Unveiling Metabolic Subtypes in Endometrial Cancer Cell Lines: Insights from Metabolomic Analysis Under Standard and Stress Conditions
Abstract
1. Introduction
- POLE-mutated: Characterized by ultramutation and good prognosis.
- Mismatch repair deficient (MMRd): Loss of MMR proteins, intermediate prognosis and responsive to immunotherapy.
- p53-abnormal (p53abn): TP53 mutations or abnormal p53 expression associated with poor outcomes.
- No specific molecular profile (NSMP): A molecular heterogeneous group that does not fit into the other three categories with intermediate prognosis.
Aim of Study
2. Results and Discussion
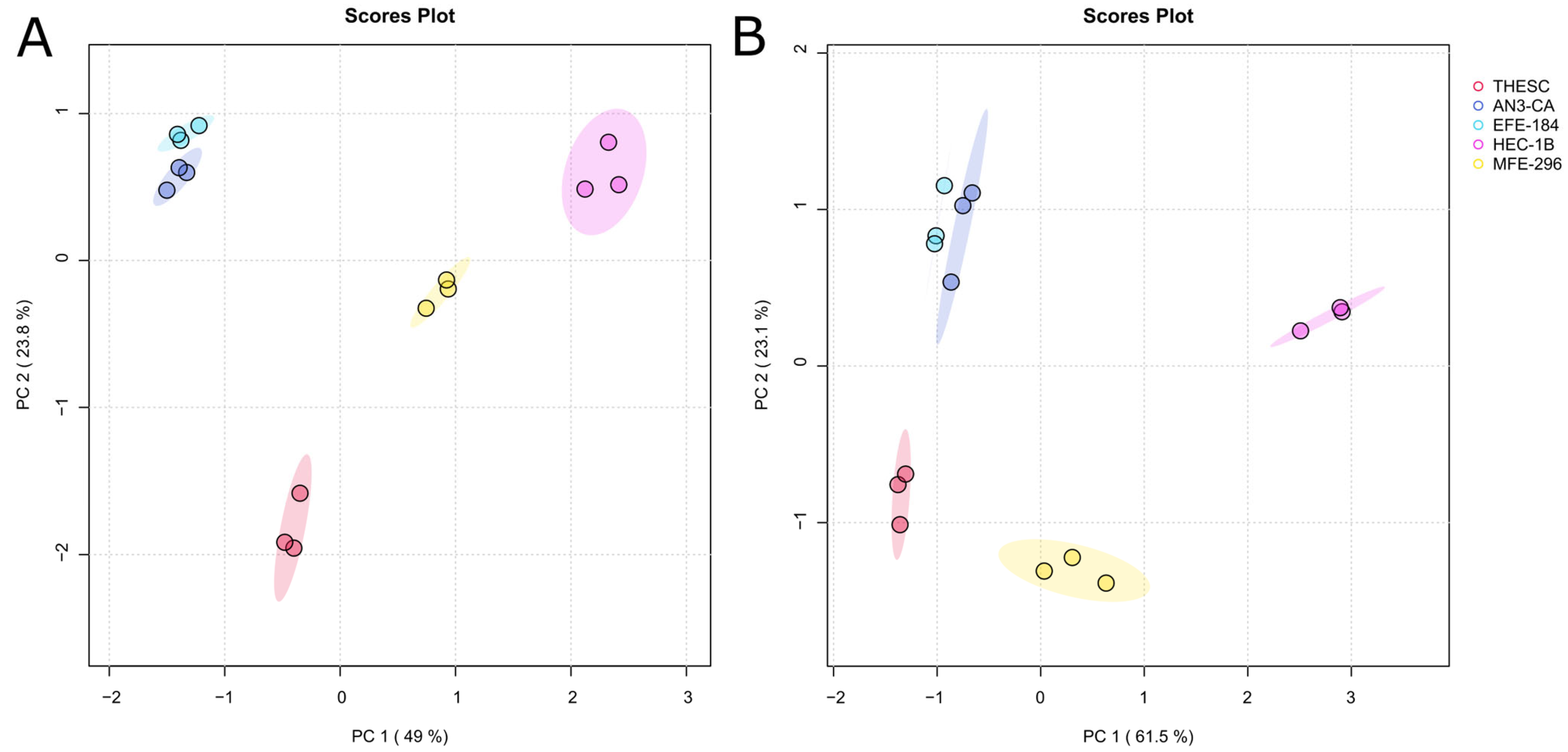
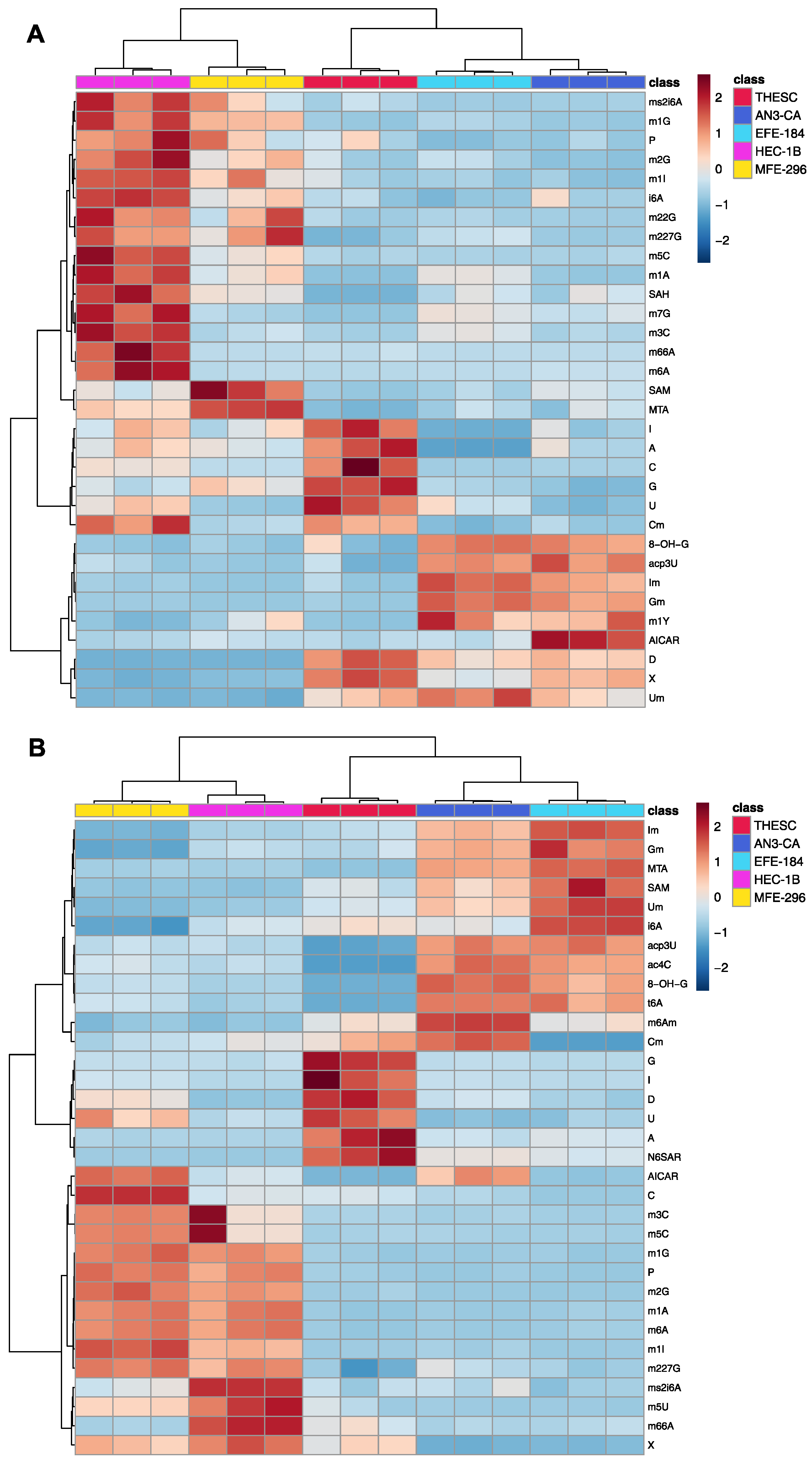
2.1. Comprehensive Exo- and Endo-Metabolomic Analysis of Endometrial Cell Lines and Their Culture Media
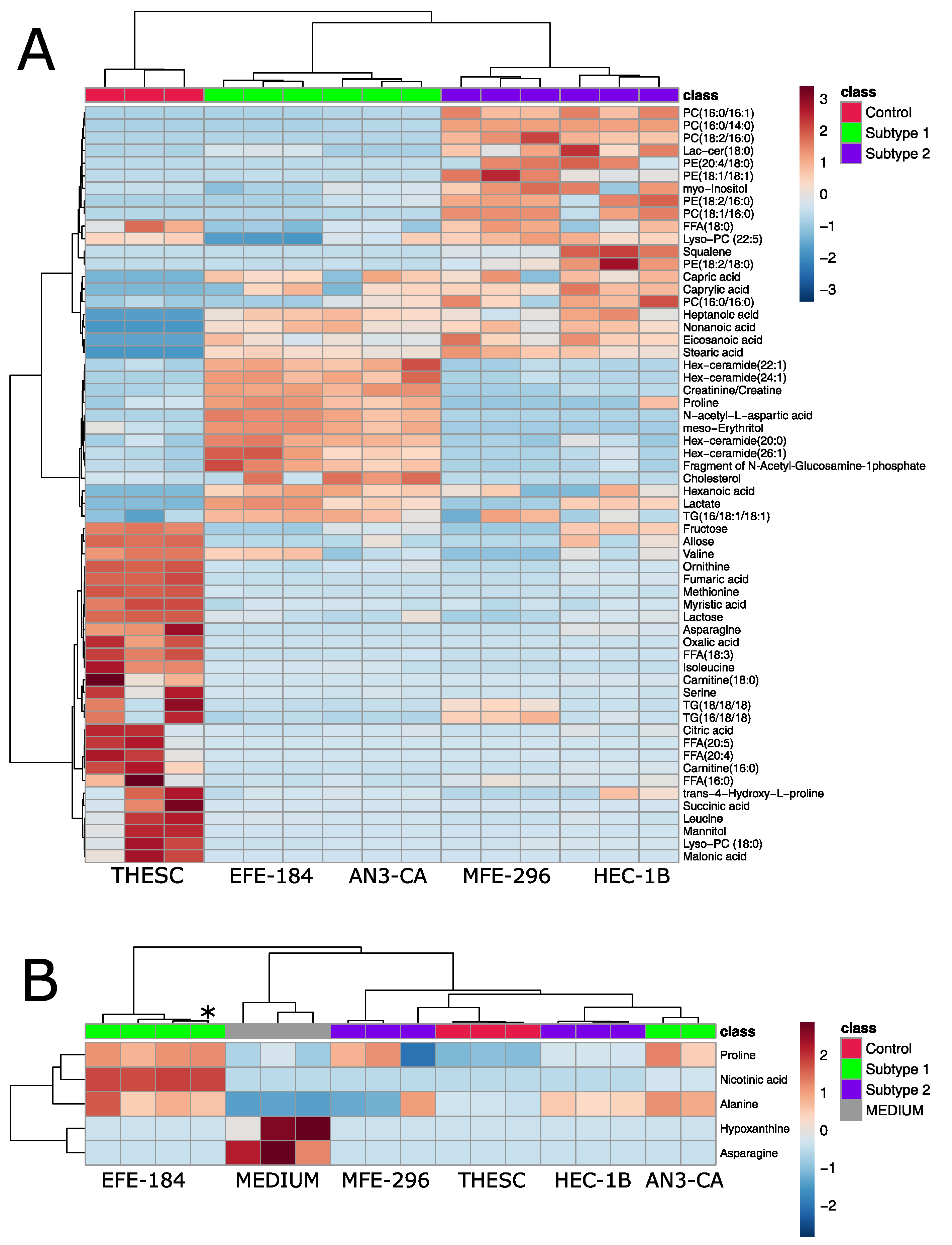
2.2. Metabolomic Analysis of Endometrial Cancer Subtypes
2.3. Validation of Metabolomic Endometrial Cancer Subtypes Using qPCR
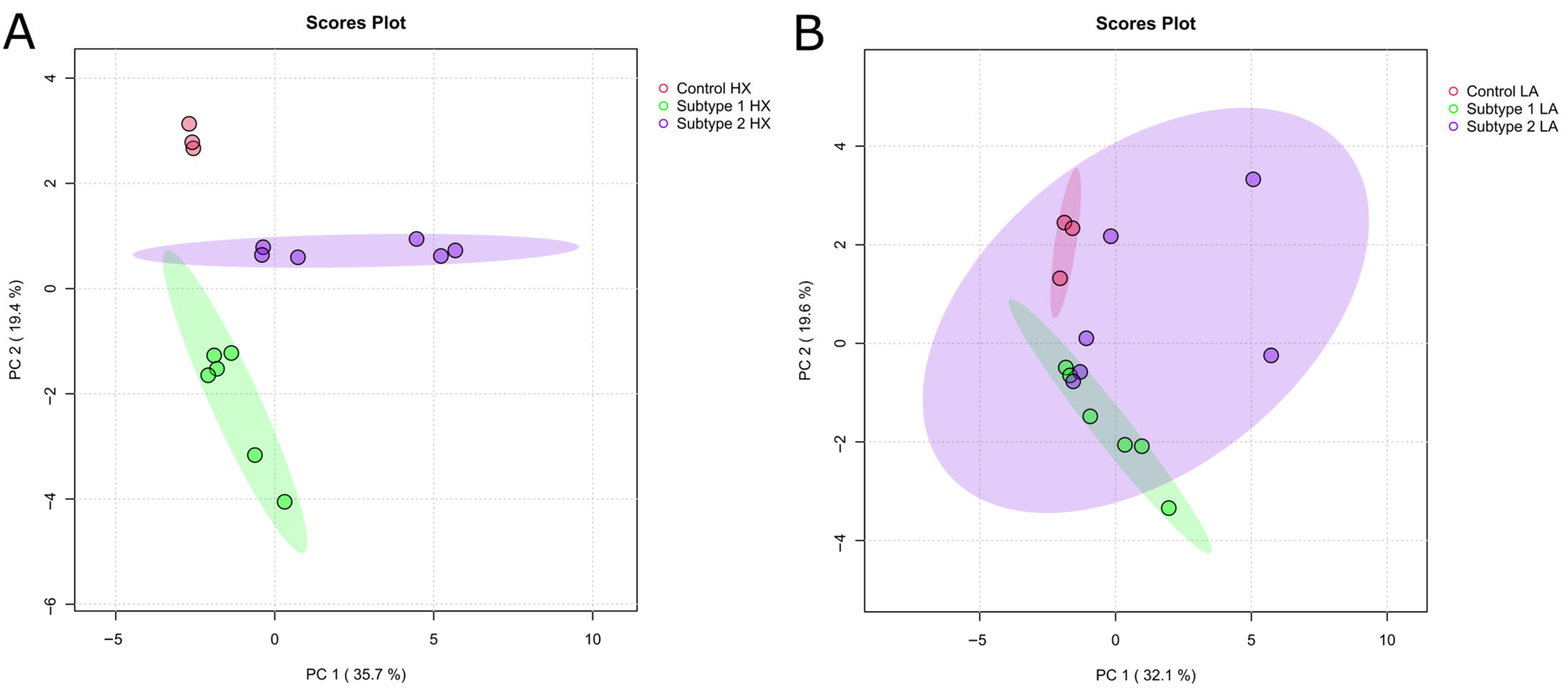
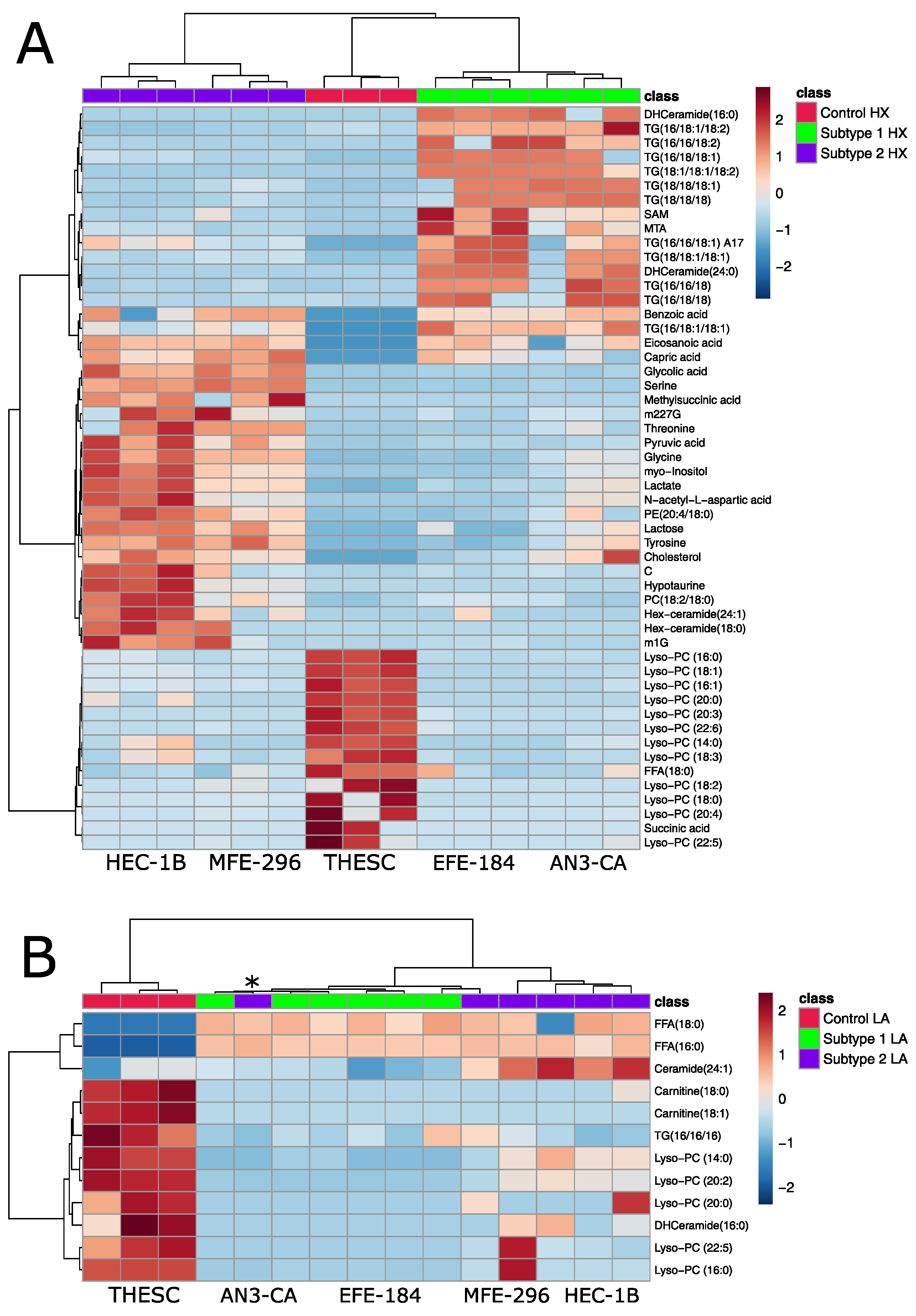
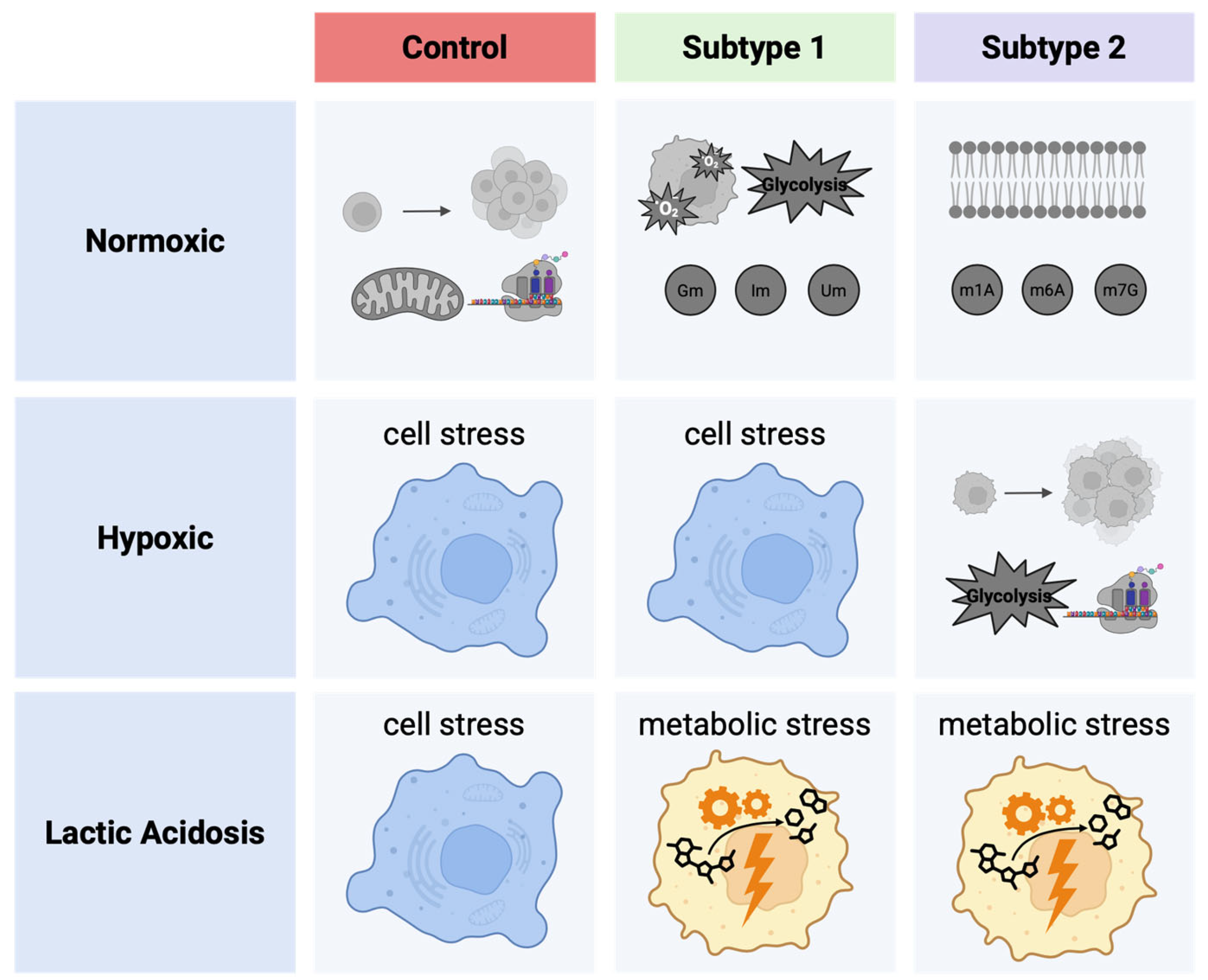
2.4. Metabolomic Analysis of Acidosis and Hypoxia-Treated Endometrial Cancer Subtypes
2.5. Establishing Clinically Relevant Context for Novel Metabolic EC Subtypes
3. Materials and Methods
3.1. Cell Culture
3.2. Cell Treatment
3.3. Cell and CCM Harvest
3.4. Preparation of CCM for MS Analysis
3.5. Preparation of Cells for MS Analysis
3.6. Untargeted GC/MS Profiling
3.7. Targeted LC/MS Analysis of Nucleosides
3.8. Targeted LC/MS Analysis of Lipids
3.9. RNA Isolation
3.10. Reverse Transcription and Quantiative PCR
3.11. Data Processing and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Chou, A.-J.; Bing, R.-S.; Ding, D.-C. Endometrial Atypical Hyperplasia and Risk of Endometrial Cancer. Diagnostics 2024, 14, 2471. [Google Scholar] [CrossRef] [PubMed]
- Langer, T. S3-Leitlinie Endometriumkarzinom. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Endometriumkarzinom/Version_2/LL_Endometriumkarzinom_Langversion_2.0.pdf (accessed on 14 November 2024).
- Skok, K.; Gradišnik, L.; Maver, U.; Kozar, N.; Sobočan, M.; Takač, I.; Arko, D.; Kavalar, R. Gynaecological cancers and their cell lines. J. Cell. Mol. Med. 2021, 25, 3680–3698. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, D.; Gong, C.; Zhang, F.; He, J.; Zhang, W.; Zhao, Y.; Sun, J. Prognostic role of hormone receptors in endometrial cancer: A systematic review and meta-analysis. World J. Surg. Oncol. 2015, 13, 208. [Google Scholar] [CrossRef]
- Mahdy, H.; Vadakekut, E.S.; Crotzer, D. Endometrial Cancer; StatPearls [Internet]: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bostan, I.-S.; Mihaila, M.; Roman, V.; Radu, N.; Neagu, M.T.; Bostan, M.; Mehedintu, C. Landscape of Endometrial Cancer: Molecular Mechanisms, Biomarkers, and Target Therapy. Cancers 2024, 16, 2027. [Google Scholar] [CrossRef]
- Faria, S.C.; Devine, C.E.; Rao, B.; Sagebiel, T.; Bhosale, P. Imaging and Staging of Endometrial Cancer. Semin. Ultrasound CT MRI 2019, 40, 287–294. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Walsh, C.S.; Hacker, K.E.; Secord, A.A.; DeLair, D.F.; McCourt, C.; Urban, R. Molecular testing for endometrial cancer: An SGO clinical practice statement. Gynecol. Oncol. 2023, 168, 48–55. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of cancer metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef]
- Finley, L.W.S. What is cancer metabolism? Cell 2023, 186, 1670–1688. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Daverio, Z.; Balcerczyk, A.; Rautureau, G.J.P.; Panthu, B. How Warburg-Associated Lactic Acidosis Rewires Cancer Cell Energy Metabolism to Resist Glucose Deprivation. Cancers 2023, 15, 1417. [Google Scholar] [CrossRef]
- Choi, S.Y.C.; Collins, C.C.; Gout, P.W.; Wang, Y. Cancer-generated lactic acid: A regulatory, immunosuppressive metabolite? J. Pathol. 2013, 230, 350–355. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Sonveaux, P.; Végran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; de Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Singh, K.K. Genetic insights into OXPHOS defect and its role in cancer. Biochim. Biophys. Acta 2011, 1807, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Muz, B.; de La Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef]
- Albertí-Valls, M.; Megino-Luque, C.; Macià, A.; Gatius, S.; Matias-Guiu, X.; Eritja, N. Metabolomic-Based Approaches for Endometrial Cancer Diagnosis and Prognosis: A Review. Cancers 2023, 16, 185. [Google Scholar] [CrossRef]
- Kozar, N.; Kruusmaa, K.; Dovnik, A.; Bitenc, M.; Argamasilla, R.; Adsuar, A.; Goswami, N.; Takač, I.; Arko, D. Identification of novel diagnostic biomarkers in endometrial cancer using targeted metabolomic profiling. Adv. Med. Sci. 2021, 66, 46–51. [Google Scholar] [CrossRef]
- Broad DepMap. DepMap 24Q4 Public; Broad Institute: Cambridge, MA, USA, 2024. [Google Scholar]
- Bairoch, A. The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- Seidel, A.; Brunner, S.; Seidel, P.; Fritz, G.I.; Herbarth, O. Modified nucleosides: An accurate tumour marker for clinical diagnosis of cancer, early detection and therapy control. Br. J. Cancer 2006, 94, 1726–1733. [Google Scholar] [CrossRef]
- Ayadi, L.; Galvanin, A.; Pichot, F.; Marchand, V.; Motorin, Y. RNA ribose methylation (2′-O-methylation): Occurrence, biosynthesis and biological functions. Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 253–269. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Q.; Chen, J.; Yu, J.; Hu, Y.; Zhang, S.; Zheng, S. 8-Hydroxyguanosine as a possible RNA oxidative modification marker in urine from colorectal cancer patients: Evaluation by ultra performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1136, 121931. [Google Scholar] [CrossRef]
- Daignan-Fornier, B.; Pinson, B. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5′-Monophosphate (AICAR), a Highly Conserved Purine Intermediate with Multiple Effects. Metabolites 2012, 2, 292–302. [Google Scholar] [CrossRef]
- Liu, J.; Eckert, M.A.; Harada, B.T.; Liu, S.-M.; Lu, Z.; Yu, K.; Tienda, S.M.; Chryplewicz, A.; Zhu, A.C.; Yang, Y.; et al. m6A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat. Cell Biol. 2018, 20, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yao, Y.; Wu, P.; Zi, X.; Sun, N.; He, J. The potential role of N7-methylguanosine (m7G) in cancer. J. Hematol. Oncol. 2022, 15, 63. [Google Scholar] [CrossRef]
- Wu, Q.; Fu, X.; Liu, G.; He, X.; Li, Y.; Ou, C. N7-methylguanosine modification in cancers: From mechanisms to therapeutic potential. J. Hematol. Oncol. 2025, 18, 12. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Marwood, S.; Bruce, M.; Constantin-Teodosiu, D.; Greenhaff, P.L. Tricarboxylic acid cycle intermediate pool size: Functional importance for oxidative metabolism in exercising human skeletal muscle. Sports Med. 2007, 37, 1071–1088. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Müller, M.; Graeve, L. Löffler/Petrides Biochemie und Pathobiochemie; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-17971-6. [Google Scholar]
- van de Poll, M.; Luiking, Y.C.; Dejong, C.; Soeters, P.B. Amino Acids: Specific Functions. In Encyclopedia of Human Nutrition; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 92–100. [Google Scholar]
- Ortiz, S.R.; Heinz, A.; Hiller, K.; Field, M.S. Erythritol synthesis is elevated in response to oxidative stress and regulated by the non-oxidative pentose phosphate pathway in A549 cells. Front. Nutr. 2022, 9, 953056. [Google Scholar] [CrossRef]
- Baenke, F.; Peck, B.; Miess, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef]
- Tomkins, G.M.; Dauben, W.G.; Sheppard, H.; Chaikoff, I.L. Squalene as a precursor of cholesterol in liver. J. Biol. Chem. 1953, 202, 487–489. [Google Scholar] [CrossRef]
- Ha, N.T.; Lee, C.H. Roles of Farnesyl-Diphosphate Farnesyltransferase 1 in Tumour and Tumour Microenvironments. Cells 2020, 9, 2352. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, H.; Bi, F.; Tang, Q.; Xu, H. Targeting the key cholesterol biosynthesis enzyme squalene monooxygenasefor cancer therapy. Front. Oncol. 2022, 12, 938502. [Google Scholar] [CrossRef]
- Lin, C.; Tian, Q.; Guo, S.; Xie, D.; Cai, Y.; Wang, Z.; Chu, H.; Qiu, S.; Tang, S.; Zhang, A. Metabolomics for Clinical Biomarker Discovery and Therapeutic Target Identification. Molecules 2024, 29, 2198. [Google Scholar] [CrossRef] [PubMed]
- Valvona, C.J.; Fillmore, H.L.; Nunn, P.B.; Pilkington, G.J. The Regulation and Function of Lactate Dehydrogenase A: Therapeutic Potential in Brain Tumor. Brain Pathol. 2016, 26, 3–17. [Google Scholar] [CrossRef] [PubMed]
- La Monica, S.; Vacondio, F.; Eltayeb, K.; Lodola, A.; Volta, F.; Viglioli, M.; Ferlenghi, F.; Galvani, F.; Galetti, M.; Bonelli, M.; et al. Targeting glucosylceramide synthase induces antiproliferative and proapoptotic effects in osimertinib-resistant NSCLC cell models. Sci. Rep. 2024, 14, 6491. [Google Scholar] [CrossRef] [PubMed]
- Horibata, Y.; Sugimoto, H. Differential contributions of choline phosphotransferases CPT1 and CEPT1 to the biosynthesis of choline phospholipids. J. Lipid Res. 2021, 62, 100100. [Google Scholar] [CrossRef]
- Saadh, M.J.; Ahmed Mustafa, M.; Yassen Qassem, L.; Ghadir, G.K.; Alaraj, M.; Shuhata Alubiady, M.H.; Zain Al-Abdeen, S.H.; Shakier, H.G.; Alshahrani, M.Y.; Zwamel, A.H. Targeting hypoxic and acidic tumor microenvironment by nanoparticles: A review. J. Drug Deliv. Sci. Technol. 2024, 96, 105660. [Google Scholar] [CrossRef]
- Chua, Y.L.; Dufour, E.; Dassa, E.P.; Rustin, P.; Jacobs, H.T.; Taylor, C.T.; Hagen, T. Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J. Biol. Chem. 2010, 285, 31277–31284. [Google Scholar] [CrossRef]
- Schänzer, A.; Döring, B.; Ondrouschek, M.; Goos, S.; Garvalov, B.K.; Geyer, J.; Acker, T.; Neubauer, B.; Hahn, A. Stress-induced upregulation of SLC19A3 is impaired in biotin-thiamine-responsive basal ganglia disease. Brain Pathol. 2014, 24, 270–279. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, W.; Chen, C.; Yan, B.; Zhu, L.; Chen, X.; Peng, C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020, 247, 117443. [Google Scholar] [CrossRef]
- Choi, K.H.; Edelstein, C.L.; Gengaro, P.; Schrier, R.W.; Nemenoff, R.A. Hypoxia induces changes in phospholipase A2 in rat proximal tubules: Evidence for multiple forms. Am. J. Physiol. 1995, 269, F846–F853. [Google Scholar] [CrossRef]
- Petan, T.; Manček-Keber, M. Half is enough: Oxidized lysophospholipids as novel bioactive molecules. Free Radic. Biol. Med. 2022, 188, 351–362. [Google Scholar] [CrossRef]
- Michel, C.; van Echten-Deckert, G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 1997, 416, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Devlin, C.M.; Lahm, T.; Hubbard, W.C.; van Demark, M.; Wang, K.C.; Wu, X.; Bielawska, A.; Obeid, L.M.; Ivan, M.; Petrache, I. Dihydroceramide-based response to hypoxia. J. Biol. Chem. 2011, 286, 38069–38078. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Tumanov, S.; Qiu, B.; Michalopoulou, E.; Spata, M.; Azzam, A.; Xie, H.; Simon, M.C.; Kamphorst, J.J. Triglycerides Promote Lipid Homeostasis during Hypoxic Stress by Balancing Fatty Acid Saturation. Cell Rep. 2018, 24, 2596–2605.e5. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Nambiar, D.K.; Ramteke, A.; Kumar, R.; Dhar, D.; Agarwal, C.; Bergman, B.; Graner, M.; Maroni, P.; Singh, R.P.; et al. Hypoxia induces triglycerides accumulation in prostate cancer cells and extracellular vesicles supporting growth and invasiveness following reoxygenation. Oncotarget 2015, 6, 22836–22856. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Lee, S.-H.; Golinska, M.; Griffiths, J.R. HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells. Cells 2021, 10, 2371. [Google Scholar] [CrossRef]
- Valli, A.; Morotti, M.; Zois, C.E.; Albers, P.K.; Soga, T.; Feldinger, K.; Fischer, R.; Frejno, M.; McIntyre, A.; Bridges, E.; et al. Adaptation to HIF1α Deletion in Hypoxic Cancer Cells by Upregulation of GLUT14 and Creatine Metabolism. Mol. Cancer Res. 2019, 17, 1531–1544. [Google Scholar] [CrossRef]
- Munksgaard Thorén, M.; Vaapil, M.; Staaf, J.; Planck, M.; Johansson, M.E.; Mohlin, S.; Påhlman, S. Myc-induced glutaminolysis bypasses HIF-driven glycolysis in hypoxic small cell lung carcinoma cells. Oncotarget 2017, 8, 48983–48995. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Mohl, D.A.; Lagies, S.; Lonzer, A.; Pfäffle, S.P.; Groß, P.; Benka, M.; Jäger, M.; Huber, M.C.; Günther, S.; Plattner, D.A.; et al. On the Quest for Biomarkers: A Comprehensive Analysis of Modified Nucleosides in Ovarian Cancer Cell Lines. Cells 2025, 14, 626. [Google Scholar] [CrossRef]
- Lagies, S.; Pichler, R.; Kaminski, M.M.; Schlimpert, M.; Walz, G.; Lienkamp, S.S.; Kammerer, B. Metabolic characterization of directly reprogrammed renal tubular epithelial cells (iRECs). Sci. Rep. 2018, 8, 3878. [Google Scholar] [CrossRef]
- Styczynski, M.P.; Moxley, J.F.; Tong, L.V.; Walther, J.L.; Jensen, K.L.; Stephanopoulos, G.N. Systematic identification of conserved metabolites in GC/MS data for metabolomics and biomarker discovery. Anal. Chem. 2007, 79, 966–973. [Google Scholar] [CrossRef]
- Lagies, S.; Pichler, R.; Vladimirov, G.; Gawron, J.; Bäzner, F.; Schreiner, A.; Kadena, D.; Plattner, D.A.; Lienkamp, S.S.; Kammerer, B. Metabolic and Lipidomic Assessment of Kidney Cells Exposed to Nephrotoxic Vancomycin Dosages. Int. J. Mol. Sci. 2021, 22, 10111. [Google Scholar] [CrossRef]
- Lagies, S.; Schlimpert, M.; Neumann, S.; Wäldin, A.; Kammerer, B.; Borner, C.; Peintner, L. Cells grown in three-dimensional spheroids mirror in vivo metabolic response of epithelial cells. Commun. Biol. 2020, 3, 246. [Google Scholar] [CrossRef]


| Cell Line | Disease (Celltype) | Donor Age (Years) | Donor Ethnicity | Genetic Alteration |
|---|---|---|---|---|
| AN3-CA | Endometrial carcinoma | 55 | Caucasian | LoF: TP53 MSI-high |
| EFE-184 | Endometrial carcinoma | 69 | Caucasian | LoF: TP53 MSI-stable |
| HEC-1B | Endometrial carcinoma | 71 | Asian | GoF: KRAS, PIK3CA, ERBB2 LoF: TP53 MSI-high |
| MFE-296 | Endometrial carcinoma | 68 | Caucasian | GoF: PIK3CA LoF: TP53 MSI-high |
| THESC | Healthy | uk | Uk | hTERT-immortalized human endometrial stromal cells (with fibroblast morphology) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCaslin, L.; Lagies, S.; Mohl, D.A.; Plattner, D.A.; Jäger, M.; Nöthling, C.; Huber, M.C.; Juhasz-Böss, I.; Kammerer, B.; Backhaus, C. Unveiling Metabolic Subtypes in Endometrial Cancer Cell Lines: Insights from Metabolomic Analysis Under Standard and Stress Conditions. Int. J. Mol. Sci. 2025, 26, 9573. https://doi.org/10.3390/ijms26199573
McCaslin L, Lagies S, Mohl DA, Plattner DA, Jäger M, Nöthling C, Huber MC, Juhasz-Böss I, Kammerer B, Backhaus C. Unveiling Metabolic Subtypes in Endometrial Cancer Cell Lines: Insights from Metabolomic Analysis Under Standard and Stress Conditions. International Journal of Molecular Sciences. 2025; 26(19):9573. https://doi.org/10.3390/ijms26199573
Chicago/Turabian StyleMcCaslin, Lana, Simon Lagies, Daniel A. Mohl, Dietmar A. Plattner, Markus Jäger, Claudia Nöthling, Matthias C. Huber, Ingolf Juhasz-Böss, Bernd Kammerer, and Clara Backhaus. 2025. "Unveiling Metabolic Subtypes in Endometrial Cancer Cell Lines: Insights from Metabolomic Analysis Under Standard and Stress Conditions" International Journal of Molecular Sciences 26, no. 19: 9573. https://doi.org/10.3390/ijms26199573
APA StyleMcCaslin, L., Lagies, S., Mohl, D. A., Plattner, D. A., Jäger, M., Nöthling, C., Huber, M. C., Juhasz-Böss, I., Kammerer, B., & Backhaus, C. (2025). Unveiling Metabolic Subtypes in Endometrial Cancer Cell Lines: Insights from Metabolomic Analysis Under Standard and Stress Conditions. International Journal of Molecular Sciences, 26(19), 9573. https://doi.org/10.3390/ijms26199573








