Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease
Abstract
1. Introduction
2. The Blood–Brain Barrier in Health and Alzheimer’s Disease
2.1. Blood–Brain Barrier Structure and Function
2.2. Blood–Brain Barrier Dysfunction in AD
3. Transferrin Receptor 1: Biology and Function
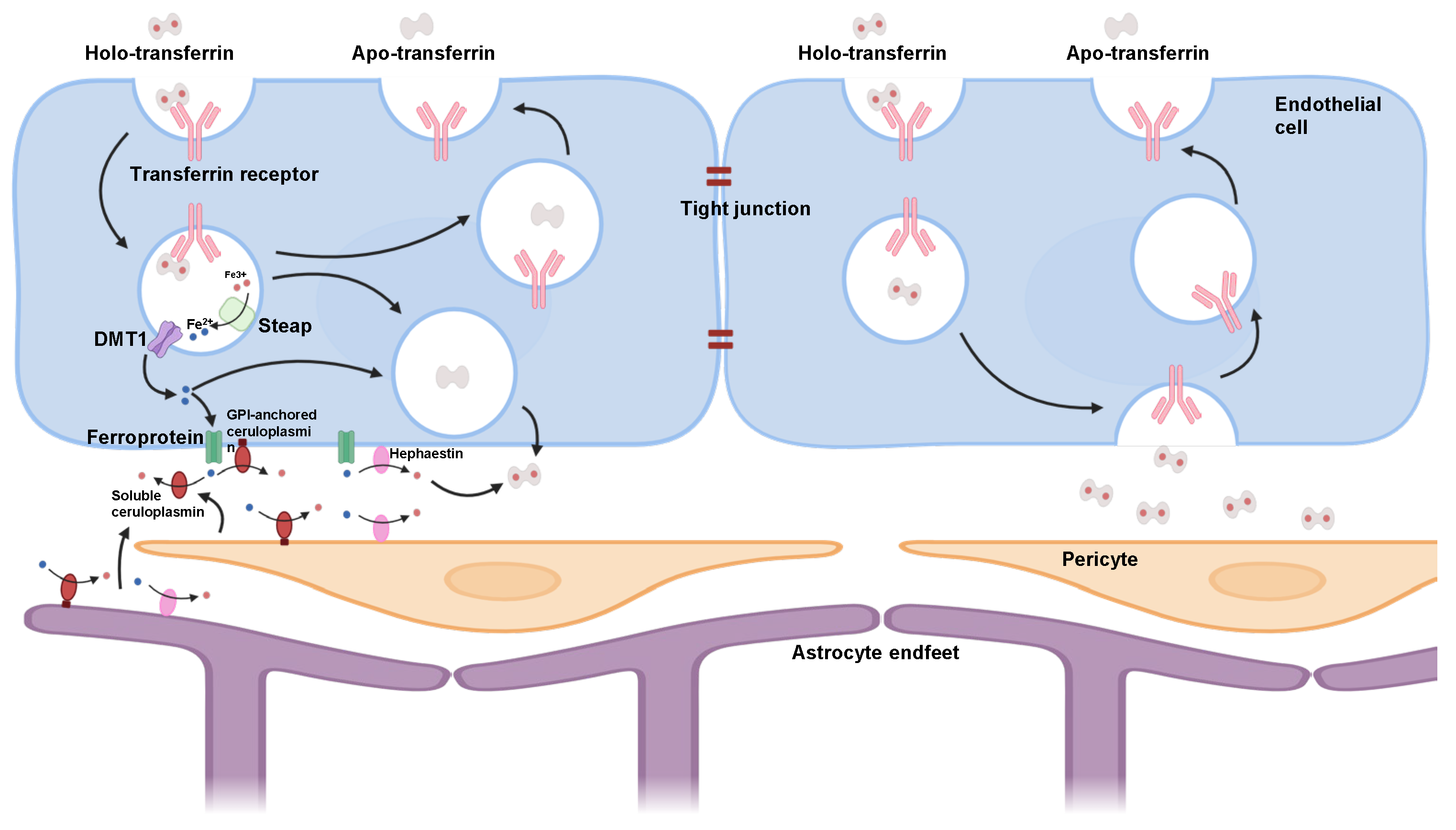
3.1. Transferrin Receptor 1 Structure and Function
3.2. Transferrin Receptor 1 in Alzheimer’s Disease
4. Transferrin Receptor 1-Mediated Drug Delivery Strategies and Challenges
4.1. Receptor-Mediated Transcytosis Principles
4.2. Antibody-Based Strategies
4.2.1. Monoclonal Antibodies
4.2.2. Antibody Fragments
4.3. Ligand-Based Strategies
4.3.1. Transferrin Conjugation
4.3.2. Peptide Mimetics
4.4. Positioning TfR1 Relative to Alternative BBB Receptors
4.5. Challenges in Translating TfR1-Targeted Therapies
4.6. Hybrid or Combination RMT Strategies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2024 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The Neuropathological Diagnosis of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s Disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Alzheimer’s Disease: Future Drug Development and the Blood-Brain Barrier. Expert Opin. Investig. Drugs 2019, 28, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.A.; Bernardes, C.; Bernardo-Castro, S.; Lino, M.; Albino, I.; Ferreira, L.; Brás, J.; Guerreiro, R.; Tábuas-Pereira, M.; Baldeiras, I.; et al. Reconsidering the Role of Blood-Brain Barrier in Alzheimer’s Disease: From Delivery to Target. Front. Aging Neurosci. 2023, 15, 1102809. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The Blood–Brain Barrier: Structure, Regulation and Drug Delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Hersh, S.D.; Wadajkar, S.A.; Roberts, B.N.; Perez, G.J.; Connolly, P.N.; Frenkel, V.; Winkles, A.J.; Woodworth, F.G.; Kim, J.A. Evolving Drug Delivery Strategies to Overcome the Blood Brain Barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Pardridge, W.M. Receptor-Mediated Drug Delivery of Bispecific Therapeutic Antibodies through the Blood-Brain Barrier. Front. Drug Deliv. 2023, 3, 1227816. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Lui, P.C.; Li, J.Y. Receptor-Mediated Therapeutic Transport Across the Blood–Brain Barrier. Immunotherapy 2009, 1, 983–993. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting Transferrin Receptors at the Blood-Brain Barrier Improves the Uptake of Immunoliposomes and Subsequent Cargo Transport into the Brain Parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef]
- Luissint, A.-C.; Artus, C.; Glacial, F.; Ganeshamoorthy, K.; Couraud, P.-O. Tight Junctions at the Blood Brain Barrier: Physiological Architecture and Disease-Associated Dysregulation. Fluids Barriers CNS 2012, 9, 23. [Google Scholar] [CrossRef]
- Huttunen, J.; Adla, S.K.; Markowicz-Piasecka, M.; Huttunen, K.M. Increased/Targeted Brain (Pro)Drug Delivery via Utilization of Solute Carriers (SLCs). Pharmaceutics 2022, 14, 1234. [Google Scholar] [CrossRef]
- Xiao, M.; Hu, G. Involvement of Aquaporin 4 in Astrocyte Function and Neuropsychiatric Disorders. CNS Neurosci. Ther. 2014, 20, 385–390. [Google Scholar] [CrossRef]
- Kaplan, L.; Chow, B.W.; Gu, C. Neuronal Regulation of the Blood–Brain Barrier and Neurovascular Coupling. Nat. Rev. Neurosci. 2020, 21, 416–432. [Google Scholar] [CrossRef]
- Nehra, G.; Bauer, B.; Hartz, A.M.S. Blood-Brain Barrier Leakage in Alzheimer’s Disease: From Discovery to Clinical Relevance. Pharmacol. Ther. 2022, 234, 108119. [Google Scholar] [CrossRef] [PubMed]
- Alruwais, N.M.; Rusted, J.M.; Tabet, N.; Dowell, N.G. Evidence of Emerging BBB Changes in Mid-age Apolipoprotein E Epsilon-4 Carriers. Brain Behav. 2022, 12, e2806. [Google Scholar] [CrossRef] [PubMed]
- Ha, I.H.; Lim, C.; Kim, Y.; Moon, Y.; Han, S.-H.; Moon, W.-J. Regional Differences in Blood-Brain Barrier Permeability in Cognitively Normal Elderly Subjects: A Dynamic Contrast-Enhanced MRI-Based Study. Korean J. Radiol. 2021, 22, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Pathak, A.; Samaiya, P.K. Alzheimer’s Disease: From Early Pathogenesis to Novel Therapeutic Approaches. Metab. Brain Dis. 2024, 39, 1231–1254. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.-Y.; Li, H.-Y.; He, C.-Y.; Shen, Y.-Y.; Zeng, G.-H.; Chen, D.-W.; Yi, X.; Ma, Y.-H.; Yu, J.-T.; et al. Dynamic Changes of CSF sPDGFRβ during Ageing and AD Progression and Associations with CSF ATN Biomarkers. Mol. Neurodegener. 2022, 17, 9. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood-Brain Barrier Breakdown Is an Early Biomarker of Human Cognitive Dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Van Hulle, C.; Ince, S.; Okonkwo, O.C.; Bendlin, B.B.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Love, S.; Blennow, K.; Zetterberg, H.; et al. Elevated CSF Angiopoietin-2 Correlates with Blood-Brain Barrier Leakiness and Markers of Neuronal Injury in Early Alzheimer’s Disease. Transl. Psychiatry 2024, 14, 3. [Google Scholar] [CrossRef]
- Cooper, J.M.; Lathuiliere, A.; Migliorini, M.; Arai, A.L.; Wani, M.M.; Dujardin, S.; Muratoglu, S.C.; Hyman, B.T.; Strickland, D.K. Regulation of Tau Internalization, Degradation, and Seeding by LRP1 Reveals Multiple Pathways for Tau Catabolism. J. Biol. Chem. 2021, 296, 100715. [Google Scholar] [CrossRef]
- Zlokovic, B.V. Cerebrovascular Effects of Apolipoprotein E: Implications for Alzheimer’s Disease. JAMA Neurol. 2013, 70, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Sagare, A.P.; Bell, R.D.; Zlokovic, B.V. Neurovascular Defects and Faulty Amyloid-β Vascular Clearance in Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 33, S87–S100. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Portugal, C.C.; Relvas, J.; Socodato, R. From Genetics to Neuroinflammation: The Impact of ApoE4 on Microglial Function in Alzheimer’s Disease. Cells 2025, 14, 243. [Google Scholar] [CrossRef]
- Li, H.; Shen, X.; Zhang, B.; Zhu, Z. Biologics as Therapeutical Agents Under Perspective Clinical Studies for Alzheimer’s Disease. Molecules 2025, 30, 3479. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; Rojas, J.C.; et al. ApoE4 Markedly Exacerbates Tau-Mediated Neurodegeneration in a Mouse Model of Tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Schmukler, E.; Solomon, S.; Simonovitch, S.; Goldshmit, Y.; Wolfson, E.; Michaelson, D.M.; Pinkas-Kramarski, R. Altered Mitochondrial Dynamics and Function in APOE4-Expressing Astrocytes. Cell Death Dis. 2020, 11, 578. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Situ, M.; Citalan-Madrid, A.F.; Stamatovic, S.M.; Xiang, J.; Keep, R.F. Blood-Brain Barrier Dysfunction in Normal Aging and Neurodegeneration: Mechanisms, Impact and Treatments. Stroke 2023, 54, 661–672. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Yang, J.; Ronaldson, P.T.; Davis, T.P. Structure, Function, and Regulation of the Blood-Brain Barrier Tight Junction in Central Nervous System Disorders. Front. Physiol. 2020, 11, 914. [Google Scholar] [CrossRef]
- Darabi, S.; Gorgich, E.A.C.; Moradi, F.; Rustamzadeh, A. Lipidopathy Disrupts Peripheral and Central Amyloid Clearance in Alzheimer’s Disease: Where Are Our Knowledge. IBRO Neurosci. Rep. 2025, 18, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Pialoux, V.; Mounier, R.; Brown, A.D.; Steinback, C.D.; Rawling, J.M.; Poulin, M.J. Relationship between Oxidative Stress and HIF-1α mRNA during Sustained Hypoxia in Humans. Free Radic. Biol. Med. 2009, 46, 321–326. [Google Scholar] [CrossRef]
- Divecha, Y.A.; Rampes, S.; Tromp, S.; Boyanova, S.T.; Fleckney, A.; Fidanboylu, M.; Thomas, S.A. The Microcirculation, the Blood-Brain Barrier, and the Neurovascular Unit in Health and Alzheimer Disease: The Aberrant Pericyte Is a Central Player. Pharmacol. Rev. 2025, 77, 100052. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ji, Y.; Wen, C.; Gan, J.; Chen, Z.; Li, R.; Lin, X.; Dou, J.; Wang, Y.; Liu, S.; et al. Tracer Kinetic Model Detecting Heterogeneous Blood–Brain Barrier Permeability to Water and Contrast Agent in Alzheimer’s Disease and Dementia with Lewy Bodies. Alzheimer’s Dement. 2025, 21, e14529. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Does Synaptic Hypometabolism or Synaptic Dysfunction, Originate Cognitive Loss? Analysis of the Evidence. Alzheimer’s Dement. 2021, 7, e12177. [Google Scholar] [CrossRef]
- van Assema, D.M.E.; Lubberink, M.; Bauer, M.; van der Flier, W.M.; Schuit, R.C.; Windhorst, A.D.; Comans, E.F.I.; Hoetjes, N.J.; Tolboom, N.; Langer, O.; et al. Blood–Brain Barrier P-Glycoprotein Function in Alzheimer’s Disease. Brain 2012, 135, 181–189. [Google Scholar] [CrossRef]
- Ramanathan, A.; Nelson, A.R.; Sagare, A.P.; Zlokovic, B.V. Impaired Vascular-Mediated Clearance of Brain Amyloid Beta in Alzheimer’s Disease: The Role, Regulation and Restoration of LRP1. Front. Aging Neurosci. 2015, 7, 136. [Google Scholar] [CrossRef]
- Miners, J.S.; Kehoe, P.G.; Love, S.; Zetterberg, H.; Blennow, K. CSF Evidence of Pericyte Damage in Alzheimer’s Disease Is Associated with Markers of Blood-Brain Barrier Dysfunction and Disease Pathology. Alzheimer’s Res. Ther. 2019, 11, 81. [Google Scholar] [CrossRef]
- Uchida, Y.; Kan, H.; Sakurai, K.; Oishi, K.; Matsukawa, N. Contributions of Blood–Brain Barrier Imaging to Neurovascular Unit Pathophysiology of Alzheimer’s Disease and Related Dementias. Front. Aging Neurosci. 2023, 15, 1111448. [Google Scholar] [CrossRef]
- Prajapati, S.K.; Shah, R.; Alford, N.; Mishra, S.P.; Jain, S.; Hansen, B.; Sanberg, P.; Molina, A.J.A.; Yadav, H. The Triple Alliance: Microbiome, Mitochondria, and Metabolites in the Context of Age-Related Cognitive Decline and Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 2187–2202. [Google Scholar] [CrossRef]
- Howe, M.D.; McCullough, L.D.; Urayama, A. The Role of Basement Membranes in Cerebral Amyloid Angiopathy. Front. Physiol. 2020, 11, 601320. [Google Scholar] [CrossRef] [PubMed]
- Eckenroth, B.E.; Steere, A.N.; Chasteen, N.D.; Everse, S.J.; Mason, A.B. How the Binding of Human Transferrin Primes the Transferrin Receptor Potentiating Iron Release at Endosomal pH. Proc. Natl. Acad. Sci. USA 2011, 108, 13089–13094. [Google Scholar] [CrossRef] [PubMed]
- Leischner, C.; Marongiu, L.; Piotrowsky, A.; Niessner, H.; Venturelli, S.; Burkard, M.; Renner, O. Relevant Membrane Transport Proteins as Possible Gatekeepers for Effective Pharmacological Ascorbate Treatment in Cancer. Antioxidants 2023, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.D.; Schmid, S.L. Differential Requirements for AP-2 in Clathrin-Mediated Endocytosis. J. Cell Biol. 2003, 162, 773–780. [Google Scholar] [CrossRef]
- Steere, A.N.; Byrne, S.L.; Chasteen, N.D.; Smith, V.C.; MacGillivray, R.T.A.; Mason, A.B. Evidence That His349 Acts as a pH-Inducible Switch to Accelerate Receptor-Mediated Iron Release from the C-Lobe of Human Transferrin. J. Biol. Inorg. Chem. 2010, 15, 1341–1352. [Google Scholar] [CrossRef][Green Version]
- Long, H.; Zhu, W.; Wei, L.; Zhao, J. Iron Homeostasis Imbalance and Ferroptosis in Brain Diseases. MedComm 2023, 4, e298. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, Y.; Kim, S.E.; An, J.-Y. Ferroptosis-Related Genes in Neurodevelopment and Central Nervous System. Biology 2021, 10, 35. [Google Scholar] [CrossRef]
- Xiang, Y.; Song, X.; Long, D. Ferroptosis Regulation through Nrf2 and Implications for Neurodegenerative Diseases. Arch. Toxicol. 2024, 98, 579–615. [Google Scholar] [CrossRef]
- Goldberg, J.M.; Loas, A.; Lippard, S.J. Metalloneurochemistry and the Pierian Spring: ‘Shallow Draughts Intoxicate the Brain’. Isr. J. Chem. 2016, 56, 791–802. [Google Scholar] [CrossRef]
- Marrocco, F.; Falvo, E.; Mosca, L.; Tisci, G.; Arcovito, A.; Reccagni, A.; Limatola, C.; Bernardini, R.; Ceci, P.; D’Alessandro, G.; et al. Nose-to-Brain Selective Drug Delivery to Glioma via Ferritin-Based Nanovectors Reduces Tumor Growth and Improves Survival Rate. Cell Death Dis. 2024, 15, 262. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Alata, W.; Tremblay, C.; Paris-Robidas, S.; Calon, F. Transferrin Receptor-Mediated Uptake at the Blood–Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Mol. Pharm. 2019, 16, 583–594. [Google Scholar] [CrossRef]
- Petralla, S.; Saveleva, L.; Kanninen, K.M.; Oster, J.S.; Panayotova, M.; Fricker, G.; Puris, E. Increased Expression of Transferrin Receptor 1 in the Brain Cortex of 5xFAD Mouse Model of Alzheimer’s Disease Is Associated with Activation of HIF-1 Signaling Pathway. Mol. Neurobiol. 2024, 61, 6383–6394. [Google Scholar] [CrossRef]
- Lemarchant, S.; Engelhardt, B.; Cicchetti, F.; Bix, G.J.; Janus, A.; Godfrin, Y.; Blasco, H.; Campbell, M.; de Rus Jacquet, A. Restoring Brain Barriers: An Innovative Approach for Treating Neurological Disorders. Fluids Barriers CNS 2025, 22, 72. [Google Scholar] [CrossRef]
- Altuwaijri, N.; Atef, E. Transferrin-Conjugated Nanostructured Lipid Carriers for Targeting Artemisone to Melanoma Cells. Int. J. Mol. Sci. 2024, 25, 9119. [Google Scholar] [CrossRef]
- Roche Initiates Phase III Clinical Trial of Actemra/RoActemra Plus Remdesivir in Hospitalised Patients with Severe COVID-19 Pneumonia. Available online: https://www.roche.com/media/releases/med-cor-2020-05-28 (accessed on 29 September 2025).
- Mayle, K.M.; Le, A.M.; Kamei, D.T. The Intracellular Trafficking Pathway of Transferrin. Biochim. Biophys. Acta (BBA)—General. Subj. 2012, 1820, 264–281. [Google Scholar] [CrossRef]
- Rossatti, P.; Redpath, G.M.I.; Ziegler, L.; Samson, G.P.B.; Clamagirand, C.D.; Legler, D.F.; Rossy, J. Rapid Increase in Transferrin Receptor Recycling Promotes Adhesion during T Cell Activation. BMC Biol. 2022, 20, 189. [Google Scholar] [CrossRef]
- Leonard, D.; Hayakawa, A.; Lawe, D.; Lambright, D.; Bellve, K.D.; Standley, C.; Lifshitz, L.M.; Fogarty, K.E.; Corvera, S. Sorting of EGF and Transferrin at the Plasma Membrane and by Cargo-Specific Signaling to EEA1-Enriched Endosomes. J. Cell Sci. 2008, 121, 3445–3458. [Google Scholar] [CrossRef]
- Trischler, M.; Stoorvogel, W.; Ullrich, O. Biochemical Analysis of Distinct Rab5- and Rab11-Positive Endosomes along the Transferrin Pathway. J. Cell Sci. 1999, 112 Pt 24, 4773–4783. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Bélanger, K.; Stanimirovic, D.B. Receptor-Mediated Transcytosis for Brain Delivery of Therapeutics: Receptor Classes and Criteria. Front. Drug Deliv. 2024, 4, 1360302. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Wang, B.; Ding, Y.; Zhang, Y.; Yu, P.; Chang, Y.-Z.; Gao, G. The Role of Iron Transporters and Regulators in Alzheimer’s Disease and Parkinson’s Disease: Pathophysiological Insights and Therapeutic Prospects. Biomed. Pharmacother. 2024, 179, 117419. [Google Scholar] [CrossRef]
- Xue, P.; Long, Z.; Jiang, G.; Wang, L.; Bian, C.; Wang, Y.; Chen, M.F.; Li, W. The Role of LRP1 in Aβ Efflux Transport across the Blood-Brain Barrier and Cognitive Dysfunction in Diabetes Mellitus. Neurochem. Int. 2022, 160, 105417. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, L.B.; Dohgu, S.; Hwang, M.C.; Farr, S.A.; Murphy, M.P.; Fleegal-DeMotta, M.A.; Lynch, J.L.; Robinson, S.M.; Niehoff, M.L.; Johnson, S.N.; et al. Testing the Neurovascular Hypothesis of Alzheimer’s Disease: LRP-1 Antisense Reduces Blood-Brain Barrier Clearance, Increases Brain Levels of Amyloid-β Protein, and Impairs Cognition. J. Alzheimer’s Dis. 2009, 17, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, T.; Bu, G. The Low-Density Lipoprotein Receptor-Related Protein 1 and Amyloid-β Clearance in Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Tremblay, C.; Bourassa, P.; Schneider, J.A.; Bennett, D.A.; Calon, F. Lower GLUT1 and Unchanged MCT1 in Alzheimer’s Disease Cerebrovasculature. J. Cereb. Blood Flow. Metab. 2024, 44, 1417–1432. [Google Scholar] [CrossRef]
- Winkler, E.A.; Nishida, Y.; Sagare, A.P.; Rege, S.V.; Bell, R.D.; Perlmutter, D.; Sengillo, J.D.; Hillman, S.; Kong, P.; Nelson, A.R.; et al. GLUT1 Reductions Exacerbate Alzheimer’s Disease Vasculoneuronal Dysfunction and Degeneration. Nat. Neurosci. 2015, 18, 521–530. [Google Scholar] [CrossRef]
- Kyrtata, N.; Emsley, H.C.A.; Sparasci, O.; Parkes, L.M.; Dickie, B.R. A Systematic Review of Glucose Transport Alterations in Alzheimer’s Disease. Front. Neurosci. 2021, 15, 626636. [Google Scholar] [CrossRef]
- Leclerc, M.; Bourassa, P.; Tremblay, C.; Caron, V.; Sugère, C.; Emond, V.; Bennett, D.A.; Calon, F. Cerebrovascular Insulin Receptors Are Defective in Alzheimer’s Disease. Brain 2022, 146, 75–90. [Google Scholar] [CrossRef]
- Sabbir, M.G. Loss of Calcium/Calmodulin-Dependent Protein Kinase Kinase 2, Transferrin, and Transferrin Receptor Proteins in the Temporal Cortex of Alzheimer’s Patients Postmortem Is Associated with Abnormal Iron Homeostasis: Implications for Patient Survival. Front. Cell Dev. Biol. 2024, 12, 1469751. [Google Scholar] [CrossRef]
- Baghirov, H. Mechanisms of Receptor-Mediated Transcytosis at the Blood-Brain Barrier. J. Control. Release 2025, 381, 113595. [Google Scholar] [CrossRef]
- Bucci, C.; Alifano, P.; Cogli, L. The Role of Rab Proteins in Neuronal Cells and in the Trafficking of Neurotrophin Receptors. Membranes 2014, 4, 642–677. [Google Scholar] [CrossRef] [PubMed]
- Stanimirovic, D.B.; Sandhu, J.K.; Costain, W.J. Emerging Technologies for Delivery of Biotherapeutics and Gene Therapy Across the Blood–Brain Barrier. BioDrugs 2018, 32, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Spieth, L.; Berghoff, S.A.; Stumpf, S.K.; Winchenbach, J.; Michaelis, T.; Watanabe, T.; Gerndt, N.; Düking, T.; Hofer, S.; Ruhwedel, T.; et al. Anesthesia Triggers Drug Delivery to Experimental Glioma in Mice by Hijacking Caveolar Transport. Neuro-Oncol. Adv. 2021, 3, vdab140. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Marsh, G.; Kusters, I.; Delincé, M.; Di Caprio, G.; Upadhyayula, S.; de Nola, G.; Hunt, R.; Ohashi, K.G.; Gray, T.; et al. Design and Validation of a Human Brain Endothelial Microvessel-on-a-Chip Open Microfluidic Model Enabling Advanced Optical Imaging. Front. Bioeng. Biotechnol. 2020, 8, 573775. [Google Scholar] [CrossRef]
- Wevers, N.R.; Kasi, D.G.; Gray, T.; Wilschut, K.J.; Smith, B.; van Vught, R.; Shimizu, F.; Sano, Y.; Kanda, T.; Marsh, G.; et al. A Perfused Human Blood–Brain Barrier on-a-Chip for High-Throughput Assessment of Barrier Function and Antibody Transport. Fluids Barriers CNS 2018, 15, 23. [Google Scholar] [CrossRef]
- Sahtoe, D.D.; Coscia, A.; Mustafaoglu, N.; Miller, L.M.; Olal, D.; Vulovic, I.; Yu, T.-Y.; Goreshnik, I.; Lin, Y.-R.; Clark, L.; et al. Transferrin Receptor Targeting by de Novo Sheet Extension. Proc. Natl. Acad. Sci. USA 2021, 118, e2021569118. [Google Scholar] [CrossRef]
- Park, T.-E.; Mustafaoglu, N.; Herland, A.; Hasselkus, R.; Mannix, R.; FitzGerald, E.A.; Prantil-Baun, R.; Watters, A.; Henry, O.; Benz, M.; et al. Hypoxia-Enhanced Blood-Brain Barrier Chip Recapitulates Human Barrier Function and Shuttling of Drugs and Antibodies. Nat. Commun. 2019, 10, 2621. [Google Scholar] [CrossRef]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered Human Blood–Brain Barrier Microfluidic Model for Vascular Permeability Analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef]
- Nair, A.L.; Groenendijk, L.; Overdevest, R.; Fowke, T.M.; Annida, R.; Mocellin, O.; de Vries, H.E.; Wevers, N.R. Human BBB-on-a-Chip Reveals Barrier Disruption, Endothelial Inflammation, and T Cell Migration under Neuroinflammatory Conditions. Front. Mol. Neurosci. 2023, 16, 1250123. [Google Scholar] [CrossRef]
- Sade, H.; Baumgartner, C.; Hugenmatter, A.; Moessner, E.; Freskgård, P.-O.; Niewoehner, J. A Human Blood-Brain Barrier Transcytosis Assay Reveals Antibody Transcytosis Influenced by pH-Dependent Receptor Binding. PLoS ONE 2014, 9, e96340. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased Brain Penetration and Potency of a Therapeutic Antibody Using a Monovalent Molecular Shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Bacyinski, A.; Xu, M.; Wang, W.; Hu, J. The Paravascular Pathway for Brain Waste Clearance: Current Understanding, Significance and Controversy. Front. Neuroanat. 2017, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.; Silva, J.; Ferreira, R.; Trigo, D. Glymphatic System, AQP4, and Their Implications in Alzheimer’s Disease. Neurol. Res. Pract. 2021, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.; Wang, M.X.; Ismail, O.; Braun, M.; Schindler, A.G.; Reemmer, J.; Wang, Z.; Haveliwala, M.A.; O’Boyle, R.P.; Han, W.Y.; et al. Loss of Perivascular Aquaporin-4 Localization Impairs Glymphatic Exchange and Promotes Amyloid β Plaque Formation in Mice. Alzheimer’s Res. Ther. 2022, 14, 59. [Google Scholar] [CrossRef]
- Kamagata, K.; Andica, C.; Takabayashi, K.; Saito, Y.; Taoka, T.; Nozaki, H.; Kikuta, J.; Fujita, S.; Hagiwara, A.; Kamiya, K.; et al. Association of MRI Indices of Glymphatic System with Amyloid Deposition and Cognition in Mild Cognitive Impairment and Alzheimer Disease. Neurology 2022, 99, e2648–e2660. [Google Scholar] [CrossRef]
- Eide, P.K.; Vatnehol, S.A.S.; Emblem, K.E.; Ringstad, G. Magnetic Resonance Imaging Provides Evidence of Glymphatic Drainage from Human Brain to Cervical Lymph Nodes. Sci. Rep. 2018, 8, 7194. [Google Scholar] [CrossRef]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.A.; Martens, E.A.; Mori, Y.; et al. The Glymphatic System: Current Understanding and Modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef]
- Hablitz, L.M.; Nedergaard, M. The Glymphatic System: A Novel Component of Fundamental Neurobiology. J. Neurosci. 2021, 41, 7698–7711. [Google Scholar] [CrossRef]
- Monfrini, E.; Baso, G.; Ronchi, D.; Meneri, M.; Gagliardi, D.; Quetti, L.; Verde, F.; Ticozzi, N.; Ratti, A.; Di Fonzo, A.; et al. Unleashing the Potential of mRNA Therapeutics for Inherited Neurological Diseases. Brain 2024, 147, 2934–2945. [Google Scholar] [CrossRef]
- Sehlin, D.; Hultqvist, G.; Michno, W.; Aguilar, X.; Dahlén, A.D.; Cerilli, E.; Bucher, N.M.; Lopes van den Broek, S.; Syvänen, S. Bispecific Brain-Penetrant Antibodies for Treatment of Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2025, 12, 100214. [Google Scholar] [CrossRef]
- Grimm, H.P.; Schumacher, V.; Schäfer, M.; Imhof-Jung, S.; Freskgård, P.-O.; Brady, K.; Hofmann, C.; Rüger, P.; Schlothauer, T.; Göpfert, U.; et al. Delivery of the BrainshuttleTM Amyloid-Beta Antibody Fusion Trontinemab to Non-Human Primate Brain and Projected Efficacious Dose Regimens in Humans. MAbs 2023, 15, 2261509. [Google Scholar] [CrossRef]
- Ward, E.S.; Ober, R.J. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol. Sci. 2018, 39, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Hultqvist, G.; Syvänen, S.; Fang, X.T.; Lannfelt, L.; Sehlin, D. Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor. Theranostics 2017, 7, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Clarke, E.; Stocki, P.; Sinclair, E.H.; Gauhar, A.; Fletcher, E.J.R.; Krawczun-Rygmaczewska, A.; Duty, S.; Walsh, F.S.; Doherty, P.; Rutkowski, J.L. A Single Domain Shark Antibody Targeting the Transferrin Receptor 1 Delivers a TrkB Agonist Antibody to the Brain and Provides Full Neuroprotection in a Mouse Model of Parkinson’s Disease. Pharmaceutics 2022, 14, 1335. [Google Scholar] [CrossRef] [PubMed]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.H.I. Avidity in Antibody Effector Functions and Biotherapeutic Drug Design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef]
- Van de Walle, I.; Silence, K.; Budding, K.; Van de Ven, L.; Dijkxhoorn, K.; de Zeeuw, E.; Yildiz, C.; Gabriels, S.; Percier, J.-M.; Wildemann, J.; et al. ARGX-117, a Therapeutic Complement Inhibiting Antibody Targeting C2. J. Allergy Clin. Immunol. 2020, 147, 1420–1429.e7. [Google Scholar] [CrossRef]
- Qi, T.; Cao, Y. In Translation: FcRn across the Therapeutic Spectrum. Int. J. Mol. Sci. 2021, 22, 3048. [Google Scholar] [CrossRef]
- Boado, R.J.; Zhang, Y.; Wang, Y.; Pardridge, W.M. Engineering and Expression of a Chimeric Transferrin Receptor Monoclonal Antibody for Blood–Brain Barrier Delivery in the Mouse. Biotechnol. Bioeng. 2009, 102, 1251–1258. [Google Scholar] [CrossRef]
- Faresjö, R.; Lindberg, H.; Ståhl, S.; Löfblom, J.; Syvänen, S.; Sehlin, D. Transferrin Receptor Binding BBB-Shuttle Facilitates Brain Delivery of Anti-Aβ-Affibodies. Pharm. Res. 2022, 39, 1509–1521. [Google Scholar] [CrossRef]
- Smith, A.J. New Horizons in Therapeutic Antibody Discovery: Opportunities and Challenges versus Small-Molecule Therapeutics. SLAS Discov. 2015, 20, 437–453. [Google Scholar] [CrossRef]
- Asaadi, Y.; Jouneghani, F.F.; Janani, S.; Rahbarizadeh, F. A Comprehensive Comparison between Camelid Nanobodies and Single Chain Variable Fragments. Biomark. Res. 2021, 9, 87. [Google Scholar] [CrossRef]
- Esparza, T.J.; Su, S.; Francescutti, C.M.; Rodionova, E.; Kim, J.H.; Brody, D.L. Enhanced in Vivo Blood Brain Barrier Transcytosis of Macromolecular Cargo Using an Engineered pH-Sensitive Mouse Transferrin Receptor Binding Nanobody. Fluids Barriers CNS 2023, 20, 64. [Google Scholar] [CrossRef]
- Liu, R.; Oldham, R.J.; Teal, E.; Beers, S.A.; Cragg, M.S. Fc-Engineering for Modulated Effector Functions—Improving Antibodies for Cancer Treatment. Antibodies 2020, 9, 64. [Google Scholar] [CrossRef]
- Lafrance-Vanasse, J.; Sadekar, S.S.; Yang, Y.; Yadav, D.B.; Meilandt, W.J.; Wetzel-Smith, M.K.; Cai, H.; Crowell, S.R.; Nguyen, V.; Lee, V.; et al. Leveraging Neonatal Fc Receptor (FcRn) to Enhance Antibody Transport Across the Blood Brain Barrier. Nat. Commun. 2025, 16, 4143. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jo, M.; Jung, S.T. Recent Achievements and Challenges in Prolonging the Serum Half-Lives of Therapeutic IgG Antibodies Through Fc Engineering. BioDrugs 2021, 35, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Pyzik, M.; Sand, K.M.K.; Hubbard, J.J.; Andersen, J.T.; Sandlie, I.; Blumberg, R.S. The Neonatal Fc Receptor (FcRn): A Misnomer? Front. Immunol. 2019, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Zarantonello, A.; Pedersen, H.; Laursen, N.S.; Andersen, G.R. Nanobodies Provide Insight into the Molecular Mechanisms of the Complement Cascade and Offer New Therapeutic Strategies. Biomolecules 2021, 11, 298. [Google Scholar] [CrossRef]
- Xenaki, K.T.; Oliveira, S.; van Bergen en Henegouwen, P.M.P. Antibody or Antibody Fragments: Implications for Molecular Imaging and Targeted Therapy of Solid Tumors. Front. Immunol. 2017, 8, 1287. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C.; Liu, J.; Wang, L.; Lei, J. Self-Assembled Targeted Nanoparticles Based on Transferrin-Modified Eight-Arm-Polyethylene Glycol–Dihydroartemisinin Conjugate. Sci. Rep. 2016, 6, 29461. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, Y.; Cai, C.; Fan, R.; Guo, R.; Xie, D. Targeted Delivery of DOX by Transferrin Conjugated DSPE-PEG Nanoparticles in Leukemia Therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 27–36. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Liu, L.; Chen, Y.-H.; You, Y.; Gao, Y.; Liu, Y.-Y.; Yang, L.; Tong, K.; Chen, D.-S.; et al. Transferrin-Pep63-Liposomes Accelerate the Clearance of Aβ and Rescue Impaired Synaptic Plasticity in Early Alzheimer’s Disease Models. Cell Death Discov. 2021, 7, 1–13. [Google Scholar] [CrossRef]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef]
- Giannetti, A.M.; Snow, P.M.; Zak, O.; Björkman, P.J. Mechanism for Multiple Ligand Recognition by the Human Transferrin Receptor. PLoS Biol. 2003, 1, e51. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, D.; Tong, Z.; Peng, B.; Cheng, X.; Esser, L.; Voelcker, N.H. Influence of Surface Ligand Density and Particle Size on the Penetration of the Blood–Brain Barrier by Porous Silicon Nanoparticles. Pharmaceutics 2023, 15, 2271. [Google Scholar] [CrossRef] [PubMed]
- Yersin, A.; Osada, T.; Ikai, A. Exploring Transferrin-Receptor Interactions at the Single-Molecule Level. Biophys. J. 2008, 94, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, Y.; Zhang, H.; Liu, Y.; Xie, R.; He, X.; Zhou, Y.; Liang, L.; Gao, H. The Protein Corona Hampers the Transcytosis of Transferrin-Modified Nanoparticles through Blood–Brain Barrier and Attenuates Their Targeting Ability to Brain Tumor. Biomaterials 2021, 274, 120888. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and Brain Uptake of Transferrin-Containing Nanoparticles by Tuning Avidity to Transferrin Receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef]
- Kawak, P.; Sawaftah, N.M.A.; Pitt, W.G.; Husseini, G.A. Transferrin-Targeted Liposomes in Glioblastoma Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 13262. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Kang, T.; Jiang, M.; Miao, D.; Gu, G.; Hu, Q.; Song, Q.; Yao, L.; Tu, Y.; et al. B6 Peptide-Modified PEG-PLA Nanoparticles for Enhanced Brain Delivery of Neuroprotective Peptide. Bioconjug. Chem. 2013, 24, 997–1007. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-Loaded PLGA-PEG Nanoparticles Conjugated with B6 Peptide for Potential Use in Alzheimer’s Disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic Acid (SA)-Modified Selenium Nanoparticles Coated with a High Blood–Brain Barrier Permeability Peptide-B6 Peptide for Potential Use in Alzheimer’s Disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Poudel, P.; Park, S. Recent Advances in the Treatment of Alzheimer’s Disease Using Nanoparticle-Based Drug Delivery Systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef] [PubMed]
- Juhairiyah, F.; de Lange, E.C.M. Understanding Drug Delivery to the Brain Using Liposome-Based Strategies: Studies That Provide Mechanistic Insights Are Essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Tian, X.; Leite, D.M.; Scarpa, E.; Nyberg, S.; Fullstone, G.; Forth, J.; Matias, D.; Apriceno, A.; Poma, A.; Duro-Castano, A.; et al. On the Shuttling across the Blood-Brain Barrier via Tubule Formation: Mechanism and Cargo Avidity Bias. Sci. Adv. 2020, 6, eabc4397. [Google Scholar] [CrossRef]
- Pardridge, W.M. Treatment of Alzheimer’s Disease and Blood–Brain Barrier Drug Delivery. Pharmaceuticals 2020, 13, 394. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.M.; Wang, Y.; Ma, Q.; Sagare, A.P.; Montagne, A.; Huuskonen, M.T.; Rege, S.V.; Kisler, K.; Dai, Z.; Körbelin, J.; et al. Endothelial LRP1 Protects against Neurodegeneration by Blocking Cyclophilin A. J. Exp. Med. 2021, 218, e20202207. [Google Scholar] [CrossRef]
- Chang, H.-Y.; Wu, S.; Li, Y.; Zhang, W.; Burrell, M.; Webster, C.I.; Shah, D.K. Brain Pharmacokinetics of Anti-Transferrin Receptor Antibody Affinity Variants in Rats Determined Using Microdialysis. MAbs 2021, 13, 1874121. [Google Scholar] [CrossRef]
- Stocki, P.; Szary, J.; Rasmussen, C.L.M.; Demydchuk, M.; Northall, L.; Logan, D.B.; Gauhar, A.; Thei, L.; Moos, T.; Walsh, F.S.; et al. Blood-Brain Barrier Transport Using a High Affinity, Brain-Selective VNAR Antibody Targeting Transferrin Receptor 1. FASEB J. 2021, 35, e21172. [Google Scholar] [CrossRef]
- Aisen, P.S. Therapeutic Effects of Intranasally-Administered Insulin in Adults with Amnestic Mild Cognitive Impairment (aMCI) or Mild Alzheimer’s Disease (AD); ClinicalTrials.gov; National Library of Medicine: Bethesda, MD, USA, 2024.
- Bien-Ly, N.; Yu, Y.J.; Bumbaca, D.; Elstrott, J.; Boswell, C.A.; Zhang, Y.; Luk, W.; Lu, Y.; Dennis, M.S.; Weimer, R.M.; et al. Transferrin Receptor (TfR) Trafficking Determines Brain Uptake of TfR Antibody Affinity Variants. J. Exp. Med. 2014, 211, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Riscado, M.; Baptista, B.; Sousa, F. New RNA-Based Breakthroughs in Alzheimer’s Disease Diagnosis and Therapeutics. Pharmaceutics 2021, 13, 1397. [Google Scholar] [CrossRef]
- Faresjö, R.; Sehlin, D.; Syvänen, S. Age, Dose, and Binding to TfR on Blood Cells Influence Brain Delivery of a TfR-Transported Antibody. Fluids Barriers CNS 2023, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, X.; Zhang, B.; Li, Y.; Alexander, C.; Harvey, P.; Zhu, Z. Brain-Targeted Intranasal Delivery of Biologics: A Perspective for Alzheimer’s Disease Treatment. RSC Pharm. 2025, 13, 3853–3875. [Google Scholar] [CrossRef]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional Morphology of the Blood-Brain Barrier in Health and Disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed]
- ALZ Forum: Roche Spells Out Phase Three Plans for Trontinemab; Global Alzheimer’s Platform Foundation: Washington, DC, USA, 2025.
- Wang, S.; He, X.; Wu, Q.; Jiang, L.; Chen, L.; Yu, Y.; Zhang, P.; Huang, X.; Wang, J.; Ju, Z.; et al. Transferrin Receptor 1-Mediated Iron Uptake Plays an Essential Role in Hematopoiesis. Haematologica 2020, 105, 2071–2082. [Google Scholar] [CrossRef]
- Chew, K.S.; Wells, R.C.; Moshkforoush, A.; Chan, D.; Lechtenberg, K.J.; Tran, H.L.; Chow, J.; Kim, D.J.; Robles-Colmenares, Y.; Srivastava, D.B.; et al. CD98hc Is a Target for Brain Delivery of Biotherapeutics. Nat. Commun. 2023, 14, 5053. [Google Scholar] [CrossRef]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Gene Delivery Targeted to the Brain Using an Angiopep-Conjugated Polyethyleneglycol-Modified Polyamidoamine Dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef]
- Bonvicini, G.; Singh, S.; Sandersjöö, L.; Sehlin, D.; Syvänen, S.; Andersson, K.G. The Effects of Dose, Valency, and Affinity on TfR-Mediated Brain Delivery in Vivo. Fluids Barriers CNS 2025, 22, 36. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Jiang, Y.; Lv, W.; Wu, L.; Wang, B.; Lv, L.; Xu, Q.; Xin, H. Enhanced Anti-Ischemic Stroke of ZL006 by T7-Conjugated PEGylated Liposomes Drug Delivery System. Sci. Rep. 2015, 5, 12651. [Google Scholar] [CrossRef]
- Yu, Y.J.; Atwal, J.K.; Zhang, Y.; Tong, R.K.; Wildsmith, K.R.; Tan, C.; Bien-Ly, N.; Hersom, M.; Maloney, J.A.; Meilandt, W.J.; et al. Therapeutic Bispecific Antibodies Cross the Blood-Brain Barrier in Nonhuman Primates. Sci. Transl. Med. 2014, 6, 261ra154. [Google Scholar] [CrossRef]
- Kariolis, M.S.; Wells, R.C.; Getz, J.A.; Kwan, W.; Mahon, C.S.; Tong, R.; Kim, D.J.; Srivastava, A.; Bedard, C.; Henne, K.R.; et al. Brain Delivery of Therapeutic Proteins Using an Fc Fragment Blood-Brain Barrier Transport Vehicle in Mice and Monkeys. Sci. Transl. Med. 2020, 12, eaay1359. [Google Scholar] [CrossRef]
- Couch, J.A.; Yu, Y.J.; Zhang, Y.; Tarrant, J.M.; Fuji, R.N.; Meilandt, W.J.; Solanoy, H.; Tong, R.K.; Hoyte, K.; Luk, W.; et al. Addressing Safety Liabilities of TfR Bispecific Antibodies That Cross the Blood-Brain Barrier. Sci. Transl. Med. 2013, 5, 183ra57. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Ito, E.; Akita, H.; Oishi, M.; Nagasaki, Y.; Futaki, S.; Harashima, H. A pH-Sensitive Fusogenic Peptide Facilitates Endosomal Escape and Greatly Enhances the Gene Silencing of siRNA-Containing Nanoparticles In Vitro and In Vivo. J. Control. Release 2009, 139, 127–132. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Li, H.; Zhang, B.; Li, Y.; Zhu, Z. Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 9793. https://doi.org/10.3390/ijms26199793
Shen X, Li H, Zhang B, Li Y, Zhu Z. Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(19):9793. https://doi.org/10.3390/ijms26199793
Chicago/Turabian StyleShen, Xinai, Huan Li, Beiyu Zhang, Yunan Li, and Zheying Zhu. 2025. "Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 19: 9793. https://doi.org/10.3390/ijms26199793
APA StyleShen, X., Li, H., Zhang, B., Li, Y., & Zhu, Z. (2025). Targeting Transferrin Receptor 1 for Enhancing Drug Delivery Through the Blood–Brain Barrier for Alzheimer’s Disease. International Journal of Molecular Sciences, 26(19), 9793. https://doi.org/10.3390/ijms26199793




