Transthyretin Amyloidosis—One of the Causes of Heart Failure in Patients with Severe Clinical Course of COVID-19
Abstract
1. Introduction
2. Results
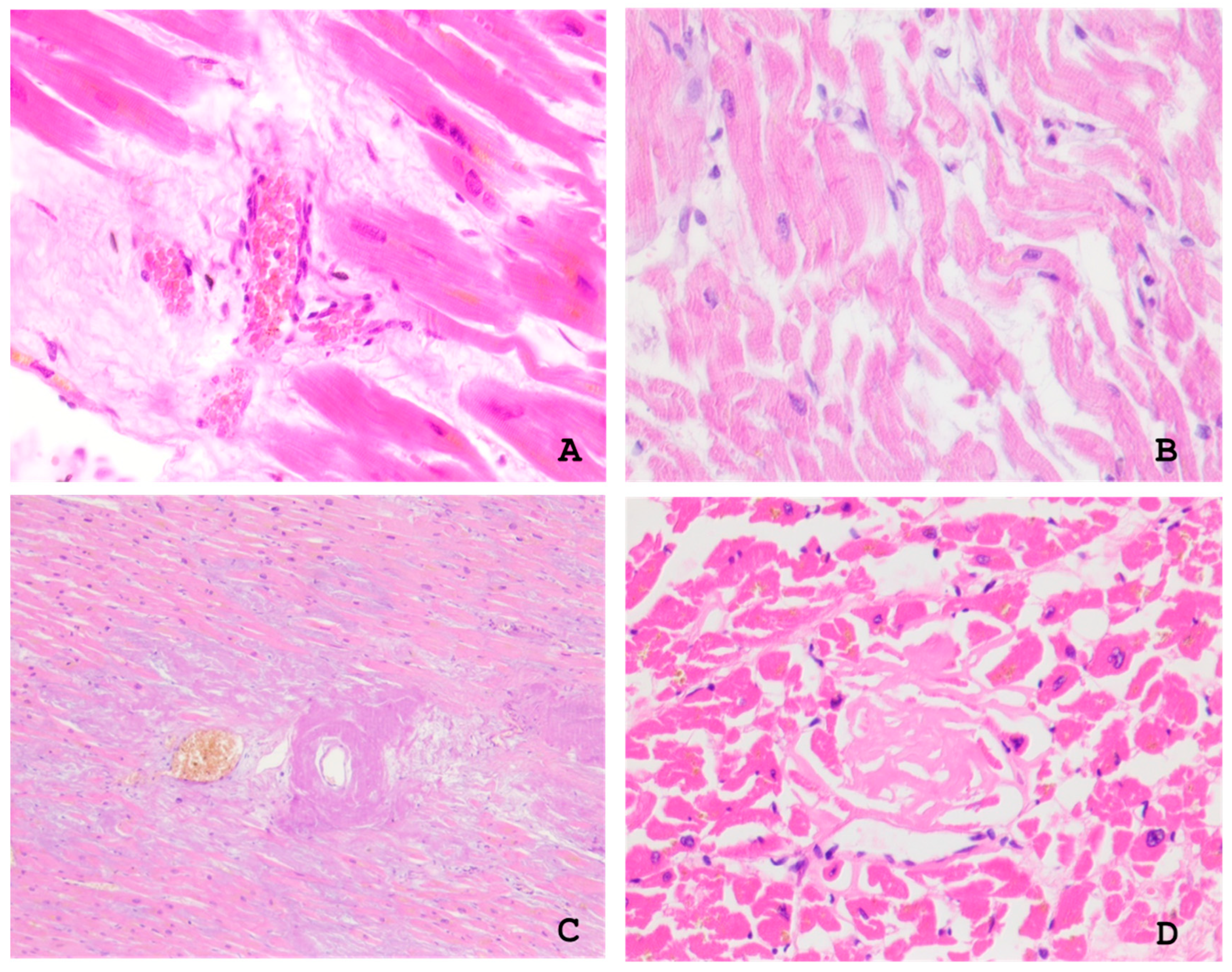
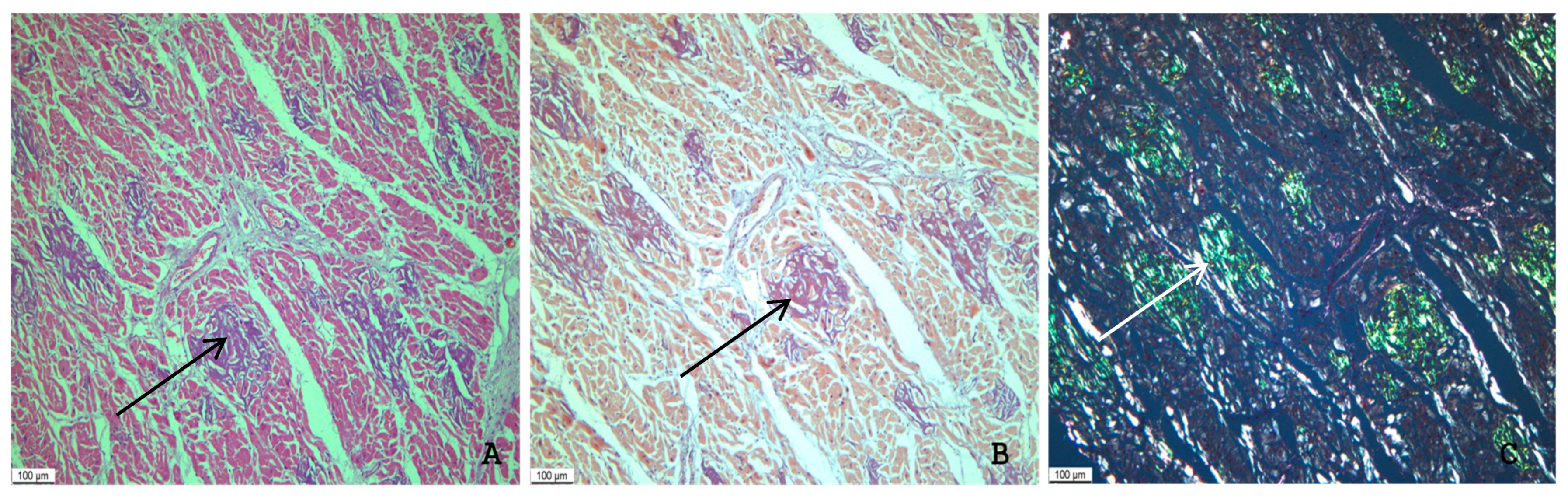
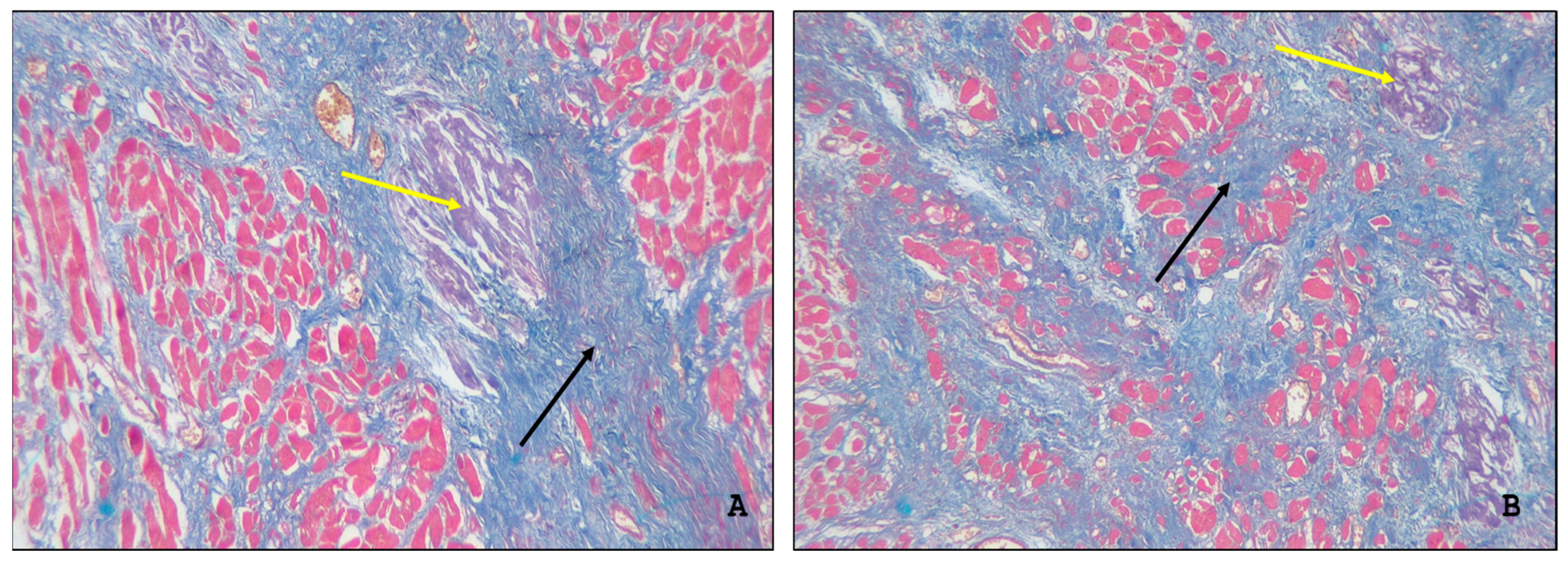
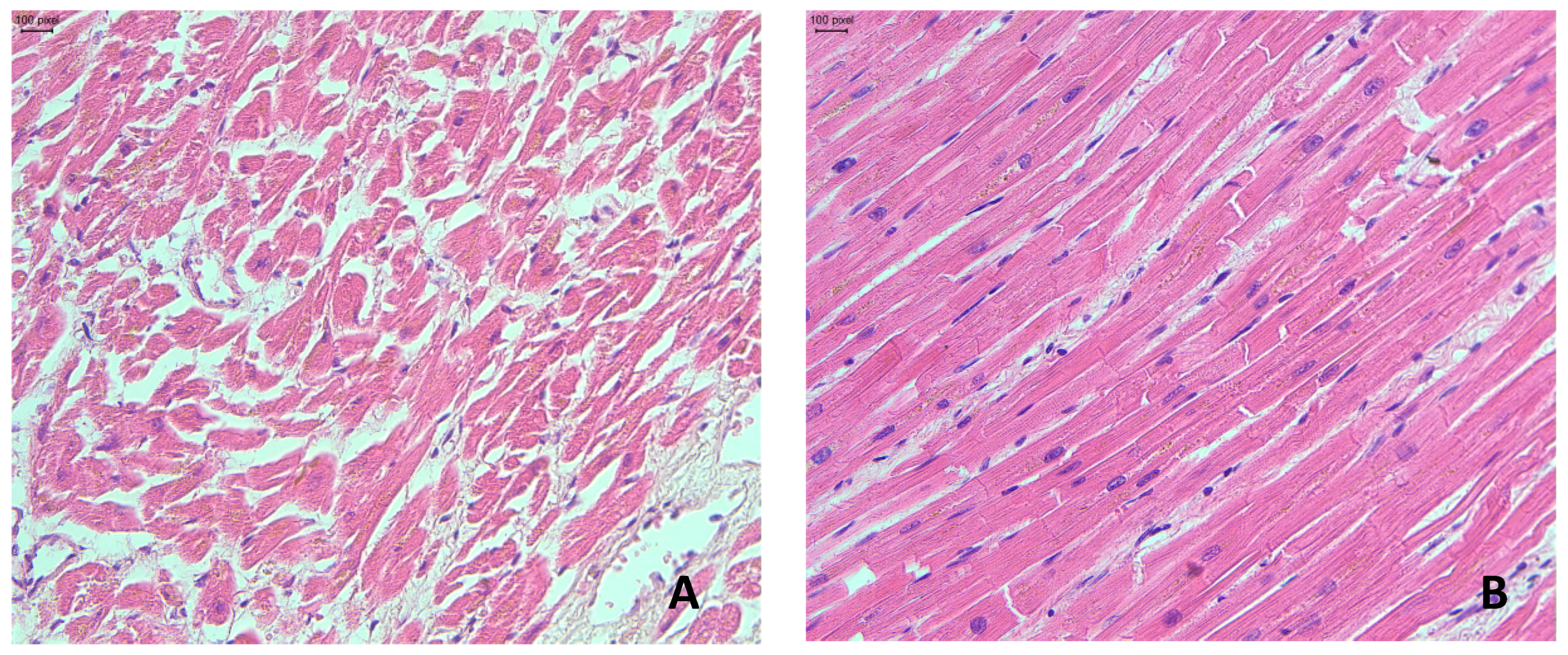
2.1. Gross and Microscopic Findings in the Myocardium
2.2. Immunohistochemical Typing of Amyloid
2.3. Assessment of the Intensity of Myocardial Damage by Amyloid
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATTR | Transthyretin amyloidosis |
| ATTR-CM | Transthyretin amyloid cardiomyopathy |
| ATTRwt | Wild-type transthyretin amyloidosis |
| ATTRv | Variant transthyretin amyloidosis |
| HF | Heart failure |
| COVID-19 | Coronavirus-19 disease |
| H&E | Hematoxylin and eosin |
| IHC | Immunohistochemistry |
References
- Bashir, Z.; Musharraf, M.; Azam, R.; Bukhari, S. Imaging modalities in cardiac amyloidosis. Curr. Probl. Cardiol. 2024, 49, 102858. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.; Albrecht, F.C.; de Arenaza, D.P.; Bart, N.; Better, N.; Carvajal-Juarez, I.; Conceição, I.; Damy, T.; Dorbala, S.; Fidalgo, J.C.; et al. World Heart Federation Consensus on Transthyretin Amyloidosis Cardiomyopathy (ATTR-CM). Glob. Heart. 2023, 18, 59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bart, N.K.; Bianchi, G.; Cuddy, S.A.M.; Goyal, P.; Griffin, J.M.; Hummel, S.L.; Macdonald, P.; Maurer, M.; Montgomery, E.; Nanne, M.G.; et al. Cardiac Amyloidosis in Older Adults with a Focus on Frailty: JACC: Advances Expert Consensus. JACC Adv. 2025, 4 Pt 1, 101784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porcari, A.; Bussani, R.; Merlo, M.; Varrà, G.G.; Pagura, L.; Rozze, D.; Sinagra, G. Incidence and Characterization of Concealed Cardiac Amyloidosis Among Unselected Elderly Patients Undergoing Post-mortem Examination. Front. Cardiovasc. Med. 2021, 8, 749523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gulati, J.S.; Pedretti, R.; Hendren, N.; Kozlitina, J.; Saelices, L.; Roth, L.R.; Grodin, J.L. Biomarkers in Subclinical Transthyretin Cardiac Amyloidosis. Curr. Heart Fail. Rep. 2025, 22, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basanta, B.; Nugroho, K.; Yan, N.L.; Kline, G.M.; Powers, E.T.; Tsai, F.J.; Wu, M.; Hansel-Harris, A.; Chen, J.S.; Forli, S.; et al. The conformational landscape of human transthyretin revealed by cryo-EM. Nat. Struct. Mol. Biol. 2025, 32, 876–883. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tschöpe, C.; Elsanhoury, A.; Kristen, A.V. Transthyretin Amyloid Cardiomyopathy-2025 Update: Current Diagnostic Approaches and Emerging Therapeutic Options. J. Clin. Med. 2025, 14, 4785. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laptseva, N.; Benz, D.C.; Schwotzer, R.; Flammer, A.J. Cardiac amyloidosis. Swiss Med. Wkly. 2024, 154, 4186. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A. Cardiac Amyloidosis. Heart Fail. Clin. 2022, 18, 479–488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukunari, A.; Matsushita, H.; Furukawa, T.; Matsuzaki, H.; Tanaka, H.; Ogawa, Y.; Sugimura, Y.; Inoue, F.; Ueda, M.; Ando, Y. Arginine: A potential prophylactic supplement for transthyretin amyloidosis. Biochem. Biophys. Res. Commun. 2024, 737, 150770. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.G.M.; Li, P.; Badrish, N.; Kesari, A.; Shah, K.B. Transthyretin Cardiac Amyloidosis: Current and Emerging Therapies. Curr. Cardiol. Rep. 2025, 27, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iino, T.; Nagao, M.; Tanaka, H.; Yoshikawa, S.; Asakura, J.; Nishimori, M.; Shinohara, M.; Harada, A.; Watanabe, S.; Ishida, T.; et al. Assessment of transthyretin instability in patients with wild-type transthyretin amyloid cardiomyopathy. Sci. Rep. 2024, 14, 20508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshinaga, T.; Yoshioka, Y.; Tsai, F.J.; Nelson, L.; Cheng, M.; Ito, R.; Fujita, S.; Ishikawa, E.; Kametani, F.; Aoyagi, R.; et al. Clinical and Biochemical Characterization of Hereditary ATTR Amyloidosis Caused by a Novel Transthyretin Variant V121A (p.V141A). Int. J. Mol. Sci. 2025, 26, 4659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obi, C.A.; Mostertz, W.C.; Griffin, J.M.; Judge, D.P. ATTR Epidemiology, Genetics, and Prognostic Factors. Methodist Debakey Cardiovasc. J. 2022, 18, 17–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ruberg, F.L.; Maurer, M.S. Cardiac Amyloidosis Due to Transthyretin Protein: A Review. JAMA 2024, 331, 778–791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koike, H.; Okumura, T.; Murohara, T.; Katsuno, M. Multidisciplinary Approaches for Transthyretin Amyloidosis. Cardiol. Ther. 2021, 10, 289–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kallash, M.; Frishman, W.H. Transthyretin Amyloid Cardiomyopathy: A Review of Approved Pharmacotherapies. Cardiol. Rev. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Briasoulis, A.; Xanthopoulos, A. Amyloids and the Heart: An Update. J. Clin. Med. 2024, 13, 7210. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia-Pavia, P.; Damy, T.; Piriou, N.; Barriales-Villa, R.; Cappelli, F.; Bahus, C.; Munteanu, C.; Keohane, D.; Mallaina, P.; Elliott, P.; et al. Prevalence and characteristics of transthyretin amyloid cardiomyopathy in hypertrophic cardiomyopathy. ESC Heart Fail. 2024, 11, 4314–4324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Movila, D.E.; Motofelea, A.C.; Cozma, D.; Albai, O.; Sima, A.C.; Andor, M.; Ciocarlie, T.; Dragan, S.R. Cardiac Amyloidosis: A Narrative Review of Diagnostic Advances and Emerging Therapies. Biomedicines 2025, 13, 1230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brannagan, T.H., 3rd; Auer-Grumbach, M.; Berk, J.L.; Briani, C.; Bril, V.; Coelho, T.; Damy, T.; Dispenzieri, A.; Drachman, B.M.; Fine, N.; et al. ATTR amyloidosis during the COVID-19 pandemic: Insights from a global medical roundtable. Orphanet J. Rare Dis. 2021, 16, 204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, K.; Sinclair, J.E.; Sadeghirad, H.; Fraser, J.F.; Short, K.R.; Kulasinghe, A. Cardiovascular disease in SARS-CoV-2 infection. Clin. Transl. Immunol. 2021, 10, e1343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruno, R.R.; Wernly, B.; Wolff, G.; Fjølner, J.; Artigas, A.; Bollen Pinto, B.; Schefold, J.C.; Kindgen-Milles, D.; Baldia, P.H.; Kelm, M.; et al. Association of chronic heart failure with mortality in old intensive care patients suffering from COVID-19. ESC Heart Fail. 2022, 9, 1756–1765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Basu-Ray, I.; Almaddah, N.K.; Adeboye, A.; Soos, M.P. Cardiac Manifestations of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Sang, C.J., 3rd; Burkett, A.; Heindl, B.; Litovsky, S.H.; Prabhu, S.D.; Benson, P.V.; Rajapreyar, I. Cardiac pathology in COVID-19: A single center autopsy experience. Cardiovasc. Pathol. 2021, 54, 107370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haslbauer, J.D.; Tzankov, A.; Mertz, K.D.; Schwab, N.; Nienhold, R.; Twerenbold, R.; Leibundgut, G.; Stalder, A.K.; Matter, M.; Glatz, K. Characterisation of cardiac pathology in 23 autopsies of lethal COVID-19. J. Pathol. Clin. Res. 2021, 7, 326–337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer-Gómez, A.; Pian-Arias, H.; Carretero-Barrio, I.; Navarro-Cantero, A.; Pestaña, D.; de Pablo, R.; Zamorano, J.L.; Galán, J.C.; Pérez-Mies, B.; Ruz-Caracuel, I.; et al. Late Cardiac Pathology in Severe COVID-19. A Postmortem Series of 30 Patients. Front. Cardiovasc. Med. 2021, 8, 748396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mikhaleva, L.; Gioeva, Z.; Varyasin, V.; Berezhnaja, E.; Vandysheva, R.; Gutyrchik, N.; Pechnikova, V.; Kontorshchikov, A.; Midiber, K.; Kakturskij, L. Pathomorphological Features of the Novel Coronavirus Disease in Patients with Systemic Amyloidosis. Biomedicines 2023, 11, 2811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Frank, S.; Turek, D.; Willi, N.; Pargger, H.; et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duca, F.; Rettl, R.; Kronberger, C.; Binder, C.; Mann, C.; Dusik, F.; Schrutka, L.; Dalos, D.; Öztürk, B.; Dachs, T.M.; et al. Myocardial structural and functional changes in cardiac amyloidosis: Insights from a prospective observational patient registry. Eur. Heart J. Cardiovasc. Imaging 2023, 25, 95–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hein, S.J.; Knoll, M.; Aus dem Siepen, F.; Furkel, J.; Schoenland, S.; Hegenbart, U.; Katus, H.A.; Kristen, A.V.; Konstandin, M. Elevated interleukin-6 levels are associated with impaired outcome in cardiac transthyretin amyloidosis. World J. Cardiol. 2021, 13, 55–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Fine, N.; Miller, R.J.H.; Hahn, C.; Chhibber, S.; Mahe, E.; Tay, J.; Duggan, P.; McCulloch, S.; Bahlis, N.; et al. Amyloidosis and COVID-19: Experience from an amyloid program in Canada. Ann. Hematol. 2022, 101, 2307–2315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al Hamed, R.; Bazarbachi, A.H.; Bazarbachi, A.; Malard, F.; Harousseau, J.L.; Mohty, M. Comprehensive Review of AL amyloidosis: Some practical recommendations. Blood Cancer J. 2021, 11, 97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| Patient No. | Gender | Age (Years) | Primary Diagnosis | Immediate Cause of Death | Comorbidities | Length of Hospital Stay (Days) | Intensity of Amyloid Deposits in the Heart * |
|---|---|---|---|---|---|---|---|
| 1 | M | 83 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Primary hypertension involving the kidneys. Cardiac amyloidosis | 11 | Grade 2 |
| 2 | M | 88 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Diffuse small-focal cardiosclerosis Cardiac amyloidosis | 3 | Grade 3 |
| 3 | F | 80 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Primary hypertension Amyloidosis involving the heart and lungs | 19 | Grade 1 |
| 4 | M | 85 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Amyloidosis involving the heart, hepatic and pulmonary vessels | 8 | Grade 3 |
| 5 | M | 87 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Diffuse small-focal cardiosclerosis. Cardiac amyloidosis | 7 | Grade 1 |
| 6 | M | 95 | Postinfarction cardiosclerosis | Myocardial infarction | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 1 | Grade 2 |
| 7 | M | 95 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Aortic atherosclerosis (Grade 3, Stage IV). Cardiac amyloidosis. Gallstone disease: chronic cholecystitis. Kidney cysts. Chronic inactive tubulointerstitial nephritis | 11 | Grade 3 |
| 8 | F | 97 | Postinfarction cardiosclerosis. | Myocardial infarction | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 6 | Grade 2 |
| 9 | F | 92 | SARS-CoV-2 infection (COVID-19) | Cardiopulmonary decompensation | Cerebral atherosclerosis, encephalopathy. Cardiac amyloidosis | 1 | Grade 3 |
| 10 | F | 90 | Postinfarction cardiosclerosis | Myocardial infarction | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 4 | Grade 1 |
| 11 | M | 94 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Diffuse small-focal cardiosclerosis. Left atrial appendage thrombosis. Cardiac amyloidosis | 8 | Grade 2 |
| 12 | M | 88 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Diffuse small-focal cardiosclerosis. Cardiac amyloidosis | 2 | Grade 3 |
| 13 | F | 85 | Infective endocarditis of the mitral valve. Atherosclerotic foot gangrene | Sepsis. Metabolic myocardial necrosis | SARS-CoV-2 infection (COVID-19). Cardiac amyloidosis. Gallbladder cancer with metastases in lymph nodes of the hepatic hilum and lesser gastric curvature | 2 | Grade 1 |
| 14 | F | 89 | Postinfarction cardiosclerosis | Myocardial infarction | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 5 | Grade 2 |
| 15 | F | 93 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Cerebral atherosclerosis, encephalopathy. Amyloidosis affecting the heart, kidneys, pancreas, and lungs | 11 | Grade 1 |
| 16 | M | 94 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Chronic cerebral ischemia. Cardiac amyloidosis. Arteriolar nephrosclerosis | 17 | Grade 1 |
| 17 | F | 89 | Ischemic infarction in the left parietotemporal area | Cerebral edema | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 2 | Grade 3 |
| 18 | M | 94 | SARS-CoV-2 infection (COVID-19) | Acute respiratory distress syndrome | Primary hypertension. Cardiac amyloidosis | 12 | Grade 2 |
| 19 | F | 89 | Postinfarction cardiosclerosis | Myocardial infarction | SARS-CoV-2 infection (COVID-19) Cardiac amyloidosis | 8 | Grade 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioeva, Z.; Mikhaleva, L.; Gutyrchik, N.; Shakhpazyan, N.; Pechnikova, V.; Midiber, K.; Kontorshchikov, A.; Zentsova, E.; Kakturskij, L. Transthyretin Amyloidosis—One of the Causes of Heart Failure in Patients with Severe Clinical Course of COVID-19. Int. J. Mol. Sci. 2025, 26, 9806. https://doi.org/10.3390/ijms26199806
Gioeva Z, Mikhaleva L, Gutyrchik N, Shakhpazyan N, Pechnikova V, Midiber K, Kontorshchikov A, Zentsova E, Kakturskij L. Transthyretin Amyloidosis—One of the Causes of Heart Failure in Patients with Severe Clinical Course of COVID-19. International Journal of Molecular Sciences. 2025; 26(19):9806. https://doi.org/10.3390/ijms26199806
Chicago/Turabian StyleGioeva, Zarina, Liudmila Mikhaleva, Nikita Gutyrchik, Nikolay Shakhpazyan, Valentina Pechnikova, Konstantin Midiber, Andrej Kontorshchikov, Elizaveta Zentsova, and Lev Kakturskij. 2025. "Transthyretin Amyloidosis—One of the Causes of Heart Failure in Patients with Severe Clinical Course of COVID-19" International Journal of Molecular Sciences 26, no. 19: 9806. https://doi.org/10.3390/ijms26199806
APA StyleGioeva, Z., Mikhaleva, L., Gutyrchik, N., Shakhpazyan, N., Pechnikova, V., Midiber, K., Kontorshchikov, A., Zentsova, E., & Kakturskij, L. (2025). Transthyretin Amyloidosis—One of the Causes of Heart Failure in Patients with Severe Clinical Course of COVID-19. International Journal of Molecular Sciences, 26(19), 9806. https://doi.org/10.3390/ijms26199806





