Enzymatic Hydrolysis of Porcine Blood as a Strategy to Obtain a Peptide-Rich Functional Ingredient
Abstract
1. Introduction
2. Results
2.1. Biological Activities of Porcine Blood Spray-Dried Hydrolysate (PBSH)
2.2. Free Amino Acid Composition of PBSH Sample
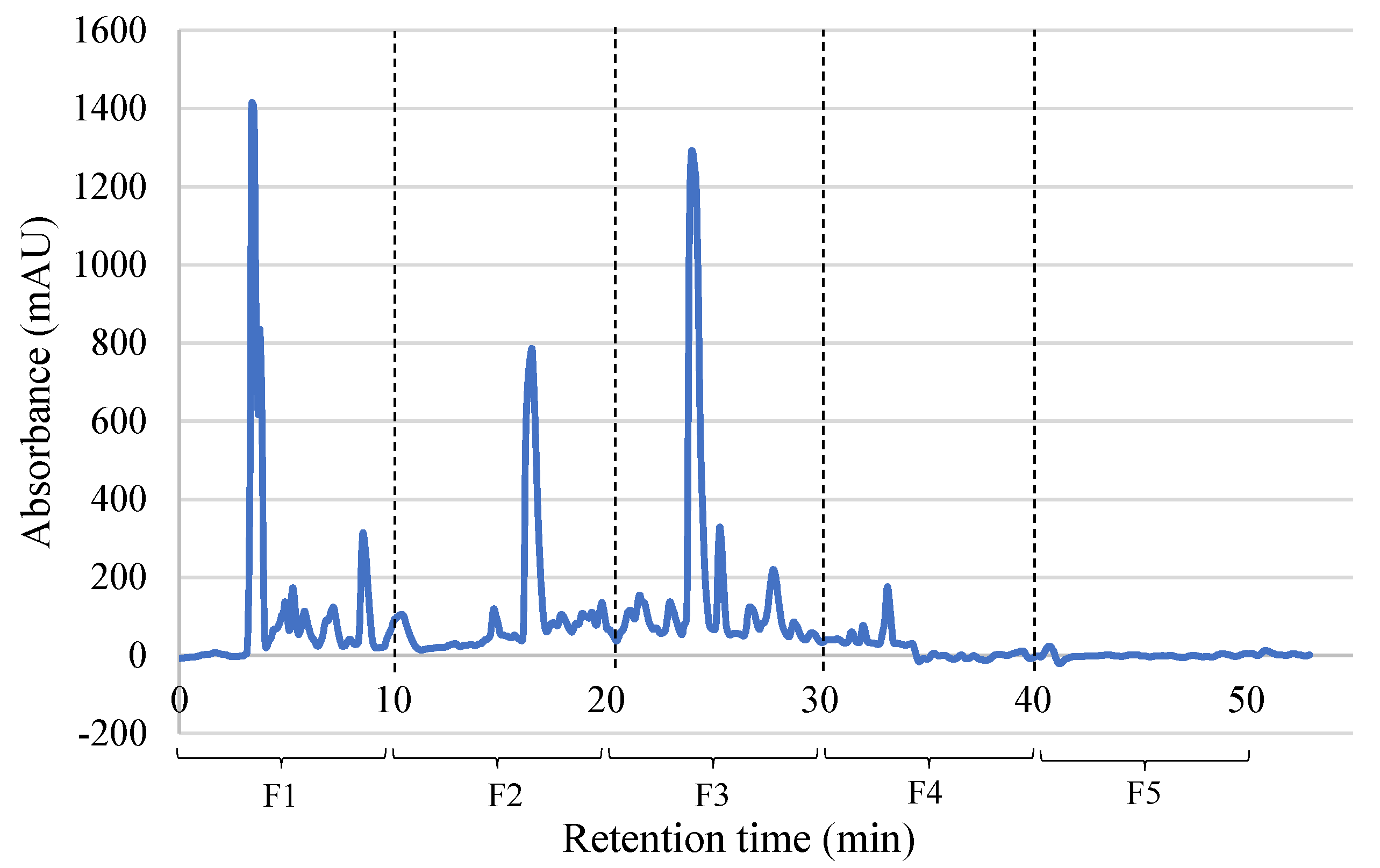
2.3. Separation and Characterization of PBSH Fractions
2.3.1. Biological Activities of PBSH Fractions Obtained by RP-HPLC
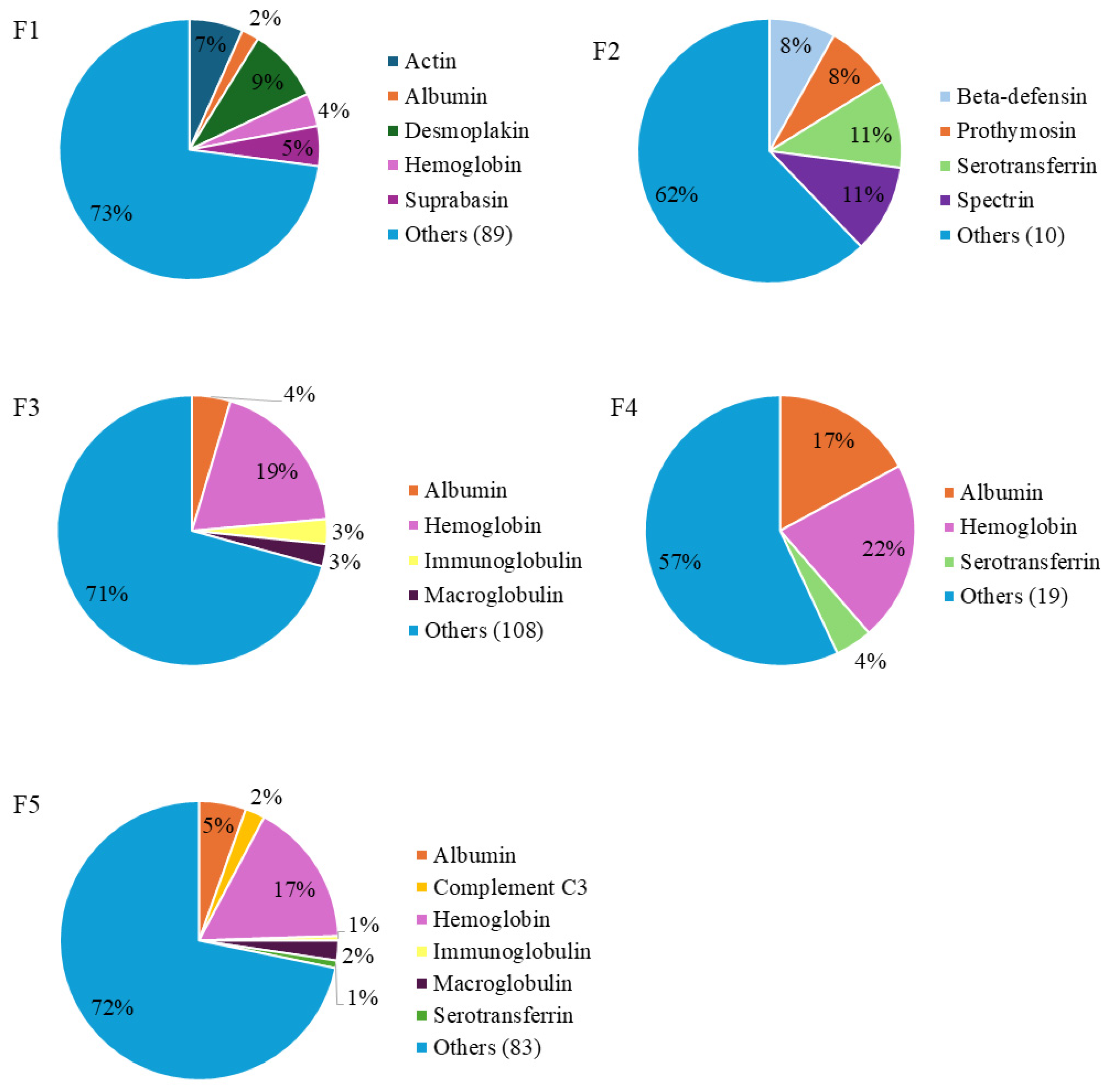
2.3.2. Protein and Peptide Identification of PBSH Fractions by LC-MS/MS
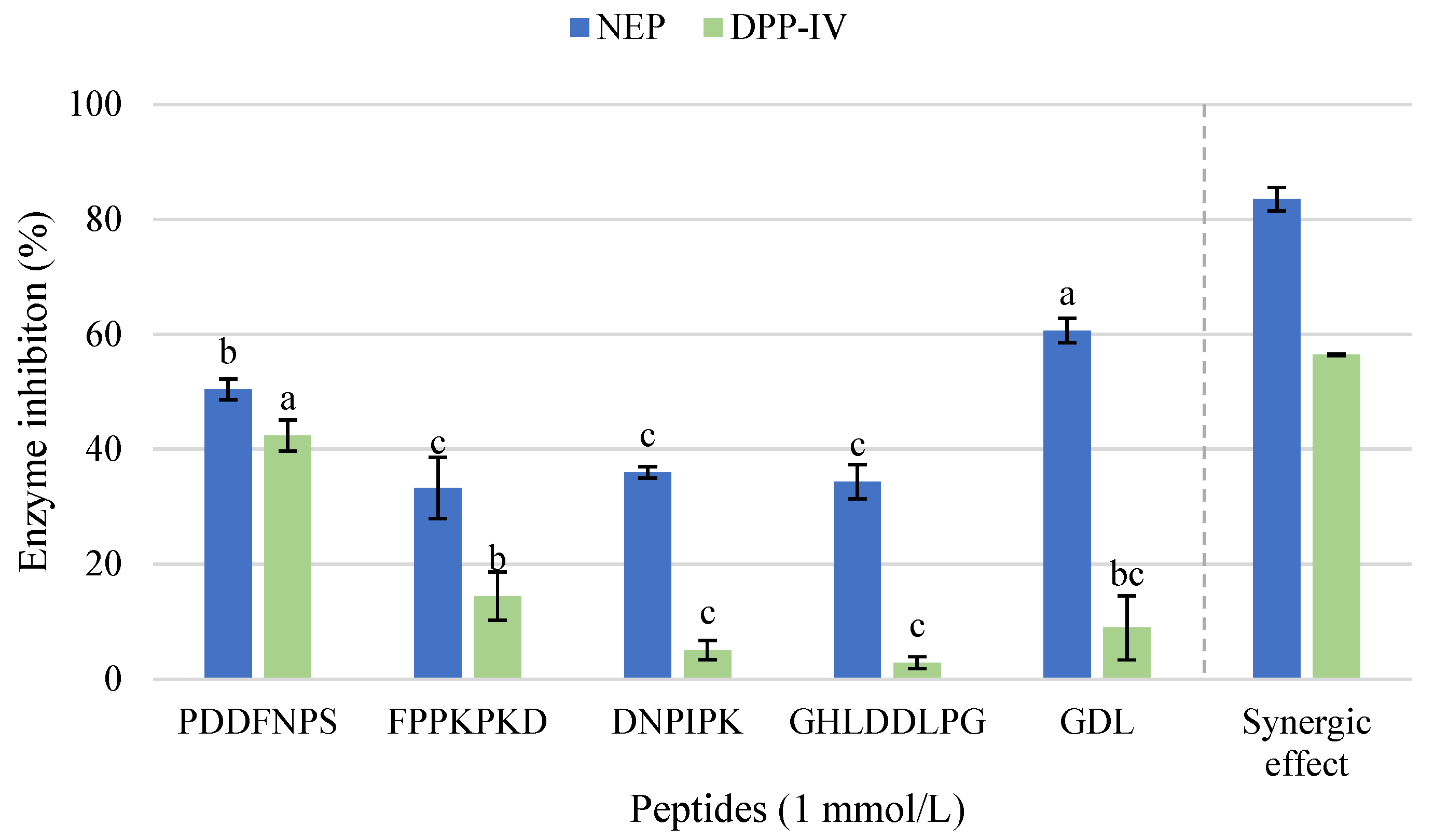
2.4. Characterization of Most Active HPLC Fraction
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
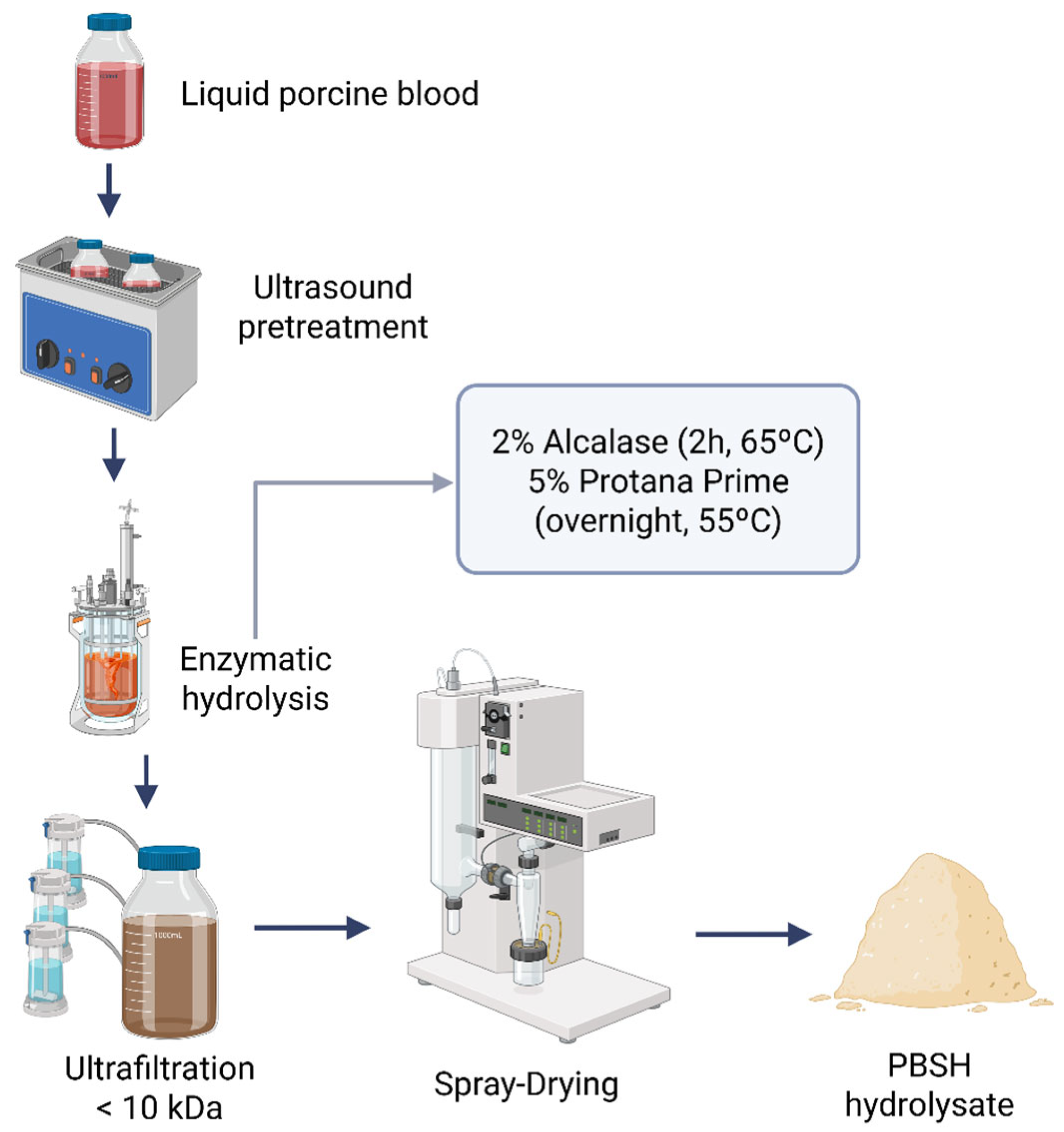
4.2. Preparation of Porcine Blood Spray-Dried Hydrolysate (PBSH)
4.3. Determination of Free Amino Acids Content (FAAs)
4.4. ABTS Radical-Scavenging Activity
4.5. Ferric Reducing Antioxidant Power (FRAP)
4.6. DPPH Free Radical-Scavenging Activity
4.7. Study of the Antioxidant Activity Stability
4.8. Determination of Dipeptidyl Peptidase IV (DPP-IV) Enzyme Inhibition
4.9. Determination of Neprilysin (NEP) Enzyme Inhibition
4.10. Determination of Tumor Necrosis α-Converting Enzyme (TACE) Inhibition
4.11. Determination of Monoacylglycerol Lipase (MGL) Inhibition
4.12. Study of Peptide Profile by RP-HPLC Separation
4.13. Peptide Identification by Mass Spectrometry in Tandem
4.14. In Silico Analysis of the Identified Peptides
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA). Animal by-Products. Available online: https://www.efsa.europa.eu/en/topics/animal-by-products (accessed on 20 May 2025).
- Ministry of Agriculture, Fisheries and Food. The Pork Meat Sector in Figures: Key Economic Indicators. Sub-Directorate General for Livestock and Game Production, Directorate General for Agricultural Production and Markets. 2023. Available online: https://www.mapa.gob.es/en/ganaderia/estadisticas/indicadoressectorporcino2023_tcm38-564427.pdf (accessed on 20 May 2025).
- Toldrá, F.; Reig, M.; Mora, L. Management of meat by- and co-products for an improved meat processing sustainability. Meat Sci. 2021, 181, 108608. [Google Scholar] [CrossRef]
- Bah, C.S.; Bekhit, A.E.A.; Carne, A.; McConnell, M.A. Slaughterhouse Blood: An Emerging Source of Bioactive Compounds. Compr. Rev. Food Sci. Food Saf. 2013, 12, 314–331. [Google Scholar] [CrossRef]
- Bułkowska, K.; Zielińska, M. Recovery of Biogas and Other Valuable Bioproducts from Livestock Blood Waste: A Review. Energies 2024, 17, 5873. [Google Scholar] [CrossRef]
- Jin, S.; Choi, J. Effects of porcine blood plasma on the emulsion stability, physicochemical characteristics and textural attributes of emulsified pork batter. J. Anim. Sci. Technol. 2021, 63, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nation. The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, Y.; Feng, T.; Gong, Z.; Dong, Z.; Wang, L.; Xu, J.; Jin, Y.; Huang, L. Replacing fishmeal with hydrolysates from whole forage-fish or pacific krill combined with soy protein concentrate in Japanese seabass (Lateolabrax japonicus) diets. Anim. Feed Sci. Technol. 2025, 327, 116440. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Chu, Y.-T.; Kuo, I.-P.; Liao, Z.-H.; Lin, Y.-R.; Liao, C.-P.; Ramadhana, A.W.; Nan, F.-H.; Hu, Y.-F. Optimizing fishmeal replacement with tilapia hydrolysate protein: Effects on growth, metabolism, and gene expression in white shrimp. Aquac. Rep. 2025, 44, 103080. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2020, 12, 703–758. [Google Scholar] [CrossRef]
- Woodgate, S.L.; Wan, A.H.L.; Hartnett, F.; Wilkinson, R.G.; Davies, S.J. The utilisation of European processed animal proteins as safe, sustainable and circular ingredients for global aquafeeds. Rev. Aquac. 2022, 14, 1572–1596. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Calduch-Giner, J.À.; Pereira, G.V.; Gonçalves, A.T.; Dias, J.; Johansen, J.; Silva, T.; Naya-Català, F.; Piazzon, C.; Sitjà-Bobadilla, A.; et al. Sustainable Fish Meal-Free Diets for Gilthead Sea Bream (Sparus aurata): Integrated Biomarker Response to Assess the Effects on Growth Performance, Lipid Metabolism, Antioxidant Defense and Immunological Status. Animals 2024, 14, 2166. [Google Scholar] [CrossRef]
- Liland, N.; Hatlen, B.; Takle, H.; Venegas, C.; Espe, M.; Torstensen, B.; Waagbø, R. Including processed poultry and porcine by-products in diets high in plant ingredients reduced liver TAG in Atlantic salmon, Salmo salar L. Aquac. Nutr. 2014, 21, 655–669. [Google Scholar] [CrossRef]
- Ye, J.-D.; Wang, K.; Li, F.-D.; Sun, Y.-Z.; Liu, X.-H. Incorporation of a mixture of meat and bone meal, poultry by-product meal, blood meal and corn gluten meal as a replacement for fish meal in practical diets of Pacific white shrimp Litopenaeus vannamei at two dietary protein levels. Aquac. Nutr. 2011, 17, e337–e347. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Costas, B.; Medale, F.; Pérez-Sánchez, J.; Glencross, B. Editorial: Feeding a sustainable blue revolution: The physiological consequences of novel ingredients on farmed fish. Front. Physiol. 2022, 13, 1092064. [Google Scholar] [CrossRef] [PubMed]
- Takakuwa, F.; Sato, H.; Mineyama, N.; Yamada, S.; Biswas, A.; Tanaka, H. Bioavailability of porcine blood meal as a fish meal substitute in the diet for red sea bream (Pagrus major, Temminck & Schlegel) fingerling. Aquac. Res. 2022, 53, 4616–4626. [Google Scholar] [CrossRef]
- Boyd, C.E.; D’Abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; McNevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R., Jr.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 57–633. [Google Scholar] [CrossRef]
- Regueiro, L.; Newton, R.; Soula, M.; Méndez, D.; Kok, B.; Little, D.C.; Pastres, R.; Johansen, J.; Ferreira, M. Opportunities and limitations for the introduction of circular economy principles in EU aquaculture based on the regulatory framework. J. Ind. Ecol. 2021, 26, 2033–2044. [Google Scholar] [CrossRef]
- Xing, L.; Li, G.; Toldrá, F.; Zhang, W. The Physiological Activity of Bioactive Peptides Obtained from Meat and Meat By-Products. Adv. Food Nutr. Res. 2021, 97, 147–185. [Google Scholar] [CrossRef]
- Kim, S. A multi-omics approach to assess production of the valuable peptides and amino acids in porcine blood protein hydrolysate. LWT 2022, 163, 113593. [Google Scholar] [CrossRef]
- Kazimierska, K.; Biel, W. Comparative Analysis of Spray-Dried Porcine Plasma and Hydrolyzed Porcine Protein as Animal-Blood-Derived Protein Ingredients for Pet Nutrition. Molecules 2023, 28, 7917. [Google Scholar] [CrossRef]
- Moreno-Mariscal, C.; Moroni, F.; Pérez-Sánchez, J.; Mora, L.; Toldrá, F. Optimization of Sequential Enzymatic Hydrolysis in Porcine Blood and the Influence on Peptide Profile and Bioactivity of Prepared Hydrolysates. Int. J. Mol. Sci. 2025, 26, 3583. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Aristoy, M.-C.; Reig, M.; Toldrá, F. Bioactive peptides. In Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 333–345. [Google Scholar]
- Zhang, Q.; Tong, X.; Sui, X.; Wang, Z.; Qi, B.; Li, Y.; Jiang, L. Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res. Int. 2018, 111, 256–264. [Google Scholar] [CrossRef]
- Brandão, M.; Bariani, R.; Rigato, I.; Bauce, B. Desmoplakin Cardiomyopathy: Comprehensive Review of an Increasingly Recognized Entity. J. Clin. Med. 2023, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Khoklang, A.; Kersanté, P.; Nontasan, S.; Sutthi, N.; Pakdeenarong, N.; Wang, T.; Wangkahart, E. Insights into the functional properties of a natural free amino acid mix: Effect on growth performance, nutrient metabolism, and immune response in a carnivorous fish, Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2023, 144, 109232. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2008, 37, 43–53. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and Functions of Amino Acids in Fish. In Amino Acids in Nutrition and Health; Wu, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1285, pp. 133–168. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khan, M.A.; Yousefi, M.; Costas, B. Roles of arginine in fish nutrition and health: Insights for future researches. Rev. Aquac. 2020, 12, 2091–2108. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Wu, G. Nutrition and metabolism of glutamate and glutamine in fish. Amino Acids 2020, 52, 671–691. [Google Scholar] [CrossRef]
- Sadri, H.; Larki, N.N.; Kolahian, S. Hypoglycemic and Hypolipidemic Effects of Leucine, Zinc, and Chromium, Alone and in Combination, in Rats with Type 2 Diabetes. Biol. Trace Elem. Res. 2017, 180, 246–254. [Google Scholar] [CrossRef]
- Ozturk-Kerimoglu, B.; Heres, A.; Mora, L.; Toldrá, F. Antioxidant peptides generated from chicken feet protein hydrolysates. J. Sci. Food Agric. 2023, 103, 7207–7217. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Borrajo, P.; Franco, D. In Search of Antioxidant Peptides from Porcine Liver Hydrolysates Using Analytical and Peptidomic Approach. Antioxidants 2021, 11, 27. [Google Scholar] [CrossRef]
- Zhan, J.; Li, G.; Dang, Y.; Pan, D. Study on the antioxidant activity of peptide isolated from porcine plasma during in vitro digestion. Food Biosci. 2021, 42, 101069. [Google Scholar] [CrossRef]
- Li, G.; Zhan, J.; Hu, L.; Yuan, C.; Takaki, K.; Ying, X.; Hu, Y. Identification of a new antioxidant peptide from porcine plasma by in vitro digestion and its cytoprotective effect on H2O2 induced HepG2 model. J. Funct. Foods 2021, 86, 104679. [Google Scholar] [CrossRef]
- Hovgaard, L.; Frokjaer, S.; van de Weert, M. (Eds.) Pharmaceutical Formulation Development of Peptides and Proteins, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; ISBN 9780429106712. [Google Scholar]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- Hu, R.; Dunmire, K.M.; Truelock, C.N.; Paulk, C.B.; Aldrich, G.; Li, Y. Antioxidant performances of corn gluten meal and DDGS protein hydrolysates in food, pet food, and feed systems. J. Agric. Food Res. 2020, 2, 10030. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Rossi, R.; Stella, S.; Ratti, S.; Maghin, F.; Tirloni, E.; Corino, C. Effects of antioxidant mixtures in the diet of finishing pigs on the oxidative status and shelf life of longissimus dorsi muscle packaged under modified atmosphere1,2. J. Anim. Sci. 2017, 95, 4986–4997. [Google Scholar] [CrossRef]
- Gasco, L.; Gai, F.; Maricchiolo, G.; Genovese, L.; Ragonese, S.; Bottari, T.; Caruso, G. Supplementation of Vitamins, Minerals, Enzymes and Antioxidants in Fish Feeds. In Feeds for the Aquaculture Sector; SpringerBriefs in Molecular Science; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Hu, X.; Ma, W.; Zhang, D.; Tian, Z.; Yang, Y.; Huang, Y.; Hong, Y. Application of Natural Antioxidants as Feed Additives in Aquaculture: A Review. Biology 2025, 14, 87. [Google Scholar] [CrossRef]
- Willard, J.R.; Barrow, B.M.; Zraika, S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia 2016, 60, 701–708. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Effect of ultrasound and enzymatic pre-treatments on the profile of bioactive peptides of beef liver hydrolysates. Food Res. Int. 2024, 197, 115240. [Google Scholar] [CrossRef]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. Effect of thermal pretreatment and gastrointestinal digestion on the bioactivity of dry-cured ham bone enzymatic hydrolyzates. Food Res. Int. 2024, 188, 114513. [Google Scholar] [CrossRef]
- Gu, Y.; Li, X.; Qi, X.; Ma, Y.; Chan, E.C.Y. In silico identification of novel ACE and DPP-IV inhibitory peptides derived from buffalo milk proteins and evaluation of their inhibitory mechanisms. Amino Acids 2023, 55, 161–171. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Wang, S.; He, R.; Wang, Z.; Zhao, L.; Ge, W. Preparation, characterization, and mechanism of DPP-IV inhibitory peptides derived from Bactrian camel milk. Int. J. Biol. Macromol. 2024, 277, 134232. [Google Scholar] [CrossRef]
- He, B.; Lian, Y.; Xue, H.; Zhou, Y.; Wei, Y.; Ma, J.; Tan, Y.; Wu, Y. DPP-IV Inhibitory Peptide against In Vitro Gastrointestinal Digestion Derived from Goat’s Milk Protein and Its Activity Enhancement via Amino Acid Substitution. Foods 2024, 13, 2721. [Google Scholar] [CrossRef]
- Arai, S.; Kurimoto, M.; Nakada, H.; Tanaka, M.; Ochi, H.; Tanaka, M.; Okochi, M. Screening of novel DPP-IV inhibitory peptides derived from bovine milk proteins using a peptide array platform. J. Biosci. Bioeng. 2023, 137, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zheng, L.; Udenigwe, C.C.; Lin, L.; Zhao, M. Enzymatic characterization and synergistic effects of protease combinations on DPP-IV inhibitory peptide release from bovine casein. Food Biosci. 2024, 60, 104276. [Google Scholar] [CrossRef]
- Akan, E. An evaluation of the in vitro antioxidant and antidiabetic potentials of camel and donkey milk peptides released from casein and whey proteins. J. Food Sci. Technol. 2020, 58, 3743–3751. [Google Scholar] [CrossRef] [PubMed]
- Kotsoni, E.; Daukšas, E.; Aas, G.H.; Rustad, T.; Tiwari, B.K.; Lammi, C.; Bollati, C.; Fanzaga, M.; D’adduzio, L.; Stangeland, J.K.; et al. Antioxidant Activity and DPP-IV Inhibitory Effect of Fish Protein Hydrolysates Obtained from High-Pressure Pretreated Mixture of Rainbow Trout (Oncorhynchus mykiss) and Atlantic Salmon (Salmo salar) Rest Raw Material. Mar. Drugs 2024, 22, 568. [Google Scholar] [CrossRef]
- Li, J.; Wei, Y.; Lei, Y.; Guo, X.; Zhao, Y.; Hao, Q.; Deng, X.; Zhang, J. Screening, identification, and mechanistic exploration of DPP-IV inhibitory peptides from collagen in Esox lucius skin. Food Biosci. 2024, 63, 105698. [Google Scholar] [CrossRef]
- Ortizo, R.G.G.; Sharma, V.; Tsai, M.-L.; Nargotra, P.; Sun, P.-P.; Chen, C.-W.; Dong, C.-D. A novel deep eutectic solvent-based green extraction and purification of DPP-IV inhibitory peptides from tilapia (Oreochromis niloticus) viscera hydrolysate. Food Biosci. 2024, 61, 104658. [Google Scholar] [CrossRef]
- Murumkar, P.R.; Ghuge, R.B.; Chauhan, M.; Barot, R.R.; Sorathiya, S.; Choudhary, K.M.; Joshi, K.D.; Yadav, M.R. Recent developments and strategies for the discovery of TACE inhibitors. Expert Opin. Drug Discov. 2020, 15, 779–801. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Omran, Z. New Disulfiram Derivatives as MAGL-Selective Inhibitors. Molecules 2021, 26, 3296. [Google Scholar] [CrossRef]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Z.; Ma, M.; Huang, X.; Guyonnet, V.; Xiong, H. Exploration of novel DPP-IV inhibitory peptides from discarded eggshell membrane: An integrated in silico and in vitro study. Food Biosci. 2024, 59, 104036. [Google Scholar] [CrossRef]
- Naya-Català, F.; Pereira, G.D.V.; Piazzon, M.C.; Fernandes, A.M.; Calduch-Giner, J.A.; Sitjà-Bobadilla, A.; Conceição, L.E.C.; Pérez-Sánchez, J. Cross-Talk Between Intestinal Microbiota and Host Gene Expression in Gilthead Sea Bream (Sparus aurata) Juveniles: Insights in Fish Feeds for Increased Circularity and Resource Utilization. Front. Physiol. 2021, 12, 748265. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Nayak, S.K. Role of gastrointestinal microbiota in fish. Aquac. Res. 2010, 41, 1553–1573. [Google Scholar] [CrossRef]
- Dawood, M.A.O. Nutritional immunity of fish intestines: Important insights for sustainable aquaculture. Rev. Aquac. 2021, 13, 642–663. [Google Scholar] [CrossRef]
- Heres, A.; Gallego, M.; Mora, L.; Toldrá, F. Identification and Quantitation of Bioactive and Taste-Related Dipeptides in Low-Salt Dry-Cured Ham. Int. J. Mol. Sci. 2022, 23, 2507. [Google Scholar] [CrossRef]
- Moreno-Mariscal, C.; Carrera-Alvarado, G.; Mora, L.; Toldrá, F. Neprilysin (NEP) and Angiotensin Converting Enzyme-I (ACE-I) inhibitory dipeptides from chicken carcass hydrolysates. LWT 2025, 221, 117591. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Features of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from dietary proteins. J. Food Biochem. 2017, 43, e12451. [Google Scholar] [CrossRef]
- Power, O.; Nongonierma, A.B.; Jakeman, P.; FitzGerald, R.J. Food protein hydrolysates as a source of dipeptidyl peptidase IV inhibitory peptides for the management of type 2 diabetes. Proc. Nutr. Soc. 2014, 73, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Alvarado, G.; Toldrá, F.; Mora, L. DPP-IV Inhibitory Peptides GPF, IGL, and GGGW Obtained from Chicken Blood Hydrolysates. Int. J. Mol. Sci. 2022, 23, 14140. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Wang, Y.; Luo, J.; Zhao, Y.; Han, G.; Han, L.; Yu, Q. Production and identification of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from discarded cowhide collagen. Food Chem. 2022, 405, 134793. [Google Scholar] [CrossRef]
- Wang, B.; Yu, Z.; Yokoyama, W.; Chiou, B.-S.; Chen, M.; Liu, F.; Zhong, F. Collagen peptides with DPP-IV inhibitory activity from sheep skin and their stability to in vitro gastrointestinal digestion. Food Biosci. 2021, 42, 101161. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods 2013, 5, 1909–1917. [Google Scholar] [CrossRef]
- 968.06; Protein (Crude) in Animal Feed, Dumas Method. AOAC International: Rockville, MD, USA, 1969.
- Aristoy, M.C.; Toldra, F. Deproteinization techniques for HPLC amino acid analysis in fresh pork muscle and dry-cured ham. J. Agric. Food Chem. 1991, 39, 1792–1795. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Effect of cooking and in vitro digestion on the antioxidant activity of dry-cured ham by-products. Food Res. Int. 2017, 97, 296–306. [Google Scholar] [CrossRef] [PubMed]
| Biological Activity | Assay | Concentration (mg/mL) | Results | IC50 (mg/mL) |
|---|---|---|---|---|
| Antioxidant | ABTS (µmol TEAC/g) | 10 | 2048.48 ± 20.01 | 2.09 |
| FRAP (µmol TE/g) | 20 | 589.99 ± 59.64 | 135.05 | |
| DPPH (%) | 20 | 35.94 ± 4.86 | 26.73 | |
| Hypoglycemic | Dipeptidylpeptidase-IV (DPP-IV) inhibition (%) | 20 | 82.78 ± 1.55 | - |
| Neprilysin inhibition (%) | 20 | 84.72 ± 1.14 | - | |
| Anti-inflammatory | TACE inhibition (%) | 100 | 50.79 ± 2.24 | - |
| Immunomodulatory | Monoacylglycerol lipase (MGL) inhibition (%) | 20 | 69.08 ± 6.93 | - |
| Antioxidant Activity | T (°C) | Storage Time (Months) | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | ||
| ABTS (µmol TEAC/g) | 4 °C | 2052.0 ± 15.3 a | 2123.5 ± 12.9 b | 2014.7 ± 4.2 b | 1917.3 ± 14.5 c | 1946.0 ± 13.8 c | 2042.3 ± 16.5 b |
| 20 °C | 2116.2 ± 16.9 a | 2057.6 ± 30.1 b | 2030.5 ± 17.8 b | 1926.0 ± 12.5 c | 2017.8 ± 8.4 b | 2056.1 ± 6.6 b | |
| FRAP (µmol TE/g) | 4 °C | 414.6 ± 16.5 c | 306.3 ± 7.2 d | 333.3 ± 20.6 d | 345.9 ± 7.2 d | 329.9 ± 20.8 d | 318.6 ± 4.6 d |
| 20 °C | 307.3 ± 32.3 d | 515.6 ± 26.0 b | 478.3 ± 34.2 b | 643.3 ± 9.0 a | 635.3 ± 28.4 a | 535.3 ± 28.4 b | |
| DPPH (% inhib) | 4 °C | 30.5 ± 1.4 g | 30.7 ± 0.7 g | 30.1 ± 2.3 g | 32.0 ± 3.9 fg | 32.0 ± 2.1 fg | 37.1 ± 2.1 ef |
| 20 °C | 40.3 ± 1.5 e | 40.6 ± 2.4 e | 46.3 ± 0.6 d | 51.9 ± 0.9 c | 59.2 ± 0.5 b | 72.3 ± 0.9 a | |
| Free Amino Acids | Concentration |
|---|---|
| (mg/g) | |
| Aspartic Acid (Asp) | 21.33 ± 0.88 |
| Glutamic Acid (Glu) | 19.88 ± 0.75 |
| Hydroxyproline (Hyp) | 0.08 ± 0.01 |
| Serine (Ser) | 12.44 ± 0.28 |
| Asparagine (Asn) | 23.40 ± 0.62 |
| Glycine (Gly) | 16.24 ± 0.57 |
| Glutamine (Gln) | 5.39 ± 0.20 |
| β-Alanine (βAla) | 0.33 ± 0.01 |
| Taurine (Tau) | 0.33 ± 0.01 |
| Histidine (His) | 40.11 ± 1.58 |
| γ-Aminobutyric acid (γAba) | 1.12 ± 0.09 |
| Threonine (Thr) | 17.29 ± 0.56 |
| Alanine (Ala) | 47.35 ± 1.43 |
| Arginine (Arg) | 6.86 ± 0.18 |
| Proline (Pro) | 4.43 ± 0.12 |
| Tyrosine (Tyr) | 5.07 ± 0.11 |
| Valine (Val) | 49.17 ± 1.27 |
| Methionine (Met) | 5.92 ± 0.16 |
| Cysteine (Cys) | 1.28 ± 0.05 |
| Isoleucine (Ile) | 3.65 ± 0.10 |
| Leucine (Leu) | 82.04 ± 2.08 |
| Phenylalanine (Phe) | 33.70 ± 0.82 |
| Tryptophan (Trp) | 12.28 ± 0.34 |
| Ornithine (Orn) | 4.84 ± 0.33 |
| Lysine (Lys) | 47.28 ± 2.05 |
| Total | 461.82 ± 14.13 |
| Biological Activity | Assay | Fraction | ||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | ||
| Antioxidant | ABTS (µmol TEAC/g) | 224.28 ± 2.13 d | 1441.31 ± 15.14 a | 1397.22 ± 12.06 b | 494.39 ± 6.92 c | 336.87 ± 22.63 d |
| FRAP (µmol TE/g) | 89.94 ± 1.90 a | 11.18 ± 4.97 b | n.d. | n.d. | 11.59 ± 1.43 b | |
| DPPH (% inhibition) | 17.01 ± 2.81 a | 7.16 ± 1.25 bc | 8.50 ± 2.12 b | 6.59 ± 0.52 bc | 3.50 ± 0.94 c | |
| Hypoglycemic | DPP IV inhibition (%) | 90.45 ± 1.16 a | n.d. | n.d. | 40.29 ± 3.99 b | n.d. |
| NEP inhibition(%) | 84.69 ± 1.68 a | 49.67 ± 0.53 b | 55.09 ± 3.78 b | 30.43 ± 2.83 c | 36.54 ± 3.92 c | |
| Anti-inflammatory | TACE inhibition (%) | 113.23 ± 5.10 a | 37.04 ± 2.42 b | 23.81 ± 4.49 c | 8.73 ± 1.12 d | 14.29 ± 2.24 cd |
| Immunomodulatory | MGL inhibition (%) | n.d. | n.d. | n.d. | 85.30 ± 3.61 | n.d. |
| Source Protein | Protein ID | Peptide Sequence | Peptide Ranker Ratio 1 |
|---|---|---|---|
| Albumin | ALBU_PIG | DNPDIPK | 0.552992 |
| DNPDIPKLKPDP | 0.501574 | ||
| EDEQKFWGK | 0.570703 | ||
| DNPDIPKLKPDPV | 0.523743 | ||
| Hemoglobin A | HBA_PIG | GHLDDLPGA | 0.535194 |
| HPDDFNPS | 0.575335 | ||
| GHLDDLPG | 0.588214 | ||
| PDDFNPS | 0.588007 | ||
| HHPDDFNPS | 0.529012 | ||
| AHHPDDFNPS | 0.517146 | ||
| GHLDDLPGAL | 0.698265 | ||
| Hemoglobin B | HBB_PIG | DPENFRL | 0.774659 |
| Immunoglobulin | IGHG1_HUMAN | FPPKPKD | 0.625664 |
| GDL | 0.546664 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Mariscal, C.; Moroni, F.; Pérez-Sánchez, J.; Mora, L.; Toldrá, F. Enzymatic Hydrolysis of Porcine Blood as a Strategy to Obtain a Peptide-Rich Functional Ingredient. Int. J. Mol. Sci. 2025, 26, 9863. https://doi.org/10.3390/ijms26209863
Moreno-Mariscal C, Moroni F, Pérez-Sánchez J, Mora L, Toldrá F. Enzymatic Hydrolysis of Porcine Blood as a Strategy to Obtain a Peptide-Rich Functional Ingredient. International Journal of Molecular Sciences. 2025; 26(20):9863. https://doi.org/10.3390/ijms26209863
Chicago/Turabian StyleMoreno-Mariscal, Cristina, Federico Moroni, Jaume Pérez-Sánchez, Leticia Mora, and Fidel Toldrá. 2025. "Enzymatic Hydrolysis of Porcine Blood as a Strategy to Obtain a Peptide-Rich Functional Ingredient" International Journal of Molecular Sciences 26, no. 20: 9863. https://doi.org/10.3390/ijms26209863
APA StyleMoreno-Mariscal, C., Moroni, F., Pérez-Sánchez, J., Mora, L., & Toldrá, F. (2025). Enzymatic Hydrolysis of Porcine Blood as a Strategy to Obtain a Peptide-Rich Functional Ingredient. International Journal of Molecular Sciences, 26(20), 9863. https://doi.org/10.3390/ijms26209863










