Comprehensive Profiling Identifies Circulating microRNA Dysregulation in Vietnamese Patients with Heart Failure
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of Study Participants
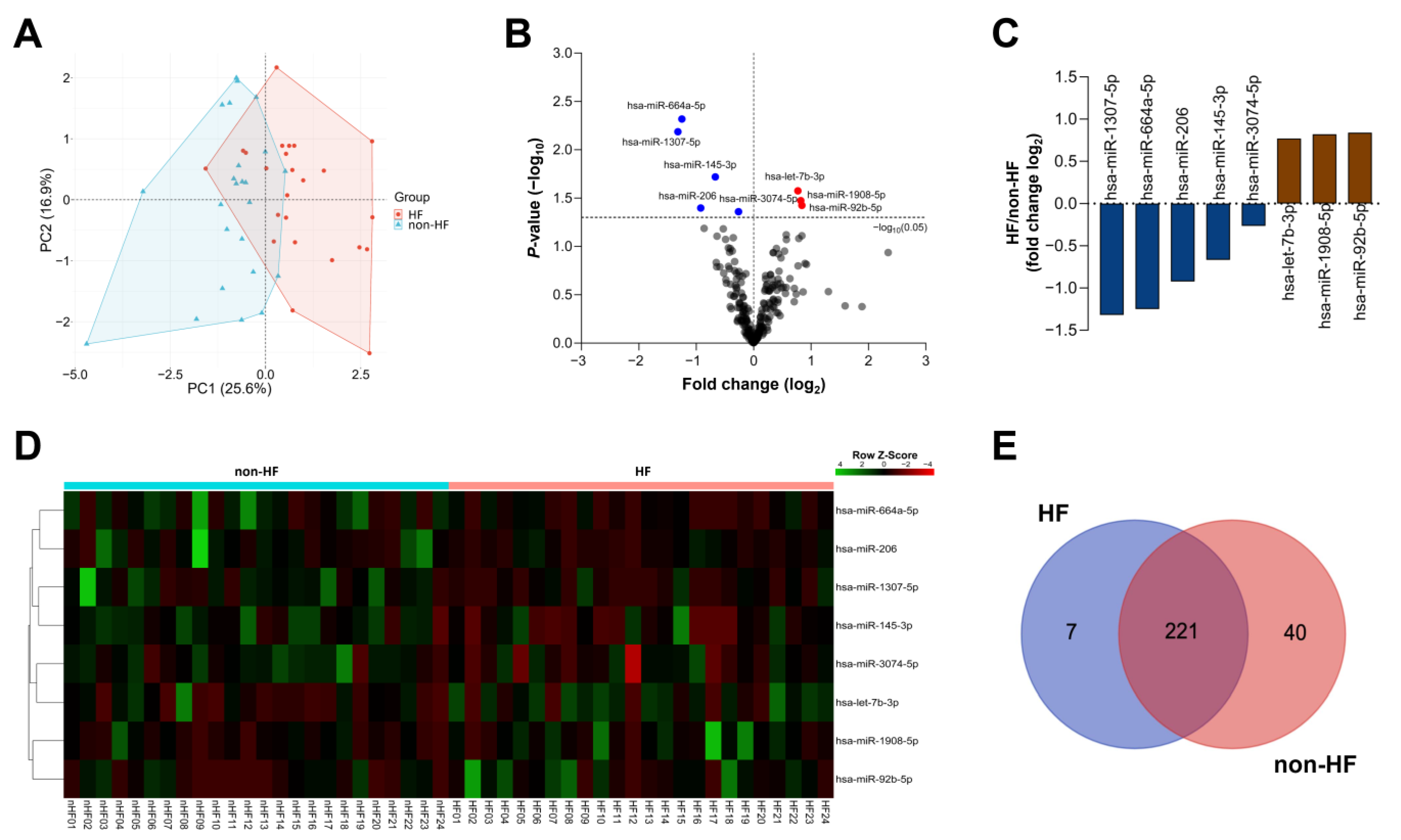
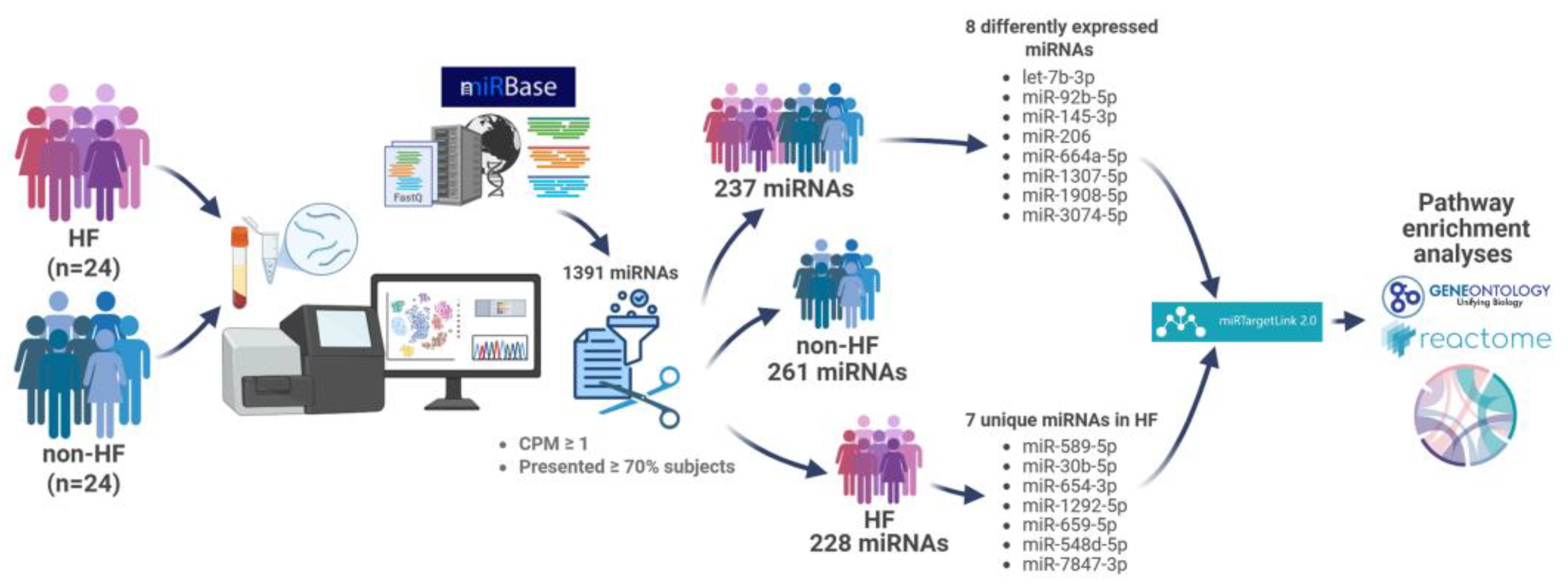
2.2. Circulating miRNA Expression Profile
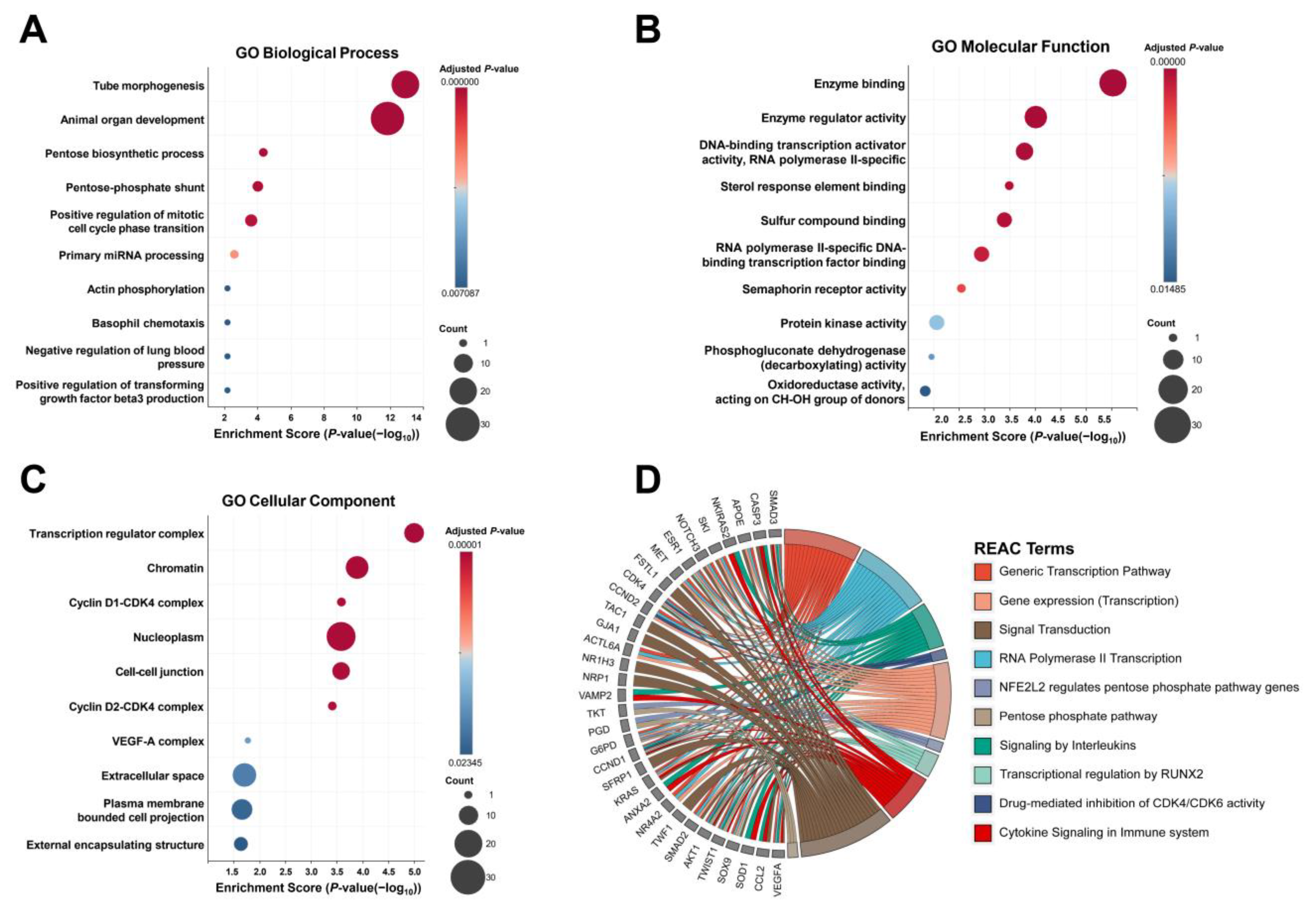
2.3. Target Gene Analysis of DE miRNAs Between the HF and Non-HF Groups
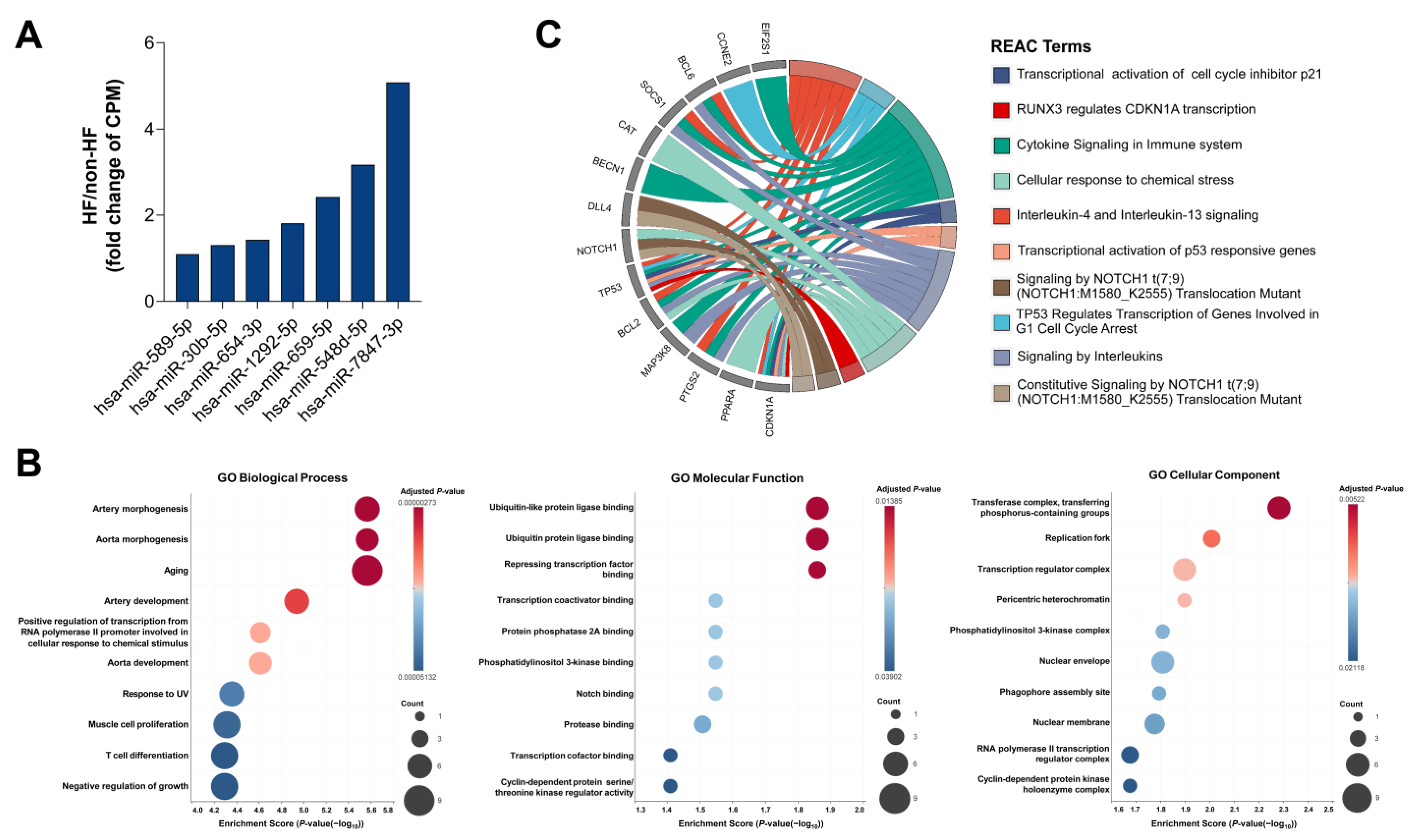
2.4. Target Gene Analysis of Unique miRNAs in HF Patients
3. Discussion
4. Patients and Methods
4.1. Study Design and Participants
4.2. Blood Sampling and miRNA Isolation
4.3. miRNA Sequencing and Target Gene Enrichment Analysis
4.4. Statistical Analyses
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2022, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics An Updated 2024 Report from the Heart Failure Society of America. J. Card. Fail. 2025, 31, 66–116. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.; Chin, J.C.; Sicignano, N.M.; Evans, A.M. Repeat Hospitalizations Predict Mortality in Patients with Heart Failure. Mil. Med. 2017, 182, e1932–e1937. [Google Scholar] [CrossRef]
- Vegter, E.L.; van der Meer, P.; de Windt, L.J.; Pinto, Y.M.; Voors, A.A. MicroRNAs in Heart Failure: From Biomarker to Target for Therapy. Eur. J. Heart Fail. 2016, 18, 457–468. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic Approaches for Cardiac Regeneration and Repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. MicroRNAs in the Human Heart: A Clue to Fetal Gene Reprogramming in Heart Failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef]
- Small, E.M.; Olson, E.N. Pervasive Roles of microRNAs in Cardiovascular Biology. Nature 2011, 469, 336–342. [Google Scholar] [CrossRef]
- Melman, Y.F.; Shah, R.; Das, S. MicroRNAs in Heart Failure: Is the Picture Becoming Less miRky? Circ. Heart Fail. 2014, 7, 203–214. [Google Scholar] [CrossRef]
- Barwari, T.; Joshi, A.; Mayr, M. MicroRNAs in Cardiovascular Disease. JACC 2016, 68, 2577–2584. [Google Scholar] [CrossRef]
- Romaine, S.P.R.; Tomaszewski, M.; Condorelli, G.; Samani, N.J. MicroRNAs in Cardiovascular Disease: An Introduction for Clinicians. Heart 2015, 101, 921–928. [Google Scholar] [CrossRef]
- Xie, J.; Hu, X.; Yi, C.; Hu, G.; Zhou, X.; Jiang, H. MicroRNA-451 Protects against Cardiomyocyte Anoxia/Reoxygenation Injury by Inhibiting High Mobility Group Box 1 Expression. Mol. Med. Rep. 2016, 13, 5335–5341. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, Z.; Dong, Z.; Liu, X.; Liu, Y.; Li, X.; Xu, Y.; Guo, Y.; Wang, N.; Zhang, M.; et al. MicroRNA-122-5p Aggravates Angiotensin II-Mediated Myocardial Fibrosis and Dysfunction in Hypertensive Rats by Regulating the Elabela/Apelin-APJ and ACE2-GDF15-Porimin Signaling. J. Cardiovasc. Trans. Res. 2022, 15, 535–547. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.-W.; Lin, J.-Y.; Miao, R.; Zhong, J.-C. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc. Toxicol. 2020, 20, 463–473. [Google Scholar] [CrossRef]
- Song, G.; Zhu, L.; Ruan, Z.; Wang, R.; Shen, Y. MicroRNA-122 Promotes Cardiomyocyte Hypertrophy via Targeting FoxO3. Biochem. Biophys. Res. Commun. 2019, 519, 682–688. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, H.; Zhao, M.; Yu, H.; Xu, W.; Wang, Z.; Xiao, H. Cardiomyocyte-Derived Small Extracellular Vesicle-Transported Let-7b-5p Modulates Cardiac Remodeling via TLR7 Signaling Pathway. FASEB J. 2024, 38, e70196. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, C. MicroRNA-21 in Cardiovascular Disease. J. Cardiovasc. Trans. Res. 2010, 3, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p As a Circulating Biomarker for Heart Failure. Circ. Res. 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.-N.; Wang, J.-L.; Fu, Y. The microRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 856358. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Li, Y.; Wang, H. Circulating microRNAs as Novel Biomarkers for Heart Failure. Hell. J. Cardiol. 2018, 59, 209–214. [Google Scholar] [CrossRef]
- Divakaran, V.; Mann, D.L. The Emerging Role of MicroRNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2008, 103, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, X.; Lu, Y.; Yang, B. miRNAs at the Heart of the Matter. J. Mol. Med. 2008, 86, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in Cardiovascular Diseases: Potential Biomarkers, Therapeutic Targets and Challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Potenza, N.; Sardu, C.; Rizzo, M.R.; Siniscalchi, M.; Musacchio, E.; Barbieri, M.; Mauro, C.; Mosca, N.; et al. Circulating microRNA Changes in Heart Failure Patients Treated with Cardiac Resynchronization Therapy: Responders vs. Non-Responders. Eur. J. Heart Fail. 2013, 15, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Armugam, A.; Sepramaniam, S.; Karolina, D.S.; Lim, K.Y.; Lim, J.Y.; Chong, J.P.C.; Ng, J.Y.X.; Chen, Y.-T.; Chan, M.M.Y.; et al. Circulating microRNAs in Heart Failure with Reduced and Preserved Left Ventricular Ejection Fraction. Eur. J. Heart Fail. 2015, 17, 393–404. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Zhou, Z.; Yang, Z. Serum Exosomal MiR-92b-5p as a Potential Biomarker for Acute Heart Failure Caused by Dilated Cardiomyopathy. Cell. Physiol. Biochem. 2018, 46, 1939–1950. [Google Scholar] [CrossRef]
- Wu, T.; Chen, Y.; Du, Y.; Tao, J.; Li, W.; Zhou, Z.; Yang, Z. Circulating Exosomal miR-92b-5p Is a Promising Diagnostic Biomarker of Heart Failure with Reduced Ejection Fraction Patients Hospitalized for Acute Heart Failure. J. Thorac. Dis. 2018, 10, 6211–6220. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Meese, E.; Abdul-Khaliq, H.; Raedle-Hurst, T. MicroRNA-183-3p Is a Predictor of Worsening Heart Failure in Adult Patients With Transposition of the Great Arteries and a Systemic Right Ventricle. Front. Cardiovasc. Med. 2021, 8, 730364. [Google Scholar] [CrossRef]
- Schoettler, F.I.; Fatehi Hassanabad, A.; Jadli, A.S.; Patel, V.B.; Fedak, P.W.M. Exploring the Role of Pericardial miRNAs and Exosomes in Modulating Cardiac Fibrosis. Cardiovasc. Pathol. 2024, 73, 107671. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhong, W.; Zhang, Q.; Zhang, Q.; Yu, Z.; Wu, H. Circulating microRNA Expression Profiling and Bioinformatics Analysis of Patients with Coronary Artery Disease by RNA Sequencing. J. Clin. Lab. Anal. 2020, 34, e23020. [Google Scholar] [CrossRef]
- Jin, P.; Gu, W.; Lai, Y.; Zheng, W.; Zhou, Q.; Wu, X. The Circulating MicroRNA-206 Level Predicts the Severity of Pulmonary Hypertension in Patients with Left Heart Diseases. Cell. Physiol. Biochem. 2017, 41, 2150–2160. [Google Scholar] [CrossRef]
- Xue, S.; Liu, D.; Zhu, W.; Su, Z.; Zhang, L.; Zhou, C.; Li, P. Circulating MiR-17-5p, MiR-126-5p and MiR-145-3p Are Novel Biomarkers for Diagnosis of Acute Myocardial Infarction. Front. Physiol. 2019, 10, 123. [Google Scholar] [CrossRef]
- Toor, S.M.; Aldous, E.K.; Parray, A.; Akhtar, N.; Al-Sarraj, Y.; Abdelalim, E.M.; Arredouani, A.; El-Agnaf, O.; Thornalley, P.J.; Pananchikkal, S.V.; et al. Identification of Distinct Circulating microRNAs in Acute Ischemic Stroke Patients with Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2022, 9, 1024790. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.-L.; Mahmood Zuhdi, A.S.; Wan Ahmad, W.A.; Vanhoutte, P.M.; de Magalhaes, J.P.; Mustafa, M.R.; Wong, P.-F. Circulating MicroRNAs in Young Patients with Acute Coronary Syndrome. Int. J. Mol. Sci. 2018, 19, 1467. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Sathyapalan, T.; Al-Rasadi, K.; Jamialahmadi, T.; Sahebkar, A. The Role of MicroRNAs in Regulating Cytokines and Growth Factors in Coronary Artery Disease: The Ins and Outs. J. Immunol. Res. 2020, 2020, 5193036. [Google Scholar] [CrossRef] [PubMed]
- Nikpay, M.; Beehler, K.; Valsesia, A.; Hager, J.; Harper, M.-E.; Dent, R.; McPherson, R. Genome-Wide Identification of Circulating-miRNA Expression Quantitative Trait Loci Reveals the Role of Several miRNAs in the Regulation of Cardiometabolic Phenotypes. Cardiovasc. Res. 2019, 115, 1629–1645. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, P.-Y.; Kong, W.-D.; Cen, W.-J.; Wang, P.-L.; Liu, C.; Zhang, W.; Li, S.-S.; Jiang, J.-W. Apoptosis-Promoting Properties of miR-3074-5p in MC3T3-E1 Cells under Iron Overload Conditions. Cell Mol. Biol. Lett. 2021, 26, 37. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Lu, M.; Cheng, C.; Feng, Z.; Zhao, R.; Liang, J.; Han, J.; Jiang, J.; Xu-Welliver, M.; et al. Let-7b-3p Inhibits Tumor Growth and Metastasis by Targeting the BRF2-Mediated MAPK/ERK Pathway in Human Lung Adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 1841–1856. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, C.; Xiong, S.; Zhou, X.; Wang, G.; Guo, J. miR-1307-5p Suppresses Proliferation and Tumorigenesis of Bladder Cancer via Targeting MDM4 and the Hippo Signaling Pathway. Discov. Oncol. 2022, 13, 57. [Google Scholar] [CrossRef]
- Cai, R.; Qimuge, N.; Ma, M.; Wang, Y.; Tang, G.; Zhang, Q.; Sun, Y.; Chen, X.; Yu, T.; Dong, W.; et al. MicroRNA-664-5p Promotes Myoblast Proliferation and Inhibits Myoblast Differentiation by Targeting Serum Response Factor and Wnt1. J. Biol. Chem. 2018, 293, 19177–19190. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, J.; Qin, D.; Xiao, S.; Yao, C. Exosomal miR-92b-5p Regulates N4BP1 to Enhance PTEN Mono-Ubiquitination in Doxorubicin-Resistant AML. Cancer Drug Resist 2025, 8, 16. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Y.; Ruan, W.; Zhu, F.; Duan, S. miR-1908 Dysregulation in Human Cancers. Front. Oncol. 2022, 12, 857743. [Google Scholar] [CrossRef]
- Wang, M.; Ji, Y.; Cai, S.; Ding, W. MiR-206 Suppresses the Progression of Coronary Artery Disease by Modulating Vascular Endothelial Growth Factor (VEGF) Expression. Med. Sci. Monit. 2016, 22, 5011–5020. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, Z.-P.; Lu, N.-N.; Xu, Q.; He, J.; Qian, X.; Yu, J.; Guan, X.; Jiang, B.-H.; Liu, L.-Z. Downregulation of miR-145 Associated with Cancer Progression and VEGF Transcriptional Activation by Targeting N-RAS and IRS1. Biochim. Biophys. Acta 2013, 1829, 239–247. [Google Scholar] [CrossRef]
- Neiburga, K.D.; Vilne, B.; Bauer, S.; Bongiovanni, D.; Ziegler, T.; Lachmann, M.; Wengert, S.; Hawe, J.S.; Güldener, U.; Westerlund, A.M.; et al. Vascular Tissue Specific miRNA Profiles Reveal Novel Correlations with Risk Factors in Coronary Artery Disease. Biomolecules 2021, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.C.; Kwee, L.C.; Neely, M.L.; Grass, E.; Jakubowski, J.A.; Fox, K.A.A.; White, H.D.; Gregory, S.G.; Gurbel, P.A.; Carvalho, L.d.P.; et al. Circulating MicroRNA Profiling in Non-ST Elevated Coronary Artery Syndrome Highlights Genomic Associations with Serial Platelet Reactivity Measurements. Sci. Rep. 2020, 10, 6169. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, S.H.; Ipe, J.; Kassab, K.; Gao, H.; Liu, Y.; Skaar, T.C.; Kreutz, R.P. Next Generation MicroRNA Sequencing to Identify Coronary Artery Disease Patients at Risk of Recurrent Myocardial Infarction. Atherosclerosis 2018, 278, 232–239. [Google Scholar] [CrossRef]
- Gutierrez-Carretero, E.; Mayoral-González, I.; Jesús Morón, F.; Fernández-Quero, M.; Domínguez-Rodríguez, A.; Ordóñez, A.; Smani, T. miR-30b-5p Downregulation as a Predictive Biomarker of Coronary In-Stent Restenosis. Biomedicines 2021, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Feng, L.; Li, Y.; Zhao, S.; Yuan, W.; Jiang, Y.; Cheng, L. MiR-30b-5p Promotes Myocardial Cell Apoptosis in Rats with Myocardial Infarction through Regulating Wnt/β-Catenin Signaling Pathway. Minerva Medica 2023, 114, 476–484. [Google Scholar] [CrossRef]
- Hildebrandt, A.; Kirchner, B.; Meidert, A.S.; Brandes, F.; Lindemann, A.; Doose, G.; Doege, A.; Weidenhagen, R.; Reithmair, M.; Schelling, G.; et al. Detection of Atherosclerosis by Small RNA-Sequencing Analysis of Extracellular Vesicle Enriched Serum Samples. Front. Cell Dev. Biol. 2021, 9, 729061. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Zhou, M.; Zhou, F.; Luo, X.; Zhong, S.; Zhou, Y.; Qin, Y.; Li, P.; Qin, C. Exosome-Derived MiRNAs as Biomarkers of the Development and Progression of Intracranial Aneurysms. J. Atheroscler. Thromb. 2020, 27, 545–610. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Liu, W.; Lin, X.Y.; Dorajoo, R.; Lee, K.W.; Richards, A.M.; Lee, C.N.; Wongsurawat, T.; Nookaew, I.; Sorokin, V. The Interaction between 30b-5p miRNA and MBNL1 mRNA Is Involved in Vascular Smooth Muscle Cell Differentiation in Patients with Coronary Atherosclerosis. Int. J. Mol. Sci. 2019, 21, 11. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhou, Y.; Ji, X.; Liu, J.; Liu, D.; Yin, P.; Peng, Y.; Hao, M.; Zhang, L.; et al. Synergistic Effects of BMP9 and miR-548d-5p on Promoting Osteogenic Differentiation of Mesenchymal Stem Cells. Biomed. Res. Int. 2015, 2015, 309747. [Google Scholar] [CrossRef] [PubMed]
- Rammah, M.; Théveniau-Ruissy, M.; Sturny, R.; Rochais, F.; Kelly, R.G. PPARγ and NOTCH Regulate Regional Identity in the Murine Cardiac Outflow Tract. Circ. Res. 2022, 131, 842–858. [Google Scholar] [CrossRef]
- Ibrahim, A.M.; Roshdy, M.; Elshorbagy, S.; Hosny, M.; Halawa, S.; Yehia, D.; Elfawy, H.A.; Eldessouki, A.; Mohamed, F.; Ellithy, A.; et al. An Investigation of Fibulin-2 in Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 7176. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, X.-C.; Chen, L.-R.; Huang, H.-Z.; Wu, W.-Y.; Wang, Y.; Li, G. Pyroptosis and Mitochondrial Function Participated in miR-654-3p-Protected against Myocardial Infarction. Cell Death Dis. 2024, 15, 393. [Google Scholar] [CrossRef]
- Jing, F.; Shi, Y.; Jiang, D.; Li, X.; Sun, J.; Guo, Q. Circ_0001944 Targets the miR-1292-5p/FBLN2 Axis to Facilitate Sorafenib Resistance in Hepatocellular Carcinoma by Impeding Ferroptosis. ImmunoTargets Ther. 2024, 13, 643–659. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, J.; Liu, S.; Shan, D.; Wang, T.; Wang, X. Urinary Exosomal Lnc-TAF12-2:1 Promotes Bladder Cancer Progression through the miR-7847-3p/ASB12 Regulatory Axis. Genes. Dis. 2025, 12, 101384. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, W.-H.; Wu, Q.; Wang, J. Two-Layer Regulation of TRAF6 Mediated by Both TLR4/NF-kB Signaling and miR-589-5p Increases Proinflammatory Cytokines in the Pathology of Severe Acute Pancreatitis. Am. J. Transl. Res. 2020, 12, 2379–2395. [Google Scholar] [PubMed]
| Characteristics | Heart Failure Patients (n = 24) | Non-Heart Failure Patients (n = 24) | p-Value |
|---|---|---|---|
| Gender, (n; % Male) | 12; 50.0 | 12; 50.0 | 1.000 |
| Age (years) | 55.0 ± 15.7 | 54.3 ± 14.6 | 0.865 |
| Body mass index (kg/m2) | 24.6 ± 3.8 | 24.5 ± 3.8 | 0.904 |
| Heart rate (beats/minute) | 77.0 ± 12.1 | 83.9 ± 12.7 | 0.060 |
| Systolic blood pressure (mm/Hg) | 120.5 ± 18.5 | 131.1 ± 13.5 | 0.028 * |
| Diastolic blood pressure (mm/Hg) a | 70.0 ± 15.0 | 79.0 ± 10.0 | 0.318 b |
| NYHA II (n; %) | 16; 66.7 | 0 | - |
| NYHA III (n; %) | 7; 29.2 | 0 | - |
| NYHA IV (n; %) | 1; 4.2 | 0 | - |
| % Left ventricular ejection fraction | 38.7 ± 13.7 | 68.7 ± 7.09 | <0.0001 * |
| Hypertension (n; %) | 21; 87.5 | 18; 75.0 | 0.461 |
| Diabetes (n; %) | 8; 33.3 | 5; 20.8 | 0.517 |
| Hyperlipidemia (n; %) | 16; 64.0 | 9; 37.5 | 0.089 |
| History of myocardial infarction (n; %) | 9; 36.0 | 0; 0.0 | 0.002 * |
| Myocarditis (n; %) | 1; 4.2 | 1; 4.2 | 1.000 |
| Ischemic heart disease (n; %) | 4; 16.0 | 1; 4.2 | 0.349 |
| Coronary syndrome (n; %) | 11; 45.8 | 0; 0.0 | 0.0002 * |
| Angina pectoris (n; %) | 1; 4.2 | 0; 0.0 | >0.999 |
| Dilated cardiomyopathy (n; %) | 8; 33.3 | 0; 0.0 | 0.004 * |
| Angiotensin receptor blocker (n; %) | 20; 83.3 | 12; 50.0 | 0.031 * |
| Betablocker (n; %) | 21; 87.5 | 4; 16.0 | <0.0001 * |
| Statin (n; %) | 18; 75.0 | 10; 41.7 | 0.039 * |
| Aspirin (n; %) | 5; 20.8 | 0; 0.0 | 0.049 * |
| Clopidogrel (n; %) | 11; 45.8 | 1; 4.2 | 0.002 * |
| Calcium channel blocker (n; %) | 1; 4.2 | 7; 29.2 | 0.048 * |
| NT-proBNP (pg/mL) a | 1806 (542–3201) | n/a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, B.-Q.; Huynh, P.A.; Nguyen, N.N.Q.; Vo, N.V.T.; Le, L.G.H.; Vu, V.H.; Nguyen, T.C.; Hoang, M.; Vu, D.M. Comprehensive Profiling Identifies Circulating microRNA Dysregulation in Vietnamese Patients with Heart Failure. Int. J. Mol. Sci. 2025, 26, 9076. https://doi.org/10.3390/ijms26189076
Vu B-Q, Huynh PA, Nguyen NNQ, Vo NVT, Le LGH, Vu VH, Nguyen TC, Hoang M, Vu DM. Comprehensive Profiling Identifies Circulating microRNA Dysregulation in Vietnamese Patients with Heart Failure. International Journal of Molecular Sciences. 2025; 26(18):9076. https://doi.org/10.3390/ijms26189076
Chicago/Turabian StyleVu, Bao-Quoc, Phuong Anh Huynh, Nhu Nhat Quynh Nguyen, Niem Van Thanh Vo, Linh Gia Hoang Le, Vu Hoang Vu, Thanh Cong Nguyen, Minh Hoang, and Diem My Vu. 2025. "Comprehensive Profiling Identifies Circulating microRNA Dysregulation in Vietnamese Patients with Heart Failure" International Journal of Molecular Sciences 26, no. 18: 9076. https://doi.org/10.3390/ijms26189076
APA StyleVu, B.-Q., Huynh, P. A., Nguyen, N. N. Q., Vo, N. V. T., Le, L. G. H., Vu, V. H., Nguyen, T. C., Hoang, M., & Vu, D. M. (2025). Comprehensive Profiling Identifies Circulating microRNA Dysregulation in Vietnamese Patients with Heart Failure. International Journal of Molecular Sciences, 26(18), 9076. https://doi.org/10.3390/ijms26189076









