Targeted Inhibition in Pediatric MET and ALK-Altered Hemispheric Gliomas: Objective Responses Followed by Treatment Resistance
Abstract
1. Introduction
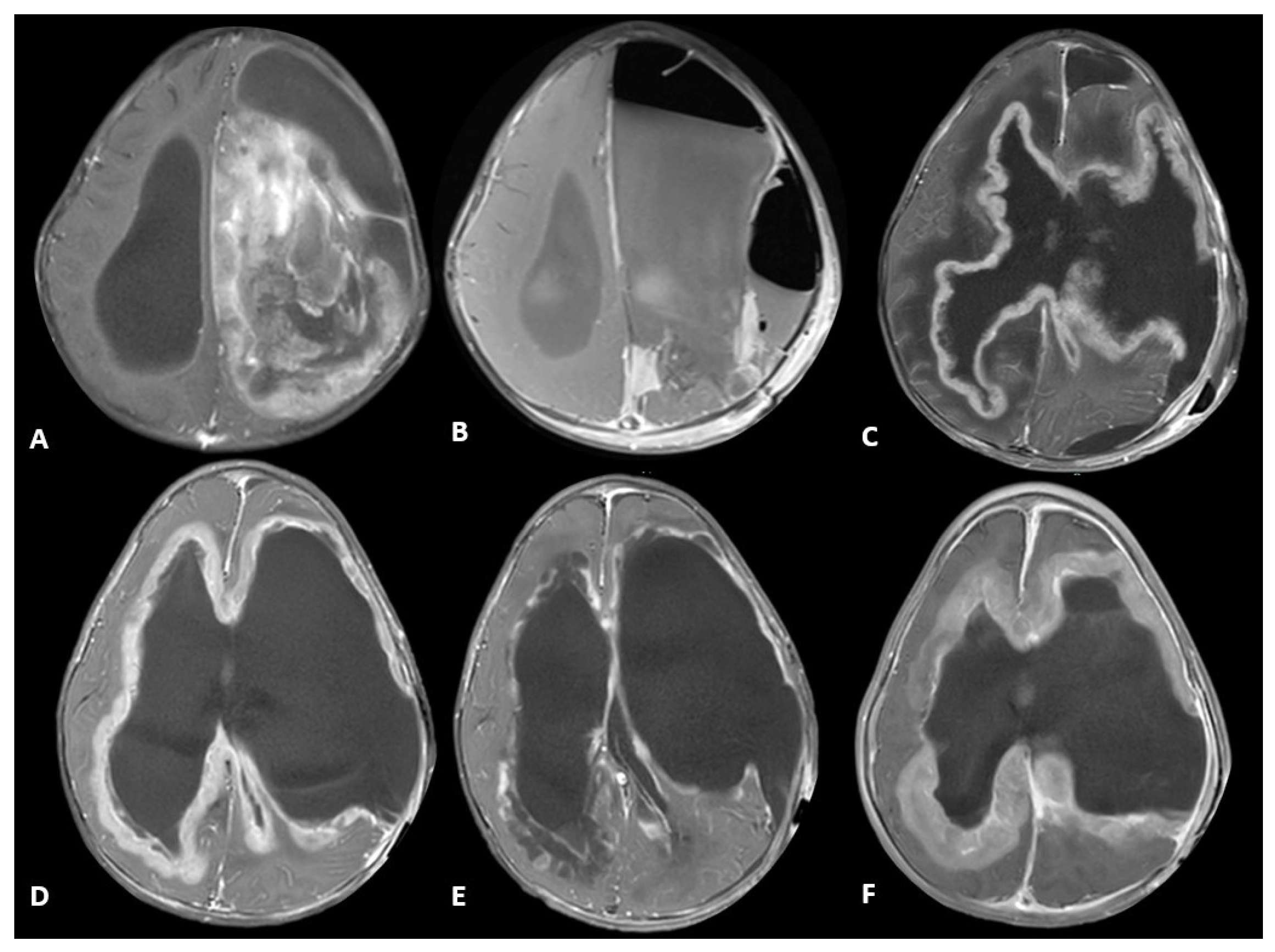
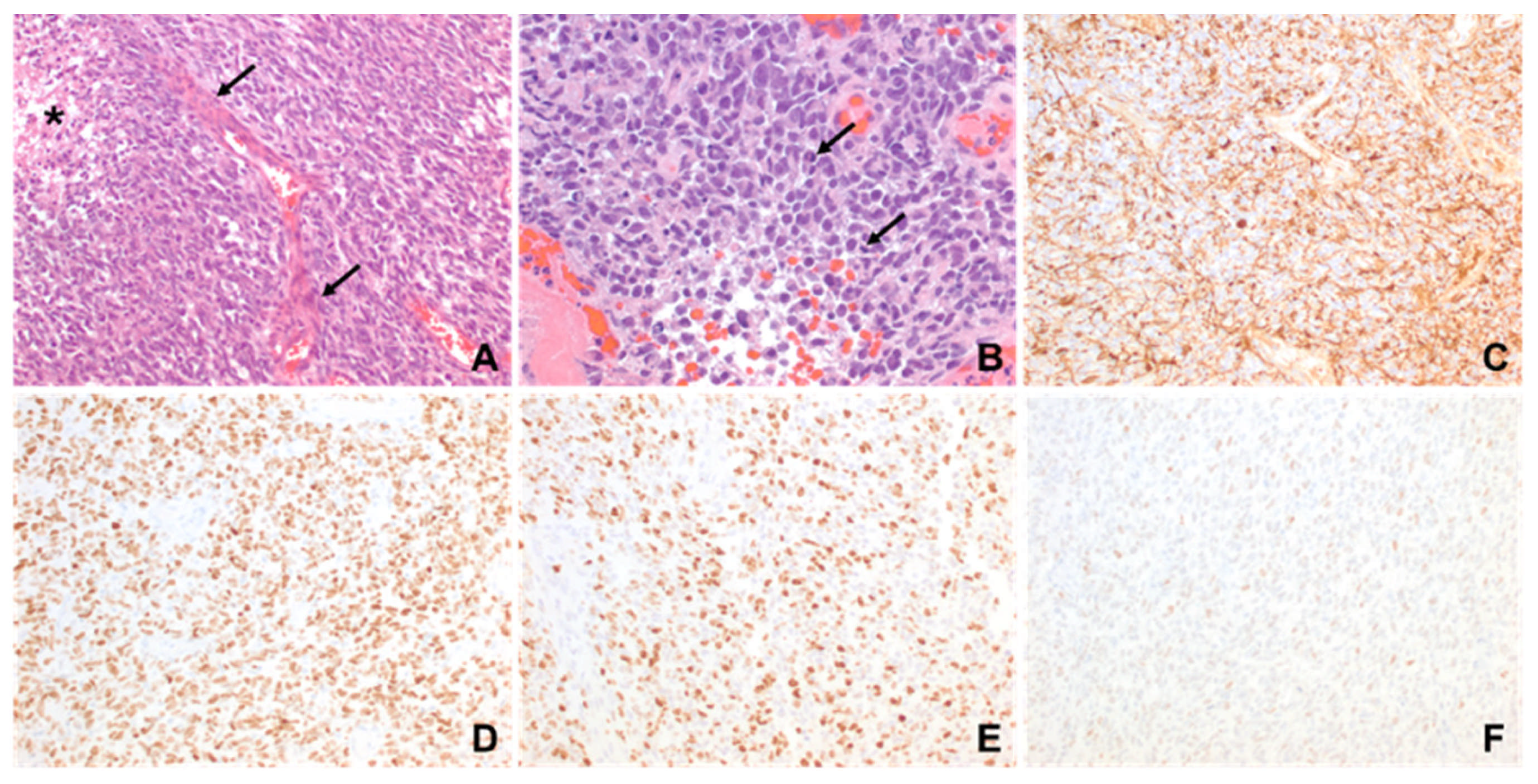
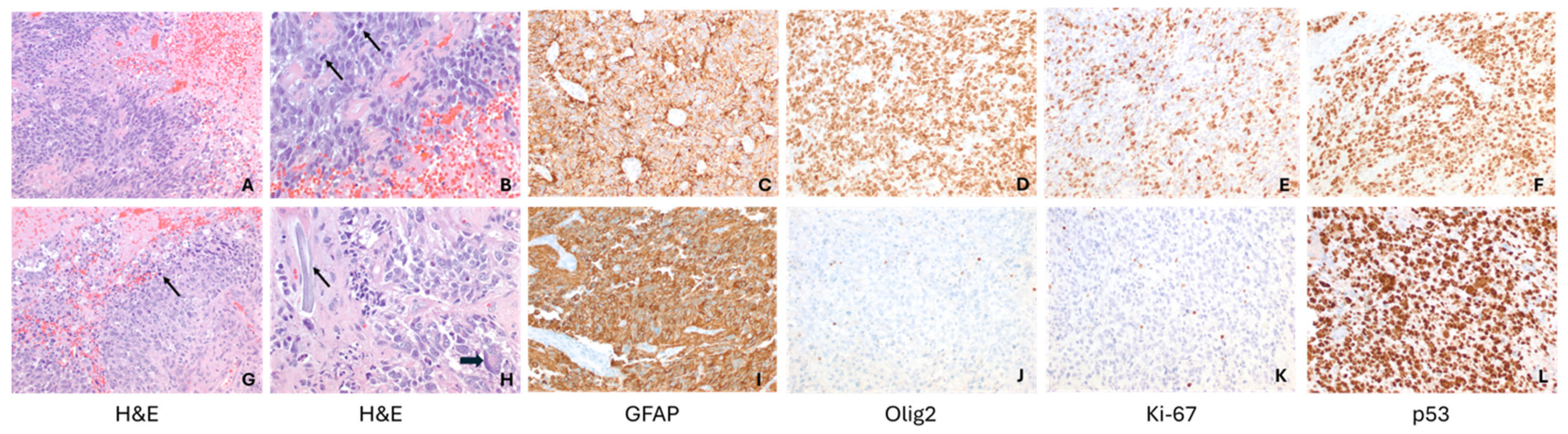
2. Case Description
3. Discussion
4. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, M.; Ballard, C.; Benedetti, J.; Neff, C.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S.; Ostrom, Q.T. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2017–2021. Neuro-Oncology 2024, 26 (Suppl. 6), vi1–vi85. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-Oncology 2015, 16 (Suppl. 10), x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Cohen, K.J.; Pollack, I.F.; Zhou, T.; Buxton, A.; Holmes, E.J.; Burger, P.C.; Brat, D.J.; Rosenblum, M.K.; Hamilton, R.L.; Lavey, R.S.; et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children’s Oncology Group. Neuro-Oncology 2011, 13, 317–323. [Google Scholar] [CrossRef]
- Jakacki, R.I.; Cohen, K.J.; Buxton, A.; Krailo, M.D.; Burger, P.C.; Rosenblum, M.K.; Brat, D.J.; Hamilton, R.L.; Eckel, S.P.; Zhou, T.; et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: A report of the Children’s Oncology Group ACNS0423 study. Neuro-Oncology 2016, 18, 1442–1450. [Google Scholar] [CrossRef]
- Dufour, C.; Grill, J.; Lellouch-Tubiana, A.; Puget, S.; Chastagner, P.; Frappaz, D.; Doz, F.; Pichon, F.; Plantaz, D.; Gentet, J.; et al. High-grade glioma in children under 5 years of age: A chemotherapy only approach with the BBSFOP protocol. Eur. J. Cancer 2006, 42, 2939–2945. [Google Scholar] [CrossRef]
- Chiang, J.; Bagchi, A.; Li, X.; Dhanda, S.K.; Huang, J.; Pinto, S.N.; Sioson, E.; Dalton, J.; Tatevossian, R.G.; Jia, S.; et al. High-grade glioma in infants and young children is histologically, molecularly, and clinically diverse: Results from the SJYC07 trial and institutional experience. Neuro-Oncology 2024, 26, 178–190. [Google Scholar] [CrossRef]
- Hargrave, D.R.; Terashima, K.; Hara, J.; Kordes, U.R.; Upadhyaya, S.A.; Sahm, F.; Bouffet, E.; Packer, R.J.; Witt, O.; Sandalic, L.; et al. Phase II Trial of Dabrafenib Plus Trametinib in Relapsed/Refractory BRAF V600-Mutant Pediatric High-Grade Glioma. J. Clin. Oncol. 2023, 41, 5174–5183. [Google Scholar] [CrossRef]
- Rosenberg, T.; Yeo, K.K.; Mauguen, A.; Alexandrescu, S.; Prabhu, S.P.; Tsai, J.W.; Malinowski, S.; Joshirao, M.; Parikh, K.; Sait, S.F.; et al. Upfront molecular targeted therapy for the treatment of BRAF-mutant pediatric high-grade glioma. Neuro-Oncology 2022, 24, 1964–1975. [Google Scholar] [CrossRef]
- Chatwin, H.V.; Cruz, J.C.; Green, A.L. Pediatric high-grade glioma: Moving toward subtype-specific multimodal therapy. FEBS J. 2021, 288, 6127–6141. [Google Scholar] [CrossRef]
- Desai, A.V.; Bagchi, A.; Armstrong, A.E.; van Tilburg, C.M.; Basu, E.M.; Robinson, G.W.; Wang, H.; Casanova, M.; André, N.; Campbell-Hewson, Q.; et al. Efficacy and safety of entrectinib in children with extracranial solid or central nervous system (CNS) tumours harbouring NTRK or ROS1 fusions. Eur. J. Cancer 2025, 220, 115308. [Google Scholar] [CrossRef] [PubMed]
- Shahab, S.W.; Schniederjan, M.; Vega, J.V.; Little, S.; Reisner, A.; MacDonald, T.; Aguilera, D. Case report: ATIC-ALK fusion in infant-type hemispheric glioma and response to lorlatinib. Front. Oncol. 2023, 13, 1123378. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.S.; Wong, M.; Mayoh, C.; Kumar, A.; Tsoli, M.; Mould, E.; Tyrrell, V.; Khuong-Quang, D.-A.; Pinese, M.; Gayevskiy, V.; et al. Brief Report: Potent clinical and radiological response to larotrectinib in TRK fusion-driven high-grade glioma. Br. J. Cancer 2018, 119, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Simoneau, J.; Robertson, P.; Muraszko, K.; Maher, C.O.; Garton, H.; Calvert, R.; Koschmann, C.; Upadhyaya, S.A.; Mody, R.; Brown, N.; et al. Long-Term Tumor Stability After First-Line Treatment With Larotrectinib in an Infant With NTRK2 Fusion-Positive High-Grade Glioma. J. Natl. Compr. Cancer Netw. 2024, 22, e247045. [Google Scholar] [CrossRef]
- Kline, C.; Felton, E.; Allen, I.E.; Tahir, P.; Mueller, S. Survival outcomes in pediatric recurrent high-grade glioma: Results of a 20-year systematic review and meta-analysis. J. Neuro-Oncol. 2018, 137, 103–110. [Google Scholar] [CrossRef]
- Strother, D.R.; Lafay-Cousin, L.; Boyett, J.M.; Burger, P.; Aronin, P.; Constine, L.; Duffner, P.; Kocak, M.; Kun, L.E.; Horowitz, M.E.; et al. Benefit from prolonged dose-intensive chemotherapy for infants with malignant brain tumors is restricted to patients with ependymoma: A report of the Pediatric Oncology Group randomized controlled trial 9233/34. Neuro-Oncology 2014, 16, 457–465. [Google Scholar] [CrossRef]
- Chuk, M.K.; Widemann, B.C.; Minard, C.G.; Liu, X.; Kim, A.; Bernhardt, M.B.; Kudgus, R.A.; Reid, J.M.; Voss, S.D.; Blaney, S.; et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children’s Oncology Group. Pediatr. Blood Cancer 2018, 65, e27077. [Google Scholar] [CrossRef]
- Gianno, F.; Giovannoni, I.; Cafferata, B.; Diomedi-Camassei, F.; Minasi, S.; Barresi, S.; Buttarelli, F.R.; Alesi, V.; Cardoni, A.; Antonelli, M.; et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica 2022, 114, 422–435. [Google Scholar] [CrossRef]
- Yoel, A.; Adjumain, S.; Liang, Y.; Daniel, P.; Firestein, R.; Tsui, V. Emerging and Biological Concepts in Pediatric High-Grade Gliomas. Cells 2024, 13, 1492. [Google Scholar] [CrossRef]
- Tauziède-Espariat, A.; Debily, M.-A.; Castel, D.; Grill, J.; Puget, S.; Roux, A.; Saffroy, R.; Pagès, M.; Gareton, A.; Chrétien, F.; et al. The pediatric supratentorial MYCN-amplified high-grade gliomas methylation class presents the same radiological, histopathological and molecular features as their pontine counterparts. Acta Neuropathol. Commun. 2020, 8, 104. [Google Scholar] [CrossRef]
- El-Ayadi, M.; Ansari, M.; Sturm, D.; Gielen, G.H.; Warmuth-Metz, M.; Kramm, C.M.; von Bueren, A.O. High-grade glioma in very young children: A rare and particular patient population. Oncotarget 2017, 8, 64564. [Google Scholar] [CrossRef]
- Bagchi, A.; Orr, B.A.; Campagne, O.; Dhanda, S.; Nair, S.; Tran, Q.; Christensen, A.M.; Gajjar, A.; Furtado, L.V.; Vasilyeva, A.; et al. Lorlatinib in a Child with ALK-Fusion–Positive High-Grade Glioma. N. Engl. J. Med. 2021, 385, 761–763. [Google Scholar] [CrossRef]
- Tsai, C.C.; Huang, M.-H.; Fang, C.-L.; Hsieh, K.L.-C.; Hsieh, T.-H.; Ho, W.-L.; Chang, H.; Tsai, M.-L.; Kao, Y.-C.; Miser, J.S.; et al. An Infant-Type Hemispheric Glioma With SOX5::ALK: A Novel Fusion. J. Natl. Compr. Cancer Netw. 2024, 22, e237102. [Google Scholar] [CrossRef]
- Lai, M.; Li, S.; Li, H.; Hu, Q.; Li, J.; Zhou, J.; Ai, R.; Zhen, J.; Zhou, Z.; Wang, L.; et al. Lorlatinib for ALK-fused, infant-type hemispheric glioma with lung metastasis: A case report. Ann. Clin. Transl. Neurol. 2023, 10, 836–841. [Google Scholar] [CrossRef]
- Yano, S.; Takeuchi, S.; Nakagawa, T.; Yamada, T. Ligand-triggered resistance to molecular targeted drugs in lung cancer: Roles of hepatocyte growth factor and epidermal growth factor receptor ligands. Cancer Sci. 2012, 103, 1189–1194. [Google Scholar] [CrossRef]

| Tumor Sample | Histopathology | Targeted Sequencing | Methylation Profiling Classification | Integrated Diagnosis | Treatment Regimen | |
|---|---|---|---|---|---|---|
| Patient 1 | Diagnostic | -Brisk mitotic activity -Gliosis in myxoid background -Vimentin positive -olig2 positive -S-100 positive | -CLIP2-MET fusion -CHEK2 frameshift alteration -CDKN2A, CDKN2B, MTAP loss | Infant-type Hemispheric Glioma | Infant-type Hemispheric Glioma, WHO-CNS Grade 4 | Systemic chemotherapy: Cyclophosphamide, Vincristine, Cisplatinum and Etoposide (2 cycles) Targeted agent: Cabozantinib |
| Patient 2 | Diagnostic | -Brisk mitotic activity -INI1 retained -P53 diffusely expressed -olig2 positive | -MBOAT2-ALK fusion -TP53 loss -MYCN copy gain | Diffuse Pediatric-type High-Grade Glioma, MYCN subtype | Diffuse Pediatric-type High-Grade Glioma, H3-wild-type and IDH-wild-type, CNS WHO Grade 4 | Radiotherapy with concurrent Temozolomide Targeted agent: Lorlatinib |
| Relapse | Similar to diagnostic sample, with olig2 loss | -MET copy gain -MBOAT2-ALK fusion -TP53 loss -MYCN copy gain | Diffuse Pediatric-type High-Grade Glioma, MYCN subtype | Diffuse Pediatric-type High-Grade Glioma, H3-wild-type and IDH-wild-type, CNS WHO Grade 4 | Target agents: Lorlatinib and Cabozantinib |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, D.; Jayappa, S.; Parker, L.; Ocal, E.; Tanaka, T.; Gokden, M.; Bielamowicz, K. Targeted Inhibition in Pediatric MET and ALK-Altered Hemispheric Gliomas: Objective Responses Followed by Treatment Resistance. Int. J. Mol. Sci. 2025, 26, 9864. https://doi.org/10.3390/ijms26209864
Wilson D, Jayappa S, Parker L, Ocal E, Tanaka T, Gokden M, Bielamowicz K. Targeted Inhibition in Pediatric MET and ALK-Altered Hemispheric Gliomas: Objective Responses Followed by Treatment Resistance. International Journal of Molecular Sciences. 2025; 26(20):9864. https://doi.org/10.3390/ijms26209864
Chicago/Turabian StyleWilson, David, Sateesh Jayappa, Lora Parker, Eylem Ocal, Tomoko Tanaka, Murat Gokden, and Kevin Bielamowicz. 2025. "Targeted Inhibition in Pediatric MET and ALK-Altered Hemispheric Gliomas: Objective Responses Followed by Treatment Resistance" International Journal of Molecular Sciences 26, no. 20: 9864. https://doi.org/10.3390/ijms26209864
APA StyleWilson, D., Jayappa, S., Parker, L., Ocal, E., Tanaka, T., Gokden, M., & Bielamowicz, K. (2025). Targeted Inhibition in Pediatric MET and ALK-Altered Hemispheric Gliomas: Objective Responses Followed by Treatment Resistance. International Journal of Molecular Sciences, 26(20), 9864. https://doi.org/10.3390/ijms26209864







