Mutation of p53 Acetylation Protects Against Angiotensin-II-Induced Cardiac Dysfunction and Fibrosis
Abstract
1. Introduction
2. Results
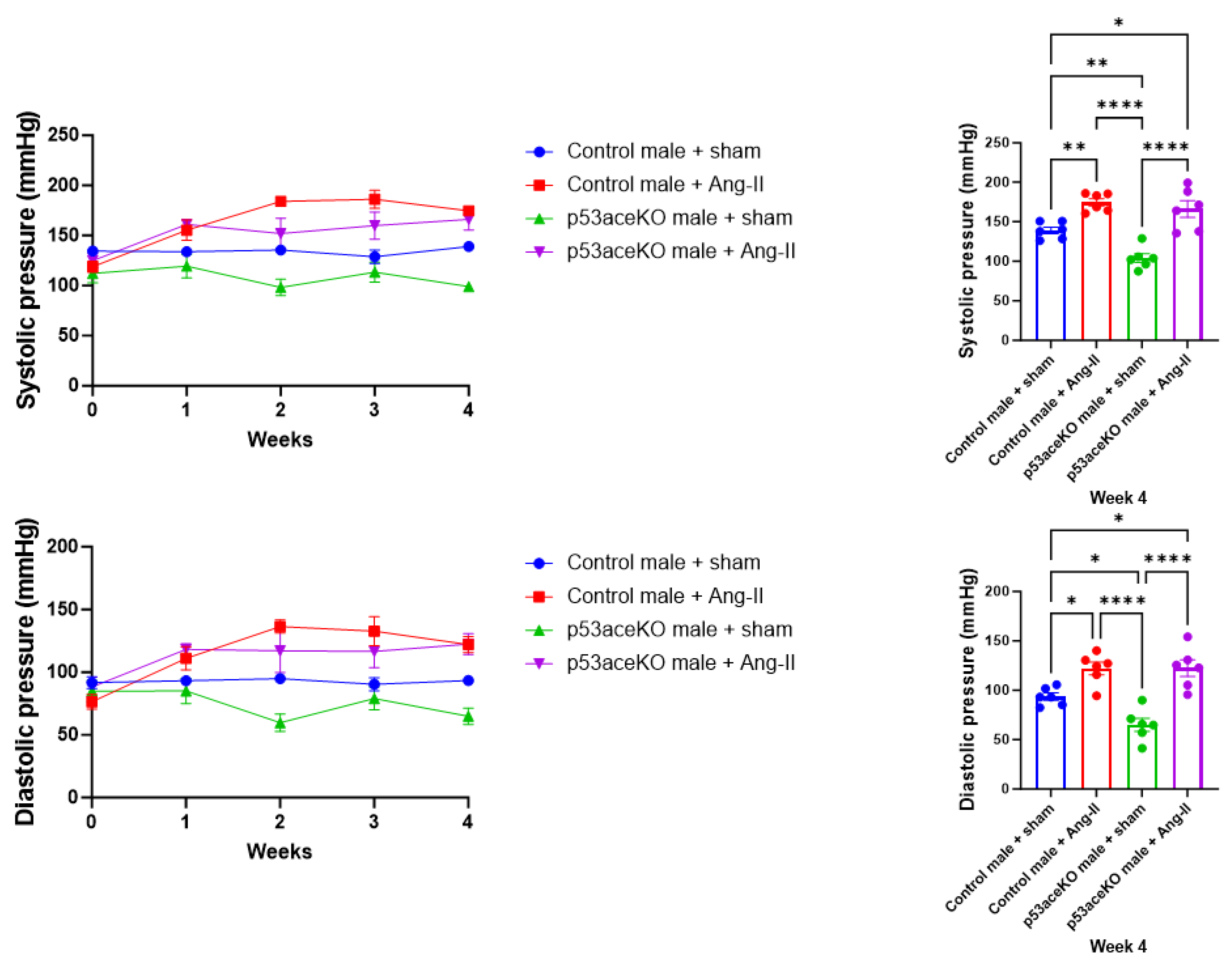
2.1. Both Control and p53aceKO Mice Exhibit Increased Blood Pressure Following Ang-II Infusion
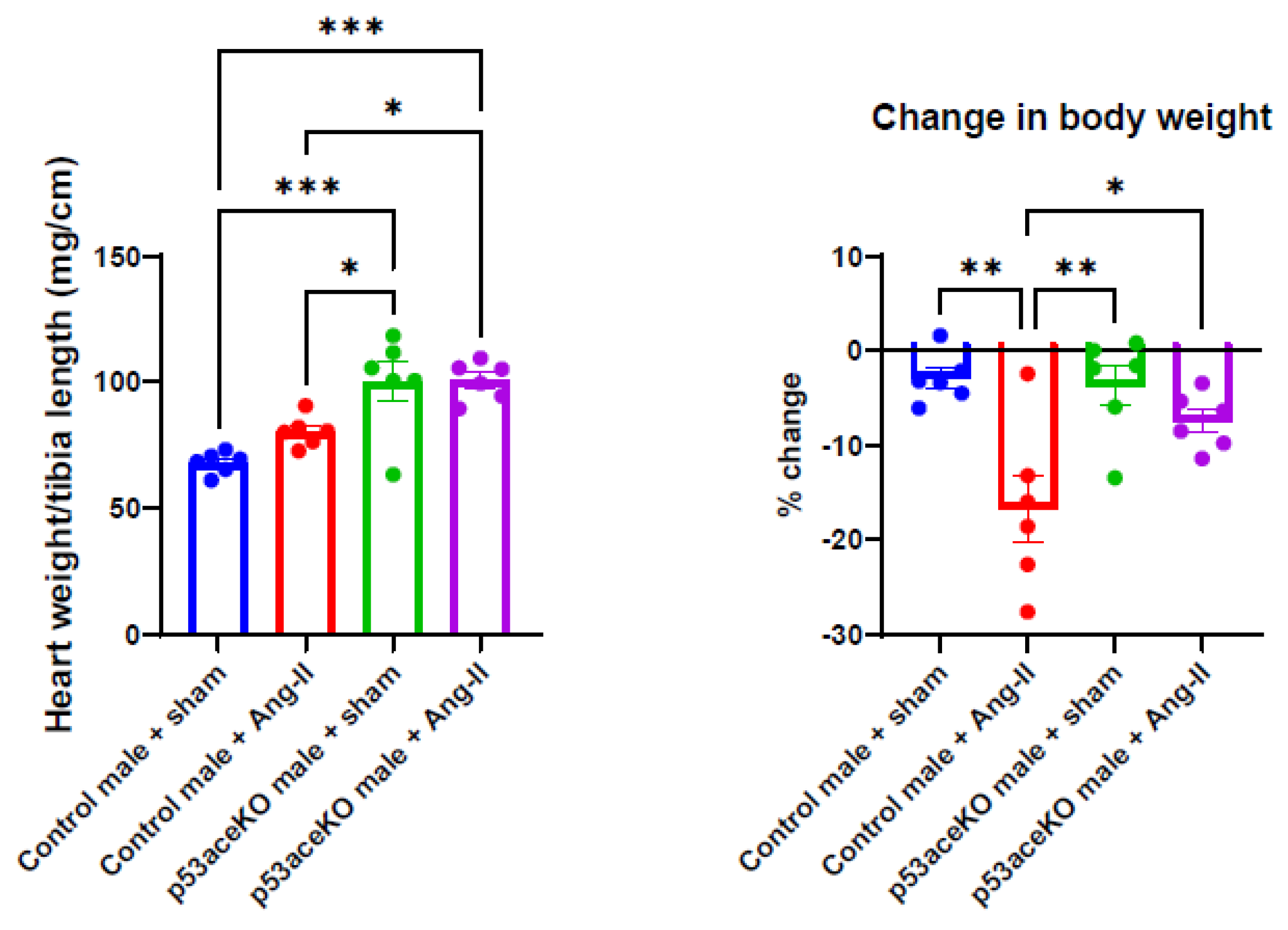
2.2. p53aceKO Mice Have a Higher Heart Weight-to-Tibia Length Ratio at Baseline and Lose Less Weight over the Course of Ang-II Infusion than Control Mice
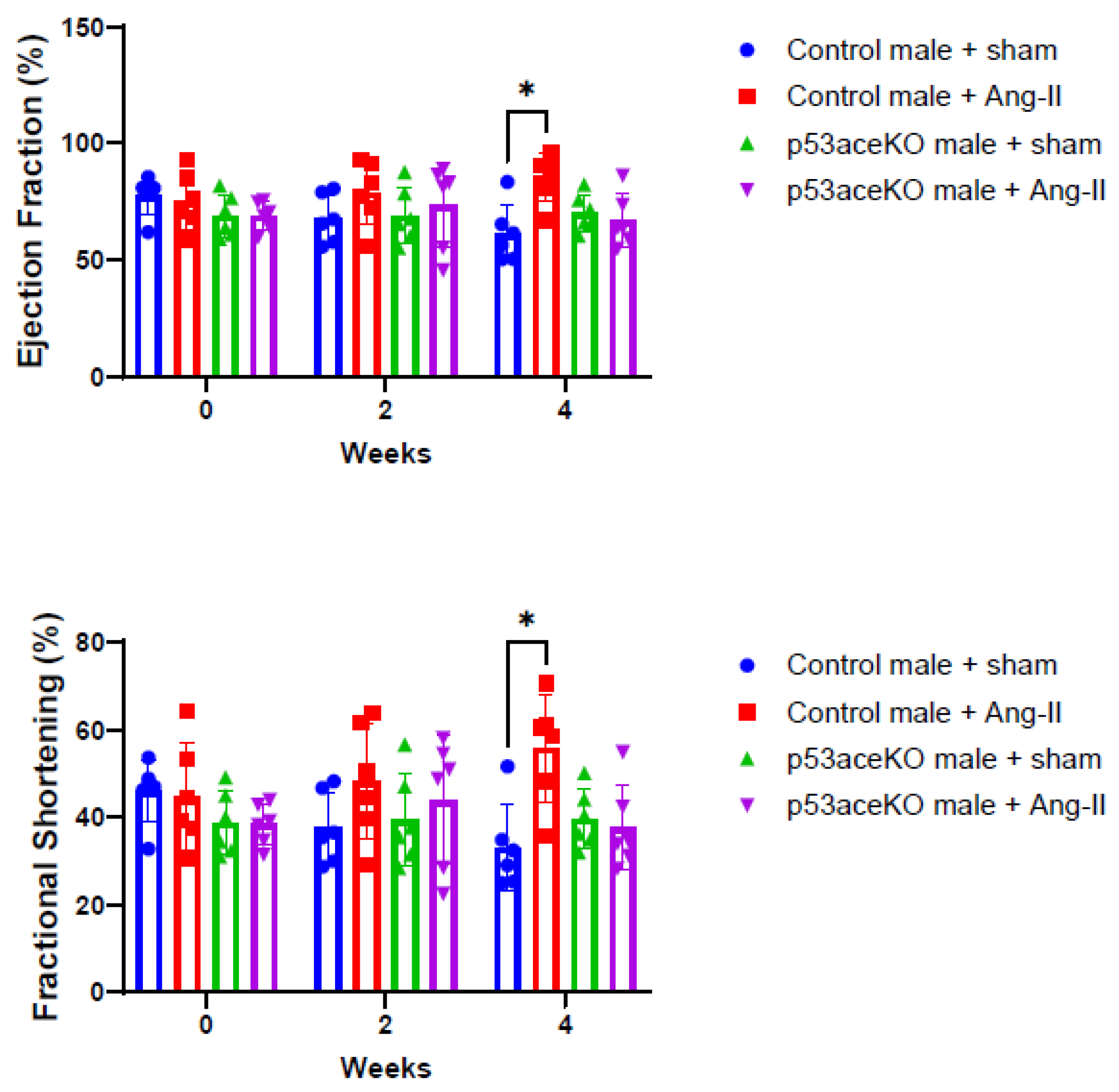
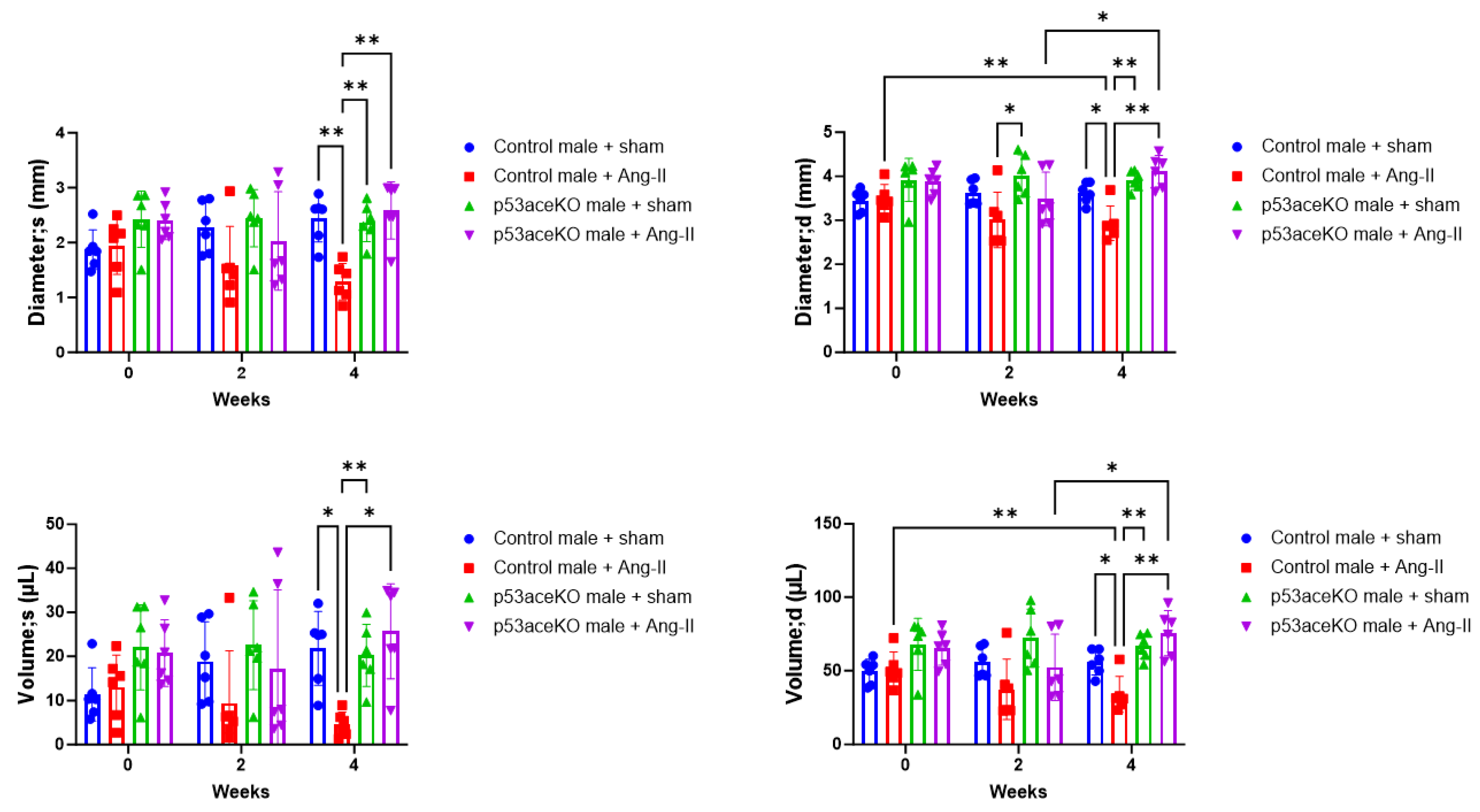
2.3. p53aceKO Mice Do Not Exhibit the Ang-II Infusion-Induced Alterations in Cardiac Function Seen in Control Mice
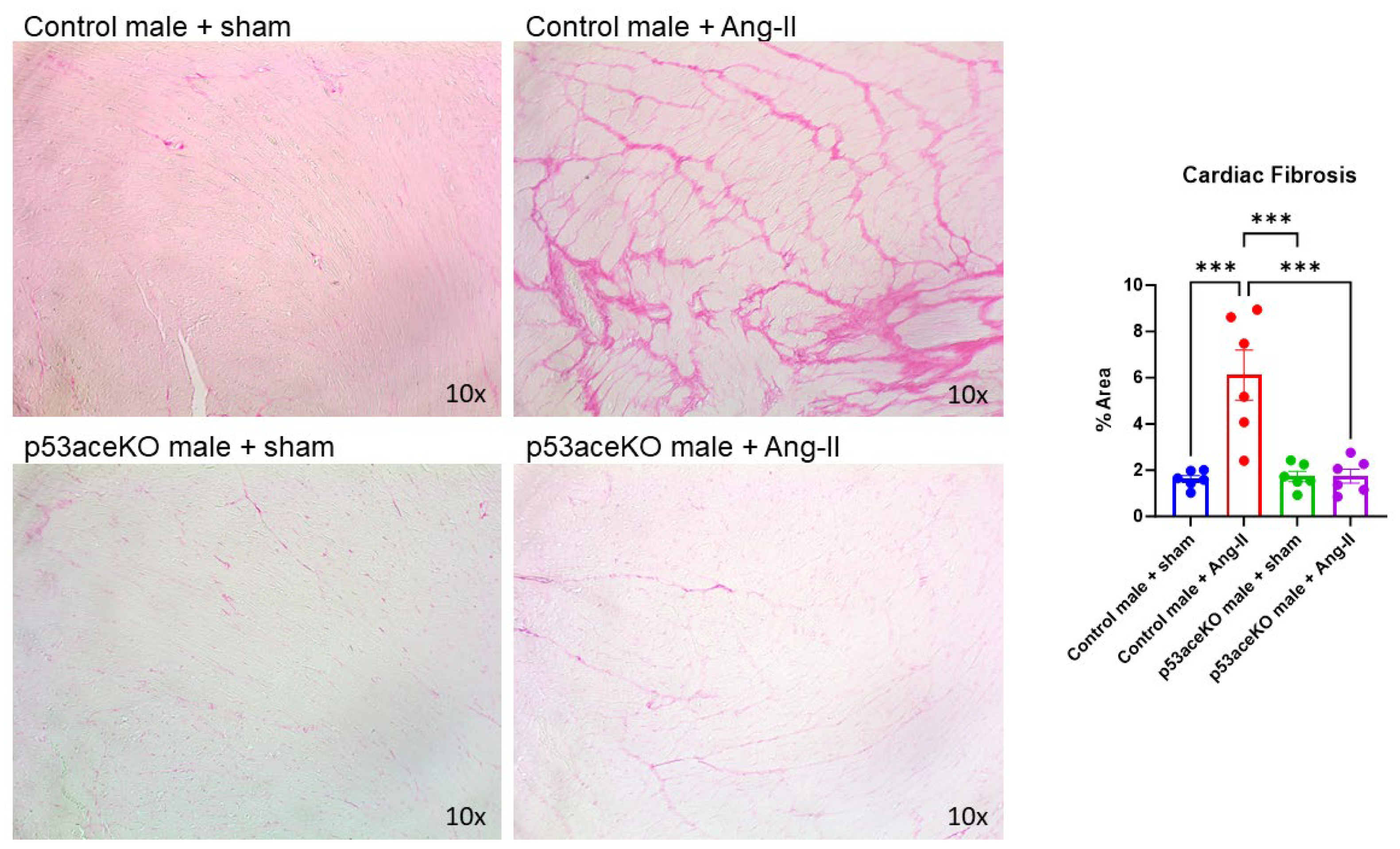
2.4. p53aceKO Mice Do Not Exhibit the Ang-II-Induced Cardiac Fibrosis Seen in Control Mice
3. Discussion
4. Methods
4.1. Experimental Animals
4.2. Micro-Osmotic Pump Implant or Sham Procedure
4.3. Blood Pressure Measurement
4.4. Transthoracic Echocardiography
4.5. Histology
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Larson, M.G.; Vasan, R.S.; Kannel, W.B.; Ho, K.K. The progression from hypertension to congestive heart failure. JAMA 1996, 275, 1557–1562. Available online: https://www.ncbi.nlm.nih.gov/pubmed/8622246 (accessed on 2 September 2025). [CrossRef] [PubMed]
- Zhu, Y.C.; Zhu, Y.Z.; Lu, N.; Wang, M.J.; Wang, Y.X.; Yao, T. Role of angiotensin AT1 and AT2 receptors in cardiac hypertrophy and cardiac remodelling. Clin. Exp. Pharmacol. Physiol. 2003, 30, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Do, Y.S.; Kawano, Y.; Starnes, V.; Barr, M.; Law, R.E.; Hsueh, W.A. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation 2000, 101, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Burke, R.M.; Lighthouse, J.K.; Baker, C.D.; Dirkx, R.A., Jr.; Kang, B.; Chakraborty, Y.; Mickelsen, D.M.; Twardowski, J.J.; Mello, S.S.; et al. p53 Regulates the Extent of Fibroblast Proliferation and Fibrosis in Left Ventricle Pressure Overload. Circ. Res. 2023, 133, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Liu, M.; Hu, L.; Guo, D.; Wang, D.; Qi, B.; Ren, G.; Hu, C.; Zhang, F.; Chun, H.J.; et al. LncRNA Airn alleviates diabetic cardiac fibrosis by inhibiting activation of cardiac fibroblasts via a m6A-IMP2-p53 axis. Biol. Direct 2022, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhou, L.; Meng, Y.; Yuan, H.; Geng, J. PKD knockdown mitigates Ang II-induced cardiac hypertrophy and ferroptosis via the JNK/P53 signaling pathway. Cell. Signal 2024, 113, 110974. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Li, D.; Ou, Y.; Jiang, L.; Chen, Y.; Zhao, Y.; Gu, W. Acetylation Is Crucial for p53-Mediated Ferroptosis and Tumor Suppression. Cell Rep. 2016, 17, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Xia, Y.; Li, L.; Li, B.; Chen, L.; Yu, W.; Ruan, Y.; Rao, T.; Zhou, X.; Cheng, F. p53 deacetylation alleviates calcium oxalate deposition-induced renal fibrosis by inhibiting ferroptosis. Biomed. Pharmacother. 2023, 164, 114925. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Cantrell, A.C.; Chen, J.X.; Gu, W.; Zeng, H. SIRT3 Deficiency Enhances Ferroptosis and Promotes Cardiac Fibrosis via p53 Acetylation. Cells 2023, 12, 1428. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Cantrell, A.C.; Williams, Q.A.; Gu, W.; Chen, Y.; Chen, J.X.; Zeng, H. p53 Acetylation Exerts Critical Roles in Pressure Overload-Induced Coronary Microvascular Dysfunction and Heart Failure in Mice. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Johnson, A.K.; Hay, M. Sex differences in angiotensin II- induced hypertension. Braz. J. Med. Biol. Res. 2007, 40, 727–734. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Williams, Q.A.; Cantrell, A.C.; Besanson, J.; Zeng, H.; Chen, J.X. TIGAR Deficiency Blunts Angiotensin-II-Induced Cardiac Hypertrophy in Mice. Int. J. Mol. Sci. 2024, 25, 2433. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.C.; Besanson, J.; Williams, Q.; Hoang, N.; Edwards, K.; Bishop, G.R.; Chen, Y.; Zeng, H.; Chen, J.X. Ferrostatin-1 specifically targets mitochondrial iron-sulfur clusters and aconitase to improve cardiac function in Sirtuin 3 cardiomyocyte knockout mice. J. Mol. Cell. Cardiol. 2024, 192, 36–47. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zeng, H.; Chen, J.X. Ablation of SIRT3 causes coronary microvascular dysfunction and impairs cardiac recovery post myocardial ischemia. Int. J. Cardiol. 2016, 215, 349–357. [Google Scholar] [CrossRef] [PubMed]
| Con + Sham | Con + Ang-II | pKO + Sham | pKO + Ang-II | Control + Sham v p53aceKO + Sham | Control + Sham v Control + Ang-II | p53aceKO + Sham v p53aceKO + Ang-II | |
|---|---|---|---|---|---|---|---|
| CO (mL/min) | 15.5 ± 1.4 | 13.5 ± 2.3 | 20.9 ± 0.8 | 23.0 ± 2.0 | * | ns | ns |
| SV (uL) | 33.9 ± 3.0 | 29.5 ± 4.9 | 46.4 ± 1.9 | 49.9 ± 4.0 | * | ns | ns |
| LVAW;s (mm) | 1.32 ± 0.04 | 1.88 ± 0.05 | 1.71 ± 0.14 | 1.55 ± 0.10 | ns | **** | ns |
| LVAW;d (mm) | 1.04 ± 0.06 | 1.33 ± 0.07 | 1.12 ± 0.08 | 1.02 ± 0.05 | ns | * | ns |
| LVPW;s (mm) | 1.17 ± 0.07 | 1.80 ± 0.10 | 1.36 ± 0.10 | 1.53 ± 0.17 | ns | ** | ns |
| LVPW;d (mm) | 0.84 ± 0.04 | 1.21 ± 0.07 | 0.93 ± 0.07 | 1.05 ± 0.10 | ns | ** | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantrell, A.C.; Williams, Q.A.; Chen, J.-X.; Zeng, H. Mutation of p53 Acetylation Protects Against Angiotensin-II-Induced Cardiac Dysfunction and Fibrosis. Int. J. Mol. Sci. 2025, 26, 9668. https://doi.org/10.3390/ijms26199668
Cantrell AC, Williams QA, Chen J-X, Zeng H. Mutation of p53 Acetylation Protects Against Angiotensin-II-Induced Cardiac Dysfunction and Fibrosis. International Journal of Molecular Sciences. 2025; 26(19):9668. https://doi.org/10.3390/ijms26199668
Chicago/Turabian StyleCantrell, Aubrey C., Quinesha A. Williams, Jian-Xiong Chen, and Heng Zeng. 2025. "Mutation of p53 Acetylation Protects Against Angiotensin-II-Induced Cardiac Dysfunction and Fibrosis" International Journal of Molecular Sciences 26, no. 19: 9668. https://doi.org/10.3390/ijms26199668
APA StyleCantrell, A. C., Williams, Q. A., Chen, J.-X., & Zeng, H. (2025). Mutation of p53 Acetylation Protects Against Angiotensin-II-Induced Cardiac Dysfunction and Fibrosis. International Journal of Molecular Sciences, 26(19), 9668. https://doi.org/10.3390/ijms26199668






