J. Exp. Med. 2000, 192, F21–F25. [Google Scholar] [CrossRef]
- Basu, A.; Castle, V.P.; Bouziane, M.; Bhalla, K.; Haldar, S. Crosstalk Between Extrinsic and Intrinsic Cell Death Pathways in Pancreatic Cancer: Synergistic Action of Estrogen Metabolite and Ligands of Death Receptor Family. Cancer Res. 2006, 66, 4309–4318. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.; Yuan, J. Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Yin, X.-M.; Wang, K.; Gross, A.; Zhao, Y.; Zinkel, S.; Klocke, B.; Roth, K.A.; Korsmeyer, S.J. Bid-Deficient Mice Are Resistant to Fas-Induced Hepatocellular Apoptosis. Nature 1999, 400, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Srairi-Abid, N.; Othman, H.; Aissaoui, D.; BenAissa, R. Anti-Tumoral Effect of Scorpion Peptides: Emerging New Cellular Targets and Signaling Pathways. Cell Calcium 2019, 80, 160–174. [Google Scholar] [CrossRef]
- Gomes, A.; Bhattacharjee, P.; Mishra, R.; Biswas, A.K.; Dasgupta, S.C.; Giri, B. Anticancer Potential of Animal Venoms and Toxins. Indian J. Exp. Biol. 2010, 48, 93–103. [Google Scholar]
- Kampo, S.; Ahmmed, B.; Zhou, T.; Owusu, L.; Anabah, T.W.; Doudou, N.R.; Kuugbee, E.D.; Cui, Y.; Lu, Z.; Yan, Q.; et al. Scorpion Venom Analgesic Peptide, BmK AGAP Inhibits Stemness, and Epithelial-Mesenchymal Transition by Down-Regulating PTX3 in Breast Cancer. Front. Oncol. 2019, 9, 21, Erratum in Front. Oncol. 2021, 11, 639813. [Google Scholar] [CrossRef]
- Dardevet, L.; Najlaoui, F.; Aroui, S.; Collot, M.; Tisseyre, C.; Pennington, M.W.; Mallet, J.-M.; De Waard, M. A Conjugate Between Lqh-8/6, a Natural Peptide Analogue of Chlorotoxin, and Doxorubicin Efficiently Induces Glioma Cell Death. Biomedicines 2022, 10, 2605. [Google Scholar] [CrossRef]
- Yu, M.; Liu, S.; Sun, P.; Pan, H.; Tian, C.; Zhang, L. Peptide Toxins and Small-Molecule Blockers of BK Channels. Acta Pharmacol. Sin. 2016, 37, 56–66. [Google Scholar] [CrossRef]
- Giangiacomo, K.M.; Sugg, E.E.; Garcia-Calvo, M.; Leonard, R.J.; McManus, O.B.; Kaczorowski, G.J.; Garcia, M.L. Synthetic Charybdotoxin-Iberiotoxin Chimeric Peptides Define Toxin Binding Sites on Calcium-Activated and Voltage-Dependent Potassium Channels. Biochemistry 1993, 32, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.-P.; Bian, S.; Tan, Z.-Y.; Takacs, Z.; Moczydlowski, E. Synthesis of a Biotin Derivative of Iberiotoxin: Binding Interactions with Streptavidin and the BK Ca2+-Activated K+ Channel Expressed in a Human Cell Line. Bioconjug Chem. 2006, 17, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Ji, Y.-H. Scorpion Venom Induces Glioma Cell Apoptosis In Vivo and Inhibits Glioma Tumor Growth In Vitro. J. Neurooncol. 2005, 73, 1–7. [Google Scholar] [CrossRef]
- Marak, B.N.; Dowarah, J.; Khiangte, L.; Singh, V.P. A Comprehensive Insight on the Recent Development of Cyclic Dependent Kinase Inhibitors as Anticancer Agents. Eur. J. Med. Chem. 2020, 203, 112571. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, C.; Chen, L.; Li, M.; Yang, J.; Nie, L.; Zhang, M.; Zhang, X.; Zhang, E. Anti-Glioma Effect of Buthus martensii Karsch (BmK) Scorpion by Inhibiting Myeloid-Derived Suppressor Cells and Activating T Cells in Tumor Microenvironment. J. Funct. Foods 2024, 116, 106163. [Google Scholar] [CrossRef]
- Delpech, B.; Girard, N.; Bertrand, P.; Courel, M.-N.; Chauzy, C.; Delpech, A. Hyaluronan: Fundamental Principles and Applications in Cancer. J. Intern. Med. 1997, 242, 41–48. [Google Scholar] [CrossRef]
- Naor, D.; Sionov, R.V.; Ish-Shalom, D. CD44: Structure, Function and Association with the Malignant Process. Adv. Cancer Res. 1997, 71, 241–319. [Google Scholar]
- Khamessi, O.; Ben Mabrouk, H.; ElFessi-Magouri, R.; Kharrat, R. RK1, the First Very Short Peptide from Buthus occitanus tunetanus Inhibits Tumor Cell Migration, Proliferation and Angiogenesis. Biochem. Biophys. Res. Commun. 2018, 499, 1–7. [Google Scholar] [CrossRef]
- Sinthuvanich, C.; Veiga, A.S.; Gupta, K.; Gaspar, D.; Blumenthal, R.; Schneider, J.P. Anticancer β-Hairpin Peptides: Membrane-Induced Folding Triggers Activity. J. Am. Chem. Soc. 2012, 134, 6210–6217. [Google Scholar] [CrossRef] [PubMed]
- Tolos (Vasii), A.M.; Moisa, C.; Dochia, M.; Popa, C.; Copolovici, L.; Copolovici, D.M. Anticancer Potential of Antimicrobial Peptides: Focus on Buforins. Polymers 2024, 16, 728. [Google Scholar] [CrossRef] [PubMed]
- DeBin, J.A.; Maggio, J.E.; Strichartz, G.R. Purification and Characterization of Chlorotoxin, a Chloride Channel Ligand from the Venom of the Scorpion. Am. J. Physiol.-Cell Physiol. 1993, 264, C361–C369. [Google Scholar] [CrossRef]
- Lippens, G.; Najib, J.; Wodak, S.J.; Tartar, A. NMR Sequential Assignments and Solution Structure of Chlorotoxin, a Small Scorpion Toxin That Blocks Chloride Channels. Biochemistry 1995, 34, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Correnti, C.E.; Gewe, M.M.; Mehlin, C.; Bandaranayake, A.D.; Johnsen, W.A.; Rupert, P.B.; Brusniak, M.-Y.; Clarke, M.; Burke, S.E.; De Van Der Schueren, W.; et al. Screening, Large-Scale Production and Structure-Based Classification of Cystine-Dense Peptides. Nat. Struct. Mol. Biol. 2018, 25, 270–278. [Google Scholar] [CrossRef]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of Chlorotoxin for Targeting of Primary Brain Tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar]
- Lyons, S.A.; O’Neal, J.; Sontheimer, H. Chlorotoxin, a Scorpion-Derived Peptide, Specifically Binds to Gliomas and Tumors of Neuroectodermal Origin. Glia 2002, 39, 162–173. [Google Scholar] [CrossRef]
- Deshane, J.; Garner, C.C.; Sontheimer, H. Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2. J. Biol. Chem. 2003, 278, 4135–4144. [Google Scholar] [CrossRef]
- Ullrich, N.; Bordey, A.; Gillespie, G.Y.; Sontheimer, H. Expression of Voltage-Activated Chloride Currents in Acute Slices of Human Gliomas. Neuroscience 1998, 83, 1161–1173. [Google Scholar] [CrossRef]
- McFerrin, M.B.; Sontheimer, H. A Role for Ion Channels in Glioma Cell Invasion. Neuron Glia Biol. 2006, 2, 39–49. [Google Scholar] [CrossRef]
- Dastpeyman, M.; Giacomin, P.; Wilson, D.; Nolan, M.J.; Bansal, P.S.; Daly, N.L. A C-Terminal Fragment of Chlorotoxin Retains Bioactivity and Inhibits Cell Migration. Front. Pharmacol. 2019, 10, 250. [Google Scholar] [CrossRef]
- Kesavan, K.; Ratliff, J.; Johnson, E.W.; Dahlberg, W.; Asara, J.M.; Misra, P.; Frangioni, J.V.; Jacoby, D.B. Annexin A2 Is a Molecular Target for TM601, a Peptide with Tumor-Targeting and Anti-Angiogenic Effects. J. Biol. Chem. 2010, 285, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Farkas, S.; Cioca, D.; Murányi, J.; Hornyák, P.; Brunyánszki, A.; Szekér, P.; Boros, E.; Horváth, P.; Hujber, Z.; Rácz, G.Z.; et al. Chlorotoxin Binds to Both Matrix Metalloproteinase 2 and Neuropilin 1. J. Biol. Chem. 2023, 299, 104998. [Google Scholar] [CrossRef] [PubMed]
- Mabunda, I.G.; Offor, B.C.; Muller, B.; Motadi, L.R.; Piater, L.A. Scorpion Venoms from the Buthidae Family: A Dual Study of Proteomic Composition and Anticancer Potentials. Toxicon 2025, 266, 108542. [Google Scholar] [CrossRef]
- Wiranowska, M.; Colina, L.O.; Johnson, J.O. Clathrin-Mediated Entry and Cellular Localization of Chlorotoxin in Human Glioma. Cancer Cell Int. 2011, 11, 27. [Google Scholar] [CrossRef]
- Barish, M.E.; Aftabizadeh, M.; Hibbard, J.; Blanchard, M.S.; Ostberg, J.R.; Wagner, J.R.; Manchanda, M.; Paul, J.; Stiller, T.; Aguilar, B.; et al. Chlorotoxin-Directed CAR T Cell Therapy for Recurrent Glioblastoma: Interim Clinical Experience Demonstrating Feasibility and Safety. Cell Rep. Med. 2025, 6, 102302. [Google Scholar] [CrossRef]
- Mamelak, A.N.; Jacoby, D.B. Targeted Delivery of Antitumoral Therapy to Glioma and Other Malignancies with Synthetic Chlorotoxin (TM-601). Expert Opin. Drug Deliv. 2007, 4, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mamelak, A.N.; Rosenfeld, S.; Bucholz, R.; Raubitschek, A.; Nabors, L.B.; Fiveash, J.B.; Shen, S.; Khazaeli, M.B.; Colcher, D.; Liu, A.; et al. Phase I Single-Dose Study of Intracavitary-Administered Iodine-131-TM-601 in Adults with Recurrent High-Grade Glioma. J. Clin. Oncol. 2006, 24, 3644–3650. [Google Scholar] [CrossRef]
- Gribbin, T.E.; Senzer, N.; Raizer, J.J.; Shen, S.; Nabors, L.B.; Wiranowska, M.; Fiveash, J.B. A Phase I Evaluation of Intravenous (IV) 131I-Chlorotoxin Delivery to Solid Peripheral and Intracranial Tumors. J. Clin. Oncol. 2009, 27, e14507. [Google Scholar] [CrossRef]
- TransMolecular, Inc. A Study of 131I-TM601 in Adults With Recurrent Malignant Glioma. ClinicalTrials.gov. U.S. National Library of Medicine. Identifier: NCT00683761. Available online: https://clinicaltrials.gov/study/NCT00683761 (accessed on 3 October 2025).
- Patil, C.G.; Walker, D.G.; Miller, D.M.; Butte, P.; Morrison, B.; Kittle, D.S.; Hansen, S.J.; Nufer, K.L.; Byrnes-Blake, K.A.; Yamada, M.; et al. Phase 1 Safety, Pharmacokinetics, and Fluorescence Imaging Study of Tozuleristide (BLZ-100) in Adults With Newly Diagnosed or Recurrent Gliomas. Neurosurgery 2019, 85, E641–E649. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Perlas, C.; Varese, M.; Guardiola, S.; García, J.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. From Venoms to BBB-Shuttles. MiniCTX3: A Molecular Vector Derived from Scorpion Venom. Chem. Commun. 2018, 54, 12738–12741. [Google Scholar] [CrossRef]
- Formicola, B.; Dal Magro, R.; Montefusco-Pereira, C.V.; Lehr, C.-M.; Koch, M.; Russo, L.; Grasso, G.; Deriu, M.A.; Danani, A.; Bourdoulous, S.; et al. The Synergistic Effect of Chlorotoxin-MApoE in Boosting Drug-Loaded Liposomes across the BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Reissmann, S.; Filatova, M.P. New Generation of Cell-penetrating Peptides: Functionality and Potential Clinical Application. J. Pept. Sci. 2021, 27, e3300. [Google Scholar] [CrossRef] [PubMed]
- Poillot, C.; Dridi, K.; Bichraoui, H.; Pêcher, J.; Alphonse, S.; Douzi, B.; Ronjat, M.; Darbon, H.; De Waard, M. D-Maurocalcine, a Pharmacologically Inert Efficient Cell-Penetrating Peptide Analogue. J. Biol. Chem. 2010, 285, 34168–34180. [Google Scholar] [CrossRef]
- Poillot, C.; Bichraoui, H.; Tisseyre, C.; Bahemberae, E.; Andreotti, N.; Sabatier, J.-M.; Ronjat, M.; De Waard, M. Small Efficient Cell-Penetrating Peptides Derived from Scorpion Toxin Maurocalcine. J. Biol. Chem. 2012, 287, 17331–17342. [Google Scholar] [CrossRef]
- Fajloun, Z.; Kharrat, R.; Chen, L.; Lecomte, C.; Di Luccio, E.; Bichet, D.; El Ayeb, M.; Rochat, H.; Allen, P.D.; Pessah, I.N.; et al. Chemical Synthesis and Characterization of Maurocalcine, a Scorpion Toxin That Activates Ca2+ Release Channel/Ryanodine Receptors. FEBS Lett. 2000, 469, 179–185. [Google Scholar] [CrossRef]
- Estève, E.; Mabrouk, K.; Dupuis, A.; Smida-Rezgui, S.; Altafaj, X.; Grunwald, D.; Platel, J.-C.; Andreotti, N.; Marty, I.; Sabatier, J.-M.; et al. Transduction of the Scorpion Toxin Maurocalcine into Cells. J. Biol. Chem. 2005, 280, 12833–12839. [Google Scholar] [CrossRef]
- Tisseyre, C.; Bahembera, E.; Dardevet, L.; Sabatier, J.-M.; Ronjat, M.; De Waard, M. Cell Penetration Properties of a Highly Efficient Mini Maurocalcine Peptide. Pharmaceuticals 2013, 6, 320–339. [Google Scholar] [CrossRef]
- Aroui, S.; Dardevet, L.; Najlaoui, F.; Kammoun, M.; Laajimi, A.; Fetoui, H.; De Waard, M.; Kenani, A. PTEN-Regulated AKT/FoxO3a/Bim Signaling Contributes to Human Cell Glioblastoma Apoptosis by Platinum-Maurocalcin Conjugate. Int. J. Biochem. Cell Biol. 2016, 77, 15–22. [Google Scholar] [CrossRef]
- Zhou, M.; Zou, X.; Cheng, K.; Zhong, S.; Su, Y.; Wu, T.; Tao, Y.; Cong, L.; Yan, B.; Jiang, Y. The Role of Cell-penetrating Peptides in Potential Anti-cancer Therapy. Clin. Transl. Med. 2022, 12, e822. [Google Scholar] [CrossRef]
- Cota-Arce, J.M.; Zazueta-Favela, D.; Díaz-Castillo, F.; Jiménez, S.; Bernáldez-Sarabia, J.; Caram-Salas, N.L.; Dan, K.W.L.; Escobedo, G.; Licea-Navarro, A.F.; Possani, L.D.; et al. Venom Components of the Scorpion Centruroides limpidus Modulate Cytokine Expression by T Helper Lymphocytes: Identification of Ion Channel-Related Toxins by Mass Spectrometry. Int. Immunopharmacol. 2020, 84, 106505. [Google Scholar] [CrossRef]
- Park, J.; Oh, J.H.; Kang, H.K.; Choi, M.-C.; Seo, C.H.; Park, Y. Scorpion-Venom-Derived Antimicrobial Peptide Css54 Exerts Potent Antimicrobial Activity by Disrupting Bacterial Membrane of Zoonotic Bacteria. Antibiotics 2020, 9, 831. [Google Scholar] [CrossRef]
- Zoccal, K.F.; Bitencourt, C.d.S.; Paula-Silva, F.W.G.; Sorgi, C.A.; de Castro Figueiredo Bordon, K.; Arantes, E.C.; Faccioli, L.H. TLR2, TLR4 and CD14 Recognize Venom-Associated Molecular Patterns from Tityus serrulatus to Induce Macrophage-Derived Inflammatory Mediators. PLoS ONE 2014, 9, e88174. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Dai, Q.; Lin, N.; Guan, T.; Song, X.; Hong, S. Scorpion Venom Polypeptide Governs Alveolar Macrophage M1/M2 Polarization to Alleviate Pulmonary Fibrosis. Tissue Cell 2022, 79, 101939. [Google Scholar] [CrossRef] [PubMed]
- Yglesias-Rivera, A.; Sánchez-Rodríguez, H.; Soto-Febles, C.; Monzote, L. Heteroctenus junceus Scorpion Venom Modulates the Concentration of Pro-Inflammatory Cytokines in F3II Tumor Cells. Life 2023, 13, 2287. [Google Scholar] [CrossRef]
- Díaz-García, A.; Ruiz-Fuentes, J.L.; Frión-Herrera, Y.; Yglesias-Rivera, A.; Garlobo, Y.R.; Sánchez, H.R.; Aurrecochea, J.C.R.; López Fuentes, L.X. Rhopalurus junceus Scorpion Venom Induces Antitumor Effect In Vitro and In Vivo Against a Murine Mammary Adenocarcinoma Model. Iran. J. Basic Med. Sci. 2019, 22, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Zoccal, K.F.; Paula-Silva, F.W.G.; Bitencourt, C.d.S.; Sorgi, C.A.; Bordon, K.d.C.F.; Arantes, E.C.; Faccioli, L.H. PPAR-γ Activation by Tityus serrulatus Venom Regulates Lipid Body Formation and Lipid Mediator Production. Toxicon 2015, 93, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ait-Lounis, A.; Laraba-Djebari, F. TNF-Alpha Modulates Adipose Macrophage Polarization to M1 Phenotype in Response to Scorpion Venom. Inflamm. Res. 2015, 64, 929–936. [Google Scholar] [CrossRef]
- Santhosh, K.N.; Ramesh, D.; Ramesh, D.; Nagaraj, U.; Shrinidhi, S.; Thippeswamy, N.B. Scorpion Venom Exhibits Adjuvant Effect by Eliciting HBsAg-Specific Th1 Immunity Through Neuro-Endocrine Interactions. Mol. Immunol. 2022, 147, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, G.B.; Hernández-López, R.A.; Rodríguez de la Vega, R.C.; Varga, Z.; Batista, C.V.F.; Salas-Castillo, S.P.; Panyi, G.; del Río-Portilla, F.; Possani, L.D. Structure, Function, and Chemical Synthesis of Vaejovis mexicanus Peptide 24: A Novel Potent Blocker of Kv1.3 Potassium Channels of Human T Lymphocytes. Biochemistry 2012, 51, 4049–4061. [Google Scholar] [CrossRef] [PubMed]
- Veytia-Bucheli, J.I.; Jiménez-Vargas, J.M.; Melchy-Pérez, E.I.; Sandoval-Hernández, M.A.; Possani, L.D.; Rosenstein, Y. Kv1.3 Channel Blockade with the Vm24 Scorpion Toxin Attenuates the CD4+ Effector Memory T Cell Response to TCR Stimulation. Cell Commun. Signal. 2018, 16, 45. [Google Scholar] [CrossRef]
- Chimote, A.A.; Hajdu, P.; Sfyris, A.M.; Gleich, B.N.; Wise-Draper, T.; Casper, K.A.; Conforti, L. Kv1.3 Channels Mark Functionally Competent CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Cancer. Cancer Res. 2017, 77, 53–61. [Google Scholar] [CrossRef]
- Magalhães, M.M.; Pereira, M.E.S.; Amaral, C.F.S.; Rezende, N.A.; Campolina, D.; Bucaretchi, F.; Gazzinelli, R.T.; Cunha-Melo, J.R. Serum Levels of Cytokines in Patients Envenomed by Tityus serrulatus Scorpion Sting. Toxicon 1999, 37, 1155–1164. [Google Scholar] [CrossRef]
- Gunas, V.; Maievskyi, O.; Synelnyk, T.; Raksha, N.; Vovk, T.; Halenova, T.; Savchuk, O.; Gunas, I. Cytokines and Their Regulators in Rat Lung Following Scorpion Envenomation. Toxicon X 2024, 22, 100198. [Google Scholar] [CrossRef]
- Zeng, L.; Sun, Y.; Zhang, H.; Yi, X.; Du, R.; Chen, Z.; Wang, Q. Scorpion Venom Peptides Enhance Immunity and Survival in Litopenaeus vannamei Through Antibacterial Action Against Vibrio parahaemolyticus. Front. Immunol. 2025, 16, 1551816. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Op den Camp, H.J.M.; De Sanctis, J.B. Specific Activation of Human Neutrophils by Scorpion Venom: A Flow Cytometry Assessment. Toxicol. Vitr. 2011, 25, 358–367. [Google Scholar] [CrossRef]
- Abdollahnia, A.; Bahmani, K.; Aliahmadi, A.; As’habi, M.A.; Ghassempour, A. Mass Spectrometric Analysis of Odonthobuthus doriae Scorpion Venom and Its Non-Neutralized Fractions after Interaction with Commercial Antivenom. Sci. Rep. 2024, 14, 10389. [Google Scholar] [CrossRef]
- Zoccal, K.F.; Bitencourt, C.d.S.; Secatto, A.; Sorgi, C.A.; Bordon, K.d.C.F.; Sampaio, S.V.; Arantes, E.C.; Faccioli, L.H. Tityus serrulatus Venom and Toxins Ts1, Ts2 and Ts6 Induce Macrophage Activation and Production of Immune Mediators. Toxicon 2011, 57, 1101–1108. [Google Scholar] [CrossRef]
- Martin-Eauclaire, M.-F.; Bougis, P.E.; de Lima, M.E. Ts1 from the Brazilian Scorpion Tityus serrulatus: A Half-Century of Studies on a Multifunctional Beta like-Toxin. Toxicon 2018, 152, 106–120. [Google Scholar] [CrossRef]
- Shariati, S.; Mafakher, L.; Shirani, M.; Baradaran, M. Unveiling New Kv1.3 Channel Blockers from Scorpion Venom: Characterization of Meuk7–3 and in Silico Design of Its Analogs for Enhanced Affinity and Therapeutic Potential. Int. J. Biol. Macromol. 2025, 319, 145327. [Google Scholar] [CrossRef] [PubMed]
- López-Giraldo, E.; Carrillo, E.; Titaux-Delgado, G.; Cano-Sánchez, P.; Colorado, A.; Possani, L.D.; del Río-Portilla, F. Structural and Functional Studies of Scorpine: A Channel Blocker and Cytolytic Peptide. Toxicon 2023, 222, 106985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Tian, C.; Zhong, C.; Li, W.; Shang, X.; Zhao, M.; Zhao, Y. Engineered Bacteria-Mediated Delivery of Scorpion Venom Peptide AGAP for Targeted Breast Cancer Therapy. Curr. Microbiol. 2025, 82, 323. [Google Scholar] [CrossRef]
- Wang, D.; Starr, R.; Chang, W.-C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-Directed CAR T Cells for Specific and Effective Targeting of Glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672. [Google Scholar] [CrossRef]
- Zargan, J.; Sajad, M.; Umar, S.; Naime, M.; Ali, S.; Khan, H.A. Scorpion (Androctonus crassicauda) Venom Limits Growth of Transformed Cells (SH-SY5Y and MCF-7) by Cytotoxicity and Cell Cycle Arrest. Exp. Mol. Pathol. 2011, 91, 447–454. [Google Scholar] [CrossRef]
- Salabi, F.; Jafari, H. Differential Venom Gland Gene Expression Analysis of Juvenile and Adult Scorpions Androctonus crassicauda. BMC Genom. 2022, 23, 636. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, M.; Gabikian, P.; Bahrami, S.-B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor Paint: A Chlorotoxin:Cy5.5 Bioconjugate for Intraoperative Visualization of Cancer Foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef]
- Butte, P.V.; Mamelak, A.; Parrish-Novak, J.; Drazin, D.; Shweikeh, F.; Gangalum, P.R.; Chesnokova, A.; Ljubimova, J.Y.; Black, K. Near-Infrared Imaging of Brain Tumors Using the Tumor Paint BLZ-100 to Achieve near-Complete Resection of Brain Tumors. Neurosurg. Focus 2014, 36, E1. [Google Scholar] [CrossRef]
- Fidel, J.; Kennedy, K.C.; Dernell, W.S.; Hansen, S.; Wiss, V.; Stroud, M.R.; Molho, J.I.; Knoblaugh, S.E.; Meganck, J.; Olson, J.M.; et al. Preclinical Validation of the Utility of BLZ-100 in Providing Fluorescence Contrast for Imaging Spontaneous Solid Tumors. Cancer Res. 2015, 75, 4283–4291. [Google Scholar] [CrossRef]
- Gordon, I.; Paoloni, M.; Mazcko, C.; Khanna, C. The Comparative Oncology Trials Consortium: Using Spontaneously Occurring Cancers in Dogs to Inform the Cancer Drug Development Pathway. PLoS Med. 2009, 6, e1000161. [Google Scholar] [CrossRef] [PubMed]
- Parrish-Novak, J.; Byrnes-Blake, K.; Lalayeva, N.; Burleson, S.; Fidel, J.; Gilmore, R.; Gayheart-Walsten, P.; Bricker, G.A.; Crumb, W.J.; Tarlo, K.S.; et al. Nonclinical Profile of BLZ-100, a Tumor-Targeting Fluorescent Imaging Agent. Int. J. Toxicol. 2017, 36, 104–112. [Google Scholar] [CrossRef]
- Kobets, A.J.; Nauen, D.; Lee, A.; Cohen, A.R. Unexpected Binding of Tozuleristide “Tumor Paint” to Cerebral Vascular Malformations: A Potentially Novel Application of Fluorescence-Guided Surgery. Neurosurgery 2021, 89, 204–211. [Google Scholar] [CrossRef]
- Yamada, M.; Miller, D.M.; Lowe, M.; Rowe, C.; Wood, D.; Soyer, H.P.; Byrnes-Blake, K.; Parrish-Novak, J.; Ishak, L.; Olson, J.M.; et al. A First-in-Human Study of BLZ-100 (Tozuleristide) Demonstrates Tolerability and Safety in Skin Cancer Patients. Contemp. Clin. Trials Commun. 2021, 23, 100830. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Saviola, A.J.; Mukherjee, A.K. Biochemical and Proteomic Characterization, and Pharmacological Insights of Indian Red Scorpion Venom Toxins. Front. Pharmacol. 2021, 12, 710680. [Google Scholar] [CrossRef] [PubMed]
- Puzari, U.; Das, B.; Mukherjee, A.K. Advancements in Diagnostic Techniques for Scorpion Venom Identification: A Comprehensive Review. Toxicon 2025, 253, 108191. [Google Scholar] [CrossRef]
- Puzari, U.; Khan, M.R.; Mukherjee, A.K. Development of a Gold Nanoparticle-Based Novel Diagnostic Prototype for In Vivo Detection of Indian Red Scorpion (Mesobuthus tamulus) Venom. Toxicon X 2024, 23, 100203. [Google Scholar] [CrossRef]
- Chauhan, S.; Mittal, R.; Kumar, M.; Mittal, A.; Kushwah, A.S. Gold Nanoparticle-Based Biosensors for Point-of-Care Diagnostics: A Review of Sensing Nanoparticle Applications and Future Prospects. Comb. Chem. High Throughput Screen. 2025, 28, 417–434. [Google Scholar] [CrossRef]
- Medley, C.D.; Smith, J.E.; Tang, Z.; Wu, Y.; Bamrungsap, S.; Tan, W. Gold Nanoparticle-Based Colorimetric Assay for the Direct Detection of Cancerous Cells. Anal. Chem. 2008, 80, 1067–1072. [Google Scholar] [CrossRef]
- Appidi, T.; Mudigunda, S.V.; Kodandapani, S.; Rengan, A.K. Development of Label-Free Gold Nanoparticle Based Rapid Colorimetric Assay for Clinical/Point-of-Care Screening of Cervical Cancer. Nanoscale Adv. 2020, 2, 5737–5745. [Google Scholar] [CrossRef] [PubMed]
- Pedarzani, P.; D’hoedt, D.; Doorty, K.B.; Wadsworth, J.D.F.; Joseph, J.S.; Jeyaseelan, K.; Kini, R.M.; Gadre, S.V.; Sapatnekar, S.M.; Stocker, M.; et al. Tamapin, a Venom Peptide from the Indian Red Scorpion (Mesobuthus tamulus) That Targets Small Conductance Ca2+-Activated K+ Channels and After Hyperpolarization Currents in Central Neurons. J. Biol. Chem. 2002, 277, 46101–46109. [Google Scholar] [CrossRef]
- Luis, E.; Anaya-Hernández, A.; León-Sánchez, P.; Durán-Pastén, M.L. The Kv10.1 Channel: A Promising Target in Cancer. Int. J. Mol. Sci. 2022, 23, 8458. [Google Scholar] [CrossRef]
- Ouadid-Ahidouch, H.; Ahidouch, A.; Pardo, L.A. Kv10.1 K+ Channel: From Physiology to Cancer. Pflug. Arch. 2016, 468, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Cázares-Ordoñez, V.; Pardo, L.A. Kv10.1 Potassium Channel: From the Brain to the Tumors. Biochem. Cell Biol. 2017, 95, 531–536. [Google Scholar] [CrossRef]
- Khamehchian, S.; Nikkhah, M.; Madani, R.; Hosseinkhani, S. Enhanced and Selective Permeability of Gold Nanoparticles Functionalized with Cell Penetrating Peptide Derived from Maurocalcine Animal Toxin. J. Biomed. Mater. Res. A 2016, 104, 2693–2700. [Google Scholar] [CrossRef]
- Kievit, F.M.; Veiseh, O.; Fang, C.; Bhattarai, N.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Chlorotoxin Labeled Magnetic Nanovectors for Targeted Gene Delivery to Glioma. ACS Nano 2010, 4, 4587–4594. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhu, J.; Sun, N.; Song, N.; Xing, Y.; Huang, H.; Zhao, J. Chlorotoxin Peptide-Functionalized Polyethylenimine-Entrapped Gold Nanoparticles for Glioma SPECT/CT Imaging and Radionuclide Therapy. J. Nanobiotechnol. 2019, 17, 30. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, Z.; Yu, Z.; Wang, H.; Lyu, X.; Han, C. Investigation on the Mechanisms of Scorpion Venom in Hepatocellular Carcinoma Model Mice via Untargeted Metabolomics Profiling. Int. Immunopharmacol. 2024, 138, 112578. [Google Scholar] [CrossRef]
- Mazhdi, Y.; Hamidi, S.M. Detection of Scorpion Venom by Optical Circular Dichroism Method. Sci. Rep. 2021, 11, 15854. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Veiseh, O.; Gunn, J.; Fang, C.; Hansen, S.; Lee, D.; Sze, R.; Ellenbogen, R.G.; Olson, J.; Zhang, M. In Vivo MRI Detection of Gliomas by Chlorotoxin-Conjugated Superparamagnetic Nanoprobes. Small 2008, 4, 372–379. [Google Scholar] [CrossRef]
- Zhao, L.; Shi, X.; Zhao, J. Chlorotoxin-Conjugated Nanoparticles for Targeted Imaging and Therapy of Glioma. Curr. Top. Med. Chem. 2015, 15, 1196–1208. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z. Chlorotoxin-Conjugated Onconase as a Potential Anti-Glioma Drug. Oncol. Lett. 2015, 9, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Cardoso, A.L.; Mendonça, L.S.; Serani, A.; Custódia, C.; Conceição, M.; Simões, S.; Moreira, J.N.; Pereira de Almeida, L.; Pedroso de Lima, M.C. Tumor-Targeted Chlorotoxin-Coupled Nanoparticles for Nucleic Acid Delivery to Glioblastoma Cells: A Promising System for Glioblastoma Treatment. Mol. Ther. Nucleic Acids 2013, 2, e100. [Google Scholar] [CrossRef] [PubMed]
- Amen, R.A.; Abd-Ellatef, G.E.F. Scorpion Venom and Its Different Peptides Aid in Treatment Focusing on Cancer Disease with the Mechanism of Action. Trends Pharm. 2025, 1, 1–11. [Google Scholar] [CrossRef]
- Rjeibi, I.; Mabrouk, K.; Mosrati, H.; Berenguer, C.; Mejdoub, H.; Villard, C.; Laffitte, D.; Bertin, D.; Ouafik, L.; Luis, J.; et al. Purification, Synthesis and Characterization of AaCtx, the First Chlorotoxin-like Peptide from Androctonus australis Scorpion Venom. Peptides 2011, 32, 656–663. [Google Scholar] [CrossRef]
- Huang, R.; Ke, W.; Han, L.; Li, J.; Liu, S.; Jiang, C. Targeted Delivery of Chlorotoxin-Modified DNA-Loaded Nanoparticles to Glioma via Intravenous Administration. Biomaterials 2011, 32, 2399–2406. [Google Scholar] [CrossRef]
- Thakur, A.; Alajangi, H.K.; Sharma, A.; Hwang, E.; Khajuria, A.; Kumari, L.; Jaiswal, P.K.; Lim, Y.; Singh, G.; Barnwal, R.P. Stigmurin Encapsulated PLA–PEG Ameliorates Its Therapeutic Potential, Antimicrobial and Antiproliferative Activities. Discov. Nano 2025, 20, 50. [Google Scholar] [CrossRef]
- Aliakbari, F.; Rahmati, S.; Ghanbari, A.; Madanchi, H.; Rashidy-Pour, A. Identification and Designing an Analgesic Opioid Cyclic Peptide from Defensin 4 of Mesobuthus martensii Karsch Scorpion Venom with More Effectiveness than Morphine. Biomed. Pharmacother. 2025, 188, 118139. [Google Scholar] [CrossRef]
- Ghodeif, S.K.; El-Fahla, N.A.; Abdel-Rahman, M.A.; El-Shenawy, N.S. Arthropod Venom Peptides: Pioneering Nanotechnology in Cancer Treatment and Drug Delivery. Cancer Pathog. Ther. 2025, 3, E1–E17. [Google Scholar] [CrossRef]
- Harrison, E.; Nicol, J.R.; Macias–Montero, M.; Burke, G.A.; Coulter, J.A.; Meenan, B.J.; Dixon, D. A Comparison of Gold Nanoparticle Surface Co-Functionalization Approaches Using Polyethylene Glycol (PEG) and the Effect on Stability, Non-Specific Protein Adsorption and Internalization. Mater. Sci. Eng. C 2016, 62, 710–718. [Google Scholar] [CrossRef]
- Gláucia-Silva, F.; Torres, J.V.P.; Torres-Rêgo, M.; Daniele-Silva, A.; Furtado, A.A.; Ferreira, S.d.S.; Chaves, G.M.; Xavier-Júnior, F.H.; Rocha Soares, K.S.; da Silva-Júnior, A.A.; et al. Tityus stigmurus-Venom-Loaded Cross-Linked Chitosan Nanoparticles Improve Antimicrobial Activity. Int. J. Mol. Sci. 2024, 25, 9893. [Google Scholar] [CrossRef]
- Rebbouh, F.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Chitosan Nanoparticles as a Delivery Platform for Neurotoxin II from Androctonus australis Hector Scorpion Venom: Assessment of Toxicity and Immunogenicity. Acta Trop. 2020, 205, 105353. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; An, N.; Li, K.; Zheng, Y.; Liang, A. Chlorotoxin-Conjugated Nanoparticles as Potential Glioma-Targeted Drugs. J. Neurooncol. 2012, 107, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; Yang, J.; Fu, S.; Zhang, S. Targeting Strategies for Superparamagnetic Iron Oxide Nanoparticles in Cancer Therapy. Acta Biomater. 2020, 102, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; He, L.; Qiu, S.; Li, Y.; Liao, Y.; Li, X.; Xie, D.; Peng, Y. OX26/CTX-Conjugated PEGylated Liposome as a Dual-Targeting Gene Delivery System for Brain Glioma. Mol. Cancer 2014, 13, 191. [Google Scholar] [CrossRef]
- Ward, M.J.; Ellsworth, S.A.; Nystrom, G.S. A Global Accounting of Medically Significant Scorpions: Epidemiology, Major Toxins, and Comparative Resources in Harmless Counterparts. Toxicon 2018, 151, 137–155. [Google Scholar] [CrossRef]
- Li, M.; Shao, X.; Wu, C.; Lu, D.; Liu, K.; Wang, W.; Liu, J.; Li, H.; Su, W.; Fang, L. Chlorotoxin-Derived Bicyclic Peptides for Targeted Imaging of Glioblastomas. Chem. Commun. 2020, 56, 9537–9540. [Google Scholar] [CrossRef]
- Baik, F.M.; Hansen, S.; Knoblaugh, S.E.; Sahetya, D.; Mitchell, R.M.; Xu, C.; Olson, J.M.; Parrish-Novak, J.; Méndez, E. Fluorescence Identification of Head and Neck Squamous Cell Carcinoma and High-Risk Oral Dysplasia With BLZ-100, a Chlorotoxin-Indocyanine Green Conjugate. JAMA Otolaryngol.–Head Neck Surg. 2016, 142, 330. [Google Scholar] [CrossRef] [PubMed]
- Dintzis, S.M.; Hansen, S.; Harrington, K.M.; Tan, L.C.; Miller, D.M.; Ishak, L.; Parrish-Novak, J.; Kittle, D.; Perry, J.; Gombotz, C.; et al. Real-Time Visualization of Breast Carcinoma in Pathology Specimens From Patients Receiving Fluorescent Tumor-Marking Agent Tozuleristide. Arch. Pathol. Lab. Med. 2019, 143, 1076–1083. [Google Scholar] [CrossRef]
- Klint, J.K.; Senff, S.; Saez, N.J.; Seshadri, R.; Lau, H.Y.; Bende, N.S.; Undheim, E.A.B.; Rash, L.D.; Mobli, M.; King, G.F. Production of Recombinant Disulfide-Rich Venom Peptides for Structural and Functional Analysis via Expression in the Periplasm of E. coli. PLoS ONE 2013, 8, e63865. [Google Scholar] [CrossRef]
- Ma, Y.; Lee, C.-J.; Park, J.-S. Strategies for Optimizing the Production of Proteins and Peptides with Multiple Disulfide Bonds. Antibiotics 2020, 9, 541. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Soares, A.G.; Stockand, J.D. Advances in Venomics: Modern Separation Techniques and Mass Spectrometry. J. Chromatogr. B 2020, 1160, 122352. [Google Scholar] [CrossRef]
- Bordon, K.; Santos, G.; Martins, J.; Wiezel, G.; Amorim, F.; Crasset, T.; Redureau, D.; Quinton, L.; Procópio, R.; Arantes, E. Pioneering Comparative Proteomic and Enzymatic Profiling of Amazonian Scorpion Venoms Enables the Isolation of Their First α-Ktx, Metalloprotease, and Phospholipase A2. Toxins 2025, 17, 411. [Google Scholar] [CrossRef]
- Daoudi, K.; Malosse, C.; Lafnoune, A.; Darkaoui, B.; Chakir, S.; Sabatier, J.; Chamot-Rooke, J.; Cadi, R.; Oukkache, N. Mass Spectrometry-based Top-Down and Bottom-Up Approaches for Proteomic Analysis of the Moroccan Buthus occitanus Scorpion Venom. FEBS Open Bio 2021, 11, 1867–1892. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.B.; de Souza, B.M.; Cocchi, F.K.; Chalkidis, H.M.; Dorce, V.A.C.; Palma, M.S. Profiling the Short, Linear, Non-Disulfide Bond-Containing Peptidome from the Venom of the Scorpion Tityus obscurus. J. Proteom. 2018, 170, 70–79. [Google Scholar] [CrossRef] [PubMed]
- García-Villalvazo, P.E.; Jiménez-Vargas, J.M.; Lino-López, G.J.; Meneses, E.P.; Bermúdez-Guzmán, M.d.J.; Barajas-Saucedo, C.E.; Delgado Enciso, I.; Possani, L.D.; Valdez-Velazquez, L.L. Unveiling the Protein Components of the Secretory-Venom Gland and Venom of the Scorpion Centruroides possanii (Buthidae) through Omic Technologies. Toxins 2023, 15, 498. [Google Scholar] [CrossRef]
- Ma, Y.; He, Y.; Zhao, R.; Wu, Y.; Li, W.; Cao, Z. Extreme Diversity of Scorpion Venom Peptides and Proteins Revealed by Transcriptomic Analysis: Implication for Proteome Evolution of Scorpion Venom Arsenal. J. Proteom. 2012, 75, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern Venomics—Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. Gigascience 2022, 11, giac048. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, C.; Yang, M.; Sun, J.; Lu, J.; Zhang, H.; Qin, J.; Zhang, W.; Jiang, Z. Unveiling the Diversity and Modifications of Short Peptides in Buthus martensii Scorpion Venom through Liquid Chromatography-High Resolution Mass Spectrometry. Toxins 2024, 16, 155. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Wang, C.K.; Craik, D.J. Chlorotoxin: Structure, Activity, and Potential Uses in Cancer Therapy. Pept. Sci. 2016, 106, 25–36. [Google Scholar] [CrossRef]
- Mamelak, A.N. Targeted Antitumor Therapy with the Scorpion Venom Chlorotoxin. Drugs Future 2011, 36, 0615. [Google Scholar] [CrossRef]
- Blaney, E.; Demeke, M.; Kamayirese, S.; Monga, L.; Hansen, L.A.; Watts, C.R.; Lovas, S. Does Chlorotoxin Target Matrix Metalloproteinase-2 in Glioblastoma? bioRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Mouchbahani-Constance, S.; Sharif-Naeini, R. Proteomic and Transcriptomic Techniques to Decipher the Molecular Evolution of Venoms. Toxins 2021, 13, 154. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Undheim, E.A.B.; Ueberheide, B.; King, G.F. Venom Peptides as Therapeutics: Advances, Challenges and the Future of Venom-Peptide Discovery. Expert Rev. Proteom. 2017, 14, 931–939. [Google Scholar] [CrossRef]
- Joglekar, A.V.; Dehari, D.; Anjum, M.M.; Dulla, N.; Chaudhuri, A.; Singh, S.; Agrawal, A.K. Therapeutic Potential of Venom Peptides: Insights in the Nanoparticle-Mediated Venom Formulations. Future J. Pharm. Sci. 2022, 8, 34. [Google Scholar] [CrossRef]
- Psenicnik, A.; Ojanguren-Affilastro, A.A.; Graham, M.R.; Hassan, M.K.; Abdel-Rahman, M.A.; Sharma, P.P.; Santibáñez-López, C.E. Optimizing Scorpion Toxin Processing through Artificial Intelligence. Toxins 2024, 16, 437. [Google Scholar] [CrossRef]
- Liscano, Y.; Oñate-Garzón, J.; Delgado, J.P. Peptides with Dual Antimicrobial–Anticancer Activity: Strategies to Overcome Peptide Limitations and Rational Design of Anticancer Peptides. Molecules 2020, 25, 4245. [Google Scholar] [CrossRef]
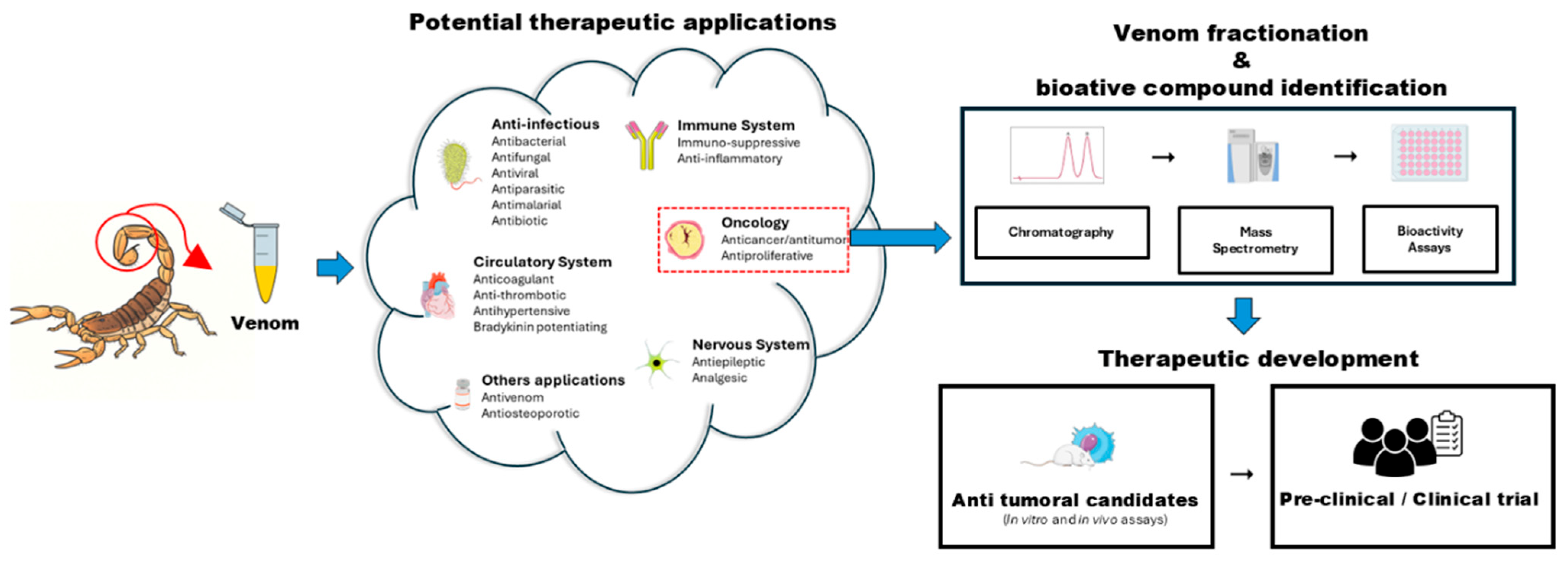
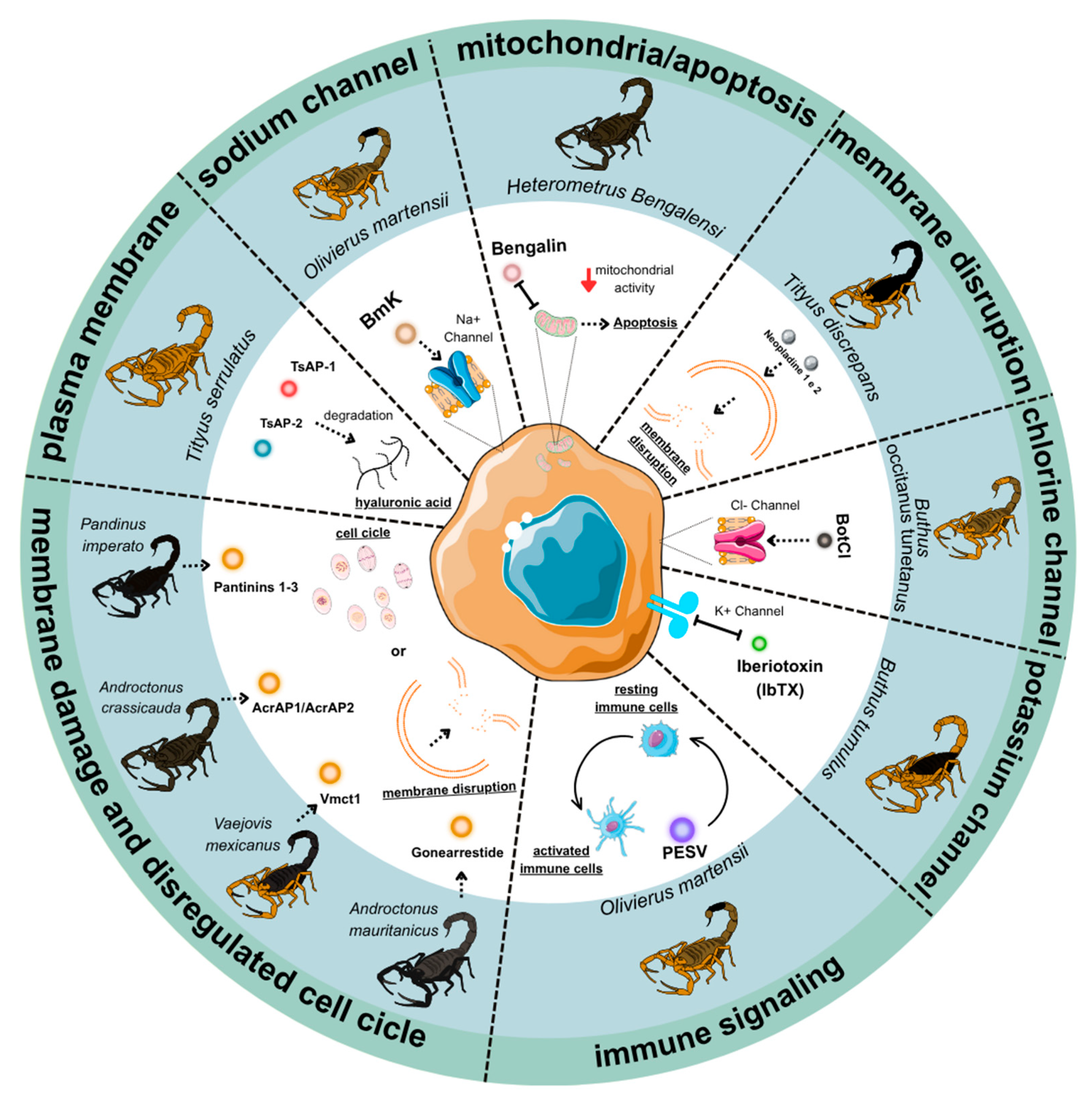
| Peptide/Protein | Scorpion Source | Molecular Mass/kDa | Molecular Target | Endpoint (Mechanism, IC50) | Primary Cancer Model(s) | Proteomic Validation | Key References |
|---|---|---|---|---|---|---|---|
| AcrAP1 | Androctonus crassicauda | 8.7 | Cell membranes | Blocks recognition and binding of target DNA by the Cascade complex. | Prostate, Lung | NR | [22,26,27,28] |
| AcrAP2 | 8.7 | ||||||
| AGAP-SYPU2 | Buthus martensii Karsch | 7.3 | Voltage-gated sodium channels (Nav1.4, Nav1.5, Nav1.7) | Induces apoptosis and inhibits proliferation and migration. | Fibrosarcoma, Colon | Yes (MALDI-TOF-MS) | [29,30,31] |
| AK and GK | Buthus martensii Karsch | - | TNF-α/EGFR/STAT3 signaling pathway | Downregulates pro-inflammatory cytokines (TNF-α) and oncogenes (c-Myc); upregulates tumor suppressors (p53/PTEN) | Gastric cancer | NR | [32] |
| Bengalin | Heterometrus bengalensis | 2.2 | Mitochondria (Bax/Bcl-2) | Induces intrinsic apoptosis via mitochondrial depolarization and cytochrome c release. | Leukemia | NR | [33,34,35,36,37] |
| BmHYA1 | Buthus martensii Karsch | - | Hyaluronic Acid (HA) in the extracellular matrix | Degrades HA, disrupting the HA-CD44 signaling axis; reduces tumor interstitial pressure, enhancing drug penetration | Breast | NR | [38,39,40,41] |
| BmK crude venom | Buthus martensii Karsch | Mixture | PI3K/Akt signaling, PTEN/p27 | Inhibits oncogenic signaling and induces apoptosis. | Glioma, Lymphoma, Breast, Hepatoma | NR | [42,43] |
| BmKn2 | Mesobuthus martensii Karsch | 8.0 | Mitochondria (Bax/Bcl-2) | Induces apoptosis, ROS generation, and mitochondrial dysfunction. | Oral Squamous Carcinoma | NR | [44,45] |
| BotCl | Buthus occitanus tunetanus | - | ClC-3, MMP-2 | A chlorotoxin-like peptide that inhibits migration and invasion. | Glioblastoma, Breast | NR | [46,47,48] |
| Chlorotoxin (CTX) | Leiurus hebraeus/quinquestriatus | 4.0 | ClC-3 chloride channels, MMP-2 | Inhibits tumor cell invasion and migration. | Malignant Glioma | NR | [47,49] |
| Cm28 | Centruroides margaritatus | 2.8 | Potassium channel Kv1.3 | Suppresses T cell activation markers (IL-2R and CD40L). | Not tested | NR | [50] |
| Crude venom | Hottentotta saulcyi | - | Na+ and K+ channels | Induces apoptosis in MCF-7 cells via TNF-α and caspase-3 upregulation. | Breast, Colon Cancer | Yes (LC-MS/MS) | [51,52] |
| Gonearestide | Androctonus mauritanicus | - | Cell membranes, CDK regulators | Induces G1 cell cycle arrest by downregulating CDK4 and upregulating p21/p27. | Prostate, Lung, Colorectal | Yes (LC-MS/MS, MALDI-TOF) | [53] |
| Hemiscorpius lepturus crude venom | Hemiscorpius lepturus | Mixture | Bax, p53, caspase-3 activation, Bcl-2 suppression | Induces apoptosis with low toxicity to normal cells. | Colorectal Cancer | NR | [54] |
| HsTX1 | Heterometrus spinnifer | 3.8 | Potassium channel Kv1.3 | Suppresses pathogenic effector memory T-lymphocytes. | Not tested | Yes (MALDI-TOF) | [55] |
| Iberiotoxin (IbTX) | Hottentotta tamulus (syn. Buthus/Mesobuthus tamulus) | 4.3 | Potassium channels Kv1.1, Kv1.3 | Blocks K+ currents, leading to Ca2+ dysregulation and apoptosis. | Breast Cancer | NR | [56,57,58,59,60]. |
| Margatoxin (MgTX) | Centruroides margaritatus | 4.2 | Potassium channel Kv1.3 | Suppresses proliferation and inhibits tumor growth. | Lung Adenocarcinoma | NR | [61,62,63,64] |
| Maurocalcine (MCa) | Scorpion maurus palmatus | 3.9 | Ryanodine receptor (RyR1) | Induces Ca2+ release; acts as a cell-penetrating peptide for drug delivery. | Breast Cancer | NR | [62,63,64,65] |
| Neopladine 1 and 2 | Tityus discrepans | - | Fas ligand → caspase-8 → Bid/tBid | Induces extrinsic apoptosis via the death receptor pathway. | Breast Cancer | Yes (MALDI-TOF) | [66] |
| Pantinins 1 | Pandinus imperator | 7.9 | Cell membranes | Destabilizes and disrupts the membrane of target cells. | Breast, Prostate Adenocarcinoma | NR | [67,68] |
| Pantinins 2 | 7.7 | ||||||
| Pantinins 3 | 7.7 | ||||||
| PESV | Buthus martensii Karsch | Mixture | Cyclin E, Kip1/p27, Bax/Bcl-2 | Induces G1-phase cell cycle arrest and apoptosis | Hepatocellular carcinoma, Prostate | NR | [69,70] |
| Smp43 | Scorpio maurus palmatus | 9.1 | Mitochondria | Induces apoptosis by activating caspase-1, triggering pyroptosis. | Lung Cancer | NR | [71,72,73,74] |
| Smp24 | Scorpio maurus palmatus | 8.9 | Mitochondria | Induces apoptosis via mitochondrial membrane potential reduction and ROS production. | Lung Cancer | NR | [75,76] |
| TsAP-1 | Tityus serrulatus | 8.4 | Cell membranes | TsAP-2 IC50 = 4 μM (SKBR3 cells); Induces apoptosis through membrane disruption | Lung, Prostate, Squamous Carcinoma | Yes (MALDI-TOF/TOF) | [77,78,79] |
| TsAP-2 | 8.4 | ||||||
| TsAP-S2 | Tityus serrulatus | 1.7 | Mitochondria | TsAP-S2 IC50 = 0.83 μM. Induces apoptosis through mitochondrial disruption. | Not tested | Yes (ESI-MS) | [78]; |
| Vm24 | Vaejovis mexicanus smithi | 3.9 | Potassium channel Kv1.3 | Suppresses pathogenic effector memory T-lymphocytes. | Breast Cancer | Yes (LC-MS/MS, MALDI-TOF) | [80] |
| Compound Name | Trial Phase | Condition | Key Findings | Status |
|---|---|---|---|---|
| Chlorotoxin (CTX)/TM-601 | Phase I/II | Recurrent high-grade glioma (glioblastoma, anaplastic astrocytoma) | Selective tumor binding; safe intratumoral use; prolonged tumor retention; limited systemic toxicity | Completed Phase I/II; discontinued for glioma therapy, redirected to imaging and drug-delivery applications |
| BLZ-100 (Tumor Paint, Tozuleristide, Chlorotoxin–ICG conjugate) | Phase I/II | Pediatric and adult brain tumors, breast cancer, sarcoma | Enabled real-time intraoperative tumor visualization; improved surgical margin delineation; well tolerated | Ongoing Phase II (pediatric brain tumors, solid tumors); active in translational oncology |
| Chlorotoxin–nanoparticle conjugates | Preclinical/Translational | Glioblastoma, metastatic tumors | Enhanced blood–brain barrier penetration; targeted imaging and therapeutic delivery in animal models | Preclinical; advancing toward first-in-human evaluation |
| Other venom-derived peptides (e.g., BmK peptides, Bengalin, Neopladines, Maurocalcine) | Preclinical | Leukemia, breast cancer, glioblastoma, colon carcinoma | Demonstrated apoptosis induction, cell cycle arrest, and tumor inhibition in vitro and xenograft models; low toxicity to normal cells | Preclinical only; not yet in clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcos, S.S.S.; Aguiar, M.R.d.C.; Oliveira, J.d.; Silva, M.R.d.; Pimentel, I.d.O.C.; dos Anjos, N.G.; Machado, G.H.R.S.; Evangelista, K.B.; Portaro, F.C.V.; Iwai, L.K. Scorpion Venom as a Source of Cancer Drugs: A Comprehensive Proteomic Analysis and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 9907. https://doi.org/10.3390/ijms26209907
Arcos SSS, Aguiar MRdC, Oliveira Jd, Silva MRd, Pimentel IdOC, dos Anjos NG, Machado GHRS, Evangelista KB, Portaro FCV, Iwai LK. Scorpion Venom as a Source of Cancer Drugs: A Comprehensive Proteomic Analysis and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(20):9907. https://doi.org/10.3390/ijms26209907
Chicago/Turabian StyleArcos, Stephanie Santos Suehiro, Mariana Ramos da Cunha Aguiar, Júlia de Oliveira, Matheus Ramos da Silva, Isabela de Oliveira Cavalcante Pimentel, Nicolas Gamboa dos Anjos, Gustavo Henrique Rohr Souza Machado, Kimberly Borges Evangelista, Fernanda Calheta Vieira Portaro, and Leo Kei Iwai. 2025. "Scorpion Venom as a Source of Cancer Drugs: A Comprehensive Proteomic Analysis and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 20: 9907. https://doi.org/10.3390/ijms26209907
APA StyleArcos, S. S. S., Aguiar, M. R. d. C., Oliveira, J. d., Silva, M. R. d., Pimentel, I. d. O. C., dos Anjos, N. G., Machado, G. H. R. S., Evangelista, K. B., Portaro, F. C. V., & Iwai, L. K. (2025). Scorpion Venom as a Source of Cancer Drugs: A Comprehensive Proteomic Analysis and Therapeutic Potential. International Journal of Molecular Sciences, 26(20), 9907. https://doi.org/10.3390/ijms26209907






