Simulated Microgravity-Induced Alterations in PDAC Cells: A Potential Role for Trichostatin A in Restoring Cellular Phenotype
Abstract
1. Introduction
2. Results
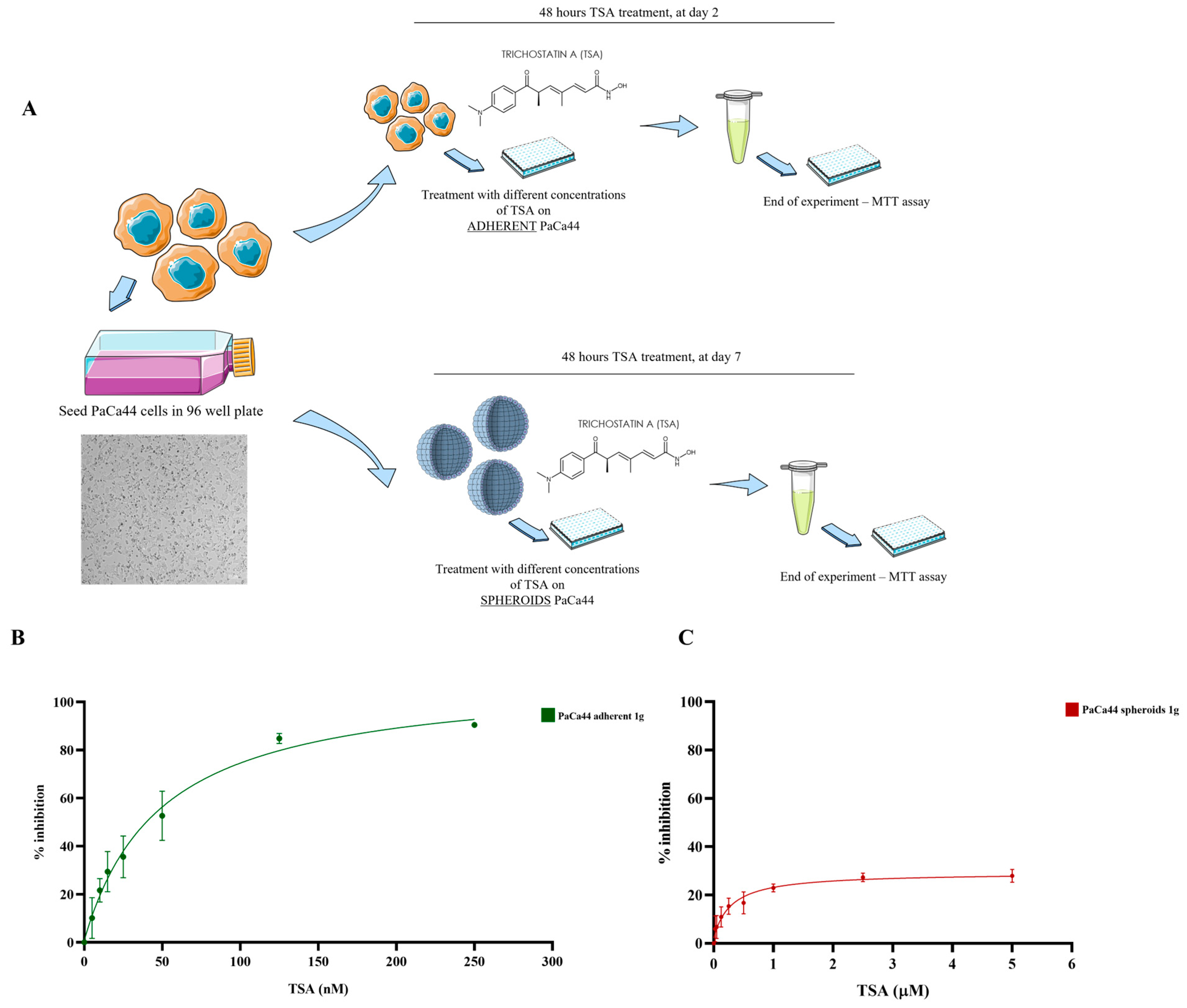
2.1. Differential Effects of Trichostatin A on 2D and 3D PaCa44 Cell Cultures at 1 g
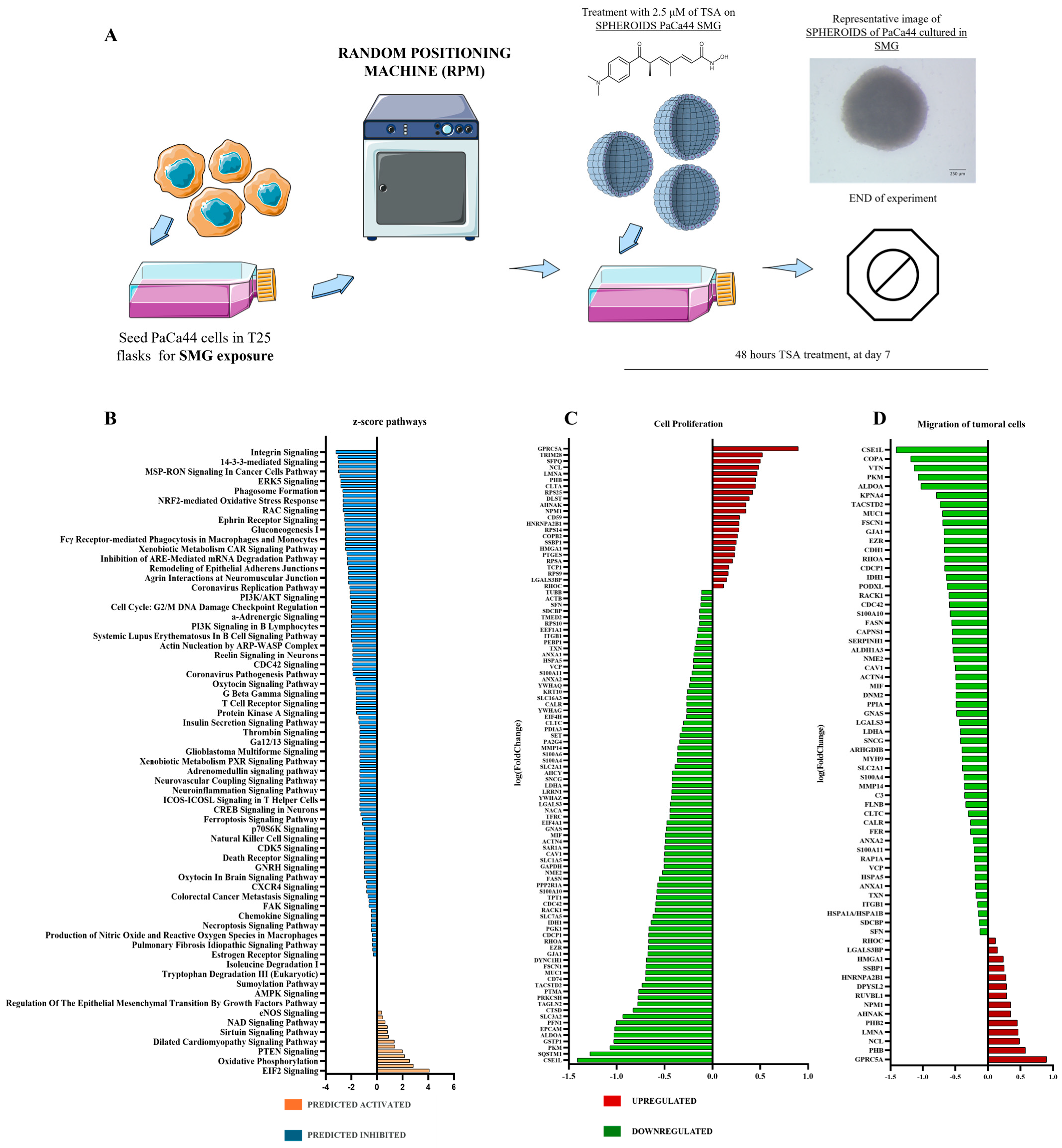
2.2. Proteomic Pathway Modulations Induced by Trichostatin A Treatment Under Simulated Microgravity in Pancreatic Cancer Spheroids
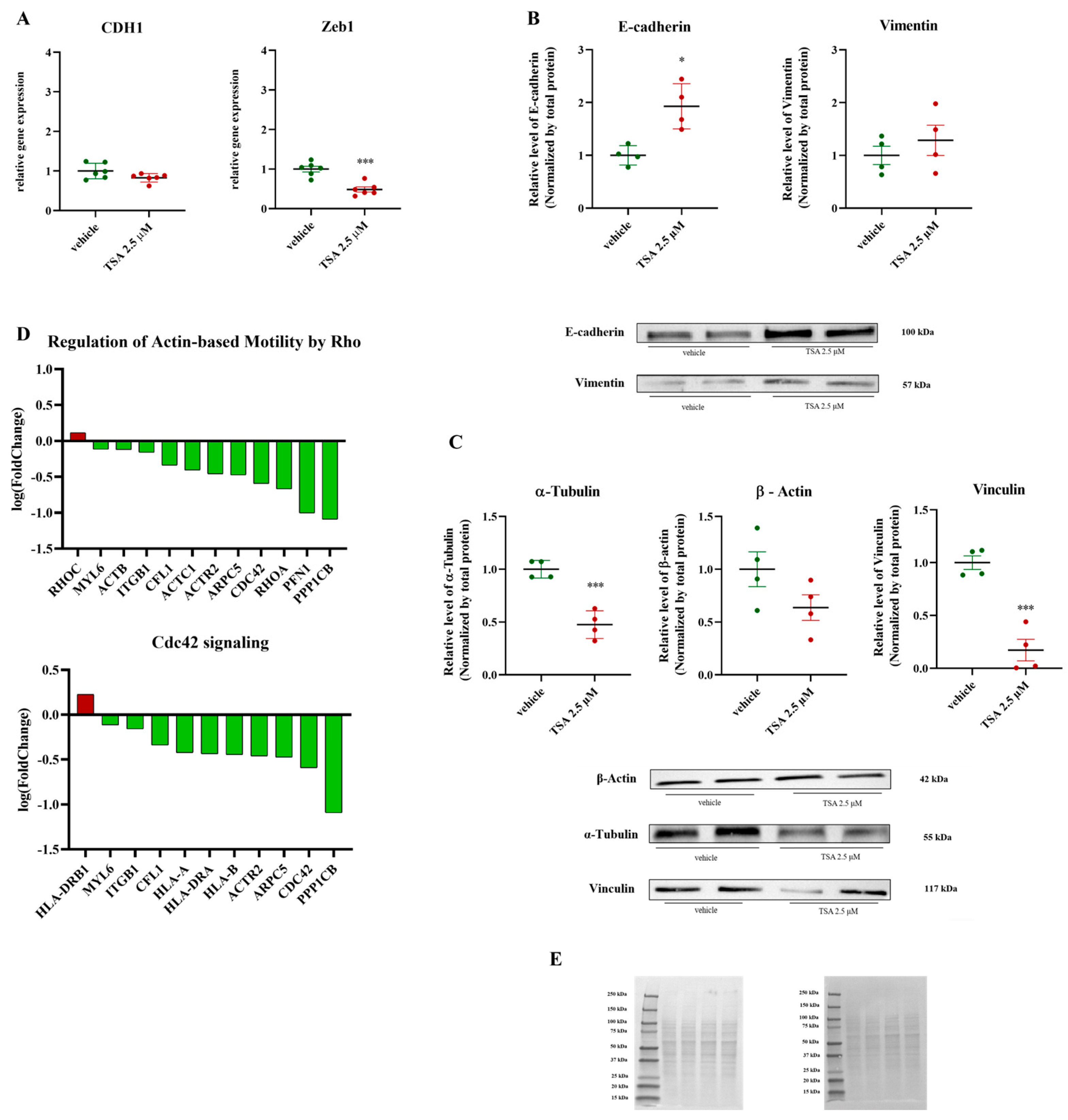
2.3. Disruption of EMT and Cytoskeletal Dynamics by Treatment: Impairment of Actin-Based Motility and Invasive Potential in Cancer Cells
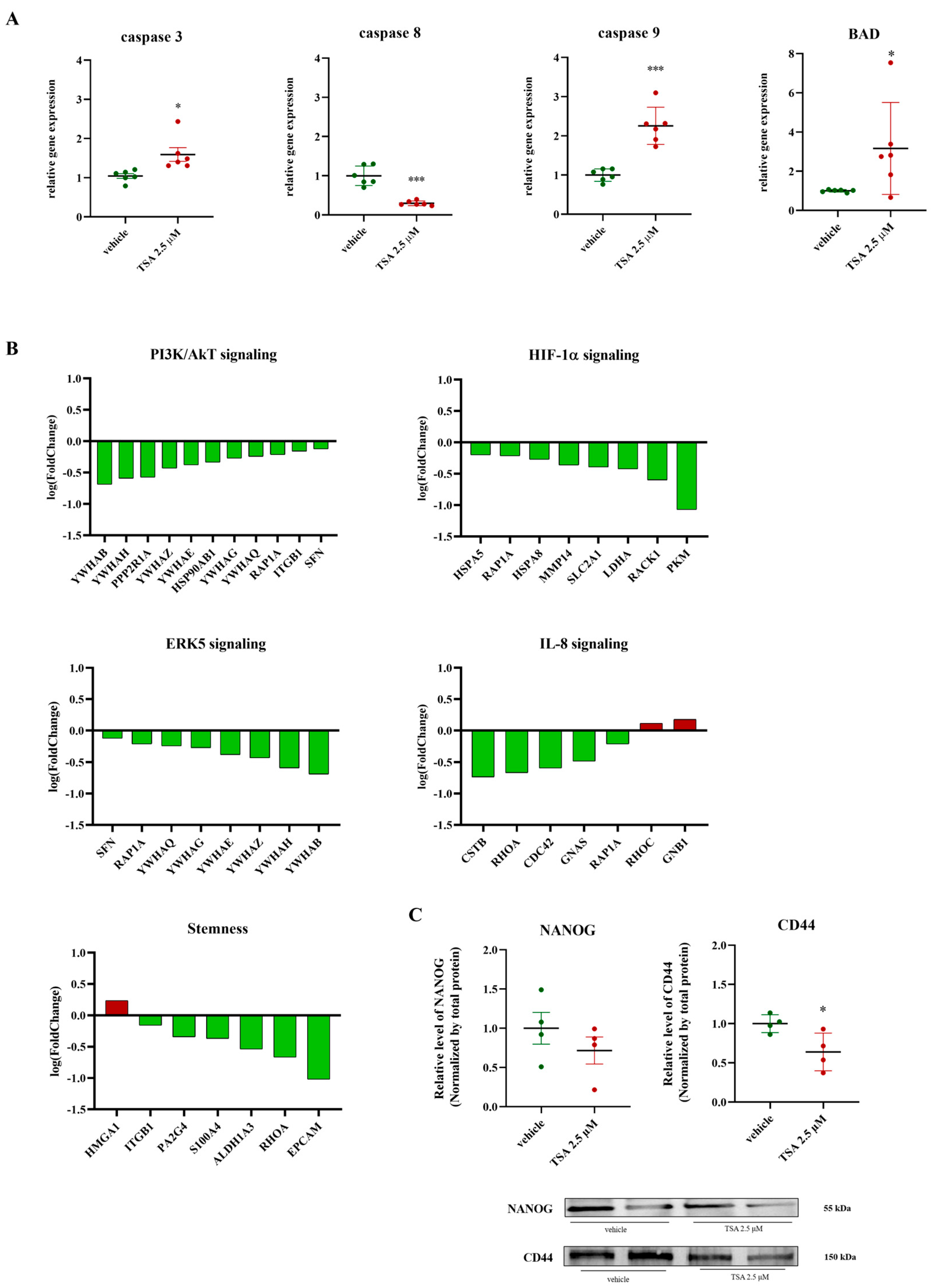
2.4. Impact of TSA (2.5 μM) on Apoptosis, PI3K/AKT, ERK, IL-8, HIF-1α Signaling, and Stemness Markers
3. Discussion
4. Materials and Methods
4.1. Chemical Agent: Trichostatin A
4.2. Cell Culture and Simulated Microgravity Exposure
4.3. Cell Proliferation MTT Assay
4.4. Proteomic Analysis
4.5. Western Blot Analysis
4.6. RNA Extraction and qRT-PCR
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cecconi, D.; Scarpa, A.; Massimo, D.; Palmieri, M.; Hamdan, M.; Astner, H.; Righetti, P.G. Proteomic Profiling of Pancreatic Ductal Carcinoma Cell Lines Treated with Trichostatin-A. Electrophoresis 2003, 24, 1871–1878. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M.A.; Kouzarides, T. Cancer Epigenetics: From Mechanism to Therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Baylin, S.B. The Epigenomics of Cancer. Cell 2007, 128, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Portela, A.; Esteller, M. Epigenetic Modifications and Human Disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Marks, P.A.; Rifkind, R.A.; Richon, V.M.; Breslow, R.; Miller, T.; Kelly, W.K. Histone Deacetylases and Cancer: Causes and Therapies. Nat. Rev. Cancer 2001, 1, 194–202. [Google Scholar] [CrossRef]
- West, A.C.; Johnstone, R.W. New and Emerging HDAC Inhibitors for Cancer Treatment. J. Clin. Investig. 2014, 124, 30–39. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. New Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2009, 2, a001008. [Google Scholar] [CrossRef]
- Collisson, E.A.; Sadanandam, A.; Olson, P.; Gibb, W.J.; Truitt, M.; Gu, S.; Cooc, J.; Weinkle, J.; Kim, G.E.; Jakkula, L.; et al. Subtypes of Pancreatic Ductal Adenocarcinoma and Their Differing Responses to Therapy. Nat. Med. 2011, 17, 500–503. [Google Scholar] [CrossRef]
- Dokmanovic, M.; Clarke, C.; Marks, P.A. Histone Deacetylase Inhibitors: Overview and Perspectives. Mol. Cancer Res. 2007, 5, 981–989. [Google Scholar] [CrossRef]
- Bolden, J.E.; Peart, M.J.; Johnstone, R.W. Anticancer Activities of Histone Deacetylase Inhibitors. Nat. Rev. Drug Discov. 2006, 5, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Kusumoto, M.; Ogawa, T.; Mizumoto, K.; Ueno, H.; Hideaki, N.; Sato, N.; Nakamura, M.; Tanaka, M. Adenovirus-Mediated P53 Gene Transduction Inhibits Telomerase Activity Independent of Its Effects on Cell Cycle Arrest and Apoptosis in Human Pancreatic Cancer Cells. Clin. Cancer Res. 1999, 5, 2140–2147. [Google Scholar] [PubMed]
- Vousden, K.H. P53. Cell 2000, 103, 691–694. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic Cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Massimo, D.; Costanzo, C.; Faggioli, L.; Maria, T.S.; Moore, P.S.; Bassi, C.; Scarpa, A.; Palmieri, M. Trichostatin A, an Inhibitor of Histone Deacetylases, Strongly Suppresses Growth of Pancreatic Adenocarcinoma Cells. Mol. Carcinog. 2003, 38, 59–69. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2008, 9, 239–252. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Sutherland, R. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Kim, K.-U.; Wilson, S.M.; Abayasiriwardana, K.S.; Collins, R.; Lars, F.; Xu, Z.; Jablons, D.M.; Nishimura, S.L.; Courtney Broaddus, V. Courtney Broaddus. A Novel in Vitro Model of Human Mesothelioma for Studying Tumor Biology and Apoptotic Resistance. Am. J. Respir. Cell Mol. Biol. 2005, 33, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Chen, Z.; Lim, W.; Cho, H.; Park, S. High-Throughput Screening of Anti-Cancer Drugs Using a Microfluidic Spheroid Culture Device with a Concentration Gradient Generator. Curr. Protoc. 2022, 2, e529. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Schreiber-Brynzak, E.; Klapproth, E.; Unger, C.; Lichtscheidl-Schultz, I.; Göschl, S.; Schweighofer, S.; Trondl, R.; Dolznig, H.; Jakupec, M.A.; Keppler, B.K. Three-Dimensional and Co-Culture Models for Preclinical Evaluation of Metal-Based Anticancer Drugs. Investig. New Drugs 2015, 33, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic In Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2018, 116, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-Based Facilities for Simulation of Microgravity: Organism-Specific Recommendations for Their Use, and Recommended Terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Calvaruso, M.; Militello, C.; Minafra, L.; La Regina, V.; Torrisi, F.; Pucci, G.; Cammarata, F.P.; Bravatà, V.; Forte, G.I.; Russo, G. Biological and Mechanical Characterization of the Random Positioning Machine (RPM) for Microgravity Simulations. Life 2021, 11, 1190. [Google Scholar] [CrossRef]
- Borst, A.G.; van Loon, J.J.W.A. Technology and Developments for the Random Positioning Machine, RPM. Microgravity Sci. Technol. 2008, 21, 287–292. [Google Scholar] [CrossRef]
- Sahebi, R.; Aghaei, M.; Halvaei, S.; Alizadeh, A. The Role of Microgravity in Cancer: A Dual-Edge Sword. Multidiscip. Cancer Investig. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Barkia, B.; Sandt, V.; Melnik, D.; Cortés-Sánchez, J.L.; Marchal, S.; Baselet, B.; Baatout, S.; Sahana, J.; Grimm, D.; Wehland, M.; et al. The Formation of Stable Lung Tumor Spheroids during Random Positioning Involves Increased Estrogen Sensitivity. Biomolecules 2024, 14, 1292. [Google Scholar] [CrossRef]
- Masini, M.A.; Bonetto, V.; Manfredi, M.; Pastò, A.; Barberis, E.; Timo, S.; Vanella, V.V.; Robotti, E.; Masetto, F.; Andreoli, F.; et al. Prolonged Exposure to Simulated Microgravity Promotes Stemness Impairing Morphological, Metabolic and Migratory Profile of Pancreatic Cancer Cells: A Comprehensive Proteomic, Lipidomic and Transcriptomic Analysis. Cell. Mol. Life Sci. 2022, 79, 226. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Grimm, D.; Corydon, T.J.; Sahana, J.; González-Torres, L.F.; Kraus, A.; Marchal, S.; Wise, P.M.; Simonsen, U.; Krüger, M. Recent Studies of the Effects of Microgravity on Cancer Cells and the Development of 3D Multicellular Cancer Spheroids. Stem Cells Transl. Med. 2025, 14, szaf008. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, D.; Suresh, S.; Prathivadhi-Bhayankaram, S.V.; Mimlitz, M.; Zetocha, N.; Bong Eun Lee; Ekpenyong, A. Microgravity Modulates Effects of Chemotherapeutic Drugs on Cancer Cell Migration. Life 2020, 10, 162. [Google Scholar] [CrossRef]
- Thiery, J.P. Epithelial–Mesenchymal Transitions in Development and Pathologies. Curr. Opin. Cell Biol. 2003, 15, 740–746. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Nieto, M.A.; Huang, R.-J.; Jackson, R.A.; Thiery, J. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Goley, E.D.; Rammohan, A.; Znameroski, E.A.; Firat-Karalar, E.N.; Sept, D.; Welch, M.D. An Actin-Filament-Binding Interface on the Arp2/3 Complex Is Critical for Nucleation and Branch Stability. Proc. Natl. Acad. Sci. USA 2010, 107, 8159–8164. [Google Scholar] [CrossRef]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases Regulate the Assembly of Multimolecular Focal Complexes Associated with Actin Stress Fibers, Lamellipodia, and Filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef]
- Barnawi, R.; Al-Khaldi, S.; Colak, D.; Tulbah, A.; Al-Tweigeri, T.; Fallatah, M.; Monies, D.; Ghebeh, H.; Al-Alwan, M. β1 Integrin Is Essential for Fascin-Mediated Breast Cancer Stem Cell Function and Disease Progression. Int. J. Cancer 2019, 145, 830–841. [Google Scholar] [CrossRef]
- Ochoa, A.; Herrera, A.; Menendez, A.; Estefanell, M.; Ramos, C.; Pons, S. Vinculin Is Required for Interkinetic Nuclear Migration (INM) and Cell Cycle Progression. J. Cell Biol. 2023, 223, e202106169. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and Their Role in Cellular Stress in Cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Raj, P.; Yao, W.; Ying, H. Glucose Metabolism in Pancreatic Cancer. Cancers 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Nieborak, A.; Saulius, L.; Jordi, C.; Heyn, P.; Gabriela, S.S.; Karsten, M.; Zeigerer, A.; Bester, R.; Protzer, U.; Schelter, F.; et al. Depletion of Pyruvate Kinase (PK) Activity Causes Glycolytic Intermediate Imbalances and Reveals a PK-TXNIP Regulatory Axis. Mol. Metab. 2023, 74, 101748. [Google Scholar] [CrossRef]
- Singh, J.K.; Simões, B.M.; Howell, S.J.; Farnie, G.; Clarke, R.B. Recent Advances Reveal IL-8 Signaling as a Potential Key to Targeting Breast Cancer Stem Cells. Breast Cancer Res. 2013, 15, 210. [Google Scholar] [CrossRef]
- Aziz, M.H.; Saida, L.; van Eijck, C.H.J.; Mustafa, D.A.M. Overexpression of the Adhesion Signaling Pathway Is Linked to Short-Term Survival in Pancreatic Ductal Adenocarcinoma. Pancreatology 2023, 24, 62–65. [Google Scholar] [CrossRef]
- Bubin, R.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7030. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Cao, X.; Tan, L.; Jia, B.; Chen, R.; Li, J. GAPDH: A Common Housekeeping Gene with an Oncogenic Role in Pan-Cancer. Comput. Struct. Biotechnol. J. 2023, 21, 4056–4069. [Google Scholar] [CrossRef]
- Vicente, J.J.; Wordeman, L. The Quantification and Regulation of Microtubule Dynamics in the Mitotic Spindle. Curr. Opin. Cell Biol. 2019, 60, 36–43. [Google Scholar] [CrossRef]
- Huang, J.; Wang, H.; Xu, Y.; Li, C.; Lv, X.; Han, X.; Chen, X.; Chen, Y.; Yu, Z. The Role of CTNNA1 in Malignancies: An Updated Review. J. Cancer 2023, 14, 219–230. [Google Scholar] [CrossRef]
- Barbosa, M.A.G.; Xavier, C.P.R.; Pereira, R.F.; Petrikaitė, V.; Vasconcelos, M.H. 3D Cell Culture Models as Recapitulators of the Tumor Microenvironment for the Screening of Anti-Cancer Drugs. Cancers 2021, 14, 190. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.-H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and Expansion of Human Colon-Cancer-Initiating Cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; El Omari, N.; Bakha, M.; Aanniz, T.; El Menyiy, N.; El Hachlafi, N.; El Baaboua, A.; El-Shazly, M.; Alshahrani, M.M.; Al Awadh, A.A.; et al. Pharmacological Properties of Trichostatin A, Focusing on the Anticancer Potential: A Comprehensive Review. Pharmaceuticals 2022, 15, 1235. [Google Scholar] [CrossRef]
- McKinley, S.; Taylor, A.; Peeples, C.; Jacob, M.; Khaparde, G.; Walter, Y.; Ekpenyong, A. Simulated Microgravity-Induced Changes to Drug Response in Cancer Cells Quantified Using Fluorescence Morphometry. Life 2023, 13, 1683. [Google Scholar] [CrossRef]
- Park, S.A.; Jung, J.M.; Park, J.S.; Lee, J.H.; Park, B.; Kim, H.J.; Park, J.-H.; Chae, W.S.; Jeong, J.H.; Choi, S.H.; et al. SWATH-MS Analysis of Cerebrospinal Fluid to Generate a Robust Battery of Biomarkers for Alzheimer’s Disease. Sci. Rep. 2020, 10, 7423. [Google Scholar] [CrossRef]
- Lakshmaiah, K.C.; Jacob, L.A.; Aparna, S.; Lokanatha, D.; Saldanha, S.C. Epigenetic Therapy of Cancer with Histone Deacetylase Inhibitors. J. Cancer Res. Ther. 2014, 10, 469–478. [Google Scholar] [CrossRef]
- Manfredi, M.; Brandi, J.; Di Carlo, C.; Vita Vanella, V.; Barberis, E.; Marengo, E.; Patrone, M.; Cecconi, D. Mining Cancer Biology through Bioinformatic Analysis of Proteomic Data. Expert Rev. Proteom. 2019, 16, 733–747. [Google Scholar] [CrossRef]
- Pollard, T.D.; Borisy, G.G. Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell 2003, 112, 453–465. [Google Scholar] [CrossRef]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane Crosstalk between the Extracellular Matrix and the Cytoskeleton. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Jordens, I.; Fernandez-Borja, M.; Marsman, M.; Dusseljee, S.; Janssen, L.; Calafat, J.; Janssen, H.; Wubbolts, R.; Neefjes, J. The Rab7 Effector Protein RILP Controls Lysosomal Transport by Inducing the Recruitment of Dynein-Dynactin Motors. Curr. Biol. 2001, 11, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Cell Adhesion: The Molecular Basis of Tissue Architecture and Morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.-T.; Wakayama, S.; Kishigami, S.; Park, K.H.; Kim, J.-H.; Van Thuan, N.; Wakayama, T. Effect of Trichostatin a on Chromatin Remodeling, Histone Modifications, DNA Replication, and Transcriptional Activity in Cloned Mouse Embryos. Biol. Reprod. 2010, 83, 454–463. [Google Scholar] [CrossRef]
- Rao, J.; Bhattacharya, D.; Banerjee, B.; Sarin, A.; Shivashankar, G.V. Trichostatin-A Induces Differential Changes in Histone Protein Dynamics and Expression in HeLa Cells. Biochem. Biophys. Res. Commun. 2007, 363, 263–268. [Google Scholar] [CrossRef]
- Barry, D.M.; Xu, K.; Meadows, S.M.; Zheng, Y.; Norden, P.R.; Davis, G.E.; Cleaver, O. Cdc42 Is Required for Cytoskeletal Support of Endothelial Cell Adhesion during Blood Vessel Formation in Mice. Development 2015, 142, 3058–3070. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. RHO GTPASES: Biochemistry and Biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Dirican, E.; Özcan, H.; Karabulut Uzunçakmak, S.; Takım, U. Evaluation Expression of the Caspase-3 and Caspase-9 Apoptotic Genes in Schizophrenia Patients. Clin. Psychopharmacol. Neurosci. 2023, 21, 171–178. [Google Scholar] [CrossRef]

| Genes | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| CDH1 | 5′-GAC ACC AAC GAT AAT CCT CCGA-3′ | 5′-GGC ACC TGA CCC TTG TAC GT-3′ |
| ZEB1 | 5′-GTT ACC AGG GAG GAG CAG TGAAA-3′ | 5′-GAC AGC AGT GTC TTG TTG TTG TAG AAA-3′ |
| caspase 3 | 5′-CTGGTTTTCGGTGGGTGT-3′ | 5′-CACTGAGTTTTCAGTGTTCTCCA-3′ |
| caspase 8 | 5′-CAGCAGCCTTGAAGGAAGTC-3′ | 5′-CGAGATTGTCATTACCCCACA-3 |
| caspase 9 | 5′-CCCAAGCTCTTTTTCATCCA-3′ | 5′-AGTGGAGGCCACCTCAAAC-3′ |
| BAD | 5′-ACCAGCAGCAGCCATCAT-3′ | 5′-GGTAGGAGCTGTGGCGACT-3′ |
| GAPDH | 5′-ATC AGC AAT GCC TCC TGC AC-3′ | 5′-TGG TCA TGA GTC CTT CCA CG-3′ |
| 18S | 5′-ACT TTC GAT GGT AGT CGC CGT-3′ | 5′-CCT TGG ATG TGG TAG CCG TTT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.A.; Masini, M.A.; Sabbatini, M.; Gribaudo, G.; Manfredi, M.; Caprì, F.G.; Bonetto, V.; Magnelli, V.; Donadelli, M.; Corino, R.; et al. Simulated Microgravity-Induced Alterations in PDAC Cells: A Potential Role for Trichostatin A in Restoring Cellular Phenotype. Int. J. Mol. Sci. 2025, 26, 4758. https://doi.org/10.3390/ijms26104758
Pagano CA, Masini MA, Sabbatini M, Gribaudo G, Manfredi M, Caprì FG, Bonetto V, Magnelli V, Donadelli M, Corino R, et al. Simulated Microgravity-Induced Alterations in PDAC Cells: A Potential Role for Trichostatin A in Restoring Cellular Phenotype. International Journal of Molecular Sciences. 2025; 26(10):4758. https://doi.org/10.3390/ijms26104758
Chicago/Turabian StylePagano, Corinna Anais, Maria Angela Masini, Maurizio Sabbatini, Giorgia Gribaudo, Marcello Manfredi, Flavia Giusy Caprì, Valentina Bonetto, Valeria Magnelli, Massimo Donadelli, Roberto Corino, and et al. 2025. "Simulated Microgravity-Induced Alterations in PDAC Cells: A Potential Role for Trichostatin A in Restoring Cellular Phenotype" International Journal of Molecular Sciences 26, no. 10: 4758. https://doi.org/10.3390/ijms26104758
APA StylePagano, C. A., Masini, M. A., Sabbatini, M., Gribaudo, G., Manfredi, M., Caprì, F. G., Bonetto, V., Magnelli, V., Donadelli, M., Corino, R., Belay, M. H., Robotti, E., & Marengo, E. (2025). Simulated Microgravity-Induced Alterations in PDAC Cells: A Potential Role for Trichostatin A in Restoring Cellular Phenotype. International Journal of Molecular Sciences, 26(10), 4758. https://doi.org/10.3390/ijms26104758









