Class 1 Sugar Beet Phytoglobin Shows Strong Affinity to Glyceraldehyde-3-Phosphate Dehydrogenase and DNA In Vitro
Abstract
1. Introduction
2. Results
2.1. BvPgb 1.2 Complex Measurements Using Isothermal Spectral Shift Assays
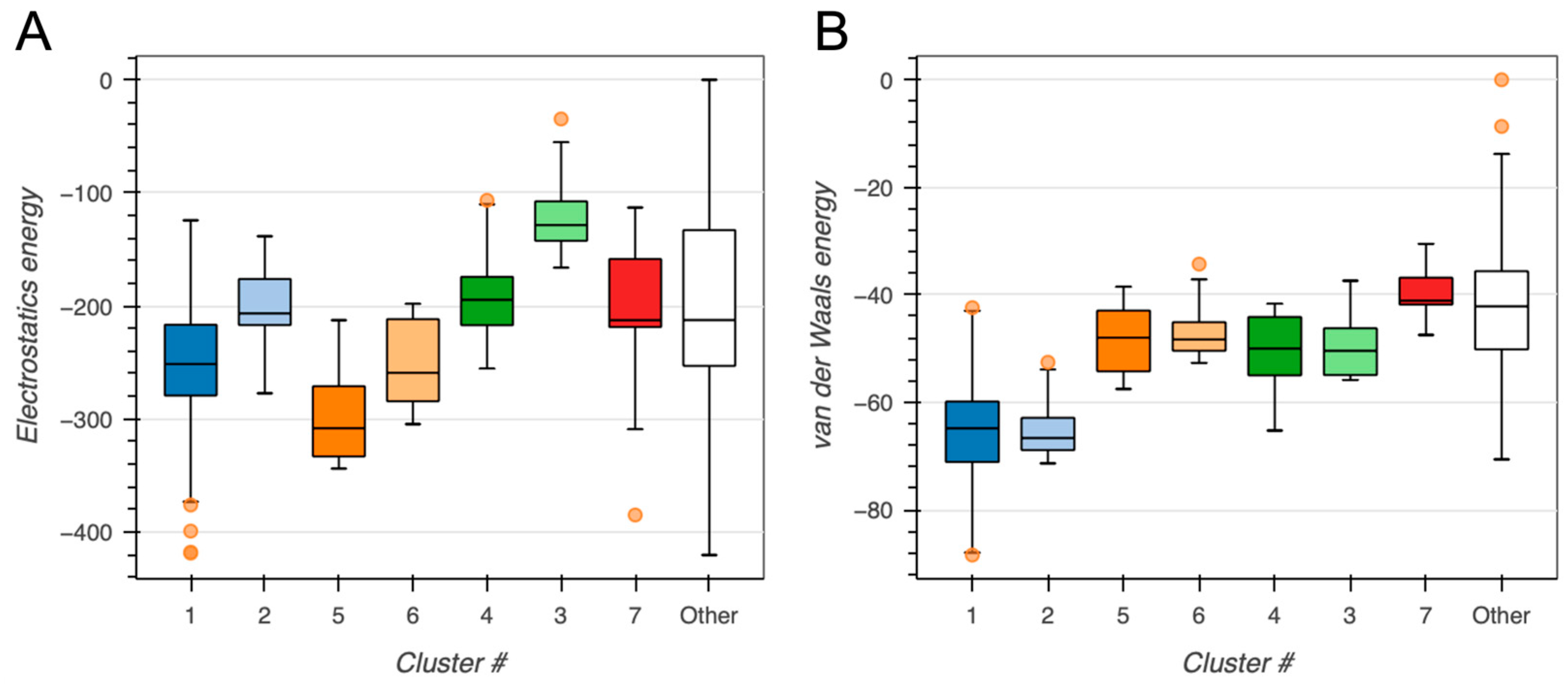
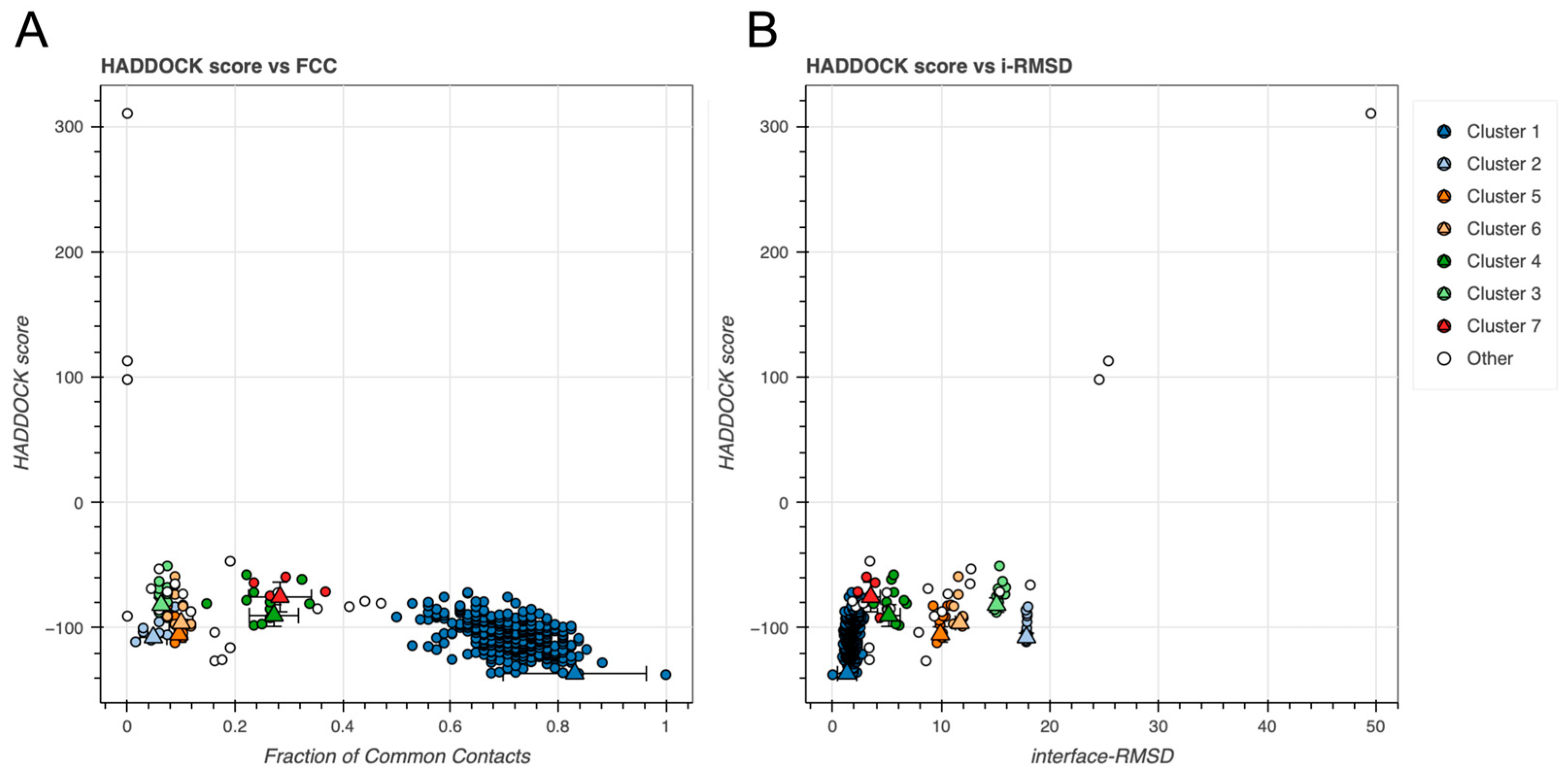
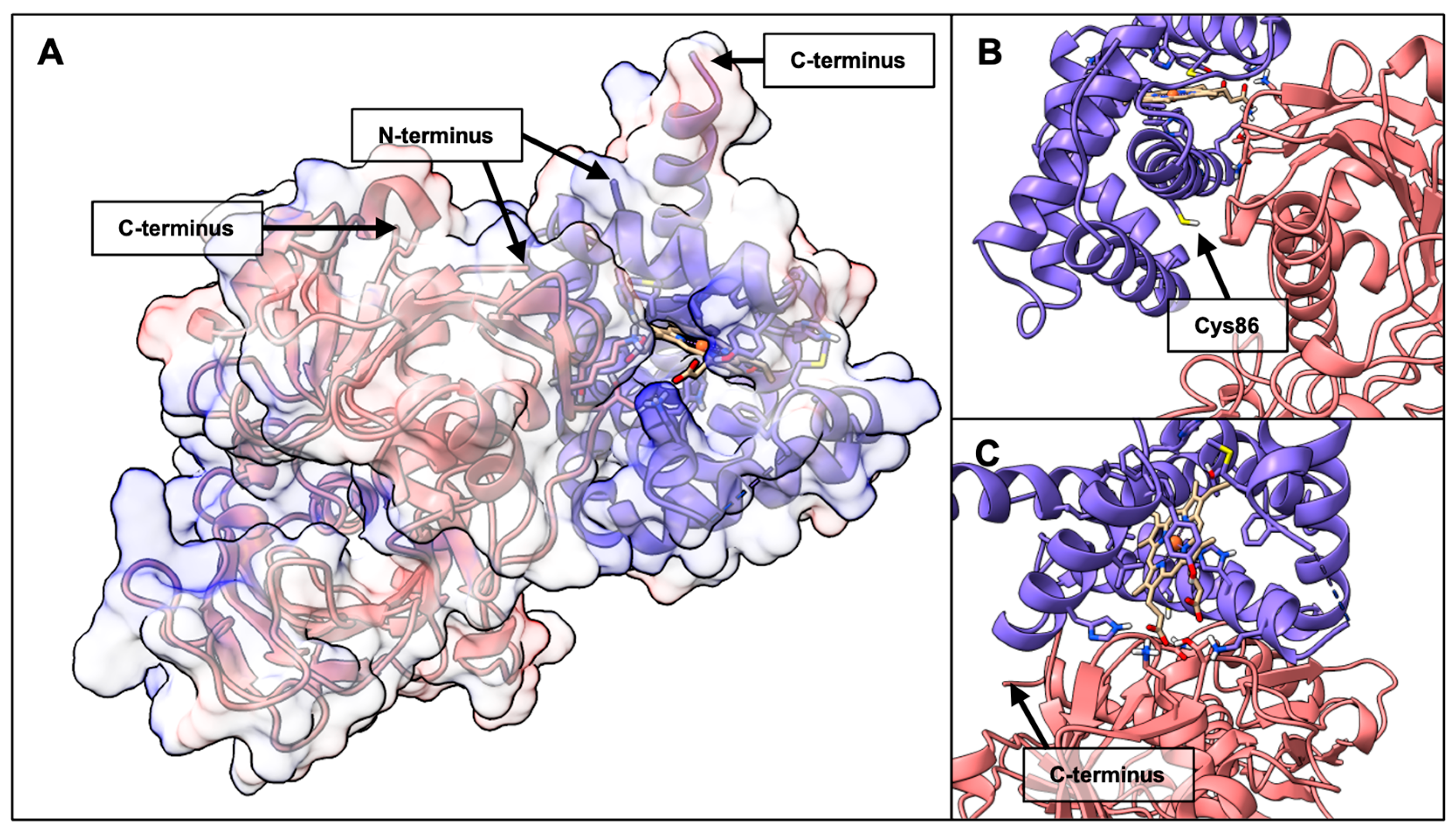
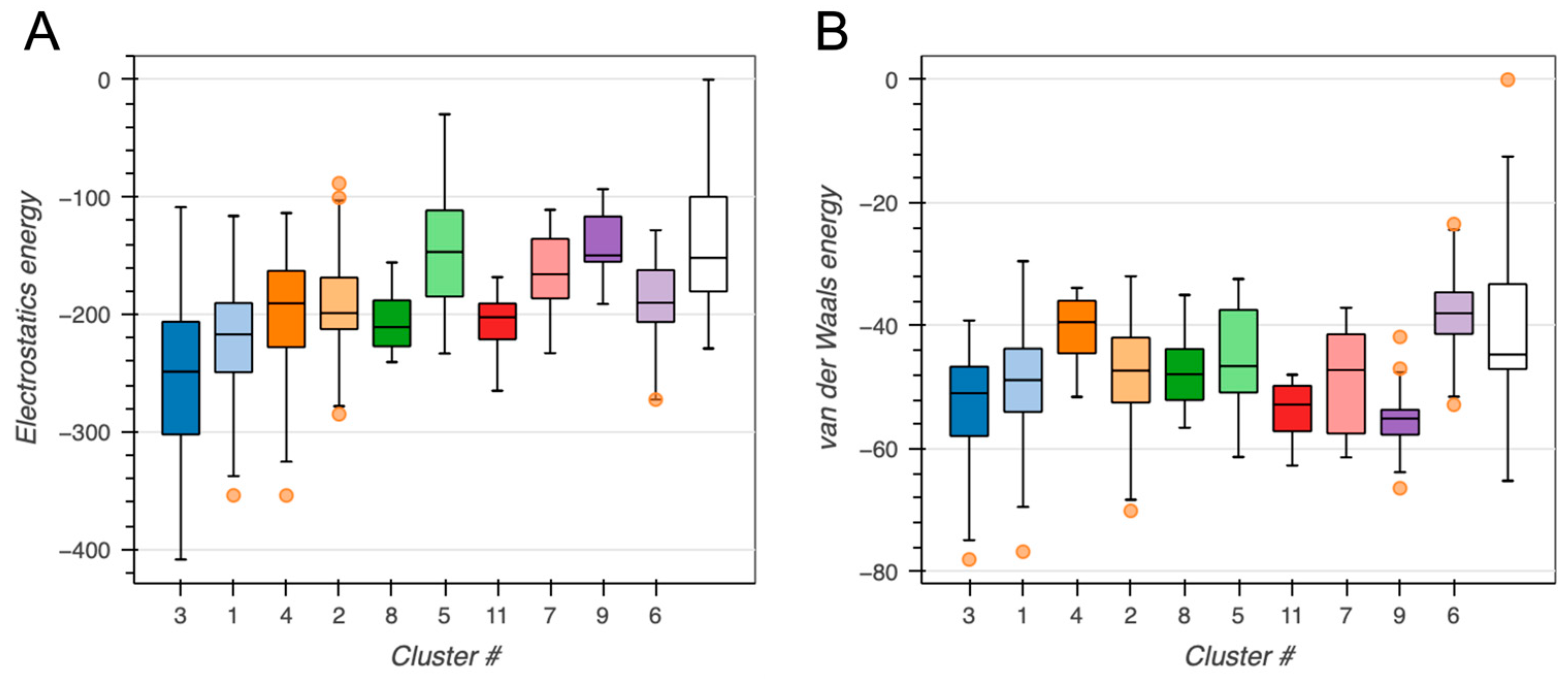
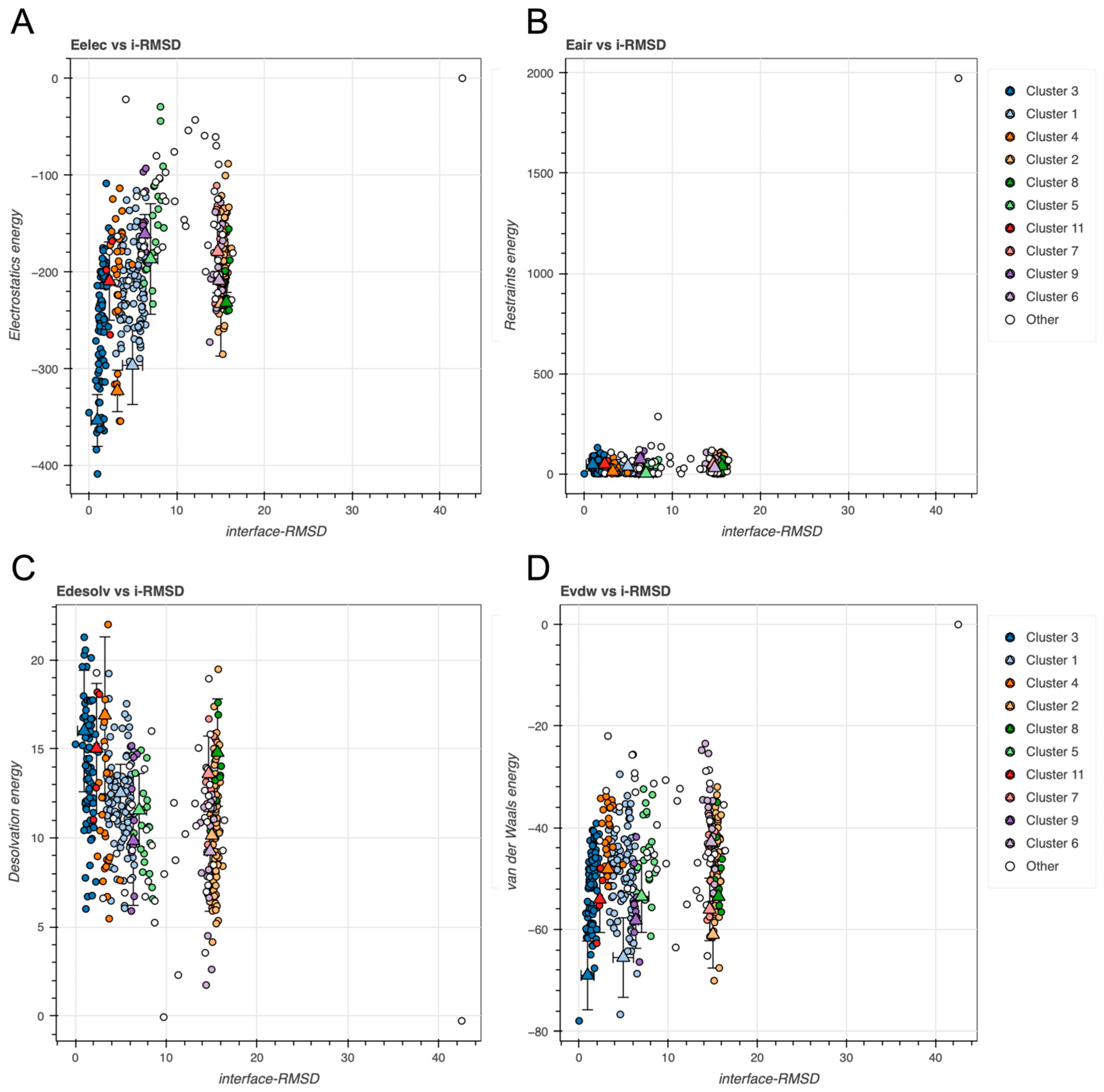
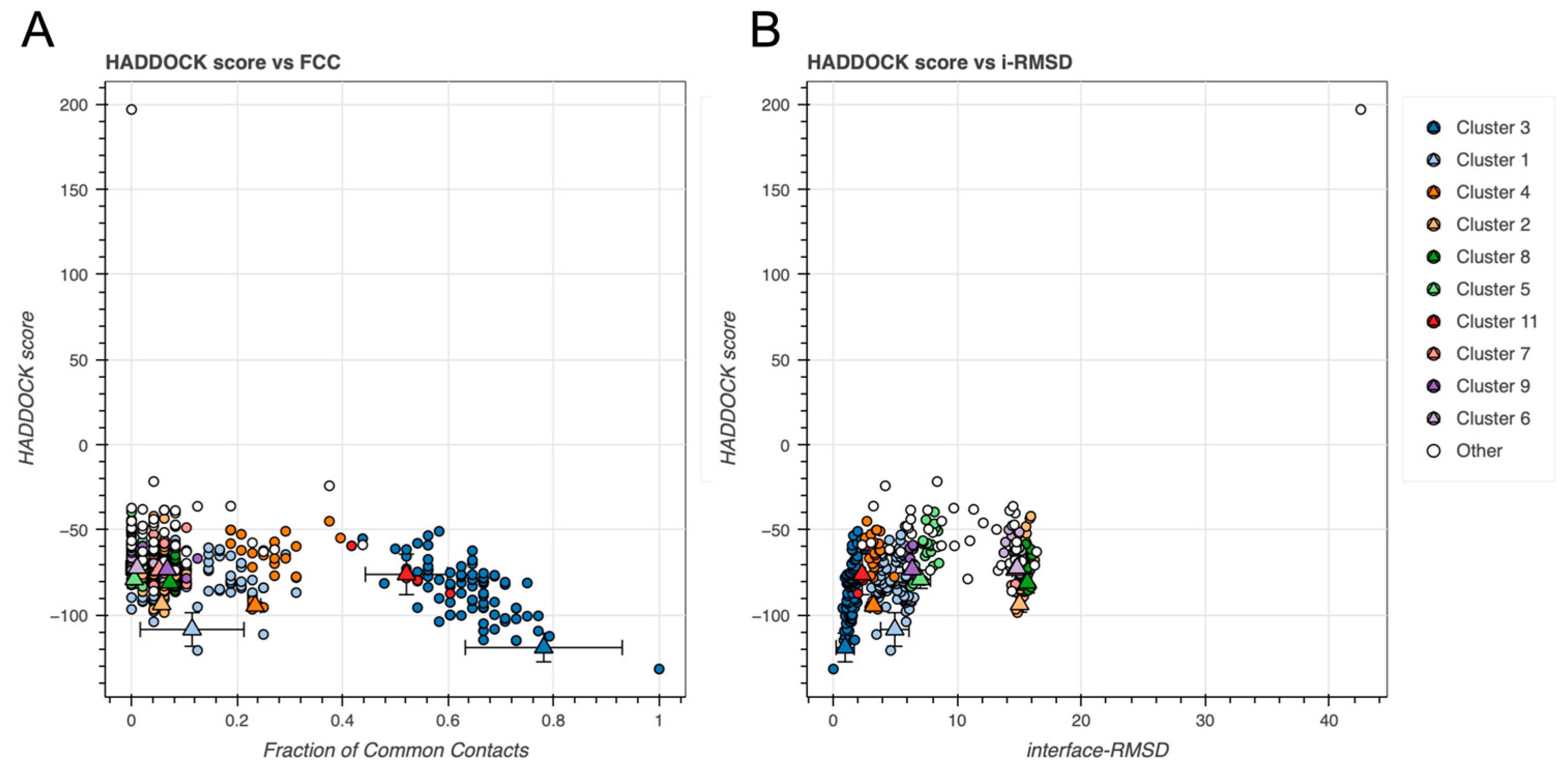
2.2. BvPgb 1.2–GAPDH Docking Analysis
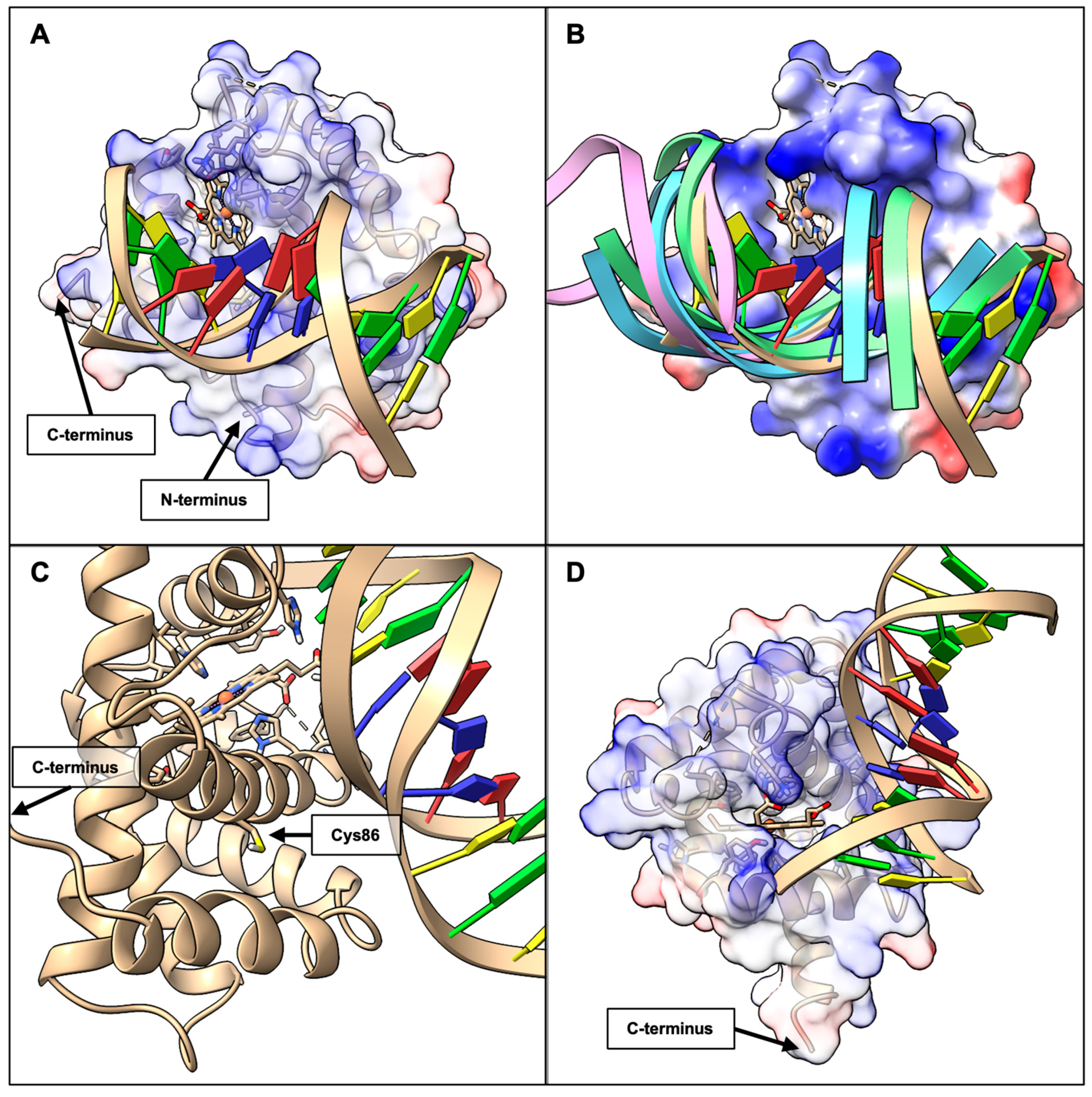
2.3. BvPgb 1.2–DNA Docking
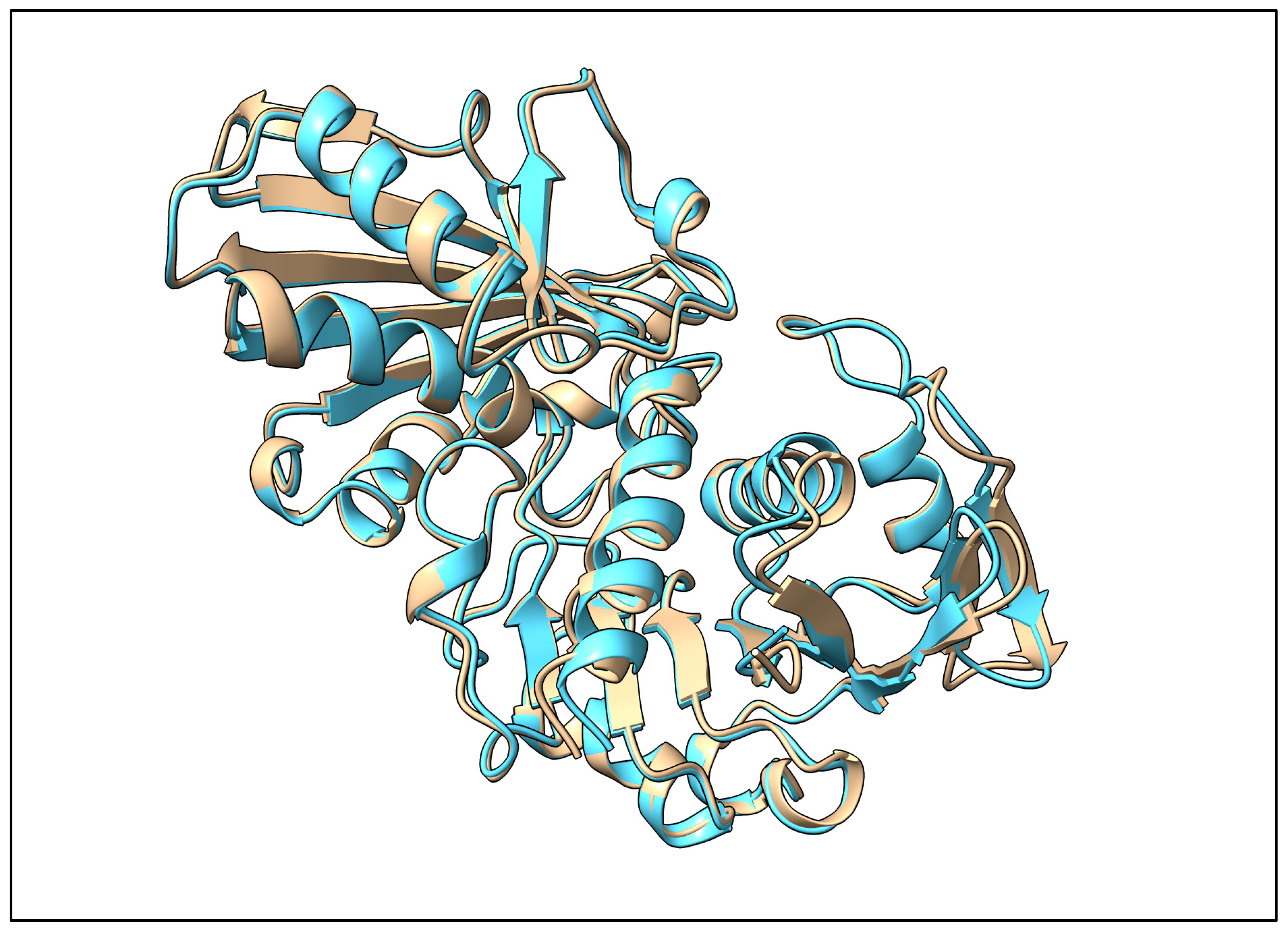
2.4. Structural Similarity of Predicted GAPC to Human GAPDH
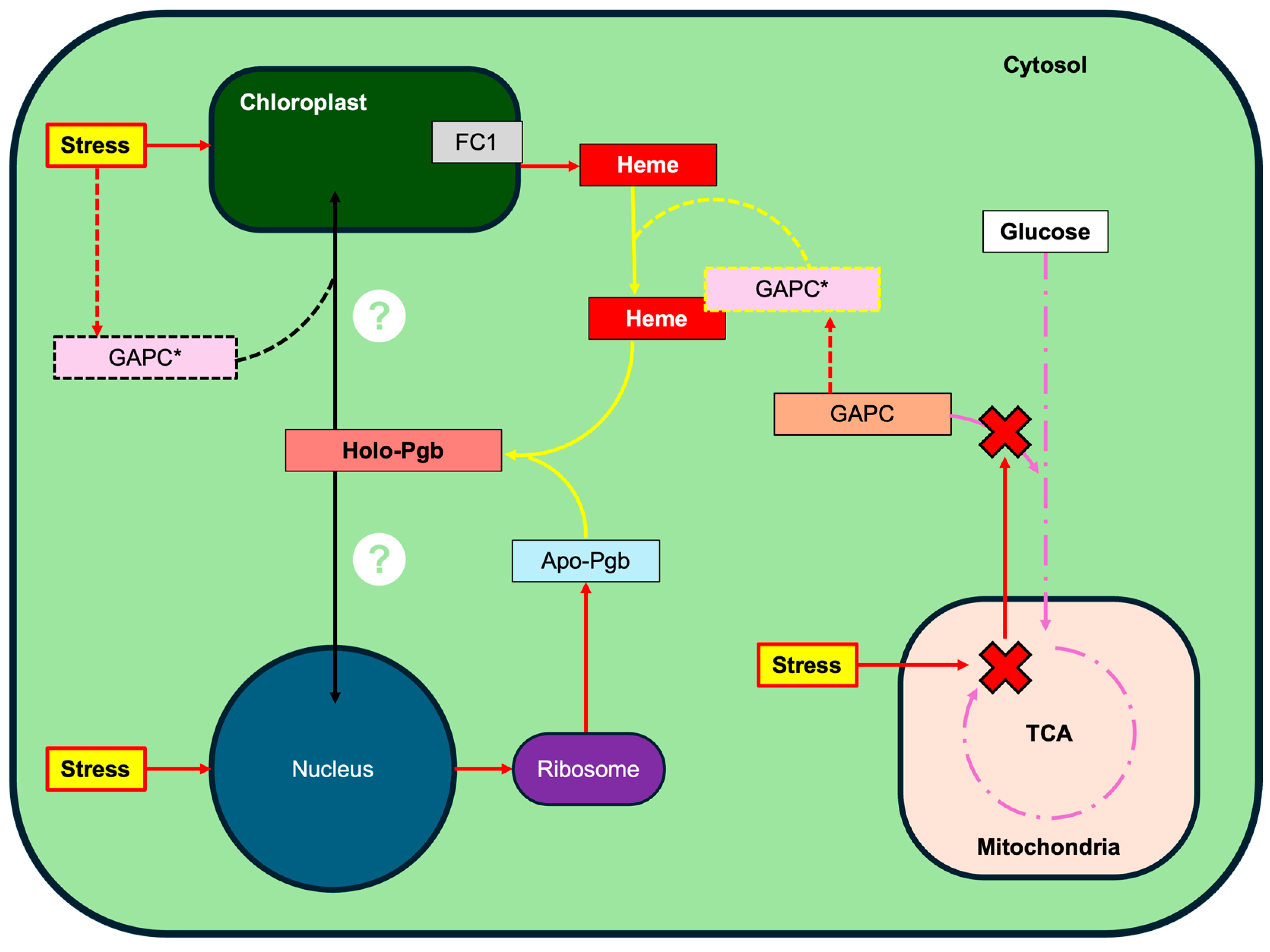
3. Discussion
4. Materials and Methods
4.1. Protein Production
4.2. Isothermal Spectral Shift Assay
4.3. Protein–Protein Docking
4.4. Protein–DNA Docking
4.5. Sequence and Structural Characterization of Putative B. vulgaris GAPC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | Buried surface area |
| BvPgb 1.2 | Class 1 phytoglobin from Beta vulgaris |
| C86A | Mutant of BvPgb 1.2 with cysteine-to-alanine substitution at position 86 |
| Eair | Restraints energy (kcal/mole) |
| Edesolv | Desolvation energy (kcal/mole) |
| Eelec | Electrostatics energy (kcal/mole) |
| Evdw | vdW energy (kcal/mole) |
| FC | Ferrochelatase |
| FCC | Fraction of common contacts |
| GAPC | Cytosolic GAPDH in plants |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| Hb | Hemoglobin |
| i-RMSD | Interface RMSD (Å) |
| ITC | Isothermal titration calorimetry |
| KD | Dissociation constant (M) |
| LjPgb 3 | Class 3 Phytoglobin from Lotus japonicus |
| Mb | Myoglobin |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| NHS | N-Hydroxysuccinimide |
| PBS-T20 | Phosphate-buffered saline supplemented with 0.05% Tween20 |
| Pgb | Phytoglobin |
| rHb1 | Class 1 phytoglobin from rice |
| RMSD | Root-mean-square deviation (Å) |
| ROS | Radical oxygen species |
| rWT | Recombinant wild type of BvPgb 1.2. |
| SpS | Spectral shift |
| vdW | van der Waal (kcal/mole) |
References
- Hebelstrup, K.H.; Shah, J.K.; Igamberdiev, A.U. The role of nitric oxide and hemoglobin in plant development and morphogenesis. Physiol. Plant. 2013, 148, 457–469. [Google Scholar] [CrossRef]
- Rubio, M.C.; Calvo-Begueria, L.; Díaz-Mendoza, M.; Elhiti, M.; Moore, M.; Matamoros, M.A.; James, E.K.; Díaz, I.; Pérez-Rontomé, C.; Villar, I.; et al. Phytoglobins in the nuclei, cytoplasm and chloroplasts modulate nitric oxide signaling and interact with abscisic acid. Plant J. 2019, 100, 38–54. [Google Scholar] [CrossRef]
- Andrzejczak, O.A.; Havelund, J.F.; Wang, W.-Q.; Kovalchuk, S.; Hagensen, C.E.; Hasler-Sheetal, H.; Jensen, O.N.; Rogowska-Wrzesinska, A.; Møller, I.M.; Hebelstrup, K.H. The hypoxic proteome and metabolome of barley (Hordeum vulgare L.) with and without phytoglobin priming. Int. J. Mol. Sci. 2020, 21, 1546. [Google Scholar] [CrossRef]
- Sirover, M.A. New insights into an old protein: The functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1999, 1432, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, E.A.; Singh, A.B.; Chakravarti, R.; Martinez-Guzman, O.; Saini, A.; Haque, M.M.; Garee, G.; Dans, P.D.; Hannibal, L.; Reddi, A.R.; et al. Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells. J. Biol. Chem. 2018, 293, 14557–14568. [Google Scholar] [CrossRef] [PubMed]
- Tupta, B.; Stuehr, E.; Sumi, M.P.; Sweeny, E.A.; Smith, B.; Stuehr, D.J.; Ghosh, A. GAPDH is involved in the heme-maturation of myoglobin and hemoglobin. FASEB J. 2022, 36, e22099. [Google Scholar] [CrossRef]
- Zheng, L.; Roeder, R.G.; Luo, Y. S phase activation of the histone H2B promoter by OCA-S, a co-activator complex that contains GAPDH as a key component. Cell 2003, 114, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Grolla, A.A.; Miggiano, R.; Di Marino, D.; Bianchi, M.; Gori, A.; Orsomando, G.; Gaudino, F.; Galli, U.; Del Grosso, E.; Mazzola, F.; et al. A nicotinamide phosphoribosyltransferase–GAPDH interaction sustains the stress-induced NMN/NAD+ salvage pathway in the nucleus. J. Biol. Chem. 2020, 295, 3635–3651. [Google Scholar] [CrossRef]
- Garten, A.; Petzold, S.; Körner, A.; Imai, S.; Kiess, W. Nampt: Linking NAD biology, metabolism and cancer. Trends Endocrinol. Metab. 2009, 20, 130–138. [Google Scholar] [CrossRef]
- Gupta, K.J.; Igamberdiev, A.U. The anoxic plant mitochondrion as a nitrite:NO reductase. Mitochondrion 2011, 11, 537–543. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Fermani, S.; Costa, A.; Lemaire, S.D.; Trost, P. Plant cytoplasmic GAPDH: Redox post-translational modifications and moonlighting properties. Front. Plant Sci. 2013, 4, 450. [Google Scholar] [CrossRef]
- Hunt, P.W.; Klok, E.J.; Trevaskis, B.; Watts, R.A.; Ellis, M.H.; Peacock, W.J.; Dennis, E.S. Increased level of hemoglobin 1 enhances survival of hypoxic stress and promotes early growth in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2002, 99, 17197–17202. [Google Scholar] [CrossRef]
- Leiva Eriksson, N.; Pin, P.; Kraft, T.; Dohm, J.; Minoche, A.; Himmelbauer, H.; Bülow, L. Differential expression patterns of non-symbiotic hemoglobins in sugar beet (Beta vulgaris ssp. vulgaris). Plant Cell Physiol. 2014, 55, 1056–1068. [Google Scholar] [CrossRef]
- Leiva Eriksson, N.; Reeder, B.J.; Wilson, M.T.; Bülow, L. Sugar beet hemoglobins: Reactions with nitric oxide and nitrite reveal differential roles for nitrogen metabolism. Biochem. J. 2019, 476, 2111–2125. [Google Scholar] [CrossRef]
- Michelet, L.; Zaffagnini, M.; Morisse, S.; Sparla, F.; Pérez-Pérez, M.E.; Francia, F.; Danon, A.; Marchand, C.; Fermani, S.; Trost, P.; et al. Redox regulation of the Calvin–Benson cycle: Something old, something new. Front. Plant Sci. 2013, 4, 470. [Google Scholar] [CrossRef]
- Martin, W.F.; Cerff, R. Physiology, phylogeny, early evolution, and GAPDH. Protoplasma 2017, 254, 1823–1834. [Google Scholar] [CrossRef]
- Hardison, R.C. Evolution of hemoglobin and its genes. Cold Spring Harb. Perspect. Med. 2012, 2, a011627. [Google Scholar] [CrossRef]
- Wajcman, H.; Kiger, L.; Marden, M.C. Structure and function evolution in the superfamily of globins. C. R. Biol. 2009, 332, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.B.; Cheng, W.; Webber, A.; Bhambhani, A.; Duff, M.R.; Kumar, C.V.; McLendon, G.L. Endonuclease-like activity of heme proteins. J. Biol. Inorg. Chem. 2005, 10, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Chakane, S.; Matos, T.; Kettisen, K.; Bülow, L. Fetal hemoglobin is much less prone to DNA cleavage compared to the adult protein. Redox Biol. 2017, 12, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, S.; Laurents, D.V.; Levitt, M. Structural similarity of DNA-binding domains of bacteriophage repressors and the globin core. Curr. Biol. 1993, 3, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Stenström, O.; Akke, M.; Bülow, L. Conformational dynamics of phytoglobin BvPgb1.2 from Beta vulgaris ssp. vulgaris. Int. J. Mol. Sci. 2023, 24, 3973. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.; Groth, L.; Leiva Eriksson, N.; Nyblom, M.; Bülow, L. Oxidative implications of substituting a conserved cysteine residue in sugar beet phytoglobin BvPgb1.2. Antioxidants 2022, 11, 1615. [Google Scholar] [CrossRef] [PubMed]
- Groth, L.; Bülow, L. Gas-binding studies of class 1 sugar beet phytoglobin and C86A mutant using isothermal spectral shifts in high-precision microliter assay. Int. J. Mol. Sci. 2025, 26, 8240. [Google Scholar] [CrossRef]
- Orlikowska, M.; Wyciszkiewicz, A.; Węgrzyn, K.; Mehringer, J.; de Souza Paiva, D.; Jurczak, P. Methods for monitoring protein-membrane binding: Comparison based on the interactions between amyloidogenic protein human cystatin C and phospholipid liposomes. Int. J. Biol. Macromol. 2024, 278, 134889. [Google Scholar] [CrossRef]
- Langer, A.; Lüdecke, A.; Bartoschik, T.; Cehlar, O.; Duhr, S.; Baaske, P.; Streicher, W. A new spectral shift-based method to characterize molecular interactions. Assay Drug Dev. Technol. 2022, 20, 83–94. [Google Scholar] [CrossRef]
- Trent, J.T.; Hvitved, A.N.; Hargrove, M.S. A model for ligand binding to hexacoordinate hemoglobins. Biochemistry 2001, 40, 6155–6163. [Google Scholar] [CrossRef]
- Smagghe, B.J.; Sarath, G.; Ross, E.; Hilbert, J.; Hargrove, M.S. Slow ligand binding kinetics dominate ferrous hexacoordinate hemoglobin reactivities and reveal differences between plants and other species. Biochemistry 2006, 45, 561–570. [Google Scholar] [CrossRef]
- Hargrove, M.S. A flash photolysis method to characterize hexacoordinate hemoglobin kinetics. Biophys. J. 2000, 79, 2733–2738. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, K.-J.; Gao, S.-Q.; He, B.; Wen, G.-B.; Tan, X.; Lin, Y.-W. Distinct mechanisms for DNA cleavage by myoglobin with a designed heme active center. J. Inorg. Biochem. 2016, 156, 113–121. [Google Scholar] [CrossRef]
- Dominguez, C.; Boelens, R.; Bonvin, A.M.J.J. HADDOCK: A protein–protein docking approach based on biochemical or biophysical information. J. Am. Chem. Soc. 2003, 125, 1731–1737. [Google Scholar] [CrossRef]
- Vangone, A.; Rodrigues, J.P.G.L.M.; Xue, L.C.; van Zundert, G.C.P.; Geng, C.; Kurkcuoglu, Z.; Nellen, M.; Narasimhan, S.; Karaca, E.; van Dijk, M.; et al. Sense and simplicity in HADDOCK scoring: Lessons from CASP-CAPRI round 1. Proteins 2017, 85, 417–423. [Google Scholar] [CrossRef]
- van Zundert, G.C.P.; Bonvin, A.M.J.J. Modeling protein–protein complexes using the HADDOCK webserver. In Protein Structure Prediction; Kihara, D., Ed.; Springer: New York, NY, USA, 2014; pp. 163–179. ISBN 978-1-4939-0366-5. [Google Scholar]
- van Dijk, M.; van Dijk, A.D.J.; Hsu, V.; Boelens, R.; Bonvin, A.M.J.J. Information-driven protein–DNA docking using HADDOCK: It is a matter of flexibility. Nucleic Acids Res. 2006, 34, 3317–3325. [Google Scholar] [CrossRef]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Carbajo Martinez, M.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An expanding genome resource for non-vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef]
- Pecherina, A.; Grinberg, M.; Ageyeva, M.; Zdobnova, T.; Ladeynova, M.; Yudintsev, A.; Vodeneev, V.; Brilkina, A. Whole-plant measure of temperature-induced changes in the cytosolic pH of potato plants using genetically encoded fluorescent sensor Pt-GFP. Agriculture 2021, 11, 1131. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Ye, W.; Kinoshita, T. Editorial: pH as a signal and secondary messenger in plant cells. Front. Plant Sci. 2023, 14, 1148689. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, P.L.; Moal, I.H.; Hwang, H.; Weng, Z.; Bates, P.A.; Bonvin, A.M.J.J.; Janin, J. A structure-based benchmark for protein–protein binding affinity. Protein Sci. 2011, 20, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Kumar, S.; Sheokand, N.; Raje, C.I.; Raje, M. The multifunctional glycolytic protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a novel macrophage lactoferrin receptor. Biochem. Cell Biol. 2012, 90, 329–338. [Google Scholar] [CrossRef]
- Lo Conte, L.; Chothia, C.; Janin, J. The atomic structure of protein–protein recognition sites. J. Mol. Biol. 1999, 285, 2177–2198. [Google Scholar] [CrossRef]
- Chothia, C.; Janin, J. Principles of protein–protein recognition. Nature 1975, 256, 705–708. [Google Scholar] [CrossRef]
- Seidel, S.A.I.; Dijkman, P.M.; Lea, W.A.; van den Bogaart, G.; Jerabek-Willemsen, M.; Lazic, A.; Joseph, J.S.; Srinivasan, P.; Baaske, P.; Simeonov, A.; et al. Microscale thermophoresis quantifies biomolecular interactions under previously challenging conditions. Methods 2013, 59, 301–315. [Google Scholar] [CrossRef]
- Timney, B.L.; Raveh, B.; Mironska, R.; Trivedi, J.M.; Kim, S.J.; Russel, D.; Wente, S.R.; Sali, A.; Rout, M.P. Simple rules for passive diffusion through the nuclear pore complex. J. Cell Biol. 2016, 215, 57–76. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, Z.; Chen, J.; Du, G. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci. Rep. 2015, 5, 8584. [Google Scholar] [CrossRef]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic heme biosynthesis: Multiple pathways to a common essential product. Microbiol. Mol. Biol. Rev. 2017, 81, e00048-16. [Google Scholar] [CrossRef]
- Espinas, N.A.; Kobayashi, K.; Sato, Y.; Mochizuki, N.; Takahashi, K.; Tanaka, R.; Masuda, T. Allocation of heme is differentially regulated by ferrochelatase isoforms in Arabidopsis cells. Front. Plant Sci. 2016, 7, 1326. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.S.; Singh, D.P.; Walker, A.R.; Smith, A.G. Two different genes encode ferrochelatase in Arabidopsis: Mapping, expression and subcellular targeting of the precursor proteins. Plant J. 1998, 15, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Masuda, T.; Singh, D.P.; Tan, F.-C.; Tsuchiya, T.; Shimada, H.; Ohta, H.; Smith, A.G.; Takamiya, K. Two types of ferrochelatase in photosynthetic and nonphotosynthetic tissues of cucumber: Their difference in phylogeny, gene expression, and localization. J. Biol. Chem. 2002, 277, 4731–4737. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Cornah, J.E.; Hadingham, S.; Smith, A.G. Expression analysis of the two ferrochelatase genes in Arabidopsis in different tissues and under stress conditions reveals their different roles in haem biosynthesis. Plant Mol. Biol. 2002, 50, 773–788. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Koide, M.; Takahashi, S.; Kikuta, A.; Aono, M.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.; Masuda, T. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol. 2007, 144, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.S.; Nägele, T.; Grimm, B.; Kaufmann, K.; Schroda, M.; Leister, D.; Kleine, T. Retrograde signaling in plants: A critical review focusing on the GUN pathway and beyond. Plant Commun. 2023, 4, 100511. [Google Scholar] [CrossRef]
- Jokipii-Lukkari, S.; Kastaniotis, A.J.; Parkash, V.; Sundström, R.; Leiva-Eriksson, N.; Nymalm, Y.; Blokhina, O.; Kukkola, E.; Fagerstedt, K.V.; Salminen, T.A.; et al. Dual targeted poplar ferredoxin NADP+ oxidoreductase interacts with hemoglobin 1. Plant Sci. 2016, 247, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Bevington, P.R.; Robinson, D.K. Data Reduction and Error Analysis for the Physical Sciences; McGraw-Hill: New York, NY, USA, 2003; pp. 36–51. ISBN 978-0-07-247227-1. [Google Scholar]
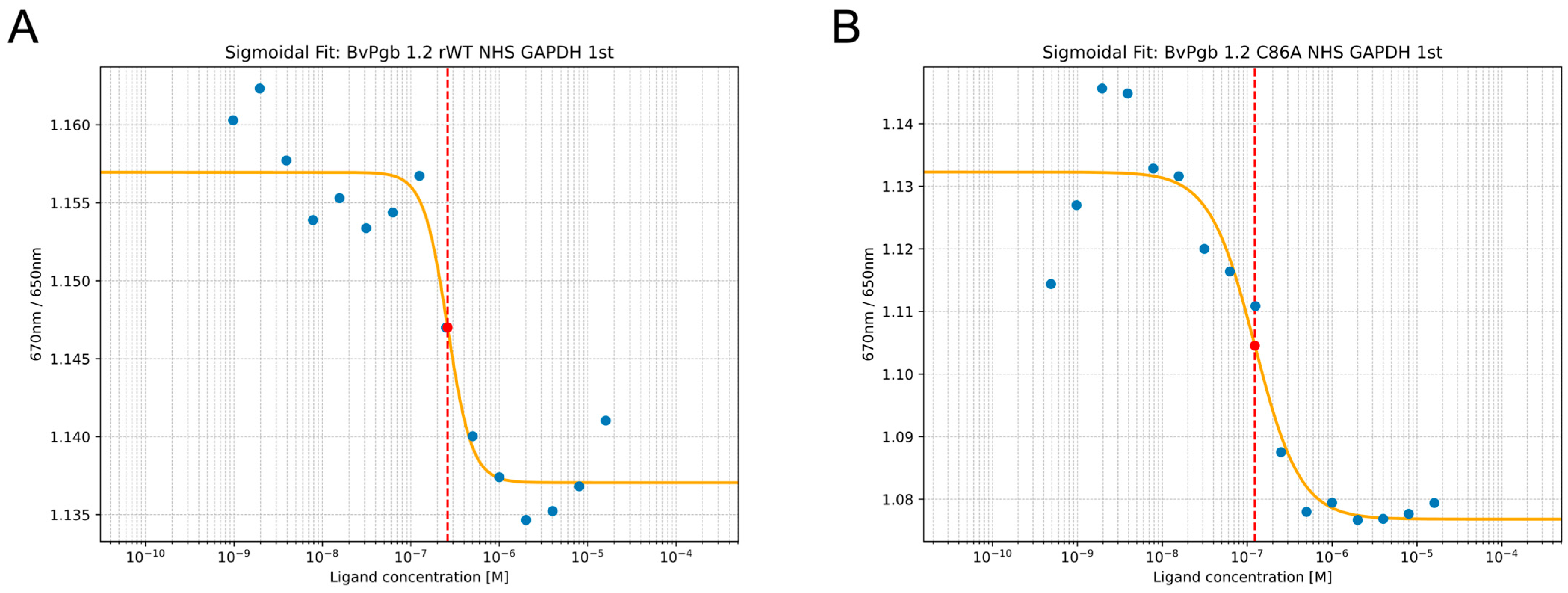
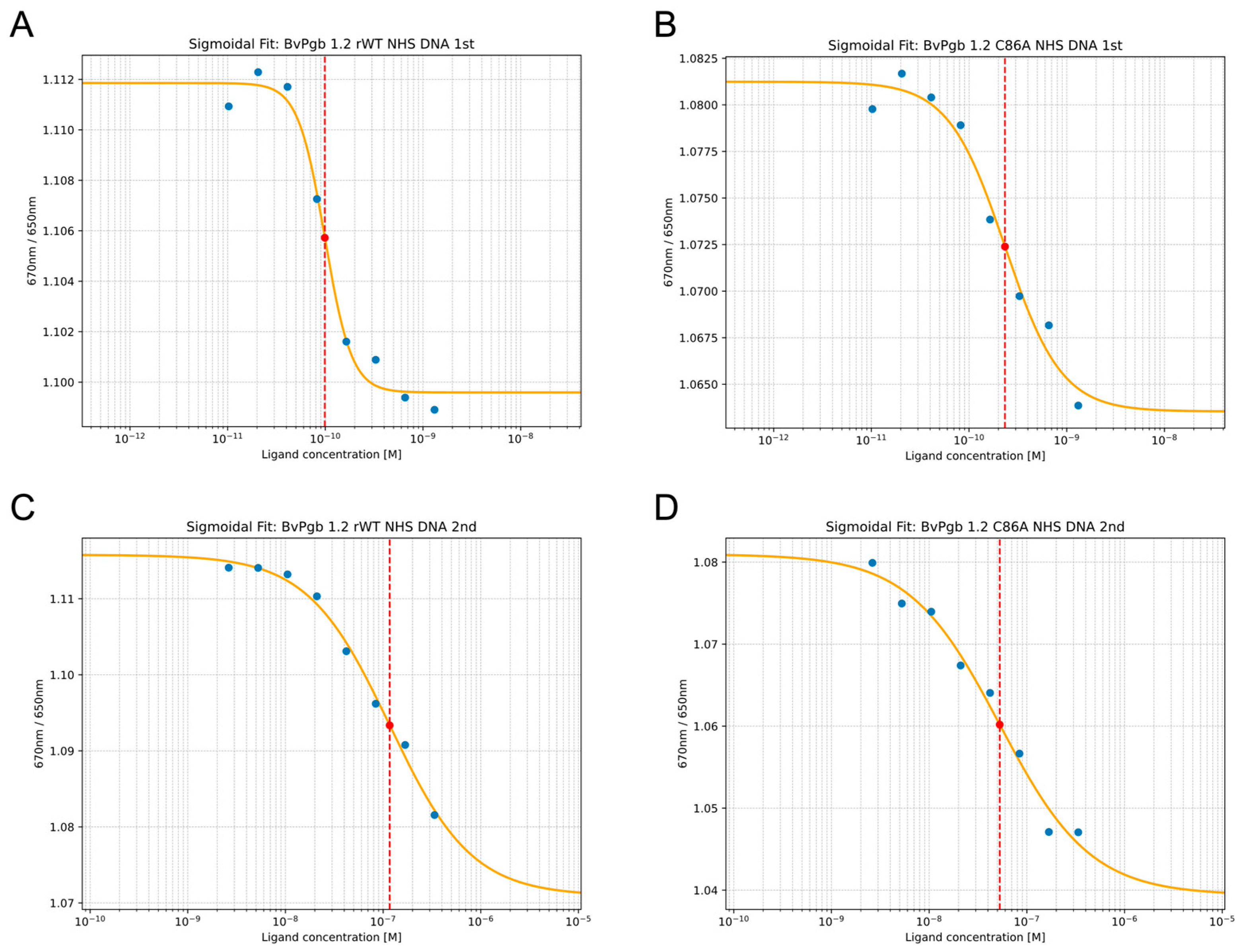

| KD(GAPDH) | KD(DNA,open) 1 | KD(DNA,closed) 1 | |
|---|---|---|---|
| Protein | [nM] | [nM] | [pM] |
| rWT | 260 ± 50 | 120 ± 50 | 100 ± 10 |
| C86A | 120 ± 40 | 50 ± 20 | 230 ± 60 |
| Cluster 1 | Cluster 2 | Cluster 5 | |
|---|---|---|---|
| HADDOCK score | −136.7 ± 0.6 | −107.8 ± 3.0 | −105.5 ± 5.3 |
| Cluster size | 324 | 11 | 10 |
| RMSD † (Å) | 1.4 ± 0.8 | 15.4 ± 0.0 | 9.7 ± 0.1 |
| van der Waals energy (kcal/mole) | −76.6 ± 4.2 | −67.5 ± 3.9 | −55.1 ± 1.5 |
| Electrostatic energy (kcal/mole) | −349.8 ± 39.9 | −236.3 ± 26.3 | −312.6 ± 40.6 |
| Desolvation energy (kcal/mole) | 9.2 ± 4.9 | 5.6 ± 1.7 | 9.6 ± 2.8 |
| Restraints violation energy (kcal/mole) | 7.0 ± 3.0 | 12.6 ± 3.2 | 24.8 ± 12.4 |
| Buried surface area (Å2) | 2463.4 ± 146.5 | 2357.1 ± 93.0 | 1984.4 ± 104.3 |
| Z-score | −2.0 | −0.5 | −0.3 |
| Cluster 3 | Cluster 1 | Cluster 4 | Cluster 2 | |
|---|---|---|---|---|
| HADDOCK score | −119.1 ± 7.3 | −108.5 ± 8.6 | −94.6 ± 1.8 | −93.7 ± 4.0 |
| Cluster size | 80 | 97 | 24 | 83 |
| RMSD † (Å) | 0.9 ± 0.6 | 4.2 ± 0.9 | 2.7 ± 0.2 | 13.6 ± 0.1 |
| van der Waals energy (kcal/mole) | −69.0 ± 5.9 | −65.5 ± 6.8 | −48.1 ± 2.3 | −61.0 ± 5.7 |
| Electrostatic energy (kcal/mole) | −353.5 ± 23.2 | −296.6 ± 35.0 | −323.0 ± 18.6 | −232.9 ± 46.9 |
| Desolvation energy (kcal/mole) | 16.0 ± 3.0 | 12.5 ± 1.4 | 16.9 ± 3.8 | 10.1 ± 3.1 |
| Restraints violation energy (kcal/mole) | 46.9 ± 26.5 | 38.4 ± 10.9 | 12.8 ± 15.7 | 37.9 ± 9.3 |
| Buried surface area (Å2) | 1701.4 ± 37.9 | 1632.2 ± 20.1 | 1497.4 ± 56.6 | 1424.3 ± 73.5 |
| Z-score | −2.1 | −1.4 | −0.5 | −0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groth, L.; Oda, M.; Bülow, L. Class 1 Sugar Beet Phytoglobin Shows Strong Affinity to Glyceraldehyde-3-Phosphate Dehydrogenase and DNA In Vitro. Int. J. Mol. Sci. 2025, 26, 9404. https://doi.org/10.3390/ijms26199404
Groth L, Oda M, Bülow L. Class 1 Sugar Beet Phytoglobin Shows Strong Affinity to Glyceraldehyde-3-Phosphate Dehydrogenase and DNA In Vitro. International Journal of Molecular Sciences. 2025; 26(19):9404. https://doi.org/10.3390/ijms26199404
Chicago/Turabian StyleGroth, Leonard, Miho Oda, and Leif Bülow. 2025. "Class 1 Sugar Beet Phytoglobin Shows Strong Affinity to Glyceraldehyde-3-Phosphate Dehydrogenase and DNA In Vitro" International Journal of Molecular Sciences 26, no. 19: 9404. https://doi.org/10.3390/ijms26199404
APA StyleGroth, L., Oda, M., & Bülow, L. (2025). Class 1 Sugar Beet Phytoglobin Shows Strong Affinity to Glyceraldehyde-3-Phosphate Dehydrogenase and DNA In Vitro. International Journal of Molecular Sciences, 26(19), 9404. https://doi.org/10.3390/ijms26199404






