Association of Elevated Galectin-4 Concentrations with Obesity, Diabetes, and Cardiovascular Diseases
Abstract
1. Introduction
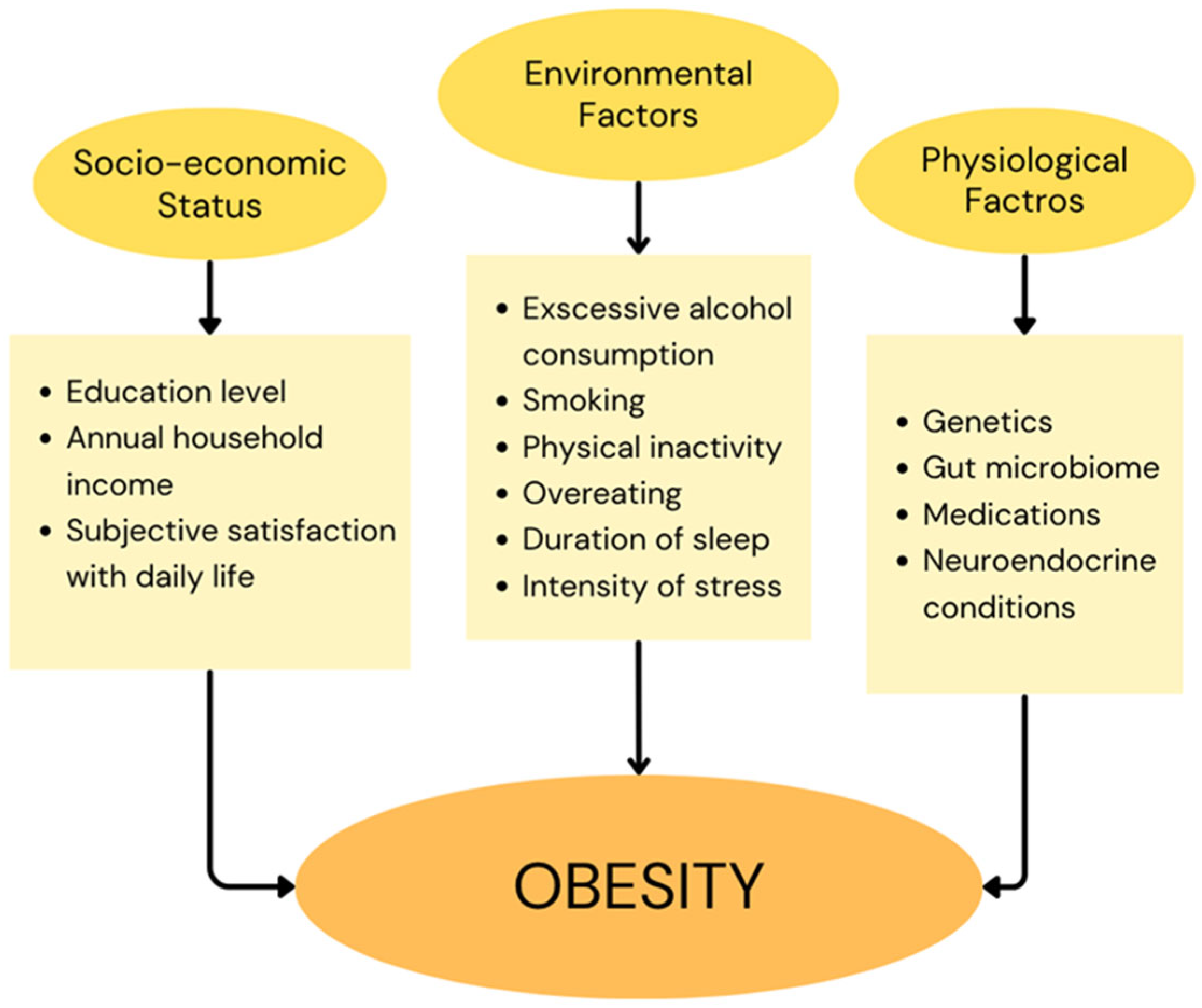
1.1. Obesity
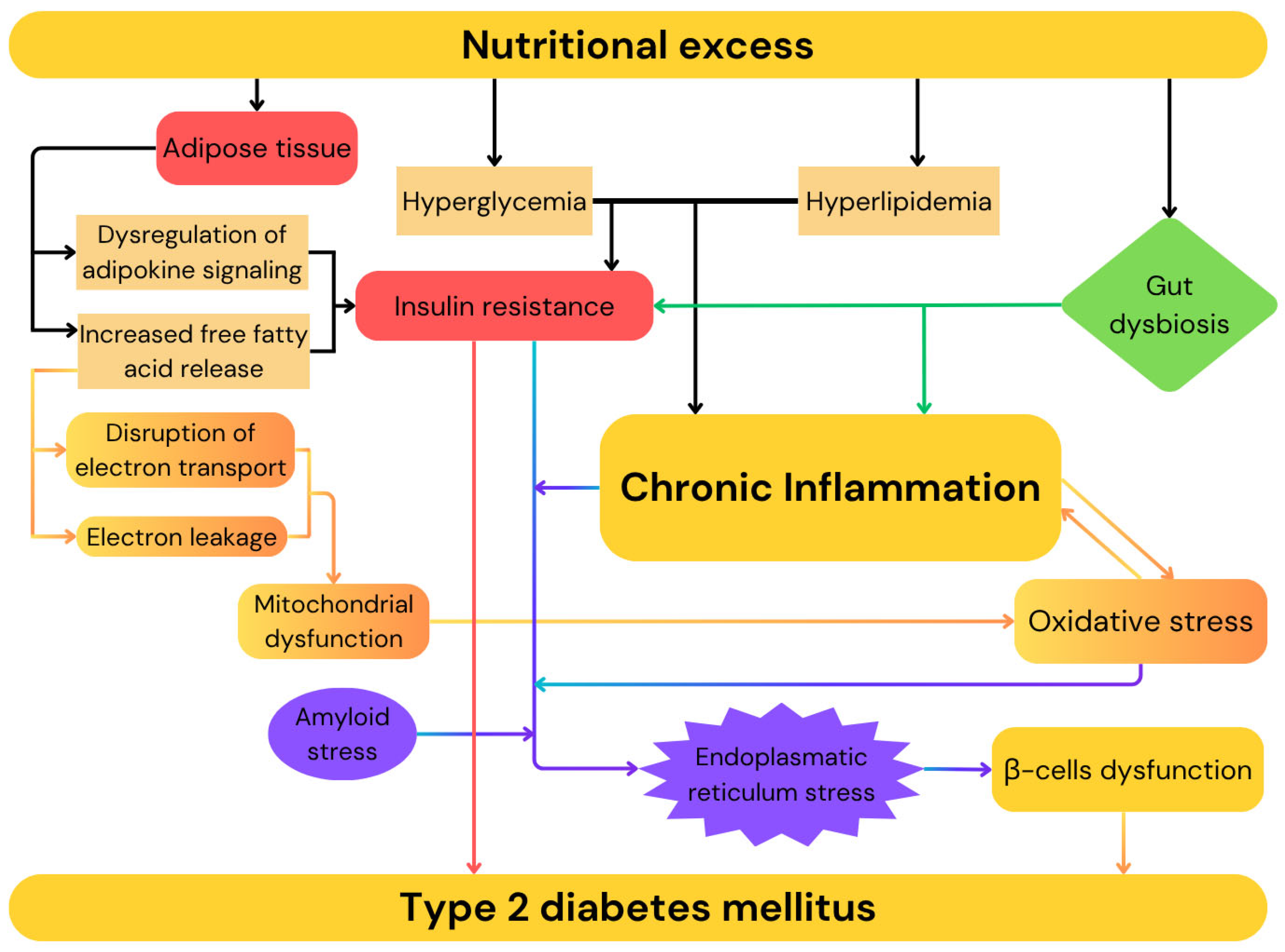
1.2. Diabetes
1.3. Cardiovascular Diseases
1.4. Galectins
1.5. Galectin-4
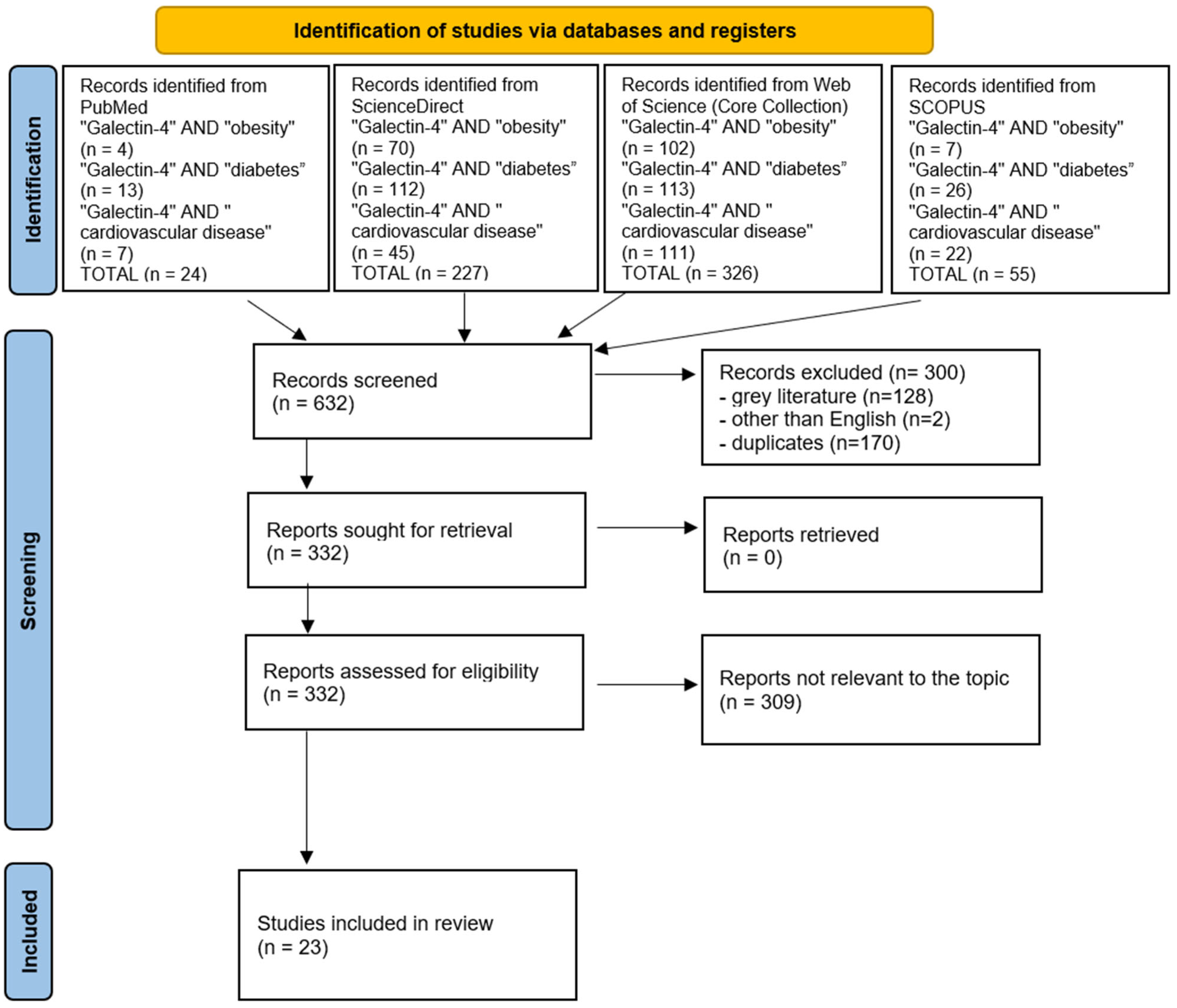
2. Methods
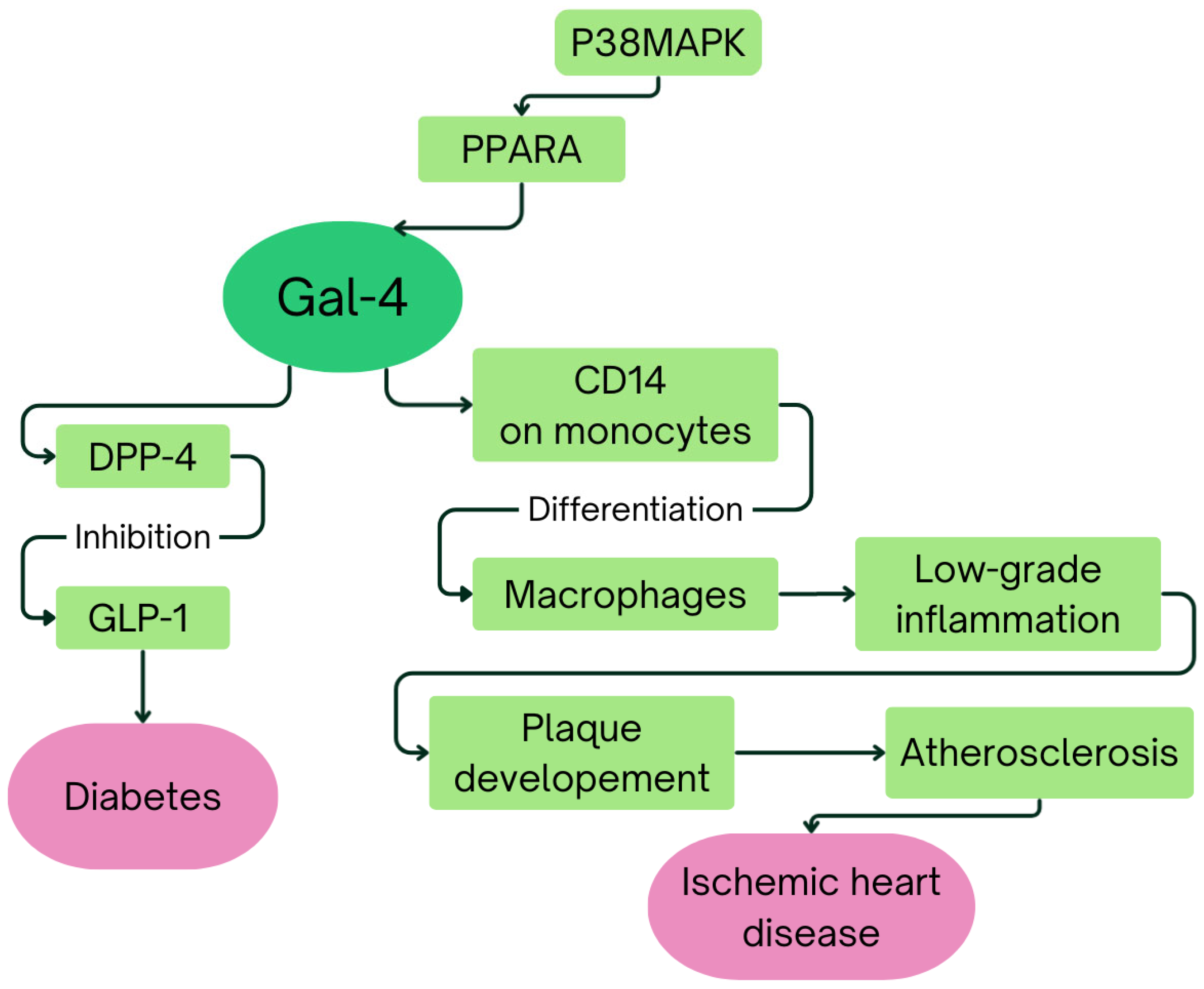
3. Galectin-4 in Obesity, Diabetes, and Cardiovascular Diseases
4. Discussion
5. Clinical Utility
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’i, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar] [CrossRef]
- Després, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Fontaine, K.R.; Redden, D.T.; Wang, C.; Westfall, A.O.; Allison, D.B. Years of life lost due to obesity. JAMA 2003, 289, 187–193. [Google Scholar] [CrossRef]
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Imaging 2012, 37, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Kadouh, H.C.; Acosta, A. Current paradigms in the etiology of obesity. Tech. Gastrointest. Endosc. 2017, 19, 2–11. [Google Scholar] [CrossRef]
- Ishida, A.; Yushuang, L.; Osami, M.; Emiko, I. Factors affecting adult overweight and obesity in urban China. Pertanika J. Soc. Sci. Humanit. 2020, 28, 503–513. [Google Scholar]
- Paolacci, S.; Borrelli, A.; Stuppia, L.; Campanile, F.C.; Dallavilla, T.; Krajčovič, J.; Veselenyiova, D.; Beccari, T.; Unfer, V.; Bertelli, M.; et al. Mendelian obesity, molecular pathways and pharmacological therapies: A review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1357–1378. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.H.; Iwakoshi, N.N.; Ozdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10 (Suppl. 2), 97S–104S. [Google Scholar] [CrossRef]
- Brandão, I.; Martins, M.J.; Monteiro, R. Metabolically Healthy Obesity-Heterogeneity in Definitions and Unconventional Factors. Metabolites 2020, 10, 48. [Google Scholar] [CrossRef]
- Majety, P.; Lozada Orquera, F.A.; Edem, D.; Hamdy, O. Pharmacological approaches to the prevention of type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1118848. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251, Erratum in Lancet 2017, 389, 2192. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef]
- Christensen, A.A.; Gannon, M. The Beta Cell in Type 2 Diabetes. Curr. Diab Rep. 2019, 19, 81. [Google Scholar] [CrossRef]
- Halban, P.A.; Polonsky, K.S.; Bowden, D.W.; Hawkins, M.A.; Ling, C.; Mather, K.J.; Powers, A.C.; Rhodes, C.J.; Sussel, L.; Weir, G.C. β-cell failure in type 2 diabetes: Postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014, 37, 1751–1758. [Google Scholar] [CrossRef]
- Yamamoto, W.R.; Bone, R.N.; Sohn, P.; Syed, F.; Reissaus, C.A.; Mosley, A.L.; Wijeratne, A.B.; True, J.D.; Tong, X.; Kono, T.; et al. Endoplasmic reticulum stress alters ryanodine receptor function in the murine pancreatic β cell. J. Biol. Chem. 2019, 294, 168–181. [Google Scholar] [CrossRef]
- Graciano, M.F.; Valle, M.M.; Kowluru, A.; Curi, R.; Carpinelli, A.R. Regulation of insulin secretion and reactive oxygen species production by free fatty acids in pancreatic islets. Islets 2011, 3, 213–223. [Google Scholar] [CrossRef]
- Li, X.; Watanabe, K.; Kimura, I. Gut Microbiota Dysbiosis Drives and Implies Novel Therapeutic Strategies for Diabetes Mellitus and Related Metabolic Diseases. Front. Immunol. 2017, 8, 1882. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Sun, T.; Huang, H.; Chen, S.; Chen, L.; Luo, C.; Yang, W.; Yang, X.; Yao, P.; Cheng, J.; et al. Association between microbiota-dependent metabolite trimethylamine-. Am. J. Clin. Nutr. 2017, 106, 888–894. [Google Scholar] [CrossRef]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Tang, C.; Ahmed, K.; Gille, A.; Lu, S.; Gröne, H.J.; Tunaru, S.; Offermanns, S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat. Med. 2015, 21, 173–177. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lui, D.T.; Lam, K.S. Non-alcoholic fatty liver disease and type 2 diabetes: An update. J. Diabetes Investig. 2022, 13, 930–940. [Google Scholar] [CrossRef]
- Sund, R.; Peltonen, T.; Lehtimäki, A.V.; Martikainen, J. Hospital treatment costs associated with incident complications in patients with type 2 diabetes-real-world study based on electronic patient information systems. BMC Health Serv. Res. 2022, 22, 469. [Google Scholar] [CrossRef]
- Cai, R.; Kong, Q.; Wang, Z.; Gao, Z.; Huo, Y. Correlation between tumor markers and type 2 diabetes mellitus complications and their related influencing factors. Ann. Palliat. Med. 2022, 11, 58–67. [Google Scholar] [CrossRef]
- Safieddine, B.; Sperlich, S.; Epping, J.; Lange, K.; Geyer, S. Development of comorbidities in type 2 diabetes between 2005 and 2017 using German claims data. Sci. Rep. 2021, 11, 11149. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2024. Diabetes Care 2024, 47 (Suppl. 1), S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Mensah, G.A.; Roth, G.A.; Fuster, V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. J. Am. Coll. Cardiol. 2019, 74, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021, Correction in J. Am. Coll. Cardiol. 2021, 77, 1958–1959. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Kim, S.J.; Mesquita, F.C.P.; Hochman-Mendez, C. New Biomarkers for Cardiovascular Disease. Tex. Heart Inst. J. 2023, 50, e238178. [Google Scholar] [CrossRef]
- Wong, Y.K.; Tse, H.F. Circulating Biomarkers for Cardiovascular Disease Risk Prediction in Patients with Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 713191. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Han, J.; Klug, M.; Kirmes, K.; Viggiani, G.; von Scheidt, M.; Schreiner, N.; Condorelli, G.; Laugwitz, K.L.; Bernlochner, I. Role of Reticulated Platelets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 527–539. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Di Fusco, S.A.; Spinelli, A.; Castello, L.; Marino, G.; Maraschi, I.; Gulizia, M.M.; Gabrielli, D.; Colivicchi, F. Do Pathophysiologic Mechanisms Linking Unhealthy Lifestyle to Cardiovascular Disease and Cancer Imply Shared Preventive Measures?—A Critical Narrative Review. Circ. J. 2024, 88, 189–197. [Google Scholar] [CrossRef]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent insights into the cellular biology of atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. The role of inflammatory cytokines in endothelial dysfunction. Basic. Res. Cardiol. 2008, 103, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Schulte, D.M.; Paulsen, K.; Türk, K.; Brandt, B.; Freitag-Wolf, S.; Hagen, I.; Zeuner, R.; Schröder, J.O.; Lieb, W.; Franke, A.; et al. Small dense LDL cholesterol in human subjects with different chronic inflammatory diseases. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 1100–1105. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Mahdinia, E.; Shokri, N.; Taheri, A.T.; Asgharzadeh, S.; Elahimanesh, M.; Najafi, M. Cellular crosstalk in atherosclerotic plaque microenvironment. Cell Commun. Signal 2023, 21, 125. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, T.; Wang, X.; Wu, M.; Zhang, R.; Yang, X.; Fu, Y.; Liu, Z. Role of the microbiota-gut-heart axis between bile acids and cardiovascular disease. Biomed. Pharmacother. 2024, 174, 116567. [Google Scholar] [CrossRef]
- Tsaneva, M.; Van Damme, E.J.M. 130 years of Plant Lectin Research. Glycoconj. J. 2020, 37, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Raposo, C.D.; Canelas, A.B.; Barros, M.T. Human Lectins, Their Carbohydrate Affinities and Where to Find Them. Biomolecules 2021, 11, 188. [Google Scholar] [CrossRef]
- Mason, C.P.; Tarr, A.W. Human lectins and their roles in viral infections. Molecules 2015, 20, 2229–2271. [Google Scholar] [CrossRef] [PubMed]
- Tobola, F.; Wiltschi, B. One, two, many: Strategies to alter the number of carbohydrate binding sites of lectins. Biotechnol. Adv. 2022, 60, 108020. [Google Scholar] [CrossRef]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef]
- Day, C.J.; Paton, A.W.; Harvey, R.M.; Hartley-Tassell, L.E.; Seib, K.L.; Tiralongo, J.; Bovin, N.; Savino, S.; Masignani, V.; Paton, J.C.; et al. Lectin activity of the pneumococcal pilin proteins. Sci. Rep. 2017, 7, 17784. [Google Scholar] [CrossRef]
- Fonseca, V.J.A.; Braga, A.L.; Filho, J.R.; Teixeira, C.S.; da Hora, G.C.A.; Morais-Braga, M.F.B. A review on the antimicrobial properties of lectins. Int. J. Biol. Macromol. 2022, 195, 163–178. [Google Scholar] [CrossRef] [PubMed]
- Haag, R. Multivalency as a chemical organization and action principle. Beilstein J. Org. Chem. 2015, 11, 848–849. [Google Scholar] [CrossRef]
- Hirabayashi, J. Application of site-directed mutagenesis to structure–function studies of carbohydrate-binding proteins. Glycosciences 1996, 355–368. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. Essentials of Glycobiology, 4th ed.Cold Spring Harbor Laboratory Press: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Blidner, A.G.; Sarrias, L.; Espelt, M.V.; Rabinovich, G.A. The universe of galectin-binding partners and their functions in health and disease. J. Biol. Chem. 2023, 299, 105400. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Quintana, J.I.; Ardá, A.; Gimeno, A.; Jiménez-Barbero, J. Targeting Galectins with Glycomimetics. Front. Chem. 2020, 8, 593. [Google Scholar] [CrossRef]
- Liu, F.T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Danielsen, E.M.; van Deurs, B. Galectin-4 and small intestinal brush border enzymes form clusters. Mol. Biol. Cell 1997, 8, 2241–2251. [Google Scholar] [CrossRef]
- Fu, Y.F.; Jiang, S.C.; Zhang, Z.W.; Yang, X.Y.; Li, Z.L.; Hu, J.; Yuan, S. Lactose and Galactose Promote the Crystallization of Human Galectin-10. Molecules 2023, 28, 1979. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.H.; Hsieh, W.C.; Lin, F.J.; Liu, F.T.; Yang, R.Y. Galectin-12 in the Regulation of Sebocyte Proliferation, Lipid Metabolism, and Immune Responses. Biomolecules 2025, 15, 837. [Google Scholar] [CrossRef]
- Wan, L.; Yang, R.Y.; Liu, F.T. Galectin-12 in Cellular Differentiation, Apoptosis and Polarization. Int. J. Mol. Sci. 2018, 19, 176. [Google Scholar] [CrossRef]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key regulators of galectin-glycan interactions. Proteomics 2016, 16, 3111–3125. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.I.; Stegmayr, J.; Grant, O.C.; Yang, Z.; Nilsson, U.J.; Boos, I.; Carlsson, M.C.; Woods, R.J.; Unverzagt, C.; Leffler, H.; et al. Galectin binding to cells and glycoproteins with genetically modified glycosylation reveals galectin-glycan specificities in a natural context. J. Biol. Chem. 2018, 293, 20249–20262. [Google Scholar] [CrossRef]
- Oda, Y.; Herrmann, J.; Gitt, M.A.; Turck, C.W.; Burlingame, A.L.; Barondes, S.H.; Leffler, H. Soluble lactose-binding lectin from rat intestine with two different carbohydrate-binding domains in the same peptide chain. J. Biol. Chem. 1993, 268, 5929–5939. [Google Scholar] [CrossRef] [PubMed]
- Huflejt, M.E.; Leffler, H. Galectin-4 in normal tissues and cancer. Glycoconj. J. 2004, 20, 247–255. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Guo, X.L. The role of galectin-4 in physiology and diseases. Protein Cell 2016, 7, 314–324. [Google Scholar] [CrossRef]
- Ideo, H.; Seko, A.; Ohkura, T.; Matta, K.L.; Yamashita, K. High-affinity binding of recombinant human galectin-4 to SO3− → 3Galbeta1 → 3GalNAc pyranoside. Glycobiology 2002, 12, 199–208. [Google Scholar] [CrossRef]
- Brewer, C.F.; Miceli, M.C.; Baum, L.G. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, E.M.; Hansen, G.H. Lipid raft organization and function in the small intestinal brush border. J. Physiol. Biochem. 2008, 64, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Stechly, L.; Morelle, W.; Dessein, A.F.; André, S.; Grard, G.; Trinel, D.; Dejonghe, M.J.; Leteurtre, E.; Drobecq, H.; Trugnan, G.; et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic 2009, 10, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Lohse, K.; Wiedenmann, B.; Dignass, A.U.; Sturm, A. Galectin-2 and -4, but not galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm. Bowel Dis. 2008, 14, 1366–1372. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Dias-Baruffi, M.; Rodrigues, L.C.; Gourdine, J.P.; Heimburg-Molinaro, J.; Ju, T.; Molinaro, R.J.; Rivera-Marrero, C.; Xia, B.; et al. Innate immune lectins kill bacteria expressing blood group antigen. Nat. Med. 2010, 16, 295–301. [Google Scholar] [CrossRef]
- Liu, F.T.; Bevins, C.L. A sweet target for innate immunity. Nat. Med. 2010, 16, 263–264. [Google Scholar] [CrossRef]
- Li, C.S.; Lo, T.H.; Tu, T.J.; Chueh, D.Y.; Yao, C.I.; Lin, C.H.; Chen, P.; Liu, F.T. Cytosolic galectin-4 enchains bacteria, restricts their motility, and promotes inflammasome activation in intestinal epithelial cells. Proc. Natl. Acad. Sci. USA 2023, 120, e2207091120. [Google Scholar] [CrossRef]
- Hokama, A.; Mizoguchi, E.; Sugimoto, K.; Shimomura, Y.; Tanaka, Y.; Yoshida, M.; Rietdijk, S.T.; de Jong, Y.P.; Snapper, S.B.; Terhorst, C.; et al. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity 2004, 20, 681–693. [Google Scholar] [CrossRef]
- Paclik, D.; Danese, S.; Berndt, U.; Wiedenmann, B.; Dignass, A.; Sturm, A. Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS ONE 2008, 3, e2629. [Google Scholar] [CrossRef]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.G. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin. Cancer Res. 2011, 17, 7035–7046. [Google Scholar] [CrossRef]
- Belo, A.I.; van der Sar, A.M.; Tefsen, B.; van Die, I. Galectin-4 Reduces Migration and Metastasis Formation of Pancreatic Cancer Cells. PLoS ONE 2013, 8, e65957. [Google Scholar] [CrossRef]
- Cai, Z.; Zeng, Y.; Xu, B.; Gao, Y.; Wang, S.; Zeng, J.; Chen, L.; Huang, A.; Liu, X.; Liu, J. Galectin-4 serves as a prognostic biomarker for the early recurrence/metastasis of hepatocellular carcinoma. Cancer Sci. 2014, 105, 1510–1517. [Google Scholar] [CrossRef]
- Hayashi, T.; Saito, T.; Fujimura, T.; Hara, K.; Takamochi, K.; Mitani, K.; Mineki, R.; Kazuno, S.; Oh, S.; Ueno, T.; et al. Galectin-4, a novel predictor for lymph node metastasis in lung adenocarcinoma. PLoS ONE 2013, 8, e81883. [Google Scholar] [CrossRef] [PubMed]
- Hippo, Y.; Yashiro, M.; Ishii, M.; Taniguchi, H.; Tsutsumi, S.; Hirakawa, K.; Kodama, T.; Aburatani, H. Differential gene expression profiles of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 2001, 61, 889–895. [Google Scholar] [PubMed]
- Rechreche, H.; Mallo, G.V.; Montalto, G.; Dagorn, J.C.; Iovanna, J.L. Cloning and expression of the mRNA of human galectin-4, an S-type lectin down-regulated in colorectal cancer. Eur. J. Biochem. 1997, 248, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Velasco, S.; Díez-Revuelta, N.; Hernández-Iglesias, T.; Kaltner, H.; André, S.; Gabius, H.J.; Abad-Rodríguez, J. Neuronal Galectin-4 is required for axon growth and for the organization of axonal membrane L1 delivery and clustering. J. Neurochem. 2013, 125, 49–62. [Google Scholar] [CrossRef]
- Wei, Q.; Eviatar-Ribak, T.; Miskimins, W.K.; Miskimins, R. Galectin-4 is involved in p27-mediated activation of the myelin basic protein promoter. J. Neurochem. 2007, 101, 1214–1223. [Google Scholar] [CrossRef]
- Stancic, M.; Slijepcevic, D.; Nomden, A.; Vos, M.J.; de Jonge, J.C.; Sikkema, A.H.; Gabius, H.J.; Hoekstra, D.; Baron, W. Galectin-4, a novel neuronal regulator of myelination. Glia 2012, 60, 919–935. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Dieden, A.; Gudmundsson, P.; Korduner, J.; Molvin, J.; Zaghi, A.; Nezami, Z.; Bachus, E.; Holm, H.; Jujic, A.; Magnusson, M. Galectin-4 is associated with diabetes and obesity in a heart failure population. Sci. Rep. 2023, 13, 20285. [Google Scholar] [CrossRef]
- Korduner, J.; Holm, H.; Jujic, A.; Melander, O.; Pareek, M.; Molvin, J.; Råstam, L.; Lindblad, U.; Daka, B.; Leosdottir, M.; et al. Galectin-4 levels in hospitalized versus non-hospitalized subjects with obesity: The Malmö Preventive Project. Cardiovasc. Diabetol. 2022, 21, 125. [Google Scholar] [CrossRef]
- Molvin, J.; Pareek, M.; Jujic, A.; Melander, O.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Using a Targeted Proteomics Chip to Explore Pathophysiological Pathways for Incident Diabetes- The Malmö Preventive Project. Sci. Rep. 2019, 9, 272. [Google Scholar] [CrossRef]
- Tromp, J.; Voors, A.A.; Sharma, A.; Ferreira, J.P.; Ouwerkerk, W.; Hillege, H.L.; Gomez, K.A.; Dickstein, K.; Anker, S.D.; Metra, M.; et al. Distinct Pathological Pathways in Patients with Heart Failure and Diabetes. JACC Heart Fail. 2020, 8, 234–242. [Google Scholar] [CrossRef]
- Beijer, K.; Nowak, C.; Sundström, J.; Ärnlöv, J.; Fall, T.; Lind, L. In search of causal pathways in diabetes: A study using proteomics and genotyping data from a cross-sectional study. Diabetologia 2019, 62, 1998–2006. [Google Scholar] [CrossRef]
- Molvin, J.; Jujić, A.; Melander, O.; Pareek, M.; Råstam, L.; Lindblad, U.; Daka, B.; Leósdóttir, M.; Nilsson, P.M.; Olsen, M.H.; et al. Proteomic exploration of common pathophysiological pathways in diabetes and cardiovascular disease. ESC Heart Fail. 2020, 7, 4151–4158. [Google Scholar] [CrossRef]
- Delacour, D.; Gouyer, V.; Zanetta, J.P.; Drobecq, H.; Leteurtre, E.; Grard, G.; Moreau-Hannedouche, O.; Maes, E.; Pons, A.; André, S.; et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J. Cell Biol. 2005, 169, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Maiseyeu, A.; Davis, S.N.; Rajagopalan, S. DPP4 in cardiometabolic disease: Recent insights from the laboratory and clinical trials of DPP4 inhibition. Circ. Res. 2015, 116, 1491–1504. [Google Scholar] [CrossRef]
- Schrader, S.; Unverdorben, L.; Hutter, S.; Knabl, J.; Schmoeckel, E.; Meister, S.; Zehni, A.Z.; Beyer, S.; Vilsmaier, T.; Mahner, S.; et al. Overexpression of galectin-4 in placentas of women with gestational diabetes. J. Reprod. Immunol. 2022, 151, 103629. [Google Scholar] [CrossRef] [PubMed]
- Elhadad, M.A.; Del C Gómez-Alonso, M.; Chen, C.W.; Neumeyer, S.; Delerue, T.; Rathmann, W.; Näbauer, M.; Meisinger, C.; Kääb, S.; Seissler, J.; et al. Plasma proteome association with coronary heart disease and carotid intima media thickness: Results from the KORA F4 study. Cardiovasc. Diabetol. 2024, 23, 181. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, C.S.; Lee, S.; Park, J.B.; Kim, H.K.; Park, S.J.; Kim, Y.J.; Lee, S.P. Systemic proinflammatory-profibrotic response in aortic stenosis patients with diabetes and its relationship with myocardial remodeling and clinical outcome. Cardiovasc. Diabetol. 2023, 22, 30. [Google Scholar] [CrossRef]
- Schroder, J.; Mygind, N.D.; Frestad, D.; Michelsen, M.; Suhrs, H.E.; Bove, K.B.; Gustafsson, I.; Kastrup, J.; Prescott, E. Pro-inflammatory biomarkers in women with non-obstructive angina pectoris and coronary microvascular dysfunction. Int. J. Cardiol. Heart Vasc. 2019, 24, 100370. [Google Scholar] [CrossRef]
- Garcia, T.; Petrera, A.; Hauck, S.M.; Baber, R.; Wirkner, K.; Kirsten, H.; Pott, J.; Tönjes, A.; Henger, S.; Loeffler, M.; et al. Relationship of proteins and subclinical cardiovascular traits in the population-based LIFE-Adult study. Atherosclerosis 2024, 398, 118613. [Google Scholar] [CrossRef]
- Dieden, A.; Girerd, N.; Ottosson, F.; Molvin, J.; Pareek, M.; Melander, O.; Bachus, E.; Råstam, L.; Lindblad, U.; Daka, B.; et al. Proteomic biomarkers and pathway analysis for progression to heart failure in three epidemiological representative cohorts. Eur. J. Heart Fail. 2024. [Google Scholar] [CrossRef] [PubMed]
- Rullman, E.; Melin, M.; Mandić, M.; Gonon, A.; Fernandez-Gonzalo, R.; Gustafsson, T. Circulatory factors associated with function and prognosis in patients with severe heart failure. Clin. Res. Cardiol. 2020, 109, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ferreira, D.; Diaz, S.O.; Ferreira, J.P.; Girerd, N.; Pellicori, P.; Mariottoni, B.; Cosmi, F.; Hazebroek, M.; Verdonschot, J.A.J.; Cuthbert, J.; et al. Phenotyping patients with ischaemic heart disease at risk of developing heart failure: An analysis of the HOMAGE trial. ESC Heart Fail. 2024, 11, 209–218. [Google Scholar] [CrossRef]
- Ljungberg, J.; Janiec, M.; Bergdahl, I.A.; Holmgren, A.; Hultdin, J.; Johansson, B.; Näslund, U.; Siegbahn, A.; Fall, T.; Söderberg, S. Proteomic Biomarkers for Incident Aortic Stenosis Requiring Valvular Replacement. Circulation 2018, 138, 590–599. [Google Scholar] [CrossRef]
- Bouwens, E.; Brankovic, M.; Mouthaan, H.; Baart, S.; Rizopoulos, D.; van Boven, N.; Caliskan, K.; Manintveld, O.; Germans, T.; van Ramshorst, J.; et al. Temporal Patterns of 14 Blood Biomarker candidates of Cardiac Remodeling in Relation to Prognosis of Patients with Chronic Heart Failure-The Bio- SH i FT Study. J. Am. Heart Assoc. 2019, 8, e009555. [Google Scholar] [CrossRef] [PubMed]
- Shavadia, J.S.; Alemayehu, W.; deFilippi, C.; Westerhout, C.M.; Tromp, J.; Granger, C.B.; Armstrong, P.W.; van Diepen, S. Novel multi-marker proteomics in phenotypically matched patients with ST-segment myocardial infarction: Association with clinical outcomes. J. Thromb. Thrombolysis 2022, 53, 841–850. [Google Scholar] [CrossRef]
- Jujic, A.; Vieira, J.P.P.; Matuskova, H.; Nilsson, P.M.; Lindblad, U.; Olsen, M.H.; Duarte, J.M.N.; Meissner, A.; Magnusson, M. Plasma Galectin-4 Levels Are Increased after Stroke in Mice and Humans. Int. J. Mol. Sci. 2023, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Gao, D.; Shi, W.; Zhao, Y.; Guo, Z.; Chen, X.; Li, W.; Liu, K.J.; Yang, J.; Zhang, Y.; et al. The Association of Serum Biomarkers with Symptomatic Hemorrhagic Transformation in Acute Ischemic Stroke Patients: A Combined Retrospective and Prospective Study. CNS Neurosci. Ther. 2025, 31, e70321. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Zethner-Moller, R.; Bové, K.B.; Mygind, N.D.; Hasbak, P.; Michelsen, M.M.; Gustafsson, I.; Kastrup, J.; Prescott, E. Protein biomarkers and coronary microvascular dilatation assessed by rubidium-82 PET in women with angina pectoris and no obstructive coronary artery disease. Atherosclerosis 2018, 275, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Toribio, M.; Fitch, K.V.; Stone, L.; Zanni, M.V.; Lo, J.; de Filippi, C.; Sponseller, C.A.; Lee, H.; Grundberg, I.; Thompson, M.A.; et al. Assessing statin effects on cardiovascular pathways in HIV using a novel proteomics approach: Analysis of data from INTREPID, a randomized controlled trial. eBioMedicine 2018, 35, 58–66. [Google Scholar] [CrossRef]
| Condition | Fasting Plasma Glucose (FPG) | Oral Glucose Tolerance Test (OGTT) | Glycated Hemoglobin (HbA1C) | Random Plasma Glucose |
|---|---|---|---|---|
| Diabetes | ≥126 mg/dL | 2 h Plasma Glucose ≥ 200 mg/dL | ≥6.5% | Classic symptoms of hyperglycemia and plasma glucose ≥ 200 mg/dL |
| Prediabetes | 100–125 mg/dL | 2 h Plasma Glucose 140–199 mg/dL | 5.7–6.4% | - |
| Galectin | Gene Symbol | Carbohydrate Preferential Affinity (β-d-Galactosides) | Organs Protein Expression |
|---|---|---|---|
| Galectin 1 | LGALS1 | LacNAc, poly-LacNAc, sulfated glycans | Bone marrow, brain, cervix, endometrium, lymph node, ovary, parathyroid gland, placenta, smooth muscle, skin, spleen, testis, tonsil, and vagina |
| Galectin 2 | LGALS2 | LacNAc, poly-LacNAc, lactose | Appendix, colon, duodenum, gallbladder, kidney, liver, lymph node, pancreas, rectum, small intestine, spleen, and tonsil |
| Galectin 3 | LGALS3 | LacNAc, poly-LacNAc, sulfated glycans, sialylated glycans | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, and skin |
| Galectin 3 binding protein | LGALS3BP | LacNAc, poly-LacNAc, lactose | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, proximal digestive tract, and skin |
| Galectin 4 | LGALS4 | LacNAc, lactose, sulfated glycans | Appendix, colon, duodenum, gallbladder, pancreas, rectum, small intestine, stomach, and neuronal cells |
| Galectin 7 | LGALS7 | LacNAc, sulfated glycans | Cervix (uterine), esophagus, oral mucosa, salivary gland, skin, tonsil, and vagina |
| Galectin 8 | LGALS8 | LacNAc, poly-LacNAc, 3′-O-sialylated and 3′-O-sulfated glycans | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, and skin |
| Galectin 9 | LGALS9 | LacNAc, poly-LacNAc, lactose, N-acetyl-LacNAc, fucosylated glycans | Adipose and soft tissue, bone marrow and lymphoid tissues, brain, endocrine tissues, female tissues, gastrointestinal tract, kidney and urinary bladder, lung, male tissues, muscle tissues, pancreas, proximal digestive tract, and skin |
| Galectin 9B | LGALS9B | LacNAc, poly-LacNAc | Appendix, bone marrow, breast, lymph node, spleen, and tonsil |
| Galectin 9C | LGALS9C | LacNAc, poly-LacNAc | Appendix, bronchus, colon, duodenum, gallbladder, lung, pancreas, spleen, stomach, and tonsil |
| Galectin 10 | LGALS10 | N-acetyl-d-glucosamine, d-mannose, weak to lactose | Lymph node, spleen, and tonsil |
| Galectin 12 | LGALS12 | β-d-galactose, lactose | Adipose tissue, low levels in heart, pancreas, spleen, and thymus |
| Galectin 13 | LGALS13 | LacNAc, N-acetyl-LacNAc, mannose, N-acetyl-galactosamine | Kidney, placenta, spleen, and urinary bladder |
| Placental Protein 13 (Galectin 14) | LGALS14 | N-acetyl-LacNAc, sulfated glycans | Adrenal gland, colon, and kidney |
| Galectin 16 | LGALS16 | N-acetyl-LacNAc, β-d-galactose, lactose | Placenta |
| GAL-4 Summary | Obesity | Diabetes | Cardiovascular Diseases |
|---|---|---|---|
| Available Studies | 1. HeArt and bRain failure inVESTigation trial (HARVEST- Malmö) [93]. 2. Malmö Preventive Project Re-Examination Study (MPP-RES) [94]. | 1. HeArt and bRain failure inVESTigation trial (HARVEST- Malmö) [93]. 2. Malmö Preventive Project Re-Examination Study (MPP-RES) [94,95,98]. 3. A Systems Biology Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT-CHF) [96]. 4. EpiHealth cohort [97]. 5. Other studies [101]. | 1. HeArt and bRain failure inVESTigation trial (HARVEST- Malmö) [93]. 2. Malmö Preventive Project Re-Examination Study (MPP-RES) [94,98,106] 3. A Systems Biology Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT-CHF) [96]. 4. KORA F4 cohort [102]. 5. LIFE-Adult study [105]. 6. STANISLAS cohort [106]. 7. HOMAGE case–control cohort [106,108]. 8. Other studies [103,104,107,109,110,111,112,113,114,115]. |
| GAL-4 Concentrations | 1. Among middle-aged and older obese individuals elevated Galectin-4 concentrations correlate with an increased probability of prior hospitalization [94]. 2. Elevated Galectin-4 concentrations correlate with higher likelihood of obesity among heart failure patients [93]. | 1. Galectin-4 levels are significantly elevated in patients with prevalent and incident diabetes compared to healthy individuals [93,94,95,96,97]. 2. Galectin-4 levels are significantly elevated in the nuclei of syncytiotrophoblasts and in both nuclei and cytoplasm of decidual cells in placentas from women with gestational diabetes mellitus [101]. | Elevated Galectin-4 concentrations are observed in patients with: 1. Coronary heart disease [93,102,108]. 2. Diabetic aortic stenosis [103]. 3. Heart failure [93]. 4. Coronary microvascular dysfunction [104]. 5. Carotid plaques [105]. 6. Ischemic stroke [112]. 7. Symptomatic intracranial hemorrhagic transformation [113]. |
| Additional Information | Both associations were statistically significant only among individuals with diabetes, meaning that the relationship between elevated Galectin-4 levels and an increased risk of obesity or prior hospitalization is primarily driven by the presence of diabetes [93,94]. | No significant differences in Galectin-4 concentrations have been observed across diabetic patient subgroups stratified by age, sex, BMI, hypertension status, estimated glomerular filtration rate, or type of antidiabetic therapy, including both oral medication and insulin [96]. | In patients with stable chronic heart failure, Galectin-4 emerged as one of several biomarkers that exhibited a marked and progressive increase preceding adverse clinical events. Baseline levels of Galectin-4, ST2, GDF-15, perlecan, and cystatin B were already significantly elevated in patients who later reached the primary endpoint [110]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozak, K.; Zajkowska, M. Association of Elevated Galectin-4 Concentrations with Obesity, Diabetes, and Cardiovascular Diseases. Int. J. Mol. Sci. 2025, 26, 9402. https://doi.org/10.3390/ijms26199402
Kozak K, Zajkowska M. Association of Elevated Galectin-4 Concentrations with Obesity, Diabetes, and Cardiovascular Diseases. International Journal of Molecular Sciences. 2025; 26(19):9402. https://doi.org/10.3390/ijms26199402
Chicago/Turabian StyleKozak, Krystian, and Monika Zajkowska. 2025. "Association of Elevated Galectin-4 Concentrations with Obesity, Diabetes, and Cardiovascular Diseases" International Journal of Molecular Sciences 26, no. 19: 9402. https://doi.org/10.3390/ijms26199402
APA StyleKozak, K., & Zajkowska, M. (2025). Association of Elevated Galectin-4 Concentrations with Obesity, Diabetes, and Cardiovascular Diseases. International Journal of Molecular Sciences, 26(19), 9402. https://doi.org/10.3390/ijms26199402







