Potential Role of Membrane Contact Sites in the Dysregulation of the Crosstalk Between Mitochondria and Lysosomes in Alzheimer’s Disease
Abstract
1. Introduction
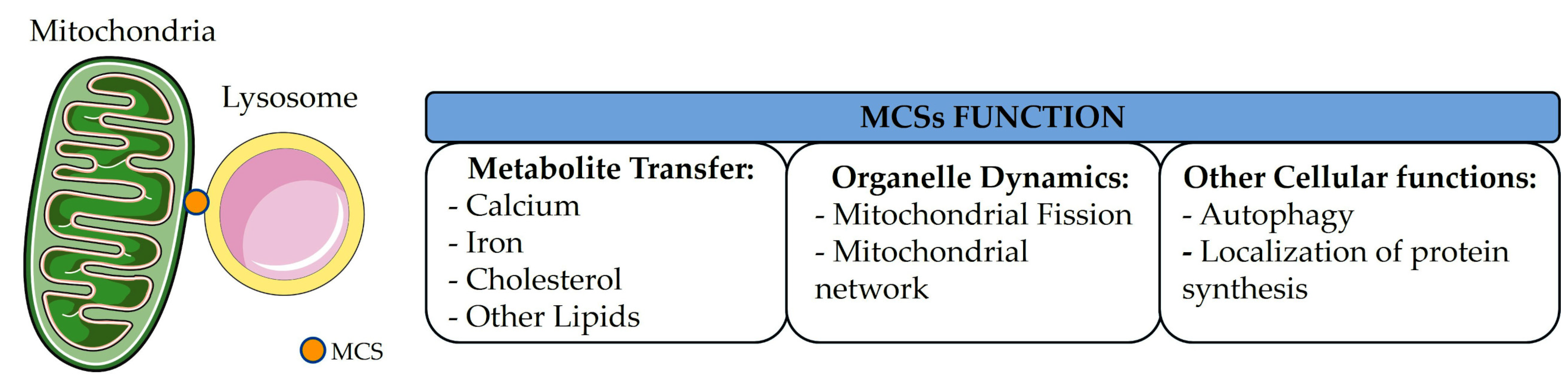
2. Mitochondria–Lysosome Contacts Formation and Dynamics
3. Functional Roles for Mitochondria–Lysosome Contacts in Regulating Cell Homeostasis
3.1. Mitochondrial Fission and Network
3.2. Calcium Exchange Between Mitochondria and Lysosomes
3.3. Iron Transfer
3.4. Cholesterol Homeostasis
3.5. Homeostasis of Other Lipids
3.6. Localization of Protein Synthesis
3.7. Regulation of Autophagy Pathways
4. Methodologies to Study Mitochondria–Lysosome Communication and Techniques for Probing Interactions
5. The Role of Mitochondrial and Lysosomal Dysfunctions in Alzheimer’s Disease
6. Potential Role of Mitochondria–Lysosome Contact Sites in Alzheimer’s Disease
7. Conclusions
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safiri, S.; Ghaffari Jolfayi, A.; Fazlollahi, A.; Morsali, S.; Sarkesh, A.; Daei Sorkhabi, A.; Golabi, B.; Aletaha, R.; Motlagh Asghari, K.; Hamidi, S.; et al. Alzheimer’s Disease: A Comprehensive Review of Epidemiology, Risk Factors, Symptoms Diagnosis, Management, Caregiving, Advanced Treatments and Associated Challenges. Front. Med. 2024, 11, 1474043. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J. ER-Organelle Contacts: A Signaling Hub for Neurological Diseases. Pharmacol. Res. 2024, 203, 107149. [Google Scholar] [CrossRef]
- Voeltz, G.K.; Sawyer, E.M.; Hajnóczky, G.; Prinz, W.A. Making the Connection: How Membrane Contact Sites Have Changed Our View of Organelle Biology. Cell 2024, 187, 257–270. [Google Scholar] [CrossRef]
- Bohnert, M. Tether Me, Tether Me Not-Dynamic Organelle Contact Sites in Metabolic Rewiring. Dev. Cell 2020, 54, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, K.M.; De Camilli, P.; Melia, T.J. Lipid Dynamics at Membrane Contact Sites. Annu. Rev. Biochem. 2025, 94, 479–502. [Google Scholar] [CrossRef]
- Rakotonirina-Ricquebourg, R.; Costa, V.; Teixeira, V. Hello from the Other Side: Membrane Contact of Lipid Droplets with Other Organelles and Subsequent Functional Implications. Prog. Lipid Res. 2022, 85, 101141. [Google Scholar] [CrossRef]
- Kozjak-Pavlovic, V. The MICOS Complex of Human Mitochondria. Cell Tissue Res. 2017, 367, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Eramo, M.J.; Lisnyak, V.; Formosa, L.E.; Ryan, M.T. The “mitochondrial Contact Site and Cristae Organising System” (MICOS) in Health and Human Disease. J. Biochem. 2020, 167, 243–255. [Google Scholar] [CrossRef]
- Rizzuto, R.; Pinton, P.; Carrington, W.; Fay, F.S.; Fogarty, K.E.; Lifshitz, L.M.; Tuft, R.A.; Pozzan, T. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2+ Responses. Science 1998, 280, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Rouiller, C. Close Topographical Relationship between Mitochondria and Ergastoplasm of Liver Cells in a Definite Phase of Cellular Activity. J. Biophys. Biochem. Cytol. 1956, 2, 73–78. [Google Scholar] [CrossRef]
- Calì, T.; Bayer, E.M.; Eden, E.R.; Hajnóczky, G.; Kornmann, B.; Lackner, L.; Liou, J.; Reinisch, K.; Rhee, H.-W.; Rizzuto, R.; et al. Key Challenges and Recommendations for Defining Organelle Membrane Contact Sites. Nat. Rev. Mol. Cell Biol. 2025, 26, 776–796. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Petkovic, M.; O’Brien, C.E.; Jan, Y.N. Interorganelle Communication, Aging, and Neurodegeneration. Genes Dev. 2021, 35, 449–469. [Google Scholar] [CrossRef]
- Prinz, W.A.; Toulmay, A.; Balla, T. The Functional Universe of Membrane Contact Sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef]
- Mc Donald, J.M.; Krainc, D. Lysosomal Proteins as a Therapeutic Target in Neurodegeneration. Annu. Rev. Med. 2017, 68, 445–458. [Google Scholar] [CrossRef]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria–Lysosome Contacts Regulate Mitochondrial Fission via RAB7 GTP Hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef]
- Deus, C.M.; Yambire, K.F.; Oliveira, P.J.; Raimundo, N. Mitochondria-Lysosome Crosstalk: From Physiology to Neurodegeneration. Trends Mol. Med. 2020, 26, 71–88. [Google Scholar] [CrossRef]
- Cisneros, J.; Belton, T.B.; Shum, G.C.; Molakal, C.G.; Wong, Y.C. Mitochondria-Lysosome Contact Site Dynamics and Misregulation in Neurodegenerative Diseases. Trends Neurosci. 2022, 45, 312–322. [Google Scholar] [CrossRef]
- Cioni, J.-M.; Lin, J.Q.; Holtermann, A.V.; Koppers, M.; Jakobs, M.A.H.; Azizi, A.; Turner-Bridger, B.; Shigeoka, T.; Franze, K.; Harris, W.A.; et al. Late Endosomes Act as mRNA Translation Platforms and Sustain Mitochondria in Axons. Cell 2019, 176, 56–72.e15. [Google Scholar] [CrossRef]
- Cantarero, L.; Juárez-Escoto, E.; Civera-Tregón, A.; Rodríguez-Sanz, M.; Roldán, M.; Benítez, R.; Hoenicka, J.; Palau, F. Mitochondria-Lysosome Membrane Contacts Are Defective in GDAP1-Related Charcot-Marie-Tooth Disease. Hum. Mol. Genet. 2021, 29, 3589–3605. [Google Scholar] [CrossRef]
- Wong, Y.C.; Peng, W.; Krainc, D. Lysosomal Regulation of Inter-Mitochondrial Contact Fate and Motility in Charcot-Marie-Tooth Type 2. Dev. Cell 2019, 50, 339–354.e4. [Google Scholar] [CrossRef]
- Burbulla, L.F.; Jeon, S.; Zheng, J.; Song, P.; Silverman, R.B.; Krainc, D. A Modulator of Wild-Type Glucocerebrosidase Improves Pathogenic Phenotypes in Dopaminergic Neuronal Models of Parkinson’s Disease. Sci. Transl. Med. 2019, 11, eaau6870. [Google Scholar] [CrossRef]
- Rabas, N.; Palmer, S.; Mitchell, L.; Ismail, S.; Gohlke, A.; Riley, J.S.; Tait, S.W.G.; Gammage, P.; Soares, L.L.; Macpherson, I.R.; et al. PINK1 Drives Production of mtDNA-Containing Extracellular Vesicles to Promote Invasiveness. J. Cell Biol. 2021, 220, e202006049. [Google Scholar] [CrossRef]
- Kim, S.; Wong, Y.C.; Gao, F.; Krainc, D. Dysregulation of Mitochondria-Lysosome Contacts by GBA1 Dysfunction in Dopaminergic Neuronal Models of Parkinson’s Disease. Nat. Commun. 2021, 12, 1807. [Google Scholar] [CrossRef]
- Höglinger, D.; Burgoyne, T.; Sanchez-Heras, E.; Hartwig, P.; Colaco, A.; Newton, J.; Futter, C.E.; Spiegel, S.; Platt, F.M.; Eden, E.R. NPC1 Regulates ER Contacts with Endocytic Organelles to Mediate Cholesterol Egress. Nat. Commun. 2019, 10, 4276. [Google Scholar] [CrossRef]
- Peng, W.; Wong, Y.C.; Krainc, D. Mitochondria-Lysosome Contacts Regulate Mitochondrial Ca2+ Dynamics via Lysosomal TRPML1. Proc. Natl. Acad. Sci. USA 2020, 117, 19266–19275. [Google Scholar] [CrossRef]
- Suomalainen, A.; Nunnari, J. Mitochondria at the Crossroads of Health and Disease. Cell 2024, 187, 2601–2627. [Google Scholar] [CrossRef]
- Rizzollo, F.; More, S.; Vangheluwe, P.; Agostinis, P. The Lysosome as a Master Regulator of Iron Metabolism. Trends Biochem. Sci. 2021, 46, 960–975. [Google Scholar] [CrossRef]
- Settembre, C.; Perera, R.M. Lysosomes as Coordinators of Cellular Catabolism, Metabolic Signalling and Organ Physiology. Nat. Rev. Mol. Cell Biol. 2024, 25, 223–245. [Google Scholar] [CrossRef]
- Sugiura, A.; McLelland, G.-L.; Fon, E.A.; McBride, H.M. A New Pathway for Mitochondrial Quality Control: Mitochondrial-Derived Vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef]
- Wong, Y.C.; Kim, S.; Peng, W.; Krainc, D. Regulation and Function of Mitochondria-Lysosome Membrane Contact Sites in Cellular Homeostasis. Trends Cell Biol. 2019, 29, 500–513. [Google Scholar] [CrossRef]
- Boutry, M.; Kim, P.K. ORP1L Mediated PI(4)P Signaling at ER-Lysosome-Mitochondrion Three-Way Contact Contributes to Mitochondrial Division. Nat. Commun. 2021, 12, 5354. [Google Scholar] [CrossRef]
- Juhl, A.D.; Heegaard, C.W.; Werner, S.; Schneider, G.; Krishnan, K.; Covey, D.F.; Wüstner, D. Quantitative Imaging of Membrane Contact Sites for Sterol Transfer between Endo-Lysosomes and Mitochondria in Living Cells. Sci. Rep. 2021, 11, 8927. [Google Scholar] [CrossRef]
- Giamogante, F.; Barazzuol, L.; Maiorca, F.; Poggio, E.; Esposito, A.; Masato, A.; Napolitano, G.; Vagnoni, A.; Calì, T.; Brini, M. A SPLICS Reporter Reveals α-Synuclein Regulation of Lysosome-Mitochondria Contacts Which Affects TFEB Nuclear Translocation. Nat. Commun. 2024, 15, 1516. [Google Scholar] [CrossRef]
- Peng, W.; Schröder, L.F.; Song, P.; Wong, Y.C.; Krainc, D. Parkin Regulates Amino Acid Homeostasis at Mitochondria-Lysosome (M/L) Contact Sites in Parkinson’s Disease. Sci. Adv. 2023, 9, eadh3347. [Google Scholar] [CrossRef]
- Guerra, F.; Bucci, C. Multiple Roles of the Small GTPase Rab7. Cells 2016, 5, 34. [Google Scholar] [CrossRef]
- Bucci, C.; Thomsen, P.; Nicoziani, P.; McCarthy, J.; van Deurs, B. Rab7: A Key to Lysosome Biogenesis. Mol. Biol. Cell 2000, 11, 467–480. [Google Scholar] [CrossRef]
- Yu, W.; Sun, S.; Xu, H.; Li, C.; Ren, J.; Zhang, Y. TBC1D15/RAB7-Regulated Mitochondria-Lysosome Interaction Confers Cardioprotection against Acute Myocardial Infarction-Induced Cardiac Injury. Theranostics 2020, 10, 11244–11263. [Google Scholar] [CrossRef]
- Onoue, K.; Jofuku, A.; Ban-Ishihara, R.; Ishihara, T.; Maeda, M.; Koshiba, T.; Itoh, T.; Fukuda, M.; Otera, H.; Oka, T.; et al. Fis1 Acts as a Mitochondrial Recruitment Factor for TBC1D15 That Is Involved in Regulation of Mitochondrial Morphology. J. Cell Sci. 2013, 126, 176–185. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Walsh, B.; Mitchell, C.A.; Rowe, T. TBC Domain Family, Member 15 Is a Novel Mammalian Rab GTPase-Activating Protein with Substrate Preference for Rab7. Biochem. Biophys. Res. Commun. 2005, 335, 154–161. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2024, 52, D368–D375. [Google Scholar] [CrossRef]
- Muñoz-Braceras, S.; Tornero-Écija, A.R.; Vincent, O.; Escalante, R. VPS13A Is Closely Associated with Mitochondria and Is Required for Efficient Lysosomal Degradation. Dis. Model. Mech. 2019, 12, dmm036681. [Google Scholar] [CrossRef]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten Lambert, H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct Fission Signatures Predict Mitochondrial Degradation or Biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef]
- Langemeyer, L.; Fröhlich, F.; Ungermann, C. Rab GTPase Function in Endosome and Lysosome Biogenesis. Trends Cell Biol. 2018, 28, 957–970. [Google Scholar] [CrossRef]
- Khalil, S.; Holy, M.; Grado, S.; Fleming, R.; Kurita, R.; Nakamura, Y.; Goldfarb, A. A Specialized Pathway for Erythroid Iron Delivery through Lysosomal Trafficking of Transferrin Receptor 2. Blood Adv. 2017, 1, 1181–1194. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Lan, B.; Gao, Y.; Cai, Y. DRP1, Fission and Apoptosis. Cell Death Discov. 2025, 11, 150. [Google Scholar] [CrossRef]
- Siow, W.X.; Kabiri, Y.; Tang, R.; Chao, Y.-K.; Plesch, E.; Eberhagen, C.; Flenkenthaler, F.; Fröhlich, T.; Bracher, F.; Grimm, C.; et al. Lysosomal TRPML1 Regulates Mitochondrial Function in Hepatocellular Carcinoma Cells. J. Cell Sci. 2022, 135, jcs259455. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A Forty-Kilodalton Protein of the Inner Membrane Is the Mitochondrial Calcium Uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Hamdi, A.; Roshan, T.M.; Kahawita, T.M.; Mason, A.B.; Sheftel, A.D.; Ponka, P. Erythroid Cell Mitochondria Receive Endosomal Iron by a “Kiss-and-Run” Mechanism. Biochim. Biophys. Acta 2016, 1863, 2859–2867. [Google Scholar] [CrossRef]
- Das, A.; Nag, S.; Mason, A.B.; Barroso, M.M. Endosome-Mitochondria Interactions Are Modulated by Iron Release from Transferrin. J. Cell Biol. 2016, 214, 831–845. [Google Scholar] [CrossRef]
- Rizzollo, F.; Agostinis, P. Mitochondria-Lysosome Contact Sites: Emerging Players in Cellular Homeostasis and Disease. Contact 2025, 8, 25152564251329250. [Google Scholar] [CrossRef]
- Bao, L.; Liu, Q.; Wang, J.; Shi, L.; Pang, Y.; Niu, Y.; Zhang, R. The Interactions of Subcellular Organelles in Pulmonary Fibrosis Induced by Carbon Black Nanoparticles: A Comprehensive Review. Arch. Toxicol. 2024, 98, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Huang, X.; Han, L.; Wang, X.; Cheng, H.; Zhao, Y.; Chen, Q.; Chen, J.; Cheng, H.; Xiao, R.; et al. Central Role of Mitofusin 2 in Autophagosome-Lysosome Fusion in Cardiomyocytes. J. Biol. Chem. 2012, 287, 23615–23625. [Google Scholar] [CrossRef] [PubMed]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From Functions to Disease. Cell Death Dis. 2018, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Rizzollo, F.; Escamilla-Ayala, A.; Fattorelli, N.; Lysiak, N.; More, S.; Barazzuol, L.; Haute, C.V.D.; Asselberghs, J.V.; Nittner, D.; et al. A Bdh2-Driven Lysosome to Mitochondria Iron Trafficking Controls Ferroptosis in Melanoma. Research Square 2024. Preprint. [Google Scholar] [CrossRef]
- Yambire, K.F.; Rostosky, C.; Watanabe, T.; Pacheu-Grau, D.; Torres-Odio, S.; Sanchez-Guerrero, A.; Senderovich, O.; Meyron-Holtz, E.G.; Milosevic, I.; Frahm, J.; et al. Impaired Lysosomal Acidification Triggers Iron Deficiency and Inflammation in Vivo. eLife 2019, 8, e51031. [Google Scholar] [CrossRef]
- Weber, R.A.; Yen, F.S.; Nicholson, S.P.V.; Alwaseem, H.; Bayraktar, E.C.; Alam, M.; Timson, R.C.; La, K.; Abu-Remaileh, M.; Molina, H.; et al. Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation. Mol. Cell 2020, 77, 645–655.e7. [Google Scholar] [CrossRef]
- Sassano, M.L.; Felipe-Abrio, B.; Agostinis, P. ER-Mitochondria Contact Sites; a Multifaceted Factory for Ca2+ Signaling and Lipid Transport. Front. Cell Dev. Biol. 2022, 10, 988014. [Google Scholar] [CrossRef]
- Charman, M.; Kennedy, B.E.; Osborne, N.; Karten, B. MLN64 Mediates Egress of Cholesterol from Endosomes to Mitochondria in the Absence of Functional Niemann-Pick Type C1 Protein. J. Lipid Res. 2010, 51, 1023–1034. [Google Scholar] [CrossRef]
- Hönscher, C.; Mari, M.; Auffarth, K.; Bohnert, M.; Griffith, J.; Geerts, W.; van der Laan, M.; Cabrera, M.; Reggiori, F.; Ungermann, C. Cellular Metabolism Regulates Contact Sites between Vacuoles and Mitochondria. Dev. Cell 2014, 30, 86–94. [Google Scholar] [CrossRef]
- González Montoro, A.; Auffarth, K.; Hönscher, C.; Bohnert, M.; Becker, T.; Warscheid, B.; Reggiori, F.; van der Laan, M.; Fröhlich, F.; Ungermann, C. Vps39 Interacts with Tom40 to Establish One of Two Functionally Distinct Vacuole-Mitochondria Contact Sites. Dev. Cell 2018, 45, 621–636.e7. [Google Scholar] [CrossRef]
- Bean, B.D.M.; Dziurdzik, S.K.; Kolehmainen, K.L.; Fowler, C.M.S.; Kwong, W.K.; Grad, L.I.; Davey, M.; Schluter, C.; Conibear, E. Competitive Organelle-Specific Adaptors Recruit Vps13 to Membrane Contact Sites. J. Cell Biol. 2018, 217, 3593–3607. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef] [PubMed]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Aston, D.; Capel, R.A.; Ford, K.L.; Christian, H.C.; Mirams, G.R.; Rog-Zielinska, E.A.; Kohl, P.; Galione, A.; Burton, R.A.B.; Terrar, D.A. High Resolution Structural Evidence Suggests the Sarcoplasmic Reticulum Forms Microdomains with Acidic Stores (Lysosomes) in the Heart. Sci. Rep. 2017, 7, 40620. [Google Scholar] [CrossRef] [PubMed]
- Martell, J.D.; Deerinck, T.J.; Lam, S.S.; Ellisman, M.H.; Ting, A.Y. Electron Microscopy Using the Genetically Encoded APEX2 Tag in Cultured Mammalian Cells. Nat. Protoc. 2017, 12, 1792–1816. [Google Scholar] [CrossRef]
- Fermie, J.; Liv, N.; Ten Brink, C.; van Donselaar, E.G.; Müller, W.H.; Schieber, N.L.; Schwab, Y.; Gerritsen, H.C.; Klumperman, J. Single Organelle Dynamics Linked to 3D Structure by Correlative Live-Cell Imaging and 3D Electron Microscopy. Traffic 2018, 19, 354–369. [Google Scholar] [CrossRef]
- Jung, M.; Mun, J.Y. Dual-Color Correlative Light and Electron Microscopy for the Visualization of Interactions between Mitochondria and Lysosomes. J. Vis. Exp. 2024, 211, e55617. [Google Scholar] [CrossRef]
- Saibil, H.R. Cryo-EM in Molecular and Cellular Biology. Mol. Cell 2022, 82, 274–284. [Google Scholar] [CrossRef]
- Ching, C.; Maufront, J.; di Cicco, A.; Lévy, D.; Dezi, M. Cool-Contacts: Cryo-Electron Microscopy of Membrane Contact Sites and Their Components. Contact 2024, 7, 25152564241231364. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, M.; Qiu, F.; Zhang, M.; Zhang, Y.-H. Cell-Permeable Organic Fluorescent Probes for Live-Cell Long-Term Super-Resolution Imaging Reveal Lysosome-Mitochondrion Interactions. Nat. Commun. 2017, 8, 1307. [Google Scholar] [CrossRef]
- Nieto-Garai, J.A.; Olazar-Intxausti, J.; Anso, I.; Lorizate, M.; Terrones, O.; Contreras, F.-X. Super-Resolution Microscopy to Study Interorganelle Contact Sites. Int. J. Mol. Sci. 2022, 23, 15354. [Google Scholar] [CrossRef]
- Lu, M.; Ward, E.; van Tartwijk, F.W.; Kaminski, C.F. Advances in the Study of Organelle Interactions and Their Role in Neurodegenerative Diseases Enabled by Super-Resolution Microscopy. Neurobiol. Dis. 2021, 159, 105475. [Google Scholar] [CrossRef]
- Cieri, D.; Vicario, M.; Giacomello, M.; Vallese, F.; Filadi, R.; Wagner, T.; Pozzan, T.; Pizzo, P.; Scorrano, L.; Brini, M.; et al. SPLICS: A Split Green Fluorescent Protein-Based Contact Site Sensor for Narrow and Wide Heterotypic Organelle Juxtaposition. Cell Death Differ. 2018, 25, 1131–1145. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, C.; Shao, X.; Guan, R.; Tian, Z.; Wang, C.; Liu, F.; Ling, P.; Guan, J.-L.; Ji, L.; et al. Super-Resolution Tracking of Mitochondrial Dynamics with An Iridium(III) Luminophore. Small 2018, 14, e1802166. [Google Scholar] [CrossRef]
- Rostagno, A.A. Pathogenesis of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 24, 107. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, L.R.; Kumsta, C.; Sandri, M.; Ballabio, A.; Hansen, M. Transcriptional and Epigenetic Regulation of Autophagy in Aging. Autophagy 2015, 11, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Cannizzo, E.S.; Clement, C.C.; Morozova, K.; Valdor, R.; Kaushik, S.; Almeida, L.N.; Follo, C.; Sahu, R.; Cuervo, A.M.; Macian, F.; et al. Age-Related Oxidative Stress Compromises Endosomal Proteostasis. Cell Rep. 2012, 2, 136–149. [Google Scholar] [CrossRef]
- Chou, C.-C.; Vest, R.; Prado, M.A.; Wilson-Grady, J.; Paulo, J.A.; Shibuya, Y.; Moran-Losada, P.; Lee, T.-T.; Luo, J.; Gygi, S.P.; et al. Proteostasis and Lysosomal Repair Deficits in Transdifferentiated Neurons of Alzheimer’s Disease. Nat. Cell Biol. 2025, 27, 619–632. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and Aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-K.; Ji, Z.-S.; Dodson, S.E.; Miranda, R.D.; Rosenblum, C.I.; Reynolds, I.J.; Freedman, S.B.; Weisgraber, K.H.; Huang, Y.; Mahley, R.W. Apolipoprotein E4 Domain Interaction Mediates Detrimental Effects on Mitochondria and Is a Potential Therapeutic Target for Alzheimer Disease. J. Biol. Chem. 2011, 286, 5215–5221. [Google Scholar] [CrossRef]
- Simonovitch, S.; Schmukler, E.; Masliah, E.; Pinkas-Kramarski, R.; Michaelson, D.M. The Effects of APOE4 on Mitochondrial Dynamics and Proteins in Vivo. J. Alzheimers Dis. 2019, 70, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Schmukler, E.; Solomon, S.; Simonovitch, S.; Goldshmit, Y.; Wolfson, E.; Michaelson, D.M.; Pinkas-Kramarski, R. Altered Mitochondrial Dynamics and Function in APOE4-Expressing Astrocytes. Cell Death Dis. 2020, 11, 578. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial Dysfunction and Oxidative Stress in Neurodegenerative Diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Wallace, D.C. A Mitochondrial Paradigm of Metabolic and Degenerative Diseases, Aging, and Cancer: A Dawn for Evolutionary Medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Wei, W.; Keogh, M.J.; Wilson, I.; Coxhead, J.; Ryan, S.; Rollinson, S.; Griffin, H.; Kurzawa-Akanbi, M.; Santibanez-Koref, M.; Talbot, K.; et al. Mitochondrial DNA Point Mutations and Relative Copy Number in 1363 Disease and Control Human Brains. Acta Neuropathol. Commun. 2017, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Coskun, P.E.; Beal, M.F.; Wallace, D.C. Alzheimer’s Brains Harbor Somatic mtDNA Control-Region Mutations That Suppress Mitochondrial Transcription and Replication. Proc. Natl. Acad. Sci. USA 2004, 101, 10726–10731. [Google Scholar] [CrossRef]
- Reiss, A.B.; Gulkarov, S.; Jacob, B.; Srivastava, A.; Pinkhasov, A.; Gomolin, I.H.; Stecker, M.M.; Wisniewski, T.; De Leon, J. Mitochondria in Alzheimer’s Disease Pathogenesis. Life 2024, 14, 196. [Google Scholar] [CrossRef]
- Reutzel, M.; Grewal, R.; Joppe, A.; Eckert, G.P. Age-Dependent Alterations of Cognition, Mitochondrial Function, and Beta-Amyloid Deposition in a Murine Model of Alzheimer’s Disease-A Longitudinal Study. Front. Aging Neurosci. 2022, 14, 875989. [Google Scholar] [CrossRef]
- D’Alessandro, M.C.B.; Kanaan, S.; Geller, M.; Praticò, D.; Daher, J.P.L. Mitochondrial Dysfunction in Alzheimer’s Disease. Ageing Res. Rev. 2025, 107, 102713. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Bharadwaj, P.; Garg, M.; Verdile, G.; Guillemin, G.J.; Martins, R.N. Targeting Mitophagy in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 1273–1297. [Google Scholar] [CrossRef]
- Kazemeini, S.; Nadeem-Tariq, A.; Shih, R.; Rafanan, J.; Ghani, N.; Vida, T.A. From Plaques to Pathways in Alzheimer’s Disease: The Mitochondrial-Neurovascular-Metabolic Hypothesis. Int. J. Mol. Sci. 2024, 25, 11720. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s Disease Mitochondrial Cascade Hypothesis: Progress and Perspectives. Biochim. Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Kametani, F.; Hasegawa, M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front. Neurosci. 2018, 12, 25. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The Role of Mitochondrial Dysfunction in Alzheimer’s Disease Pathogenesis. Alzheimers Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef]
- Spina, E.; Ferrari, R.R.; Pellegrini, E.; Colombo, M.; Poloni, T.E.; Guaita, A.; Davin, A. Mitochondrial Alterations, Oxidative Stress, and Therapeutic Implications in Alzheimer’s Disease: A Narrative Review. Cells 2025, 14, 229. [Google Scholar] [CrossRef]
- McGill Percy, K.C.; Liu, Z.; Qi, X. Mitochondrial Dysfunction in Alzheimer’s Disease: Guiding the Path to Targeted Therapies. Neurotherapeutics 2025, 22, e00525. [Google Scholar] [CrossRef] [PubMed]
- Colacurcio, D.J.; Nixon, R.A. Disorders of Lysosomal Acidification-The Emerging Role of v-ATPase in Aging and Neurodegenerative Disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised Autophagy and Neurodegenerative Diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.E.; Oddo, S. Autophagic/Lysosomal Dysfunction in Alzheimer’s Disease. Alzheimers Res. Ther. 2013, 5, 53. [Google Scholar] [CrossRef]
- Heo, H.; Park, H.; Lee, M.S.; Kim, J.; Kim, J.; Jung, S.-Y.; Kim, S.K.; Lee, S.; Chang, J. TRIM22 Facilitates Autophagosome-Lysosome Fusion by Mediating the Association of GABARAPs and PLEKHM1. Autophagy 2024, 20, 1098–1113. [Google Scholar] [CrossRef]
- Nixon, R.A.; Wegiel, J.; Kumar, A.; Yu, W.H.; Peterhoff, C.; Cataldo, A.; Cuervo, A.M. Extensive Involvement of Autophagy in Alzheimer Disease: An Immuno-Electron Microscopy Study. J. Neuropathol. Exp. Neurol. 2005, 64, 113–122. [Google Scholar] [CrossRef]
- Lo, C.H.; Zeng, J. Defective Lysosomal Acidification: A New Prognostic Marker and Therapeutic Target for Neurodegenerative Diseases. Transl. Neurodegener. 2023, 12, 29. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yang, D.-S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty Autolysosome Acidification in Alzheimer’s Disease Mouse Models Induces Autophagic Build-up of Aβ in Neurons, Yielding Senile Plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef]
- Cataldo, A.M.; Nixon, R.A. Enzymatically Active Lysosomal Proteases Are Associated with Amyloid Deposits in Alzheimer Brain. Proc. Natl. Acad. Sci. USA 1990, 87, 3861–3865. [Google Scholar] [CrossRef]
- Nixon, R.A. Autophagy, Amyloidogenesis and Alzheimer Disease. J. Cell Sci. 2007, 120, 4081–4091. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, Z.P.; Bretou, M.; Annaert, W. Endo-Lysosomal Dysregulations and Late-Onset Alzheimer’s Disease: Impact of Genetic Risk Factors. Mol. Neurodegener. 2019, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Ditaranto, K.; Tekirian, T.L.; Yang, A.J. Lysosomal Membrane Damage in Soluble Abeta-Mediated Cell Death in Alzheimer’s Disease. Neurobiol. Dis. 2001, 8, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Kosenko, E.; Poghosyan, A.; Kaminsky, Y. Subcellular Compartmentalization of Proteolytic Enzymes in Brain Regions and the Effects of Chronic β-Amyloid Treatment. Brain Res. 2011, 1369, 184–193. [Google Scholar] [CrossRef]
- Zaretsky, D.V.; Zaretskaia, M.V.; Molkov, Y.I. Membrane Channel Hypothesis of Lysosomal Permeabilization by Beta-Amyloid. Neurosci. Lett. 2022, 770, 136338. [Google Scholar] [CrossRef]
- Sanyal, A.; Scanavachi, G.; Somerville, E.; Saminathan, A.; Nair, A.; Bango Da Cunha Correia, R.F.; Aylan, B.; Sitarska, E.; Oikonomou, A.; Hatzakis, N.S.; et al. Neuronal Constitutive Endolysosomal Perforations Enable α-Synuclein Aggregation by Internalized PFFs. J. Cell Biol. 2025, 224, e202401136. [Google Scholar] [CrossRef]
- Rose, K.; Jepson, T.; Shukla, S.; Maya-Romero, A.; Kampmann, M.; Xu, K.; Hurley, J.H. Tau Fibrils Induce Nanoscale Membrane Damage and Nucleate Cytosolic Tau at Lysosomes. Proc. Natl. Acad. Sci. USA 2024, 121, e2315690121. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ha, T.-Y.; Lee, M.-S.; Chang, K.-A. Regulatory Mechanisms and Therapeutic Implications of Lysosomal Dysfunction in Alzheimer’s Disease. Int. J. Biol. Sci. 2025, 21, 1014–1031. [Google Scholar] [CrossRef]
- Angst, G.; Jia, N.; Esqueda, L.E.T.; Fan, Y.; Cai, Q.; Wang, C. Autophagy in Alzheimer Disease Pathogenesis and Its Therapeutic Values. Autophagy Rep. 2025, 4, 2471677. [Google Scholar] [CrossRef] [PubMed]
- Demers-Lamarche, J.; Guillebaud, G.; Tlili, M.; Todkar, K.; Bélanger, N.; Grondin, M.; Nguyen, A.P.; Michel, J.; Germain, M. Loss of Mitochondrial Function Impairs Lysosomes. J. Biol. Chem. 2016, 291, 10263–10276. [Google Scholar] [CrossRef]
- Baixauli, F.; Acín-Pérez, R.; Villarroya-Beltrí, C.; Mazzeo, C.; Nuñez-Andrade, N.; Gabandé-Rodriguez, E.; Ledesma, M.D.; Blázquez, A.; Martin, M.A.; Falcón-Pérez, J.M.; et al. Mitochondrial Respiration Controls Lysosomal Function during Inflammatory T Cell Responses. Cell Metab. 2015, 22, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Mosquera, L.; Yambire, K.F.; Couto, R.; Pereyra, L.; Pabis, K.; Ponsford, A.H.; Diogo, C.V.; Stagi, M.; Milosevic, I.; Raimundo, N. Mitochondrial Respiratory Chain Deficiency Inhibits Lysosomal Hydrolysis. Autophagy 2019, 15, 1572–1591. [Google Scholar] [CrossRef]
- Joshi, A.U.; Ebert, A.E.; Haileselassie, B.; Mochly-Rosen, D. Drp1/Fis1-Mediated Mitochondrial Fragmentation Leads to Lysosomal Dysfunction in Cardiac Models of Huntington’s Disease. J. Mol. Cell Cardiol. 2019, 127, 125–133. [Google Scholar] [CrossRef]
- Girolimetti, G.; Gagliardi, S.; Cordella, P.; Bramato, G.; Di Corato, R.; Romano, R.; Guerra, F.; Bucci, C. Induced Mitochondrial Deficit by NDUFS3 Transient Silencing Reduces RAB7 Expression and Causes Lysosomal Dysfunction in Pancreatic Cancer Cells. Cell Commun. Signal. 2025, 23, 224. [Google Scholar] [CrossRef]
- Hughes, A.L.; Gottschling, D.E. An Early Age Increase in Vacuolar pH Limits Mitochondrial Function and Lifespan in Yeast. Nature 2012, 492, 261–265. [Google Scholar] [CrossRef]
- Jiang, Y.; Sato, Y.; Im, E.; Berg, M.; Bordi, M.; Darji, S.; Kumar, A.; Mohan, P.S.; Bandyopadhyay, U.; Diaz, A.; et al. Lysosomal Dysfunction in Down Syndrome Is APP-Dependent and Mediated by APP-βCTF (C99). J. Neurosci. 2019, 39, 5255–5268. [Google Scholar] [CrossRef]
- Bonda, D.J.; Wang, X.; Perry, G.; Smith, M.A.; Zhu, X. Mitochondrial Dynamics in Alzheimer’s Disease: Opportunities for Future Treatment Strategies. Drugs Aging 2010, 27, 181–192. [Google Scholar] [CrossRef]
- Lian, W.-W.; Zhou, W.; Zhang, B.-Y.; Jia, H.; Xu, L.-J.; Liu, A.-L.; Du, G.-H. DL0410 Ameliorates Cognitive Disorder in SAMP8 Mice by Promoting Mitochondrial Dynamics and the NMDAR-CREB-BDNF Pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired Mitochondrial Dynamics and Abnormal Interaction of Amyloid Beta with Mitochondrial Protein Drp1 in Neurons from Patients with Alzheimer’s Disease: Implications for Neuronal Damage. Hum. Mol. Genet. 2011, 20, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Mufson, E.J.; Counts, S.E.; Wuu, J.; Alldred, M.J.; Nixon, R.A.; Che, S. Regional Selectivity of Rab5 and Rab7 Protein Upregulation in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimers Dis. 2010, 22, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Mufson, E.J.; Alldred, M.J.; Counts, S.E.; Wuu, J.; Nixon, R.A.; Che, S. Upregulation of Select Rab GTPases in Cholinergic Basal Forebrain Neurons in Mild Cognitive Impairment and Alzheimer’s Disease. J. Chem. Neuroanat. 2011, 42, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Alldred, M.J.; Counts, S.E.; Cataldo, A.M.; Neve, R.L.; Jiang, Y.; Wuu, J.; Chao, M.V.; Mufson, E.J.; Nixon, R.A.; et al. Microarray Analysis of Hippocampal CA1 Neurons Implicates Early Endosomal Dysfunction during Alzheimer’s Disease Progression. Biol. Psychiatry 2010, 68, 885–893. [Google Scholar] [CrossRef]
- Tiernan, C.T.; Ginsberg, S.D.; Guillozet-Bongaarts, A.L.; Ward, S.M.; He, B.; Kanaan, N.M.; Mufson, E.J.; Binder, L.I.; Counts, S.E. Protein Homeostasis Gene Dysregulation in Pretangle-Bearing Nucleus Basalis Neurons during the Progression of Alzheimer’s Disease. Neurobiol. Aging 2016, 42, 80–90. [Google Scholar] [CrossRef]
- Armstrong, A.; Mattsson, N.; Appelqvist, H.; Janefjord, C.; Sandin, L.; Agholme, L.; Olsson, B.; Svensson, S.; Blennow, K.; Zetterberg, H.; et al. Lysosomal Network Proteins as Potential Novel CSF Biomarkers for Alzheimer’s Disease. Neuromolecular Med. 2014, 16, 150–160. [Google Scholar] [CrossRef]
- Rodriguez, L.; Mohamed, N.-V.; Desjardins, A.; Lippé, R.; Fon, E.A.; Leclerc, N. Rab7A Regulates Tau Secretion. J. Neurochem. 2017, 141, 592–605. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Yan, S.S. PINK1 Activation Attenuates Impaired Neuronal-Like Differentiation and Synaptogenesis and Mitochondrial Dysfunction in Alzheimer’s Disease Trans-Mitochondrial Cybrid Cells. J. Alzheimers Dis. 2021, 81, 1749–1761. [Google Scholar] [CrossRef]
- Han, S.; Nandy, P.; Austria, Q.; Siedlak, S.L.; Torres, S.; Fujioka, H.; Wang, W.; Zhu, X. Mfn2 Ablation in the Adult Mouse Hippocampus and Cortex Causes Neuronal Death. Cells 2020, 9, 116. [Google Scholar] [CrossRef]
- Chen, H.; Xing, H.; Zhong, C.; Lin, X.; Chen, R.; Luo, N.; Chen, L.; Huang, Y. METTL3 Confers Protection against Mitochondrial Dysfunction and Cognitive Impairment in an Alzheimer Disease Mouse Model by Upregulating Mfn2 via N6-Methyladenosine Modification. J. Neuropathol. Exp. Neurol. 2024, 83, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.S.; Adriaanse, B.A.; Greig, N.H.; Mattson, M.P.; Cader, M.Z.; Bohr, V.A.; Fang, E.F. Mitophagy and Alzheimer’s Disease: Cellular and Molecular Mechanisms. Trends Neurosci. 2017, 40, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular Interplay between Mammalian Target of Rapamycin (mTOR), Amyloid-Beta, and Tau: Effects on Cognitive Impairments. J. Biol. Chem. 2010, 285, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, A.; Yamamoto, S.; Ting, T.; Fan, Y.; Sadleir, K.; Wang, Y.; Zhang, W.; Huang, S.; Levine, B.; Vassar, R.; et al. A Becn1 Mutation Mediates Hyperactive Autophagic Sequestration of Amyloid Oligomers and Improved Cognition in Alzheimer’s Disease. PLoS Genet. 2017, 13, e1006962. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Song, Y.-Q.; Tu, J. Autophagy in Alzheimer’s Disease Pathogenesis: Therapeutic Potential and Future Perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef]
- Nilsson, P.; Loganathan, K.; Sekiguchi, M.; Matsuba, Y.; Hui, K.; Tsubuki, S.; Tanaka, M.; Iwata, N.; Saito, T.; Saido, T.C. Aβ Secretion and Plaque Formation Depend on Autophagy. Cell Rep. 2013, 5, 61–69. [Google Scholar] [CrossRef]
- Bordi, M.; Berg, M.J.; Mohan, P.S.; Peterhoff, C.M.; Alldred, M.J.; Che, S.; Ginsberg, S.D.; Nixon, R.A. Autophagy Flux in CA1 Neurons of Alzheimer Hippocampus: Increased Induction Overburdens Failing Lysosomes to Propel Neuritic Dystrophy. Autophagy 2016, 12, 2467–2483. [Google Scholar] [CrossRef]
- Chung, K.M.; Hernández, N.; Sproul, A.A.; Yu, W.H. Alzheimer’s Disease and the Autophagic-Lysosomal System. Neurosci. Lett. 2019, 697, 49–58. [Google Scholar] [CrossRef]
- Lachance, V.; Wang, Q.; Sweet, E.; Choi, I.; Cai, C.-Z.; Zhuang, X.-X.; Zhang, Y.; Jiang, J.L.; Blitzer, R.D.; Bozdagi-Gunal, O.; et al. Autophagy Protein NRBF2 Has Reduced Expression in Alzheimer’s Brains and Modulates Memory and Amyloid-Beta Homeostasis in Mice. Mol. Neurodegener. 2019, 14, 43. [Google Scholar] [CrossRef]
- Pickford, F.; Masliah, E.; Britschgi, M.; Lucin, K.; Narasimhan, R.; Jaeger, P.A.; Small, S.; Spencer, B.; Rockenstein, E.; Levine, B.; et al. The Autophagy-Related Protein Beclin 1 Shows Reduced Expression in Early Alzheimer Disease and Regulates Amyloid Beta Accumulation in Mice. J. Clin. Investig. 2008, 118, 2190–2199. [Google Scholar] [CrossRef]
- Sharoar, M.G.; Palko, S.; Ge, Y.; Saido, T.C.; Yan, R. Accumulation of Saposin in Dystrophic Neurites Is Linked to Impaired Lysosomal Functions in Alzheimer’s Disease Brains. Mol. Neurodegener. 2021, 16, 45. [Google Scholar] [CrossRef]
- Barrachina, M.; Maes, T.; Buesa, C.; Ferrer, I. Lysosome-Associated Membrane Protein 1 (LAMP-1) in Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2006, 32, 505–516. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Yuan, P.; Wu, Y.; Schrag, M.; Paradise, S.; Grutzendler, J.; De Camilli, P.; Ferguson, S.M. Massive Accumulation of Luminal Protease-Deficient Axonal Lysosomes at Alzheimer’s Disease Amyloid Plaques. Proc. Natl. Acad. Sci. USA 2015, 112, E3699–E3708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xiao, D.; Zhao, Y.; Tan, B.; Long, Z.; Yu, L.; He, G. Enhanced Autolysosomal Function Ameliorates the Inflammatory Response Mediated by the NLRP3 Inflammasome in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 629891. [Google Scholar] [CrossRef] [PubMed]
- León, M.; Prieto, J.; Molina-Navarro, M.M.; García-García, F.; Barneo-Muñoz, M.; Ponsoda, X.; Sáez, R.; Palau, F.; Dopazo, J.; Izpisua Belmonte, J.C.; et al. Rapid Degeneration of iPSC-Derived Motor Neurons Lacking Gdap1 Engages a Mitochondrial-Sustained Innate Immune Response. Cell Death Discov. 2023, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. VDAC1, Mitochondrial Dysfunction, and Alzheimer’s Disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Kmita, H.; Messina, A.A.; De Pinto, V. VDAC as a Cellular Hub: Docking Molecules and Interactions. Int. J. Mol. Sci. 2023, 24, 6649. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.J.; Ponce, D.P.; Aranguiz, A.; Behrens, M.I.; Quintanilla, R.A. Mitochondrial Permeability Transition Pore Contributes to Mitochondrial Dysfunction in Fibroblasts of Patients with Sporadic Alzheimer’s Disease. Redox Biol. 2018, 19, 290–300. [Google Scholar] [CrossRef]
- Song, L.; Tang, Y.; Law, B.Y.K. Targeting Calcium Signaling in Alzheimer’s Disease: Challenges and Promising Therapeutic Avenues. Neural Regen. Res. 2024, 19, 501–502. [Google Scholar] [CrossRef]
- Chaudhary, B.; Kumari, S.; Dhapola, R.; Sharma, P.; Paidlewar, M.; Vellingiri, B.; Medhi, B.; HariKrishnaReddy, D. Calcium Dysregulation in Alzheimer’s Disease: Unraveling the Molecular Nexus of Neuronal Dysfunction and Therapeutic Opportunities. Biochem. Pharmacol. 2025, 242, 117211. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-Mediated Mitophagy in Neurodegenerative Diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef]
- Mattson, M.P. ER Calcium and Alzheimer’s Disease: In a State of Flux. Sci. Signal. 2010, 3, pe10. [Google Scholar] [CrossRef]
- Calvo-Rodriguez, M.; Hou, S.S.; Snyder, A.C.; Kharitonova, E.K.; Russ, A.N.; Das, S.; Fan, Z.; Muzikansky, A.; Garcia-Alloza, M.; Serrano-Pozo, A.; et al. Increased Mitochondrial Calcium Levels Associated with Neuronal Death in a Mouse Model of Alzheimer’s Disease. Nat. Commun. 2020, 11, 2146. [Google Scholar] [CrossRef]
- Curcio-Morelli, C.; Charles, F.A.; Micsenyi, M.C.; Cao, Y.; Venugopal, B.; Browning, M.F.; Dobrenis, K.; Cotman, S.L.; Walkley, S.U.; Slaugenhaupt, S.A. Macroautophagy Is Defective in Mucolipin-1-Deficient Mouse Neurons. Neurobiol. Dis. 2010, 40, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Cheng, X.; Lian, Y.; Xu, H.; Zeng, Z.; Zhu, H. TRPML1 Participates in the Progression of Alzheimer’s Disease by Regulating the PPARγ/AMPK/Mtor Signalling Pathway. Cell Physiol. Biochem. 2017, 43, 2446–2456. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Area-Gomez, E.; Del Carmen Lara Castillo, M.; Tambini, M.D.; Guardia-Laguarta, C.; de Groof, A.J.C.; Madra, M.; Ikenouchi, J.; Umeda, M.; Bird, T.D.; Sturley, S.L.; et al. Upregulated Function of Mitochondria-Associated ER Membranes in Alzheimer Disease. EMBO J. 2012, 31, 4106–4123. [Google Scholar] [CrossRef] [PubMed]
- Barbero-Camps, E.; Fernández, A.; Baulies, A.; Martinez, L.; Fernández-Checa, J.C.; Colell, A. Endoplasmic Reticulum Stress Mediates Amyloid β Neurotoxicity via Mitochondrial Cholesterol Trafficking. Am. J. Pathol. 2014, 184, 2066–2081. [Google Scholar] [CrossRef] [PubMed]
- Murley, A.; Nunnari, J. The Emerging Network of Mitochondria-Organelle Contacts. Mol. Cell 2016, 61, 648–653. [Google Scholar] [CrossRef]
- Vrijsen, S.; Vrancx, C.; Del Vecchio, M.; Swinnen, J.V.; Agostinis, P.; Winderickx, J.; Vangheluwe, P.; Annaert, W. Inter-Organellar Communication in Parkinson’s and Alzheimer’s Disease: Looking Beyond Endoplasmic Reticulum-Mitochondria Contact Sites. Front. Neurosci. 2022, 16, 900338. [Google Scholar] [CrossRef] [PubMed]

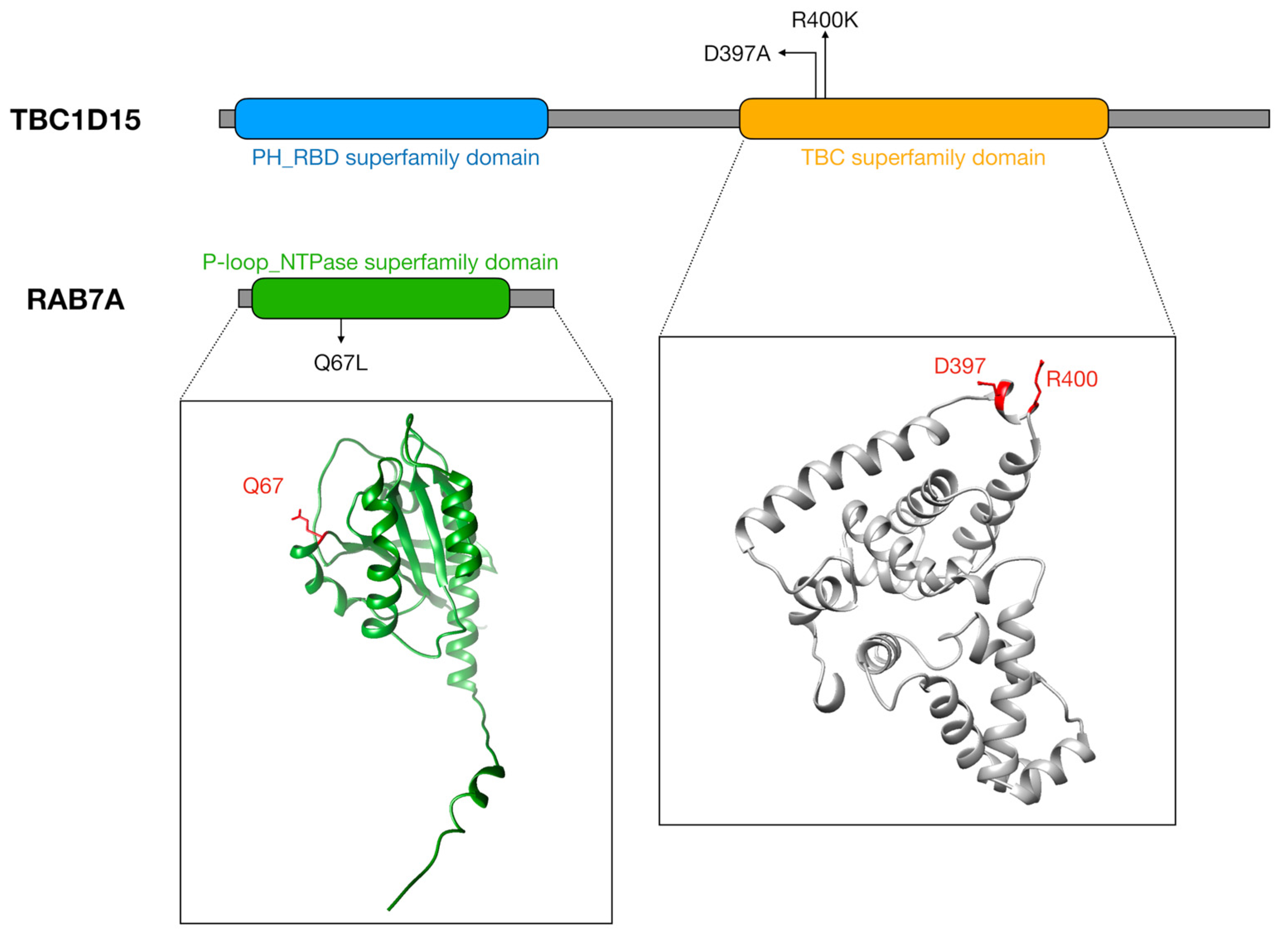

| Methods | Benefits | Disadvantages | References |
|---|---|---|---|
| Electron Microscopy-based techniques | |||
| EM | High-resolution imaging and magnification | Requires chemical fixation that may induce artifacts Inability to image live cells Creation of monochromatic images | [16] |
| TEM | High-resolution ultrastructure imaging Provides information on both the structure and elemental composition | Low-throughput technique Potential for artifacts Inability to image live cells | [46,67] |
| FIB-SEM | 3D reconstruction through sequential imaging for detailed structural analysis Allows the study of contact dynamics High resolution | Slow data acquisition High-maintenance equipment Technically demanding | [69] |
| ET | Visualization of the full, native 3D architecture of complex samples like organelles Artifacts minimization | Limited tilting range of sample holder Technically demanding Inability to observe live cells | [67] |
| Super Resolution Fluorescent Microscopy-based techniques | |||
| SIM | Live cell imaging and dynamics Fast image acquisition speed Low phototoxicity 3D structure imaging Compatibility with conventional fluorescent probes | Lower lateral resolution Creation of artifacts during image reconstruction | [16,73,77] |
| STORM | Live-cell imaging and time-lapse High spatial resolution (10–30 nm) Individual multicolor fluorophore localization Compatibility with conventional fluorescent probes and organic dyes | Slow image acquisition Potential for photobleaching and phototoxicity | [52] |
| CLEM | Combination of fluorescence microscopy (multicolor labels) and electron microscopy (high resolution) Localization of molecules with the corresponding ultrastructural context | Low efficiency of available combinatorial probes Different requirements for sample preparation for the two techniques | [16,69,70] |
| Proximity-based biotinylation and fluorescence techniques | |||
| APEX2 | Rapid reaction and high spatiotemporal resolution High activity and specificity | High oxidative stress and toxicity due to H2O2 | [70] |
| FRET | Measures molecular distances in living cells in real-time High spatial resolution and sensitivity Can be used to study the dynamics of MCS | Requires the same expression of the donor and acceptor Possible photobleaching, cross-excitation, autofluorescence from cellular components, and bleed-through emission | [16] |
| SPLICS | In vitro and in vivo applications | Irreversibility of the GFP reconstitution | [34] |
| Tethering Proteins at MCS | Protein Alteration in AD | Evidence for Dysfunction | Investigated Models | Reference | |
|---|---|---|---|---|---|
| RAB7A-TBC1D15-FIS1 | FIS1 | Increased levels | Abnormal mitochondrial dynamics (fission) | AD brains and APP transgenic mice primary hippocampal neurons | [126,127] |
| RAB7A | Increased levels | Excessive mitochondrial fission Regulation of tau secretion | Neurons and CSF of MCI and AD patients | [129,130,131,132,133] | |
| TBC1D15 | No information | ||||
| Mfn2 | Reduced levels | Abnormal mitochondrial fusion and fragmentation Impaired iron transfer | AD brains and primary hippocampal neurons from APP transgenic mice, AD cybrids | [126,127,134] | |
| LAMP1-GDAP1 | LAMP1 | Increased levels | Impaired lysosomal functions Accumulation of lysosomes in amyloid plaques | CSF, neurons, glial cells of AD patients; AD mouse models, | [132,146,147,148] |
| LAMP1 | Reduced levels | Lysosomal dysfunction | AD mouse and cell models | [149] | |
| GDAP1 | No information | ||||
| VDAC1-TRPML1 | VDAC1 | Increased levels | Impaired calcium signaling Mitochondrial dysfunction | AD brains and cortical tissues from AD mouse models | [151,152] |
| TRPML1 | Reduced levels | Impaired calcium signaling Autophagy dysregulation | AD mouse models | [159,160] | |
| STAD3 | Increased levels | Increased mitochondrial cholesterol levels | AD mouse models | [163] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolimetti, G.; Calcagnile, M.; Bucci, C. Potential Role of Membrane Contact Sites in the Dysregulation of the Crosstalk Between Mitochondria and Lysosomes in Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9858. https://doi.org/10.3390/ijms26209858
Girolimetti G, Calcagnile M, Bucci C. Potential Role of Membrane Contact Sites in the Dysregulation of the Crosstalk Between Mitochondria and Lysosomes in Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(20):9858. https://doi.org/10.3390/ijms26209858
Chicago/Turabian StyleGirolimetti, Giulia, Matteo Calcagnile, and Cecilia Bucci. 2025. "Potential Role of Membrane Contact Sites in the Dysregulation of the Crosstalk Between Mitochondria and Lysosomes in Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 20: 9858. https://doi.org/10.3390/ijms26209858
APA StyleGirolimetti, G., Calcagnile, M., & Bucci, C. (2025). Potential Role of Membrane Contact Sites in the Dysregulation of the Crosstalk Between Mitochondria and Lysosomes in Alzheimer’s Disease. International Journal of Molecular Sciences, 26(20), 9858. https://doi.org/10.3390/ijms26209858







