Venous Thrombogenesis and Cervical Cancer: Plasma MicroRNAs as Prognostic Indicators of Tumor Behavior
Abstract
1. Introduction
2. Results
2.1. Correlation Between Baseline miRNA Expression
2.2. MiRNA Expression Levels and Patients’ Characteristics
2.3. MiRNA Expression Levels and CC-Related VTE Incidence
2.4. VTE Status and Patients’ Prognosis
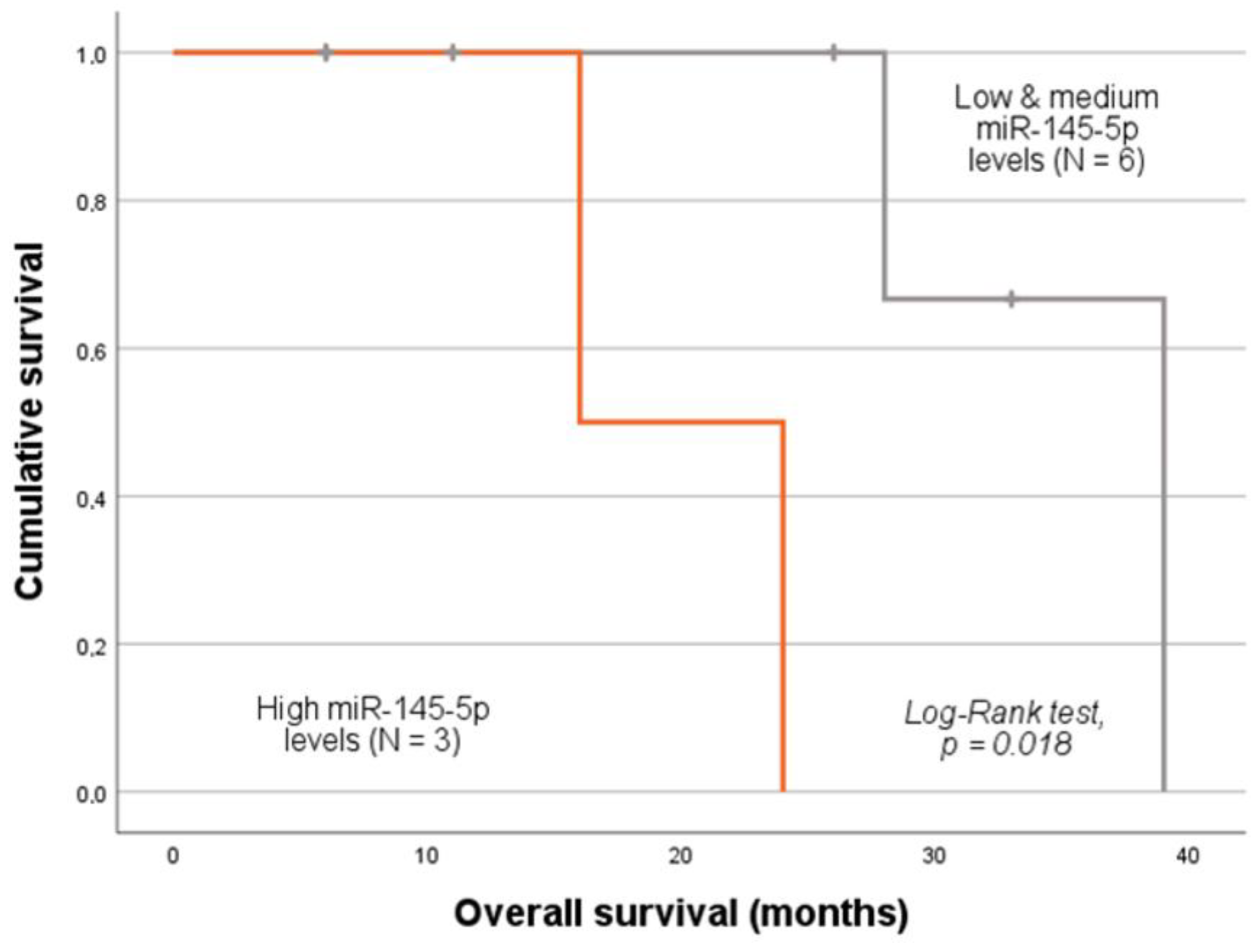
2.5. MiRNA Expression Levels and Patients’ Prognosis
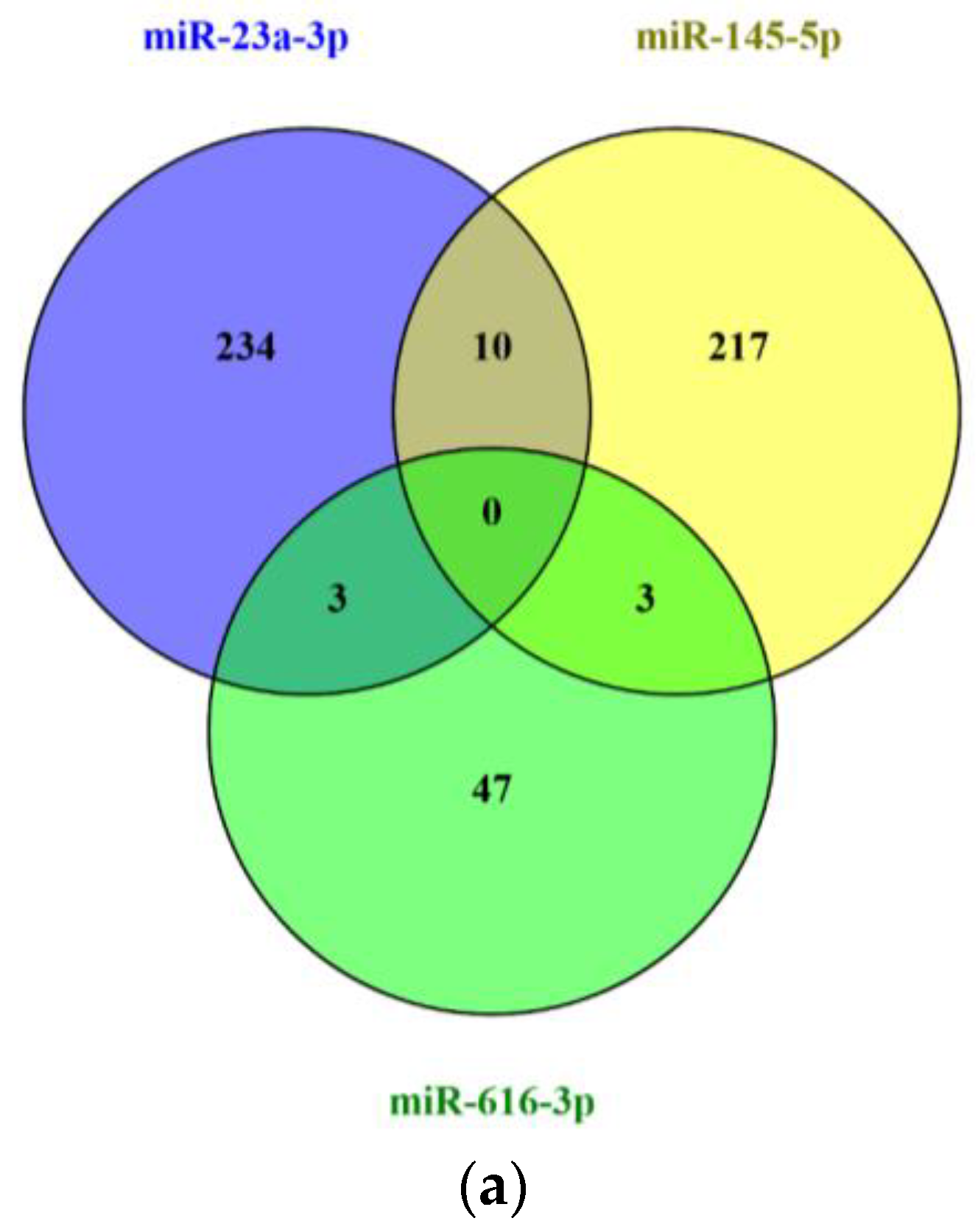
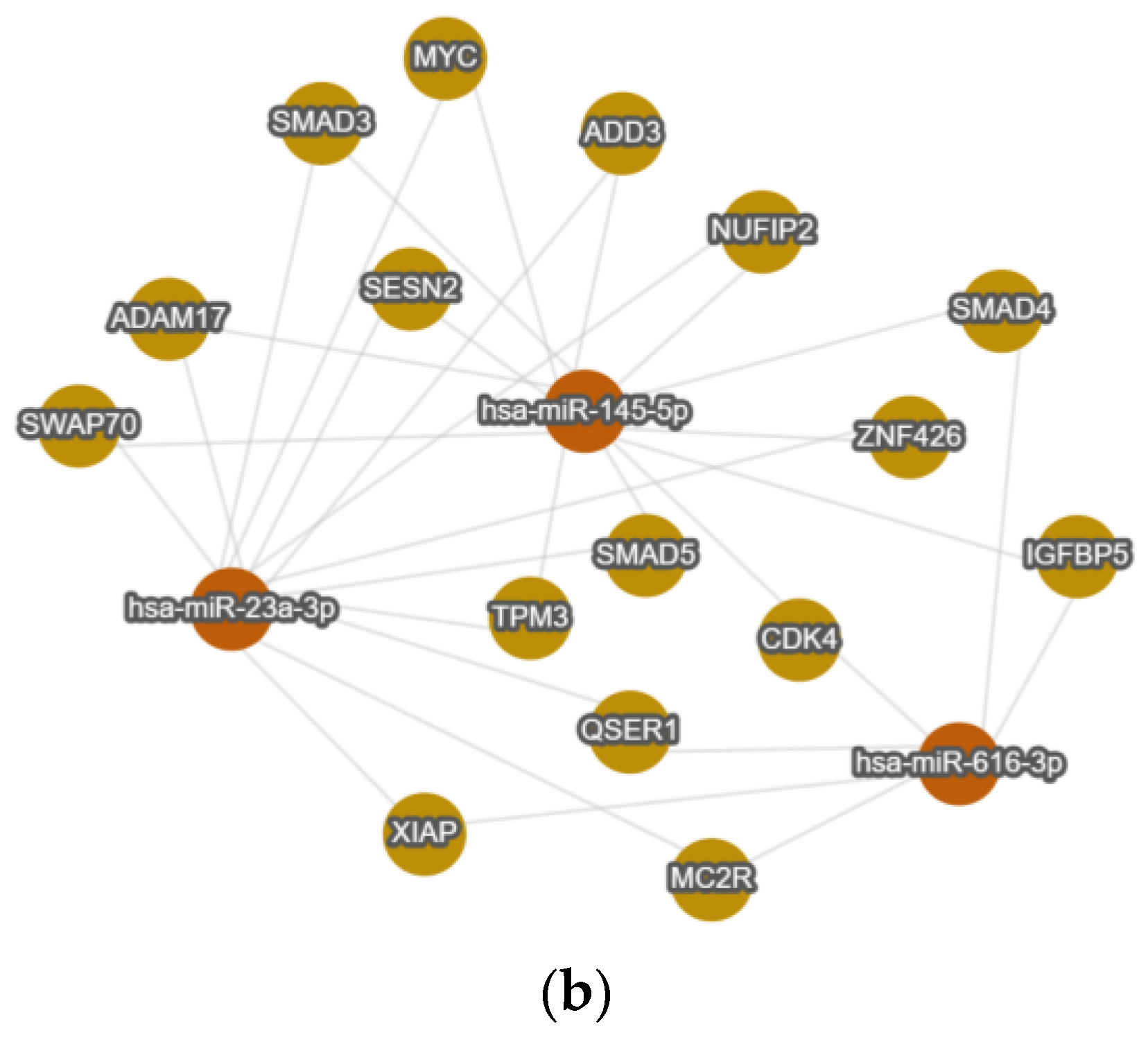
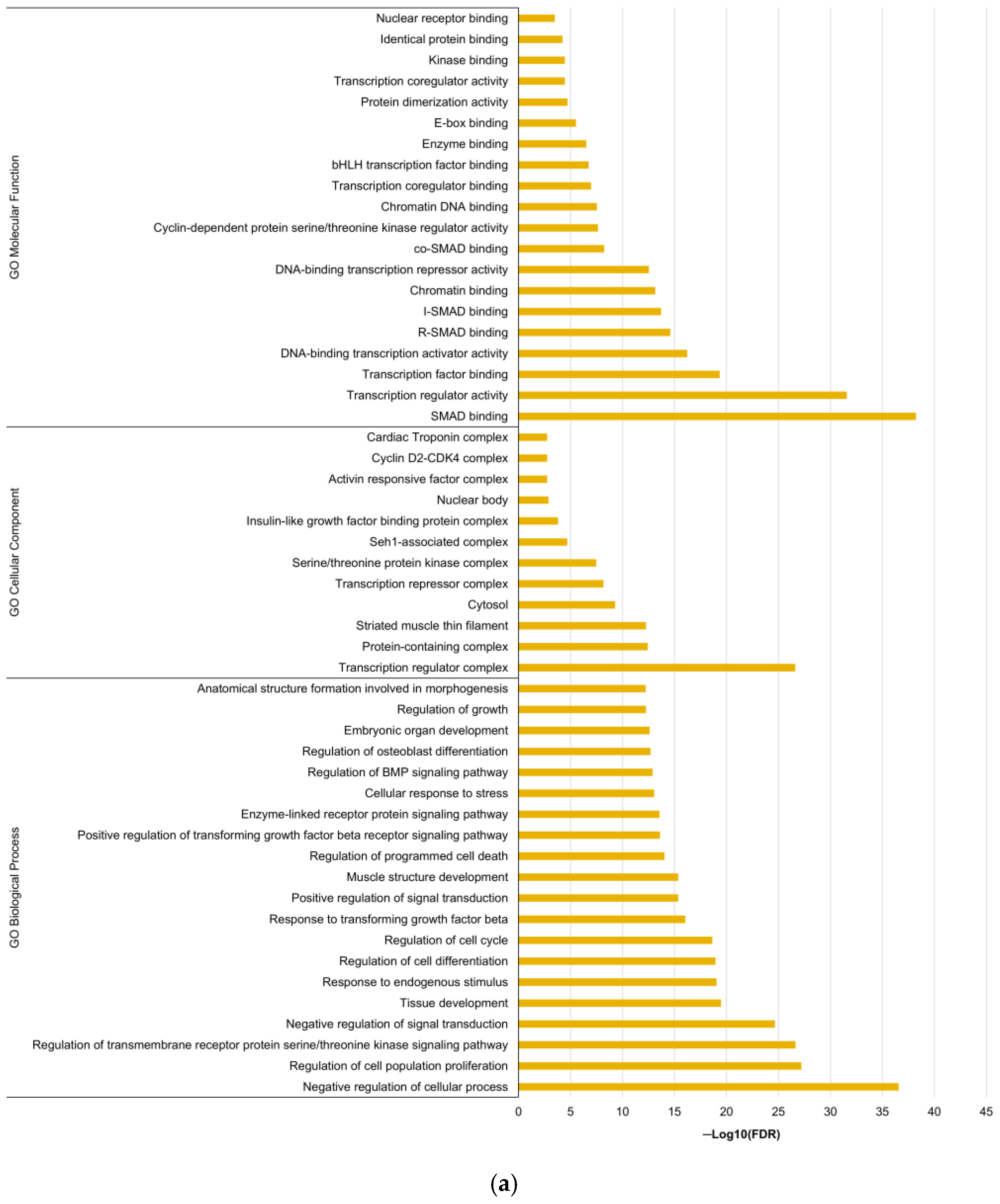
2.6. In Silico Analysis
3. Discussion
4. Materials and Methods
4.1. Population Description
4.2. Sample Collection and Processing
4.3. MiRNA Selection
4.4. Total RNA Extraction
4.5. cDNA Synthesis
4.6. Relative Quantification of miRNAs
4.7. Statistical Analysis
4.8. In Silico Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Cervical Adenocarcinoma |
| ATG7 | Autophagy-related protein 7 |
| CAT | Cancer-Associated Thrombosis |
| CC | Cervical Cancer |
| CCRT | Concurrent chemoradiotherapy |
| cDNA | Complementary DNA |
| CI | Confidence Interval |
| CSCC | Cervical Squamous Cell Carcinoma |
| Ct | Cycle threshold |
| EAC | Esophageal Adenocarcinoma |
| EC | Esophageal Cancer |
| EDTA | Ethylenediaminetetraacetic acid |
| ERK | Extracellular signal-regulated kinase |
| ESCC | Esophageal Squamous Cell Carcinoma |
| F3 | Coagulation Factor 3 |
| FDR | False Discovery Rate |
| FIGO | International Federation of Gynecology and Obstetrics |
| FSCN1 | Fascin actin-bundlin protein 1 |
| FVIIIa | Activated Coagulation Factor VIII |
| FXa | Activated Coagulation Factor X |
| FXI | Coagulation Factor XI |
| FXIa | Activated Coagulation Factor XI |
| GO | Gene Ontology |
| HLTF | Helicase-Like Transcription Factor |
| HPV | Human Papillomavirus |
| HR | Hazard Ratio |
| IPO Porto | Portuguese Institute of Oncology of Porto |
| MEK | Mitogen-activated protein kinase |
| mRNAs | Messenger RNAs |
| miRs or miRNAs | microRNAs |
| N | number |
| ncRNAs | Non-coding RNAs |
| ns | Non-significant |
| OS | Overall Survival |
| PAI-1 | Plasminogen Activator Inhibitor-1 |
| PDCD6 | Programmed Cell Death 6 |
| pKLK | Plasma Kallikrein |
| PPI | Protein–protein interaction |
| RCAN3 | Regulators of Calcineurin 3 |
| RT-qPCR | Real-Time quantitative Polymerase Chain Reaction |
| RUNX1 | Runt-related Transcription Factor |
| SD | Standard Deviation |
| SIP1 | SMAD-Interacting Protein 1 |
| TF | Tissue Factor |
| TFPI1 | Tissue Factor Pathway Inhibitor 1 |
| TFPI2 | Tissue Factor Pathway Inhibitor 2 |
| THBS2 | Thrombospondin 2 |
| TIMP2 | Tissue Inhibitor of Metalloproteinase 2 |
| VEGF | Vascular Endothelial Growth Factor |
| VTE | Venous Thromboembolism |
| WNT2B | Wnt family member 2B |
Appendix A
| Validated Targets According to MirTargetLink 2.0 * | ||||||
|---|---|---|---|---|---|---|
| miR-23a-3p | miR-145-5p | miR-616-3p | ||||
| APAF1 | DNAJC21 | PTPDC1 | ABCC1 | NAIP | IVNS1ABP | PON1 |
| ATAT1 | DOCK4 | PTPN14 | ABHD17C | NANOG | KIF21A | TFPI2 |
| CDH1 | DSTN | QSER1 | ABRACL | NDRG2 | LMNB2 | BBOX1 |
| CHUK | DYNC2LI1 | RNF111 | ADAM17 | NDUFA4 | MAP1LC3B | C5orf46 |
| CXCL12 | DYNLL2 | RNF38 | ADD3 | NEDD9 | MAP2K4 | CAPRIN2 |
| CXCL8 | EGLN3 | RNMT | AKR1B10 | NFATC1 | MAP3K11 | CARNS1 |
| FANCG | EN2 | RPL7L1 | ALDH3A1 | NIPSNAP1 | MAP3K3 | CCSAP |
| FAS | ERBIN | RPP14 | ALPPL2 | NRAS | MAP4K2 | CDK4 |
| FOXA1 | ETNK1 | RPRD2 | ANGPT2 | NUDT1 | MUC19 | CEP350 |
| FOXO3 | FAM222B | RPS4X | AP1G1 | PAK4 | MUC4 | CNNM2 |
| FZD5 | FAM91A1 | RPS5 | APH1A | PARP8 | NDUFS2 | COIL |
| G6PC | FASTKD1 | RPSAP58 | ARF6 | PIGF | NR1D2 | CREBZF |
| GJA1 | FASTKD5 | S100A7A | ARL6IP5 | PODXL | NUFIP2 | CSGALNACT1 |
| GLS | FAU | SCAMP2 | BNIP3 | POU5F1 | NUP43 | CXCL5 |
| HES1 | FBN2 | SDHD | BRAF | PPP3CA | ORC4 | DCTPP1 |
| HMGB2 | FKBP4 | SEMA6D | C11orf65 | PTP4A2 | P4HA1 | DLGAP3 |
| HMGN2 | FLNA | SEPT2 | CAMK1D | PXN | PADI1 | FSHB |
| HNF1B | FNIP1 | SERINC3 | CBFB | ROBO2 | PANK1 | HLA-DOB |
| HOXB4 | FOXM1 | SESN2 | CCDC43 | ROCK1 | PDGFD | IGFBP5 |
| HSP90AA1 | FOXR2 | SLC27A4 | CD28 | RPA1 | PHACTR2 | INTS6 |
| IL6R | FSD1L | SLC35G3 | CD40 | RPS6KB1 | PLAGL2 | KMT2A |
| IRF1 | FUT4 | SLC6A15 | CD44 | RREB1 | PLEKHM1 | LGALS3BP |
| KLF3 | GGA3 | SLC6A6 | CDH2 | RTKN | PNMA3 | LPAR2 |
| LAMP1 | GMPS | SNTG1 | CDK4 | SENP1 | PRDM2 | MAL2 |
| LDHA | GNAI3 | SOCS6 | CDK6 | SERINC5 | PSAT1 | MC2R |
| LDHB | GNB2 | SOD2 | CDKN1A | SERPINE1 | RAB3IP | MED17 |
| LPAR1 | GRK2 | SPTY2D1 | CEP19 | SET | RBM18 | MIEF1 |
| LRP5 | GRTP1 | SSR1 | CFTR | SMAD2 | REL | MIER3 |
| MEF2C | GTF3C4 | SSRP1 | CLINT1 | SMAD3 | RPS6KA3 | MRO |
| MT2A | HAPLN1 | STAMBPL1 | COL5A1 | SOCS7 | RRAGC | MTHFD1 |
| MYH1 | HCFC1 | STARD7 | CRNDE | SOX2 | SAMD5 | PAFAH1B1 |
| MYH2 | HIST1H3B | STS | CTGF | SOX9 | SESN2 | PRKAG1 |
| MYH4 | HIVEP1 | STT3B | CTNND1 | SP1 | SLC16A10 | PRPF4B |
| NEK6 | HMGCS1 | SWAP70 | DDC | SP7 | SLC16A5 | PSMA2 |
| POU4F2 | HNRNPUL1 | TBL2 | DDX17 | SPTBN1 | SLC22A9 | PTMA |
| PPARGC1A | HRG | TCF25 | DDX6 | SPTLC1 | SLC26A2 | QSER1 |
| PPP2R5E | HS2ST1 | THUMPD3 | DFFA | SRGAP1 | SMAD4 | RBM26 |
| PTEN | IMPDH2 | TLR6 | DTD1 | STAT1 | SMAD5 | RBM43 |
| PTPN11 | ITPKC | TMED7 | E2F3 | SWAP70 | SMIM17 | SERTAD2 |
| RGS5 | JMJD1C | TMEM170A | EGFR | TGFB2 | SNTB1 | SFMBT2 |
| SMAD3 | KDM3B | TNFAIP3 | EIF4E | TGFBR2 | SNX24 | SIK1 |
| SMAD5 | KIAA1210 | TNFAIP8 | EPAS1 | TIRAP | SOX11 | SLFN12 |
| SPRY2 | KIAA1551 | TNPO1 | ERG | TMEM9B | SRPX2 | SLFN12L |
| ST7L | KIF20A | TNRC6A | ETS1 | TNFSF13 | TGFBI | SMAD4 |
| STAT3 | KIF22 | TOPBP1 | F11R | TPM3 | THSD7A | TMEM59 |
| TERF2 | KLF10 | TOR1AIP1 | FAM3C | TPRG1 | TMOD3 | TOR4A |
| TMEM64 | KLF12 | TPM3 | FAM45A | TSPAN6 | TNR | TRMT112 |
| TOP1 | LEMD2 | TRIM59 | FLI1 | TUG1 | UBR7 | USP37 |
| TSC1 | LMAN2 | TRIM63 | FSCN1 | VEGFA | UBXN2A | XIAP |
| XIAP | LMNB1 | TRRAP | FXN | VPS51 | UNC5D | ZNF347 |
| ABCD1 | MAP1B | TSNAX | GMFB | YES1 | UTP15 | ZNF35 |
| ACSS3 | MC2R | TXNIP | GOLM1 | AAED1 | VGLL4 | ZNF460 |
| ACTN4 | MCFD2 | TXNL4A | HDAC11 | ACTB | WASHC2C | ZNF850 |
| ADAM17 | MCU | UBALD1 | HDAC2 | AGPS | WSB1 | PON1 |
| ADAM28 | MGAT5 | UQCRFS1 | HLTF | AGTRAP | ZBTB25 | |
| ADAM9 | MMGT1 | USP34 | HMGA2 | ALG9 | ZFAND3 | |
| ADD3 | MPP6 | USP5 | IFNB1 | ANKRD28 | ZFYVE9 | |
| ARL6IP1 | MRPS34 | VAV3 | IGF1R | AQR | ZNF100 | |
| ASNS | MTAP | VCAM1 | ILK | BCLAF3 | ZNF426 | |
| ATP5A1 | MYC | WEE1 | IRS1 | BTG1 | ZNF445 | |
| ATXN7L3B | MYH10 | ZBTB10 | IRS2 | C1orf27 | ZNF451 | |
| BAZ2A | NACC1 | ZCCHC2 | ITGB8 | CCDC80 | ZNF660 | |
| BCAP29 | NDUFV3 | ZIK1 | JADE1 | CCDC85C | ZNF678 | |
| BRWD1 | NLGN4X | ZNF117 | KLF4 | CLSTN2 | ZNF772 | |
| BSDC1 | NLK | ZNF138 | KLF5 | CRAMP1 | ||
| BTLA | NOL11 | ZNF253 | KREMEN1 | CRYBG1 | ||
| C2orf69 | NUFIP2 | ZNF257 | LYPLA2 | CSRNP3 | ||
| C8orf58 | NUP153 | ZNF267 | MAP2K6 | CTNNBIP1 | ||
| CAMKV | P2RY11 | ZNF268 | MCM2 | CYP2C19 | ||
| CCL8 | PAFAH1B3 | ZNF273 | MDM2 | DDI2 | ||
| CCT5 | PDCD6IP | ZNF275 | MEST | DEK | ||
| CD302 | PDIA6 | ZNF281 | MIXL1 | DMXL1 | ||
| CELF1 | PEX26 | ZNF319 | MMP1 | DNAJC28 | ||
| CENPM | PIGM | ZNF426 | MMP12 | DUSP6 | ||
| CEP57L1 | PIK3R1 | ZNF485 | MMP14 | EID2B | ||
| CNN2 | PKM | ZNF550 | MSH3 | ERBB4 | ||
| CNOT1 | PLAG1 | ZNF578 | MTDH | FZD6 | ||
| CNOT6 | PNRC2 | ZNF669 | MTMR14 | HBEGF | ||
| CNOT9 | POM121C | ZNF682 | MUC1 | HIF1A | ||
| CRLS1 | PPIC | ZNF701 | MYC | HIST1H2AH | ||
| CSDE1 | PSAP | ZNF704 | MYO5A | HIST1H2BF | ||
| DCP2 | PSMC3 | MYO6 | IGFBP5 | |||
| DDX5 | PTGFRN | MYOCD | IMMP2L | |||
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Q.; Zhang, Y.; Ji, Y.; Li, J.; Liu, X.; Duan, H.; Feng, Z.; Liu, Y.; Zhang, Y.; et al. Global Burden of Cervical Cancer: Current Estimates, Temporal Trend and Future Projections Based on the GLOBOCAN 2022. J. Natl. Cancer Cent. 2025, 5, 322–329. [Google Scholar] [CrossRef]
- Salarzaei, M.; van de Laar, R.L.O.; Ewing-Graham, P.C.; Najjary, S.; van Esch, E.; van Beekhuizen, H.J.; Mustafa, D.A.M. Unraveling Differences in Molecular Mechanisms and Immunological Contrasts between Squamous Cell Carcinoma and Adenocarcinoma of the Cervix. Int. J. Mol. Sci. 2024, 25, 6205. [Google Scholar] [CrossRef]
- Na, J.; Li, Y.; Wang, J.; Wang, X.; Lu, J.; Han, S. The Correlation between Multiple HPV Infections and the Occurrence, Development, and Prognosis of Cervical Cancer. Front. Microbiol. 2023, 14, 1220522. [Google Scholar] [CrossRef]
- Meng, Y.; Chu, T.; Lin, S.; Wu, P.; Zhi, W.; Peng, T.; Ding, W.; Luo, D.; Wu, P. Clinicopathological Characteristics and Prognosis of Cervical Cancer with Different Histological Types: A Population-Based Cohort Study. Gynecol. Oncol. 2021, 163, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, Z.; Xu, X.; Yin, Z.; Lou, H. Comparison of Adenocarcinoma and Adenosquamous Carcinoma Prognoses in Chinese Patients with FIGO Stage IB-IIA Cervical Cancer Following Radical Surgery. BMC Cancer 2020, 20, 664. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, W.; Wen, Z.; Li, F.; Tong, L.; Tang, W. Does Adenocarcinoma Have a Worse Prognosis than Squamous Cell Carcinoma in Patients with Cervical Cancer? A Real-World Study with a Propensity Score Matching Analysis. J. Gynecol. Oncol. 2020, 31, e80. [Google Scholar] [CrossRef] [PubMed]
- Arip, M.; Tan, L.F.; Jayaraj, R.; Abdullah, M.; Rajagopal, M.; Selvaraja, M. Exploration of Biomarkers for the Diagnosis, Treatment and Prognosis of Cervical Cancer: A Review. Discov. Oncol. 2022, 13, 91. [Google Scholar] [CrossRef]
- Dasari, S.; Wudayagiri, R.; Valluru, L. Cervical Cancer: Biomarkers for Diagnosis and Treatment. Clin. Chim. Acta 2015, 445, 7–11. [Google Scholar] [CrossRef]
- Cohen, A.; Lim, C.S.; Davies, A.H. Venous Thromboembolism in Gynecological Malignancy. Int. J. Gynecol. Cancer 2017, 27, 1970–1978. [Google Scholar] [CrossRef]
- Khorana, A.A.; Francis, C.W.; Culakova, E.; Kuderer, N.M.; Lyman, G.H. Thromboembolism Is a Leading Cause of Death in Cancer Patients Receiving Outpatient Chemotherapy. J. Thromb. Haemost. 2007, 5, 632–634. [Google Scholar] [CrossRef]
- Fernandes, C.J.; Morinaga, L.T.K.; Alves, J.L.; Castro, M.A.; Calderaro, D.; Jardim, C.V.P.; Souza, R. Cancer-Associated Thrombosis: The When, How and Why. Eur. Respir. Rev. 2019, 28, 180119. [Google Scholar] [CrossRef]
- Mantha, S.; Rak, J. Cancer Genetic Alterations and Risk of Venous Thromboembolism. Thromb. Res. 2022, 213, S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Kacimi, S.E.O.; Moeinafshar, A.; Haghighi, S.S.; Saghazadeh, A.; Rezaei, N. Venous Thromboembolism in Cancer and Cancer Immunotherapy. Crit. Rev. Oncol. Hematol. 2022, 178, 103782. [Google Scholar] [CrossRef]
- Neto, B.V.; Tavares, V.; da Silva, J.B.; Liz-Pimenta, J.; Marques, I.S.; Carvalho, L.; Salgado, L.; Pereira, D.; Medeiros, R. Thrombogenesis-Associated Genetic Determinants as Predictors of Thromboembolism and Prognosis in Cervical Cancer. Sci. Rep. 2023, 13, 9519. [Google Scholar] [CrossRef]
- Jacobson, G.; Lammli, J.; Zamba, G.; Hua, L.; Goodheart, M.J. Thromboembolic Events in Patients with Cervical Carcinoma: Incidence and Effect on Survival. Gynecol. Oncol. 2009, 113, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Eckert, L.; Wang, Y.; Wang, H.; Cohen, A. Venous Thromboembolism Risk in Patients With Cancer Receiving Chemotherapy: A Real-World Analysis. Oncologist 2013, 18, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.S.; Mousa, S.A. Cancer-Associated Thrombosis: Risk Factors, Molecular Mechanisms, Future Management. Clin. Appl. Thromb. Hemost. 2020, 26, 1076029620954282. [Google Scholar] [CrossRef]
- Sevestre, M.A.; Soudet, S. Epidemiology and Risk Factors for Cancer-Associated Thrombosis. J. Med. Vasc. 2020, 45, 6S3–6S7. [Google Scholar] [CrossRef]
- Neto, B.V.; Tavares, V.; Santos, J.M.O.; Cerqueira, F.; Pereira, D.; Medeiros, R. Map of Thrombogenesis in Viral Infections and Viral-Driven Tumours. Discov. Oncol. 2023, 14, 3. [Google Scholar] [CrossRef]
- Scridon, A. Platelets and Their Role in Hemostasis and Thrombosis—From Physiology to Pathophysiology and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 12772. [Google Scholar] [CrossRef]
- Pablo-Moreno, J.A.D.; Serrano, L.J.; Revuelta, L.; Sánchez, M.J.; Liras, A. The Vascular Endothelium and Coagulation: Homeostasis, Disease, and Treatment, with a Focus on the Von Willebrand Factor and Factors VIII and V. Int. J. Mol. Sci. 2022, 23, 8283. [Google Scholar] [CrossRef]
- Minors, D.S. Haemostasis, Blood Platelets and Coagulation. Anaesth. Intensive Care Med. 2007, 8, 214–216. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.M.; Levi, M.; Reitsma, P.H. New Fundamentals in Hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Sira, J.; Eyre, L. Physiology of Haemostasis. Anaesth. Intensive Care Med. 2016, 17, 79–82. [Google Scholar] [CrossRef]
- Hiller, E. Basic Principles of Hemostasis. In Modern Hematology; Humana Press: Totowa, NJ, USA, 2007; pp. 327–345. [Google Scholar]
- Chapin, J.C.; Hajjar, K.A. Fibrinolysis and the Control of Blood Coagulation. Blood Rev. 2015, 29, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Olgasi, C.; Assanelli, S.; Cucci, A.; Follenzi, A. Hemostasis and Endothelial Functionality: The Double Face of Coagulation Factors. Haematologica 2024, 109, 2041–2048. [Google Scholar] [CrossRef]
- Tavares, V.; Neto, B.V.; Marques, I.S.; Assis, J.; Pereira, D.; Medeiros, R. Cancer-Associated Thrombosis: What about MicroRNAs Targeting the Tissue Factor Coagulation Pathway? Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189053. [Google Scholar] [CrossRef]
- Vadivel, K.; Ponnuraj, S.M.; Kumar, Y.; Zaiss, A.K.; Bunce, M.W.; Camire, R.M.; Wu, L.; Evseenko, D.; Herschman, H.R.; Bajaj, M.S.; et al. Platelets Contain Tissue Factor Pathway Inhibitor-2 Derived from Megakaryocytes and Inhibits Fibrinolysis. J. Biol. Chem. 2014, 289, 31647–31661. [Google Scholar] [CrossRef]
- Miyake, R.; Yamada, Y.; Yamanaka, S.; Kawaguchi, R.; Ootake, N.; Myoba, S.; Kobayashi, H. Tissue Factor Pathway Inhibitor 2 as a Serum Marker for Diagnosing Asymptomatic Venous Thromboembolism in Patients with Epithelial Ovarian Cancer and Positive D-Dimer Results. Mol. Clin. Oncol. 2022, 16, 46. [Google Scholar] [CrossRef]
- Hao, R.; Li, H.; Li, X.; Liu, J.; Ji, X.; Zhang, H.; Zhang, Z.; Yang, P.; Zhai, Z. Transcriptomic Profiling of LncRNAs and MRNAs in a Venous Thrombosis Mouse Model. iScience 2025, 28, 111561. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, J.K.; Rao, D.S. MiRNA Dysregulation in Cancer: Towards a Mechanistic Understanding. Front. Genet. 2014, 5, 54. [Google Scholar] [CrossRef]
- Toden, S.; Zumwalt, T.J.; Goel, A. Non-Coding RNAs and Potential Therapeutic Targeting in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188491. [Google Scholar] [CrossRef]
- Morelli, V.M.; Brækkan, S.K.; Hansen, J.B. Role of MicroRNAs in Venous Thromboembolism. Int. J. Mol. Sci. 2020, 21, 2602. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating MicroRNAs as Stable Blood-Based Markers for Cancer Detection. Proc. Nat. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef]
- Teruel-Montoya, R.; Rosendaal, F.R.; Martínez, C. MicroRNAs in Hemostasis. J. Thromb. Haemost. 2015, 13, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Hernando, C.; Suárez, Y. MicroRNAs in Endothelial Cell Homeostasis and Vascular Disease. Curr. Opin. Hematol. 2018, 25, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Mukaka, M.M. Statistics Corner: A Guide to Appropriate Use of Correlation Coefficient in Medical Research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Ali, K.; Al-Hameed, A. Spearman’s Correlation Coefficient in Statistical Analysis. Int. J. Nonlinear Anal. Appl. 2022, 13, 2008–6822. [Google Scholar] [CrossRef]
- Langer, F.; Bokemeyer, C. Crosstalk between Cancer and Haemostasis: Implications for Cancer Biology and Cancer-Associated Thrombosis with Focus on Tissue Factor. Hamostaseologie 2012, 32, 95–104. [Google Scholar] [PubMed]
- Sharma, B.K.; Flick, M.J.; Palumbo, J.S. Cancer-Associated Thrombosis: A Two-Way Street. Semin. Thromb. Hemost. 2019, 45, 559–568. [Google Scholar] [CrossRef]
- Morgan, M.A.; Iyengar, T.D.; Napiorkowski, B.E.; Rubin, S.C.; Mikuta, J.J. The Clinical Course of Deep Vein Thrombosis in Patients with Gynecologic Cancer. Gynecol. Oncol. 2002, 84, 67–71. [Google Scholar] [CrossRef]
- Wentzensen, N.; Silver, M.I. Biomarkers for Cervical Cancer Prevention Programs: The Long and Winding Road From Discovery to Clinical Use. J. Low. Genit. Tract. Dis. 2016, 20, 191–194. [Google Scholar] [CrossRef]
- Hassan, N.; Efing, J.; Kiesel, L.; Bendas, G.; Götte, M. The Tissue Factor Pathway in Cancer: Overview and Role of Heparan Sulfate Proteoglycans. Cancers 2023, 15, 1524. [Google Scholar] [CrossRef]
- Sahu, A.; Jha, P.K.; Prabhakar, A.; Singh, H.D.; Gupta, N.; Chatterjee, T.; Tyagi, T.; Sharma, S.; Kumari, B.; Singh, S.; et al. MicroRNA-145 Impedes Thrombus Formation via Targeting Tissue Factor in Venous Thrombosis. eBioMedicine 2017, 26, 175–186. [Google Scholar] [CrossRef]
- Wang, W.; Ning, J.; Tang, Z.; He, Y.; Yao, L.-C.; Ye, L.; Wu, L. MicroRNA-23a Acts as an Oncogene in Pancreatic Carcinoma by Targeting TFPI-2. Exp. Ther. Med. 2020, 20, 53. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Shigetomi, H.; Imanaka, S. Reassessing the Role of Tissue Factor Pathway Inhibitor 2 in Neoplastic and Non-Neoplastic Lesions. Cancers 2025, 17, 1447. [Google Scholar] [CrossRef]
- Wojtukiewicz, M.Z.; Mysliwiec, M.; Tokajuk, A.; Kruszewska, J.; Politynska, B.; Jamroze, A.; Wojtukiewicz, A.M.; Tang, D.G.; Honn, K.V. Tissue Factor Pathway Inhibitor-2 (TFPI-2)—An Underappreciated Partaker in Cancer and Metastasis. Cancer Metastasis Rev. 2024, 43, 1185–1204. [Google Scholar] [CrossRef]
- Fullár, A.; Karászi, K.; Hollósi, P.; Lendvai, G.; Oláh, L.; Reszegi, A.; Papp, Z.; Sobel, G.; Dudás, J.; Kovalszky, I. Two Ways of Epigenetic Silencing of TFPI2 in Cervical Cancer. PLoS ONE 2020, 15, e0234873. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Imanaka, S.; Matsubara, S.; Shigetomi, H.; Yoshimoto, C. Dual Role of Tissue Factor Pathway Inhibitor 2—A Novel Serodiagnostic Marker for Ovarian Cancer—In Human Cancers. Int. J. Transl. Med. 2024, 4, 419–438. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Wang, S.Z.; Wang, N.; Jiang, W.G.; Ji, Y.H.; Zhang, S.L. Reduced Expression of Tissue Factor Pathway Inhibitor-2 Contributes to Apoptosis and Angiogenesis in Cervical Cancer. J. Exp. Clin. Cancer Res. 2012, 31, 1. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wen-Ming, C.; Jun-Bo, J. Plasma MiR-145 as a Novel Biomarker for the Diagnosis and Radiosensitivity Prediction of Human Cervical Cancer. J. Int. Med. Res. 2017, 45, 1054–1060. [Google Scholar] [CrossRef]
- Tavares, V.; Savva-Bordalo, J.; Rei, M.; Liz-Pimenta, J.; Assis, J.; Pereira, D.; Medeiros, R. Plasma MicroRNA Environment Linked to Tissue Factor Pathway and Cancer-Associated Thrombosis: Prognostic Significance in Ovarian Cancer. Biomolecules 2024, 14, 928. [Google Scholar] [CrossRef]
- Balia, C.; Giordano, M.; Scalise, V.; Neri, T.; Fontanini, G.; Basolo, F.; Celi, A.; Pedrinelli, R. MiR-19a and MiR-20a and Tissue Factor Expression in Activated Human Peripheral Blood Mononuclear Cells. Thrombosis 2017, 2017, 1076397. [Google Scholar] [CrossRef]
- Teruel, R.; Pérez-Sánchez, C.; Corral, J.; Herranz, M.T.; Pérez-Andreu, V.; Saiz, E.; García-Barberá, N.; Martínez-Martínez, I.; Roldán, V.; Vicente, V.; et al. Identification of MiRNAs as Potential Modulators of Tissue Factor Expression in Patients with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. J. Thromb. Haemost. 2011, 9, 1985–1992. [Google Scholar] [CrossRef]
- Zhu, X. MiR-125b but Not MiR-125a Is Upregulated and Exhibits a Trend to Correlate with Enhanced Disease Severity, Inflammation, and Increased Mortality in Sepsis Patients. J. Clin. Lab. Anal. 2020, 34, e23094. [Google Scholar] [CrossRef]
- Wang, G.; Chai, B.; Yang, L. MiR-128 and MiR-125 Regulate Expression of Coagulation Factor IX Gene with Nonsense Mutation by Repressing Nonsense-Mediated MRNA Decay. Biomed. Pharmacother. 2016, 80, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Sousa, H. MicroRNAs as Biomarkers of Cervical Cancer Development: A Literature Review on MiR-125b and MiR-34a. Mol. Biol. Rep. 2014, 41, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.W.; Liu, T.L.; Wong, C.W.; Liu, S.K.; Lum, Y.L.; Ming, W.K. The Dysregulation of MicroRNAs in the Development of Cervical Pre-Cancer—An Update. Int. J. Mol. Sci. 2022, 23, 7126. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, J.; Chen, A.; Zhou, J.; Liu, J.; Cheng, J.; Qiu, J.; Zhang, J. Downregulation of MicroRNA-145 Is Associated with Aggressive Progression and Poor Prognosis in Human Cervical Cancer. Tumour Biol. 2015, 36, 3703–3708. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, D.S.; Chen, J.Y.; Ding, N. Aberrant Expression of MiR-20a and MiR-203 in Cervical Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yao, D.; Chen, J.; Ding, N.; Ren, F. MiR-20a Promotes Cervical Cancer Proliferation and Metastasis in Vitro and in Vivo. PLoS ONE 2015, 10, e0120905. [Google Scholar] [CrossRef] [PubMed]
- Hackl, M.; Brunner, S.; Fortschegger, K.; Schreiner, C.; Micutkova, L.; Mück, C.; Laschober, G.T.; Lepperdinger, G.; Sampson, N.; Berger, P.; et al. MiR-17, MiR-19b, MiR-20a, and MiR-106a Are down-Regulated in Human Aging. Aging Cell 2010, 9, 291–296. [Google Scholar] [CrossRef]
- Derouet, M.F.; Liu, G.; Darling, G.E. MiR-145 Expression Accelerates Esophageal Adenocarcinoma Progression by Enhancing Cell Invasion and Anoikis Resistance. PLoS ONE 2014, 9, e115589. [Google Scholar] [CrossRef]
- Derouet, M.F.; Dakpo, E.; Wu, L.; Zehong, G.; Conner, J.; Keshavjee, S.; de Perrot, M.; Waddell, T.; Elimova, E.; Yeung, J.; et al. MiR-145 Expression Enhances Integrin Expression in SK-GT-4 Cell Line by down-Regulating c-Myc Expression. PLoS ONE 2018, 9, e115589. [Google Scholar] [CrossRef]
- Chen, F.; Chen, L.; Zhang, Y.; Shi, L.; Xu, H.; Song, T. Survival Comparison Between Squamous Cell Carcinoma and Adenocarcinoma for Radiotherapy-Treated Patients with Stage IIB-IVA Cervical Cancer. Front. Oncol. 2022, 12, 895122. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Song, K.; Cui, B.; Jiang, J.; Yang, X.; Kong, B. A Comparison of the Prognosis between Adenocarcinoma and Squamous Cell Carcinoma in Stage IB–IIA Cervical Cancer. Int. J. Clin. Oncol. 2018, 23, 522–531. [Google Scholar] [CrossRef]
- Campos-Parra, A.D.; Pérez-Quintanilla, M.; Martínez-Gutierrez, A.D.; Pérez-Montiel, D.; Coronel-Martínez, J.; Millan-Catalan, O.; De León, D.C.; Pérez-Plasencia, C. Molecular Differences between Squamous Cell Carcinoma and Adenocarcinoma Cervical Cancer Subtypes: Potential Prognostic Biomarkers. Curr. Oncol. 2022, 29, 4689–4702. [Google Scholar] [CrossRef]
- Grossi, I.; Salvi, A.; Baiocchi, G.; Portolani, N.; De Petro, G. Functional Role of MicroRNA-23b-3p in Cancer Biology. MicroRNA 2018, 7, 156–166. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, L.; Wang, W.; Cheng, K.; Wang, H.; Ji, Y.; Su, X.; Hao, M. Down-Regulation of MiR-125b by HPV16 E6 Might Promote Cervical Cancer Progression through TAZ/TEAD. Front. Oncol. 2025, 15, 1444874. [Google Scholar] [CrossRef]
- Mei, L.L.; Wang, W.J.; Qiu, Y.T.; Xie, X.F.; Bai, J.; Shi, Z.Z. MiR-125b-5p Functions as a Tumor Suppressor Gene Partially by Regulating HMGA2 in Esophageal Squamous Cell Carcinoma. PLoS ONE 2017, 12, e0185636. [Google Scholar] [CrossRef] [PubMed]
- Saijo, Y.; Furumoto, H.; Yoshida, K.; Nishimura, M.; Irahara, M. Clinical Significance of Vascular Endothelial Growth Factor Expression and Microvessel Density in Invasive Cervical Cancer. J. Med. Investig. 2015, 62, 154–160. [Google Scholar] [CrossRef]
- Xu, Z.; Maiti, D.; Kisiel, W.; Duh, E.J. Tissue Factor Pathway Inhibitor-2 Is Upregulated by Vascular Endothelial Growth Factor and Suppresses Growth Factor-Induced Proliferation of Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Piškur, I.; Topolovec, Z.; Bakula, M.; Zagorac, I.; Milić Vranješ, I.; Vidosavljević, D. Expression of Vascular Endothelial Growth Factor-A (VEGF-A) in Adenocarcinoma and Squamous Cell Cervical Cancer and Its Impact on Disease Progression: Single Institution Experience. Medicina 2023, 59, 1189. [Google Scholar] [CrossRef] [PubMed]
- Prat, J.; Belhadj, H.; Berek, J.; Bermudez, A.; Bhatla, N.; Cain, J.; Denny, L.; Fujiwara, K.; Hacker, N.; Åvall-Lundqvist, E.; et al. Figo’s Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum: Abridged Republication. J. Gynecol. Oncol. 2015, 26, 87–89. [Google Scholar] [CrossRef]
- Chen, J.; Yao, D.; Li, Y.; Chen, H.; He, C.; Ding, N.; Lu, Y.; Ou, T.; Zhao, S.; Li, L.; et al. Serum MicroRNA Expression Levels Can Predict Lymph Node Metastasis in Patients with Early-Stage Cervical Squamous Cell Carcinoma. Int. J. Mol. Med. 2013, 32, 557–567. [Google Scholar] [CrossRef]
- Cao, M.M.; Liu, Q.X.; Zou, X.; Fan, X.C.; Liu, C.; Zhang, S.Y.; Wang, T.S.; Li, C.Y.; Zhang, C.; Geng, X.N.; et al. Identification of a Serum Three-MicroRNA Signature for Cervical Cancer Diagnosis. Chin. Med. J. 2021, 134, 1756–1758. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Wu, Q.Y.; Zhang, C.X.; Wang, Q.; Ling, J.; Huang, X.T.; Sun, X.; Yuan, M.; Wu, D.; Yin, H.F. MiR-20a Inhibits the Killing Effect of Natural Killer Cells to Cervical Cancer Cells by Downregulating RUNX1. Biochem. Biophys. Res. Commun. 2018, 505, 309–316. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, J.; Luo, R.; Zhou, X.; Wang, J.; Chen, F. MicroRNA-20a Regulates Cell Proliferation, Apoptosis and Autophagy by Targeting Thrombospondin 2 in Cervical Cancer. Eur. J. Pharmacol. 2019, 844, 102–109. [Google Scholar] [CrossRef]
- Liu, X. Up-Regulation of MiR-20a by HPV16 E6 Exerts Growth-Promoting Effects by Targeting PDCD6 in Cervical Carcinoma Cells. Biomed. Pharmacother. 2018, 102, 996–1002. [Google Scholar] [CrossRef]
- Ma, S.; Chan, Y.P.; Kwan, P.S.; Lee, T.K.; Yan, M.; Tang, K.H.; Ling, M.T.; Vielkind, J.R.; Guan, X.Y.; Chan, K.W. MicroRNA-616 Induces Androgen-Independent Growth of Prostate Cancer Cells by Suppressing Expression of Tissue Factor Pathway Inhibitor TFPI-2. Cancer Res. 2011, 71, 583–592. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, L.; Li, L. Human Papillomavirus Type 16 E7 Promotes Cell Viability and Migration in Cervical Cancer by Regulating the MiR-23a/HOXC8 Axis. J. Obstet. Gynaecol. 2024, 44, 2311658. [Google Scholar] [CrossRef]
- He, X.W.; Shi, Y.H.; Zhao, R.; Liu, Y.S.; Li, G.F.; Hu, Y.; Chen, W.; Cui, G.H.; Su, J.J.; Liu, J.R. Plasma Levels of MiR-125b-5p and MiR-206 in Acute Ischemic Stroke Patients After Recanalization Treatment: A Prospective Observational Study. J. Stroke Cerebrovasc. Dis. 2019, 28, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.; Al Masry, H.; Hussein, M.; Abd Elkareem, R.M.; Masoud, M.M. The Potential Role of Micro-RNA 125b-5p Level in Predicting Outcome from Thrombolytic Therapy in Patients with Acute Ischemic Stroke. J. Thromb. Thrombolysis 2023, 56, 275–282. [Google Scholar] [CrossRef]
- Peng, B.; Theng, P.Y.; Le, M.T.N. Essential Functions of MiR-125b in Cancer. Cell Prolif. 2021, 54, e12913. [Google Scholar] [CrossRef]
- Wang, X.; Tang, S.; Le, S.Y.; Lu, R.; Rader, J.S.; Meyers, C.; Zheng, Z.M. Aberrant Expression of Oncogenic and Tumor-Suppressive MicroRNAs in Cervical Cancer Is Required for Cancer Cell Growth. PLoS ONE 2008, 3, e2557. [Google Scholar] [CrossRef]
- Nuovo, G.J.; Wu, X.; Volinia, S.; Yan, F.; Di Leva, G.; Chin, N.; Nicol, A.F.; Jiang, J.; Otterson, G.; Schmittgen, T.D.; et al. Strong Inverse Correlation between MicroRNA-125b and Human Papillomavirus DNA in Productive Infection. Diagn. Mol. Pathol. 2010, 19, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.M.; Snir, O.; Hindberg, K.D.; Hveem, K.; Brækkan, S.K.; Hansen, J.-B. High MicroRNA-145 Plasma Levels Are Associated with Decreased Risk of Future Incident Venous Thromboembolism: The HUNT Study. Blood 2024, 143, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Sennblad, B.; Basu, S.; Mazur, J.; Suchon, P.; Martinez-Perez, A.; Vlieg, A. van H.; Truong, V.; Li, Y.; Gådin, J.R.; Tang, W.; et al. Genome-Wide Association Study with Additional Genetic and Post-Transcriptional Analyses Reveals Novel Regulators of Plasma Factor XI Levels. Hum. Mol. Genet. 2017, 26, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Nourse, J.; Danckwardt, S. A Novel Rationale for Targeting FXI: Insights from the Hemostatic MicroRNA Targetome for Emerging Anticoagulant Strategies. Pharmacol. Ther. 2021, 218, 107676. [Google Scholar] [CrossRef] [PubMed]
- Villadsen, S.B.; Bramsen, J.B.; Ostenfeld, M.S.; Wiklund, E.D.; Fristrup, N.; Gao, S.; Hansen, T.B.; Jensen, T.I.; Borre, M.; Rntoft, T.F.; et al. The MiR-143/-145 Cluster Regulates Plasminogen Activator Inhibitor-1 in Bladder Cancer. Br. J. Cancer 2012, 106, 366–374. [Google Scholar] [CrossRef]
- Meijers, J.C.; Tekelenburg, W.L.; Bouma, B.N.; Bertina, R.M.; Rosendaal, F.R. High Levels of Coagulation Factor XI as a Risk Factor for Venous Thrombosis. N. Engl. J. Med. 2000, 342, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayanan, A.; Chandrasekaran, K.S.; Karunagaran, D. MicroRNA-145 Modulates Epithelial-Mesenchymal Transition and Suppresses Proliferation, Migration and Invasion by Targeting SIP1 in Human Cervical Cancer Cells. Cell Oncol. 2017, 40, 119–131. [Google Scholar] [CrossRef]
- Ye, C.; Sun, N.X.; Ma, Y.; Zhao, Q.; Zhang, Q.; Xu, C.; Wang, S.B.; Sun, S.H.; Wang, F.; Li, W. MicroRNA-145 Contributes to Enhancing Radiosensitivity of Cervical Cancer Cells. FEBS Lett. 2015, 589, 702–709. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Yang, L. MiR-145 Inhibits Cervical Cancer Progression and Metastasis by Targeting WNT2B by Wnt/β-Catenin Pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 3740–3751. [Google Scholar]
- Zhang, X.Y.; Ma, H.; Li, J.; Lu, X.R.; Li, J.Q.; Yuan, N.; Zhang, Z.L.; Xue, X.Y. Functional Implications of MiR-145/RCAN3 Axis in the Progression of Cervical Cancer. Reprod. Biol. 2020, 20, 140–146. [Google Scholar] [CrossRef]
- Ma, L.; Li, L.L. MiR-145 Contributes to the Progression of Cervical Carcinoma by Directly Regulating FSCN1. Cell Transplant. 2019, 28, 1299–1305. [Google Scholar] [CrossRef]
- Silva, J.; Tavares, V.; Afonso, A.; Garcia, J.; Cerqueira, F.; Medeiros, R. Plasmatic MicroRNAs and Treatment Outcomes of Patients with Metastatic Castration-Resistant Prostate Cancer: A Hospital-Based Cohort Study and In Silico Analysis. Int. J. Mol. Sci. 2023, 24, 9101. [Google Scholar] [CrossRef]
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing Sample and MiRNA Profile Quality in Serum and Plasma or Other Biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of Methodologies to Detect Low Levels of Hemolysis in Serum for Accurate Assessment of Serum MicroRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef]
- Tavares, V.; Savva-Bordalo, J.; Rei, M.; Liz-Pimenta, J.; Assis, J.; Pereira, D.; Medeiros, R. Haemostatic Gene Expression in Cancer-Related Immunothrombosis: Contribution for Venous Thromboembolism and Ovarian Tumour Behaviour. Cancers 2024, 16, 2356. [Google Scholar] [CrossRef]
- Tsai, S.J.; Ruan, Y.X.; Lee, C.C.; Lee, M.S.; Chiou, W.Y.; Lin, H.Y.; Hsu, F.C.; Su, Y.C.; Hung, S.K. The incidence of venous thromboembolism in cervical cancer: A nationwide population-based study. BMC Res. Notes 2012, 5, 316. [Google Scholar] [CrossRef]
- Satoh, T.; Matsumoto, K.; Tanaka, Y.O.; Akiyama, A.; Nakao, S.; Sakurai, M.; Ochi, H.; Onuki, M.; Minaguchi, T.; Sakurai, H.; et al. Incidence of Venous Thromboembolism before Treatment in Cervical Cancer and the Impact of Management on Venous Thromboembolism after Commencement of Treatment. Thromb. Res. 2013, 131, e127–e132. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Krishna, M.; Subbalakshmi, A.R.; Ramisetty, S.; Mohanty, A.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging Biomarkers and Molecular Targets for Precision Medicine in Cervical Cancer. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189106. [Google Scholar] [CrossRef] [PubMed]
- Fourie, L.; Christowitz, C.; Eksteen, C.; van der Merwe, H.; Botha, H.; Venter, C.; Engelbrecht, A.M. Inflammation and Thrombotic Risk in Late-Stage Cervical Cancer: An Exploratory Study of Coagulation and Cytokine Profiles in a South African Cohort. Cytokine 2024, 184, 156782. [Google Scholar] [CrossRef] [PubMed]
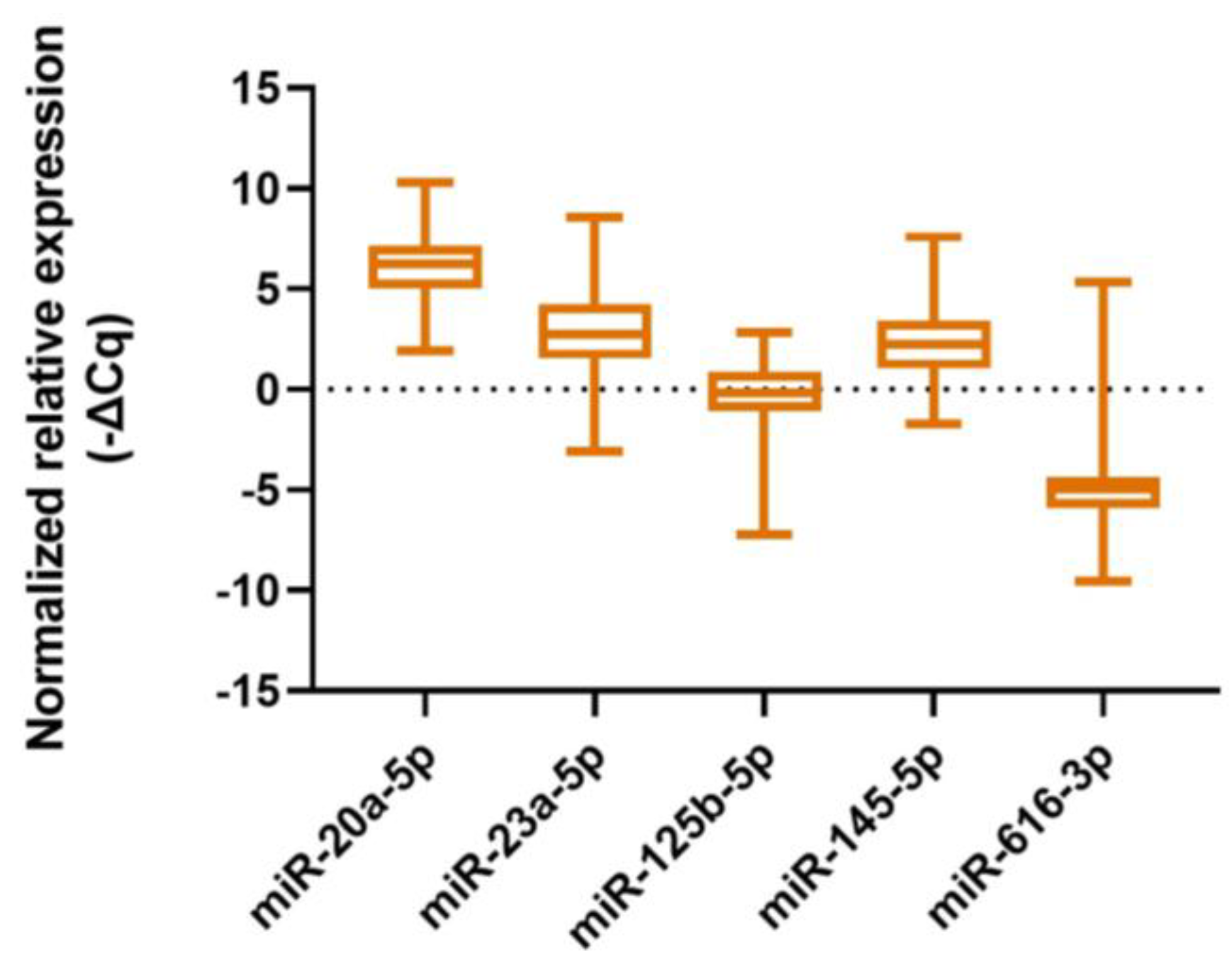
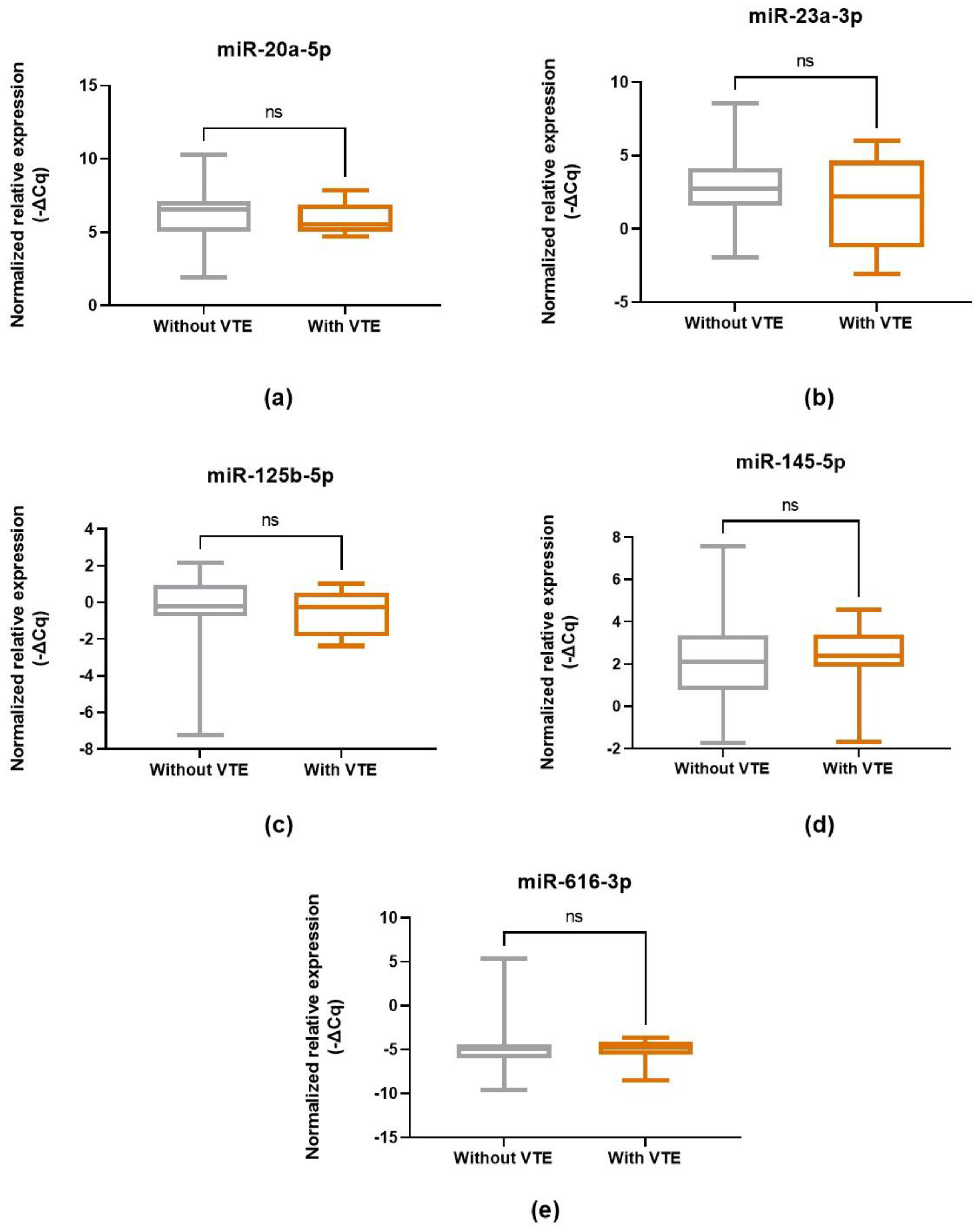
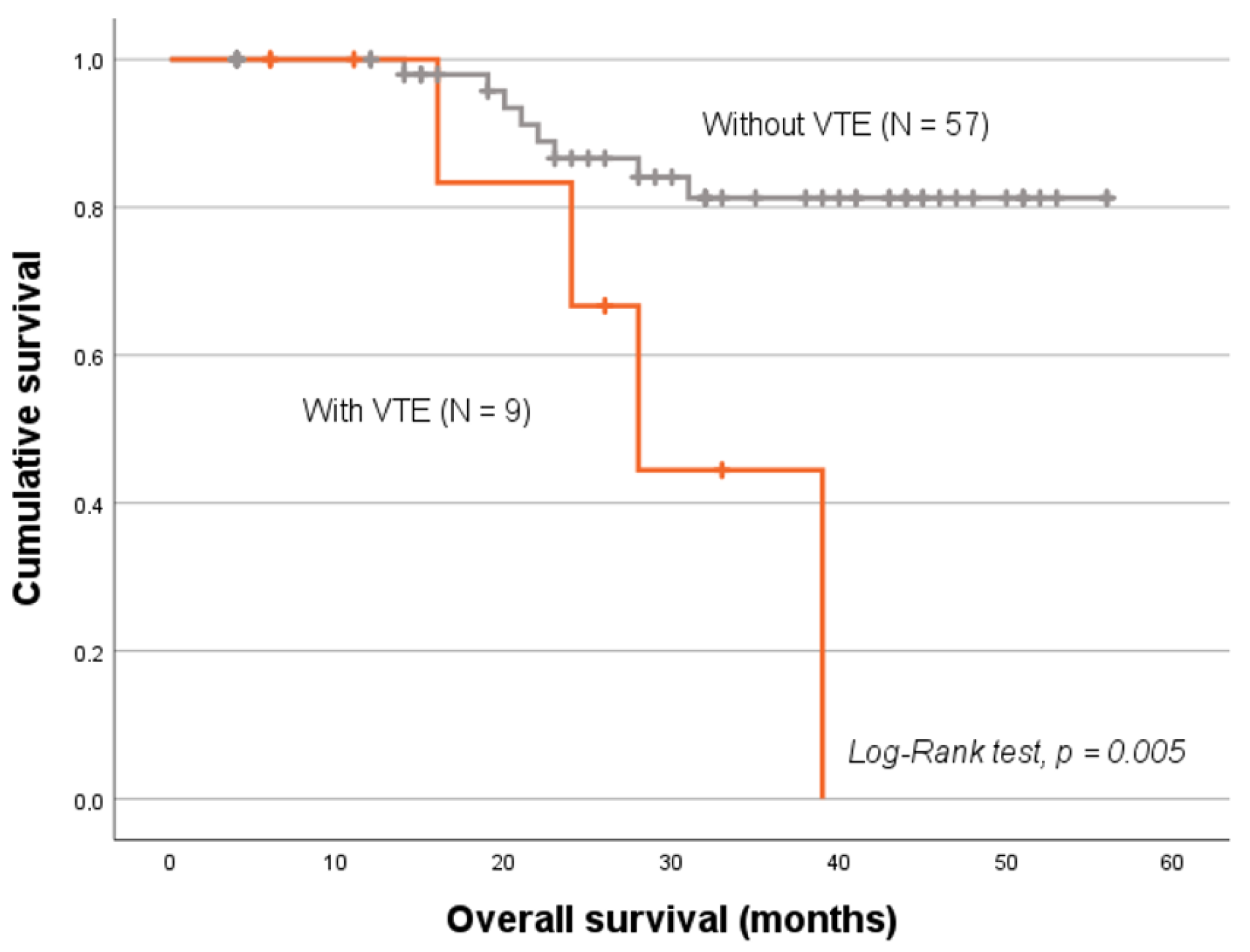
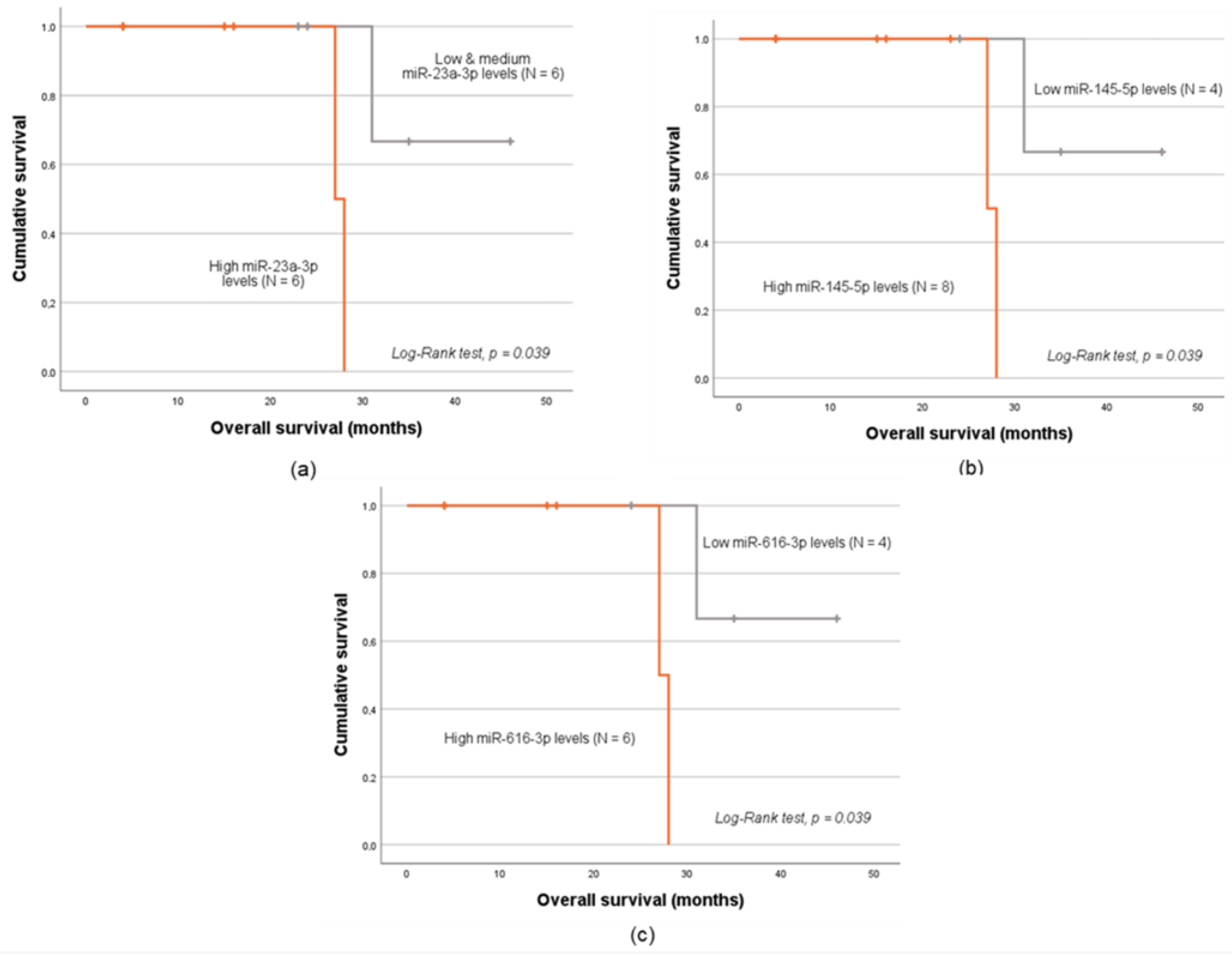

| miRNA | miR-20a-5p | miR-23a-3p | miR-125b-5p | miR-145-5p | miR-616-3p | |
|---|---|---|---|---|---|---|
| miR-20a-5p | Coefficient test | - | 0.659 * | 0.598 | 0.662 * | 0.555 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| miR-23a-3p | Coefficient test | 0.659 * | - | 0.546 | 0.700 * | 0.305 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| miR-125b-5p | Coefficient test | 0.598 | 0.546 | - | 0.660 | 0.332 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| miR-145-5p | Coefficient test | 0.662 * | 0.700 * | 0.660 | - | 0.434 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | ||
| miR-616-3p | Coefficient test | 0.555 | 0.305 | 0.332 | 0.434 | - |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| miRNA | Age (Years) | Histology | FIGO Stage | ||||
|---|---|---|---|---|---|---|---|
| <52 | ≥52 | CSCC | ADC | Other | <IIB | ≥IIB | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| miR-20a-5p | |||||||
| Low | 7 (21.2%) | 15 (48.4%) | 31 (60.8%) | 2 (20%) | 0 (0%) | 6 (66.7%) | 27 (49.1%) |
| High | 26 (78.8%) | 16 (51.6%) | 20 (39.2%) | 8 (80%) | 3 (100%) | 3 (33.3%) | 28 (50.9%) |
| p-value | 0.043 * | 0.003 | 0.332 | ||||
| miR-23a-3p | |||||||
| Low | 13 (39.4%) | 20 (58.8%) | 28 (53.8%) | 4 (33.3%) | 1 (33.3%) | 9 (100%) | 36 (62.1%) |
| High | 20 (60.6%) | 14 (41.2%) | 24 (46.2%) | 8 (66.7%) | 2 (66.7%) | 0 (0%) | 22 (37.9%) |
| p-value | 0.178 | 0.194 | 0.025 ** | ||||
| miR-125b-5p | |||||||
| Low | 12 (36.4%) | 21 (61.8%) | 21 (40.4%) | 1 (8.3%) | 0 (0%) | 5 (55.6%) | 28 (48.3%) |
| High | 21 (63.6%) | 13 (38.2%) | 31 (59.6%) | 11 (91.7%) | 3 (100%) | 4 (44.4%) | 30 (51.7%) |
| p-value | 0.067 | 0.018 * | 0.687 | ||||
| miR-145-5p | |||||||
| Low | 13 (39.4%) | 20 (58.8%) | 29 (55.8%) | 4 (33.3%) | 0 (0%) | 8 (88.9%) | 25 (43.1%) |
| High | 20 (60.6%) | 14 (41.2%) | 23 (44.2%) | 8 (66.7%) | 3 (100%) | 1 (11.1%) | 33 (56.9%) |
| p-value | 0.178 | 0.027 | 0.011 | ||||
| miR-616-3p | |||||||
| Low | 14 (43.8%) | 17 (54.8%) | 27 (54%) | 4 (40%) | 0 (0%) | 5 (62.5%) | 26 (47.3%) |
| High | 18 (56.2%) | 14 (45.2%) | 23 (46%) | 6 (60%) | 3 (100%) | 3 (37.5%) | 29 (52.7%) |
| p-value | 0.530 | 0.070 | 0.425 | ||||
| Variable | N (%) |
|---|---|
| Age at CC diagnosis (years) | 51.9 ± 13.4 * |
| <52 | 33 (47.8) |
| ≥52 | 34 (49.3) |
| Missing data | 2 (2.9) |
| Histology | |
| Squamous Cell Carcinoma | 52 (75.4) |
| Adenocarcinoma | 12 (17.4) |
| Other | 3 (4.3) |
| Missing data | 2 (2.9) |
| FIGO Stage | |
| <IIB | 9 (13.0) |
| ≥IIB | 58 (84.1) |
| Missing data | 2 (2.9) |
| VTE Status | |
| No | 58 (84.1) |
| Yes | 9 (13.0) |
| Missing data | 2 (2.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.T.; Neto, B.V.; da Silva, J.B.; Carvalho, L.; Salgado, L.; Pereira, D.; Adega, F.; Tavares, V.; Medeiros, R. Venous Thrombogenesis and Cervical Cancer: Plasma MicroRNAs as Prognostic Indicators of Tumor Behavior. Int. J. Mol. Sci. 2025, 26, 9796. https://doi.org/10.3390/ijms26199796
Costa MT, Neto BV, da Silva JB, Carvalho L, Salgado L, Pereira D, Adega F, Tavares V, Medeiros R. Venous Thrombogenesis and Cervical Cancer: Plasma MicroRNAs as Prognostic Indicators of Tumor Behavior. International Journal of Molecular Sciences. 2025; 26(19):9796. https://doi.org/10.3390/ijms26199796
Chicago/Turabian StyleCosta, Mariana Teixeira, Beatriz Vieira Neto, José Brito da Silva, Luísa Carvalho, Lurdes Salgado, Deolinda Pereira, Filomena Adega, Valéria Tavares, and Rui Medeiros. 2025. "Venous Thrombogenesis and Cervical Cancer: Plasma MicroRNAs as Prognostic Indicators of Tumor Behavior" International Journal of Molecular Sciences 26, no. 19: 9796. https://doi.org/10.3390/ijms26199796
APA StyleCosta, M. T., Neto, B. V., da Silva, J. B., Carvalho, L., Salgado, L., Pereira, D., Adega, F., Tavares, V., & Medeiros, R. (2025). Venous Thrombogenesis and Cervical Cancer: Plasma MicroRNAs as Prognostic Indicators of Tumor Behavior. International Journal of Molecular Sciences, 26(19), 9796. https://doi.org/10.3390/ijms26199796






