Pattern of Regulatory T Cells, Resident Memory T Cells, and Exhausted T Cells in Human Pericardial Fluid Samples of Cardiovascular Patients
Abstract
1. Introduction
2. Results
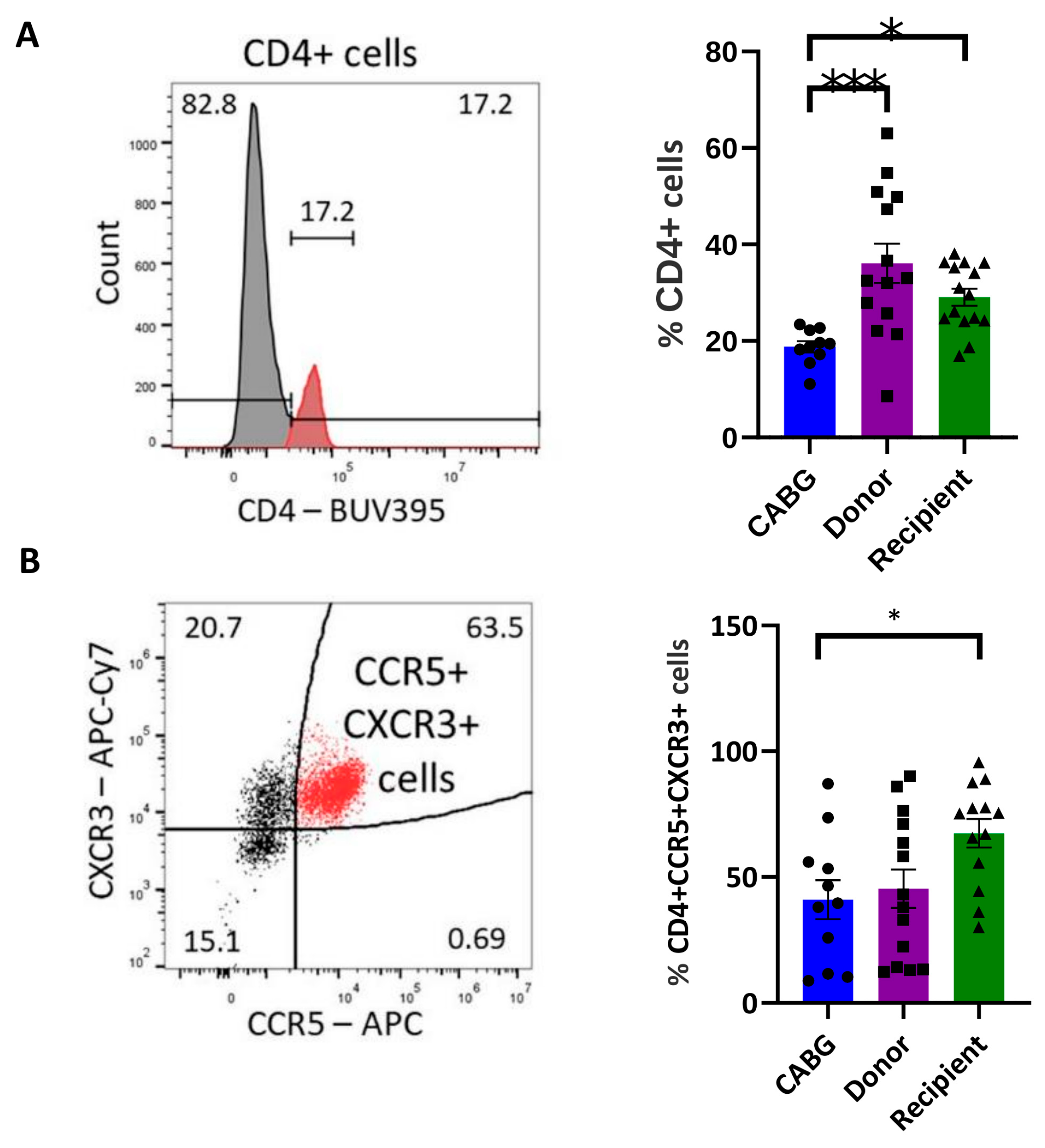
2.1. Enrichment of Inflammatory-Homing T Cells in Transplantation Recipients
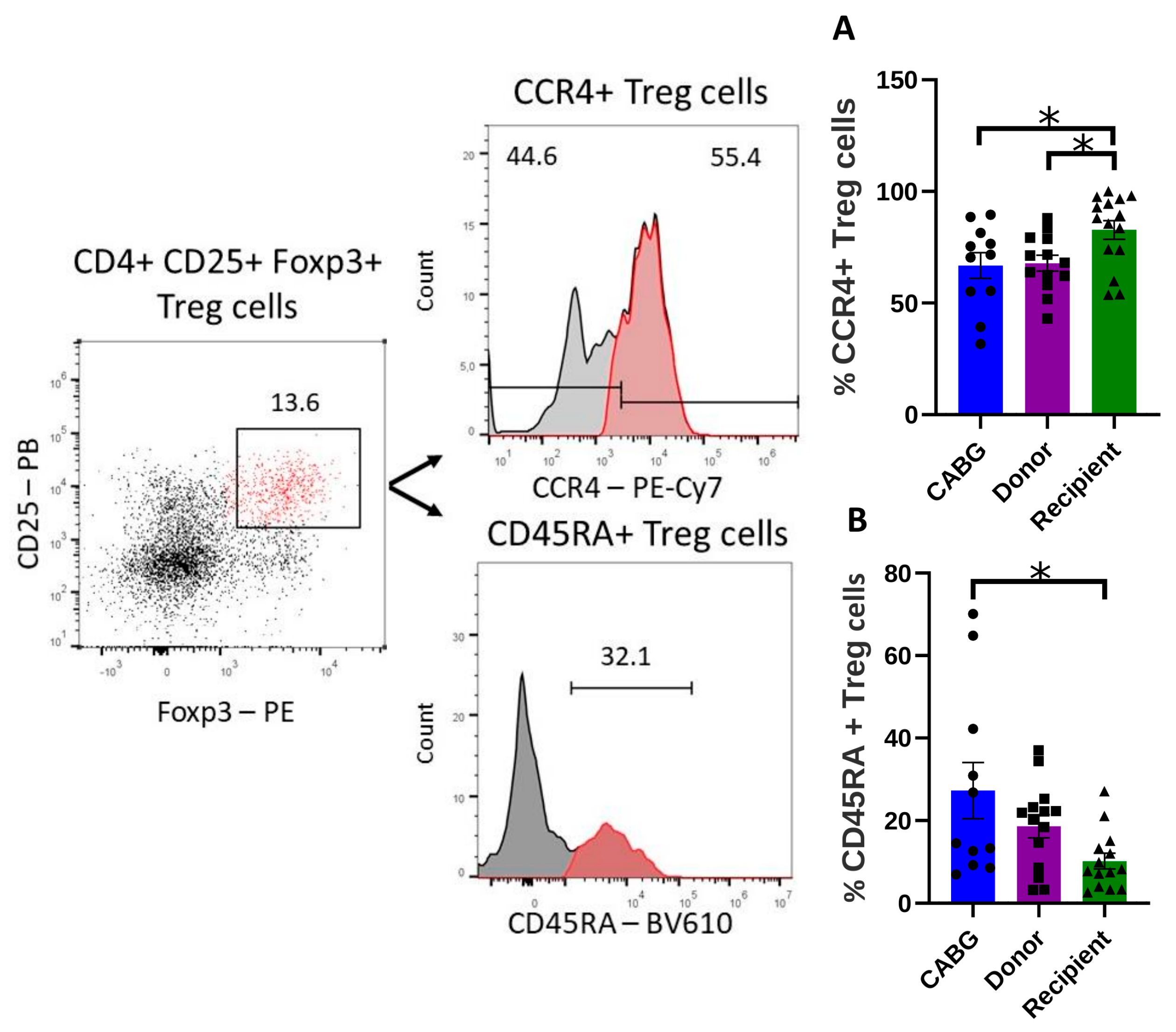
2.2. Increased Infiltration but Decreased Treg Function in Transplant Recipients
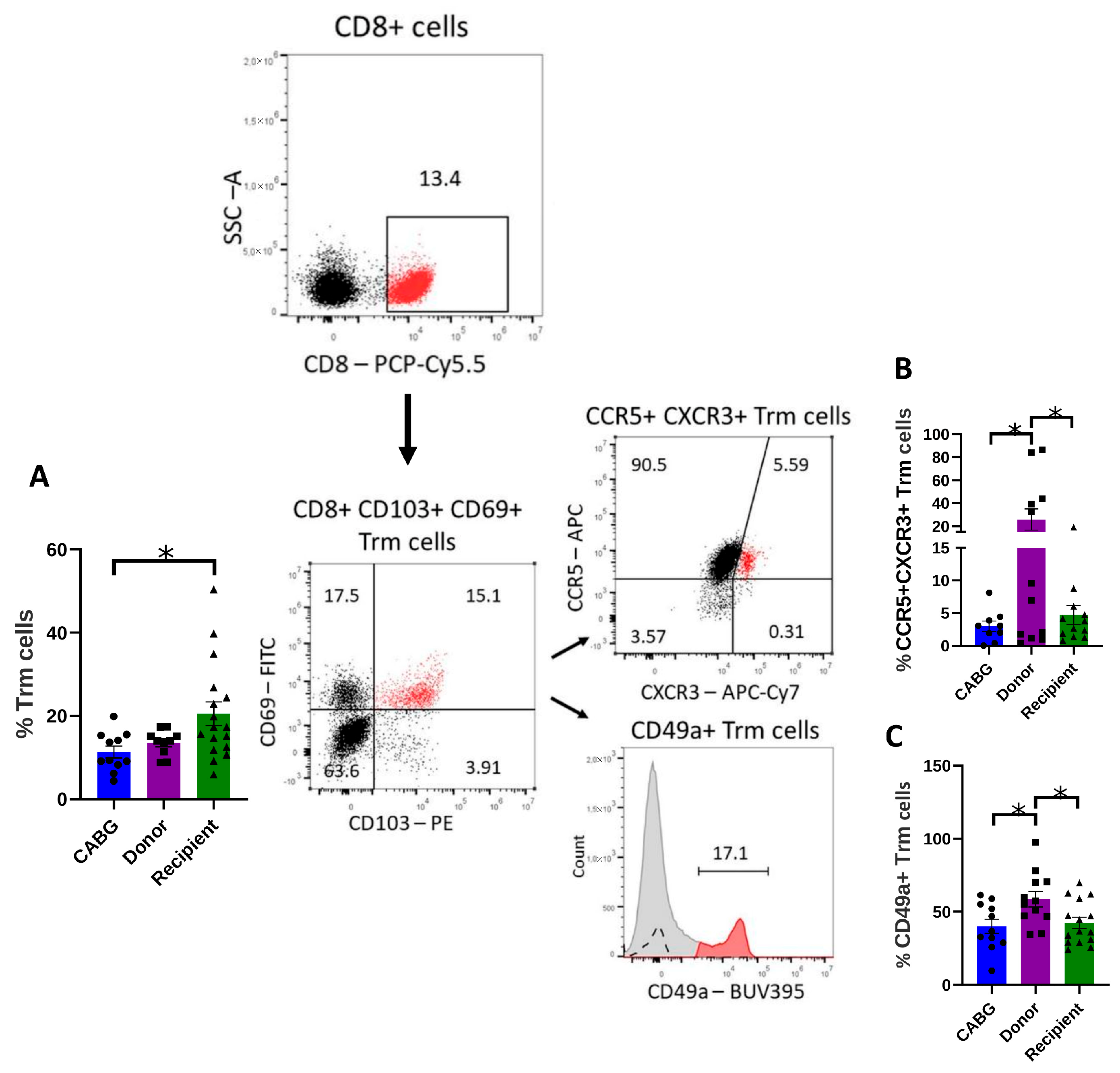
2.3. Tissue Resident Memory T Cells (Trms) Are Enriched in Patients with DCM, but Their Homing and Retention May Be Impaired in the Pericardial Fluid
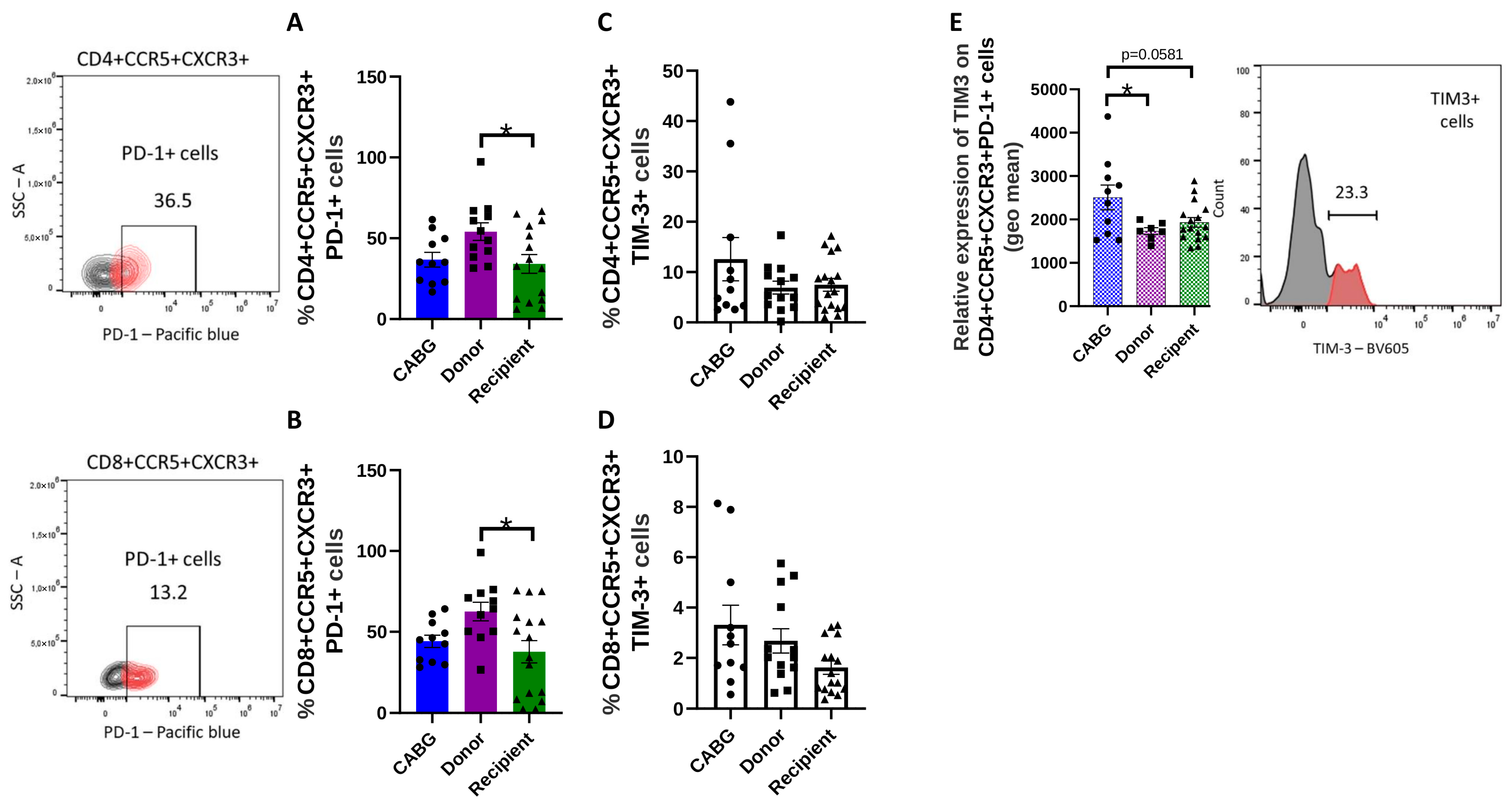
2.4. Reduced Number of Transiently Exhausted but Increased Number of Terminally Exhausted T Cells in the Cardiac Patients
2.5. Clinical Variables
3. Discussion
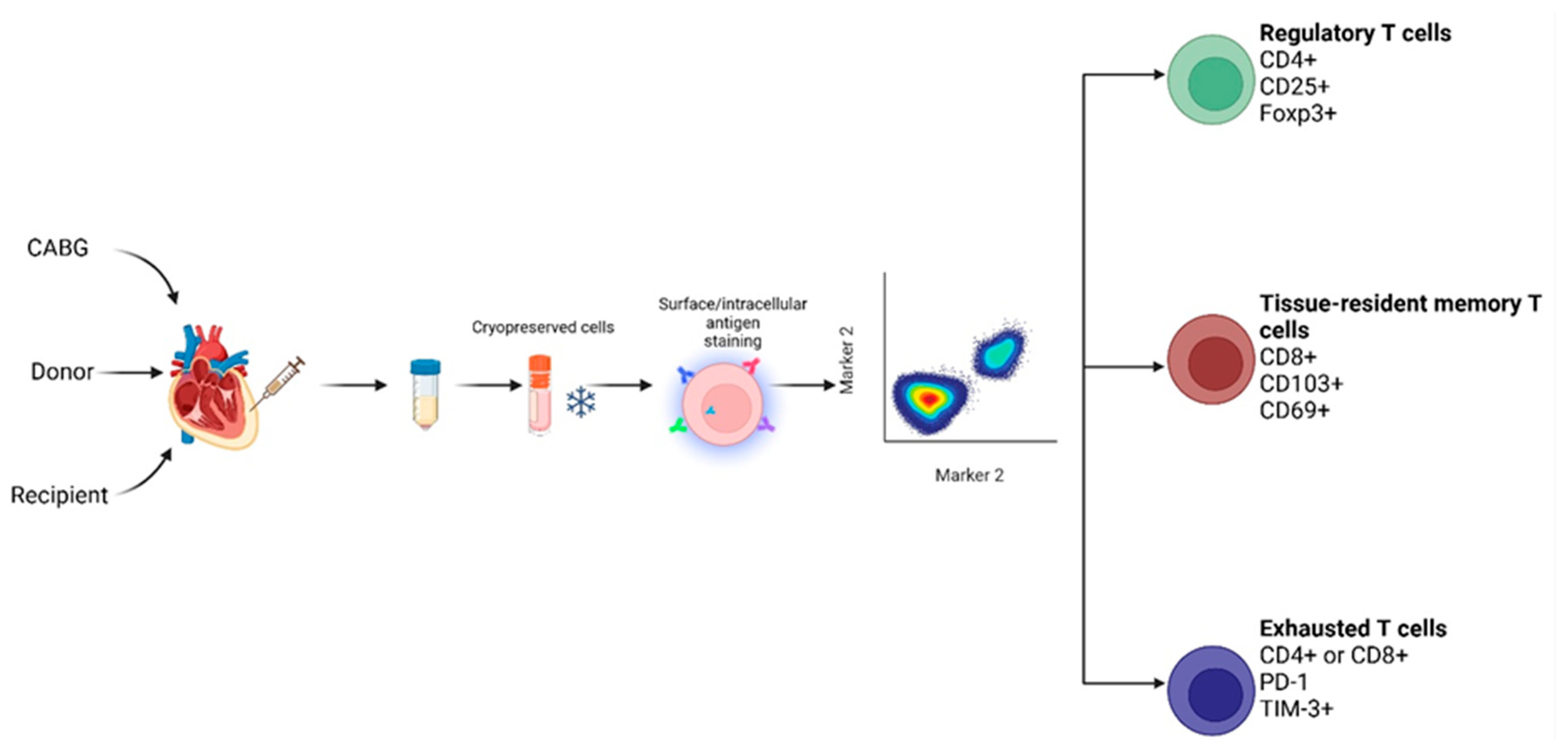
4. Materials and Methods
4.1. Study Design, Setting, and Participants
4.2. Variables and Definitions
4.3. Sample Collection and Preparation
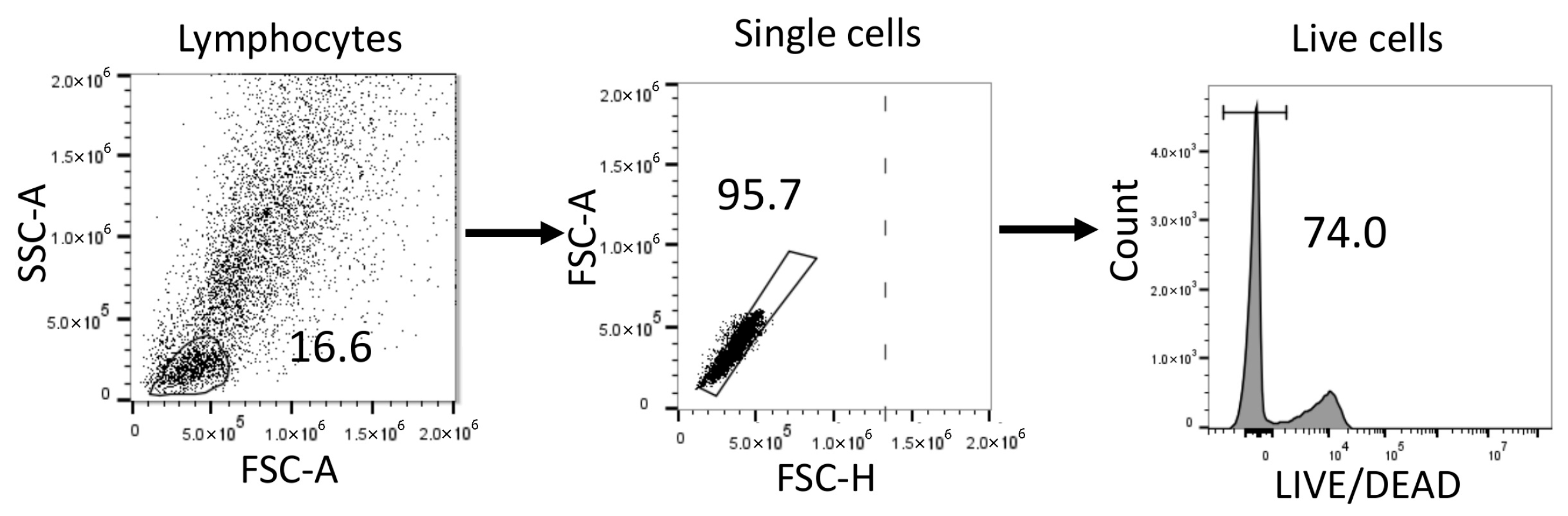
4.4. Flow Cytometry
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epelman, S.; Liu, P.P.; Mann, D.L. Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat. Rev. Immunol. 2015, 15, 117–129. [Google Scholar] [CrossRef]
- Weirather, J.; Hofmann, U.D.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef]
- Kino, T.; Khan, M.; Mohsin, S. The Regulatory Role of T Cell Responses in Cardiac Remodeling Following Myocardial Infarction. Int. J. Mol. Sci. 2020, 21, 5013. [Google Scholar] [CrossRef] [PubMed]
- Deniset, J.F.; Belke, D.; Lee, W.Y.; Jorch, S.K.; Deppermann, C.; Hassanabad, A.F.; Turnbull, J.D.; Teng, G.; Rozich, I.; Hudspeth, K.; et al. Gata6+ Pericardial Cavity Macrophages Relocate to the Injured Heart and Prevent Cardiac Fibrosis. Immunity 2019, 51, 131–140.e5. [Google Scholar] [CrossRef] [PubMed]
- Dobaczewski, M.; Xia, Y.; Bujak, M.; Gonzalez-Quesada, C.; Frangogiannis, N.G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am. J. Pathol. 2010, 176, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Frantz, S. Role of T-cells in myocardial infarction. Eur. Heart J. 2016, 37, 873–879. [Google Scholar] [CrossRef]
- Szentes, V.; Gazdag, M.; Szokodi, I.; Dezsi, C.A. The Role of CXCR3 and Associated Chemokines in the Development of Atherosclerosis and During Myocardial Infarction. Front. Immunol. 2018, 9, 1932. [Google Scholar] [CrossRef]
- Macias, C.; Villaescusa, R.; del Valle, L.; Boffil, V.; Cordero, G.; Hernandez, A.; Hernandez, P.; Ballester, J.M. Endothelial adhesion molecules ICAM-1, VCAM-1 and E-selectin in patients with acute coronary syndrome. Rev. Esp. Cardiol. 2003, 56, 137–144. [Google Scholar] [CrossRef]
- Reiss, Y.; Engelhardt, B. T cell interaction with ICAM-1-deficient endothelium in vitro: Transendothelial migration of different T cell populations is mediated by endothelial ICAM-1 and ICAM-2. Int. Immunol. 1999, 11, 1527–1539. [Google Scholar] [CrossRef][Green Version]
- Carman, C.V.; Martinelli, R. T Lymphocyte-Endothelial Interactions: Emerging Understanding of Trafficking and Antigen-Specific Immunity. Front. Immunol. 2015, 6, 603. [Google Scholar] [CrossRef]
- Walling, B.L.; Kim, M. LFA-1 in T Cell Migration and Differentiation. Front. Immunol. 2018, 9, 952. [Google Scholar] [CrossRef]
- Arimura, T.; Hayashi, T.; Kimura, A. Molecular etiology of idiopathic cardiomyopathy. Acta Myol. 2007, 26, 153–158. [Google Scholar]
- Ferreira, A.; Ferreira, V.; Antunes, M.M.; Lousinha, A.; Pereira-da-Silva, T.; Antunes, D.; Cunha, P.S.; Oliveira, M.; Ferreira, R.C.; Rosa, S.A. Dilated Cardiomyopathy: A Comprehensive Approach to Diagnosis and Risk Stratification. Biomedicines 2023, 11, 834. [Google Scholar] [CrossRef]
- Caforio, A.L.; Mahon, N.G.; Baig, M.K.; Tona, F.; Murphy, R.T.; Elliott, P.M.; McKenna, W.J. Prospective familial assessment in dilated cardiomyopathy: Cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation 2007, 115, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Johnson, R.; Birch, S.; Zentner, D.; Hershberger, R.E.; Fatkin, D. Familial Dilated Cardiomyopathy. Heart Lung Circ. 2020, 29, 566–574. [Google Scholar] [CrossRef]
- Kearney, M.T.; Cotton, J.M.; Richardson, P.J.; Shah, A.M. Viral myocarditis and dilated cardiomyopathy: Mechanisms, manifestations, and management. Postgrad. Med. J. 2001, 77, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhou, R.; Li, T.; Hua, Y.; Zhou, K.; Li, Y.; Luo, S.; An, Q. The Molecular Role of Immune Cells in Dilated Cardiomyopathy. Medicina 2023, 59, 1246. [Google Scholar] [CrossRef]
- Horn, S.; Lueking, A.; Murphy, D.; Staudt, A.; Gutjahr, C.; Schulte, K.; Konig, A.; Landsberger, M.; Lehrach, H.; Felix, S.B.; et al. Profiling humoral autoimmune repertoire of dilated cardiomyopathy (DCM) patients and development of a disease-associated protein chip. Proteomics 2006, 6, 605–613. [Google Scholar] [CrossRef][Green Version]
- Nussinovitch, U.; Shoenfeld, Y. The clinical and diagnostic significance of anti-myosin autoantibodies in cardiac disease. Clin. Rev. Allergy Immunol. 2013, 44, 98–108. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wei, J.; Zheng, H.; Zhou, N.; Xu, X.; Deng, X.; Liu, T.; Zou, Y. The immune system in cardiovascular diseases: From basic mechanisms to therapeutic implications. Signal Transduct. Target. Ther. 2025, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Li, H.; Chen, C.; Wang, D.W. Inflammatory Cytokines, Immune Cells, and Organ Interactions in Heart Failure. Front. Physiol. 2021, 12, 695047. [Google Scholar] [CrossRef]
- Fatehi Hassanabad, A.; Fedak, P.W.M.; Deniset, J.F. Acute Ischemia Alters Human Pericardial Fluid Immune Cell Composition. JACC Basic Transl. Sci. 2021, 6, 765–767. [Google Scholar] [CrossRef]
- Isidoro, C.A.; Deniset, J.F. Pericardial Immune Cells and Their Evolving Role in Cardiovascular Pathophysiology. Can. J. Cardiol. 2023, 39, 1078–1089. [Google Scholar] [CrossRef]
- Steffens, S.; Nahrendorf, M.; Madonna, R. Immune cells in cardiac homeostasis and disease: Emerging insights from novel technologies. Eur. Heart J. 2022, 43, 1533–1541. [Google Scholar] [CrossRef]
- Noutsias, M.; Rohde, M.; Goldner, K.; Block, A.; Blunert, K.; Hemaidan, L.; Hummel, M.; Blohm, J.H.; Lassner, D.; Kuhl, U.; et al. Expression of functional T-cell markers and T-cell receptor Vbeta repertoire in endomyocardial biopsies from patients presenting with acute myocarditis and dilated cardiomyopathy. Eur. J. Heart Fail. 2011, 13, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.T.; Ding, Y.J.; Liao, Y.H.; Yu, X.; Xiao, H.; Xie, J.J.; Yuan, J.; Zhou, Z.H.; Liao, M.Y.; Yao, R.; et al. Defective circulating CD4+CD25+Foxp3+CD127low regulatory T-cells in patients with chronic heart failure. Cell Physiol. Biochem. 2010, 25, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, W.; Xiong, S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS ONE 2013, 8, e74955. [Google Scholar] [CrossRef]
- Zou, S.; Liu, B.; Feng, Y. CCL17, CCL22 and their receptor CCR4 in hematologic malignancies. Discov. Oncol. 2024, 15, 412. [Google Scholar] [CrossRef]
- Feng, G.; Bajpai, G.; Ma, P.; Koenig, A.; Bredemeyer, A.; Lokshina, I.; Lai, L.; Forster, I.; Leuschner, F.; Kreisel, D.; et al. CCL17 Aggravates Myocardial Injury by Suppressing Recruitment of Regulatory T Cells. Circulation 2022, 145, 765–782. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Liu, S.; Lv, Y.; Zhang, R.; Zhou, X.; Zhang, Y.; Weng, S.; Xu, H.; Ba, Y.; et al. Infiltrating treg reprogramming in the tumor immune microenvironment and its optimization for immunotherapy. Biomark. Res. 2024, 12, 97. [Google Scholar] [CrossRef]
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950. [Google Scholar] [CrossRef]
- Booth, N.J.; McQuaid, A.J.; Sobande, T.; Kissane, S.; Agius, E.; Jackson, S.E.; Salmon, M.; Falciani, F.; Yong, K.; Rustin, M.H.; et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J. Immunol. 2010, 184, 4317–4326. [Google Scholar] [CrossRef] [PubMed]
- Valmori, D.; Merlo, A.; Souleimanian, N.E.; Hesdorffer, C.S.; Ayyoub, M. A peripheral circulating compartment of natural naive CD4 Tregs. J. Clin. Investig. 2005, 115, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N.; Mackay, L.K. Tissue-resident memory T cells: Local specialists in immune defence. Nat. Rev. Immunol. 2016, 16, 79–89. [Google Scholar] [CrossRef]
- Szabo, P.A.; Miron, M.; Farber, D.L. Location, location, location: Tissue resident memory T cells in mice and humans. Sci. Immunol. 2019, 4, eaas9673. [Google Scholar] [CrossRef]
- Vyas, V.; Sandhar, B.; Keane, J.M.; Wood, E.G.; Blythe, H.; Jones, A.; Shahaj, E.; Fanti, S.; Williams, J.; Metic, N.; et al. Tissue-resident memory T cells in epicardial adipose tissue comprise transcriptionally distinct subsets that are modulated in atrial fibrillation. Nat. Cardiovasc. Res. 2024, 3, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Kalinoski, H.; Daoud, A.; Rusinkevich, V.; Jurcova, I.; Talor, M.V.; Welsh, R.A.; Hughes, D.; Zemanova, K.; Striz, I.; Hooper, J.E.; et al. Injury-induced myosin-specific tissue-resident memory T cells drive immune checkpoint inhibitor myocarditis. Proc. Natl. Acad. Sci. USA 2024, 121, e2323052121. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, S.; Schlums, H.; Gallais Serezal, I.; Martini, E.; Chiang, S.C.; Marquardt, N.; Gibbs, A.; Detlofsson, E.; Introini, A.; Forkel, M.; et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity 2017, 46, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Reilly, E.C.; Lambert Emo, K.; Buckley, P.M.; Reilly, N.S.; Smith, I.; Chaves, F.A.; Yang, H.; Oakes, P.W.; Topham, D.J. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. USA 2020, 117, 12306–12314. [Google Scholar] [CrossRef]
- de Jong, M.J.M.; Depuydt, M.A.C.; Schaftenaar, F.H.; Liu, K.; Maters, D.; Wezel, A.; Smeets, H.J.; Kuiper, J.; Bot, I.; van Gisbergen, K.; et al. Resident Memory T Cells in the Atherosclerotic Lesion Associate with Reduced Macrophage Content and Increased Lesion Stability. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1318–1329. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Sun, B.; Xun, Z.; Zhou, Z.; Zhang, N.; Piao, M.; Li, C.; Li, J.; Li, S.; Zhang, L.; Chen, X.; et al. Single-cell transcriptomic analysis deciphers the inflammatory microenvironment characterized by CXCL9+ fibroblasts and ACKR1+ endothelial cells in immune-related myocarditis. J. Transl. Med. 2025, 23, 555. [Google Scholar] [CrossRef]
- Jin, H.T.; Anderson, A.C.; Tan, W.G.; West, E.E.; Ha, S.J.; Araki, K.; Freeman, G.J.; Kuchroo, V.K.; Ahmed, R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. USA 2010, 107, 14733–14738. [Google Scholar] [CrossRef]
- Inston, N.; Drayson, M.; Ready, A.; Cockwell, P. Serial changes in the expression of CXCR3 and CCR5 on peripheral blood lymphocytes following human renal transplantation. Exp. Clin. Transplant. 2007, 5, 638–642. [Google Scholar]
- Melter, M.; Exeni, A.; Reinders, M.E.; Fang, J.C.; McMahon, G.; Ganz, P.; Hancock, W.W.; Briscoe, D.M. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation 2001, 104, 2558–2564. [Google Scholar] [CrossRef]
- Altara, R.; Mallat, Z.; Booz, G.W.; Zouein, F.A. The CXCL10/CXCR3 Axis and Cardiac Inflammation: Implications for Immunotherapy to Treat Infectious and Noninfectious Diseases of the Heart. J. Immunol. Res. 2016, 2016, 4396368. [Google Scholar] [CrossRef]
- Iskandar, R.; Liu, S.; Xiang, F.; Chen, W.; Li, L.; Qin, W.; Huang, F.; Chen, X. Expression of pericardial fluid T-cells and related inflammatory cytokines in patients with chronic heart failure. Exp. Ther. Med. 2017, 13, 1850–1858. [Google Scholar] [CrossRef]
- Alexander, M.R.; Dale, B.L.; Smart, C.D.; Elijovich, F.; Wogsland, C.E.; Lima, S.M.; Irish, J.M.; Madhur, M.S. Immune Profiling Reveals Decreases in Circulating Regulatory and Exhausted T Cells in Human Hypertension. JACC Basic Transl. Sci. 2023, 8, 319–336. [Google Scholar] [CrossRef]
- Hudson, W.H.; Gensheimer, J.; Hashimoto, M.; Wieland, A.; Valanparambil, R.M.; Li, P.; Lin, J.X.; Konieczny, B.T.; Im, S.J.; Freeman, G.J.; et al. Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1+ Stem-like CD8+ T Cells during Chronic Infection. Immunity 2019, 51, 1043–1058.e4. [Google Scholar] [CrossRef]
- Fender, E.A.; Zack, C.J. Shining a new light on pericardial fluid. Heart 2021, 107, 1528–1529. [Google Scholar] [CrossRef]
- Kobashigawa, J.; Khush, K.; Colvin, M.; Acker, M.; Van Bakel, A.; Eisen, H.; Naka, Y.; Patel, J.; Baran, D.A.; Daun, T.; et al. Report from the American Society of Transplantation Conference on Donor Heart Selection in Adult Cardiac Transplantation in the United States. Am. J. Transplant. 2017, 17, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, R.J.; Wong, N.; Liu, D.H.; Chua, C.; William, J.; Tee, S.L.; Sata, Y.; Bergin, P.; Hare, J.; Leet, A.; et al. Vasoplegia Following Orthotopic Heart Transplantation: Prevalence, Predictors and Clinical Outcomes. J. Card. Fail. 2022, 28, 617–626. [Google Scholar] [CrossRef]
- Kilic, A.; Allen, J.G.; Weiss, E.S. Validation of the United States-derived Index for Mortality Prediction After Cardiac Transplantation (IMPACT) using international registry data. J. Heart Lung Transpl. 2013, 32, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, J.R.; Cheng, A.; Ising, M.; Lenneman, A.; Birks, E.; Slaughter, M.S. Heart Transplant Survival Based on Recipient and Donor Risk Scoring: A UNOS Database Analysis. Asaio J. 2016, 62, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Sharples, L.D.; Nilsson, J.; Smith, C.; Goldstone, A.R.; Lockowandt, U. EuroSCORE II. Eur. J. Cardio-Thorac. Surg. 2012, 41, 734–744. [Google Scholar] [CrossRef]
- Lucien, C. Letter: Grading of angina pectoris. Circulation 1976, 54, 522–523. [Google Scholar] [CrossRef]
| Recipient Characteristics | CABG Patients Characteristics | ||
|---|---|---|---|
| Age (year) | 51.00 (30.00–58.50) | 69.00 (63.00–74.00) | |
| BMI (kg/m2) | 24.90 (21.00–25.79) | - | |
| Sex (Female/Male) | 5 (29.41%)/12 (70.59%) | 3 (27.27%)/8 (72.73%) | |
| Comorbidities | Hypertonia: 7 (42.18%) DM: 2 (11.76%) Hypothyreosis: 3 (17.65%) | Hyperlipidemia: 3 (27.27%) DM: 5 (45.45%) Hypothyreosis: 3 (27.27%) | |
| Etiology | DCM | Non-DCM | Ischemic Heart Disease |
| Familial: 3 (17.65%) Viral: 1 (5.88%) Ischemic: 2 (11.76%) Non-ischemic: 7 (42.18%) | Restrictive: 1 (5.88%) Ischemic: 1 (5.88%) HCM: 2 (11.76%) | 11 (100%) | |
| Scores | UNOS recipient score | EuroSCORE | |
| Very low risk: 2 (11.76%) Low risk: 8 (47.06%) Intermediate risk: 2 (11.76%) High risk: 2 (11.76%) Very high risk: 3 (17.65%) | 2: 2 (18.18%) 4: 1 (9.09%) 5: 1 (9.09%) 6: 3 (27.27%) | ||
| IMPACT | |||
| 4.00 (1.25–5.00) | |||
| Classification | NYHA | CCS | |
| II: 4 (23.53%) III: 3 (17.65%) IV: 10 (58.82%) | I: 1 (9.09%) II: 2 (18.18%) III: 2 (18.18%) IV: 2 (18.18%) | ||
| Pre-op. laboratory values | |||
| CRP (mg/L) CK (IU/L) BUN (mmol/L) Creatinine (µmol/L) GFR (mL/min/1.73m2) AST (UI/L) ALT (UI/L) GGT (UI/L) ALP (UI/L) LDH (UI/L) Total Bilirubin (µmol/L) Total Protein (g/L) Albumin (g/L) Cholesterol (mmol/L) Triglycerides (mmol/L) HDL (mmol/L) LDL (mmol/L) PLT (G/L) Haemoglobin (g/L) WBC (G/L) Neutrophil % Neutrophil Count (109/L) Lymphocyte % Lymphocyte Count (109/L) Eosinophil % Eosinophil (109/L) Monocyte % Monocyte Count (109/L) Basophil % Basophil Count (109/L) | 2.35 (1.10–8.31) 115.50 (59.50–200.25) 9.90 (5.50–12.80) 114.00 (84.25–155.50) 79.89 (48.11–90.00) 23.00 (18.00–32.00) 29.00 (13.00–34.00) 81.00 (37.00–160.00) 77.00 (60.00–131.00) 417.50 (330.00–506.75) 11.00 (6.55–18.78) 71.50 (65.60–77.60) 46.50 (40.30–49.20) 3.80 (3.50–4.80) 1.18 (0.94–2.32) 1.36 (0.95–1.43) 2.92 (2.21–3.71) 206.00 (144.80–240.00) 130.50 (94.00–137.75) 8.74 (7.40–9.99) 73.00 (68.05–77.20) 5.76 (5.04–6.92) 16.10 (13.75–22.10) 1.25 (0.96–1.62) 1.20 (0.63–2.10) 0.11 (0.06–0.15) 7.85 (7.15–9.68) 0.72 (0.50–0.86) 0.35 (0.20–0.78) 0.03 (0.02–0.06) | 2.31 (0.50–4.85) 94.50 (48.75–197.25) 6.50 (4.45–8.20) 70.50 (67.00–95.75) 88.60 (75.90–90.00) 21.00 (19.00–28.75) 31.00 (14.25–39.25) 27.50 (14.25–47.75) 81.00 (63.50–89.00) 316.00 (225.25–366.75) 9.20 (6.70–12.38) 69.60 (68.00–76.20) – 3.85 (3.08–4.83) 1.24 (0.93–1.69) 1.22 (0.95–1.44) 2.04 (1.57–3.16) 254.50 (176.75–279.75) 148.00 (136.00–158.00) 7.05 (6.24–7.56) 60.75 (56.75–68.10) 4.17 (3.94–4.62) 26.85 (21.68–30.98) 1.83 (1.55–2.16) 2.35 (1.85–4.83) 0.17 (0.11–0.34) 7.75 (6.80–8.35) 0.54 (0.41–0.60) 0.50 (0.50–0.68) 0.03 (0.03–0.05) | |
| Preoperative echocardiography parameters and hemodynamic measurement | Echocardiography parameters | Echocardiography parameters | |
| EF (%): 22.50 (20.00–25.75) | EF (%): 56.5 (43.75–59.75) LVEDD (mm): 50.0 (42.0–50.0) LVESD (mm): 35.0 (28.0–38.0) LVPWd (mm): 10.0 (9.00–11.0) AscAoD (mm): 30.5 (29.0–32.0) IVSd (mm): 11.0 (9.0–14.0) Systolic AoD (mm): 21.0 (17.0–22.0) AoVmax (m/s): 1.30 (1.05–1.43) TAPSE (mm): 22.0 (20.0–24.0) | ||
| Hemodynamic measurement | |||
| PVR (Wood Units): 2.40 (1.76–2.85) sPAP (mmHg): 45.00 (39.00–55.50) dPAP (mmHg): 22.00 (16.50–25.50) mPAP (mmHg): 33.00 (25.00–37.00) PAWP (mmHg): 22.00 (17.00–24.50) | |||
| Perioperative complications | BTT: 3 (17.65%) Post-op MCS: 2 (11.8%) 1 year mortality: 0 (0%) 2 year mortality: 2 (11.8%) 5 year mortality: 2 (11.8%) | Vasoplegia: 4 (23.5%) PGD: 3 (17.6%) Rejection: 1 (5.9%) Reoperation: 1 (5.9%) Retransplant.: 0 (0%) | - |
| Postoperative Inotropic Support | Max-IS: 37.50 (16.10–66.38) Max-VIS: 41.00 (29.40–72.33) Norepinephrine equivalence: 0.31 (0.20–0.66) | - | |
| Donor Characteristics | |
|---|---|
| Age (year) | 39.50 (31.50–47.50) |
| BMI (kg/m2) | 26.40 (22.48–29.65) |
| Sex (Female/Male) | 2 (14.29%)/12 (85.71%) |
| Cause of death | Intracerebral hemorrhage: 7 (50.00%) Traumatic subdural hemorrhage: 1 (7.14%) Traumatic subarachnoid hemorrhage: 1 (7.14%) Diffuse brain injury: 4 (28.57%) Anoxia: 1 (7.14%) |
| UNOS donor risk score | Low risk: 6 (42.86%) Intermediate risk: 8 (57.14%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Érsek, B.; Opra, J.; Fekete, N.; Ifju, M.; Molnár, V.; Bugyik, E.; Pállinger, É.; Székely, A.; Radovits, T.; Merkely, B.; et al. Pattern of Regulatory T Cells, Resident Memory T Cells, and Exhausted T Cells in Human Pericardial Fluid Samples of Cardiovascular Patients. Int. J. Mol. Sci. 2025, 26, 9852. https://doi.org/10.3390/ijms26209852
Érsek B, Opra J, Fekete N, Ifju M, Molnár V, Bugyik E, Pállinger É, Székely A, Radovits T, Merkely B, et al. Pattern of Regulatory T Cells, Resident Memory T Cells, and Exhausted T Cells in Human Pericardial Fluid Samples of Cardiovascular Patients. International Journal of Molecular Sciences. 2025; 26(20):9852. https://doi.org/10.3390/ijms26209852
Chicago/Turabian StyleÉrsek, Barbara, Júlia Opra, Nóra Fekete, Mandula Ifju, Viktor Molnár, Edina Bugyik, Éva Pállinger, Andrea Székely, Tamás Radovits, Béla Merkely, and et al. 2025. "Pattern of Regulatory T Cells, Resident Memory T Cells, and Exhausted T Cells in Human Pericardial Fluid Samples of Cardiovascular Patients" International Journal of Molecular Sciences 26, no. 20: 9852. https://doi.org/10.3390/ijms26209852
APA StyleÉrsek, B., Opra, J., Fekete, N., Ifju, M., Molnár, V., Bugyik, E., Pállinger, É., Székely, A., Radovits, T., Merkely, B., & Buzás, E. I. (2025). Pattern of Regulatory T Cells, Resident Memory T Cells, and Exhausted T Cells in Human Pericardial Fluid Samples of Cardiovascular Patients. International Journal of Molecular Sciences, 26(20), 9852. https://doi.org/10.3390/ijms26209852







