microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting
Abstract
1. Introduction
2. Results
2.1. Anti-miR-22 ASO Mediates miR-22 Inhibition in Muscles In Vitro and In Vivo
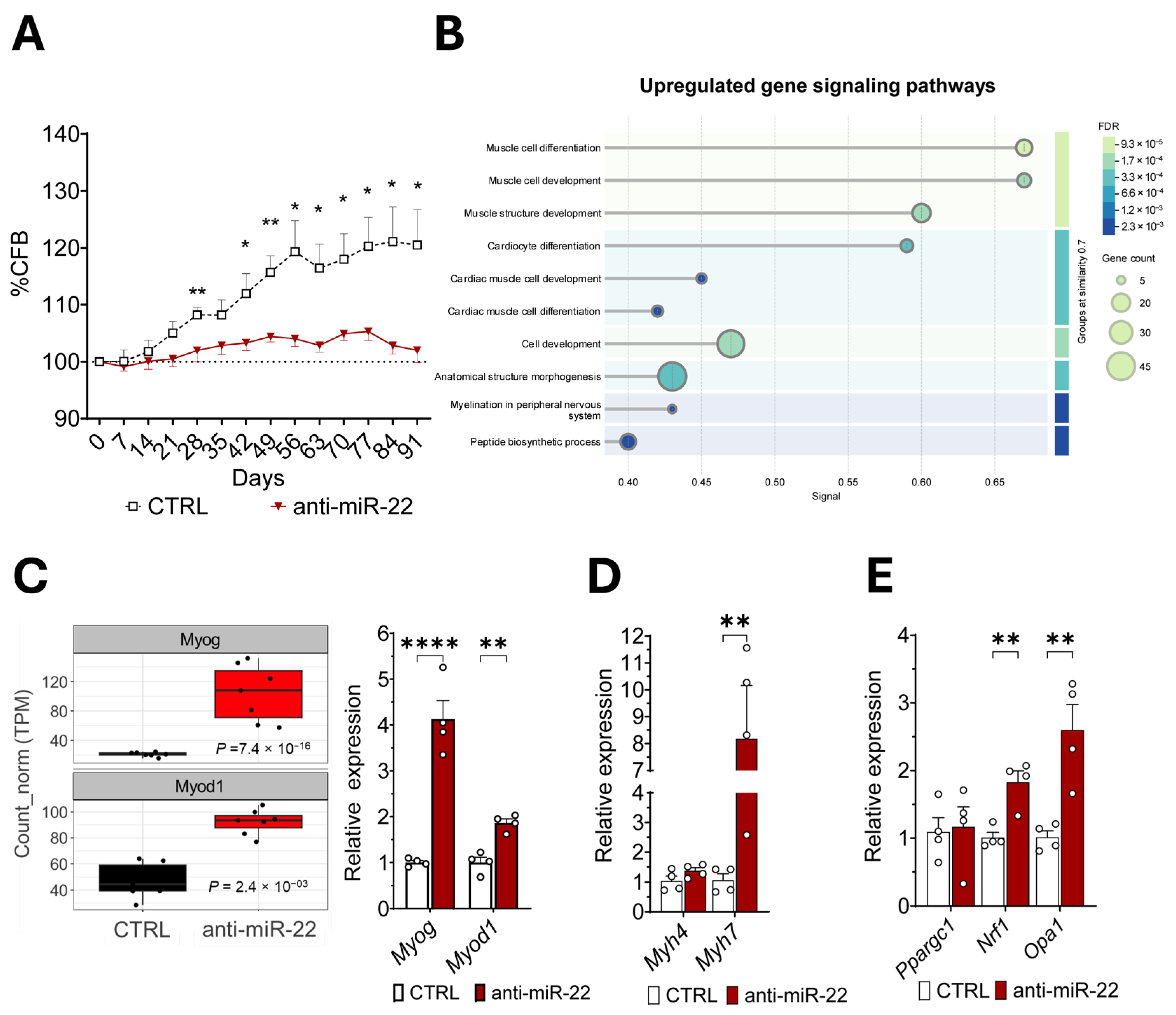
2.2. miR-22 Inhibition Promotes Transcriptional Activation of Myogenic Programs and Mitochondrial Homeostasis Regulation in Skeletal Muscles
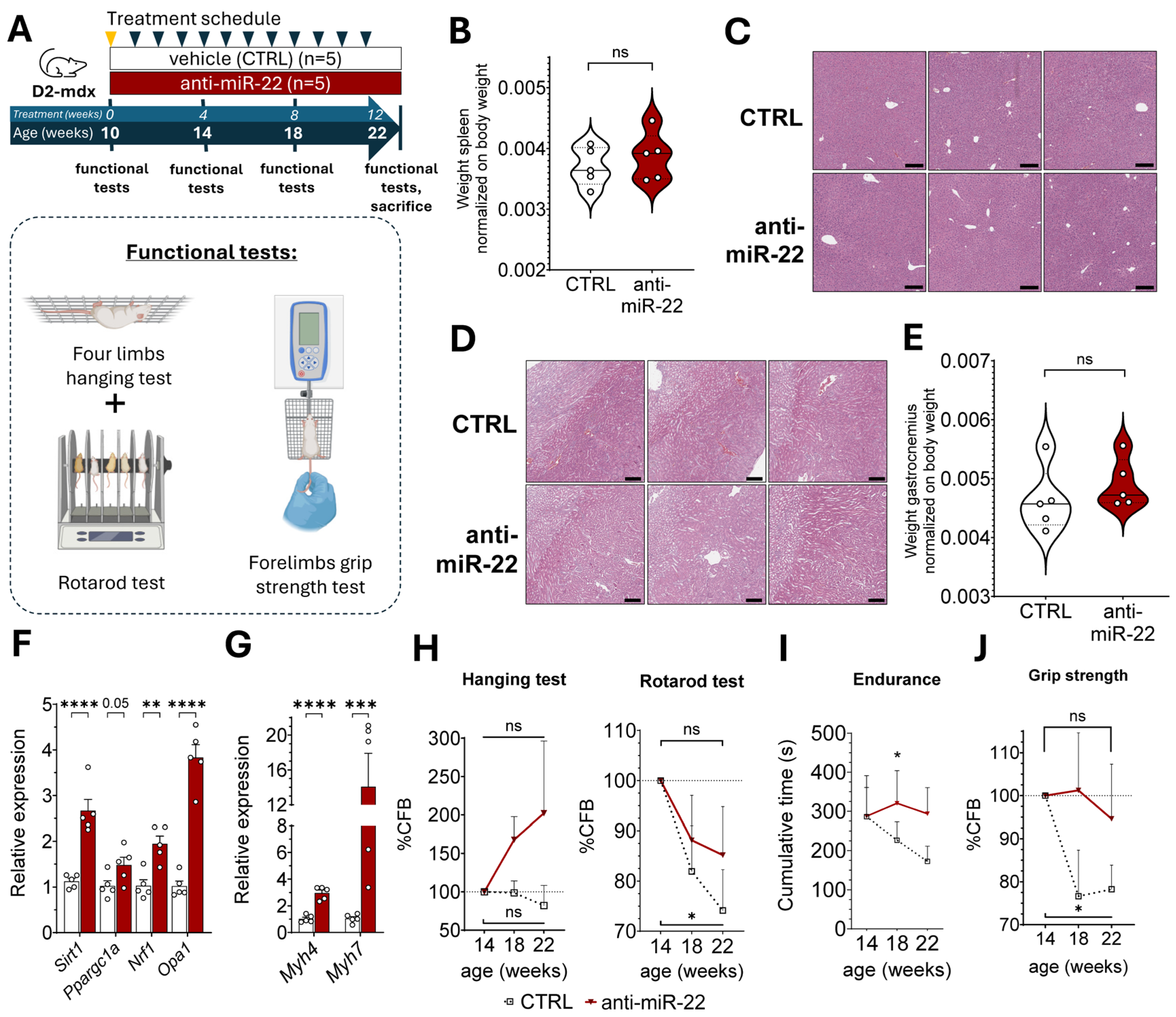
2.3. miR-22 Inhibition Supports Oxidative Remodeling to Limit Fibrosis and Enhance Muscle Function in Muscular Dystrophy
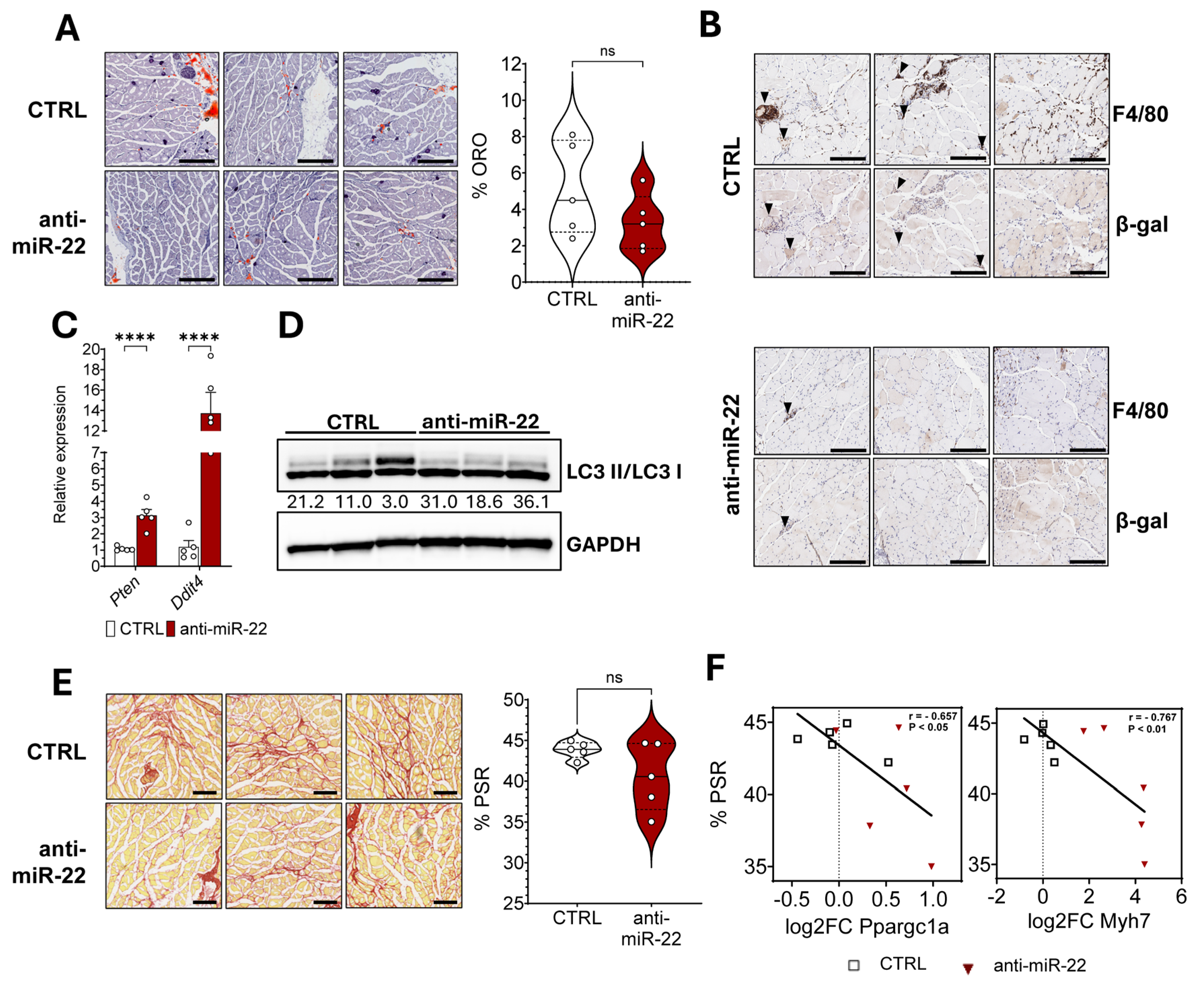
2.4. miR-22 Inhibition Limits Macrophage Infiltration in Dystrophic Skeletal Muscles by Inducing Autophagy Through mTOR Inhibition
3. Discussion
4. Materials and Methods
4.1. Characterization of the Antisense Nucleotide Compound
4.2. Cell Culture and Treatment
4.3. Animals and Treatments
4.4. Muscle Function Evaluation
4.5. Blood Collection and Plasma Processing
4.6. Histological Analysis
4.7. RNA Extraction, cDNA Preparation and Quantitative Real Time (qRT)-PCR
4.8. ELISA for Quantification of Anti-miR-22 ASO in Liver and Skeletal Muscle
4.9. Library Preparation and Sequencing
4.10. RNA Sequencing Analysis
- I.
- Preprocess and perform quality control for sequencing data using the FastQC tool (v. 0.12.0) (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 8 October 2025) and the MultiQC framework (v. 1.29) (https://github.com/MultiQC/MultiQC, accessed on 8 October 2025).
- II.
- Trimming of the sequencing reads for adapter/low-quality sequence using the fastp tool (v. 1.0.0) (https://github.com/OpenGene/fastp, accessed on 8 October 2025) with default parameters.
- III.
- Mapping the filtered reads to GRcm39 genome using STAR with the following options: --outSAMtype BAM SortedByCoordinate --outSAMunmapped Within --chimOutType WithinBAM --outFilterType BySJout --outFilterMultimapNmax 200 --alignSJoverhangMin 8 --alignSJDBoverhangMin 1 --outFilterMismatchNmax 999 --outFilterMismatchNoverReadLmax 0.04 --alignIntronMin 20 --alignIntronMax 1,000,000 --alignMatesGapMax
- IV.
- Quantify the number of reads mapping to each gene using gene annotation from Gencode M25 using the FeatureCounts utility of the Subread package [70] with parameters set to -t exon --extraAttributes gene_name -p -C --countReadPairs. Counts were restricted to the last 500 bp of the 3′UTR of each gene to handle biases in sequencing coverage.
- V.
- Perform read counts normalization and differential gene expression analysis using DESeq2 [71] with its default settings to fit the negative binomial model and perform Wald tests, identifying genes that are differentially expressed between the anti-miR-22 ASO-treated and control muscles. After calculating the initial results, the lfcShrink function of DESEq2 with the “ashr” method was used to moderate extreme Log Fold Change estimates, reducing the impact of low-count genes and leading to more reliable effect size estimates for ranking and interpretation.
4.11. Protein Extraction and Immunoblotting
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | serine/threonine (Ser/Thr) kinase |
| ASO | antisense oligonucleotide |
| ATP | adenosine triphosphate |
| DDIT4 | DNA-damage-inducible transcript 4 |
| DEGs | differentially expressed genes |
| DIO | diet-induced obesity |
| DMD | Duchenne muscular dystrophy |
| FAO | fatty acid oxidation |
| FOXO | forkhead box O |
| GAS | gastrocnemius |
| H&E | Hematoxylin and eosin |
| HFD | high-fat diet |
| HSkMC | human skeletal muscle cells |
| IMAT | intramuscular adipose tissue |
| MAP1LC3B/LC3 | microtubule-associated protein 1 light chain 3 beta |
| LNA | locked nucleic acid |
| MetS | metabolic syndrome |
| miR-22 | microRNA-22 |
| miRNAs | microRNAs |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mammalian target of rapamycin complex 1 |
| MYH | myosin heavy chain |
| MyoD1 | myoblast determination protein 1 |
| MYOG | myogenin |
| myomiRs | muscle-enriched microRNAs |
| NRF1 | nuclear respiratory factor 1 |
| OPA1 | optic atrophy 1 |
| ORO | oil red O |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PPARGC1a | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PSR | picrosirius red |
| PTEN | phosphatase and tensin homolog |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| SEM | standard error of the mean |
| UTR | untranslated region |
| β-gal | beta-galactosidase |
| %CFB | percentage of change from baseline |
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Glancy, B.; Balaban, R.S. Energy metabolism design of the striated muscle cell. Physiol. Rev. 2021, 101, 1561–1607. [Google Scholar] [CrossRef]
- Periasamy, M.; Herrera, J.L.; Reis, F.C.G. Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism. Diabetes Metab. J. 2017, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Hepple, R.T.; Burelle, Y. Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: Tailoring the organelle for optimal function. Am. J. Physiol. Cell Physiol. 2012, 302, C629–C641. [Google Scholar] [CrossRef]
- Pette, D.; Spamer, C. Metabolic properties of muscle fibers. Fed. Proc. 1986, 45, 2910–2914. [Google Scholar] [PubMed]
- CohenSolal, A. Exercise to prevent cardiotoxicity in cancer: Ready for implementation? Eur. J. Prev. Cardiol. 2022, 29, 462. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Ljubicic, V.; Burt, M.; Jasmin, B.J. The therapeutic potential of skeletal muscle plasticity in Duchenne muscular dystrophy: Phenotypic modifiers as pharmacologic targets. FASEB J. 2014, 28, 548–568. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Coelho-Junior, H.J.; Landi, F.; Picca, A. Mitochondrial Quantity and Quality in Age-Related Sarcopenia. Int. J. Mol. Sci. 2024, 25, 2052. [Google Scholar] [CrossRef]
- Liao, T.; Xiong, L.; Wang, X.; Yang, S.; Liang, Z. Mitochondrial disorders as a mechanism for the development of obese Sarcopenia. Diabetol. Metab. Syndr. 2023, 15, 224. [Google Scholar] [CrossRef]
- Bonato, A.; Raparelli, G.; Caruso, M. Molecular pathways involved in the control of contractile and metabolic properties of skeletal muscle fibers as potential therapeutic targets for Duchenne muscular dystrophy. Front. Physiol. 2024, 15, 1496870. [Google Scholar] [CrossRef]
- Marini, J.F.; Pons, F.; Leger, J.; Loffreda, N.; Anoal, M.; Chevallay, M.; Fardeau, M.; Leger, J.J. Expression of myosin heavy chain isoforms in Duchenne muscular dystrophy patients and carriers. Neuromuscul. Disord. 1991, 1, 397–409. [Google Scholar] [CrossRef]
- Mogharehabed, F.; Czubryt, M.P. The role of fibrosis in the pathophysiology of muscular dystrophy. Am. J. Physiol. Cell Physiol. 2023, 325, C1326–C1335. [Google Scholar] [CrossRef] [PubMed]
- Flores-Opazo, M.; Kopinke, D.; Helmbacher, F.; Fernandez-Verdejo, R.; Tunon-Suarez, M.; Lynch, G.S.; Contreras, O. Fibro-adipogenic progenitors in physiological adipogenesis and intermuscular adipose tissue remodeling. Mol. Aspects Med. 2024, 97, 101277. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, H.; Wang, T.; Xia, Y.; Jin, L.; Jiang, A.; Zhu, L.; Chen, L.; Li, R.; Li, X. Co-methylated genes in different adipose depots of pig are associated with metabolic, inflammatory and immune processes. Int. J. Biol. Sci. 2012, 8, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.; Macias, E.; Zarini, S.; Garfield, A.; Zemski Berry, K.; Gerszten, R.; Schoen, J.; Cree-Green, M.; Bergman, B.C. Quantifying the inflammatory secretome of human intermuscular adipose tissue. Physiol. Rep. 2022, 10, e15424. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Bergman, B.C.; Brennan, A.M.; Sparks, L.M. Intermuscular adipose tissue in metabolic disease. Nat. Rev. Endocrinol. 2023, 19, 285–298. [Google Scholar] [CrossRef]
- Sampson, M.; Bailey, M.; Clark, J.; Evans, M.L.; Fong, R.; Hall, H.; Hambling, C.; Hadley-Brown, M.; Morrish, N.; Murphy, H.; et al. A new integrated care pathway for ambulance attended severe hypoglycaemia in the East of England: The Eastern Academic Health Science Network (EAHSN) model. Diabetes Res. Clin. Pract. 2017, 133, 50–59. [Google Scholar] [CrossRef]
- Kelley, D.E.; Goodpaster, B.; Wing, R.R.; Simoneau, J.A. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am. J. Physiol. 1999, 277, E1130–E1141. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef]
- Pereyra, A.S.; Lin, C.T.; Sanchez, D.M.; Laskin, J.; Spangenburg, E.E.; Neufer, P.D.; Fisher-Wellman, K.; Ellis, J.M. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 2022, 59, 101456. [Google Scholar] [CrossRef] [PubMed]
- Henique, C.; Mansouri, A.; Vavrova, E.; Lenoir, V.; Ferry, A.; Esnous, C.; Ramond, E.; Girard, J.; Bouillaud, F.; Prip-Buus, C.; et al. Increasing mitochondrial muscle fatty acid oxidation induces skeletal muscle remodeling toward an oxidative phenotype. FASEB J. 2015, 29, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Sebori, R.; Kuno, A.; Hosoda, R.; Hayashi, T.; Horio, Y. Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice. Oxid. Med. Cell. Longev. 2018, 2018, 9179270. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Fukumura, S.; Nikaido, K.; Tachi, N.; Kozuka, N.; Seino, T.; Hatakeyama, K.; Mori, M.; Ito, Y.M.; Takami, A.; et al. Resveratrol improves motor function in patients with muscular dystrophies: An open-label, single-arm, phase IIa study. Sci. Rep. 2020, 10, 20585. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef]
- Agagunduz, D.; Celik, M.N.; Deniz Gunes, B.; Atabilen, B.; Sarikaya, B.; Icer, M.A.; Budan, F. Involvement of miRNAs in the Cluster of Metabolic Factors of MetS: Nutrition-Genome-MetS Axis. J. Clin. Med. 2025, 14, 4234. [Google Scholar] [CrossRef]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes. 2023, 14, 1375. [Google Scholar] [CrossRef]
- Kimura, M.; Kothari, S.; Gohir, W.; Camargo, J.F.; Husain, S. MicroRNAs in infectious diseases: Potential diagnostic biomarkers and therapeutic targets. Clin. Microbiol. Rev. 2023, 36, e0001523. [Google Scholar] [CrossRef]
- Koopmans, P.J.; Ismaeel, A.; Goljanek-Whysall, K.; Murach, K.A. The roles of miRNAs in adult skeletal muscle satellite cells. Free Radic. Biol. Med. 2023, 209, 228–238. [Google Scholar] [CrossRef]
- Alteri, A.; De Vito, F.; Nessina, G.; Pompili, M.; Calconi, A.; Visca, P.; Mottolese, M.; Presutti, C.; Grossi, M. Cyclin D1 is a major target of miR-206 in cell differentiation and transformation. Cell Cycle 2013, 12, 3781–3790. [Google Scholar] [CrossRef]
- Small, E.M.; O’Rourke, J.R.; Moresi, V.; Sutherland, L.B.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. Regulation of PI3-kinase/Akt signaling by muscle-enriched microRNA-486. Proc. Natl. Acad. Sci. USA 2010, 107, 4218–4223. [Google Scholar] [CrossRef]
- Panella, R.; Petri, A.; Desai, B.N.; Fagoonee, S.; Cotton, C.A.; Nguyen, P.K.; Lundin, E.M.; Wagshal, A.; Wang, D.Z.; Näär, A.M.; et al. MicroRNA-22 Is a Key Regulator of Lipid and Metabolic Homeostasis. Int. J. Mol. Sci. 2023, 24, 2870. [Google Scholar] [CrossRef]
- Thibonnier, M.; Esau, C.; Ghosh, S.; Wargent, E.; Stocker, C. Metabolic and energetic benefits of microRNA-22 inhibition. BMJ Open Diabetes Res. Care 2020, 8. [Google Scholar] [CrossRef]
- Kaur, K.; Vig, S.; Srivastava, R.; Mishra, A.; Singh, V.P.; Srivastava, A.K.; Datta, M. Elevated Hepatic miR-22-3p Expression Impairs Gluconeogenesis by Silencing the Wnt-Responsive Transcription Factor Tcf7. Diabetes 2015, 64, 3659–3669. [Google Scholar] [CrossRef] [PubMed]
- Bar, N.; Dikstein, R. miR-22 forms a regulatory loop in PTEN/AKT pathway and modulates signaling kinetics. PLoS ONE 2010, 5, e10859. [Google Scholar] [CrossRef] [PubMed]
- Song, S.J.; Ito, K.; Ala, U.; Kats, L.; Webster, K.; Sun, S.M.; Jongen-Lavrencic, M.; Manova-Todorova, K.; Teruya-Feldstein, J.; Avigan, D.E.; et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 2013, 13, 87–101. [Google Scholar] [CrossRef]
- Xu, D.; Takeshita, F.; Hino, Y.; Fukunaga, S.; Kudo, Y.; Tamaki, A.; Matsunaga, J.; Takahashi, R.U.; Takata, T.; Shimamoto, A.; et al. miR-22 represses cancer progression by inducing cellular senescence. J. Cell Biol. 2011, 193, 409–424. [Google Scholar] [CrossRef]
- Prakash, T.P.; Mullick, A.E.; Lee, R.G.; Yu, J.; Yeh, S.T.; Low, A.; Chappell, A.E.; Ostergaard, M.E.; Murray, S.; Gaus, H.J.; et al. Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle. Nucleic Acids Res. 2019, 47, 6029–6044. [Google Scholar] [CrossRef] [PubMed]
- Amirouche, A.; Jahnke, V.E.; Lunde, J.A.; Koulmann, N.; Freyssenet, D.G.; Jasmin, B.J. Muscle-specific microRNA-206 targets multiple components in dystrophic skeletal muscle representing beneficial adaptations. Am. J. Physiol. Cell Physiol. 2017, 312, C209–C221. [Google Scholar] [CrossRef]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J. Clin. Investig. 2012, 122, 2054–2065. [Google Scholar] [CrossRef]
- Taetzsch, T.; Shapiro, D.; Eldosougi, R.; Myers, T.; Settlage, R.E.; Valdez, G. The microRNA miR-133b functions to slow Duchenne muscular dystrophy pathogenesis. J. Physiol. 2021, 599, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Q.; Wang, B.; Wu, W.; Wei, J.; Li, P.; Huang, R. miR-22 regulates C2C12 myoblast proliferation and differentiation by targeting TGFBR1. Eur. J. Cell Biol. 2018, 97, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.P.; Wang, D.Z. miR-22 in cardiac remodeling and disease. Trends Cardiovasc. Med. 2014, 24, 267–272. [Google Scholar] [CrossRef]
- Wen, W.; Chen, X.; Huang, Z.; Chen, D.; Zheng, P.; He, J.; Chen, H.; Yu, J.; Luo, Y.; Yu, B. miR-22-3p regulates muscle fiber-type conversion through inhibiting AMPK/SIRT1/PGC-1alpha pathway. Anim. Biotechnol. 2021, 32, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel′in, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Lima, V.M.; Liu, J.; Brandão, B.B.; Lino, C.A.; Balbino-Silva, C.S.; Ribeiro, M.A.C.; Oliveira, T.E.; Real, C.C.; De Paula Faria, D.; Cederquist, C. miRNA-22 deletion limits white adipose expansion and activates brown fat to attenuate high-fat diet-induced fat mass accumulation. Metab. Clin. Exp. 2021, 117, 154723. [Google Scholar] [CrossRef]
- Katare, P.B.; Dalmao-Fernandez, A.; Mengeste, A.M.; Hamarsland, H.; Ellefsen, S.; Bakke, H.G.; Kase, E.T.; Thoresen, G.H.; Rustan, A.C. Energy metabolism in skeletal muscle cells from donors with different body mass index. Front. Physiol. 2022, 13, 982842. [Google Scholar] [CrossRef]
- Mengeste, A.M.; Rustan, A.C.; Lund, J. Skeletal muscle energy metabolism in obesity. Obesity 2021, 29, 1582–1595. [Google Scholar] [CrossRef]
- Long, K.; Su, D.; Li, X.; Li, H.; Zeng, S.; Zhang, Y.; Zhong, Z.; Lin, Y.; Li, X.; Lu, L.; et al. Identification of enhancers responsible for the coordinated expression of myosin heavy chain isoforms in skeletal muscle. BMC Genom. 2022, 23, 519. [Google Scholar] [CrossRef]
- Hughes, S.M.; Chi, M.M.; Lowry, O.H.; Gundersen, K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J. Cell Biol. 1999, 145, 633–642. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Desbats, M.A.; Fadini, G.P.; Albiero, M.; Favaro, G.; Ciciliot, S.; Soriano, M.E.; Morbidoni, V.; Cerqua, C.; et al. Age-Associated Loss of OPA1 in Muscle Impacts Muscle Mass, Metabolic Homeostasis, Systemic Inflammation, and Epithelial Senescence. Cell Metab. 2017, 25, 1374–1389 e1376. [Google Scholar] [CrossRef] [PubMed]
- Morena, F.; Lim, S.; Cabrera, A.R.; Chambers, T.L.; Koopmans, P.J.; Tsitkanou, S.; Khadgi, S.; Peterson, C.; Schrems, E.R.; Muhyudin, R.; et al. Promoting mitochondrial fusion is protective against cancer-induced muscle detriments in males and females. BMC Cancer 2025, 25, 1300. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bibee, K.P.; Cheng, Y.-J.; Ching, J.K.; Marsh, J.N.; Li, A.J.; Keeling, R.M.; Connolly, A.M.; Golumbek, P.T.; Myerson, J.W.; Hu, G.; et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014, 28, 2047. [Google Scholar] [CrossRef]
- Eghtesad, S.; Jhunjhunwala, S.; Little, S.R.; Clemens, P.C. Rapamycin ameliorates dystrophic phenotype in mdx mouse skeletal muscle. Mol. Med. 2011, 17, 917–924. [Google Scholar] [CrossRef]
- Tao, Z.; Feng, C.; Mao, C.; Ren, J.; Tai, Y.; Guo, H.; Pu, M.; Zhou, Y.; Wang, G.; Wang, M. MiR-4465 directly targets PTEN to inhibit AKT/mTOR pathway–mediated autophagy. Cell Stress. Chaperones 2019, 24, 105–113. [Google Scholar] [CrossRef]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2–mTOR signaling and tumor suppression through REDD1-mediated 14–3–3 shuttling. Genes. Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Hammers, D.W.; Hart, C.C.; Matheny, M.K.; Wright, L.A.; Armellini, M.; Barton, E.R.; Sweeney, H.L. The D2.mdx mouse as a preclinical model of the skeletal muscle pathology associated with Duchenne muscular dystrophy. Sci. Rep. 2020, 10, 14070. [Google Scholar] [CrossRef]
- Gordon, B.S.; Delgado-Diaz, D.C.; Carson, J.; Fayad, R.; Wilson, L.B.; Kostek, M.C. Resveratrol improves muscle function but not oxidative capacity in young mdx mice. Can. J. Physiol. Pharmacol. 2014, 92, 243–251. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Hollinger, K.; Selsby, J.T. PGC-1alpha gene transfer improves muscle function in dystrophic muscle following prolonged disease progress. Exp. Physiol. 2015, 100, 1145–1158. [Google Scholar] [CrossRef]
- Yiangou, L.; Ross, A.D.B.; Goh, K.J.; Vallier, L. Human Pluripotent Stem Cell-Derived Endoderm for Modeling Development and Clinical Applications. Cell Stem Cell 2018, 22, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.A. The role of mTORC1 in regulating protein synthesis and skeletal muscle mass in response to various mechanical stimuli. Rev. Physiol. Biochem. Pharmacol. 2014, 166, 43–95. [Google Scholar] [CrossRef]
- Tu, J.; Fang, Y.; Han, D.; Tan, X.; Xu, Z.; Jiang, H.; Wang, X.; Hong, W.; Wei, W. Correction: MicroRNA-22 represses glioma development via activation of macrophage-mediated innate and adaptive immune responses. Oncogene 2022, 41, 2526–2527. [Google Scholar] [CrossRef]
- Shen, C.; Chen, M.T.; Zhang, X.H.; Yin, X.L.; Ning, H.M.; Su, R.; Lin, H.S.; Song, L.; Wang, F.; Ma, Y.N.; et al. The PU.1-Modulated MicroRNA-22 Is a Regulator of Monocyte/Macrophage Differentiation and Acute Myeloid Leukemia. PLoS Genet. 2016, 12, e1006259. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; van Putten, M. Assessing functional performance in the mdx mouse model. J. Vis. Exp. 2014, 85, 51303. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5439. [Google Scholar] [CrossRef]
- Balcells, I.; Cirera, S.; Busk, P.K.; Balcells, I.; Cirera, S.; Busk, P.K. Specific and sensitive quantitative RT-PCR of miRNAs with DNA primers. BMC Biotechnol. 2011, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed]

| Gene | Species | Forward Primer | Reverse Primer |
|---|---|---|---|
| Ddit4 | mouse | GCCGGAGGAAGACTCCTCATA | CATCAGGTTGGCACACAGGT |
| Fbxo32 | mouse | CGACCTGCCTGTGTGCTTAC | CTTGCGAATCTGCCTCTCTG |
| Foxo1 | mouse | CGTGCCCTACTTCAAGGATAA | GCACTCGAATAAACTTGCTGTG |
| Foxo3 | mouse | GTGTGCCCTACTTCAAGGATAA | TCATTCTGAACGCGCATGA |
| Gapdh | mouse | TGACCACAGTCCATGCCATC | GACGGACACATTGGGGGTAG |
| Myh4 | mouse | GCCTCCTTCTTCATCTGGTAA | CGATTCGCTCCTTTTCAGAC |
| Myh7 | mouse | TACTTGCTACCCTCAGGTGG | ATGGCTGAGCCTTGGATTCTC |
| Myod1 | mouse | TACGACACCGCCTACTACA | GGAGATGCGCTCCACTATG |
| Myog | mouse | AGTGAATGCAACTCCCACAG | GACGTAAGGGAGTGCAGATTG |
| Nrf1 | mouse | ACAGATAGTCCTGTCTGGGGAAA | TGGTACATGCTCACAGGGATCT |
| Opa1 | mouse | CCGACCTGGACAAGATTACTG | CCATGATCTGTTGCTCGAAATG |
| Ppargc1 | mouse | CCCATACACAACCGCAGTC | GAACCCTTGGGGTCATTTG |
| Pten | mouse | AGGCACAAGAGGCCCTAGAT | CTGACTGGGAATTGTGACTCC |
| Sirt1 | mouse | CAGTGAGAAAATGCTGGCCTA | TTGGTGGTACAAACAGGTATTGA |
| Tet2 | mouse | GTGGACTGCGAGGCTGAG | AGTCTTGGGAGGGCAAGC |
| Trim63 | mouse | GGTGCCTACTTGCTCCTTGT | CTGGTGGCTATTCTCCTTGG |
| GAPDH | human | GATTCCACCCATGGCAAATTC | GTCATGAGTCCTTCCACGATAC |
| NRF1 | human | GTATCTCACCCTCCAAACCTAAC | CCAGGATCATGCTCTTGTACTT |
| OPA1 | human | CTCACCATGTGGCCCTATTT | ACGGTACAGCCTTCTTTCAC |
| PPARGC1 | human | ACGAAGAGCTCTCCTCCTTC | CAGCATAGAGTTGCTCCTCC |
| PTEN | human | TCCACAAACAGAACAAGATGCTA | CGATTTCTTGATCACATAGACTTCC |
| SIRT1 | human | AAATGCTGGCCTAATAGAGTGG | TGGCAAAAACAGATACTGATTACC |
| TET2 | human | GAAAAAGATGAAGGTCCTTTTTATACC | TTTACCCTTCTGTCCAAACCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomasini, S.; Monteleone, E.; Altieri, A.; Margiotta, F.; Dardmeh, F.; Alipour, H.; Holm, A.; Kauppinen, S.; Panella, R. microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting. Int. J. Mol. Sci. 2025, 26, 9900. https://doi.org/10.3390/ijms26209900
Tomasini S, Monteleone E, Altieri A, Margiotta F, Dardmeh F, Alipour H, Holm A, Kauppinen S, Panella R. microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting. International Journal of Molecular Sciences. 2025; 26(20):9900. https://doi.org/10.3390/ijms26209900
Chicago/Turabian StyleTomasini, Simone, Emanuele Monteleone, Anna Altieri, Francesco Margiotta, Fereshteh Dardmeh, Hiva Alipour, Anja Holm, Sakari Kauppinen, and Riccardo Panella. 2025. "microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting" International Journal of Molecular Sciences 26, no. 20: 9900. https://doi.org/10.3390/ijms26209900
APA StyleTomasini, S., Monteleone, E., Altieri, A., Margiotta, F., Dardmeh, F., Alipour, H., Holm, A., Kauppinen, S., & Panella, R. (2025). microRNA-22 Inhibition Stimulates Mitochondrial Homeostasis and Intracellular Degradation Pathways to Prevent Muscle Wasting. International Journal of Molecular Sciences, 26(20), 9900. https://doi.org/10.3390/ijms26209900








